Graphical abstract
Keywords: Black tea, Rolling pressure, Untargeted metabolomics, Catechins, Flavonol/flavone glycosides, Phenolic acids, Tea taste
Highlights
-
•
The effect of rolling pressure on black tea quality was systematically studied.
-
•
The metabolic profiles were fluctuated as the rolling pressure increased.
-
•
Forty-seven most prominently altered metabolites were identified.
-
•
Rolling pressure affect the enzymatic oxidation degree of phenolics and amino acids.
-
•
An appropriate rolling pressure is beneficial to black tea taste and liquor color.
Abstract
Rolling represents an essential stage in congou black tea processing. However, the influence of rolling pressure on tea flavor and non-volatile compounds remains unclear. Herein, a combination of untargeted metabolomics, tea pigments quantification, E-tongue, colorimeter and sensory evaluation was used to evaluate the effect of rolling pressure on black tea quality. As the rolling pressure increased, theaflavins (TFs), thearubigins (TRs), and theabrownins (TBs) significantly elevated. The tea metabolic profiles fluctuated and 47 metabolites were identified as key differential metabolites including flavan-3-ols, flavonol/flavone glycosides, phenolic acids, amino acids. These substances altered possibly due to the variations in enzymatic oxidation of tea phenolics and amino acids. Overall, black tea with moderate rolling pressure presented higher sweetness, lower bitterness, and higher quality index (10 TFs + TRs)/TBs. The results were verified by a validation batch. This study provided new insights into the regulation of rolling pressure and a guidance for black tea processing.
1. Introduction
Congou black tea is a unique and traditional tea type derived from China, which is manufactured using tea shoots or leaves (Camellia sinensis L.) through complicated post-harvest processing (plucking, withering, rolling, fermentation, and drying) (Hua et al., 2021). Instead of breaking into small granular particles like the crush-tear-curl (CTC) black tea, the leaves of congou black tea are tight, thin and strip-sharped after processing. High-quality congou black tea imparts reddish-brown and wiry appearance, sweet-like aroma, orange-red and bright liquor color, and sweet mellow-like taste (J. Li et al., 2019). Congou black tea is rich in multiple beneficial phytochemicals including flavan-3-ols, flavonol/flavone glycosides, amino acids, phenolic acids, organic acids, and other components, which show a variety of health benefits in humans such as antioxidant, anti-cancer, anti-microbial, anti-inflammatory, inhibiting-obesity properties, and cardioprotective effect (Sharma & Rao, 2009). Because of these remarkable characteristics, congou black tea has been popularly favored by consumers around the world. The flavor quality of finished black tea largely relies on several factors during tea processing, i.e., the tenderness of fresh tea leaves (plucking), the dehydration of tea leaves (withering), the deconstruction of leave tissues (rolling), the enzyme-driven reactions (fermentation), and the termination of biochemical conversions (drying) (Bortolini, Windson Isidoro Haminiuk, Cristina Pedro, de Andrade Arruda Fernandes, & Maria Maciel, 2021). Given the importance of tea processing, an in-depth understanding of the influences of different manufacturing technologies on black tea quality is significant to the quality control and fundamental research in the field of black tea processing.
Substantial studies have been conducted in this context. These studies mostly concentrated on the main biochemical changes and their influences on the flavor quality in the crucial stages of black tea processing. Among all manufacturing stages, fermentation is usually regarded as the decisive stage, because most significant chemical transformations occur in this step. There has been a large number of researches focusing on the regulation of fermentation technology, such as fermentation time (Qu, Zeng, Tong, Feng, Chen, & Ni, 2020), fermentation temperature (Zhu et al., 2022), and fermentation humidity (Saikia, Boruah, & Sarma, 2015). Besides, other steps such as withering and drying have also been studied extensively (Omiadze et al., 2014, Ye et al., 2021, Essiedu et al., 2023). In contrast, studies focusing on the step of rolling are relatively limited.
Rolling is the initial stage of fermentation, which is not only a physical but also a chemical event occurring in the congou black tea production. The two primary objectives of rolling are to mix the biochemical compounds with enzymes adequately, as well as to shape the tea leaves into tight and curled strips (Jia, Zhang, Yuan, Chen, & Chen, 2021). Before rolling, the substrates such as phenolic compounds are mainly located in the vacuoles while oxidases are presented in the cytoplasm, thereby the enzyme-mediated oxidation reaction of polyphenols are hardly proceeded due to the spatial separation (Chiang, Yang, Wang, & Chen, 2022). Upon rolling, the tea leaves are twisted and crushed, the cell sap are squeezed out and coat on the leaves surface, leading to the adequate mixing of substrates with enzymes and promoting the subsequent biochemical reactions. In the manufacturing of Chinese congou black tea, a rolling apparatus is generally adopted, which has a circle steel table with many pleats and on top of it there’s a bucket covered with a press cap (Jia et al., 2021). The withered tea leaves are rolled in this equipment with an adjustable rolling pressure and frequency for a required time period, until the leaves are twisted and the tissues are disrupted to a desired degree. Generally speaking, an appropriate setting of rolling parameters enables a moderate cellular deconstruction, which is a prerequisite for the fermentation and biochemical transformations during tea processing and positively affects the black tea quality.
So far, only a few studies have been reported on the rolling techniques in black tea processing. The effect of different rolling systems including the orthodox, the CTC, and their effects on the tea biochemical components have been studied (Das and Datta, 2018, Ozdemir et al., 2017). Wu et al., 2022, Wu et al., 2022) investigated the influences of various rolling times and frequencies on the flavor substances and taste, color qualities of black tea, and explored the parameters which rendered the optimal sensory quality. Zhu et al. (2017) reported that rolling at a low temperature (20 ± 2℃) could retain the activity of polyphenol oxidase (PPO) and peroxidase (POD) to the maximum extent, which can be used as an effective technology to improve black tea quality. However, the rolling pressure, a vital parameter which significantly affect the cellular deconstruction degree, has still not been carefully explored.
The metabolomics analysis using liquid chromatography coupled to mass spectrometry (LC-MS) enables the comprehensive qualitative and quantitative determination of metabolites in tea (J. Li et al., 2022). The high-performance liquid chromatography (HPLC) is usually used for the quantitative analysis of major compounds in tea by referring to the authentic standards (J. Li et al., 2019). A comprehensive evaluation of sensory indicators of tea (appearance, color, taste) is thought to be reliable by a combination of human sensory assessment, E-tongue, and chromatic differences measurement. At present, an integrated analytical approach of biochemical analysis and sensory indicator evaluation has been widely performed in the field of tea processing and quality control research (Li et al., 2020, Li et al., 2022, Wu et al., 2022).
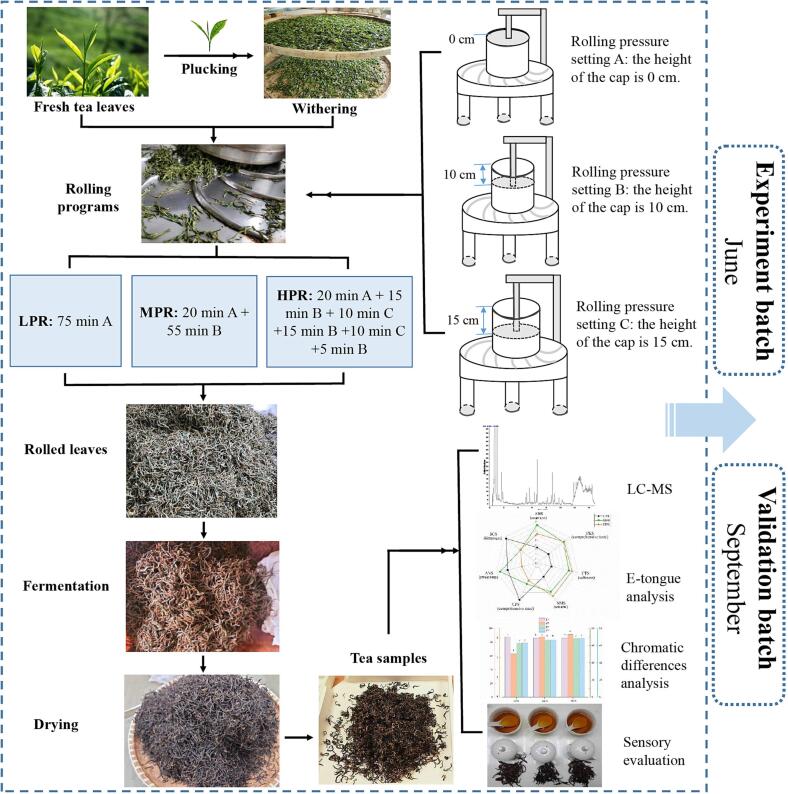
The aim of this study was to unravel the variations of non-volatile compounds and flavor characteristics of congou black tea in response to the treatments of different rolling pressures. To this end, black tea samples were manufactured using three different rolling pressures, i.e., light-pressure rolling (LPR), moderate-pressure rolling (MPR), and heavy-pressure rolling (HPR). Comprehensive molecular and sensory evaluation was performed by a combination of LC-MS based untargeted metabolomics, tea pigments quantification analysis, panelist sensory evaluation, E-tongue response, and chromatic differences analysis. To ensure the reliability, independent experiment and validation batch were conducted, respectively. The study design and workflow are illustrated in Fig. 1.
