Highlights
-
•
Study on the relationship between lipids and VOCs.
-
•
Identified 13 key VOCs in grouper lipid.
-
•
PUFA, mainly DHA and EPA, could be the primary source of key VOCs.
Keywords: Grouper, HS-SPME-GC–MS, Volatile compounds, Lipid, Fatty acid, Cold storage
Abstract
To investigate the relationship between lipid oxidation and the development of volatile compounds (VOCs) in grouper lipid during cold storage, lipids were extracted from grouper as a single-factor study to avoid the complex interactions between microorganisms and proteins. Lipid oxidation during storage and the content of 12 long-chain fatty acids (FAs) in grouper lipids were evaluated. The HS-SPME-GC–MS technique was used to analyze the VOCs in grouper lipids, and a total of 13 key VOCs, primarily comprising alcohols and aldehydes, were screened. Pearson correlation analysis showed a strong acorrelation between these 13 key VOCs, which influenced the overall flavor of grouper lipids, and lipid oxidation, mainly involving secondary oxidation of lipids and the oxidation of long-chain polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Possible solutions for grouper lipid deterioration were proposed, providing a reference for maintaining the overall quality of grouper and regulating flavor formation.
1. Introduction
According to a report by the Food and Agriculture Organization (FAO), global per capita consumption and availability of seafood have increased by approximately 1.3 times compared to 20 years ago and continue to rise annually (Wang et al., 2022, Wang et al., 2022). Additionally, Naylor et al. (2021) predicted that fish demand will further increase double by the mid-21st century. Grouper (Epinephelus coioides), ranked as the third most economically significant fish in China in terms of annual production, is favored by consumers due to its high content of unsaturated fatty acids, various amino acids, and rich flavor. However, these qualities also contribute to the challenge of grouper storage and logistics. To inhibit microbial metabolism and enzyme activity, as well as reduce the rate of biochemical reactions, cold storage (0–4 °C) is a commonly used technique due to its simplicity and cost-effectiveness (Wang et al., 2022). Nevertheless, even during cold storage, proteins, lipids, and other substances can deteriorate and even lead to spoilage of aquatic products.
The evaluation of aquatic product quality based on odor has been one of the choices for consumers (Li et al., 2022). Previous studies have separately examined the impact of protein changes and VOCs alterations on quality deterioration (Chu, Mei, et al., 2023). Another crucial endogenous factor is lipid oxidation. Lipid oxidation affects the color, texture, nutritional value, taste, and aroma of aquatic products, resulting in off-flavors and undesirable tastes (Chu et al., 2022). This is a significant motive for scientists to investigate methods for inhibiting lipid oxidation and preserving the quality of aquatic products. Lipid oxidation is a complex process involving enzymatic oxidation of fatty acids by lipoxygenases, leading to the formation of hydroperoxides and peroxides with conjugated double bonds due to oxygen addition to the hydrocarbon chain (Amaral et al., 2018). Additionally, the chain reaction induced by free radicals causes lipid autoxidation (Mariutti & Bragagnolo, 2017). The autoxidation of unsaturated fatty acids (UFA) primarily occurs due to the presence of numerous oxidative break sites in UFAs, which react with oxygen to generate primary oxidation products and subsequently decompose into alkoxy and peroxy radicals (Wang et al., 2022). Some secondary compounds, including aldehydes and alcohols, are formed through reactions with various substances at β-breaks of free radicals (Bassam et al., 2022, Chang et al., 2020). Thus, the formation of VOCs is closely associated with lipid oxidation, particularly the oxidation and degradation of UFAs in fish, where long-chain fatty acids are known to be highly susceptible to oxidation (Shchepinov, 2020). Moreover, lipid oxidation products such as alcohols and aldehydes have lower odor thresholds compared to other types of VOCs and contribute more to the flavor of aquatic products (Zhang et al., 2009). Furthermore, since most volatile odors are lipophilic, lipids can retain volatile gases (Ammari & Schroen, 2018), underscoring the significance of studying lipid oxidation and VOC changes.
As described in previous studies, food matrices have a very complex microenvironment, where various interactions occur between microorganisms, proteins, and lipids (Chu, Mei, et al., 2023). This complexity poses challenges in determining the true underlying causes of quality deterioration and odor changes in aquatic products. To date, extensive research has focused on analyzing the volatile organic compounds (VOCs) of aquatic products and evaluating product quality based on relatively quantitative concentrations of individual VOCs (Dai et al., 2022, Lou et al., 2023, Mishra et al., 2023). However, limited information exists regarding the relationship between lipid oxidation and VOCs. Furthermore, no relevant research has been conducted to explore the development of lipid components during cold storage by excluding the influence of microorganisms or proteins.
In summary, this study aimed to investigate lipid oxidation in grouper by conducting a univariate analysis that focused solely on lipids and excluded the influence of microorganisms and proteins. Various parameters including peroxide values (POV), thiobarbituric acid-reactive substances (TBARs), total oxidation values (TOTOX), acid value (AV), and anisidine value (AnV) were evaluated to assess lipid oxidation in grouper during cold storage. The study also examined volatile compounds (VOCs) present in grouper lipids and established a relationship between VOCs and lipid oxidation by considering changes in free fatty acids. By focusing on both the oxidation and degradation of grouper lipids during cold storage, as well as the relationship between fatty acid changes and VOCs, this study aimed to enhance the monitoring of quality. Furthermore, the investigation into the dynamic changes of lipid composition in grouper provides insights for future research on the quality and nutritional value of aquatic food. These findings can serve as a reference for implementing appropriate measures to reduce lipid oxidation and the production of undesirable VOCs in the future.
2. Materials and methods
2.1. Preparation of grouper samples and lipid extraction
The live groupers weighing approximately 650 g ± 50 g were procured from the Luchao Harbor Seafood Market, situated near the school. To minimize sample variability, lipids from a total of 20 groupers were utilized in this study. First, with the help of seafood market staff, the live groupers were beaten to death on the head. Subsequently, the head, viscera, skin, and bones were carefully removed, leaving only the muscle tissue. Finally, the muscle tissue was placed in crushed ice and transported to the laboratory within 30 min for lipid extraction.
Lipids were extracted by referring to the method of Carvalho Barros et al. (2020) with slight modifications. Briefly, 100 g of grouper muscle was thoroughly mixed with 300 mL of chloroform–methanol (2:1, v/v) and homogenized in an ice-water bath using a high-speed homogenizer (HR-500, Shanghai, China). The resulting mixture was then transferred to a sealed container and kept at 4 °C for 3 h. Afterward, the mixture was filtered using a double-layer qualitative filter paper. To the filtrate, a volume of 1/5 (v/v) physiological saline was added, and the resulting solution was subjected to centrifugation at 8000 g for 20 min. The lipid solution was collected, and the organic solvents were evaporated using nitrogen gas. The extracted 85 mL of lipids from the grouper were aliquoted into capped centrifuge tubes, stored in a light-protected environment, and kept in a refrigerator at 4 °C for 15 d. To ensure the exclusion of microbial influence during storage, all sampling procedures were conducted in a sterile environment.
