Highlights
-
•
The stability of anthocyanins are affected by adverse environmental factors and gastrointestinal conditions.
-
•
Encapsulations increase the stability and bioavailability of anthocyanins.
-
•
Encapsulations enhance the biological functions of anthocyanins.
-
•
Encapsulation provides an effective solution for stability and controlled delivery.
Keywords: Anthocyanins, Stability, Bioactivity, Bioavailability, Encapsulation systems
Abstract
The health benefits of anthocyanins have attracted extensive research interest. However, anthocyanins are sensitive to certain environmental and gastrointestinal conditions and have low oral bioavailability. It has been reported that delivery systems made in different ways could improve the stability, bioavailability and bioactivity of anthocyanins. This present review summarizes the factors affecting the stability of anthocyanins and the reasons for poor bioavailability, and various technologies for encapsulation of anthocyanins including microcapsules, nanoemulsions, microemulsions, Pickering emulsions, nanoliposomes, nanoparticles, hydrogels and co–assembly with amphiphilic peptides were discussed. In particular, the effects of these encapsulation technologies on the stability, bioavailability and bioactivities of anthocyanins in vitro and in vivo experiments are reviewed in detail, which provided scientific insights for anthocyanins encapsulation methods. However, the application of anthocyanins in food industry as well as the biological fate and functional pathways in vivo still need to be further explored.
1. Introduction
Anthocyanins are glycoconjugates of flavonoids, secondary metabolites of many plants. They are members of one of the natural pigment families produced in the plant kingdom and are responsible for blue, purple, red, and orange coloration of many fruits and vegetables such as grapes, blackcurrants, elderberries, raspberries, red cabbage, carrots, and purple potatoes (Li et al., 2017, Wu et al., 2023). Anthocyanins are safe, non–toxic, and rich in sources (Wang et al., 2014, Chen et al., 2015). There are more than 700 different anthocyanins in nature, among which six anthocyanins pelargonidin, cyanidin, delphinidin, peonidin, petunidin and malvidin are common in plants (Saha, Singh, Paul, Sarkar, Khan, & Banerjee, 2020), their diverse functions in plants include attracting pollination from insects and seed dispersal by other animals, preventing UV damage, ameliorating different abiotic and biological stresses, and participating in physiological processes (Vidana Gamage et al., 2021, Ahammed and Yang, 2022, Jezek et al., 2023). Anthocyanins can not only reflect the coloration of plants, but also have been traditionally used for medicinal purposes because of their low cytotoxicity and extensive bioactivities such as antioxidation, anti-inflammation, anti-aging, lipid-lowering, anti-cancer, and neuroprotective effects (Tsuda, 2012, Li et al., 2022, Escalante-Aburto et al., 2023). As a result, the extraction of anthocyanins from edible plants has received increasing interest as a potential source of medicinal components (Khoo, Azlan, Tang, & Lim, 2017). However, application of anthocyanins in foods is constrained by a number of factors. For example, the stability of anthocyanin is poor and can easily deteriorate by pH, light, temperature, and oxygen, all of these well accelerate their degradation during long-term storage (Eker et al., 2019, Barbosa et al., 2021). Furthermore, only a small portion of anthocyanins can be absorbed by the human body because of the harsh environment in the gastrointestinal tract and the low permeabilities of anthocyanins, which leads to the low bioavailability of anthocyanins (Fernandes et al., 2019, Tena et al., 2020). To date, many technologies have been developed and applied to these bioactive ingredients to overcome these issues. For example, modifying the structure of bioactive components can improve their stability. However, there are shortcomings in modification technologies, such as low conversion rate of acylation modification, poor solubility of esterification modification, and the long time required for pyranylation modification. Similarly, although auxiliary color can enhance the color of anthocyanins, their protective effect is unstable because of the poor stability and susceptibility to dissociation of auxiliary pigments (Liu et al., 2020).
