Highlights
-
•
Water holding capacity was increased from 3 d in both yak and cattle.
-
•
Cattle showed richer water holding capacity than yak.
-
•
Postmortem aging significantly affected muscle protein oxidation and denaturation.
-
•
The myofibrillar proteins were degraded during postmortem aging.
-
•
Protein-related properties would affect water holding capacity in postmortem muscle.
Keywords: Water distribution, Protein oxidative, Protein denaturation, Protein degradation, Muscle, Postmortem aging
Abstract
The water distribution have a profound influence on meat quality, and proteins play a critical role in water distribution. The water distribution detected with proton NMR and its relationship with protein related properties were investigated. Three populations of water were detected: bound water (T21, P21), immobilized water (T22, P22), and free water (T23, P23). The decreased T22 and T23 indicated an increase in water-holding capacity in both muscles from 3 days of aging. The P22 in cattle was higher than that in yak and the P23 in cattle was lower than that in yak, suggesting that cattle exhibited a greater water-holding capacity compared to yak. Moreover, postmortem aging affected muscle protein oxidation, denaturation, and degradation. Correlation analysis suggested that protein oxidation and denaturation caused muscle water loss and protein degradation could allow the muscle to retain water. It provides a basis for the optimization of quality of meat and products.
1. Introduction
The distribution of water in skeletal muscle and meat has been receiving increasing attention, as it significantly influences essential meat quality factors, including tenderness, juiciness, water-holding capacity and appearance (Qian et al., 2020). Muscle protein oxidation, denaturation, and degradation are considered to be the main determinants of water-holding capacity in meat (Bowker & Zhuang, 2015). Postmortem aging is a complex biochemical process that significantly impacts the location, content and mobility of water and affects biochemical reactions of proteins during conversion of muscle to meat (Huff-Lonergan and Lonergan, 2005, Pan et al., 2022). Yak (Bos grunniens) meat and products are attractive due to their richness in protein and low fat content (Zuo et al., 2016). However, it is generally accepted that poor water-holding capacity of yak meat during postmortem aging is a common problem, resulting in financial losses for the food industry due to poor processability and palatability (Li et al., 2019, Zuo et al., 2016). The exact mechanisms of water-holding capacity of yak meat during postmortem aging are far from fully understood.
Muscle proteins may undergo a series of physical and chemical changes attributes like denaturation, oxidation and degradation during postmortem aging (Bowker & Zhuang, 2015). The extended postmortem pH decline accelerates partial protein denaturation, leading to diminished water-holding capacity (Yang et al., 2022). The degree of protein denaturation is often determined by protein solubility (Bowker & Zhuang, 2015). Protein oxidation involves the modifications of amino acid side chains by free radicals, such as formation of sulfhydryl and carbonyl compounds (Delles & Xiong, 2014). Studies have shown that oxidation induces protein cross-linking resulting in the changes in myofilament spacing, water in muscle is lost from the changing spacing leading to the reduced water-holding capacity (Pan et al., 2022). The degradation of muscle proteins, primarily of myofibrillar proteins, has been proven to be correlated with water-holding capacity (Xing et al., 2016). Lawson (2004) reported that degradation of desmin leads to the swelling of muscle cells, which helps muscle structure retain water. Qian et al. (2020) revealed that integrin degradation might restrain the formation of drip channel in postmortem pork muscle. Overall, the protein denaturation, oxidation, and degradation are closely related to water-holding capacity. Although water-holding capacity reflects the ability of meat to retain its water, the distribution and mobility pattern of water, especially in postmortem yak meat is unclear. Furthermore, the relationship between those proteins- related properties and water properties (distribution and mobility) remains to be investigated.
Therefore, the objective of this study was to determine the distribution and mobility of water and its relationship with proteins-related properties in postmortem yak muscle. The differences of water properties and proteins-related properties between yak and cattle were also investigated. This novel observation will provide scientific insights about the mechanism of water distribution and mobility pattern and its relationship with protein-related properties. It is of great significance to improve the market value and processing performance of meat.
2. Materials and methods
2.1. Meat samples
Ten male yaks and cattle (Simmental crossbreeding local yellow cattle) with age of 36 ∼ 48 months fed on the same diet in the same batch were slaughtered by trained personnel at the commercial breeding farms of Qinghai (Qinghai Baide Investment Development Co. Ltd., Xining City, Qinghai Province, China) and Gansu (Gansu Bofeng Cattle Development Co. Ltd., Zhangye City, Gansu Province, China), respectively. The protocols and procedures employed were reviewed and approved by the Institutional Animal Care and Use Committee of Gansu Agricultural University (approved ID: 2012-2-159). After bleeding, the longissimus lumborum (LL) muscle was immediately removed, vacuum packed, and transferred to the laboratory at 4 °C. After removing visible connective tissues and fat, the LL muscles were cut into chops with an average weight of 20 g and stored at 4 °C for 0, 0.5, 1, 3, 5, and 7 days. A portion of samples randomly taken from each aging period were used to determine relaxation. The remainder of samples from each aging period were stored at −80 °C for protein related properties analysis.
2.2. Low-field nuclear magnetic resonance (LF-NMR)
The relaxation measurements were conducted according to Li et al. (2014) by using a PQ001 LF-NMR analyzer (Niumag Electric Co., Shanghai, China) with a magnetic field strength of 0.5 T and spectrometer frequency of 23 MHz at 32 °C. Approximately 2 g of muscle sample were placed in a 40-mm glass tube and inserted into the NMR probe. T2 was measured using the Carr-Purcell-Meiboom-Gill (CPMG) sequences with a τ value of 250 μs. A total of 3000 echoes were acquired with 16 scan repetitions. The data were analyzed by using MultiExp Inv Analysis software (Niumag Electric Corporation, Shanghai, China). Three relaxation T2 water populations (bound water T21, immobilized water T22, and free water T23) were determined using the cumulative integration method (Shao et al., 2016).
2.3. Magnetic resonance imaging (MRI)
The assay was performed according to Li et al. (2022) by using a NMI20-060HeI magnetic resonance imager (Niumag Electric Co., Shanghai, China). Proton density images were obtained using multiple-spin-echo (MSE) sequences with a TR value of 1000 ms and TE value of 18.2 ms. Pseudo-color maps were derived to visualize the water distribution, and the gray intensity values were analyzed by using the Image Evaluation software (Niumag Electric Co., Shanghai, China).
2.4. Extraction of myofibrillar protein
The extraction of myofibrillar protein was done as described by Bu et al. (2023) with minor modifications. In brief, meat samples were homogenized with 8-fold volume of buffer (0.025 M K3PO4, 0.15 M KCl, 2.5 mM EGTA, 2.5 mM MgCl2, pH 7.0) and then centrifuged at 3000 g for 15 min. The sediment was resuspended with an 8-fold volume of 1% Triton X-100 and centrifuged at 3000 g for 15 min. The sediment was again resuspended with an 8-fold volume of 0.1 M KCl and then centrifuged at 3000 g for 15 min. The obtained myofibrillar protein concentration was detected through the Biuret method.
2.5. Oxidative properties of myofibrillar proteins
2.5.1. Protein carbonyl content determination
The determination of protein carbonyl content was performed by using 2, 4-dinitrophenylhydrazine (DNPH) method (Levine, Williams, Stadtman, & Shacter, 1994). Briefly, myofibrillar protein (100 μL) was incubated with 500 μL 2 M HCl containing 0.02 M DNPH for 60 min. Subsequently, 500 μL of 25% trichloroacetic acid was added and the mixture was centrifuged at 10,000g for 8 min. The pellet was washed with ethanol ethyl acetate solution (1:1, v:v) thrice and then incubated with 10 mL of 5 M guanidine hydrochloride for 30 min at 37 °C. The absorbance was measured at 370 nm.