2.2. Evaluation of lipid oxidation
2.2.1. Determination of POV
The determination of POV was carried out in an environment protected from direct sunlight, referring to the method of Zhang et al. (2022) with slight modifications. A total of 1 g of grouper lipid was mixed thoroughly with 15 mL of a trichloromethane-glacial acetic acid mixture (1:1, v/v) until complete dissolution. Next, 1.00 mL of saturated potassium iodide solution was accurately added to the mixture and shaken for 30 s. After allowing it to stand in the dark for 3 min, 50 mL of ultrapure water was added. The iodine precipitate was then titrated using a 0.002 mol/L Na2S2O3 standard solution until the solution turned light yellow. Subsequently, 1 mL of starch indicator was added, and the titration was continued until the blue color disappeared. Blank experiments were also conducted. The POV was expressed as the mass fraction of peroxide equivalent to iodine and calculated using the following formula:
where V is the volume of Na2S2O3 standard solution consumed, mL; V0 is the volume of Na2S2O3 standard solution consumed in the blank experiment, mL; c is the concentration of Na2S2O3 standard solution, mol/L; 0.1269 is the mass of iodine equivalent to 1.00 mL of Na2S2O3 standard solution (1.000 mol/L), g; and m is the mass of the sample, g. The result of POV is expressed as g/100 g.
2.2.2. Determination of TBARs
The determination of TBARs in grouper lipid was performed referring to the method of Agyare et al. (2022) with slight modifications. Briefly, 0.2 g of the sample was accurately weighed and then dissolved and diluted to a volume of 25 mL by 1-butanol. Next, 5 mL of the solution was mixed with 5 mL of TBA reagent (0.2 g/100 mL, dissolved in 1-butanol). The mixture was then heated in a water bath at 95 °C for 2 h. After cooling the mixture to room temperature, the absorbance was measured at a wavelength of 530 nm. Blank experiments were conducted alongside to account for any background interference or non-specific absorbance. The TBARs value was calculated by the following formula:
where A is the absorbance value of the solution under test; B is the absorbance value of the blank reagent; and m is the mass of the sample, g.
2.2.3. The calculation of TOTOX
TOTOX is a significant indicator that allows for a comprehensive assessment of both primary and secondary oxidation, as mentioned by Hu et al. (2022). It was calculated based on the POV and TBARs, according to the following equation:
2.2.4. Determination of AV
The determination of AV in grouper lipid was referred to the method of Zhang et al. (2021). Briefly, 0.2 g of lipid was accurately weighed and dissolved in 50 mL of an isopropanol-ether mixture (1:1, v/v). After sufficient shaking to ensure thorough mixing, the solution was titrated with 0.1 mol/L NaOH, and the volume of NaOH consumed was recorded. The AV was then calculated based on the volume of KOH consumed (V) and the mass of the sample (m), and the result was expressed as mg KOH/g lipid. The calculation of AV was as follows:
2.2.5. Determination of AnV
The determination of AnV in grouper lipid was conducted following the method described by Hwang et al. (2017). Specifically, 1 mL of sample was mixed with 25 mL of isooctane, followed by centrifugation at 4000 r/min for 20 min at 4 °C. The absorbance value (A1) of the supernatant was then measured at 350 nm. Additionally, 5 mL of the sample solution was mixed with 1 mL of p-methoxyaniline solution (0.25 g/100 mL, dissolved in acetic acid) and allowed to stand for 10 min before measuring the absorbance value (A2) at 350 nm. The calculation of AnV was as follows:
2.3. Quantitative analysis of FFAs
FFAs were quantified using UPLC -QTOFMS analysis methods (Waters, Milford, CT). The entire analysis was carried out at 45 °C. The mobile phases were 0.1 % formic acid (v/v, A) and acetonitrile/methanol/isopropanol (2:2:1, v/v/v, B), and the concentration of buffer B was tapered from 5 % to 100 % during elution. The flow rate was 0.4 mL/min.
Data collection was conducted in both positive and negative ion modes using the following parameter settings: scanning range of 50–1000 amu, scanning rate of 0.2 s/scan, capillary voltage of 2 kV, source temperature of 115 °C, and desolventizing gas temperature of 450 °C. The data were acquired using UNIFI 1.9.4 software (Waters, Milford, CT). The obtained results were expressed in mg/g.
2.4. Relative quantification of volatile compounds
VOCs in lipids were extracted using the HS-SPME method. In the case of grouper lipids, this study characterized and relatively quantified the VOCs by referencing previous methods (Chu, Mei, et al., 2023). Briefly, 1 mL of lipid was placed in a headspace injection vial, then an internal standard (IS) of 10 μL aliquot of 2,4,6-trimethyl-pyridine (HPLC, 1 mg/100 mL) was added. The headspace injection vial was then sealed and equilibrated in a water bath to facilitate proper headspace formation. Subsequently, the VOCs were extracted using an extraction needle (50/30UMDVB/CARonPDMS) for 40 min. The VOCs were characterized using a GC–MS system (Agilent 7890A-5975C, US). The specific machine settings referred to the study by Chu, Ding, et al. (2023).
The identity of each peak in the VOC analysis was determined through a combination of matching and comparison with the NIST 18 mass spectral library, the Wiley version 6.0 database for GC–MS, and published VOC retention indices (RI). In addition to identification, the relative content of VOCs was determined by considering the internal standard (IS) addition and mass ion response intensity, and the calculation was as follows.
where Cn and CIS are the concentrations of the detected VOC and IS, respectively; and In and IIS are the mass-ion response strengths of the detected VOC and IS, respectively.
The odor activity value (OAV) is the ratio of the detected VOC concentration to its odor threshold. The thresholds of detected VOC were obtained from “Handbook of Odor”.
2.5. Statistical data
All experiments were conducted with three replications, and the results were presented as mean ± standard deviation (SD). The collected data were subjected to one-way analysis of variance (ANOVA) using SPSS software 26.0, and significance was determined using Duncan's test. Different lowercase letters were used to indicate significant differences (p < 0.05) between the samples. To analyze the relationship and variability of VOCs and FFAs, dimensionality reduction techniques such as PCA and OPLS-DA were employed. Furthermore, to understand the impact of lipid oxidation and FFAs on the formation of VOCs in grouper during cold storage, Pearson correlation analysis was conducted to uncover FFAs associated with the production of key VOCs.
3. Results and discussion
3.1. Evaluation of grouper lipid oxidation
3.1.1. POV
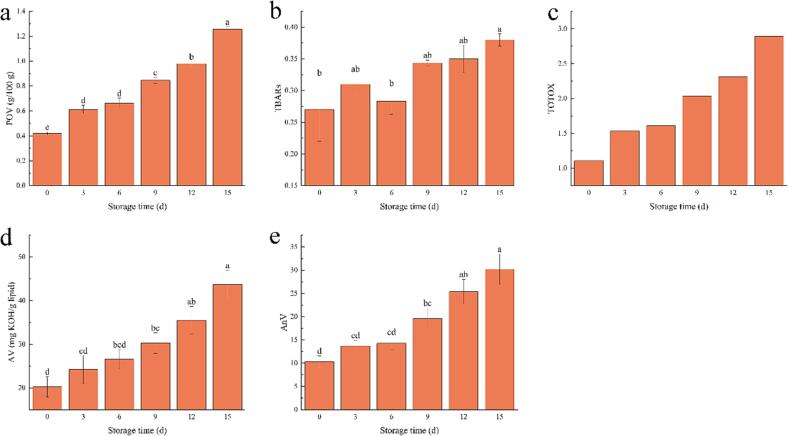
The lipids present in aquatic products are susceptible to oxidation reactions when stored in cold conditions. These reactions give rise to intermediate products, such as peroxides. Assessing the degree of lipid oxidation in aquatic products at the primary level can be achieved by utilizing POV. It reflects the concentration of primary oxidation products of lipids during the oxidation process (Zhou et al., 2019). Fig. 1(a) displays the changes in POV of grouper lipids during cold storage. The initial POV was 0.42 g/100 g, which gradually increased to 1.26 g/100 g on day 15, indicating a continuous production of peroxides during cold storage. This may be the key role played by lipoxygenase in promoting the primary oxidation of grouper lipids (Cao et al., 2020).
