Highlights
-
•
Eleven major constituents were identified from methanol extract of Noni fruit.
-
•
The methanol extract of Noni fruit exhibited obvious laxative activity.
-
•
This extract showed low toxicity to liver, kidney, and gut microbiome.
Keywords: Noni fruit, Constituent analysis, Laxative activity, Toxicity
Abstract
Noni fruits have gained considerable popularity as dietary supplements. However, the major constituents, the laxative activity, and the toxicity of Noni fruit remains still unknown. The purpose of the present study was, therefore, to analyze the constituents of methanol extract of Noni fruit by UPLC-MS, and further evaluate laxative activity and safety aspects of this Noni fruit-derived products in mice. UPLC-MS analysis identified eleven major constituents from this Noni fruit extract. Administration of this extract obviously shortened the time of first fecal excrement, significantly increased the total number and the weight of stools, and remarkably restored the intestinal transit to normal level in the constipated mice, with low toxicity to liver and kidney, and meanwhile, the abundance, composition, and function of gut microbiota remained homeostasis. These results revealed the laxative activity of the methanol extract of Noni fruit with low toxicity and no influence on gut microbiota.
1. Introduction
Constipation, characterized by inactive intestinal mobility, excretion difficulty, and incomplete bowel evacuation, is a common gastrointestinal disorder prevalent in individuals worldwide (Bharucha, Pemberton, & Locke, 2013). Increasing studies on constipation have clarified its associations with increased incidence of colorectal cancer, chronic kidney disease, Parkinson disease, and also some other functional gastrointestinal disorders that may impair health‐related quality of life (Eor et al., 2019, Simons et al., 2010).
Botanicals and their active ingredient such as senna, cascara, and frangula, are widely prescribed to patients with constipation (Zihad et al., 2018). Generally, these interventions acting as stimulants are known to exert strong purgative activities by promoting stool elimination and triggering bowel movements (Alsalimy, Madi, & Awaisu, 2018). However, evidence is accumulating that the long-term use of these laxatives may result in drug resistance, whereby the purgative activity is reduced. Moreover, most laxatives are reported to accompany various undesirable side effects, including flatulence, abdominal pain, or damage of gut microbiota (Huang et al., 2012). Given evidence that most laxatives are unsuitable for long-term use, there is, therefore, a medical need to improve therapeutic strategies to alleviate constipation and associated disorders.
The primary focus for constipation treatment remains on the identification of novel laxatives derived from natural products with strong therapeutic response and less side effect. Noni (Morinda citrifolia L., Rubiaceae) has been used for a long history as a dietary supplement and herbal remedy for a wide range of diseases (Almeida, de Oliveira, & Hotza, 2019). Since various biological activities, including analgesic, anti-cancer, anti-inflammatory, antioxidant, and anti-diabetic, have been claimed for the fruit, leave, stem, and root extracts of Noni, it has gained considerable popularity as beneficial functional food worldwide (Hong et al., 2019, Lin et al., 2013). Fruit juice of Noni, in particular, was approved by the European Commission in 2003 as a Novel Food. Presently, the scientific evidence on the laxative activity of Noni fruit remains still unknown, which inspires the idea of exploring the functional benefits of Noni as prokinetic supplementation to extend its beneficial effects.
This exploratory study was therefore intended to investigate the major constituents, the laxative activity, and the toxicity of the methanol extract of Noni fruit. This study revealed 11 major constituents of this Noni fruit extract, and further demonstrated its laxative activity with low toxicity to liver, kidney, and gut microbiota.
2. Materials and methods
2.1. Plant material and extraction
Noni fruits were collected in April 2013 from Nanning, Guangxi Province, China and identified by Prof. Jing-Quan Yuan at the Department of Pharmaceutical Chemistry, Guangxi Botanical Garden of Medicinal Plants, where a voucher specimen (No. n130426) was deposited for future reference. The air-dried Noni fruit (1.0 kg) were powdered and extracted with MeOH (3 × 8 L, 2 h each) at room temperature. Removal of MeOH under reduced pressure yielded the solid powder of 105.4 g.
2.2. Sample preparation, chromatography, and mass spectrometry conditions
The extraction was dissolved in 50 % methanol and then transferred to a 10-mL volumetric flask. Additional solvent was added to the line of the flask. The sample solution was passed through a 0.22-µm filter prior to injection.
The chromatographic analysis using an Acquity UPLCTM system (Waters Corp., USA) was conducted with an Agilent phenyl column (100 mm × 2.1 mm i.d., 1.7 µm) at 25 °C and a flow rate of 0.3 mL/min. The mobile phase consisted of acetonitrile (A) and water with 0.2 % phosphoric acid (B) with a gradient elution: 0–15 min, 2–10.0 % (A); 15–30 min, 10.0–25.0 % (A).
Mass spectrometry detection was performed on a Synapt G2 MS system (Waters Corp., USA) equipped with an ESI source. Nitrogen gas was used for nebulization. The detection mode of the flight tube was selected to be a “V” pattern. Negative ion spectra of the column elute were recorded in the range of m/z 50–1200. The optimized conditions of the ESI source were as follows: capillary voltage, 2.5 kV; sampling cone voltage, 40 V; extraction cone voltage, 3.0 V; ESI source temperature, 120 °C; desolvation temperature, 450 °C; cone gas flow, 30 L/h; desolvation gas flow, 800 L/h; collision gas flow, 0.5 mL/min; collision energy for MSE acquisition mode, 4.0 eV for low energy scans and 15–40 eV for high energy scans; and dynamic adjustment of the fragmentor voltage range from 25 to 40 V for MS/MS acquisition mode. The lock mass compound was leucine enkephalin (m/z 556.2771), and the interval scan time was 0.02 s. Masslynx 4.1 (Waters Corp.) was used to control the instrument.