2.5.2. Protein sulfhydryl content determination
The assay was performed as described by Srinivasan and Hultin (1997) with minor modifications. In brief, 250 μL of myofibrillar protein (2 mg/mL) was incubated with 2 mL of 0.01 M 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB) for 30 min at 25 °C. The absorbance was measured at 412 nm. The sulfhydryl content was calculated using a molar absorbance coefficient of 13600 mol/(L cm) and the results are expressed as nmol sulfhydryl/mg protein.
Where A412 is the absorbance of the solution at 412 nm. D and C are dilution ratio and protein concentration, respectively.
2.6. Hydration properties of myofibrillar proteins
2.6.1. Protein solubility assay
The protocol was performed according to a previous study (Bowker & Zhuang, 2015) with minor modifications. Sarcoplasmic protein solubility was conducted by homogenizing 2 g of muscle samples in 15 mL of cold 20 mM potassium phosphate buffer (pH 7.2). The homogenates were placed on a rocker plate at 4 °C for 12 h and then centrifuged at 4000g for 20 min. The protein concentration of the supernatant was detected through the Biuret method. Total protein solubility was performed by homogenizing 2 g of muscle samples in 15 mL of cold 100 mM potassium phosphate buffer (pH 7.2) containing 1.1 M KI. The following procedure was similar to the sarcoplasmic protein solubility determination. Myofibrillar protein solubility was expressed as the difference between total protein solubility and sarcoplasmic protein solubility.
2.6.2. Protein surface hydrophobicity assay
The assay was performed as described by Chelh et al. (2006) with slight modifications. The protein concentration of myofibrillar protein was adjusted to 1.5 mg/mL with phosphate buffer (pH 7.4). Subsequently, 50 μL of 1.8 mg/mL bromophenol blue solution was added, and the mixture was centrifuged at 4500g for 20 min. The absorbance was measured at 595 nm.
2.7. Differential scanning calorimeter (DSC)
The thermal properties of the meat samples were measured using DSC (DSC25, TA company, America) according to Shang et al. (2022). Approximately 15 mg of the samples were placed and sealed in an aluminum crucible and then put into the DSC. The measurement was conducted under following conditions: the DSC was balanced at 20 °C for 2 min, and the temperature was then raised from 20 °C to 100 °C at the rate of 5 °C/min. The DSC map was obtained, and the transition temperature was measured.
2.8. SDS-PAGE
The assay was performed according to a previous study (Sun & Arntfield, 2012) with slight modifications. In brief, 1 g minced meat sample was homogenized with 10-fold volume of isolation buffer (0.15 M KCl, 2.5 mM EDTA, 2.5 mM MgCl2, 1.5 mM NaN3, 2.5 mM Na4P2O7, 1.5 mM DTT, 15 mM Tris–maleate, pH 6.8) and then centrifuged at 1000g for 10 min. The supernatant was discarded. Subsequently, the above procedures were repeated for 5 times. The protein concentration was detected through the Biuret method. The concentration of gel sample was adjusted to 4 mg/mL. A 12% polyacrylamide separating gel and a 5% stacking gel were applied, and 20 μL of the sample was loaded onto the gel. The electrophoresis was carried out at 80 V for the stacking gel and 120 V for the separating gel. The amount of target protein was calculated by gray scanning using Image J software (NIH Image, MD, USA).
2.9. Statistical analysis
The data from different aging times (0, 0.5, 1, 3, 5, and 7 d) were analyzed by using Analysis of Variance Procedure. Student’s t test was used for different species (yak and cattle) comparison (*P < 0.05, **P < 0.01). The differences between the mean values were compared by Duncan’s multiple range test (P < 0.05). Each assay was performed at least thrice.
3. Results and discussion
3.1. Water distribution by LF-NMR and MRI
3.1.1. LF-NMR analysis
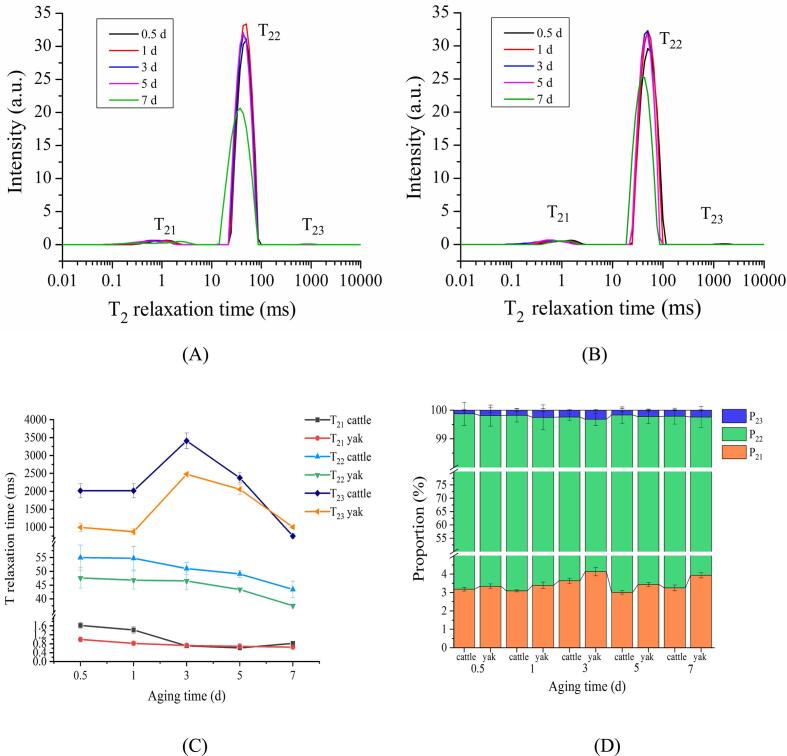
LF-NMR provides useful information regarding interactions between exchangeable protons and water protons in proteins, thus providing insight into the physical–chemical state of water in skeletal muscle (Pearce, Rosenvold, Andersen, & Hopkins, 2011). LF-NMR is an excellent technique for quantifying the water distribution and mobility in muscle and meat without destroying the muscle structure (Zhao et al., 2023). The T2 relaxation time curves obtained from yak and cattle are given in Fig. 1A and 1B, respectively. Three distinct peaks were observed at 0.01 ∼ 10 ms (T21), 10 ∼ 100 ms (T22), and 100 ∼ 10000 ms (T23). T21 represents bound water, which is tightly bound to other macromolecular constituents by molecular force; T22 represents immobilized water, which is related to the muscle fiber structure and susceptible to loss due to myofibrillar protein degradation; T23 represents free water, which is mainly found on the surface of the myofibrillar lattice and can move freely (Li et al., 2022). A slower relaxation time indicates the limited water mobility in muscle (Xing et al., 2016). The T21 values were too low to reflect water migration in postmortem muscle. Besides, T21 was not affected by postmortem aging due to its strong binding connection to proteins (Bi et al., 2023). It is suggested that muscle samples with longer T22 and T23 relaxation time exhibit poor water-holding capacity (Zhang et al., 2019). Fig. 1C displays the T2 relaxation time of both muscles for different aging periods, and the corresponding area proportions are shown in Fig. 1D. In Fig. 1C, no significant changes were observed in T21 in either yak or cattle (P > 0.05). T22 decreased from 5 to 7 d in yak and from 3 to 7 d in cattle (P < 0.05). T23 reached its highest value at 3 d postmortem in both yak and cattle and then decreased at 3 to 7 d (P < 0.05). Overall, the current study indicates that the water-holding capacity increased from 3 d in both yak and cattle. In China, the postmortem aging technology is extensively applicated to the processing of cattle under the guidance of the national standard (GB/T 29392). However, as the yak industry started relatively late, the application of postmortem aging on yak is very limited (Bai et al., 2023). The biochemical factors that affect water distribution in yak and cattle at different aging periods should be further explored.