Fig. 1.
POV (a), TBARs (b), TOTOX (c), AV (d), and AnV (e) of grouper lipids during cold storage.
3.1.2. TBARs
The decomposition of hydroperoxides gives rise to secondary oxidation products, such as ketones, aldehydes, and alcohols. Malondialdehyde (MDA), which is a significant oxidation product of polyunsaturated fatty acids during secondary lipid oxidation (Bian et al., 2022, Huang et al., 2023). The extent of secondary lipid oxidation can be evaluated by measuring the quantity of characteristic substances generated through their reaction with chromogenic compounds. Typically, higher TBARs values indicate elevated production of MDA and increased levels of lipid oxidation. The changes in TBARs values of grouper lipids during cold storage are presented in Fig. 1(b). Initially, the TBARs value was 0.27, and it gradually increased to 0.38 (15 d) over the storage period, indicating the continuous production of secondary oxidation products. This phenomenon can be attributed to the high instability of free radicals, which act as primary oxidation products of lipids. These free radicals further initiate chain reactions, leading to the occurrence of secondary lipid oxidation. This further led to the generation of VOCs such as ketones, aldehydes, and alcohols, which had an adverse impact on the odor of grouper.
TOTOX serves as a comprehensive indicator for assessing both initial and late-stage lipid oxidation, providing a better evaluation of the progressive oxidative deterioration of lipids (Tenyang et al., 2017). Consistent with the observations of POVs and TBARs, the refrigeration process notably increased (p < 0.05) the TOTOX value of grouper lipids, rising from 1.11 (0 d) to 2.89 (15 d). Taken together, these findings strongly indicate that cold storage significantly induces the oxidation of grouper lipids, as evidenced by the changes in POV and TBARs values.
3.1.3. AV
AV serves as another crucial indicator for assessing the extent of lipid oxidation. It is commonly utilized to measure the quantity of free carboxyl groups present in lipids. There exists a strong positive correlation between AV and the number of free carboxylic acid groups (Wang et al., 2021, Wang et al., 2021). According to the Guidelines for Characterizing Food Grade Fish Oils, the acceptable limit for AV is 7–8 mg KOH/g lipid. Fig. 1(d) displays the variation in AV of grouper lipid during cold storage. Initially, the AV was recorded as 2.35 mg KOH/g lipid, which gradually increased over the storage period and reached 3.28 mg KOH/g lipid at 15 d. Although a significant increase (p < 0.05) was observed during cold storage, the AV remained well below the unacceptable limit value. Based on the report by Boran et al. (2006), the observed increase in AV is attributed to the accumulation of chemical components that arise from the breakdown of primary lipid oxidation products. This finding suggests that grouper lipids generate –COOH groups during primary oxidation, but in small quantities.
3.1.4. AnV
AnV is an indicator that reflects the presence of aldehydes, particularly α, β-unsaturated aldehydes, generated during the oxidation of grouper lipids. It also serves as a measure of the extent of secondary lipid oxidation (Faas et al., 2020). Fig. 1(e) illustrates the significant increase in AnV from 10.28 (0 d) to 30.16 (15 d), indicating substantial oxidation of grouper lipids and the production of a large quantity of aldehydes even under refrigerated conditions. This observation aligns with a similar trend identified in the study conducted by Ochrem et al. (2015).
3.2. GC–MS
3.2.1. Qualitative and quantitative analysis of VOCs
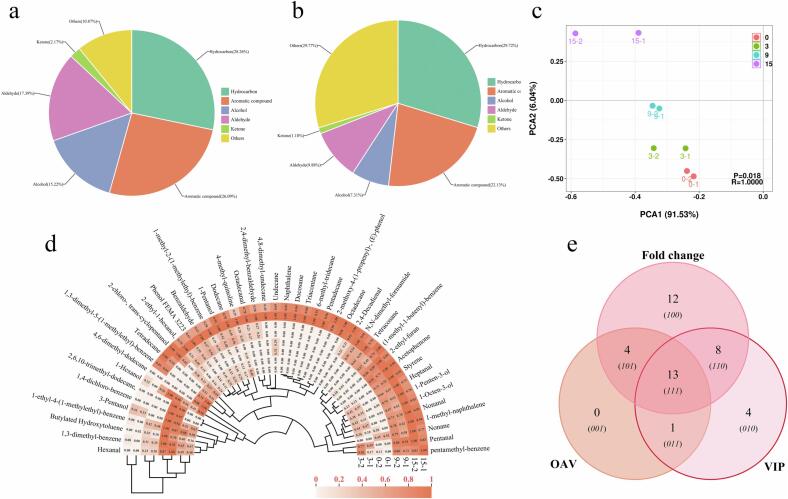
A total of 46 potential VOCs were detected in grouper lipids during cold storage, and their individual quantification was performed using an IS. The content of each VOC is presented in Table 1. And the detected VOCs consist of 13 hydrocarbons, 12 aromatic compounds, 7 alcohols, 8 aldehydes, 1 ketone, and 5 other VOCs. Fig. 2(a-b) illustrate the number and content (at the end of storage) of the six types of detected VOCs. Hydrocarbons and aromatic compounds constituted over half of the total number (54.35 %) and content (51.85 %). However, their impact on the overall odor development of grouper lipids during cold storage is minimal, as most of them do not possess a distinct flavor or have very high odor thresholds. On the other hand, alcohols, aldehydes, and ketones, despite being detected in relatively low quantities (34.78 %) and contents (18.37 %), play a significant role in the odor development of grouper lipids due to their low odor thresholds. Additionally, other VOCs like N,N-dimethyl-formamide, naphthalene, and 4-methyl-quinoline, with low odor thresholds and high contents, also contribute to some extent to the odor development of grouper lipids. Principal component analysis (PCA) results (Fig. 2(c)) demonstrated distinct differences in the VOC profiles of grouper lipids during cold storage, indicating significant variability at different storage times. To visualize changes in the abundance of individual VOCs during cold storage and their clustering relationships, a clustered heat map was generated using normalized data (Fig. 2(d)). Clustering trees were constructed for both sample groups and VOCs based on similarity of abundance. The results, similar to those of PCA, classify the sample groups into four categories after three clusters, highlighting the influence of storage time on the evolution of VOCs. Additionally, the samples from 0 d and 3 d, observed in the third cluster, were grouped together, suggesting that the evolution of VOCs in grouper lipids was relatively less pronounced in the early storage period, with significant production and increase occurring mainly in the middle and late storage periods.
Table 1.