2.3. Animals and treatments
Male ICR mice (18–22 g) were purchased from Hebei Medical University (Shijiazhuang, China). All mice were fed with a standard chow diet (calories provided by 22.8 % protein, 13.8 % fat, and 63.4 % carbohydrate) and water ad libitum, housed under standard conditions at temperature of 24 ± 1 °C, 55 ± 1 % humidity and 12 h light/dark cycle. The animal experiments were approved by the Institutional Animal Care and Use Committee of Hebei University (Approval Number: IACUC-2018012). The animals were allowed to acclimate for one week, and then randomly assigned to normal control group (N, n = 10), model group (M, n = 10), or Noni extract treated group (G1, 0.625 g/kg, n = 10), (G2, 1.25 g/kg, n = 10), (G3, 2.5 g/kg, n = 10), 10 mice per cage. Mice were orally administered with vehicle (distilled water) or Noni extract (0.625, 1.25, 2.5 g/kg), respectively, for 21 days. Solid powder of Noni extract was solved with distilled water. Body weight, food intake and water consumption of mice in each group (10 mice) were measured every two days throughout the experiment. All mice were sacrificed by cervical dislocation after anesthesia with isoflurane.
2.4. Measurement of fecal output and fecal parameters
After the last dose of drug, mice were fasted for 16 h prior to the experiment, and then orally administered with or without diphenoxylate at concentration of 5 mg/kg to induce constipation as described elsewhere (Luo et al., 2017). Mice in each group were assigned into individual cage to collect feces, and each individual was observed for 6 h. The time for the first black feces, the number, and weight of the feces per animal were measured during the experimental period. After the wet weight was weighed, the feces were then dried at 60 ℃ for 12 h to obtain the fecal dry weight. The analysis for fecal water content was based on the following formula: Fecal water content= (Fecal wet weight -Fecal dry weight)/Fecal wet weight × 100 %. Each experiment was performed in triplicate.
2.5. Effect of methanol extract of Noni fruit on intestinal transit
The animals were orally administered with vehicle or methanol extract of Noni fruit (0.625, 1.25, 2.5 g/kg) for 21 days. The effect of methanol extract of Noni fruit on intestinal transit were evaluated according to a previously described method (Kim et al., 2019), with minor modification. After the last administration, mice were fasted for 16 h, and then orally administered with diphenoxylate (5 mg/kg) to induce constipation. After 30 min of diphenoxylate treatment, the mice were fed with carbon solution containing 5 % charcoal powder and 10 % Acacia gum. The mice were then sacrificed after feeding with the carbon for 25 min, and the small intestines were rapidly dissected out. The length of the small intestines and the distance of carbon transit were measured. The intestinal carbon transit ratio was calculated as follows: (distance of the carbon traveled) / (length of the small intestine) × 100 % (Hsieh et al., 2016). Each experiment was performed in triplicate.
2.6. Assessment of liver and kidney functions
Blood was taken from the mice eyeballs, which was then centrifuged at 3000 rpm for 10 min to obtain supernatant. Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine were assessed using commercially available diagnostic kits (Nanjing Jiancheng Bioengineering Institute, China).
2.7. Histological analysis of liver and kidney
Tissues of liver and kidney, fixed in 10 % formaldehyde solution, were embedded in paraffin, and then cut into 3 µm sections, de-paraffinized in xylene, and rehydrated in a graded series of ethanol. The sections of these tissues were assessed by morphometric evaluation with hematoxylin and eosin (H&E) staining.
2.8. Sample collection and analysis of gut microbiota
Fresh fecal samples were collected 24 h after the last dose of drugs and were stored at −80 °C. Total bacterial DNA was extracted from fecal samples using a QIAamp DNA stool Mini Kit from Qiagen (Germany). The V3-V4 region of the bacterial 16S rRNA gene was amplified with the common primer pair 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′- GGACTACHVGGGTWTCTAAT-3′) by PCR (95 °C for 5 min, followed by 15 cycles at 95 °C for 1 min, 50 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min). High-throughput sequencing analysis of bacterial rRNA genes was performed using the Illumina Hiseq 2500 platform (2 × 250 paired ends) at Biomarker Technologies Co, Ltd. (Beijing, China) (Xu et al., 2017). Paired-end reads were merged using FLASH v1.2.7 and allocated to samples based on their unique barcodes. High-quality reads were obtained based on the QIIME (V1.7.0), quality control process and bioinformatics analysis of these reads from each sample was performed using the QIIME software package. Sequences with ≥97 % similarity were clustered into the same operational taxonomic units (OTUs). α-diversity, β-diversity, principal component analysis (PCA), non-metric multidimensional scaling (NMDS), and UniFrac distance-based principal coordinate analysis (PCoA) and principal component analysis (PCA) were conducted by QIIME. KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of the function of gut microbiota was examined by the software of PICRUSt, BugBase, Tax4Fun, and FAPROTAX.
