Abstract
Aims.
Astaxanthin is a natural carotenoid with antioxidant and anti-inflammatory effects. We conducted a double blind, placebo-controlled, randomized trial to determine the effects of astaxanthin treatment on lipids, CVD markers, glucose tolerance, insulin action, and inflammation in individuals with prediabetes and dyslipidemia.
Materials and Methods.
Adult participants with dyslipidemia and prediabetes (n = 34) underwent baseline blood draw, oral glucose tolerance test (OGTT), and a one-step hyperinsulinemic-euglycemic clamp. They were then randomized (n=22 treated, 12 placebo) to receive astaxanthin 12 mg daily or placebo for 24 weeks. Baseline studies were repeated after 12 and 24 weeks of therapy.
Results.
After 24 weeks, astaxanthin treatment significantly decreased LDL (−0.33 ± 0.11 mM) and total cholesterol (−0.30 ± 0.14 mM) (both p<0.05). Astaxanthin also reduced levels of the CVD risk markers fibrinogen (−473 ± 210 ng/mL), L-selectin (−0.08 ± 0.03 ng/mL), and fetuin A (−10.3 ± 3.6 ng/mL), (all p < 0.05). While the effects of astaxanthin treatment did not reach statistical significance, there were trends towards improvements in the primary outcome measure, insulin-stimulated whole body glucose disposal (+0.52 ±0.37 mg/m2/min, p=0.078), as well as fasting [Insulin] (−5.6 ± 8.4 pM, p=0.097), and HOMA2-IR (−0.31 ± 0.16, p = 0.060), suggesting improved insulin action. No consistent significant differences from baseline were observed for any of these outcomes in the placebo group. Astaxanthin was safe and well tolerated with no clinically significant adverse events.
Conclusions.
Although the primary endpoint did not meet the prespecified significance level, these data suggest that astaxanthin is a safe OTC supplement that improves lipid profiles and markers of CVD risk in individuals with prediabetes and dyslipidemia.
Introduction.
Prediabetes represents a major and growing health concern worldwide (1). Around 5-10% of individuals who meet these criteria progress to diagnosed type 2 diabetes per year (2). Even individuals who retain their prediabetic status are at increased risk for multiple comorbidities, including macro- and microvascular complications (3–5). Results from the Diabetes Prevention Program Outcomes Study indicate lifestyle interventions have the strongest effect on preventing progression to type 2 diabetes (6, 7). However, effective lifestyle interventions are difficult for the majority of patients to achieve and maintain over the long term, driving the search for additional therapies to prevent deterioration of glucose control and reduce vascular complications.
One molecule with promise in this area is astaxanthin, a xanthophyll carotenoid with demonstrated antioxidant activity (reviewed in (8)). Already approved for use as a food supplement, astaxanthin has been studied as a treatment for skin aging (9), oxidative stress and inflammation related to osteoarthritis (10), diabetic retinopathy (11), Alzheimer’s (reviewed in (12)), cognitive performance (reviewed in (13)), and dyslipidemia (14–16). Astaxanthin has been shown to improve glycemic control in individuals with prediabetes (15), postmenopausal women (17), and subjects at risk for metabolic syndrome (18), with mixed effects on lipid levels (14–16).
Astaxanthin is now widely used with an estimated global market for health care of nearly 500 million dollars. However, the metabolic and cardiovascular effects have never been comprehensively examined. Therefore, we sought to address this gap in scientific knowledge with the current study. Here we describe the results of our randomized, placebo-controlled, double-blind study in which we evaluated the effects of 24 weeks astaxanthin treatment on lipids, markers of cardiovascular disease (CVD) risk (4,5), glycemic control, insulin action, substrate oxidation, inflammation, and oxidative stress in individuals with prediabetes and dyslipidemia. Because dyslipidemia is so common in prediabetes, we elected to study subjects with prediabetes and dyslipidemia to represent the most realistic target population for astaxanthin use.
Materials and Methods
Participants.
Thirty-six men and women with prediabetes and dyslipidemia participated in the trial. Eligibility criteria included: age 18-75 years; BMI 25-39 kg/m2; impaired fasting glucose (5.33-6.89 mM) or elevated HbA1c (5.7-6.4%); dyslipidemia ([LDL] >2.59 mM, [HDL] < 1.04 mM for males or < 1.29 mM for females, [TG] > 3.88 mM, or taking a statin or fibrate medication); and stable concomitant medication use for 30 days. Exclusion criteria included: diagnosis or treatment for type 1 or type 2 diabetes; capable of becoming pregnant; liver transaminase > 3x ULN; eGFR < 30 mL/min/1.73m2; myocardial infarction or stroke within 6 months; blood pressure > 160 mmHg systolic or > 100 mmHg diastolic; or use of steroids, anti-depressants, weight loss medications, OTC antioxidants, or thiazolidinediones. All participants provided informed consent before participating in the study.
Study Design.
The study was designed as a double-blind, randomized, parallel, placebo-controlled, single dose study. After baseline testing including blood draw, oral glucose tolerance test (OGTT), indirect calorimetry (IDC), and one-step hyperinsulinemic-euglycemic clamp (HEC) participants were randomly assigned 2:1 in a double-blind fashion to receive either astaxanthin 12 mg daily, a commonly used dose (15–17), or placebo for 24 weeks. Assignment to placebo or test substance was performed by the Research Pharmacist using the Research Randomizer software (www.randomizer.org). All study staff and subjects were blinded as to the treatment assignment. Test material and placebo were provided in gelatin capsules. Participants were instructed to take the capsule at approximately the same time each day with food. Participants returned to the research unit for repeat metabolic testing after 12 and 24 weeks of treatment.