Fig. 1.
The study design and workflow.
2. Materials and methods
2.1. Chemicals and reagents
E-tongue correction diagnostic solution was obtained from Evans Technology Co., Ltd (Tianjin, China). Oxalic acid and sodium bicarbonate (analytical pure) were purchased from Maclin Biochemical Technology Co., Ltd (Shanghai China). Ice acetic acid (chromatographic pure), 95 % ethanol, n-butanol (analytical pure) and ethyl acetate (superior grade pure) were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Formic acid (chromatographic pure) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol and acetonitrile (chromatographic pure) were obtained from Merck (Darmstadt, Germany). Authentic standards were acquired from Yuanye bio-technology (Shanghai, China), J&K scientific (Beijing, China), or ZZBIO (Shanghai, China). Detailed information of the standards is provided in Table S1.
2.2. Sample preparation
Fresh leaves of C. sinensis cv. Jiukeng (one bud and two leaves) were plucked from tea garden in Shengzhou, Zhejiang Province, China (30◦30′N, 120◦10′E). The tea plant variety has been authenticated by the Tea Research Institute, Chinese Academy of Agricultural Sciences. The harvested tea leaves were spread and withered until the moisture content of tea leaves was lowered to about 60 %. Then the withered tea leaves were divided into three equal portions (7 kg) and treated with three different rolling pressure programs (LPR, MPR, HPR) in an automatic rolling machine (6CRN-35, Xiangfeng Machinery Co. Ltd., China), respectively. The rolling parameters were pre-set as shown in Fig. 1. First, three levels of rolling pressure were set by adjusting the height of the press cap: 0 cm (A), 10 cm (B), 15 cm (C). By setting the height as 0 cm (A), the cap just touched the tea leaves and no pressure was exerted. As the height increased to 10 cm (B) and 15 cm (C), the press of the cap on tea leaves was increasingly tight and heavy, and the rolling pressure was accordingly increased. Then, the three different rolling pressure programs with same time (75 min) and frequency (45 rpm) were set as the combinations of setting A, B, and C: 75 min A (LPR), 20 min A + 55 min B (MPR), 20 min A + 15 min B + 10 min C + 15 min B + 10 min C + 5 min B (HPR). After rolling, the leaves were placed in an environment-controlled cabin with a temperature of 28 ℃, and relative humidity of 90 % for 3 h. Next, the fermented tea leaves were firstly dried at 110 ℃ until the moisture level reached to 20 ∼ 25 %, and then dried at 90 ℃ until the moisture content was reduced to about 5 %, as described in previous studies (Hua et al., 2021, Zhu et al., 2022, Wu et al., 2022, Wang et al., 2022). The detailed workflow of black tea processing using three different rolling pressure programs is schematically shown in Fig. 1.
Two independent batches of black tea processing including experiment and validation batch were performed following the same procedure in June and September, respectively.
2.3. Human sensory evaluation
The sensory evaluation was conducted by five certificated assessors according to the China National Methodology for Tea Sensory Evaluation (GB/T 23776–2018). All tea samples were presented in a random order and all assessors had no prior knowledge of tea samples. First, 200 g of each tea sample was put into a white square plate for the dry tea appearance evaluation. Second, 3 g of each tea sample was put into a white porcelain cup and brewed with 150 mL boiled water for 5 min. Then, the tea infusion was filtered into a porcelain bowl for the evaluation of liquor color, aroma, and taste. Next, the infused leaf was placed into a white enamel plate for the evaluation of infused leaf appearance. The final score of each sensory factor was obtained from the average of the scores given by all reviewers. The overall quality score was calculated according to a 100-point system: total quality score (100 %) = dry tea appearance (25 %) + liquor color (10 %) + aroma (25 %) + taste (30 %) + infused leaf appearance (10 %). The informed content was obtained for all assessors. The sensory evaluation was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.4. Color measurement of tea liquor
A colorimeter apparatus (CM-5, Konica Minolta (China) Investment Co., Ltd.) was used to analyze the chromatic differences of tea liquors. The chromatic system contains four color indexes, i.e., L*, a*, b*, and C*, representing the luminance (bright: 100, dark: 0), red-green degree (red: +, green: −), yellow-blue degree (yellow: +, blue: -), and color saturation (saturated: 100, unsaturated: 0), respectively. Each tea sample was brewed twice according to the method in section 2.3. Deionized water was used as a blank. Each tea infusion was measured for three times.
2.5. E-tongue-based taste evaluation
The taste profiling of tea infusion was estimated by an E-tongue system (α-Astree II, Alpha MOS, Toulouse, France). The apparatus was equipped with seven sensors including AHS, NMS, CTS, ANS, SCS, PKS and CPS, which allowed to characterize the sourness, umami, saltiness, sweetness, bitterness, and comprehensive taste in tea infusion, respectively. The measurement was carried out according to a previous study (Wu et al., 2022b). Every black tea sample was brewed twice as the section 2.3. And each tea liquor was tested with three technical replicates.
2.6. The quantitative determination of tea pigments
The quantification of tea pigments (TFs, TRs, and TBs) was performed as previously described (Wang et al., 2022). Briefly, first, each tea sample (3 g) was extracted with 125 mL of boiling water for 10 min in a boiling bath. Second, 30 mL of ethyl acetate was added into 30 mL of tea extract in a separatory funnel followed by shaking, and the ethyl acetate layer and water layer were separated and acquired. Then, 2 mL of ethyl acetate layer was diluted using 95 % ethanol to a volume of 25 mL to obtain solution A. 2 mL of water layer was diluted using saturated oxalic acid, pure water and 95 % ethanol (v/v) to a volume of 25 mL to obtain solution B. And then, 15 mL of 2.5 % sodium bicarbonate (v/v) was added into 15 mL of ethyl acetate layer in a separatory funnel. After shaking and layering, 4 mL of ethyl acetate layer was retained and diluted using 95 % ethanol (v/v) to a volume of 25 mL to obtain solution C. For solution D, 15 mL of n-butanol was added to 15 mL of the tea extract in a separatory funnel. After shaking and layering, 2 mL of water layer was retained and diluted using saturated oxalic acid, pure water and 95 % ethanol (v/v) to a volume of 25 mL to obtain solution D. The absorbance of AA, AB, AC, and AD of four solutions were acquired using a UV–visible spectrophotometer at 380 nm. The contents of TFs, TRs, and TBs were calculated according to the equations: TFs (%) = AC × 2.25; TRs (%) = 7.06 × (2AA + 2AB - AC − 2AD); TBs (%) = 2 × 7.06 × AD.
The determination of four major TFs compositions, comprising theaflavin (TF), theaflavin-3-gallate (TF-3-G), theaflavin-3′-gallate (TF-3′-G), and theaflavin-3,3′-digallate (TF-D-G), were performed using an HPLC system (LC-20A, Shimadzu, Kyoto, Japan) as previously described (Li et al., 2019). A Waters-C18 column (4.6 mm × 250 mm, 5 µm) at 35 ℃ was used for the separation. Ultrapure water with 2 % acetic acid (v/v) and 100 % acetonitrile (v/v) were used as the mobile phase A and B, respectively. The detection wavelength was set to 380 nm. The quantification was performed using calibration curves of standards.