Fig. 1.
Water distribution of yak and cattle during aging. (A) Representative water distribution curve of yak. (B) Representative water distribution curve of cattle. (C) Changes in T2 relaxation time of yak and cattle. T21: the T2 relaxation time of bound water; T22: the T2 relaxation time of immobilized water; T23: the T2 relaxation time of free water. (D) Changes in water ratio of yak and cattle. P21: the percent of bound water; P22: the percent of immobilized water; P23: the percent of free water.
The peak area proportion represented the content of the three components of water in muscles. P21, P22, and P23 respectively were related to the areas of relaxation times T21, T22, and T23. In Fig. 1D, it can be seen that the area proportions of P21 (bound water) and P23 (free water) were <5% for both yak and cattle samples during aging, while the area proportion of P22 (immobilized water) was >95%. This suggests that immobilized water is the main water component in skeletal muscle, which is supported by a previous study (Zhao et al., 2023). At 3 d postmortem, the P22 in both yak and cattle was lowest (P < 0.05), and the P23 in both muscles was highest (P < 0.05), indicating that some of the immobilized water could be transformed into free water at 3 d postmortem. This reveals that poor water-holding capacity had occurred at early 3 d postmortem aging stage. These results are supported by Zhang et al. (2019), who demonstrated the high water mobility or a high proportion of free water suggests poor water-holding capacity. Additionally, the P22 in cattle was higher than that in yak, and the P23 in cattle was lower than that in yak (P < 0.05), suggesting that cattle have a richer water-holding capacity than yak. In general, yaks mature later than cattle. It is well established that yak and cattle tend to accumulate more intramuscular fat as the animal ages, and the meat should contain more juice due to intramuscular fat (Bai et al., 2023). We therefore speculated that cattle showed more intramuscular fat and exhibited richer water-holding capacity compared to yak at the same ages. It has been reported that water distribution and water-holding capacity are closely related to rigor mortis. The pH decrease that occurs due to rigor mortis can result in myofibrillar lateral shrinkage, leading to differences in water distribution and decreased water-holding capacity at 3 d postmortem (Pearce et al., 2011, Sun et al., 2019). Overall, the LF-NMR results indicate that poor and rich water-holding capacity appeared at 3 d and 5 d postmortem in both yak and cattle, respectively. In addition, cattle exhibited a richer water-holding capacity than yak.
3.1.2. MRI analysis
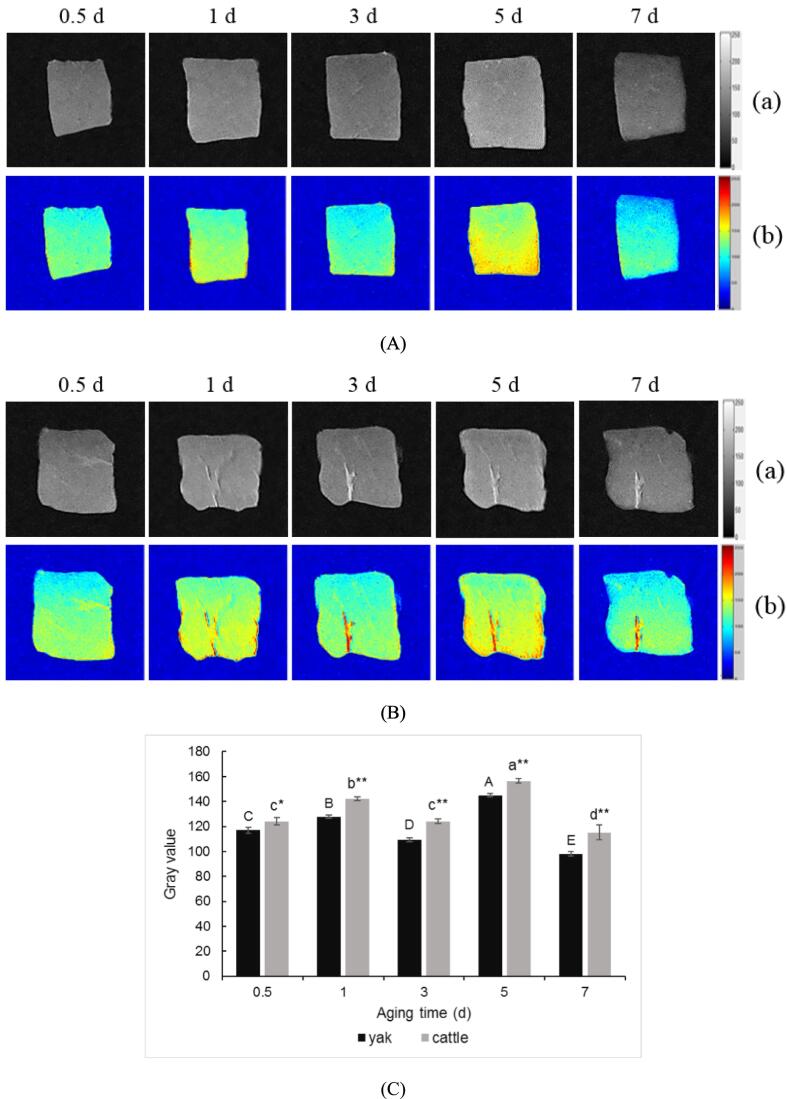
LF-NMR results confirmed significant differences in water distribution between yak and cattle muscle samples during postmortem aging, so MRI was used to further analyze them visually. LF-NMR combined with MRI is a common method widely used to evaluate the distribution and mobility pattern of water (Li et al., 2022, Zhao et al., 2023). The pseudo-color maps of both yak and cattle during postmortem aging are shown in Fig. 2 (Ab and Bb), where the red area corresponds to a high density of H proton, which reflects the bound or immobile water, while the blue area corresponds to a low density of H proton, which reflects the free water in skeletal muscle (Zhao et al., 2023). The blue area was found to be distributed more at 3 d postmortem, whereas the red area was more at 5 d postmortem in both yak and cattle. The gray value of grey-scale maps (Fig. 2 Aa and Ba) is shown in Fig. 2C, reflecting the contents of immobilized water in the muscle sample. A high proportion of immobilized water suggests rich water-holding capacity. In this study, the gray value in both yak and cattle was lowest (P < 0.05) at 3 d postmortem, and it was highest (P < 0.05) in both muscles at 5 d postmortem. The results of pseudo-color maps and gray value indicated a high content of free water and low water-holding capacity at 3 d, while a high content of immobilized water and rich water-holding capacity at 5 d postmortem. Postmortem muscle pH development is closely related to water-holding capacity. A fast decrease in pH at 3 d postmortem in skeletal muscle (Zhang et al., 2017) results in myofibrillar lateral shrinkage, leading to water loss. With the extension of aging, an increase in pH at 5 d deviates from the isoelectric point of muscle fibers, and the dissociation of myosin enlarges the spatial structure to retain more water (Sun et al., 2019). Furthermore, the gray value in cattle was higher than that in yak, suggesting that cattle exhibited a richer water-holding capacity than yak. The MRI results were in accordance with the aforementioned changes in T2 relaxation time and their area proportion.