The primary outcome of the study was insulin sensitivity for whole body glucose disposal rate (GDR) as determined with the HEC. Variability for determination of insulin-stimulated glucose disposal has been reported to be 10% (19). To detect a treatment effect of this magnitude would require at least 22 evaluable subjects. An evaluable subject is one who has completed all procedures. To maximize the number of treated subjects, we applied the general inflation factor formula, resulting in a distribution of 22 subjects in the treated group and 12 receiving placebo, for a total of 34.
Safety Measures.
At baseline, weeks 6, 12, 18, 24, and end-of-study, participants underwent a safety evaluation that included vital signs, weight, safety labs, and review of concomitant medications and adverse events. A physical exam was completed at baseline, week 12, week 24, and end-of-study, Twenty-four-hour blood pressure was measured at baseline, week 12, and week 24. An electrocardiogram was completed at baseline and end-of-study.
Oral Glucose Tolerance Test (OGTT).
Each participant was admitted to the Altman Clinical and Translational Research Institute (ACTRI) the morning after an overnight (~10 hr) fast. An OGTT was then performed as follows: Blood samples were obtained through an intravenous catheter prior to and at 30, 60, 90 and 120 minutes after ingestion of a 75-g glucose load. After the OGTT, each participant remained in the ACTRI overnight. In the evening, they were given a standardized meal and then underwent an overnight (~10 hour) fast in preparation for the HEC procedure on the second day of testing.
Hyperinsulinemic/euglycemic clamp (HEC).
Following an overnight fast in the ACTRI, each participant underwent determination of in vivo insulin action as assessed by a 3-hour hyperinsulinemic (120 mU/m2/min) euglycemic (5 mM) clamp as described previously (20). The whole-body glucose disposal rate (GDR) in each participant was determined from the values obtained during the steady-state period, the average of the values between the 140th and 160th minute and the 160th and 180th minute.
Indirect calorimetry (IDC).
Resting energy expenditure (REE) and carbohydrate and fat oxidation were determined by IDC employing previously described methodologies (20). IDC was performed in conjunction with the clamp procedure before the start of insulin infusion (time −30 to 0 min) and during the last 30 min of the glucose/insulin infusion.
COVID-Related Modifications.
The study was initiated before the onset of the COVID pandemic. With the initiation of COVID-mandated restrictions, the following change was made in the protocol: After the OGTT evaluation, participants were discharged from the ACTRI with a standardized meal, which they consumed at home. After an overnight (~10 hr) fast, they returned to the ACTRI for the HEC procedure the following day. Five subjects followed the modified study protocol.
Assays
Insulin and c-peptide were quantitated by immunoassay, performed by Quest Diagnostics (San Juan Capistrano, CA). Lipids were measured as part of a standard lipid panel by Quest, employing spectrophotometric assays.
Circulating serum adiponectin was measured using a commercially available ELISA kit (Millipore/Sigma, Burlington, MA). The lower limit of detection with this assay was 0.2 ng/mL; the inter- and intra-assay coefficients of variation were 7.4 and 8.4% respectively. FGF21 was measured using an ELISA kit from R & D Systems (Minneapolis, MN). The sensitivity was 8.69 pg/mL with inter- and intra-assay coefficients of variation of 3.9 and 10.9% respectively.
Multiplex analysis was performed using MILLIPLEX MAP magnetic bead kits from Millipore employing a Bio-Rad Bioplex 200 instrument. Inflammatory factors were measured with a human Inflammation Magnetic Bead Panel. Analytes included: GRO, IFNγ, IL-1ß, IL-1RA, IL-6, IL-8, IL-10, IL-15, IL-17A, MCP-1, MIP1a, TNFα, VEGF-A. Cardiovascular disease markers were measured using CVD magnetics bead Panel 1: BNP, NT-Pro-BNP, FABP4, Oncostatin, and Troponin 1. Acute phase CVD markers were measured using Panel 3: adipsin, α2-macroglobulin, CRP, fetuin A, fibrinogen, L-selectin, and haptoglobin.
Protein carbonylation was measured using a colorimetric kit from Cayman Chemical (Ann Arbor, MI).
Calculations.
Area under the Curve (AUC) for glucose, insulin and c-peptide were determined employing the trapezoidal rule. HOMA2-IR and HOMA-%B (21) were determined using the on-line calculator (https://www.dtu.ox.ac.uk/homacalculator/download.php). The Matsuda insulin sensitivity index (ISI) was calculated as ISI = 10,000/√(FPG x FPI) x (GOGTT x IOGTT), while the Matsuda hepatic sensitivity index = [glucose]t=0 x [insulin]t=0 (22). Early-phase insulin secretion (InsAUC30/GluAUC30) was calculated with data from the OGTT as: [insulin]t=0 + [insulin]t=30/[glucose]t=0 + [glucose]t=30 (23). Adipose tissue insulin sensitivity (AT Si) = [FFA]fasting x [insulin]fasting. Metabolic flexibility (MF) was calculated from IDC data as MF = RQclamp-RQfasting.
Data analysis.
Within-group treatment effects were evaluated using paired Student’s t test analysis and repeated measures ANOVA. Comparisons between groups were made using t tests for independent samples. Where variables were not normally distributed, significance was confirmed by a Mann-Whitney U test. Data are presented as mean ± SEM with two-tailed P < 0.05 considered statistically significant. All analyses were run using Prism 9 statistical software, version 9.3.1 (GraphPad Inc., San Diego, CA). Secondary outcomes were considered exploratory and evaluated for possible statistical significance (no adjustment for multiple comparisons) to support further research on this intervention.