2.7. LC-MS based metabolomics analysis
Tea samples were fully crushed and then 10.0 ± 0.1 mg of grounded power was used for extraction. The non-volatile compounds were extracted using 70 % methanol solution (v/v). The detailed methods referred to an early study (Li et al., 2020). Three replicates were performed for each sample.
The untargeted metabolomics was conducted using an ultra-high performance liquid chromatography (UHPLC) system coupled to Q-Exactive mass spectrometry (Q-Exactive/MS) (Thermo Fisher, CA, USA) as previously reported (Li et al., 2020, Li et al., 2022). Chromatographic separation was achieved by an ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm, Waters, MA, USA) using a gradient elution program. The ultrapure water containing 0.1 % formic acid (v/v), and acetonitrile containing 0.1 % formic acid (v/v) were used as the mobile phase A and B, respectively. High resolution mass spectrometry data were collected in positive and negative mode by full scan mode with mass-to-charge ratio (m/z) range as 100–1000. MS/MS fragmentation was acquired using the higher energy collisional dissociation with an energy of 40 eV. To ensure the stability of the LC-MS working, the samples of quality control were executed from pooled samples and were analyzed every 10 samples in the whole running batch. Metabolites were structurally or tentatively identified based on exact mass measurement (mass error < 5 ppm), MS/MS fragments, and authentic standards confirmation.
2.8. Data processing and statistical analysis
Ion feature extraction and peak alignment was performed using XCMS (version 3.4.1) software to generate a data file including the information of retention time (RT), m/z, and intensity of all detected ion peaks. The obtained data matrix was normalized to the total ion intensities (as relative abundance of the ions) and were processed using 80 % rule to exclude missing values subsequently. Then, QC samples were applied to evaluate the reproducibility of the data and ions with RSDs less than 20 % were used for the further analysis (J. Li et al., 2022). The partial least squares-discriminant analysis (PLS-DA) and variable importance in the projection (VIP) were performed using the SIMCA-P 14.1 software (Umetrics, Sweden). One-way analysis of variance (one Way-ANOVA, LSD multiple test) was conducted by SPSS 19.1 software (IBM, USA), with p < 0.05 as significant. Heat-map analysis was achieved by TBtools (Toolbox for biologists).
3. Results and discussion
3.1. Rolling pressure influenced the tea sensory quality, especially liquor color and taste
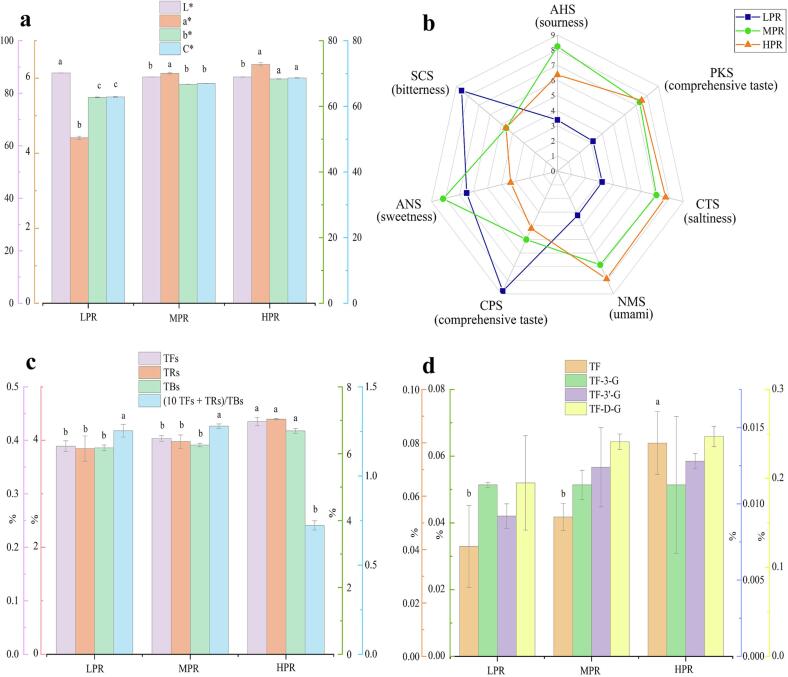
Different rolling pressure treatments demonstrated various effects on the sensory quality of congou black tea, especially reflected in the taste and liquor color (Table S2). Congou black tea treated by MPR exhibited a sweet mellow-like taste with highest score, followed by LPR, while the tea treated with HPR presented an unsatisfied flavor. In terms of liquor color, MPR-treated tea showed a bright orange-red liquor with highest score, followed by HPR and LPR. The results indicated that black tea treated with moderate rolling pressure exhibited the best flavor quality. In order to further characterize the sensory changes, the chromatic differences and E-tongue analysis were performed.
Compared to LPR, the congou black tea produced by HPR and MPR showed significantly higher values of a* (redness), b* (yellowness) and C* (color saturation), which were steadily rose along with the increased pressure. However, the value of L* (luminance) was significantly reduced with the upward rolling pressure (Fig. 2a). Therefore, we speculated that the heavier rolling pressure resulted in massive formation of tea pigments, which enabled the tea liquor to present lower value of luminance, and higher values of redness, yellowness, and color saturation.
Fig. 2.
Color attributes (a), radar charts of taste intensities (b) for congou black tea infusion produced by different rolling pressures. The total tea pigments contents of TFs, TBs, and TRs, and the index of (10 TFs + TRs)/TBs (c), four major components of TFs (d) in black tea samples treated with different rolling pressures. Different letters indicate significant differences between two groups (p < 0.05).
The taste profiles characterized by E-tongue are presented as a radar map (Fig. 2b). There were distinctions in the intensities of taste sensors between congou black tea infusions treated with different rolling pressures. The tea infusions in MPR rendered the highest intensity of ANS (sweetness, 8.2) and higher intensity of NMS (umami, 6.9), but lowest intensity of SCS (bitterness, 4.6). On the other hand, the tea infusions with HPR showed the lowest ANS (sweetness, 3.3) and reached the highest SCS (bitterness, 8.5) in LPR. In one word, MPR-treated tea imparted higher sweetness and umami scores and lower bitter score, which was consistent with the result of human sensory evaluation.
3.2. Dynamic changes in tea pigments
During rolling and fermentation, the catechins are oxidized and further polymerized to tea pigments (TFs, TRs, and TBs), which are remarkably related with the flavor, color, and beneficial function of black tea (Tanaka et al., 2022). TFs, mainly consisting of TF, TF-3-G, TF-3′-G, and TF-D-G enable the tea liquor to exhibit a brightness and yellowish color, and present an astringency and briskness taste (Zhang, Ho, Zhou, Santos, Armstrong, & Granato, 2019). As shown in Fig. 2c, the total TFs, TRs, and TBs contents increased gradually (p < 0.05) as the rolling pressure increased. Simultaneously, the contents of four major TFs components also showed a rising tendency, although a significant difference was only observed for TF (Fig. 2d). The results suggested that with an enhanced rolling pressure, a significant accumulation of tea pigments was obtained. TRs account for the thickness taste and red-brown color of the tea (Tanaka et al., 2022). Whereas, TBs determine the darkness of black tea infusion (Zhang et al., 2019). Moreover, the ratio of (10 TFs + TRs)/TBs is an important index for estimating the flavor quality of black tea (Samanta, Cheeni, Das, Roy, Ghosh, & Mitra, 2015). It was observed that the tea in LPR treatment showed lower contents of TFs and TRs, while the tea in HPR treatment showed highest content of TBs (Fig. 2c), both of which were not conducive to the tea quality. Instead, MPR-treated tea showed a highest ratio of (10 TFs + TRs)/TBs compared with the other two treatments (Fig. 2c), implying that the moderate rolling pressure may be beneficial for tea quality formation. The results were in agreement with the analysis of sensory evaluation and chromatic differences.