2.9. Statistical analysis
All data were presented as the means ± standard error of the mean (SEM). Differences between groups were analyzed using One-way Analysis of Variance (ANOVA) followed by Duncan’s test for multiple comparison, and a p value < 0.01 or p value < 0.05 was considered statistically significant.
3. Results
3.1. Constituent analysis of methanol extract of Noni fruit by UPLC-MS
UPLC-MS analysis was initially conducted to understand the major constituents of methanol extract of Noni fruit, which may account for its bioactivity. The total chromatogram extracts from Noni fruit were illustrates in Fig. 1. Eleven major constituents were identified from their ion fragments, including coumalic acid, p-hydroxybenzoic acid, kaempferol-3-rutinoside, ferulic acid, etc. (Table 1).
Fig 1.
(A) UPLC spectrum of extracts from Noni fruit. (B) The constituents of methanol extract of Noni fruit.
Table 1.
The constituents of methanol extract of Noni fruit.
| No. | tR | m/z | name | Chemical Formula | Ionic Fragment |
|---|---|---|---|---|---|
| 1 | 5.398 | 353.0871 | chlorogenic acid | C16H18O9 | 605/371 |
| 2 | 6.130 | 179.0332 | caffeic acid | C9H8O4 | 135/106 |
| 3 | 6.378 | 193.0498 | ferulic acid | C10H10O4 | 177 |
| 4 | 7.818 | 137.0214 | p-Hydroxybenzoic acid | C7H6O3 | 119/109/83 |
| 5 | 8.998 | 139.0021 | coumalic acid | C6H4O4 | 107/93 |
| 6 | 11.098 | 161.0212 | 4-hydroxycoumarin | C9H6O3 | 147/119/93 |
| 7 | 16.444 | 593.1522 | Kaempferol-3-rutinoside | C27H30O15 | 285 |
| 8 | 21.741 | 463.0821 | isoquercetin | C21H20O12 | 301/179/151 |
| 9 | 24.583 | 609.1423 | rutinum | C27H30O16 | 301/271/179/151 |
| 10 | 25.717 | 301.0312 | quercetin | C15H10O7 | 179/151 |
| 11 | 27.213 | 343.0811 | obtusin | C18H16O7 | 313/242/214 |
3.2. Effect of methanol extract of Noni fruit on fecal excretion-related parameters
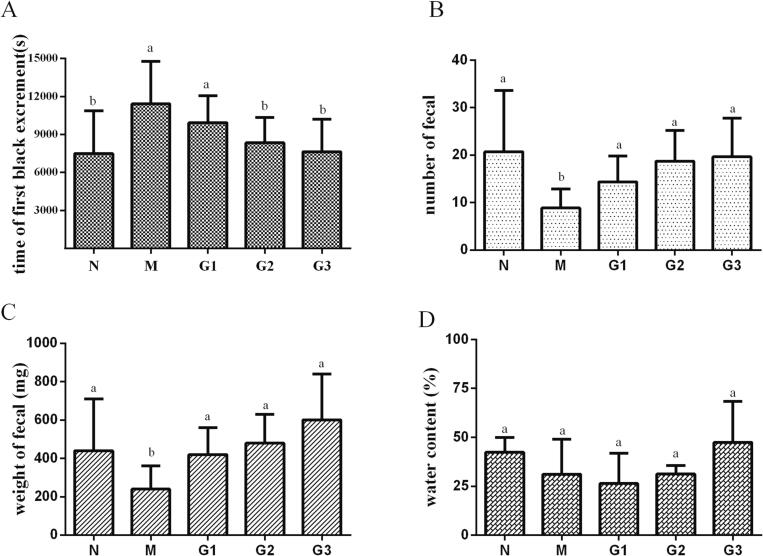
To investigate the beneficial effects of methanol extract of Noni fruit on fecal excretion parameters, alterations in the time for the first black feces, the fecal number, the fecal weight, and water content of feces in each group were investigated in mice with diphenoxylate-induced constipation after administering Noni fruit extract. As shown in Fig. 2, diphenoxylate administration remarkably delayed the time for the first black feces and significantly reduced the fecal number and weight. Fortunately, oral administration of this Noni fruit extract exhibited an obvious ameliorating effect in diphenoxylate-induced constipation dose-dependently, with shortened time for the first black feces and increased fecal number and weight (Fig. 2A-C). Also, dry and hard feces in constipated mice were changed into soft feces after Noni fruit extract administration, accompanied by a mild increase of water content in feces of Noni fruit extract-treated mice compared with that of diphenoxylate treated mice though without significant (Fig. 2D). Apparently, the above results suggested that the methanol extract of Noni fruit treatment showed potent beneficial effect against diphenoxylate-induced constipation with obviously restored fecal output.
Fig 2.
Fecal excretion parameters in mice with or without Noni fruit extract administration. (A) The time for the first black feces after diphenoxylate (5 mg/kg) administration were recorded, and the (B) number and (C) weight of the feces per animal were measured during the experiment. (D) The analysis of fecal water content was conducted after the feces were dried at 60 ℃ for 12 h to obtain the fecal dry weight. Values were presented as mean ± S.E.M (n = 10). Different lower-case letters indicate significant difference (p < 0.05). Groups represented, N: Saline; M: saline + diphenoxylate; G1: Noni fruit extract 0.625 g/kg + diphenoxylate; G2: Noni fruit extract 1.25 g/kg + diphenoxylate; G3: Noni fruit extract 2.5 g/kg + diphenoxylate.