Fig. 2.
Changes in grey-scale (a) and pseudo-color (b) maps of (A) yak and (B) cattle during aging and (C) gray value of yak and cattle during aging. The capital letters represent the difference within the yak and the lowercase letters represent the difference within the cattle (P < 0.05). ** means the differences between yak and cattle (P < 0.01). * means the differences between yak and cattle (P < 0.05).
3.2. Changes in oxidative and denaturation properties of proteins in postmortem muscle
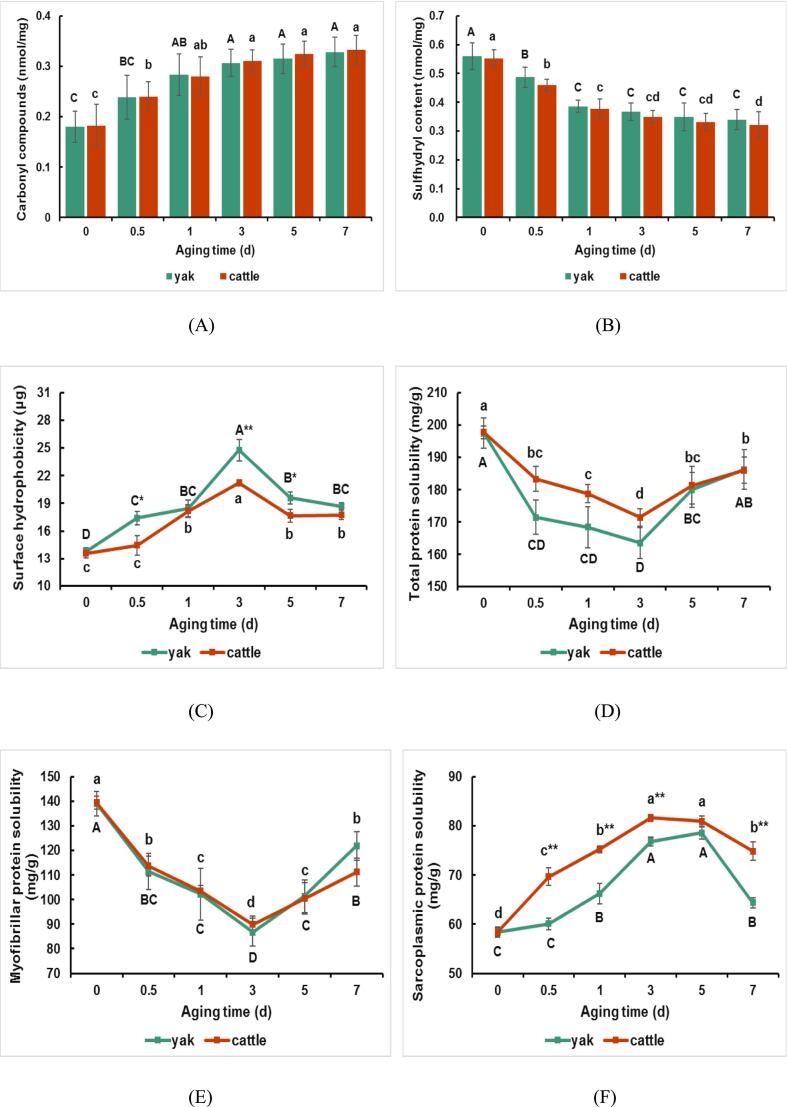
Protein oxidation is an irreversible oxidative modification that inevitably occurs in postmortem muscle. Protein oxidation might lead to the reduction of water-holding capacity and deterioration of meat tenderness (Pan et al., 2022). Protein carbonyl and sulfhydryl have been widely used to evaluate muscle protein oxidation (Liu, Liu, Zheng, & Ma, 2022). Protein oxidation leads to the generation of a variety of oxidation products, among which the carbonyl compounds are considered to be one of the most important products. The sulfhydryl group, with high antioxidant reactivity, is easily oxidized to –SOH, –SOOH, and –SS–, leading to a decrease in sulfhydryl content (Pan et al., 2022). In this study, protein carbonyl content increased at 0 to 1 d (P < 0.05) in yak and increased at 0 to 0.5 d in cattle (P < 0.05; Fig. 3A). The sulfhydryl content decreased in both yak and cattle at 0 to 1 d (P < 0.05; Fig. 3B), suggesting the occurrence of protein oxidation in postmortem muscles. Thus, postmortem aging significantly affected muscle oxidative status. Nevertheless, no differences in carbonyl and sulfhydryl content were found between yak and cattle during postmortem aging. The relationship between protein oxidation and water-holding capacity was demonstrated by the increased formation of carbonyl compounds, decreased formation of sulfhydryl compounds. During the postmortem aging, muscle is attacked by reactive oxygen species, which leads to increased oxidation capacity of muscle tissue. Meanwhile, protein oxidation destroys muscle protein structure, which leads to protein aggregation and reduced activity of related enzymes (Liu, Liu, Zheng, & Ma, 2022). Bao and Ertbjerg (2019) proposed that protein oxidative may disrupt the order and integrity of muscle cells and limit the ability of myogenic fibres to absorb water, reducing the water-holding capacity of the meat. The surface hydrophobicity of myofibrillar proteins reflects the hydration properties of proteins, which could be used to evaluate the effect of conformational stability of myofibrillar proteins on water-holding capacity in meat. The lower the surface hydrophobicity of proteins, the stronger the water-binding ability, representing the richer water-holding capacity (Wang, Zhang, Fang, & Bhandari, 2016). In the present study, the surface hydrophobicity in both muscles increased at 0.5 to 3 d and then decreased at 3 to 5 d (P < 0.05; Fig. 3C). Furthermore, the surface hydrophobicity in yak was higher than that in cattle at 3 d (P < 0.01) and 5 d (P < 0.05). The increase in surface hydrophobicity of protein will weaken its hydration ability, causing bound and immobilized water to change into free water, severely reducing muscle water-holding capacity (Xu et al., 2023). The results indicated the poor water-holding capacity at 3 d postmortem, and cattle showed a richer water-holding capacity than yak.
Fig. 3.
Change in oxidative and denaturation properties of proteins of yak and cattle during aging. (A) Carbonyl compounds. (B) Sulfhydryl content. (C) Surface hydrophobicity. (D) Total protein solubility. (E) Myofibrillar protein solubility. (F) Sarcoplasmic protein solubility. The capital letters represent the difference within the yak and the lowercase letters represent the difference within the cattle (P < 0.05). ** means the differences between yak and cattle (P < 0.01). * means the differences between yak and cattle (P < 0.05).