3.3. Dynamic changes in metabolic profiles
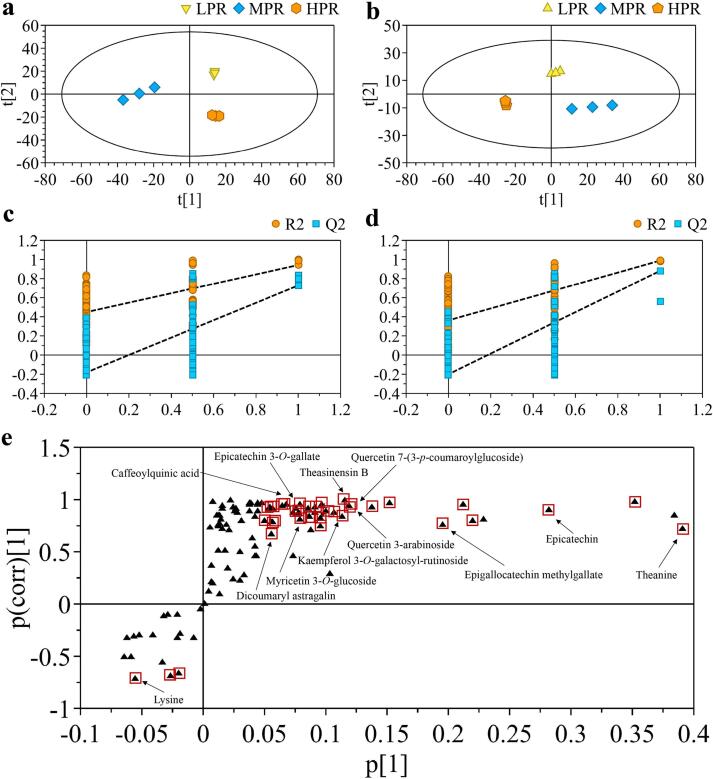
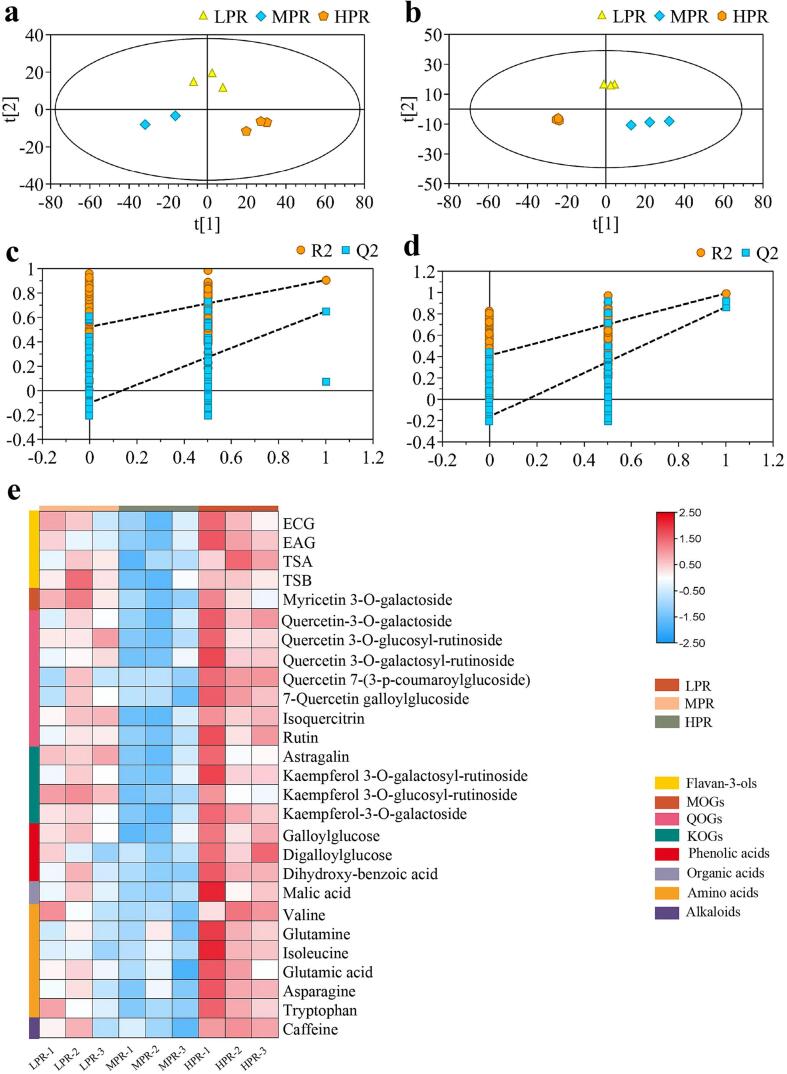
The phytochemical changes associated with the effect of rolling pressure were explored by analyzing the nonvolatile components using UHPLC-Q-Exactive/MS-based untargeted metabolomics. A total of 2587 and 2491 ion features were generated in either ESI (+) or ESI (−) mode, respectively. The high coefficient (R2 > 0.99) of two replicate QC samples in ESI (+) and ESI (−) demonstrated the precision of the metabolomics analysis in this study (Fig. S1). The score plots of PLS-DA model in ESI (+) (Fig. 3a, R2X = 0.673, R2Y = 0.966, Q2 = 0.816) and ESI (−) (Fig. 3b, R2X = 0.704, R2Y = 0.961, Q2 = 0.875) clearly differentiated black tea samples treated with three rolling pressure programs. The permutation test with intercepts of ESI (+) as R2 = (0.0, 0.449), Q2 = (0.0, − 0.181), and ESI (−) as R2 = (0.0, 0.362), Q2 = (0.0, − 0.201) indicated that the models were reliable (Fig. 3c, 3d). The results indicated a significant impact of rolling pressure on the chemical compositions in black tea. Generally, it was revealed that MPR samples were distinguished from LPR and HPR in the PC1 direction, while LPR and HPR samples were separated in PC2 direction. Such dynamic changing pattern suggested that the metabolic profiles of tea leaves were fluctuated in response to increasing rolling pressure, rather than a steady changing trend.
Fig. 3.
PLS-DA score plot and cross-validation of metabolites of congou black tea by different rolling pressures in ESI positive (a, c) and negative mode (b, d), respectively. S-plot of PLS-DA model for all identified metabolites (e). Black triangles represent ions included in PLS-DA model, and ions highlighted with red square represent most significantly altered ions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Key differential metabolites
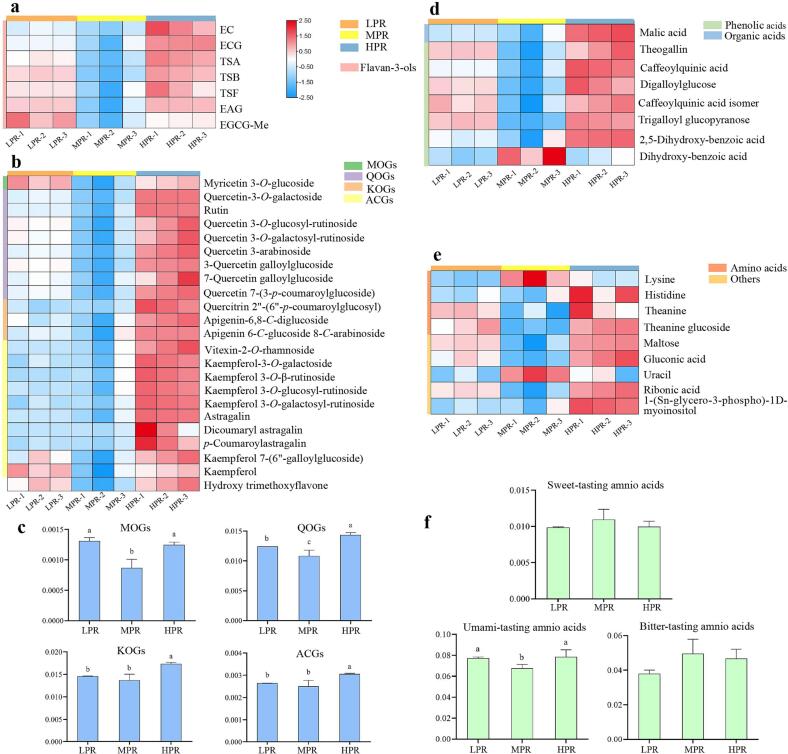
Based on one-way ANOVA and VIP analysis, 47 key differential metabolites (p < 0.05, VIP > 1) were screened, and were annotated by exact mass, MS/MS fragments, retention time rule, and standards validation. The detailed information of the differential metabolites including 7 flavan-3-ols and their dimers, 23 flavonol and flavonol/flavone glycosides, 7 phenolic acids, 4 free amino acids and derivative, 2 organic acids, and 4 other compounds is shown in Table 1. On the other hand, S-plot was generated by PLS-DA model of all identified 105 metabolites, in which metabolites significantly contributed to the classification were highlighted in red (Fig. 3e). The result of S-plot further verified the contribution of the differential metabolites, particularly flavan-3-ols, dimeric catechins, flavonol glycosides. The content levels of the 47 key differential compounds are demonstrated in heatmap (Fig. 4).