3.3. Effect of methanol extract of Noni fruit on gastrointestinal motility
To evaluate if enhancement of gastrointestinal motility was responsible for the laxative activity, effects of methanol extract of Noni fruit on small intestinal transit were detected. The results showed that the administration of methanol extract of Noni fruit dose-dependently enhanced the propulsion of carbon through the gastrointestinal tract (Fig. 3A). Particularly, Noni fruit extract at the dose of 2.5 g/kg (95.6 %) and 1.25 g/kg (88.1 %) significantly increased the push rates in comparison to the model mice induced by diphenoxylate (67.8 %) (Fig. 3B). Presumably, the laxative activity of methanol extract of Noni fruit resulted from the acceleration of small intestinal transit in diphenoxylate-induced mice.
Fig 3.
Gastrointestinal motility in mice with or without Noni fruit extract administration. (A) Carbon push in gut 25 min later after diphenoxylate (5 mg/kg) administered. (B) Analysis of push rates (%). Values are presented as mean ± S.E.M (n = 10). Different lower-case letters indicate significant difference (p < 0.05). Groups represented, N: Saline; M: saline + diphenoxylate; G1: Noni fruit extract 0.625 g/kg + diphenoxylate; G2: Noni fruit extract 1.25 g/kg + diphenoxylate; G3: Noni fruit extract 2.5 g/kg + diphenoxylate.
3.4. Body weight and food intake
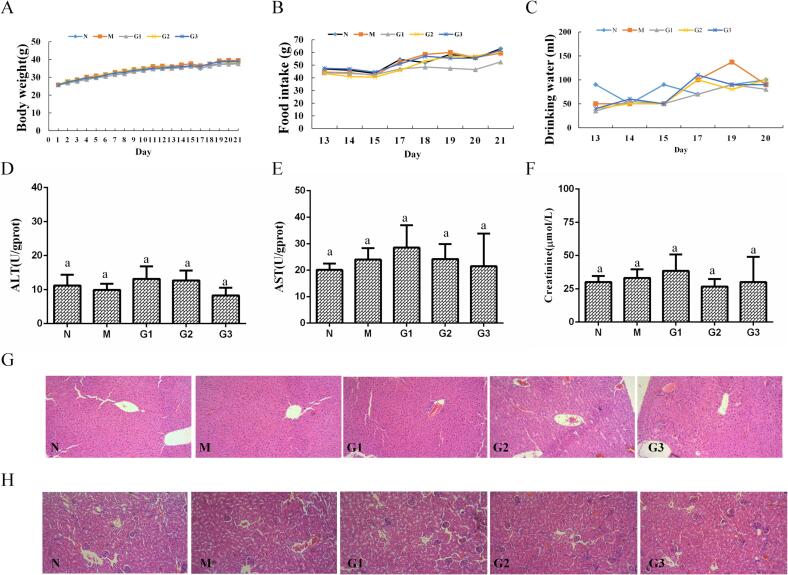
The body weight of Noni fruit extract-treated groups showed no significant differences compared to the normal group during the experiment (Fig. 4A). Consistently, the current data showed that food intake and water drinking were not significantly changed between mice in the Noni fruit extract-treated groups and control group (Fig. 4B and Fig. 4C).
Fig 4.
Toxicity analysis of Noni fruit extract administration. Statistics of (A) body weight, (B) food intake, and (C) drinking water in mice with or without Noni fruit extract administration. Body weight, food intake, and drinking water of all mice in each group (10 mice) were recorded and analyzed during the experiment. (D-F) Serum ALT, AST, and creatinine levels. (G-H) Hematoxylin and eosin (H&E) staining of liver and kidney tissues (Magnification 100×). Values were presented as mean ± SEM (n = 10). Groups represented, N: Saline; M: saline + diphenoxylate; G1: Noni fruit extract 0.625 g/kg + diphenoxylate; G2: Noni fruit extract 1.25 g/kg + diphenoxylate; G3: Noni fruit extract 2.5 g/kg + diphenoxylate.
3.5. Toxicity assessment of liver and kidney
To further examine if methanol extract of Noni fruit administration resulted in liver and kidney damage, the levels of AST, ALT, creatinine in serum were measured, and pathological examination of liver and kidney were detected by morphological observation using H&E-stained tissues. As presented in Fig. 4D-F, there was no statistical significance in terms of AST, ALT, and creatinine levels between mice administered with or without Noni fruit extract. Results of the histological examination in Fig. 4G and H showed normal morphological character both in liver and kidney tissues in all groups. Taken together, these results suggested low toxicity of this methanol extract of Noni fruit to liver and kidney.