Data and Resource Availability.
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Results
Participants.
Fifty-eight participants were screened for eligibility. Thirty-six participants met all the inclusion criteria and were enrolled/randomized in the study. The most common reasons for screen failure were fasting glucose/HbA1c or lipid levels that did not meet the inclusion criteria. Two participants, both in the placebo group, were withdrawn after the 3-month evaluation visit due to noncompliance with the study medication or study visits: their data are not included in the analysis. Characteristics at the baseline/randomization visit of the participants who completed all evaluations (n=34) are presented in Table 1. The groups were well matched for age, BMI, heart rate and 24 hour blood pressure (BP). Statistically significant differences in baseline values for the various outcomes between groups were present only for IL1ß and IL-17A levels (Sup. Table 1)
Table 1.
Baseline participant characteristics
| Group | Treated | Placebo |
|---|---|---|
| Number (F/M) | 22 (7/15) | 12 (3/9) |
| Age (yr) | 51 ± 3 | 53 ± 3 |
| BMI (kg/m2) | 31.81 ± 0.88 | 31.89 ± 0.98 |
| [Glucose]f (mM) | 5.87 ± 0.11 | 5.64 ± 0.10 |
| [Insulin]f (pM) | 95.9 ± 11.0 | 73.4 ± 10.8 |
| HOMA-IR | 2.13 ± 0.23 | 1.52 ± 0.21 |
| HR (bpm) | 70 ± 2 | 65 ± 2 |
| 24 hr BP (mm Hg) | 136 ± 4/79 ± 2 | 137 ± 5/79 ± 4 |
| Total [cholesterol]f (mM) | 4.71 ± 0.17 | 4.84 ± 0.36 |
| [LDL]f (mM) | 2.90 ± 0.16 | 2.98 ± 0.31 |
| [HDL]f (mM) | 1.13 ± 0.05 | 1.17 ± 0.08 |
| [TG]f (mM) | 1.68 ± 0.26 | 1.58 ± 0.17 |
| [FFA]f (mM) | 0.56 ± 0.03 | 0.57 ± 0.06 |
Safety.
Body weight and BMI were stable over the study period in both the astaxanthin treatment and placebo groups. Twenty-four-hour BP was also unaltered in either group (Fig. 1). When considering the relative (%) change from baseline, there was a trend toward reduced resting heart rate over time in the ASTX treatment group (p=0.053). This behavior differed significantly from that in the placebo group, where resting HR increased (p=0.01 and 0.016 ASTA Rx vs placebo, at 12 and 24 weeks, respectively). There was a statistically significant improvement in alkaline phosphatase in the astaxanthin treatment group. The treatment was well tolerated, as noted earlier the only dropouts were in the placebo group. No treatment-related adverse events were reported.
Figure 1.
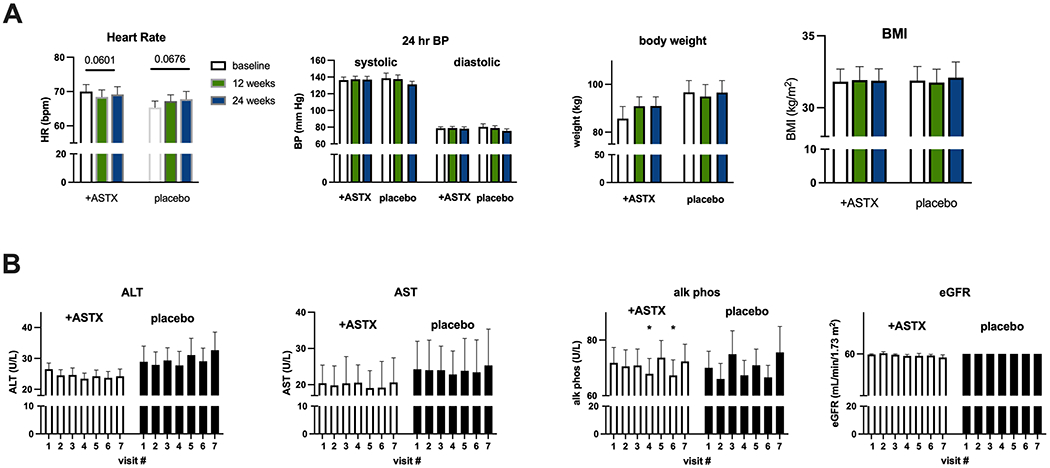
Safety Measures. A. Vital signs. Open bars – baseline, green bars – 12 weeks evaluation, blue bars – 24 weeks.
B. Safety laboratory values. Visit #1 – baseline, visit #2 – randomization, visit #3 - 6 weeks, visit #4 – 12 weeks evaluation, visit #5 - 18 weeks, visit #6 – 24 weeks evaluation, visit #7 – follow-up
Results are Ave + SEM
*p<0.05 vs visit #1
eGFR reported as value <60 or >60
Lipids.
Astaxanthin treatment improved the lipid profile, resulting in significant decreases in total cholesterol (−0.30 ± 0.14 mM, p=0.027 by ANOVA vs p=0.558 for placebo) and LDL (−0.33 ± 0.11 mM, p=0.030 vs 0.969 for placebo). There was a non-statistically significant trend for (p=0.12) for HDL to increase (+0.059 ± 0.031 mM,) with astaxanthin treatment. When considered as % change from baseline, the increase in HDL levels was significant (p=0.044 vs 0.803 in placebo). Triglyceride and FFA levels were unchanged. There were no changes in lipids in the placebo group (Fig. 2).
Figure 2.