The denaturation properties of proteins are usually determined by detecting protein solubility in postmortem muscle (Bowker & Zhuang, 2015). Total protein, myofibrillar protein, and sarcoplasmic protein solubility are shown in Fig. 3. The total protein solubility decreased at 0 to 0.5 d and then increased at 3 to 5 d in yak, and it decreased at 0 to 3 d and then increased at 3 to 5 d in cattle (P < 0.05; Fig. 3D). The myofibrillar protein solubility in both yak and cattle decreased at 0 to 3 d and then increased at 3 to 7 d (P < 0.05; Fig. 3E). The low protein solubility indicated a high extent of protein denaturation (Choi et al., 2010). The majority of water in the muscle fiber is trapped within the myofibrils, and denaturation of myofibrillar proteins can be linked to a reduction in the amount of water that can be held by myofibrillar proteins, and thereby leading to reduction of water-holding capacity (Zhang, Puolanne, & Ertbjerg, 2021). The results of this study suggested the poor water-holding capacity at 3 d postmortem, which was consistent with the previous study that reported that decreased myofibrillar protein solubility was closely related to low water-holding capacity (Choi et al., 2010). In addition to myofibrillar protein, studies have shown that sarcoplasmic protein solubility also plays a crucial role in water-holding capacity. It has been demonstrated that the high sarcoplasmic protein solubility reflects rich water-holding capacity (Bowker & Zhuang, 2015). The denatured sarcoplasmic proteins could alter the gelation progress of muscle proteins which in turns increases the water-holding capacity (Yang et al., 2022). Consistently, the present study found that the sarcoplasmic protein solubility in both yak and cattle increased at 0 to 3 d and then decreased at 5 to 7 d (P < 0.05; Fig. 3F). Moreover, the cattle showed higher sarcoplasmic protein solubility than yak from 0.5 to 7 d (except for 5 d), which was also supported by the results of surface hydrophobicity. The pH value of yak meat is lower than that of cattle meat. Lower pH will likely lead to more severe protein denaturation and thus contribute to reducing the amount of water that are held within myofibrils (Zhang, Puolanne, & Ertbjerg, 2021). It may explain the reasons that cattle show a richer water-holding capacity than yak.
3.3. DSC analysis
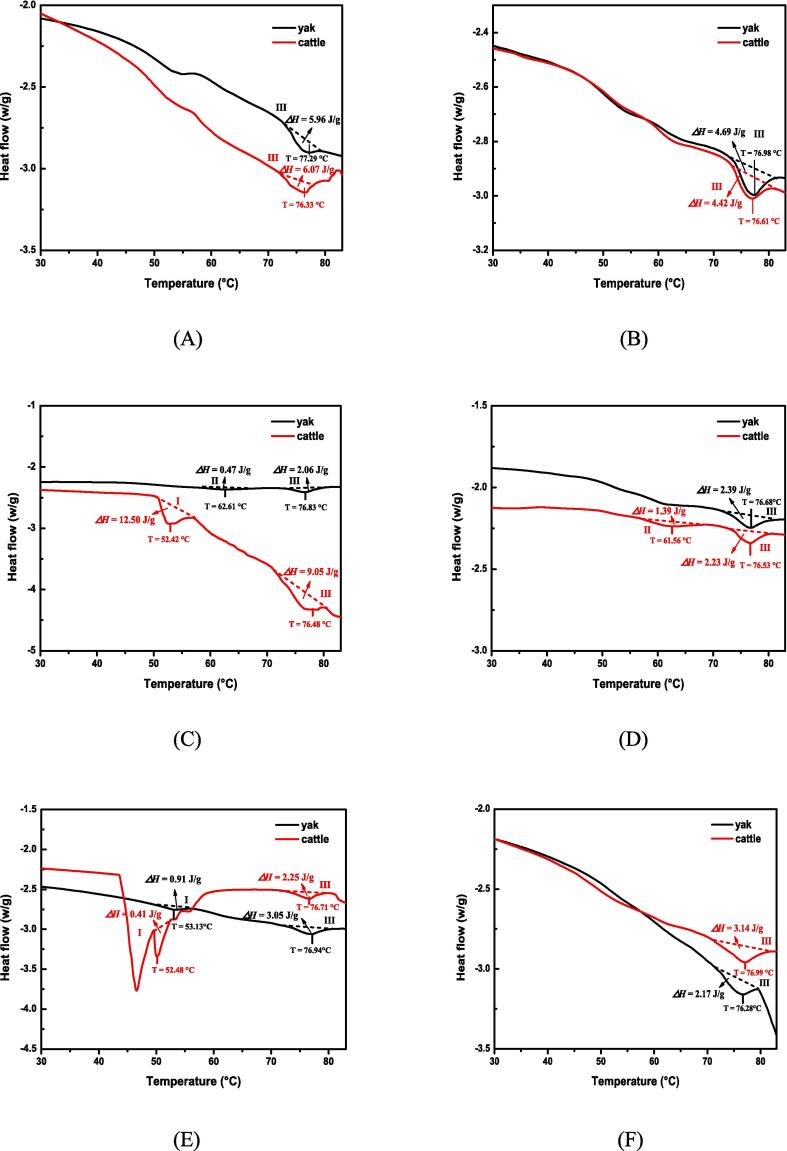
In general, three endothermic transitions usually occur in muscle proteins, representing the unfolding of myosin (peak I, 50 ∼ 60 °C), a mixture of collagen/sarcoplasmic protein (peak II, 60 ∼ 70 °C), and actin (peak III, 70 ∼ 80 °C) (Vaskoska et al., 2021). In this study, three major peaks were found in both yak and cattle during postmortem aging (Fig. 4 and Table S1). The peak I was observed at 5 d postmortem in yak, while it was found at 1 d and 5 d postmortem in cattle. Besides, the enthalpy (ΔH) of myosin in cattle at 1 d postmortem was 12.50 J/g, which was noticeably higher than that of 3 d postmortem (0.41 J/g). These results indicated the total denaturation of myosin at 5 d postmortem in both yak and cattle. Myosin accounts for approximately 40% of myofibrillar proteins, which has a remarkable impact on muscle water-holding capacity. Peak II was detected at 1 d postmortem in yak, while it was observed at 3 d postmortem in cattle, suggesting the denaturation of collagen/sarcoplasmic protein after 1 d in yak and 3 d in cattle. Sun et al. (2019) indicated that protein was more prone to denaturation at low pH, which played an important part in lateral shrinkage of myofibrils resulting in a decreased intra-myofibrillar space. Accordingly, the denaturation of collagen/sarcoplasmic protein occurred earlier in yak than in cattle. Moreover, there were no differences in the thermal denaturation temperature of actin in both yak and cattle (P > 0.05), indicating that the actin was stable in both muscles during postmortem aging. Overall, DSC results demonstrated that myosin and collagen/sarcoplasmic protein was denatured during aging. The myosin and collagen/sarcoplasmic protein denaturation likely shrinks the myofilamental lattice spacing thereby reducing the water-holding capacity (Liu, Puolanne, & Ertbjerg, 2014).
Fig. 4.
DSC curves of yak and cattle during aging. (A) 0 d. (B) 0.5 d. (C) 1 d. (D) 3 d. (E) 5 d. (F) 7 d. T: temperature; ΔH: enthalpy.