VOCs content (μg/kg) of grouper lipids during cold storage.
| NO. | VOCs | Storage time (d) |
Threshold (µg/kg) | OAV |
VIP | FC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 9 | 15 | 0 d | 3 d | 9 d | 15 d | |||||
| Hydrocarbon | ||||||||||||
| 1 | Nonane | 13.54 ± 0.95c | ND | 19.2 ± 2.9b | 24.73 ± 3.18 a | 650 | 0.02 | 0.00 | 0.03 | 0.04 | 0.70 | 1.83 |
| 2 | Undecane | 18.31 ± 0.63b | ND | ND | 50.92 ± 9.2 a | 5.6 | 3.27 | 0.00 | 0.00 | 9.09 | 0.43 | 2.78 |
| 3 | Dodecane | 87.66 ± 9.91c | 116.79 ± 4.93c | 209.74 ± 15.26b | 311.08 ± 28.44 a | >13000 | 1.17 | 3.55 | ||||
| 4 | Tetradecane | ND | 45.97 ± 0.1b | ND | 76.47 ± 13.33 a | >13000 | 0.62 | >2 | ||||
| 5 | Pentadecane | ND | ND | ND | 244.06 ± 13.41 a | >13000 | 0.92 | >2 | ||||
| 6 | Octadecane | ND | ND | ND | 128.53 ± 1.34 a | 0.92 | >2 | |||||
| 7 | Docosane | ND | ND | ND | 93.97 ± 8.03 a | 0.92 | >2 | |||||
| 8 | Tetracosane | ND | ND | 55.21 ± 3.28b | 84.05 ± 10.03 a | 1.10 | >2 | |||||
| 9 | Triacontane | ND | ND | ND | 66.11 ± 7.48 a | 0.92 | >2 | |||||
| 10 | 4,6-Dimethyl-dodecane | ND | 38.63 ± 2.46 a | ND | ND | 0.30 | >2 | |||||
| 11 | 2,6,10-Trimethyl-dodecane, | 21.45 ± 1.09 a | ND | ND | ND | 0.92 | <0.5 | |||||
| 12 | 6-Methyl-tridecane | ND | ND | ND | 156.76 ± 7.59 a | 0.92 | >2 | |||||
| 13 | 4,8-Dimethyl-undecane | ND | ND | ND | 46.56 ± 15.74 a | 0.91 | >2 | |||||
| Aromatic compound | ||||||||||||
| 14 | 1-Methyl-2-(1-methylethyl)-benzene | 45.14 ± 2.93c | 73.8 ± 5.06b | 75.37 ± 11.77b | 132.47 ± 24.03 a | 1.10 | 2.93 | |||||
| 15 | 1,3-Dimethyl-benzene | 211.48 ± 4.38 a | 163.94 ± 9.44b | 138.08 ± 2.24c | 104.59 ± 4.41 d | 1.18 | 0.49 | |||||
| 16 | Styrene | ND | 47.66 ± 2.7 | 276.6 ± 6.53 | 363.39 ± 14.5 | 12.2 | 0.00 | 3.91 | 22.67 | 29.79 | 1.13 | >2 |
| 18 | 1,4-Dichloro-benzene | 74.28 ± 1.28 a | ND | ND | ND | 0.92 | <0.5 | |||||
| 19 | 1-Ethyl-4-(1-methylethyl)-benzene | 31.03 ± 1.03 a | 27.77 ± 2.44b | 23.31 ± 0.49b | 16.7 ± 0.63c | 1.14 | 0.54 | |||||
| 20 | 1,3-Dimethyl-5-(1-methylethyl)-benzene | ND | 46.16 ± 5.48 a | ND | 42.91 ± 0.01 a | 0.52 | >2 | |||||
| 21 | (1-Methyl-1-butenyl)-benzene | 14.02 ± 0.11b | 15.04 ± 0.77b | 20 ± 0.98 a | 23.33 ± 1.1 a | 1.14 | 1.66 | |||||
| 22 | Pentamethyl-benzene | 43.67 ± 1.84c | 58.25 ± 10.88b | 67.12 ± 1.29 ab | 71.89 ± 3.19 a | 1.04 | 1.65 | |||||
| 23 | Butylated Hydroxytoluene | 281.11 ± 34.38 a | 149.09 ± 27.83b | 124.12 ± 17.37b | 86.18 ± 2.1c | 1.11 | 0.31 | |||||
| 24 | Phenol FEMA 3223 | 16.21 ± 1.89c | 19.57 ± 0.83c | 29.87 ± 0.9b | 44.62 ± 6.98 a | 9.1 | 1.78 | 2.15 | 3.28 | 4.90 | 1.15 | 2.75 |
| 25 | 2-Methoxy-4-(1-propenyl)-, (E)-phenol | ND | ND | ND | 69.23 ± 3.49 a | 0.91 | >2 | |||||
| Alcohol | ||||||||||||
| 26 | 1-Pentanol | 5.97 ± 0.68 d | 10.09 ± 0.86c | 14.13 ± 0.54b | 24.21 ± 0.8 a | 0.47 | 12.70 | 21.47 | 30.07 | 51.52 | 1.18 | 4.06 |
| 27 | 3-Pentanol | 24.99 ± 0.83 a | 14.85 ± 1.41b | ND | 9.63 ± 1.05c | 4.125 | 6.06 | 3.60 | 0.00 | 2.34 | 0.90 | 0.39 |
| 28 | 1-Hexanol | 13.74 ± 0.94 a | 13.49 ± 1.38 a | 14.06 ± 1.14 a | 10.56 ± 0.59b | 102 | 0.13 | 0.13 | 0.14 | 0.10 | 0.68 | 0.77 |
| 29 | 1-Penten-3-ol | 12.63 ± 1.13c | 20.96 ± 0.41b | 29.13 ± 1.64 a | 34.53 ± 3.18 a | 0.4 | 31.57 | 52.40 | 72.82 | 86.32 | 1.15 | 2.73 |
| 30 | 1-Octen-3-ol | 22.98 ± 1.25 d | 40.25 ± 6.24c | 56.17 ± 4.21b | 74.4 ± 5.3 a | 0.9 | 25.54 | 44.73 | 62.42 | 82.67 | 1.16 | 3.24 |
| 31 | 2-Ethyl-1-hexanol | 53.72 ± 3.42b | 57.33 ± 1.39b | 64.49 ± 3.1b | 80.31 ± 5.31 a | 0.4 | 134.30 | 143.33 | 161.22 | 200.79 | 1.11 | 1.50 |
| 32 | 2-Chloro-, trans-cyclopentanol | 53.4 ± 1.02c | 64.28 ± 1.44b | 51.46 ± 13.58c | 81.89 ± 0.08 a | 0.56 | 1.53 | |||||
| Aldehyde | ||||||||||||
| 33 | Pentanal | 18.11 ± 1.93c | 46.41 ± 3.87b | 55.94 ± 0.77 a | 56.93 ± 3.68 a | 0.24 | 75.45 | 193.36 | 233.07 | 237.20 | 1.02 | 3.14 |
| 34 | Hexanal | 89.53 ± 6.35 a | 46.26 ± 0.47b | 24.5 ± 2.45c | ND | 0.67 | 133.62 | 69.04 | 36.57 | 0.00 | 1.15 | <0.5 |
| 35 | Heptanal | ND | 16.11 ± 0.78c | 27.28 ± 0.16b | 35.46 ± 5.44 a | 0.25 | 0.00 | 64.42 | 109.12 | 141.85 | 1.13 | >2 |
| 36 | Nonanal | ND | 10.54 ± 0.37c | 16.69 ± 0.34b | 22.43 ± 0.89 a | 0.1 | 0.00 | 105.39 | 166.87 | 224.35 | 1.16 | >2 |
| 37 | Benzaldehyde | 23.87 ± 1.98c | 34.69 ± 3.56c | 47.86 ± 1.67b | 63.93 ± 9.55 a | 3.5 | 6.82 | 9.91 | 13.67 | 18.26 | 1.17 | 2.68 |
| 38 | 2,4-Decadienal | ND | ND | 11.98 ± 0.37b | 53.49 ± 2.19 a | 0.01 | 0.00 | 0.00 | 1197.97 | 5348.79 | 1.08 | >2 |
| 39 | 2,4-Dimethyl-benzaldehyde | ND | ND | 19.22 ± 0.26b | 57.34 ± 15.16 a | 1.12 | >2 | |||||
| 40 | Octadecanal | ND | ND | 38.68 ± 3.28b | 137.04 ± 17.28 a | 1.13 | >2 | |||||
| Ketone | ||||||||||||
| 41 | Acetophenone | ND | ND | 43.64 ± 3.63b | 51.16 ± 0.93 a | 5.629 | 0.00 | 0.00 | 7.75 | 9.09 | 1.08 | >2 |
| other | ||||||||||||
| 42 | 2-Ethyl-furan | ND | ND | 64.46 ± 1.96 a | 72.01 ± 6.24 a | 1.07 | >2 | |||||
| 43 | N,N-dimethyl-formamide | ND | ND | 37.54 ± 1.69b | 63.5 ± 3.15 a | 0.026 | 0.00 | 0.00 | 1443.92 | 2442.27 | 1.11 | >2 |
| 44 | Naphthalene | ND | ND | ND | 78.77 ± 7.21 a | 0.45 | 0.00 | 0.00 | 0.00 | 175.05 | 0.92 | >2 |
| 45 | 1-Methyl-naphthalene | 29.65 ± 8.23c | 40.83 ± 10.73c | 79.47 ± 6.34b | 101.91 ± 2.44 a | 1.10 | 3.44 | |||||
| 46 | 4-Methyl-quinoline | 484.69 ± 39.27c | 664.25 ± 138.88b | 687.19 ± 27.2b | 969.29 ± 250.1 a | 28 | 17.31 | 23.72 | 24.54 | 34.62 | 0.98 | 2.00 |
All results are expressed as mean ± standard deviation. ND means not detected. The thresholds of compounds obtained from “Handbook of Odor”.