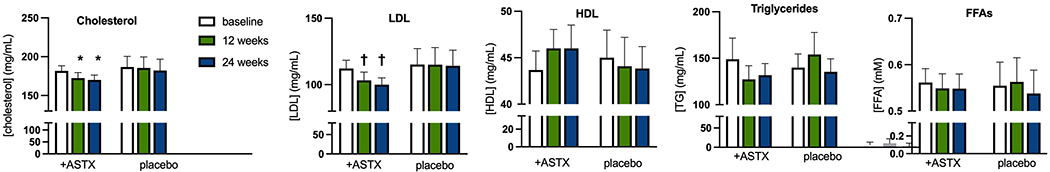
Treatment effects on fasting lipid levels.
Open bars – baseline, green bars – 12 weeks evaluation, blue bars – 24 weeks evaluation. Results are Ave + SEM.
* p<0.05 vs paired baseline value
† p<0.01 vs paired baseline value
CVD markers.
To evaluate the potential for astaxanthin to reduce CVD risk, we monitored circulating levels of multiple markers for CVD risk. With astaxanthin treatment there were statistically significant and consistent decreases in fetuin A, fibrinogen, and L-selectin levels (Fig. 3), suggestive of a reduction in CVD risk. Levels of the other markers were unchanged. In the placebo group there were transient decreases in fetuin A and L-selectin at 12 weeks, values that returned to not different from baseline at 24 weeks.
Figure 3.
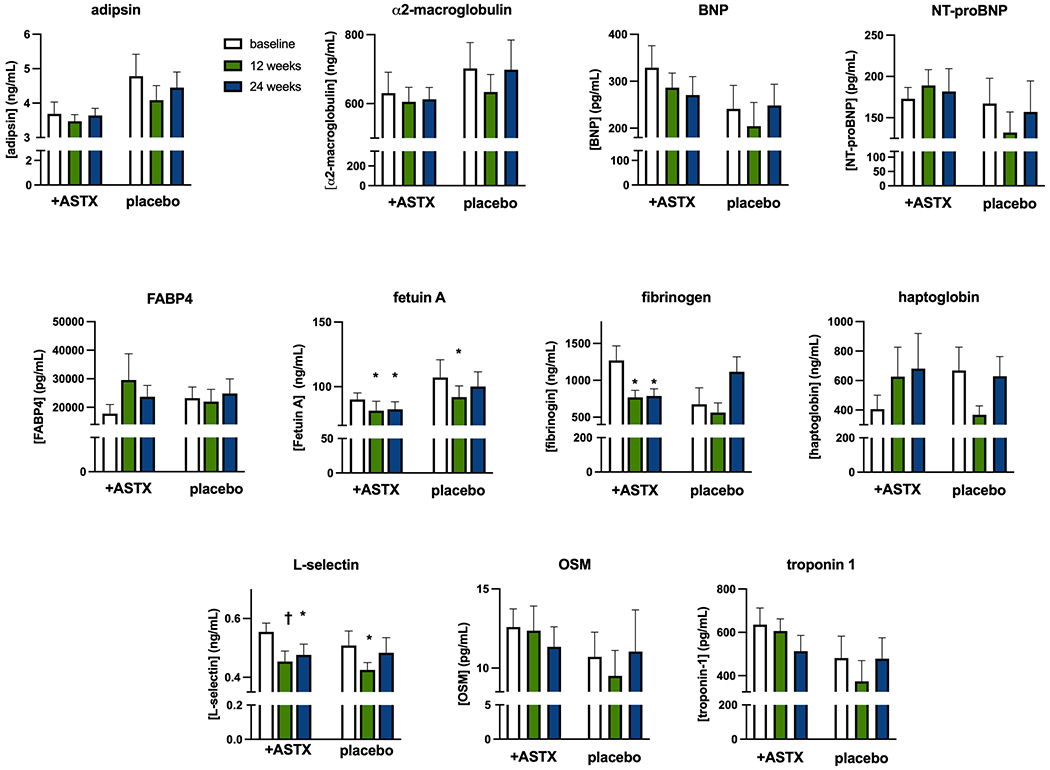
Treatment effects on selected circulating markers for CVD.
Open bars – baseline, green bars – 12 weeks evaluation, blue bars – 24 weeks evaluation. Results are Ave + SEM.
* p<0.05 vs paired baseline value
†p<0.01 vs paired baseline value
Glycemic control.
Long term glycemic control, as indicated by HbA1c levels, was unaltered by astaxanthin treatment, as were fasting glucose levels (Fig. 4A). Fasting insulin levels trended (p=0.097) toward a reduction by 24 weeks of treatment. There were also trends for several indices of insulin action in the fasting state, HOMA2-IR (p=0.060, 24 weeks vs baseline, p=0.991 for placebo) and Hepatic Si (p=0.090, 24 weeks vs baseline, p=0.759 for placebo), to improve with treatment (Fig. 4B), while adipose tissue insulin sensitivity was unaltered. These indices were stable with time in the placebo group. Responses of both glucose and insulin to an OGTT were unaltered over the 24-week period in either the ASTX or placebo groups (Fig. 4C). This included the Matsuda index. Insulin secretion in both the fasting state (HOMA-%ß, Fig. 4B) and in response to the oral glucose challenge (early-phase insulin release, Fig. 4C), were unaltered by astaxanthin treatment and stable across time in the placebo group.
Figure 4.