3.4. Myofibrillar protein degradation in postmortem muscle
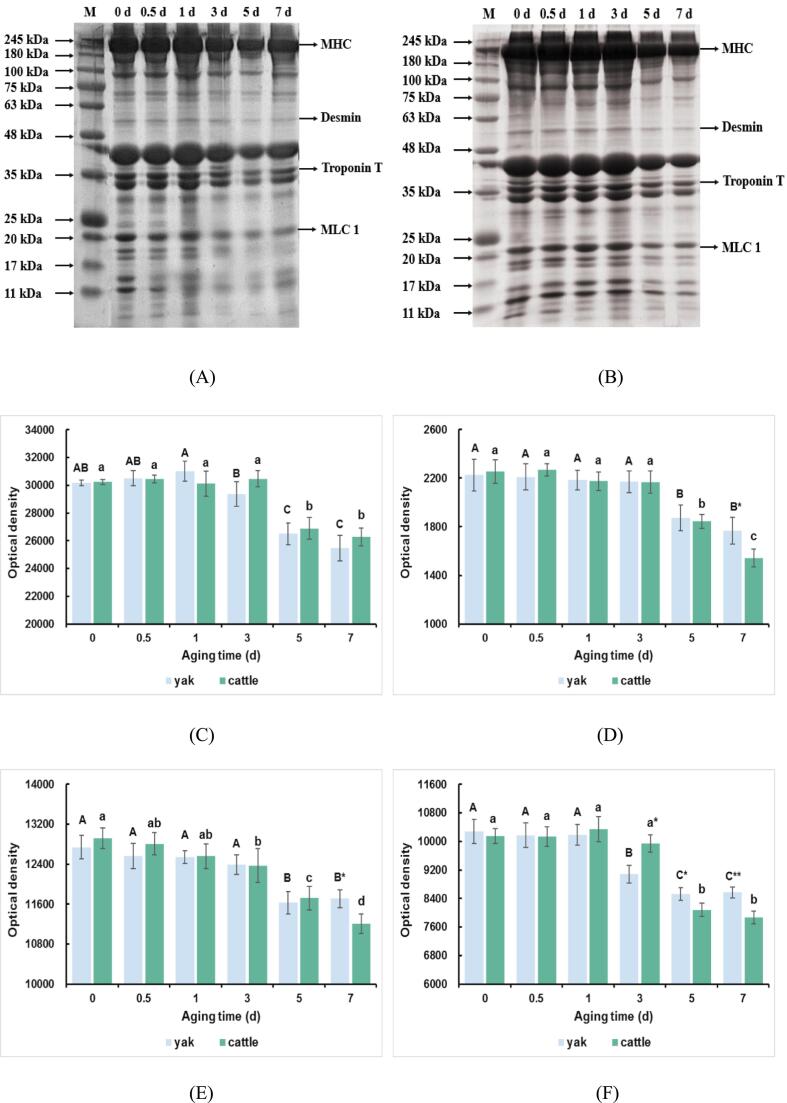
Fig. 5 (A and B) displays the protein separation by SDS-PAGE to evaluate the degradation of myofibrillar proteins of yak and cattle during postmortem aging. The myofibrillar proteins comprise 220 kDa of myosin heavy chain (MHC), 54 kDa of desmin, 35 kDa of troponin-T, and 23 kDa of myosin light chain 1 (MLC1). It has been reported that enzymatic degradation of key myofibrillar proteins, such as MHC, desmin, troponin-T, and others, would cause the weakening of intra and inter myofibrillar bonds which would result in improvement of water-holding capacity and tenderness development (Zhang et al., 2020). MHC is the main structural and functional protein in muscle, which responsible for the structural integrity of skeletal muscle involved in muscle contraction and relaxation. The degradation of MHC directly leads to the disruption of muscle structure and softening of skeletal muscle (Sun et al., 2023). In this study, the intensity of MHC decreased at 3 d to 5 d in both cattle and yak (P < 0.05; Fig. 5C), suggesting a stepwise escalation in MHC degradation after 3 d postmortem in both muscle. Besides, Xue et al. (2012) suggested that MHC is the major component of thick filament in sarcomeres, and postmortem aging could cause cross-linking in MHC through carbonyl and disulfide bonds. Thus, the results of MHC degradation were in accordance with the increased carbonyl formation (Fig. 3A). Desmin and troponin-T play a key role in maintaining myofibrillar structure, and the degradation of desmin and troponin-T positively impacts on water-holding capacity (Zhang et al., 2020). The present study showed that the intensity of desmin and troponin-T decreased at 3 d to 5 d in yak and decreased at 3 d to 7 d in cattle (P < 0.05; Fig. 5D and E). This result indicated that significant degradation of desmin and troponin-T occurred in yak and cattle after 3 d postmortem, which could be explained by residual calpain-1 activity at late postmortem aging (Lomiwes, Farouk, Wu, & Young, 2014). It has been suggested that low desmin and troponin T degradation results in severe shrinkage of muscle cells and increases the distance between those, which further leads to a high drip loss of the postmortem muscles during storage (Huff-Lonergan & Lonergan, 2005). Therefore, significant degradation of desmin and troponin-T in this study indicated the rich water-holding capacity after 3 d postmortem. MLC1 is closely related to the head region of the myosin molecule, and it was reported to modulate the myosin motor (Anderson, Lonergan, & Huff-Lonergan, 2012). The intensity of MLC1 decreased at 1 d to 5 d in yak and decreased at 3 d to 5 d in cattle (P < 0.05; Fig. 5F), suggesting the degradation of MLC1 in postmortem yak and cattle muscle. Degradation of MHC during aging could potentially cause release of MLC1 into the sarcoplasmic fraction, and the release of MLC1 weakens the actomyosin bond. During postmortem aging, the weakening of the bond between actin and myosin could cause the disruption of the actomyosin crossbridges and result in an increase in water-holding capacity (Anderson, Lonergan, & Huff-Lonergan, 2012). In addition, the intensity of desmin and troponin-T in cattle was lower than that of yak at 7 d postmortem (P < 0.05; Fig. 5D and 5E). Besides, the cattle showed a lower intensity of MLC1 than yak at 5 d (P < 0.05) and 7 d postmortem (P < 0.01; Fig. 5F). These results indicated that the degradation rate of desmin, troponin-T, and MLC1 in cattle was higher than that of yak. The degradation of myofibrillar proteins within the myofibril at rigor at low pH may reduce the myofibrillar lattices' resistance to external forces, which could irreversibly change the spacing between the myofibrils, resulting in increased drip loss (Bond & Warner, 2007). This could explain why the cattle show the higher degradation of myofibrillar proteins and richer water-holding capacity. Furthermore, the ongoing degradation of these myofibrillar proteins resulted in consistent changes in muscle water-holding capacity. Therefore, the cattle exhibited a richer water-holding capacity than yak, which was also supported by the results of water distribution and protein-related properties.
Fig. 5.
Representative SDS-PAGE of myofibrillar proteins of (A) yak and (B) cattle during aging. (C) Optical density analysis of MHC. (D) Optical density analysis of desmin. (E) Optical density analysis of troponin T. (F) Optical density analysis of MLC1. The capital letters represent the difference within the yak and the lowercase letters represent the difference within the cattle (P < 0.05). ** means the differences between yak and cattle (P < 0.01). * means the differences between yak and cattle (P < 0.05).