Encapsulation has the potential to enhance the stability of anthocyanins by establishing a functional barrier between anthocyanins and external environmental factors (such as oxygen, light, temperature, water, enzymes, and bioactive compounds) (Ghosh, Sarkar, Das, & Chakraborty, 2022). Encapsulation is the process by which the bioactive material is captured by the coating material and transported to the right place and released at the right time (Jafari et al., 2008, Rosales and Fabi, 2022). Encapsulation provides outstanding advantages for internal bioactive agents, including enhanced oxidative thermal and light stability, bioavailability, flavor masking, and sustained and controlled release (Alu'datt et al., 2022, Weng et al., 2023). Therefore, encapsulation is an important method to improve the stability and bioavailability of anthocyanins, enabling to expand their application under various processing conditions.
In this review, the problems in the practical application of anthocyanins were reviewed. The mechanism of improving anthocyanins stability, the latest innovation and advanced strategies were introduced. These include the preparations and characterizations of anthocyanins carrier systems and the use of various in vitro and in vivo models to assess the performance improvements of these systems in terms of anthocyanin stability, bioactivity and bioavailability. This review has an important reference value for the applications of anthocyanins in the food industry.
2. Problems with the application of anthocyanins
2.1. Poor in vitro stability
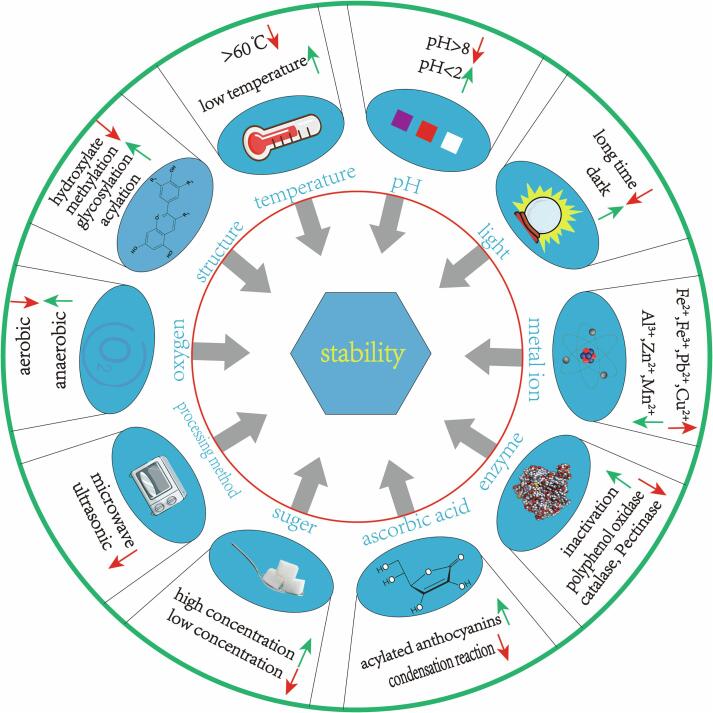
Monomer anthocyanins generally have a low degree of stability, which are directly related to their structure, and depend on the quantity, type, and mode of ligand interactions (Sun, Cao, Bai, Liao, & Hu, 2010). They can be decomposed into colorless, brown, soluble, or insoluble compounds in different ways. In general, the stability of anthocyanins decreases quicker when glycoside ligands are more extensively hydroxylated (Oliveira et al., 2020). In addition, anthocyanins are also affected by the external environment, and easily degraded under different environmental conditions. Structure, pH, temperature, light, metal ions, enzymes, oxygen, and ascorbic acid can all lead to the degradation of anthocyanins and the deterioration of food color (Fig. 1) (Tan et al., 2021, Cai et al., 2022, Yuan et al., 2023). Therefore, the direct use of anthocyanins as a natural bioactive substance in the food industry faces certain limitations.
Fig. 1.
Schematic picture of factors affecting the stability of anthocyanins.