Table 1.
Differential metabolites identified by comparative metabolomics in congou black tea treated with different rolling pressures.
| No. | Metabolite | Ionization | m/z | TR/min | p | VIP | Folds change (HPR/MPR) | Folds change (LPR/MPR) | MS/MS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Epigallocatechin methylgallate (EGCG-Me) b | M-H | 471.0932 | 8.21 | 0.004 | 1.5 | 1.48 | 1.82 | 125, 161, 183, 305 |
| 2 | Epiafzelechin 3-gallate (EAG) b | M-H | 425.0878 | 10.52 | 0.005 | 1.4 | 1.38 | 1.14 | 169, 255, 273 |
| 3 | Epicatechin 3-O-gallate (ECG) a | M-H | 441.0823 | 8.87 | 0.006 | 1.3 | 1.44 | 1.40 | 169, 289, 331, 271, 193, 397 |
| 4 | Epicatechin (EC) a | M-H | 289.0713 | 6.62 | 0.001 | 1.0 | 1.24 | 1.07 | 125, 179, 205, 245 |
| 5 | Theasinensin B (TSB) b | M-H | 761.1359 | 4.64 | <0.001 | 1.5 | 1.52 | 1.42 | 423, 483, 575, 593, 609, 635, 743 |
| 6 | Theasinensin A (TSA) b | M-H | 913.1469 | 5.90 | <0.001 | 1.4 | 1.47 | 1.27 | 285, 423, 573, 591, 743, 761 |
| 7 | Theasinensin F (TSF) b | M-H | 897.1520 | 7.30 | 0.038 | 1.4 | 1.42 | 1.30 | 407, 727, 745 |
| 8 | 3-Quercetin galloylglucoside b | M-H | 615.1027 | 8.48 | <0.001 | 1.5 | 1.57 | 1.32 | 463, 300, 301 |
| 9 | 2″-(6″-p-Coumaroylglucosyl) quercitrin b | M-H | 755.1873 | 11.80 | <0.001 | 1.5 | 1.40 | 1.01 | 609, 591, 301, 271 |
| 10 | 7-Quercetin galloylglucoside b | M-H | 615.1027 | 8.23 | 0.024 | 1.5 | 1.48 | 1.21 | 463, 300, 301 |
| 11 | Myricetin 3-O-glucoside b | M-H | 479.0825 | 7.80 | 0.002 | 1.4 | 1.44 | 1.51 | 316, 317, 271 |
| 12 | Kaempferol 7-(6″-galloylglucoside) b | M-H | 599.1075 | 9.90 | 0.016 | 1.4 | 1.26 | 1.15 | 125, 169, 313, 285, 447 |
| 13 | Rutin a | M-H | 609.1461 | 8.70 | 0.001 | 1.4 | 1.29 | 1.10 | 301, 343 |
| 14 | Quercetin 3-O-glucosyl-rutinoside b | M-H | 771.1989 | 8.20 | 0.005 | 1.3 | 1.31 | 1.18 | 301, 343, 609 |
| 15 | Hydroxy trimethoxyflavone b | M-H | 327.0893 | 8.20 | 0.003 | 1.3 | 1.30 | 1.23 | 237, 211, 265 |
| 16 | Quercetin 3-O-galactosyl-rutinoside b | M-H | 771.1989 | 8.00 | 0.005 | 1.3 | 1.31 | 1.17 | 301, 343, 609 |
| 17 | Kaempferol a | M-H | 285.0414 | 12.24 | 0.026 | 1.3 | 1.42 | 1.51 | 227, 239, 211 |
| 18 | Quercetin 7-(3-p-coumaroylglucoside) b | M-H | 609.1279 | 11.80 | <0.001 | 1.2 | 1.29 | 1.13 | 463, 300, 301 |
| 19 | Dicoumaryl astragalin b | M-H | 739.1675 | 12.20 | 0.040 | 1.2 | 2.55 | 0.99 | 145, 285, 453, 593 |
| 20 | Vitexin-2-O-rhamnoside a | M-H | 577.1563 | 8.60 | 0.005 | 1.2 | 1.21 | 1.02 | 413, 293, 457 |
| 21 | p-Coumaroylastragalin b | M-H | 593.1306 | 11.90 | 0.002 | 1.2 | 2.78 | 1.96 | 285, 307, 447 |
| 22 | Kaempferol 3-O-β-rutinoside a | M-H | 593.1506 | 9.79 | 0.001 | 1.2 | 1.21 | 0.99 | 285, 327 |
| 23 | Apigenin-6,8-C-diglucoside b | M-H | 593.1512 | 6.60 | 0.009 | 1.2 | 1.24 | 1.07 | 473, 353, 503, 383, 575 |
| 24 | Astragalin a | M-H | 447.0933 | 10.20 | 0.001 | 1.2 | 1.22 | 1.03 | 255, 284, 285, 327, 357 |
| 25 | Kaempferol 3-O-galactosyl-rutinoside b | M-H | 755.2040 | 8.70 | <0.001 | 1.1 | 1.22 | 1.00 | 285 |
| 26 | Quercetin-3-O-galactoside b | M-H | 463.0882 | 8.90 | <0.001 | 1.1 | 1.34 | 1.12 | 301, 300, 293 |
| 27 | Kaempferol 3-O-glucosyl-rutinoside b | M-H | 755.2040 | 9.10 | 0.004 | 1.1 | 1.20 | 1.02 | 285 |
| 28 | Apigenin 6-C-glucoside 8-C-arabinoside b | M-H | 563.1406 | 7.50 | 0.020 | 1.1 | 1.22 | 1.07 | 353, 383, 524, 443, 473, 503, 545 |
| 29 | Quercetin 3-arabinoside b | M-H | 433.0799 | 9.77 | <0.001 | 1.0 | 1.42 | 1.17 | 300, 271, 301, 255 |
| 30 | Kaempferol-3-O-galactoside b | M-H | 447.0933 | 9.80 | 0.003 | 1.0 | 1.22 | 1.01 | 255, 284, 285, 327, 357 |
| 31 | Caffeoylquinic acid b | M-H | 353.0868 | 4.50 | 0.002 | 1.4 | 1.39 | 1.16 | 135,173,179,191 |
| 32 | Theogallin a | M-H | 343.0671 | 1.70 | 0.004 | 1.4 | 1.53 | 1.43 | 191 |
| 33 | Digalloylglucose b | M-H | 483.078 | 5.22 | <0.001 | 1.3 | 1.56 | 1.39 | 125, 169, 211,271, 313, 331 |
| 34 | Caffeoylquinic acid isomer b | M-H | 353.0868 | 5.70 | 0.007 | 1.3 | 1.55 | 1.45 | 135,173,179,191 |
| 35 | Trigalloyl glucopyranose b | M-H | 635.0883 | 7.24 | <0.001 | 1.3 | 1.84 | 1.70 | 125, 169, 313, 465, 483 |
| 36 | Dihydroxy-benzoic acid b | M-H | 153.0182 | 6.30 | 0.007 | 1.2 | 0.82 | 0.78 | 109 |
| 37 | 2,5-Dihydroxy-benzoic acid a | M-H | 153.0182 | 5.30 | 0.016 | 1.0 | 1.21 | 1.07 | 109 |
| 38 | Gluconic acid b | M-H | 195.0510 | 0.69 | <0.001 | 1.6 | 1.22 | 1.13 | 129, 159, 177 |
| 39 | Maltose a | M-H | 341.1089 | 0.76 | 0.002 | 1.2 | 1.49 | 1.37 | 113, 119, 143, 161, 179 |
| 40 | Ribonic acid b | M-H | 165.0398 | 0.70 | <0.001 | 1.0 | 1.44 | 1.30 | 75, 105, 129, 147 |
| 41 | Lysine a | M + H | 147.1133 | 0.59 | 0.004 | 1.6 | 0.83 | 0.75 | 84, 130 |
| 42 | Theanine a | M + H | 175.1082 | 0.77 | 0.034 | 1.5 | 1.24 | 1.21 | 129, 158 |
| 43 | Histidine a | M + H | 156.0772 | 0.61 | 0.037 | 1.1 | 1.15 | 1.02 | 95, 110 |
| 44 | Uracil b | M + H | 113.0346 | 1.09 | 0.015 | 1.5 | 0.88 | 0.85 | 70, 96, 113 |
| 45 | Malic acid a | M-H | 133.0137 | 0.77 | 0.002 | 1.1 | 1.20 | 1.03 | 71, 89, 115 |
| 46 | Theanine glucoside b | M + H | 337.1615 | 0.77 | <0.001 | 1.6 | 1.51 | 1.40 | 253, 301, 319 |
| 47 | 1-(Sn-glycero-3-phospho)-1D-myoinositol b | M-H | 333.0592 | 0.67 | 0.003 | 1.1 | 1.19 | 1.00 | 153, 241 |
confirmed by standards;
, identified based on exact mass and MS/MS.