3.6. Changes in gut microbiota
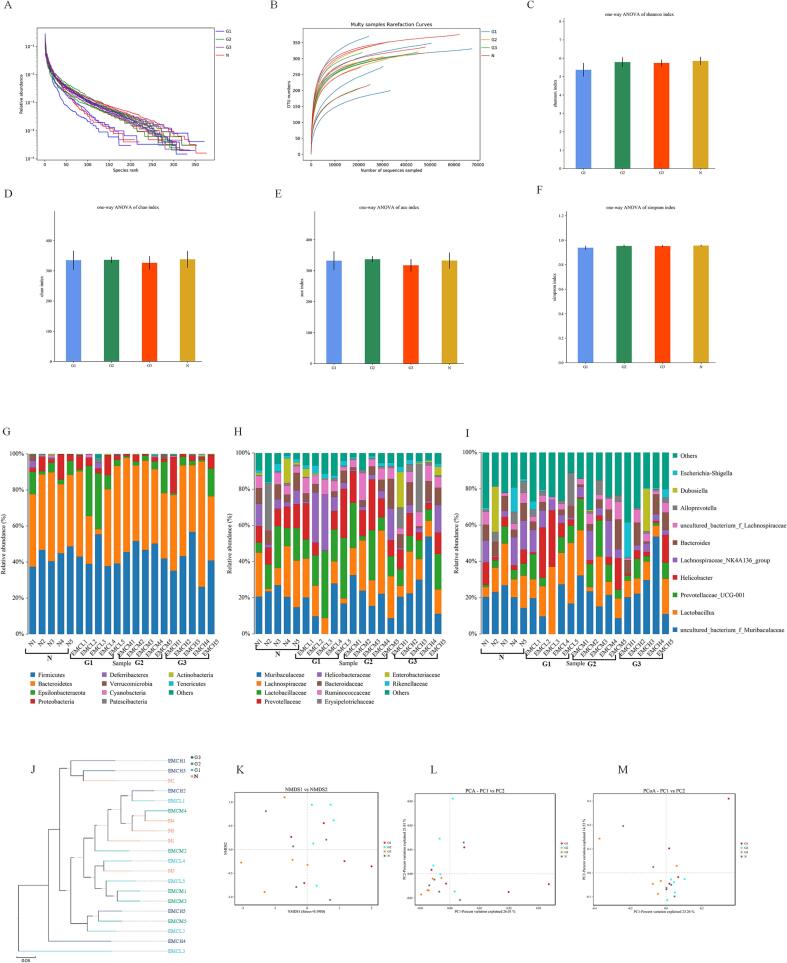
To clarify whether administration of methanol extract of Noni fruit altered gut microbial composition, the composition of fecal microbiota was assessed by sequencing the bacterial 16S rRNA V3 + V4 region. Results in Fig. S1 revealed weak modulating effects of methanol extract of Noni fruit (G1, G2, G3) on the number of operational taxonomic unit (OTUs). The observed OTU changes in the α-diversity between control mice or Noni fruit extract administered mice were assessed to evaluate the relative abundance of fecal microbiota, using the OTU rank curves, rarefaction curves, Shannon curves, Shannon index, Chao1 index, ACE index, and Simpson index. As shown in Fig. 5A-F, the richness and diversity of the gut microbiota remained unchanged with the treatment of methanol extract of Noni fruit, showing results similar to the number of OTUs. Notably, these results demonstrated that the abundance of gut microbiota was barely changed by the administration of methanol extract of Noni fruit.
Fig 5.
The abundance and structure of the gut microbiota in mice with or without Noni fruit extract administration. (A) OTU rank curves, and (B) rarefaction curves in normal mice or Noni fruit extract -administrated mice, respectively; (C-F) shows the Shannon index, Chao1 index, Simpson index, and ACE index of each group. Values were presented as mean ± S.E.M (n = 5). Relative abundances of the gut microbiota at phylum level (G), genus level (H) and family level (I). (J) Weighted Unifrac cluster tree based on UPGMA. (K) NMDS score plot based on Bray-Curtis, (L) PCoA score plot, and (M) PCA score plot based on binary jaccard. Groups represented, N: Saline; G1: Noni fruit extract 0.625 g/kg; G2: Noni fruit extract 1.25 g/kg; G3: Noni fruit extract 2.5 g/kg.
Additionally, the differences in the gut microbial composition were taxonomically assessed at the phylum level, the genus level, and the family level. The results of the phylum level analysis (Fig. 5G) showed that the fecal microbiota of mice was mainly composed of Bacteroidetes, Firmicutes, Proteobacteria, and Epsilonbacteraeota. The relative abundance of fecal microbiota was similar between the normal control mice and Noni fruit extract treated mice. The taxonomic changes in the microbial community evaluated at the family and genus levels (Fig. 5H-I) were consistent with those observed at the phylum level. The fecal microbiota of mice was mainly composed of Muribaculaceae, Lachnospiraceae, Lactobacillaceae, Prevotellaceae, and Helicobacteraceae at the family level, and mainly composed of Bacteroides, Lactobacillus, Lachnospiraceae, Helicobacter, and Prevotellaceae at the genus level.
Also, the overall composition of the gut microbiome was evaluated using β-diversity indices. The results of Weighted Unifrac cluster tree based on UPGMA showed that Noni fruit extract administration barely altered the gut microbiota structure (Fig. 5J). Structural changes of the gut microbiota analyzed by NMDS, PCoA and PCA also revealed that the Noni fruit extract treated groups (G1, G2, G3) were assigned proximate to the cluster of normal mice without Noni fruit extract supplementation (Fig. 5K-M). Totally, the composition of gut microbiota in mice with Noni fruit extract administration was similar to that of mice in the normal control group without Noni fruit extract supplementation, indicating that Noni fruit extract supplementation exhibited no influence on abundance and composition of gut microbiota.