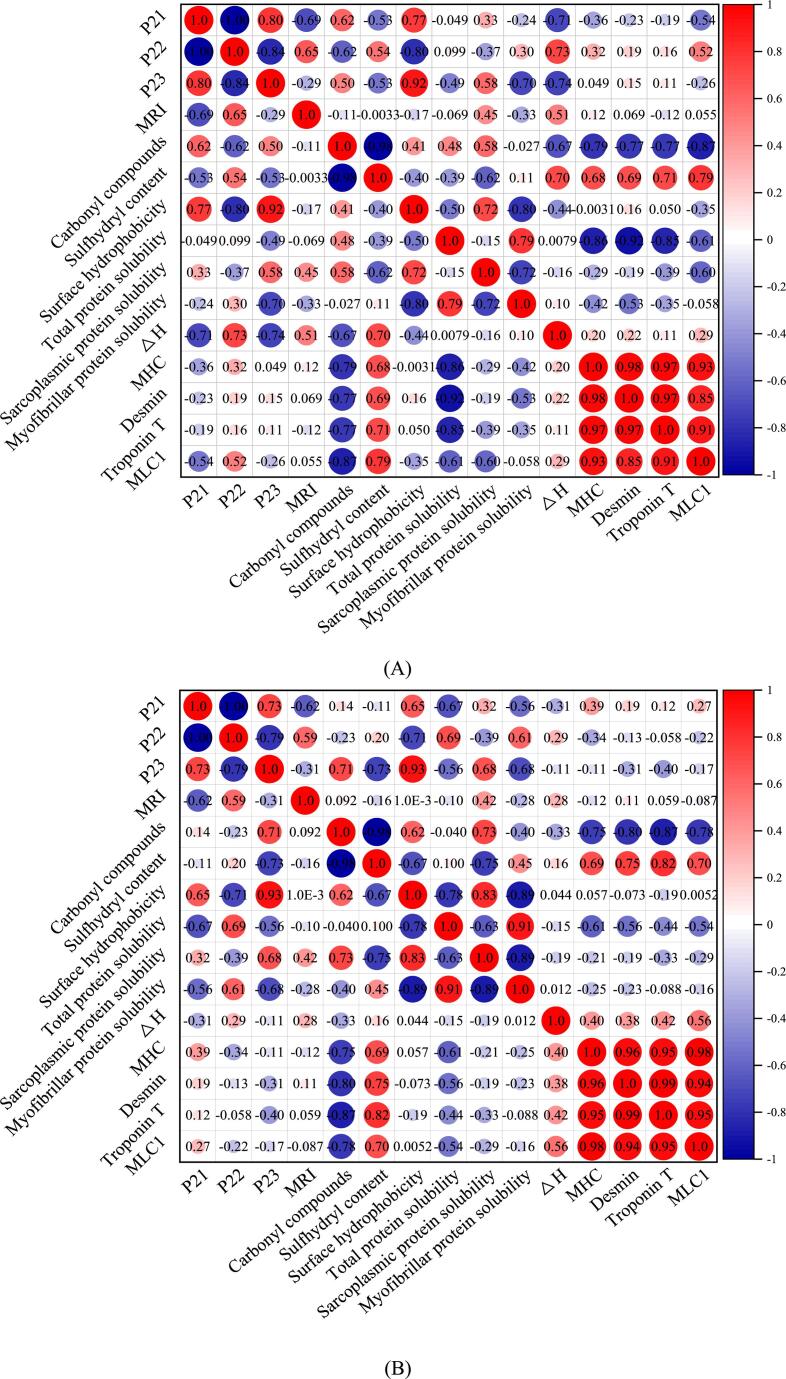
3.5. Correlation analysis
Protein oxidation, denaturation, and degradation are regarded as the main determinants of water-holding capacity in meat (Bowker & Zhuang, 2015). Therefore, correlation analysis was applied to study the relationship between protein oxidation and denaturation, degradation of myofibrillar proteins, and water distribution in yak (Fig. 6A) and cattle (Fig. 6B). The Pearson correlation coefficients in yak indicate that P21 and P23 were positively correlated with changes in carbonyl compounds and surface hydrophobicity. They were negatively correlated with sulfhydryl content and ΔH. P21 was also negatively correlated with the degradation of MLC1. Besides, P22 had positive correlations with sulfhydryl content, ΔH, and degradation of MLC1, and negative correlations with carbonyl compounds and surface hydrophobicity. This study also found positive correlations between MRI and ΔH. Fig. 6B in cattle showed that P21 and P23 had positive correlations with surface hydrophobicity and negative correlations with total protein solubility and myofibrillar protein solubility. In addition, P22 was positively correlated with total protein solubility and myofibrillar protein solubility. It was also negatively correlated with surface hydrophobicity. P23 represents free water, and a high proportion of free water suggests low water-holding capacity. P22 represents immobilized water, and a high proportion of immobilized water suggests rich water-holding capacity. The generation of carbonyl compounds in the process of protein oxidation and increases of surface hydrophobicity lead to the reduction of water-holding capacity. The higher the ΔH, the better the thermal stability of proteins, indicating the reducing of loss of free water. The degradation of MLC1 results in rich muscle water-holding capacity. This could explain the correlation between protein-related properties and water distribution. These correlation analysis results indicate protein-related properties, including protein oxidation, denaturation, and degradation, could affect water-holding capacity in postmortem muscle, which is in agreement with previous findings in pork muscle (Zhang et al., 2020). Overall, the results of correlation between protein-related properties and water-holding capacity provided a theoretical basis for the improvement of meat quality.
Fig. 6.
Correlation analysis of the water distribution and protein-related properties of (A) yak and (B) cattle performed by Pearson’s correlation analysis. The correlation coefficient is shown in different colors in the Figure; the colored boxes on the right indicate the different correlation coefficients. The correlation coefficient is proportional to the color depth. A correlation coefficient closer to −1 (blue) indicated a negative correlation, and that closer to +1 indicated a positive correlation (red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
In summary, the present study indicated, using LF-NMR, that poor water-holding capacity had occurred at 3 d postmortem aging, and it increased in both yak and cattle after 3 d. Additionally, the cattle showed richer water-holding capacity than yak. MRI results confirmed the changes observed with LF-NMR. Moreover, postmortem aging affected muscle protein oxidation, denaturation, and degradation, which then affected the muscle water distribution. The correlation analysis results further confirmed the relationship between these protein-related properties and water-holding capacity in postmortem muscle. Understanding protein-related properties in postmortem skeletal muscle is essential to improve water-holding capacity and its quality to expand the development and consumption of meat products in the market. In the future, the molecular connections between biochemical reactions of proteins and water distribution in skeletal muscles should be further investigated.
CRediT authorship contribution statement
Zhaobin Guo: Investigation, Data curation, Formal analysis, Funding acquisition, Writing – original draft. Cheng Chen: Formal analysis, Writing – review & editing. Guoyuan Ma: Writing – review & editing. Qunli Yu: Supervision. Li Zhang: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the University Innovation Fund of Gansu Province (2022A-056) and China National Modern Agriculture (Beef Cattle and Yak) Industrial Technology System Funding Project (CARS-37).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100891.
Contributor Information
Zhaobin Guo, Email: guozhb007@163.com.