Fig. 4.
Heat-map of dynamic changes of flavan-3-ols and their derivatives (a), flavonols and flavonol/flavone glycosides (b), phenolic acids and organic acids (d), amino acid and other components (e) in congou black tea treated with different rolling pressures. Levels of MOGs, QOGs, KOGs, ACGs (c), bitter-tasting, sweet-tasting, and umami-tasting amino acids (f) in three rolling pressures. Different letters indicate significant differences between two groups (p < 0.05).
3.4.1. Effect of rolling pressure on flavan-3-ols and their derivatives
Flavan-3-ols (catechins), including epicatechin (EC), epicatechin 3-O-gallate (ECG), epigallocatechin (EGC), epigallocatechin gallate (EGCG), etc. are the most abundant phenolic compounds, making up 25 % to 35 % dried weight in the tender tea shoots (Jia Li et al., 2020). Theasinensins (TSs) containing theasinensin A (TSA), theasinensin B (TSB), and theasinensin F (TSF) is a group of dimeric catechins formed via enzyme-catalyzed reactions during fermentation (oxidation), due to the contact of catechins and enzymes in the tea processing (Tan et al., 2016). Epigallocatechin methylgallate (EGCG-Me) and epiafzelechin 3-gallate (EAG) are also the derived compounds of flavan-3-ols (Wu et al., 2022b). Under the treatments of different rolling pressures, the significantly differential catechins and their derivatives comprised of EC, ECG, EAG, EGCG-Me, TSA, TSB, and TSF (p < 0.05, VIP > 1) in this study (Table 1). As shown in Fig. 4a, the contents of EC, ECG, EGCG-Me, EAG, TSA, TSB, and TSF in MPR-treated black tea were significantly lower than that of other two groups, especially ECG, EGCG-Me and TSB, which contents were lower more than 1.4-time compared with HPR and LPR (Table 1). While EC, ECG, TSA, TSB, TSF and EAG were higher in HPR, and EGCG-Me was higher in LPR-treated tea. The results showed that applying a moderate rolling pressure could lead to a reduction of catechins, while an insufficient or excessive rolling pressure resulted in a relatively higher content of catechins. During black tea processing, a series of oxidation reactions take place, which are primarily catalyzed by endogenous enzymes. As the dominating substrates, catechins are generally oxidized by PPO to o-quinones, and further polymerized to dimeric catechins of TFs and TSs (Xu et al., 2021). Meanwhile, the catechins and dimeric catechins can also be oxidized by PPO and/or POD to form the polymeric catechins of TRs and TBs (L. Chen et al., 2021) (Fig. S2). It was supposed that the moderate rolling pressure of MPR allowed an appropriate degree of cellular deconstruction, which permitted a sufficient enzymatic oxidation of catechins, leading to the lower levels of flavan-3-ols and their derivatives in MPR (Fig. 4a). Flavan-3-ols and their derivatives have been considered as contributors to the bitterness and astringent taste for black tea (Zhang et al., 2019). Therefore, the contents decrease of flavan-3-ols and their derivatives in MPR-treated black tea might induce a more delightful taste with less bitterness and astringency, which were in accordance with the results of human sensory evaluation and E-tongue analysis.
3.4.2. Effect of rolling pressure on flavonol/flavone glycosides
The compounds of flavonols and flavones are usually found in tea bounded to a glycoside moiety in the forms of O-glycosides or C-glycosides (Wu et al., 2022b). According to the aglycones, the flavonols/flavone glycosides can be divided into kaempferol-O-glycosides (KOGs), quercetin-O-glycosides (QOGs), myricetin-O-glycosides (MOGs), and apigenin-C-glycosides (ACGs), etc. (Fang, Song, Xu, & Ye, 2019). The flavonol existing as the form of O-glycosides (KOGs, QOGs, MOGs) are the main phenolics second only to flavan-3-ols, accounting for about 13 % tea polyphenols in tea fresh leaves (Guo et al., 2021). In this study, 23 flavonol and flavonol/flavone glycosides showed significant differences (p < 0.05, VIP > 1). Generally, the contents of flavonol/flavone glycosides in black tea produced by HPR were significantly higher than those of MPR and LPR, except for a few species (Fig. 4b). The MPR-treated tea presented significantly lower levels of MOGs, QOGs, KOGs and ACGs, especially the MOGs and QOGs, whose reduction in MPR were much pronounced than that of KOGs (Fig. 4c). For instance, the contents of myricetin 3-O-glucoside, kaempferol and p-coumaroylastragalin with MPR were lower 1.44, 1.42 and 1.96 times, respectively, compared to HPR and/or LPR. Flavonol glycosides have been found to decline during fermentation (oxidation) of black tea by PPO and POD-catalyzed flavonol glycosides oxidations (Guo et al., 2021) (Fig. S2). Our result suggested an enhanced flavonol glycosides oxidations in MPR group. Flavonol/flavone glycosides are reported to be responsible for the astringent and bitter taste in tea, and induce a mouth-coating/drying astringency sensation (Scharbert, Holzmann, & Hofmann, 2004). Their taste thresholds have been found ranging from 0.001 to 19.8 µmol/L, which are markedly lower than those of catechins (Scharbert, et al., 2004). Moreover, flavonol/flavone glycosides can enhance the bitterness sensation of caffeine, and negatively affect the tea taste through the synergistic effect (Scharbert et al., 2005). Thus, the low concentrations of flavonol/flavone glycosides in MPR-treated tea were expected to reduce the bitterness and astringency in tea infusion, which agreed with the results of sensory evaluation and E-tongue.
3.4.3. Effect of rolling pressure on phenolic acids and organic acids
Phenolic acids present as primary precursors for catechins and flavonol glycosides and are one important class of phenolic constituents in tea, which are responsible for the sourness and astringency taste in black tea (Tan et al., 2016). Organic acids are the crucial intermediate compounds involving in the citric acid cycle and phenylpropanoid metabolic pathway, which play a role in the formation of acid taste in tea infusion (Zhang et al., 2023). As shown in Fig. 4d, MPR resulted in the lowest concentrations of phenolic acids, such as digalloylglucose, caffeoylquinic acid, theogallin and trigalloyl glucopyranose (folds change > 1.39 compared with LPR and HPR) (Table 1), as well as malic acid. While the contents of these compounds in HPR group were evidently higher than the other two treatments. Phenolic acids are unstable and can be easily oxidized by enzymes during black tea processing, accompanied by the catechin-coupled oxidation (L. Chen et al., 2021) (Fig. S2). Our result implied that the phenolic acids oxidation was altered in response to different rolling pressures. Previous study has shown that caffeoylquinic acid and theogallin are the most abundant phenolic acid derivatives in tea liquor (Huang et al., 2021). Malic acid is one species of major organic acids dominating sourness in tea infusion (F. Yu et al., 2022). The results suggested that applying an appropriate rolling pressure of MPR tended to improve the delightful taste of black tea by the reduction of phenolic acids and organic acids in tea samples.