3.7. Functional changes in gut microbiota
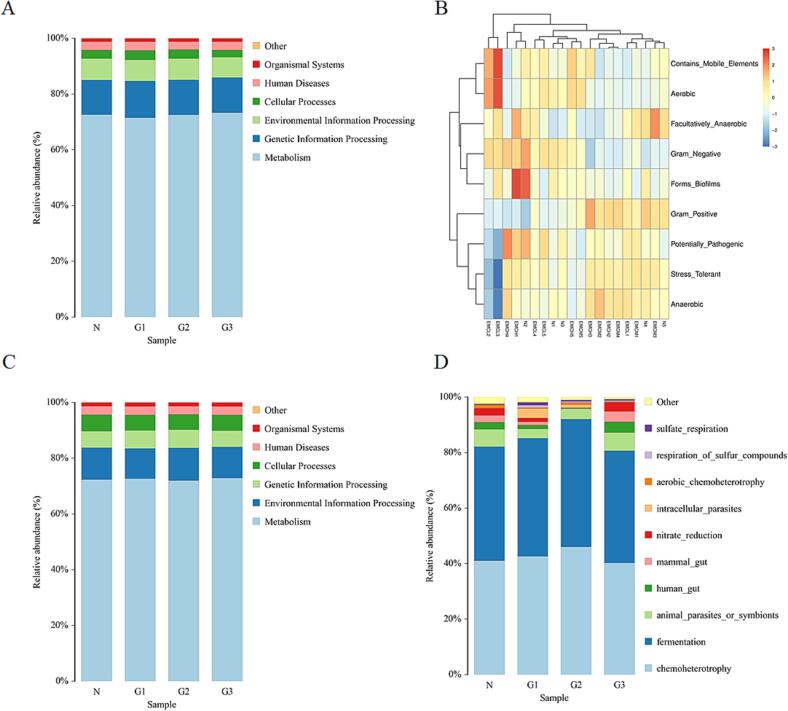
The software of PICRUSt, BugBase, Tax4Fun, and FAPROTAX were used to examined potential differences in the function of the gut microbiota between all mice. The results of KEGG enrichment analysis shown in Fig. 6 demonstrated that the supplementation of Noni fruit extract did not change the function of the gut microbiota. As shown in Fig. 6A, functions of known microbes initially predicted by PICRUSt software showed that fecal microbiota of all mice was mainly associated with functions of metabolism, genetic information, environmental information processing, cellular processes, human diseases, and organismal systems. Particularly, there was no significant difference in the abundance of bacteria enriched to function in human diseases, which was consistent with that predicted by TaxFun (Fig. 6C). Phenotypes of prokaryotic microorganisms predicted by BugBase showed that the phenotypes of fecal microbiota mainly included aerobic, anaerobic, contains mobile elements, facultatively anaerobic, forms biofilms, gram negative, gram positive, potentially pathogenic, and stress tolerant (Fig. 6B). Functions predicted by FAPROTAX showed that fecal microbiota of mice was mainly associated with chemoheterotrophy, fermentation, and animal parasites or symbionts (Fig. 6D). Further study of functional classifications also confirmed that the functional diversities were assigned to clusters that do not differ significantly between the normal groups (N) or Noni fruit extract treated groups (G1, G2, G3) when p value < 0.05 Collectively, these results indicated that supplementation of this Noni fruit extract had no influence on the function of gut microbiota.
Fig 6.
KEGG enrichment analysis of the functional composition of the gut microbiota in mice with or without Noni fruit extract administration. KEGG enrichment analysis of the function of gut microbiota was examined by the software of (A) PICRUSt, (B) BugBase, (C) Tax4Fun, and (D) FAPROTAX. Groups represented, N: Saline; G1: Noni fruit extract 0.625 g/kg; G2: Noni fruit extract 1.25 g/kg; G3: Noni fruit extract 2.5 g/kg.
4. Discussion and conclusion
Tremendous efforts have been focused on evaluating the benefits of Noni as functional food. Here, the present study evaluated the laxative activity of Noni fruit using the methanol extract. The results of our research showed, for the first time, that the Noni fruit extract exhibited obviously laxative activity, which worked by influencing bowel movements and facilitating defecation, with low toxicity to liver and kidney, and no significant influence on gut microbiome homeostasis. Moreover, UPLC-MS analysis identified eleven major constituents from this Noni fruit extract which may account for its bioactivity.
Since the fruit juice was approved by the European Commission as a Novel Food in 2003, Noni fruits have been commercialized as natural functional healthy food worldwide (Potterat & Hamburger, 2007). Unfortunately, a test of chronic oral toxicity of Noni fruit and leaf extracts was reported, and a presumed causal link between consumption of Noni juice and hepatotoxicity was suggested in previously published clinical case reports (Millonig et al., 2005, Mohamad Shalan et al., 2017, Stadlbauer et al., 2005). Also, the laxative activity of Noni has never been investigated detailly. Herein, in the present study, we investigated the laxative activity of methanol extract of Noni fruit and evaluated its toxicity. Our findings demonstrated the laxative activity of this extract in diphenoxylate-induced mice which was related to the acceleration of small intestinal transit. Toxic study clarified that no changes on serum levels of AST, ALT, and creatinine, and on pathological result of liver and kidney were observed after Noni extract administration, thereby preliminary indicating its safety for oral administration.