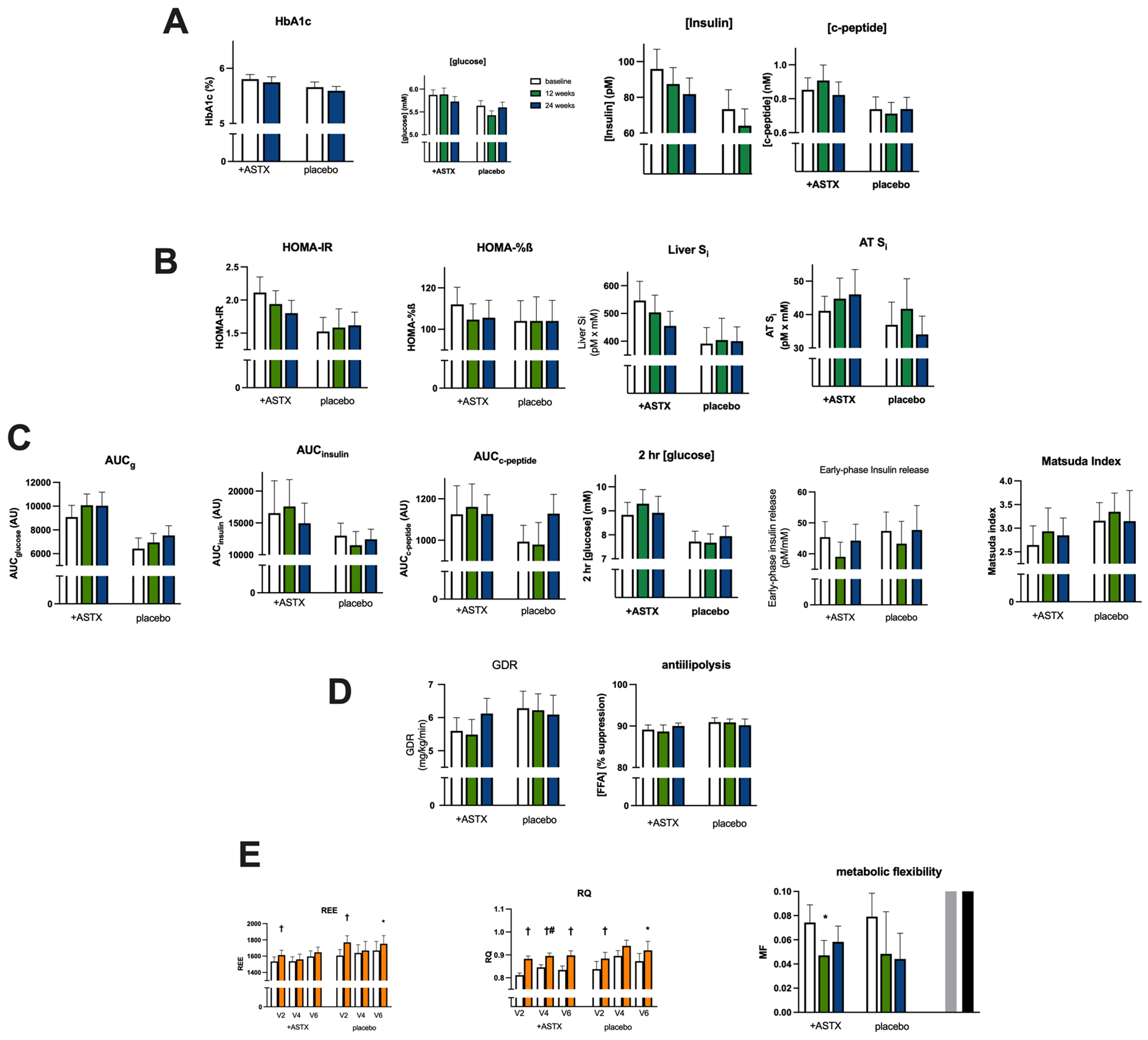
Treatment effects on measures of glycemic control, insulin secretion, insulin action and substrate oxidation. A. Fasting values: HbA1c, glucose, insulin, c-peptide. B. Fasting-derived indices of insulin action and secretion: HOMA-IR, HOMA-%B, liver insulin sensitivity index, adipose tissue insulin sensitivity index. C. OGTT-derived indices of insulin action and secretion: AUCglucose, AUCinsulin, AUCc-peptide, 2 hr [glucose], early-phase insulin release (InsAUC30/GluAUC30), Matsuda index. D. Clamp-derived values: insulin-stimulated glucose disposal rate, suppression of FFA levels. E. Indirect calorimetry-derived substrate oxidation: resting energy expenditure, respiratory quotient, metabolic flexibility.
For panels A-D: Open bars – baseline, green bars – 12 weeks evaluation, blue bars – 24 weeks. For panel E: open bars – fasting state, orange bars – clamp state.
* p<0.05 vs paired baseline value
† p<0.01 vs paired fasting state value
#p< 0.05 vs paired V2 value
Results from the HEC procedure showed that insulin stimulation of whole-body GDR, the primary outcome measure, (Fig. 4D) and reduction of FFA levels (antilipolysis) were similar at baseline between groups. Effects of ASTX treatment did not attain statistical significance for either outcome. However, when determined as the change from baseline, there was a trend (p=0.078) toward an improvement in GDR with treatment, unlike in the placebo group (p=0.753). Substrate (carbohydrate and fat) oxidation was measured by IDC before and during the HEC. Resting energy expenditure in the fasting state (basal) was comparable between groups and did not change over time in either group (Fig. 4E). At baseline there was a modest, but statistically significant (p<0.01), increase in REE during insulin infusion that was observed in both groups. This response was also seen at the 24-week visit but was absent at the 12-week visit, a behavior observed in both groups. Substrate oxidation, as represented by the respiratory quotient (RQ), was similar in the fasting state (basal) between groups and did not change with time, even with astaxanthin treatment (Fig. 4E). As anticipated, glucose + insulin infusion increased RQ in both groups. With astaxanthin treatment there was a transient further increase in RQ over baseline. The ability to shift between utilizing fat and carbohydrate is termed “metabolic flexibility” (MF) and in the current situation can be expressed as the individual difference in RQ between the fasting and clamp states. While there was considerable intra-subject variability in MF, it was not altered in a statistically significant manner with astaxanthin treatment or over time in the placebo group.