Fig. 2.
The number (a) and content (b) of detected VOCs, PCA scores plot (c), circular clustering heat map of VOCs (d), and Venn diagram of key VOCs (e).
Among the detected VOCs, aldehydes, alcohols, and ketones are the primary contributors to the overall flavor of grouper lipids during oxidation. Alcohols, specifically, have been identified as secondary metabolites resulting from lipid oxidation, primarily generated from the oxidation of unsaturated fatty acids (Cheng et al., 2023). Of the seven alcohols detected, their levels showed a gradual increase with storage time, resulting in a total content rise from 187.43 μg/kg to 315.54 μg/kg. Periche et al. (2016) have reported that unsaturated alcohols generally have much lower odor thresholds than saturated alcohols, thus exerting a significant impact on the flavor of foods. Within this experiment, the unsaturated alcohols detected were 1-penten-3-ol and 1-octen-3-ol, accounting for a relatively high proportion of the alcohols (ranging from 19.00 % to 34.52 %). Particularly noteworthy is 1-octen-3-ol, which was identified as a VOC responsible for providing fishy, fatty, mushroomy, and grassy flavors in aquatic products (Li et al., 2020). It plays a crucial role in shaping the flavor profile of grouper lipids during cold storage and contributes significantly to the development of aquatic product flavors.
Aldehydes in grouper lipids are primarily formed through the decomposition of alkoxy and unsaturated fatty acids (Wang et al., 2021). Among the 8 aldehydes detected, pentanal, hexanal, heptanal, and nonanal are compounds known to exhibit oily and fatty odors, while these saturated linear aldehydes are associated with unpleasant, irritating, and spicy odors (Cheng et al., 2023, Sérot et al., 2001). Most of the aldehydes were not detectable in the early stages of storage and gradually increased as the storage time progressed, resulting in a total content ranging from 131.51 μg/kg to 426.62 μg/kg.
In addition to aldehydes and alcohols, ketones can also be formed through the oxidation of unsaturated fatty acids or the degradation of amino acids. Ketones are known for their lower odor thresholds and play a significant role in contributing to the complex odors of aquatic products. In this study, only one ketone, acetophenone, was detected. Its presence was observed at 9 d with a content of 43.64 μg/kg, which increased to 51.16 μg/kg at 15 d. The content of VOCs in these three main categories (aldehydes, alcohols, and ketones) exhibited similar trends during storage, which also aligned with the clustering of the sample groups. Notably, significant changes in VOCs occurred mainly in the middle and late stages of storage. This phenomenon can be attributed to the production of certain VOCs with lower thresholds and higher contents, which is consistent with the findings of previous analyses.
Lastly, it is important to mention that remarkably high levels of 4-methyl-quinoline were observed among the other 5 VOCs detected. Quinoline is a compound that contains an aromatic ring and is often described as having a faintly smoky, bitter, or peculiarly pungent odor similar to petroleum (Ben Khemis & Ben Lamine, 2021). During the storage of grouper, the content of 4-methyl-quinoline increased significantly, rising from 484.69 μg/kg to 969.29 μg/kg, making it one of the most abundant compounds detected. Furthermore, N,N-dimethyl-formamide and naphthalene also contributed to the development of VOCs during the lipid oxidation of grouper due to their lower odor thresholds. These compounds, along with 4-methyl-quinoline, play an important role in shaping the overall odor profile of grouper lipids during cold storage.
3.2.2. Screening of key VOCs
To assess the contribution of grouper lipid oxidation to the overall flavor, the OAV of each VOC needs to be considered. The “Handbook of Odor” was referred to obtain the odor thresholds of 23 compounds among the detected VOCs, and their corresponding OAVs were computed and presented in Table 1. According to Chu, Ding, et al. (2023), VOCs with OAV > 1 are considered characteristic odorants that significantly contribute to the overall odor of grouper. Among these VOCs, aldehydes and alcohols had notably higher OAVs due to their extremely low odor thresholds. Additionally, other detected VOCs also exhibited high OAVs. To scientifically identify key VOCs related to lipid oxidation in grouper, variables important in projection (VIP) values and fold change (FC) were calculated, and the results are shown in Table 1. Based on criteria such as OAV > 1, FC > 2, or FC < 0.5, and VIP > 1, a total of 13 key VOCs were screened (Fig. 2(e)). These key VOCs include stylene, phenol FEMA 3223, 1-pentanol, 1-penten-3-ol, 1-octen-3-ol, pentanol, hexanal, heptanal, nonanal, benzaldehyde, 2,4-decadienal, acetophenone, and N,N-dimethylformamide. They are likely involved in various biological and chemical pathways, including the oxidation of grouper lipids, and may significantly impact the overall flavor of grouper.