All colors of the rainbow can be reproduced by dissolving anthocyanins in aqueous solutions with a pH range of 1.0–14.0. At pH = 1.0, the flavylium cation is the predominant species in anthocyanins solutions, and it is primarily responsible for purple and red hues. The blue quinone cation predominates at pH levels of 2.0–4.0. The two main species (i.e., chalcone and carbinol pseudobase) are both colorless at pH 5.0–6.0. At pH values over 7.0, anthocyanins degrade into various compounds depending on the substituent group (Tanaka, Sasaki, & Ohmiya, 2008). Co-pigmentation is a phenomenon in which pigments and other colorless chemical molecules develop intricate relationships that may boost the color intensity. It is widely acknowledged that the primary process sustaining color in plants is the interaction between anthocyanins and co-pigments (Davies & Mazza, 1993). When exposed to ultraviolet or visible light, or other kinds of ion rays, anthocyanins are typically unstable. However, anthocyanins with a C5 hydroxyl group replacement are more susceptible to degradation than anthocyanins without such a substitution. The rate of anthocyanins degradation increases with increasing temperature. Hydroxylation of glycoside ligands decreases the thermal stability of anthocyanins, whereas methylation, glycosylation, and acylation increase thermal stability. Plant anthocyanins are polyphenols with one or more phenolic hydroxyl groups that oxidize easily when exposed to oxidizing substances, which causes anthocyanins to deteriorate, leading to discoloration (Delgado-Vargas & Paredes-Lopez, 2002). Anthocyanins can also be immediately degraded by molecular oxygen, resulting in colorless or brown substances. Additionally, anthocyanins enzymes such as glycosidase and polyphenol oxidase existing in plant tissues can break down anthocyanins, resulting in loss of coloration. In certain cases, anthocyanins can also be hydrolyzed, which can lead to their oxidation and degradation. While K+ has no discernible impact on the stability of blueberry anthocyanins and did not enhance their absorbance, addition of Mg2+, Ca2+, Cu2+, and Al3+ enhances color without clearly affecting the stability of blackberry anthocyanins. Furthermore, high levels of Na+, Zn2+, and Mn2+ can improve the stability and pigmentation of blueberry anthocyanins; the stability of blueberry anthocyanins is decreased by Fe2+, Fe3+, and Pb2+, and white precipitation is produced in solutions of blueberry anthocyanins that contain Fe3+ and Pb2+ (Li, Meng, & Zhou, 2009). Beyond a certain level, sugar and its degradation products accelerate the degradation of anthocyanins, especially under low sugar concentrations. More deterioration is caused by fructose, arabinose, and lactose than by glucose, sucrose, or maltose (Sigurdson, Tang, & Giusti, 2017). In summary, these external factors affect the stability and reduce the wide application of anthocyanins in different fields.
2.2. Low in vivo bioavailability
Anthocyanins are gaining popularity as bioactive components in a variety of foods, medications, cosmetics, and nutritional supplements as their roles receive increasing attention. As a result, anthocyanins consumption has increased in recent years. The biological activity of anthocyanins depends on their bioavailability (Kalt et al., 2020, Alvarez-Suarez et al., 2021). To play a pharmacological role in vivo, drugs must first be absorbed and then transported through the bloodstream to target organs (Agulló, Villaño, García-Viguera, & Domínguez-Perles, 2020). However, the use of anthocyanins is limited by their low bioavailability. The general bioavailability of dietary anthocyanins has been reported to be around 1–2 % (Lila, Burton-Freeman, Grace, & Kalt, 2016), and maximum plasma concentrations were reached within 0.5–2 h after consumption of anthocyanins-rich fruits. In animal studies, the systemic bioavailability of anthocyanins was estimated to be 0.26–1.8 %, and in human studies, maximum plasma levels of total anthocyanins after consumption of berries or grapes ranged from 1 to 100 nmol/L (Fang, 2014). After ingestion, anthocyanins are rapidly absorbed in the stomach and small intestine by various specific enzymes. The absorbed anthocyanins are then metabolized by metabolic enzymes to glucuronide, sulphate, and methylate in the intestinal epithelium, liver, and kidneys (Agulló et al., 2020).