Adiponectin and FGF21 are circulating factors with important roles in metabolic regulation (reviewed in (24, 25)). Levels of both factors were stable throughout the study, but for a transient, but statistically significant, increase in FGF21 after 12 weeks of astaxanthin treatment (Supplemental Material, Table 1).
Inflammation.
Chronic, low-grade systemic inflammation is a common feature of pre-diabetes and other insulin resistant states (reviewed in (26)). Multiple circulating markers of inflammatory status were evaluated (Sup. Table 1). In the astaxanthin -treated group there were no statistically significant changes in the levels of either pro- or anti-inflammatory cytokines and chemokines. In the placebo group there was no consistent changes in levels of these factors, only transient increases in GRO, IFNγ and TNFα at 12 weeks, that returned to not different from baseline at 24 weeks.
Protein carbonylation is accepted as a marker for systemic oxidative stress (reviewed in (27)). Protein carbonylation in the serum was stable across the study period and unaltered by astaxanthin treatment (Sup. Table 1).
Discussion
Prediabetes is growing in prevalence worldwide (reviewed in (1)). Identification and treatment of individuals with prediabetes is of paramount importance as the condition represents a state of heightened risk for CVD and progression to type 2 diabetes. However, current treatment options are limited with the focus on diet and lifestyle interventions. Specifically, the American Diabetes Association currently recommends that patients with prediabetes obtain a 7% reduction in body weight and exercise 150 min/week. While the evidence supports these interventions, they are practically difficult to achieve. Thus, there is a large unmet need to develop therapies that reduce CVD risk and progression to type 2 diabetes in individuals with prediabetes and dyslipidemia. Our study suggests that astaxanthin may represent such a therapy as treatment improved lipid profiles, markers of CVD risk, and showed trends toward improving insulin sensitivity.
Astaxanthin is a xanthophyll carotenoid with documented antioxidant properties (reviewed in (8)). Astaxanthin is being evaluated in clinical trials for effects on several conditions including osteoarthritis, Alzheimer’s, diabetic neuropathy, stroke, Polycystic Ovary Syndrome, and exercise capacity. Astaxanthin is widely available online and in retail stores with a reported market for natural astaxanthin from microalgae, as was used in the current study, of nearly $500 million USD in 2020. The appeal of a compound such as astaxanthin is that it is readily available, over the counter, and well tolerated. Additionally, patients are increasingly interested in “natural” treatments for a variety of conditions, particularly for preventative therapies. Therefore, astaxanthin represents a therapeutic intervention that could be attractive to both patients and providers.
Published work has reported astaxanthin effects on metabolic control and inflammation in subjects across a range of insulin sensitivities (15–17). However, these studies have been limited by their short duration, small sample sizes, and/or paucity of clinical endpoints. Therefore, for the millions of patients taking astaxanthin we lack appropriate data to inform their use. To address this gap in our knowledge, we conducted a comprehensive randomized, placebo-controlled clinical trial in 36 subjects with prediabetes to evaluate the effects of astaxanthin treatment on glycemic control, dyslipidemia, insulin action, systemic inflammation, and CVD biomarkers.
Regarding effects on lipids, astaxanthin treatment improved aspects of the pro-atherogenic lipid profile characteristic of prediabetes. Total cholesterol and LDL levels were reduced, while HDL levels increased, relative to baseline (Fig, 2). Specifically, astaxanthin treatment reduced LDL by 0.33 mM. To put this reduction into context, it has previously been reported that the risk of a major cardiovascular event is reduced by 23% for every 1.00 mM reduction in LDL (28). Extrapolating from these data, the LDL reduction observed here could result in a 7.5% relative reduction in CV events.
In addition to improving features of the pro-atherogenic lipid profile, astaxanthin induced stable reductions in several markers of CVD risk. Specifically, astaxanthin treatment resulted in statistically significant and consistent decreases in fetuin A, fibrinogen, and L-selectin levels. These CVD markers have not been assessed in other astaxanthin trials. Elevated fetuin-A levels are associated with both increased risk of type 2 diabetes (29) and increased severity of coronary artery disease (30). Therapies with positive effects on CVD reduction have been shown to reduce fetuin-A levels. These include statins (31), bariatric surgery, weight loss, exercise, metformin, and pioglitazone (reviewed in (32)). We mention these therapies to frame the effects of astaxanthin on fetuin-A versus other, commonly studied CVD interventions.
Fibrinogen, has long been known as a CVD risk marker (33) and levels are elevated in type 2 diabetes (34). Both fibrates and statins reduce fibrinogen levels (reviewed in (35)). L-selectin levels are elevated in patients with type 2 diabetes (36). Taken together, astaxanthin-mediated reduction in these markers of CVD risk, a property shared to some extent with cardioprotective agents, would suggest improvements in cardiovascular health.
Regarding astaxanthin actions on systemic inflammation in human subjects, we found no treatment effects on multiple pro-inflammatory cyto- and chemokines (Sup. Table 1). Previously published data on inflammatory markers has been widely variable. For example, prior studies reported TNFα levels were decreased with astaxanthin treatment in subjects at risk for metabolic syndrome (18), but unchanged in healthy subjects (37). And, while we found no treatment effect on protein carbonylation, a marker of systemic oxidative stress, reports have showed improvement in markers of oxidative stress in overweight subjects (14) and post-menopausal women (38).