3.3. Changes in fatty acids
Polyunsaturated fatty acids (PUFAs) in aquatic products, especially omega-3 long-chain PUFAs (n-3 LC-PUFAs), have garnered significant attention due to their high biological activity and health benefits (Shchepinov, 2020). However, lipids rich in PUFAs have been reported to be highly prone to oxidation, leading to a decrease in the nutritional value of aquatic products (Zhao et al., 2021). As mentioned in the analysis of VOCs, alcohols, aldehydes, and ketones can all be generated through PUFAs oxidation, resulting in negative effects on the overall odor of aquatic products. To evaluate the changes in fatty acids in grouper lipids during cold storage, the focus was on long-chain fatty acids, and the content of 12 specific fatty acids was determined (as shown in Table 2). It was observed that palmitic acid (C16:0) and stearic acid (C18:0) were the predominant saturated fatty acids (SFA), while linoleic acid (C18:2 n6c), eicosapentaenoic acid (EPA, C20:6 n3), and docosahexaenoic acid (DHA, C22:6 n3) were the most abundant PUFAs. Among the 12 detected fatty acids, the content of unsaturated fatty acids (UFA) consistently remained higher than that of saturated fatty acids (SFA) throughout the storage period. Notably, a substantial amount of PUFAs (30.66 g/100 g lipid) was observed in fresh grouper lipids (0 d), accounting for 58.65 % of the total fatty acids, particularly EPA (6.84 g/100 g lipid) and DHA (19.18 g/100 g lipid). However, all fatty acids exhibited a declining trend during storage, and the loss of these fatty acid substrates reflected the degree of lipid oxidation (Hu et al., 2022). The reduction in PUFAs content was particularly pronounced, reaching 17.13 g/100 g lipid by the end of the storage period. Similar findings were reported by Yu et al. (2020), who observed a decrease in total fatty acids, ΣSFA, ΣMUFA, and ΣPUFA in salmon during storage, aligning with the results of this study. In fact, the rate of fatty acid degradation is closely related to the number of double bonds in the carbon chain, and PUFAs, especially EPA and DHA, exhibit more significant oxidative losses compared to other fatty acids (Biandolino et al., 2021).
Table 2.
FAs content (g/100 g lipid) of grouper lipids during cold storage.
| NO. | FAs | Storage time (d) |
|||
|---|---|---|---|---|---|
| 0 | 3 | 9 | 15 | ||
| 1 | C16:0 | 9.74 ± 0.47a | 8.99 ± 0.40b | 6.91 ± 0.75c | 4.94 ± 0.14d |
| 2 | C17:0 | 1.27 ± 0.03a | 1.07 ± 0.07b | 0.88 ± 0.03b | 0.90 ± 0.03b |
| 3 | C18:0 | 4.41 ± 0.38a | 3.66 ± 0.18b | 3.36 ± 0.13b | 2.62 ± 0.11c |
| 4 | C20:0 | 0.72 ± 0.02a | 0.61 ± 0.05b | 0.49 ± 0.01c | 0.35 ± 0.01d |
| 5 | C21:0 | 0.17 ± 0.04a | ND | ND | ND |
| ΣSFA | 16.31 | 14.34 | 11.63 | 8.81 | |
| 6 | C16:1 | 0.75 ± 0.03a | 0.74 ± 0.03a | 0.6 ± 0.06b | 0.64 ± 0.02b |
| 7 | C18:1 n9t | 4.41 ± 0.02a | 3.23 ± 0.01b | 2.43 ± 0.01c | 1.9 ± 0.01d |
| 8 | C20:1 | 0.15 ± 0.01a | ND | ND | ND |
| ΣMUFA | 5.31 | 3.98 | 3.03 | 2.54 | |
| 9 | C18:2 n6c | 4.59 ± 0.03a | 4.09 ± 0.06b | 3.61 ± 0.02c | 2.84 ± 0.05d |
| 10 | C20:2 | 0.04 ± 0.01a | 0.03 ± 0a | ND | ND |
| 11 | C20:5 n3 | 6.84 ± 0.59a | 5.27 ± 0.12b | 4.15 ± 0.12c | 3.21 ± 0.02d |
| 12 | C22:6 n3 | 19.18 ± 0.03a | 18.4 ± 0.84a | 14.68 ± 0.01b | 11.08 ± 0.08c |
| ΣEPA + DHA | 26.03 | 23.67 | 18.83 | 14.29 | |
| ΣPUFA | 30.66 | 27.79 | 22.44 | 17.13 | |
| ΣUFA | 35.97 | 31.77 | 25.46 | 19.67 | |
| Total FA | 52.28 | 46.1 | 37.1 | 28.49 | |
All results are expressed as mean ± standard deviation. ND means not detected.
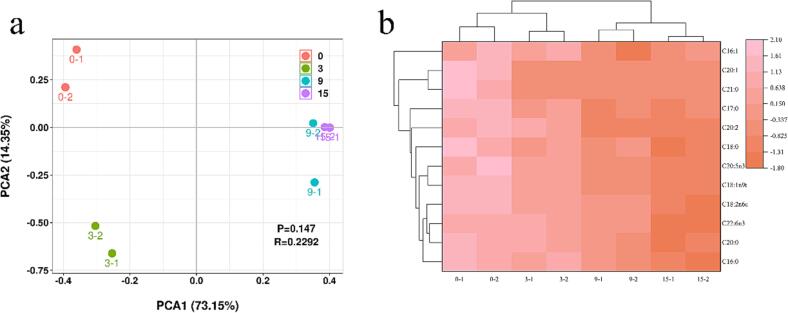
Meanwhile, PCA (Fig. 3(a)) demonstrated the differences in fatty acids of grouper lipids during the cold storage, revealing distinct variations in VOCs generated at different storage times. Furthermore, a cluster heatmap (Fig. 3(b)) was generated to visualize the clustering relationships of fatty acids in grouper lipids. It was evident that the major changes in fatty acids occurred in the mid to late stages of storage, which aligned with the variations in VOCs. This observation further highlighted the correlation between changes in VOCs and fatty acid oxidation.
Fig. 3.
PCA scores plot (a), and cluster heat map (b) of FAs.
3.4. Prediction of the potential pathways for key VOCs production in grouper lipids during storage
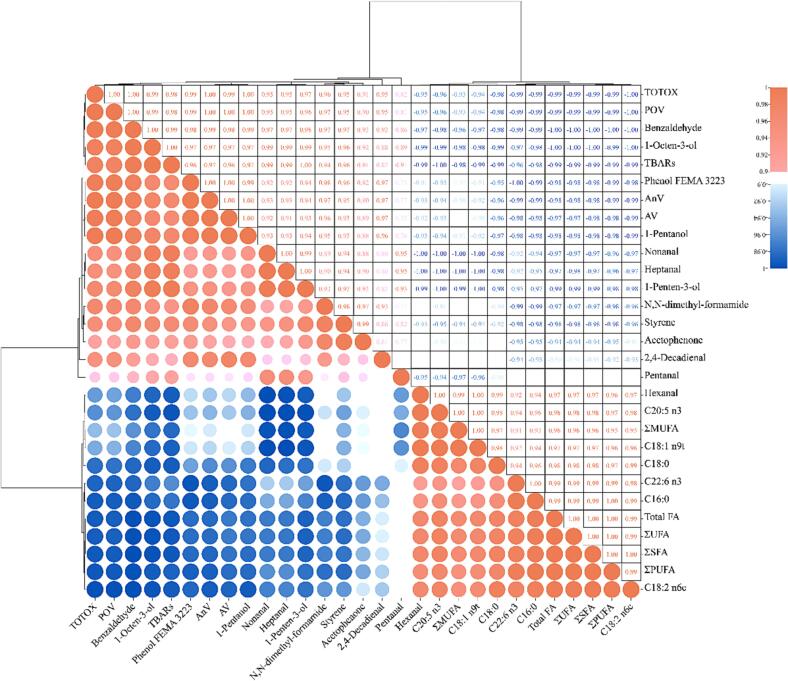
To assess the interrelationships between the identified key VOCs and oxidation of grouper lipids, Pearson correlation analysis was conducted between the 13 key VOCs and the lipid oxidation-related data (POV, TBARs, TOTOX, AV, AnV, and FAs). A correlation heatmap was generated and depicted in Fig. 4, revealing significant positive and negative correlations (>0.8 or < -0.8) among almost all the data.