Changes in the chemical structure of anthocyanins, such as in the number of hydroxyl groups and the presence of glucose, can alter the absorption process and lead to changes in potential antioxidant capacity (Kuntz et al., 2015). In the oral cavity, anthocyanins may combine with cells in the oral epithelium and saliva, and may also be exposed to various enzyme activities, which may lead to early anthocyanins degradation (Alvarez-Suarez et al., 2021). Because of the special acidic environment in the stomach and the small absorption area of gastric mucosa, the absorption effect of most substances in the stomach is relatively poor. However, cations expressed in stomach tissue are dominant in the acidic stomach environment, and it was found that after 30 mins of in situ gastric absorption, lingonberry anthocyanins in gastric juice were reduced by 19–37 %. These reductions can be attributed to absorption, as anthocyanins do not degrade in simulated acidic gastric juice, and the presence of anthocyanins in bile was used to confirm the gastric absorption of anthocyanins, which identified a 20 % absorption of examined anthocyanins (Talavera, Felgines, Texier, Besson, Lamaison, & Rémésy, 2003). Depending on their size and composition, food passes from the stomach to the small intestine in a few hours (Chai et al., 2018, Oteiza et al., 2022). When anthocyanins bind to intestinal cells, they have microvilli on one side of the intestinal lumen and are absorbed by bioactive transport or passive diffusion, the two processes that constitute important absorption pathways for anthocyanins (Tarone, Cazarin, & Junior, 2020). According to a study by He, Wallace, Keatley, Failla, and Giusti (2009), approximately 7.5 % of intact anthocyanins can be detected after 2 h ingestion in small intestinal tissues. Meanwhile, anthocyanins were also found to be able to enter Caco-2 cell monolayers (Steinert, Ditscheid, Netzel, & Jahreis, 2008). In addition to degradation, anthocyanins are also affected by autoxidation and isomerization induced by residual dissolved oxygen in the gastrointestinal tract. Microorganisms are also involved in the metabolism of anthocyanins, as they degrade unstable forms of anthocyanins into phenolic acids. Felgines, Texier, Besson, Fraisse, Lamaison and Remesy (2002) showed that blackberry anthocyanins are excreted through the urine in both intact and methylated forms, but no aglycones or conjugated forms were detected. In addition, small amounts of anthocyanins and aglycones have been detected in fecal contents, suggesting that the microbial community has adapted to the degradation of anthocyanins (Shen et al., 2022). The concentration of anthocyanins extract from Jatropha curcas was 20–1500 μM; however, according to a gastric epithelial cell transport test, the cell transport rate is only 0.14–0.25 % (McGhie & Walton, 2007). Anthocyanins need to be released slowly to retain their concentration or efficacy over time, which is necessary so that they can perform their bioactive roles. Furthermore, controlled release can fulfil environmental stimulation requirements by providing beneficial timing, target, and conditions for release (McGhie & Walton, 2007).
3. Encapsulations enhance the stability and bioactivities of anthocyanins
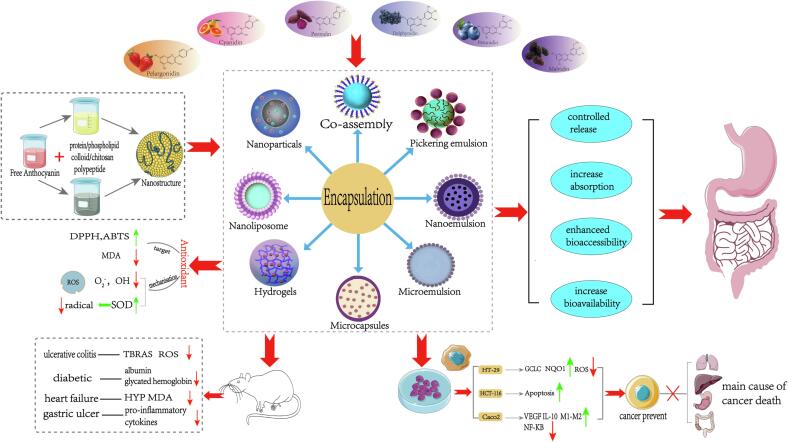
Encapsulation technology can embed anthocyanins inside nanoparticles, protecting them from adverse environmental factors during processing, storage, and digestion, thereby improving their physicochemical properties and enhancing their health benefits. The most promising in vitro and in vivo studies on the effects of encapsulated anthocyanins on human health are summarized in Fig. 2 and discussed below.