With regard to glucose metabolism, we found trends towards reductions in HOMA2-IR and hepatic Si with astaxanthin treatment (Fig. 4), suggesting improvements in insulin action in the fasting state. For whole body insulin action determined from the HEC, the primary outcome of the study, there was a non-statistically significant trend toward improvement with treatment. Insulin action in adipose tissue, insulin secretion, and insulin-induced metabolic flexibility were not influenced by astaxanthin treatment. These data suggest that the liver may represent a major target tissue for astaxanthin effects on glucose metabolism. Attention regarding potential mechanisms of the beneficial effects of astaxanthin have focused on the strong antioxidant capacity of the molecule (8, 39, 40). Antioxidant-independent actions of astaxanthin have also been proposed on mitochondrial biogenesis and function (41, 42) and as a modulator of peroxisome proliferator-activated receptor (PPAR) activity (43), predominately PPARα, which would be consistent with a primarily hepatic site of action.
Variability in the nature and magnitude of responses to astaxanthin treatment could be due to multiple factors, including dose selection, treatment duration, and patient population (reviewed in (44)). We chose to evaluate individuals with prediabetes and dyslipidemia as this population could benefit from a non-pharmacologic intervention to bolster lifestyle modifications. The dose selected, 12 mg/day, is in the range investigated previously (15-17, reviewed in (45)), and is the commonly available dose commercially. Therefore, the dose chosen represents the current “real-world” use of astaxanthin. The study duration, 6 months, was chosen to reveal possible delayed-onset responses and was considered long enough to detect potential “deterioration” in glycemic control or conversion to type 2 diabetes in the placebo group (2). A variety of outcomes were monitored to track multiple features of the prediabetes phenotype(s).
A limitation of the current study could be sample size, as several potential treatment effects, including the primary outcome measure, approached but did not attain statistical significance. It should also be noted that the presentation of the results is that of a per-protocol analysis rather than of an intention-to-treat basis. The number of subjects was also insufficient to support a sub-analysis to determine if the responses to astaxanthin treatment were due to the heterogeneity of the prediabetic phenotypes, impaired fasting glucose vs impaired glucose tolerance (reviewed in (46)). Additional studies would be valuable in determining potential differential benefits of astaxanthin on these phenotypes.
In summary, our study represents the most comprehensive examination to date of astaxanthin, a commonly used nutraceutical, in individuals with prediabetes. We found that treatment reduced total and LDL cholesterol, increased HDL cholesterol, and improved markers of CVD risk including fetuin A, fibrinogen, and L-selectin levels. Treatment also trended to improve insulin sensitivity as measured by HOMA2-IR and hepatic Si. Treatment was well tolerated with no clinically apparent side effects. Our findings suggest that astaxanthin represents a promising supplement to improve the metabolic and CVD risk profile for individuals with prediabetes and dyslipidemia.
Supplementary Material
Acknowledgements.
We thank Todd May and Adrienne Armstrong for their work as study coordinators, Debra Armstrong and Paivi Burke for their nursing expertise in assisting with study visits including clamp studies, and Leslie Carter for technical assistance.
Funding and Assistance.
This work was supported by an award from Fuji Chemical Industries Co., LTD. The sponsors had no involvement in the design and execution of the study or the analysis and interpretation of the results.
The project described was partially supported by the National Institutes of Health, Grant UL1TR001442. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Duality of Interest Disclosure. No relevant conflicts to disclose.
Clinical trial reg. no. NCT03310359, clinicaltrials.gov
References
- 1.Rett K, Gottwald-Hostalek U. Understanding prediabetes: definition, prevalence, burden and treatment options for an emerging disease. Current Med Res Opin. 2019;35:1529–34. [DOI] [PubMed] [Google Scholar]
- 2.Group DPPR. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniele G, Abdul-Ghani MA, DeFronzo RA. What are the pharmacotherpy options for treating prediabetes? Expert Opin Pharmacother. 2014;14:2003–18. [DOI] [PubMed] [Google Scholar]
- 4.Vistisen D, Kivimaki M, Perreault L, Hulman A, Witte DR, Brunner EJ, et al. Reversion from prediabetes to normoglycemia and risk of cardiovascular disease and mortality: the Whitehall II cohort study. Diabetologia. 2019;62:1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg RB, Orchard TJ, Crandall JP, Boyko EJ, Budoff M, Dabela D, et al. Effects of long-term metformin and lifestyle interventions on cardiovascular events in the diabetes prevention program and its outcome study. Circulation. 2022;145:1632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galaviz KI, Weber MB, Suvada K, Gujral UP, Wei J, Merchant R, et al. Interventions for reversing prediabetes: A systemic review and meta-analysis. Am J Prev Med. 2022;62:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Buckley Woods H, et al. Preventing the progression to Type 2 diabetes mellitus in adults at high risk: A systematic review and network meta-analysis of lifestyle, pharnacological and surgical interventions. Diab Res Clin Prac. 2015;107:320–31. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Kumar R, Diksha, Kumari A, Panwar A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J Basic Microbiol. 2021. [DOI] [PubMed] [Google Scholar]