The gut microbiome, as an ecosystem embodying trillions of microorganisms, is a key contributor in shaping the physiology of the healthy host (Cani, 2018, Cryan et al., 2020). Indeed, the gut microbiota has mutual relationship and co-exist in harmony with the host, producing metabolites that contribute many beneficial effects such as maintenance of normal immunity, prevention of pathogen colonization, and improvement of nutrient absorption (Kau, Ahern, Griffin, Goodman, & Gordon, 2011). Moreover, growing knowledge with respect to the gut microbiome has linked the composition and function of human gut microbiome to pathophysiological processes of numerous common diseases and phenotypes, including obesity, immune dysregulation, liver cirrhosis, type 2 diabetes, and inflammatory bowel disease (Falony et al., 2016, Goodrich et al., 2014, Schmidt et al., 2018). Thus, the maintenance of gut microbiome homeostasis both in terms of bacterial diversity but also function is critical for maintaining healthy host physiology. Nevertheless, in recent years, increasing evidence has clarified that many drugs can damage intestinal commensal microbes, thus changing microbiome composition and function (Forslund et al., 2015). These changes can directly influence health outcomes or dramatically reduce drug efficacy (Weersma, Zhernakova, & Fu, 2020). Therapeutic strategies that have no influence on gut microbiome homeostasis, therefore, become ever more attractive. Fortunately, in the present study, we found that the fecal microbial composition was consistent among all mice administered with or without Noni extract when microbiota was assessed at the phylum, genus, and family level. KEGG enrichment analysis conducted by the software of PICRUSt, BugBase, Tax4Fun, and FAPROTAX indicated that the supplementation of Noni fruit extract did not change the function of the fecal microbiota. Particularly, fecal microbiota associated with functions of human diseases kept consistent among all mice. Thus, our present study demonstrated that mice administered orally with methanol extract of Noni fruit did not induce compositional or functional changes of the gut microbiota when compared with the normal control mice without Noni fruit extract supplementation.
UPLC-MS analysis identified a total of eleven previously known compounds from the methanol extract of Noni fruit, including chlorogenic acid, caffeic acid, ferulic acid, p-Hydroxybenzoic acid, coumalic acid, 4-hydroxycoumarin, kaempferol-3-rutinoside, isoquercetin, rutinum, quercetin, and obtusin. These monomers are reported to have anti-inflammatory (Gegentana et al., 2020), carbonic anhydrase inhibitory (Pontecorvi et al., 2022), and antioxidant activities (Sun et al., 2021). However, other biological activities of these monomers still remain unclear, it is difficult to infer which monomer may be exactly responsible for the laxative activity. Here, we demonstrated the laxative activity of this Noni fruit extract, indicating that the joint action of the identified monomers might presumably account for the laxative activity. The real evidence for the toxicity of these monomers has not been exactly established (Petric, Ruzic, & Zuntar, 2021). Fortunately, in the present study, we found that the methanol extract of Noni fruit containing eleven monomers exhibited no toxicity to mice.
Collectively, the results from our present study demonstrated that the methanol extract of Noni fruit could improve constipation by promoting defecation and gastrointestinal motility in mice with low toxicity to liver and kidney, and meanwhile, Noni fruit extract administration maintained gut microbiota homeostasis which confers health benefits to the host. Additionally, eleven major constituents were identified from this Noni fruit extract, which may account for its bioactivity. These outcomes therefore indicated that this methanol extract of Noni fruit could be used as an ideal supplementation for constipation.
Compliance with ethical standard
Funding: This research was supported by the Hebei Natural Science Foundation (H2022201051), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-071), the Innovation Capacity Improvement Plan of Hebei Province (20567605H), and Institute of Life Science and Green Development of Hebei University.
Ethical approval: This article does not contain any studies with human subjects. All animal studies were conducted following the guidance of the animal wel-fare committee of Hebei University.
Author contributions: Huo X., Liu M., and Ma G. designed and wrote the manuscript; Sun S., Zhang J., Gao H., and Cao Z. performed the experiments; Li K., Sun S., Gao H., Zhang J., Wang Y., and Xu X. analyzed the data; Li K., Wang Y., and Xu X. carefully reviewed the manuscript. All the listed authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100925.
Contributor Information
Guoxu Ma, Email: mgxfl8785@163.com.
Mengmeng Liu, Email: mmliu@hbu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
(A) OTU number and (B) Shannon curves of gut microbiota in normal mice or Noni fruit extract-administrated mice.
Data availability
Data will be made available on request.