Li Zhang, Email: zhanglwubd@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Anderson M.J., Lonergan S.M., Huff-Lonergan E. Myosin light chain 1 release from myofibrillar fraction during postmortem aging is a potential indicator of proteolysis and tenderness of beef. Meat Science. 2012;90:345–351. doi: 10.1016/j.meatsci.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Bai X., Yin F., Ru A., Tian W., Chen Q., Chai R., et al. Effect of slaughter age and postmortem aging time on tenderness and water-holding capacity of yak (Bos grunniens) longissimus thoracis muscle. Meat Science. 2023;202 doi: 10.1016/j.meatsci.2023.109201. [DOI] [PubMed] [Google Scholar]
- Bao Y., Ertbjerg P. Effects of protein oxidation on the texture and water-holding of meat: A review. Critical Reviews in Food Science and Nutrition. 2019;59:3564–3578. doi: 10.1080/10408398.2018.1498444. [DOI] [PubMed] [Google Scholar]
- Bi Y., Shan Q., Luo R., Bai S., Ji C., Wang Y., et al. Dynamic changes in water mobility and taste substances of cooked Tan lamb meat after chilled storage. Journal of Food Composition and Analysis. 2023;117 [Google Scholar]
- Bond J.J., Warner R.D. Ion distribution and protein proteolysis affect water-holding capacity of longissimus dorsi et lumborum in meat lamb subjected to antemortem exercise. Meat Science. 2007;75:406–414. doi: 10.1016/j.meatsci.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poultry Science. 2015;94:1657–1664. doi: 10.3382/ps/pev120. [DOI] [PubMed] [Google Scholar]
- Bu X., Wang H., Wang Y., Ojangba T., Nan H., Zhang L., et al. Effects of iron-catalyzed oxidation and methemoglobin oxidation systems on endogenous enzyme activity and myofibrillar protein degradation in yak meat. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134647. [DOI] [PubMed] [Google Scholar]
- Chelh I., Gatellier P., Santé-Lhoutellier V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Science. 2006;74:681–683. doi: 10.1016/j.meatsci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Lee S.H., Choe J.H., Rhee M.S., Lee S.K., Joo S.T., et al. Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livestock Science. 2010;127:183–191. [Google Scholar]
- Delles R.M., Xiong Y.L. The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Science. 2014;97:181–188. doi: 10.1016/j.meatsci.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lawson M.A. The role of integrin degradation in post-mortem drip loss in pork. Meat Science. 2004;68:559–566. doi: 10.1016/j.meatsci.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Levine R.L., Williams J.A., Stadtman E.P., Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Li S., Yu Q., Han L., Zhang Y., Tian X., Zhao S. Effects of proteome changes on the tenderness of yak rumen smooth muscle during postmortem storage based on the label-free mass spectrometry. Food Research International. 2019;116:1336–1343. doi: 10.1016/j.foodres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu D., Zheng A., Ma Q. Haem-mediated protein oxidation affects water-holding capacity of beef during refrigerated storage. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Puolanne E., Ertbjerg P. Temperature induced denaturation of myosin: Evidence of structural alterations of myosin subfragment-1. Meat Science. 2014;98:124–128. doi: 10.1016/j.meatsci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Li X., Fan M., Huang Q., Zhao S., Xiong S., Yin T., et al. Effect of micro and nano-starch on the gel properties, microstructure and water mobility of myofibrillar protein from grass carp. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130579. [DOI] [PubMed] [Google Scholar]
- Li Y., Li X., Wang J.Z., Zhang C.H., Xie X.L. Effects of oxidation on water distribution and physicochemical properties of porcine myofibrillar protein gel. Food Biophysics. 2014;9:169–178. [Google Scholar]
- Lomiwes D., Farouk M.M., Wu G., Young O.A. The development of meat tenderness is likely to be compartmentalised by ultimate pH. Meat Science. 2014;96:646–651. doi: 10.1016/j.meatsci.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Pan J., Li C., Liu X., He L., Zhang M., Huang S., et al. A multivariate insight into the organoleptic properties of porcine muscle by ultrasound-assisted brining: Protein oxidation, water state and microstructure. LWT–Food Science and Technology. 2022;159 [Google Scholar]
- Pearce K.L., Rosenvold K., Andersen H.J., Hopkins D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes — A review. Meat Science. 2011;89:111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Qian S., Li X., Wang H., Wei X., Mehmood W., Zhang C., et al. Contribution of calpain to protein degradation, variation in myowater properties and the water-holding capacity of pork during postmortem ageing. Food Chemistry. 2020;324 doi: 10.1016/j.foodchem.2020.126892. [DOI] [PubMed] [Google Scholar]
- Shang S., Wu B., Fu B., Jiang P., Liu Y., Qi L., et al. Enzyme treatment-induced tenderization of puffer fish meat and its relation to physicochemical changes of myofibril protein. LWT–Food. Science and Technology. 2022;155 [Google Scholar]
- Shao J.H., Deng Y.M., Jia N., Li R.R., Cao J.X., Liu D.Y., et al. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chemistry. 2016;200:308–314. doi: 10.1016/j.foodchem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Hultin H.O. Chemical, physical, and functional properties of cod proteins modified by a nonenzymic free-radical-generating system. Journal of Agricultural and Food Chemistry. 1997;45:310–320. [Google Scholar]
- Sun K., Pan C., Chen S., Liu S., Hao S., Huang H., et al. Quality changes and indicator proteins of Litopenaeus vannamei based on label-free proteomics analysis during partial freezing storage. Current Research in Food Science. 2023;6 doi: 10.1016/j.crfs.2022.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.B., Huang J.C., Li T.T., Ang Y., Xu X.L., Huang M. Effects of preslaughter shackling on postmortem glycolysis, meat quality, changes of water distribution, and protein structures of broiler breast meat. Poultry Science. 2019;98:4212–4220. doi: 10.3382/ps/pez175. [DOI] [PubMed] [Google Scholar]
- Sun X.D., Arntfield S.D. Gelation properties of myofibrillar pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocolloids. 2012;27:394–400. [Google Scholar]
- Vaskoska R., Venien A., Ha M., White J.D., Unnithan R.R., Astruc T., et al. Thermal denaturation of proteins in the muscle fbre and connective tissue from bovine muscles composed of type I (masseter) or type II (cutaneous trunci) fibres: DSC and FTIR microspectroscopy study. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128544. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang M., Fang Z., Bhandari B. Gelation properties of myofibrillar protein under malondialdehyde induced oxidative stress. Journal of the Science of Food and Agriculture. 2016;97:50–57. doi: 10.1002/jsfa.7680. [DOI] [PubMed] [Google Scholar]
- Xing T., Li Y.H., Li M., Jiang N.N., Xu X.L., Zhou G.H. Influence of transport conditions and pre-slaughter water shower spray during summer on protein characteristics and water distribution of broiler breast meat. Animal Science Journal. 2016;87:1413–1420. doi: 10.1111/asj.12593. [DOI] [PubMed] [Google Scholar]
- Xue M., Huang F., Huang M., Zhou G. Influence of oxidation on myofibrillar proteins degradation from bovine via μ-calpain. Food Chemistry. 2012;134:106–112. [Google Scholar]
- Xu Y., Zhang D., Xie F., Li X., Schroyen M., Chen L., et al. Changes in water-holding capacity of chilled fresh pork in controlled freezing-point storage assisted by different modes of electrostatic field action. Meat Science. 2023;204 doi: 10.1016/j.meatsci.2023.109269. [DOI] [PubMed] [Google Scholar]
- Yang N., Liang X., Cao J., Zhang Q., Tan Y., Xu B., et al. Denaturation manner of sarcoplasmic proteins in Pale, Soft and Exudative meat determines their positive impacts on myofibrillar water-holding capacity. Meat Science. 2022;185 doi: 10.1016/j.meatsci.2021.108723. [DOI] [PubMed] [Google Scholar]
- Zhang D., Li H., Emara A.M., Hu Y., Wang Z., Wang M., et al. Effect of in vitro oxidation on the water retention mechanism of myofibrillar proteins gel from pork muscles. Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2020.126226. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu Q., Han L., Chen C., Li H., Han G. Study on the apoptosis mediated by cytochrome c and factors that affect the activation of bovine longissimus muscle during postmortem aging. Apoptosis. 2017;22:777–785. doi: 10.1007/s10495-017-1374-2. [DOI] [PubMed] [Google Scholar]
- Zhang M., Xia X., Liu Q., Chen Q., Kong B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Science. 2019;151:24–32. doi: 10.1016/j.meatsci.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Puolanne E., Ertbjerg P. Mimicking myofibrillar protein denaturation in frozen-thawed meat: Effect of pH at high ionic strength. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128017. [DOI] [PubMed] [Google Scholar]
- Zhao X., Guo R., Li X., Wang X., Zeng L., Weng X., et al. Effect of oil-modifed crosslinked starch as a new fat replacer on gel properties, water distribution, and microstructures of pork meat batter. Food Chemistry. 2023;409 doi: 10.1016/j.foodchem.2022.135337. [DOI] [PubMed] [Google Scholar]
- Zuo H., Han L., Yu Q., Niu K., Zhao S., Shi H. Proteome changes on water-holding capacity of yak longissimus lumborum during postmortem aging. Meat Science. 2016;121:409–419. doi: 10.1016/j.meatsci.2016.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.