3.4.4. Effect of rolling pressure on amino acids
Amino acids, which account for 1 % to 4 % of the dry weight in tea, are generally considered to be the important contributors to the umami, sweet, and mellow taste of tea infusion (Y. Chen et al., 2020). A total of 18 amino acids were identified, among which 4 amino acids and derivatives (i.e., theanine, histidine, lysine, and theanine glucoside) had significant differences (p < 0.05, VIP > 1) among the three treatments (Table 1, Fig. 4e). Based on the taste characteristics, these amino acids can be divided into sweet-tasting (threonine, serine, proline, and methionine), bitter-tasting (valine, tyrosine, phenylalanine, lysine, leucine, isoleucine, histidine, and arginine), and umami-tasting amino acids (theanine, glutamine, glutamic acid, asparagine, and aspartic acid) (Z. Yu & Yang, 2020). In this study, the level of total sweet-tasting amino acids was highest in the tea samples of MPR treatment although not statistically significant (Fig. 4f), that might be beneficial for the sweet mellow-like taste of congou black tea, which was supported by the high ANS (sweetness, 8.2) intensity analyzed by E-tongue. The total content of umami-tasting amino acids was significantly higher in HPR group (Fig. 4f), that was considered possibly responsible for the high intensity of NMS (umami, 7.9) in HPR treatment. Besides, the total content of bitter-tasting amino acids exhibited higher level in MPR group, but showed no significant difference (Fig. 4f).
In summary, the MPR-treated black tea presented lowest levels of some astringent and bitter substances, while HPR and LPR resulted in the accumulation of those unpleasant substances. Among which, ECG, EGCG-Me, TSB, myricetin 3-O-glucoside, kaempferol, p-coumaroylastragalin, and digalloylglucose, caffeoylquinic acid, theogallin, etc. might be the potential maker compounds with relatively larger folds change and higher value of VIP.
3.5. Independent validation batch at a different tea season
Aimed to verify the influence of rolling pressure on the black tea quality, an independent validation batch was implemented in a different tea season following the same conditions. The sensory changes analyzed by human sensory evaluation, tea liquor color chromatic analysis, and E-tongue were largely verified in the validation batch (Table S3, Fig. S3). The quantitative contents of TRs, TBs, as well as TFs and its four main components of TF, TF-3-G, TF-3′-G, and TF-D-G, showed the similar variations as the experiment batch (Fig. S4). The high coefficient (R2 > 0.98) in two parallel QC samples suggested the high precision of metabolomics analysis in validation batch (Fig. S5). Meanwhile, the supervised PLS-DA score plots of metabolomics data in validation batch revealed the similar separation and clustering pattern as the experiment batch (Fig. 5a, 5b). The cross-validation with 200 permutation tests indicated the reliability of the models (Fig. 5c, 5d). Moreover, a total of 27 key differential metabolites (p < 0.05, VIP > 1) were identified among the three treatments in validation batch, including 4 flavan-3-ols and their derivatives, 12 flavonol/flavone glycosides, 3 phenolic acids, 6 amino acids, 1 alkaloid and 1 organic acid. Among them, 17 differential metabolites were shared in both experimental and validation batch, such as ECG, EAG, TSA, TSB, myricetin 3-O-galactoside, rutin, astragalin, digalloylglucose and malic acid, etc. The variations of key differential metabolites were visualized by a heatmap (Fig. 5e), which were generally consistent with that of experiment batch.
Fig. 5.
PLS-DA score plot and cross-validation of metabolites of congou black tea in validation batch in ESI positive (a, c) and negative mode (b, d), respectively. Heat-map of the differential metabolites of tea samples (e) in validation batch.
4. Conclusions
In this study, a comprehensive comparison by human sensory evaluation, E-tongue, chromatic differences, tea pigments, and metabolomics were carried out to systematically study the effects of rolling pressure on congou black tea quality. The tea flavor quality, particularly liquor color and taste, were significantly influenced by rolling pressure. With the increase of rolling pressure, the global metabolic profiles presented a fluctuation in black tea, among which the contents of tea pigments, flavan-3-ols, flavonol/flavone glycosides, phenolic acids, organic acids, and amino acids were varied most prominently. Overall, MPR rendered the most premium flavor quality with highest sweetness, higher umami, lowest bitterness, and highest value of (10 TFs + TRs)/TBs, that possibly due to the appropriate degree of oxidative reactions in tea phenolics and amino acids during black tea manufacturing. The results were further verified by the validation batch performed in the different season. In the future, to further validate the role of key differential metabolites in taste changes during black tea processing, validation will be performed by accurately quantifying the important differential compounds, combined with taste reconstitution/omission experiments. The study provided new insights into the regulation of rolling pressure for the flavor substances and quality of congou black tea, which could afford a comprehensive rationale for manipulating rolling pressure as a beneficial tool in the quality control of tea processing.
CRediT authorship contribution statement
Shan Zhang: Investigation, Formal analysis, Visualization, Writing – original draft. Shimin Wu: Investigation, Methodology, Writing – original draft. Qinyan Yu: Investigation, Formal analysis, Methodology. Xujiang Shan: Formal analysis, Visualization. Le Chen: Investigation, Resources. Yuliang Deng: Investigation, Resources. Jinjie Hua: Investigation, Resources. Jiayi Zhu: Investigation, Resources. Qinghua Zhou: Methodology, Writing – review & editing. Yongwen Jiang: Project administration, Funding acquisition. Haibo Yuan: Supervision, Project administration, Data curation. Jia Li: Conceptualization, Investigation, Formal analysis, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants of the China Agriculture Research System of MOF and MARA (CARS-19), the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (No. CAAS-ASTIP-TRICAAS), and the Natural Science Foundation of China (No. 42277275).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100989.
Contributor Information
Haibo Yuan, Email: 192168092@tricaas.com.