References
- Almeida E.S., de Oliveira D., Hotza D. Properties and applications of Morinda citrifolia (Noni): A review. Comprehensive Reviews in Food Science and Food Safety. 2019;18(4):883–909. doi: 10.1111/1541-4337.12456. [DOI] [PubMed] [Google Scholar]
- Alsalimy N., Madi L., Awaisu A. Efficacy and safety of laxatives for chronic constipation in long-term care settings: A systematic review. Journal of Clinical Pharmacy and Therapeutics. 2018;43(5):595–605. doi: 10.1111/jcpt.12721. [DOI] [PubMed] [Google Scholar]
- Bharucha A.E., Pemberton J.H., Locke G.R., 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144(1):218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D. Human gut microbiome: Hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., O'Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurology. 2020;19(2):179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- Eor J.Y., Tan P.L., Lim S.M., Choi D.H., Yoon S.M., Yang S.Y., Kim S.H. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Research International. 2019;116:1173–1182. doi: 10.1016/j.foodres.2018.09.062. [DOI] [PubMed] [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K.…Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S.…Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegentana X., Li F.C., Zhang Y.F., Shen S.J., Yang P., Cai S.Q. Discovery of the active compounds of Smilacis Glabrae Rhizoma by utilizing the relationship between the individual differences in blood drug concentration and the pharmacological effect in rats. Journal of Ethnopharmacology. 2020;258 doi: 10.1016/j.jep.2020.112886. [DOI] [PubMed] [Google Scholar]
- Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R.…Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.H., Yi Y.S., Han S.Y., Aziz N., Kim H.G., Park S.H.…Cho J.Y. Morinda citrifolia noni water extract enhances innate and adaptive immune responses in healthy mice, ex vivo, and in vitro. Phytotherapy Research. 2019;33(3):676–689. doi: 10.1002/ptr.6256. [DOI] [PubMed] [Google Scholar]
- Hsieh S.K., Xu J.R., Lin N.H., Li Y.C., Chen G.H., Kuo P.C.…Tzen J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu'er tea (Camellia sinensis) Journal of Food and Drug Analysis. 2016;24(4):722–729. doi: 10.1016/j.jfda.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.H., Lin J.S., Li T.C., Lee S.C., Wang H.P., Lue H.C., Su Y.C. Comparison of a Chinese Herbal Medicine (CCH1) and lactulose as first-line treatment of constipation in long-term care: A randomized, double-blind, double-dummy, and placebo-controlled trial. Evidence-based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/923190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Park J.W., Kang M.J., Choi H.J., Bae S.J., Choi Y.S.…Hwang D.Y. Anti-inflammatory response and muscarinic cholinergic regulation during the laxative effect of asparagus cochinchinensis in loperamide-induced constipation of SD rats. International Journal of Molecular Sciences. 2019;20(4) doi: 10.3390/ijms20040946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.L., Chang Y.Y., Yang D.J., Tzang B.S., Chen Y.C. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chemistry. 2013;140(1–2):31–38. doi: 10.1016/j.foodchem.2013.02.035. [DOI] [PubMed] [Google Scholar]
- Luo D., Qu C., Zhang Z., Xie J., Xu L., Yang H.…Su Z. Granularity and laxative effect of ultrafine powder of dendrobium officinale. Journal of Medicinal Food. 2017;20(2):180–188. doi: 10.1089/jmf.2016.3827. [DOI] [PubMed] [Google Scholar]
- Millonig G., Stadlmann S., Vogel W. Herbal hepatotoxicity: Acute hepatitis caused by a Noni preparation (Morinda citrifolia) European Journal of Gastroenterology & Hepatology. 2005;17(4):445–447. doi: 10.1097/00042737-200504000-00009. [DOI] [PubMed] [Google Scholar]
- Mohamad Shalan N.A.A., Mustapha N.M., Mohamed S. Chronic toxicity evaluation of Morinda citrifolia fruit and leaf in mice. Regulatory Toxicology and Pharmacology. 2017;83:46–53. doi: 10.1016/j.yrtph.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Petric Z., Ruzic J., Zuntar I. The controversies of parabens - an overview nowadays. Acta Pharmaceutica. 2021;71(1):17–32. doi: 10.2478/acph-2021-0001. [DOI] [PubMed] [Google Scholar]
- Pontecorvi V., Mori M., Picarazzi F., Zara S., Carradori S., Cataldi A.…Supuran C.T. Novel insights on human carbonic anhydrase inhibitors based on coumalic acid: Design, synthesis, molecular modeling investigation, and biological studies. International Journal of Molecular Sciences. 2022;23(14) doi: 10.3390/ijms23147950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterat O., Hamburger M. Morinda citrifolia (Noni) fruit–phytochemistry, pharmacology, safety. Planta Medica. 2007;73(3):191–199. doi: 10.1055/s-2007-967115. [DOI] [PubMed] [Google Scholar]
- Schmidt T.S.B., Raes J., Bork P. The human gut microbiome: from association to modulation. Cell. 2018;172(6):1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Simons C.C., Schouten L.J., Weijenberg M.P., Goldbohm R.A., van den Brandt P.A. Bowel movement and constipation frequencies and the risk of colorectal cancer among men in the Netherlands Cohort Study on Diet and Cancer. American Journal of Epidemiology. 2010;172(12):1404–1414. doi: 10.1093/aje/kwq307. [DOI] [PubMed] [Google Scholar]
- Stadlbauer V., Fickert P., Lackner C., Schmerlaib J., Krisper P., Trauner M., Stauber R.E. Hepatotoxicity of NONI juice: Report of two cases. World Journal of Gastroenterology. 2005;11(30):4758–4760. doi: 10.3748/wjg.v11.i30.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Guo F., Peng X., Cheng K., Xiao L., Zhang H.…Deng Z. Metabolism of phenolics of tetrastigma hemsleyanum roots under in vitro digestion and colonic fermentation as well as their in vivo antioxidant activity in Rats. Foods. 2021;10(9) doi: 10.3390/foods10092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersma R.K., Zhernakova A., Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Wang J., Hong F., Wang S., Jin X., Xue T.…Zhai Y. Melatonin prevents obesity through modulation of gut microbiota in mice. Journal of Pineal Research. 2017;62(4) doi: 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- Zihad S., Saha S., Rony M.S., Banu H., Uddin S.J., Shilpi J.A., Grice I.D. Assessment of the laxative activity of an ethanolic extract of Bambusa arundinacea (Retz.) Willd. shoot. Journal of Ethnopharmacology. 2018;214:8–12. doi: 10.1016/j.jep.2017.11.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.