Fig. 4.
A correlation heatmap of 13 key VOCs and the lipid oxidation-related data.
It was observed that the three alcohols exhibited a strong correlation with TBARs, DHA, EPA, and PUFAs, which was consistent with previous research findings (Chu, Ding, et al., 2023). This suggests that during the storage of grouper lipids, the production of alcohols may largely stem from the secondary oxidation of lipids, especially from the oxidation of long-chain polyunsaturated fatty acids such as EPA and DHA. Furthermore, the five aldehydes were found to be potentially involved in both primary and secondary lipid oxidation. Among the 12 detected fatty acids, an interesting finding was that MUFA showed the highest correlation with the five aldehydes, distinguishing it from other studies (Sérot et al., 2001). The other three VOCs also exhibited strong correlations with DHA, likely attributable to the high susceptibility of long-chain PUFAs to oxidation due to their longer carbon chains.
4. Conclusion
In order to investigate the relationship between lipid oxidation and VOCs development in grouper during cold storage, this study focused on extracting lipids from grouper as a single-factor study, excluding the influence of microorganisms and proteins. The primary and secondary oxidation of grouper lipids was characterized using POV, TBARs, TOTOX, AV, and AnV to assess the extent of lipid oxidation during cold storage. Simultaneously, the changes in 12 important long-chain fatty acids in grouper lipids were examined, primarily characterized by the oxidation of polyunsaturated fatty acids, especially DHA and EPA. Meanwhile, using SPME-GC–MS, 13 significant VOCs that influenced the overall flavor of grouper lipids were selected, including three alcohols, six aldehydes, one ketone, and three other compounds. Pearson correlation analysis revealed a high correlation between the development of these VOCs and lipid changes in grouper. Specifically, alcohols produced during the storage of grouper lipids may stem from the secondary oxidation of lipids, especially DHA and EPA as the primary polyunsaturated fatty acids. Aldehydes, on the other hand, may primarily arise from the oxidation of monounsaturated fatty acids. Furthermore, the oxidation of some longer-chain polyunsaturated fatty acids could generate compounds with low odor thresholds and high content. This study provides new insights into the relationship between compound changes and odor in grouper lipids, and serves as an important basis for linking lipid changes during cold storage to grouper quality deterioration. Based on the comprehensive identification of grouper lipid quality and fatty acids in this study, a possible approach has been suggested, and corresponding methods need to be discovered to inhibit and slow down the oxidation of long-chain polyunsaturated fatty acids, such as EPA and DHA.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD2100101), China Agriculture Research System of MOF and MARA (CARS-47), and the Shanghai Professional Technology Service Platform on Cold Chain Equipment Performance and Energy Saving Evaluation (19DZ2284000). All authors have read and agreed to the published version of the manuscript.
CRediT authorship contribution statement
Yuanming Chu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Jun Mei Data analysis: Validation. Jing Xie: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The authors do not have permission to share data.
References
- Agyare A.N., An C.H., Liang Q. Goji Berry (Lycium Barbarum L.) carotenoids enrichment through ‘Green’ extraction method improves oxidative stability and maintains fatty acids of yak ghee with microwave heating and storage. Foods. 2022;11(3):369. doi: 10.3390/foods11030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral A.B., da Silva M.V., Lannes S.C. da S. Lipid oxidation in meat: Mechanisms and protective factors – a review. Food Science and Technology. 2018;38(suppl 1):1–15. doi: 10.1590/fst.32518. [DOI] [Google Scholar]
- Ammari A., Schroen K. Flavor retention and release from beverages: A kinetic and thermodynamic perspective. Journal of Agricultural and Food Chemistry. 2018;66(38):9869–9881. doi: 10.1021/acs.jafc.8b04459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassam S.M., Noleto-Dias C., Farag M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131139. [DOI] [PubMed] [Google Scholar]
- Ben Khemis I., Ben Lamine A. Physico-chemical investigations of human olfactory receptors OR10G4 and OR2B11 activated by vanillin, ethyl vanillin, coumarin and quinoline molecules using statistical physics method. International Journal of Biological Macromolecules. 2021;193:915–922. doi: 10.1016/j.ijbiomac.2021.10.155. [DOI] [PubMed] [Google Scholar]
- Bian C., Yu H., Yang K., Mei J., Xie J. Effects of single-, dual-, and multi-frequency ultrasound-assisted freezing on the muscle quality and myofibrillar protein structure in large yellow croaker (Larimichthys crocea) Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biandolino F., Parlapiano I., Denti G., Di Nardo V., Prato E. Effect of different cooking methods on lipid content and fatty acid profiles of Mytilus galloprovincialis. Foods. 2021;10(2):416. doi: 10.3390/foods10020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boran G., Karaçam H., Boran M. Changes in the quality of fish oils due to storage temperature and time. Food Chemistry. 2006;98(4):693–698. doi: 10.1016/j.foodchem.2005.06.041. [DOI] [Google Scholar]
- Cao X., Islam M.N., Duan Z., Pan X., Xu W., Wei X., Zhong S. Chlorogenic acid osmosis of snakehead fish: A novel approach to maintain quality and suppress deterioration during storage. International Journal of Food Properties. 2020;23(1):387–399. doi: 10.1080/10942912.2020.1732409. [DOI] [Google Scholar]
- Carvalho Barros J., Munekata P.E.S., de Carvalho F.A.L., Pateiro M., Barba F.J., Domínguez R.…Lorenzo J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods. 2020;9(1):44. doi: 10.3390/foods9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Wu G., Zhang H., Jin Q., Wang X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Critical Reviews in Food Science and Nutrition. 2020;60(9):1496–1514. doi: 10.1080/10408398.2019.1575792. [DOI] [PubMed] [Google Scholar]
- Cheng H., Wang J., Xie J. Progress on odor deterioration of aquatic products: Characteristic volatile compounds, analysis methods, and formation mechanisms. Food Bioscience. 2023;53 doi: 10.1016/j.fbio.2023.102666. [DOI] [Google Scholar]
- Chu Y., Ding Z., Wang J., Xie J. Exploration of the evolution and production of volatile compounds in grouper (Epinephelus coioides) during cold storage. Food Bioscience. 2023;52 doi: 10.1016/j.fbio.2023.102496. [DOI] [Google Scholar]
- Chu Y., Mei J., Xie J. Integrated volatile compounds and non-targeted metabolomics analysis reveal the characteristic flavor formation of proteins in grouper (Epinephelus coioides) during cold storage. Food Research International. 2023;172 doi: 10.1016/j.foodres.2023.113145. [DOI] [PubMed] [Google Scholar]