Jia Li, Email: jiali1986@tricaas.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Chen L., Liu F., Yang Y., Tu Z., Lin J., Ye Y., Xu P. Oxygen-enriched fermentation improves the taste of black tea by reducing the bitter and astringent metabolites. Food Research International. 2021;148 doi: 10.1016/j.foodres.2021.110613. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zeng L., Liao Y., Li J., Zhou B., Yang Z., Tang J. Enzymatic reaction-related protein degradation and proteinaceous amino acid metabolism during the black tea (Camellia sinensis) manufacturing process. Foods. 2020;9(1) doi: 10.3390/foods9010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S.-H., Yang K.-M., Wang S.-Y., Chen C.-W. Enzymatic treatment in black tea manufacturing processing: Impact on bioactive compounds, quality, and bioactivities of black tea. Lwt. 2022;163 doi: 10.1016/j.lwt.2022.113560. [DOI] [Google Scholar]
- Das S., Datta A.K. Mass transfer coefficient and mass diffusivity of O2 and CO2 during oxidation of macerated CTC and rolled orthodox leaves in black tea manufacturing. Journal of Food Process Engineering. 2018;41(8) doi: 10.1111/jfpe.12875. [DOI] [Google Scholar]
- Essiedu J.A., Gonu H., Adadi P., Withayagiat U. Polyphenols and antioxidant activity of thunbergia laurifolia infused tea under drying conditions. Journal of Food Quality. 2023;2023 doi: 10.1155/2023/5046880. [DOI] [Google Scholar]
- Fang Z.-T., Song C.-J., Xu H.-R., Ye J.-H. Dynamic changes in flavonol glycosides during production of green, yellow, white, oolong and black teas from Camellia sinensis L. (cv. Fudingdabaicha) International Journal of Food Science & Technology. 2019;54(2):490–498. doi: 10.1111/ijfs.13961. [DOI] [Google Scholar]
- Goncalves Bortolini D., Windson Isidoro Haminiuk C.I., Cristina Pedro A., de Andrade Arruda Fernandes I., Maria Maciel G. Processing, chemical signature and food industry applications of Camellia sinensis teas: An overview. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.Y., Lv Y.Q., Ye Y., Liu Z.Y., Zheng X.Q., Lu J.L.…Ye J.H. Polyphenol oxidase dominates the conversions of flavonol glycosides in tea leaves. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128088. [DOI] [PubMed] [Google Scholar]
- Hua J., Xu Q., Yuan H., Wang J., Wu Z., Li X., Jiang Y. Effects of novel fermentation method on the biochemical components change and quality formation of congou black tea. Journal of Food Composition and Analysis. 2021;96 doi: 10.1016/j.jfca.2020.103751. [DOI] [Google Scholar]
- Huang A., Jiang Z., Tao M., Wen M., Xiao Z., Zhang L.…Zhang L. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129950. [DOI] [PubMed] [Google Scholar]
- Jia J., Zhang C., Yuan B., Chen Z., Chen J. Development and process parameter optimization with an integrated test bench for rolling and forming strips of oolong tea. Journal of Food Process Engineering. 2021;44(12) doi: 10.1111/jfpe.13901. [DOI] [Google Scholar]
- Li J., Wang J., Yao Y., Hua J., Zhou Q., Jiang Y.…Dong C. Phytochemical comparison of different tea (Camellia sinensis) cultivars and its association with sensory quality of finished tea. Lwt. 2020;117 doi: 10.1016/j.lwt.2019.108595. [DOI] [Google Scholar]
- Li J., Wu S., Yu Q., Wang J., Deng Y., Hua J.…Jiang Y. Chemical profile of a novel ripened Pu-erh tea and its metabolic conversion during pile fermentation. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132126. [DOI] [PubMed] [Google Scholar]
- Li J., Yao Y., Wang J., Hua J., Wang J., Yang Y.…Yuan H. Rutin, gamma-aminobutyric acid, gallic acid, and caffeine negatively affect the sweet-mellow taste of congou black tea infusions. Molecules. 2019;24(23) doi: 10.3390/molecules24234221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiadze N.T., McHedlishvili N.I., Rodrigez-Lopez J.N., Abutidze M.O., Sadunishvili T.A., Pruidze N.G. Biochemical processes at the stage of withering during black tea production. Applied Biochemistry and Microbiology. 2014;50(4):394–397. doi: 10.1134/s0003683814040103. [DOI] [PubMed] [Google Scholar]
- Ozdemir F., Tontul I., Balci-Torun F., Topuz A. Effect of rolling methods and storage on volatile constituents of Turkish black tea. Flavour and Fragrance Journal. 2017;32(5):362–375. doi: 10.1002/ffj.3385. [DOI] [Google Scholar]
- Qu F., Zeng W., Tong X., Feng W., Chen Y., Ni D. The new insight into the influence of fermentation temperature on quality and bioactivities of black tea. Lwt. 2020;117 doi: 10.1016/j.lwt.2019.108646. [DOI] [Google Scholar]
- Saikia D., Boruah P.K., Sarma U. A sensor network to monitor process parameters of fermentation and drying in black tea production. Mapan. 2015;30(3):211–219. doi: 10.1007/s12647-015-0142-4. [DOI] [Google Scholar]
- Samanta T., Cheeni V., Das S., Roy A.B., Ghosh B.C., Mitra A. Assessing biochemical changes during standardization of fermentation time and temperature for manufacturing quality black tea. Journal of Food Science and Technology. 2015;52(4):2387–2393. doi: 10.1007/s13197-013-1230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharbert S., Holzmann N., Hofmann T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. Journal of Agricultural and Food Chemistry. 2004;52(11):3498–3508. doi: 10.1021/jf049802u. [DOI] [PubMed] [Google Scholar]
- Scharbert S., Holzmann N., Hofmann T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. Journal of Agriculture and Food Chemistry. 2005;53:5377–5384. doi: 10.1021/jf050294d. [DOI] [PubMed] [Google Scholar]
- Sharma V., Rao L.J. A thought on the biological activities of black tea. Critical Reviews in Food Science and Nutrition. 2009;49(5):379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- Tan J., Dai W., Lu M., Lv H., Guo L., Zhang Y.…Lin Z. Study of the dynamic changes in the non-volatile chemical constituents of black tea during fermentation processing by a non- targeted metabolomics approach. Food Research International. 2016;79:106–113. doi: 10.1016/j.foodres.2015.11.018. [DOI] [Google Scholar]
- Tanaka T., Yasumatsu M., Hirotani M., Matsuo Y., Li N., Zhu H.T.…Zhang Y.J. New degradation mechanism of black tea pigment theaflavin involving condensation with epigallocatechin-3-O-gallate. Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.131326. [DOI] [PubMed] [Google Scholar]
- Wang H., Shen S., Wang J., Jiang Y., Li J., Yang Y.…Yuan H. Novel insight into the effect of fermentation time on quality of Yunnan Congou black tea. LWT. 2022;155 doi: 10.1016/j.lwt.2021.112939. [DOI] [Google Scholar]
- Wu S., Yu Q., Zhu J., Hua J., Shen S., Jiang Y. Analysis on the effect of different rolling frequencies on the Congou black tea quality based on electronic tongue and metabolomics. Food Science. 2022 https://kns.cnki.net/kcms/detail/11.2206.TS.20220628.1538.032.html In Chinese. [Google Scholar]
- Wu S., Yu Q., Shen S., Shan X., Hua J., Zhu J.…Li J. Non-targeted metabolomics and electronic tongue analysis reveal the effect of rolling time on the sensory quality and nonvolatile metabolites of congou black tea. Lwt. 2022;169 doi: 10.1016/j.lwt.2022.113971. [DOI] [Google Scholar]
- Xu A., Lai W., Chen P., Awasthi M.K., Chen X., Wang Y., Xu P. A comprehensive review on polysaccharide conjugates derived from tea leaves: Composition, structure, function and application. Trends in Food Science & Technology. 2021;114:83–99. doi: 10.1016/j.tifs.2021.05.020. [DOI] [Google Scholar]
- Ye F., Qiao X., Gui A., Wang S., Liu P., Wang X.…Zheng P. Metabolomics provides a novel interpretation of the changes in main compounds during black tea processing through different drying methods. Molecules. 2021;26(21) doi: 10.3390/molecules26216739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Chen C., Chen S., Wang K., Huang H., Wu Y.…Li B. Dynamic changes and mechanisms of organic acids during black tea manufacturing process. Food Control. 2022;132 doi: 10.1016/j.foodcont.2021.108535. [DOI] [Google Scholar]
- Yu Z., Yang Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Critical Reviews in Food Science and Nutrition. 2020;60(5):844–858. doi: 10.1080/10408398.2018.1552245. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ho C.T., Zhou J., Santos J.S., Armstrong L., Granato D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Comprehensive Reviews in Food Science & Food Safety. 2019;18(5):1474–1495. doi: 10.1111/1541-4337.12479. [DOI] [PubMed] [Google Scholar]
- Zhang S., Shan X., Niu L., Chen L., Wang J., Zhou Q.…Wu T. The Integration of Metabolomics, Electronic Tongue, and Chromatic Difference Reveals the Correlations between the Critical Compounds and Flavor Characteristics of Two Grades of High-Quality Dianhong Congou Black Tea. Metabolites. 2023;13(7) doi: 10.3390/metabo13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., He H., Ye Y., Dong C., Gui A., Gao M., Chen L. Influence of rolling temperature on physicochemical quality of congou black tea. Modern. Food Science and Technology. 2017;33(5):168–175. doi: 10.13982/j.mfst.1673-9078.2017.5.027. In Chinese. [DOI] [Google Scholar]
- Zhu J., Wang J., Yuan H., Ouyang W., Li J., Hua J., Jiang Y. Effects of fermentation temperature and time on the color attributes and tea pigments of Yunnan congou black tea. Foods. 2022;11(13) doi: 10.3390/foods11131845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.