- 9.Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair and disease: A comprehensive review. Nutrients. 2018;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L-j Chen W-P. Astaxanthin ameloriates cartilage damage in experimental osteoarthritis. Mol Rheumatol. 2015;25:768–71. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Wang Y. Recent advances and the mechanisms of astaxanthin in ophthalmological diseases. J Ophthalmol. 2022;2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balendra V, Singh SK. Therapeutic potential of astaxanthin and superoxide dismutase in Alzheimer’s disease. Open Biol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimmig B, Kim S-H, Nash K, Bickford PC, Shytle RD. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognative function in age and neurodegeneration. Geroscience. 2017;39:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. 2011;66:363–9. [DOI] [PubMed] [Google Scholar]
- 15.Urkaze M, Kobashi C, Satou Y, Shigeta K, Toshima M, Takagi M, et al. The benneficial effects of astaxanthin on glucose metabolism and modified low-density lipoprotein in healthy volunteers and subjects with prediabetes. Nutrients. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hypertension. Atherosclerosis. 2010;209:520–3. [DOI] [PubMed] [Google Scholar]
- 17.Iwabayashi M, Fujioka N, Nomoto K, Miyazaki R, Takahashi H, Hibino S, et al. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-Aging Med. 2009;6:15–21. [Google Scholar]
- 18.Uchiyama A, Okada Y. Clinical efficacy of astaxanthin-containing Haematococcus Pluvialis extract for volunteers at risk of metabolic syndrome. J Clin Biochem Nutr. 2008;43:38–43. [Google Scholar]
- 19.Le DSNT, Brookshire T, Krakoff J, Bunt JC. Repeatability and reproducibility of the hyperinsulinemic-euglycemic clamp and tracer dilution technique in a controlled inpatient setting. Metabolism. 2009;58:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorburn AW, Gumbiner B, Flynn T, Henry RR. Substrate oxidation errors during combined indirect calorimetry-hyperinsulinemic glucose clamp studies. Metabolism. 1991;40:391–8. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insuloin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 23.Stancakova A, Javorsky M, Kuulasmaa T, Haffner SM, Kuusisto J, Laasko M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes. 2009;58:1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng L, Lam KSL, Xu A. The therapeitic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–67. [DOI] [PubMed] [Google Scholar]
- 26.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. [DOI] [PubMed] [Google Scholar]
- 27.Akagawa M Protein carbonylation: molecular mechanisms, biological implications, and analytical approaches. Free Radic Res. 2021;55:307–20. [DOI] [PubMed] [Google Scholar]
- 28.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction amomg different therapeutic interventions A systematic review and meta-ana;ysis. JAMA. 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- 29.Guo VY, Cao B, Cai C, Cheng KK-Y, Cheung BMY. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2018;55:87–98. [DOI] [PubMed] [Google Scholar]
- 30.Afrisham R, Paknejad M, Ilbeigi D, Sadegh-Nejadi S, Gorgani-Firuzajee S, Vahidi M. Positive correlation between circulating Fetuin-A and severity of coronary artery disease in men. Endo Metab Immune Disord Drug Targets. 2021;21:338–44. [DOI] [PubMed] [Google Scholar]
- 31.Kadoglou NPE, K0ttas G, Lampropoulos S, Vitta I, Liapis CD. Aerum levels of fetuin-A, osteoprotegerin and osteopontin in patients with coronary artery disease: effects of statin (HMGCoA-reductase inhibitor) therapy. Clin Drug Invest. 2014;34:165–71. [DOI] [PubMed] [Google Scholar]
- 32.Trepanowski JF, Mey J, Varady KA. Fetuin-a: a novel link between obesity and related complications. Int J Obes. 2015;39:734–41. [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB, Wolf PA, Castelli WP, D’’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham study. JAMA. 1987;258:1183–6. [PubMed] [Google Scholar]
- 34.Aziz IA, Fawwad A, Siddiqui IA, Perveen K, Nangrejo R, Waris N, et al. Association of fibrinogen and plasminogen activator inhibitor-1 with diabetes mellitus. Pak J Pharm Sci. 2022;35:165–9. [PubMed] [Google Scholar]
- 35.SahebKkar A, Serban M-C, Mikhailidis DP, Toth PP, Munter P, Ursoniu S, et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol Res. 2016;103:236–52. [DOI] [PubMed] [Google Scholar]
- 36.Chu JW, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Tsao PS. Effect of rosiglitazone treatment on circulating vascular and inflammatory markers in insulin-resistant subjects. Diab Vasc Dis Res. 2005;2:37–41. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Natur Metab. 2010;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YK, Chyun J-H. The effects of astaxanthin supplements on lipid peroxidation and antioxidant status in postmenopausal women. Nutritional Sci. 2004;7:41–6. [Google Scholar]
- 39.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR 1990;2227–38. [PubMed] [Google Scholar]
- 40.Gowd V, Xiao J, Wang M, Chen F, Cheng K-W. Multi-mechanistic antidiabetic potentialof astaxanthin: An update on preclinical and clinical evidence. Mol Nutr Food Res 2021;e2100252: doi: 10.1002/mnfr.202100252. [DOI] [PubMed] [Google Scholar]
- 41.Nishida Y, Nawaz A, Kado T, Takikawa A, Igarashi Y, Onogi Y, et al. Astaxanthin stimulated mitochiondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. 2020;11:241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida Y, Nawaz A, Hecht K, Tobe K. Astaxanthin as a novel mitochondrial regulator: A new aspect of carotenoids, beyond antioxidents. Nutrients 2022;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi C-K. Astaxanthin as a peroxisome proliferator-activated receptor (PPAR) modulator: Its therapeutic implications. MArine Drugs 2019;17:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung LY-L, Chan SM-N, Tam H-L. Astaxanthin influence on health outcomes of adults at risk of metabolic syndrome: A systematic review and meta-analysis. Nutrients. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brendler T, Willianson EM. Astaxanthin: How much is too much? A safety review. Phyotherapy Research. 2019;33:3090–111. [DOI] [PubMed] [Google Scholar]
- 46.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9:193–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