- Chu Y., Tan M., Bian C., Xie J. Effect of ultrasonic thawing on the physicochemical properties, freshness, and protein-related properties of frozen large yellow croaker (Pseudosciaena crocea) Journal of Food Science. 2022;87(1):52–67. doi: 10.1111/1750-3841.15983. [DOI] [PubMed] [Google Scholar]
- Dai W., Wang W., Gu S., Xu M., Yao H., Zhou X., Ding Y. Effect of chitosan-epigallocatechin gallate coating on volatile flavor compounds retention in bighead carp (Aristichthys nobilis) fillets during chilled storage. LWT. 2022;169 doi: 10.1016/j.lwt.2022.114027. [DOI] [PubMed] [Google Scholar]
- Faas N., Röcker B., Smrke S., Yeretzian C., Yildirim S. Prevention of lipid oxidation in linseed oil using a palladium-based oxygen scavenging film. Food Packaging and Shelf Life. 2020;24 doi: 10.1016/j.fpsl.2020.100488. [DOI] [Google Scholar]
- Hu Y., Zhao G., Yin F., Liu Z., Wang J., Qin L.…Zhu B. Effects of roasting temperature and time on aldehyde formation derived from lipid oxidation in scallop (Patinopecten yessoensis) and the deterrent effect by antioxidants of bamboo leaves. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130936. [DOI] [PubMed] [Google Scholar]
- Huang X., Tu Z., Liu W., Wu C., Wang H. Effect of three culture patterns on quality changes of crayfish meats during partial freezing storage. Food Chemistry. 2023;414 doi: 10.1016/j.foodchem.2023.135683. [DOI] [PubMed] [Google Scholar]
- Hwang J.-Y., Ha H.-K., Lee M.-R., Kim J.W., Kim H.-J., Lee W.-J. Physicochemical Property and Oxidative Stability of Whey Protein Concentrate Multiple Nanoemulsion Containing Fish Oil. Journal of Food Science. 2017;82(2):437–444. doi: 10.1111/1750-3841.13591. [DOI] [PubMed] [Google Scholar]
- Li H., Xi B., Yang X., Wang H., He X., Li W., Gao Y. Evaluation of change in quality indices and volatile flavor components in raw milk during refrigerated storage. LWT. 2022;165 doi: 10.1016/j.lwt.2022.113674. [DOI] [Google Scholar]
- Li P., Chen Z., Tan M., Mei J., Xie J. Evaluation of weakly acidic electrolyzed water and modified atmosphere packaging on the shelf life and quality of farmed puffer fish (<scp> Takifugu obscurus </scp>) during cold storage. Journal of Food Safety. 2020;40(3) doi: 10.1111/jfs.12773. [DOI] [Google Scholar]
- Lou X., Wen X., Chen L., Shu W., Wang Y., Hoang T.T., Yang H. Changes in texture, rheology and volatile compounds of golden pomfret sticks inoculated with Shewanella baltica during spoilage. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134616. [DOI] [PubMed] [Google Scholar]
- Mariutti L.R.B., Bragagnolo N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Research International. 2017;94:90–100. doi: 10.1016/j.foodres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Mishra P.K., Kakatkar A.S., Kamal Gautam R., Kumar V., Debbarma A., Chatterjee S. Effect of ajwain (Trachyspermum ammi) extract and gamma irradiation on the shelf-life extension of rohu (Labeo rohita) and seer (Scomberomorus guttatus) fish steaks during chilled storage. Food Research International. 2023;163 doi: 10.1016/j.foodres.2022.112149. [DOI] [PubMed] [Google Scholar]
- Naylor R.L., Kishore A., Sumaila U.R., Issifu I., Hunter B.P., Belton B.…Crona B. Blue food demand across geographic and temporal scales. Nature Communications. 2021;12(1):5413. doi: 10.1038/s41467-021-25516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochrem A.S., Żychliñska-Buczek J., Zapletal P. Carp (Cyprinus carpio L.) lipid oxidation during cold storage. Archives of Polish. Fisheries. 2015;23(2):101–106. doi: 10.1515/aopf-2015-0011. [DOI] [Google Scholar]
- Periche A., Castelló M.L., Heredia A., Escriche I. Effect of different drying methods on the phenolic, flavonoid and volatile compounds of Stevia rebaudiana leaves. Flavour and Fragrance Journal. 2016;31(2):173–177. doi: 10.1002/ffj.3298. [DOI] [Google Scholar]
- Sérot T., Regost C., Prost C., Robin J., Arzel J. Effect of dietary lipid sources on odour-active compounds in muscle of turbot (Psetta maxima) Journal of the Science of Food and Agriculture. 2001;81(14):1339–1346. doi: 10.1002/jsfa.950. [DOI] [Google Scholar]
- Shchepinov M.S. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends in Pharmacological Sciences. 2020;41(4):236–248. doi: 10.1016/j.tips.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Tenyang N., Ponka R., Tiencheu B., Djikeng F.T., Azmeera T., Karuna M.S.L.…Womeni H.M. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in Far-North Region of Cameroon. Food Chemistry. 2017;221:1308–1316. doi: 10.1016/j.foodchem.2016.11.025. [DOI] [PubMed] [Google Scholar]
- Wang F., Gao Y., Wang H., Xi B., He X., Yang X., Li W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS) Meat Science. 2021;175 doi: 10.1016/j.meatsci.2021.108449. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang Q., Xu L., Sun D.-W. Effects of extremely low frequency pulsed electric field (ELF-PEF) on the quality and microstructure of tilapia during cold storage. LWT. 2022;169 doi: 10.1016/j.lwt.2022.113937. [DOI] [Google Scholar]
- Wang X.-Y., Xie J., Chen X.-J. Differences in lipid composition of Bigeye tuna (Thunnus obesus) during storage at 0 °C and 4 °C. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110233. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang H., Wu Y., Xiang H., Zhao Y., Chen S.…Li L. Insights into lipid oxidation and free fatty acid profiles to the development of volatile organic compounds in traditional fermented golden pomfret based on multivariate analysis. LWT. 2022;171 doi: 10.1016/j.lwt.2022.114112. [DOI] [Google Scholar]
- Yu Y.-J., Yang S.-P., Lin T., Qian Y.-F., Xie J., Hu C. Effect of cold chain logistic interruptions on lipid oxidation and volatile organic compounds of salmon (Salmo salar) and their correlations with water dynamics. Frontiers in Nutrition. 2020;7 doi: 10.3389/fnut.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhen Z., Zhang W., Zeng T., Zhou G. Effect of intensifying high-temperature ripening on proteolysis, lipolysis and flavor of Jinhua ham. Journal of the Science of Food and Agriculture. 2009;89(5):834–842. doi: 10.1002/jsfa.3521. [DOI] [Google Scholar]
- Zhang Q., Chen X., Ding Y., Ke Z., Zhou X., Zhang J. Diversity and succession of the microbial community and its correlation with lipid oxidation in dry-cured black carp (Mylopharyngodon piceus) during storage. Food Microbiology. 2021;98 doi: 10.1016/j.fm.2020.103686. [DOI] [PubMed] [Google Scholar]
- Zhang X., Lu N., Li Z., Meng X., Cao W., Xue Y.…Tang Q. Effects of curcumin-mediated photodynamic treatment on lipid degradation of oysters during refrigerated storage. Journal of the Science of Food and Agriculture. 2022;102(5):1978–1986. doi: 10.1002/jsfa.11536. [DOI] [PubMed] [Google Scholar]
- Zhao G.-H., Hu Y.-Y., Liu Z.-Y., Xie H., Zhang M., Zheng R.…Zhou D.-Y. Simultaneous quantification of 24 aldehydes and ketones in oysters (Crassostrea gigas) with different thermal processing procedures by HPLC-electrospray tandem mass spectrometry. Food Research International. 2021;147 doi: 10.1016/j.foodres.2021.110559. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhou D.-Y., Liu Z.-Y., Yin F.-W., Liu Z.-Q., Li D.-Y., Shahidi F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chemistry. 2019;272:109–116. doi: 10.1016/j.foodchem.2018.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.