Fig. 2.
The production process of anthocyanin nanostructures and ways to improve their bioactivity and bioavailability. DPPH: 2,2-Diphenyl-1-picrylhydrazyl; ABTS: 2,20-Azinobis-3-ethylbenzthiazoline-6-sulfonic acid; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TBARS: Thiobarbituric acid reactive ubstances; HYP: Hydroxyproline; MAD: Malondialdehyde; GCLC: Glutamate cysteine ligase catalytic subunit; NQO1: Quinone oxidoreductase 1; VEGF: Vascular endothelial growth factor. NF-kB: nuclear factor kappa-B signal pathway; IL-10: Interleukin-10; M1-M2: The transformation of macrophages from M1 to M2 phenotypes. The red arrows indicate a decrease or suppression; Green arrows indicate rise or promote. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Microcapsules
Microcapsule technology has been newly developed in recent years. It mainly involves dispersing small reactive, sensitive, or volatile droplets, solid particles or gas by using certain physical or chemical methods with the goal to form a thin film encapsulated microcapsule (Zhang et al., 2015, Feitosa et al., 2023). On the one hand, these microcapsules can store sensitive bioactive compounds, slow down the degradation process, and improve delivery to specific sites. On the other hand, they can also isolate the interaction between materials, and prolong storage time (He et al., 2015, Zhang et al., 2020). These properties have prompt the wide acceptance of microcapsules in catalysis, drug delivery, sensors, and other fields. As well as contribute to an improvement of the stability of bioactive compounds, maximizing their retention and controlling their release in vivo (Liu, Chen, & He, 2019). Therefore, microcapsule treatment can be effective in stabilizing anthocyanins against degradation by light and oxygen exposure, thus enhancing their application value in functional products.
The stability, permeability, and embedding efficiency of microcapsules are decided by the type of wall materials. The main wall materials need to meet several basic characteristics, such as emulsification, film formation, water solubility, and high stability, and these materials include proteins, carbohydrates, gum, fiber, and their mixtures at different proportions (Cai et al., 2022, Neuenfeldt et al., 2022). Among them, the popular single wall materials are maltodextrin, β-cyclodextrin, β-glucan, gelatin, soy protein isolate, etc. For example, when maltodextrin is used as carrier and loaded with Clitoria ternatea anthocyanins, the encapsulation rate can reach 87.34 ± 5.9 %, with the best color stability in the temperature range of −20 °C to 4 °C, and physical stability with good light resistance (Ab Rashid et al., 2021). Another study showed that the encapsulation efficiency of β-glucan microcapsules and β-cyclodextrin microcapsules loaded with saffron anthocyanins could reach 63.25 % and 45.00 %, respectively. Meanwhile, the content of anthocyanins, antioxidants, and phenolics were found to be high level under intestinal conditions, indicating that both wall materials had the potential to encapsulate saffron bioactive substances (Ahmad, Ashraf, Gani, & Gani, 2018). A comparison of the effects of gelatin, soy protein isolate, maltodextrin, and acacia gum encapsulation on the release rate of anthocyanins showed that after 2.5 h of in vitro digestion, the release rate of anthocyanins in microcapsules encapsulated with soy protein isolate was the lowest (27.1 % of total anthocyanins), followed by microcapsules encapsulated with gelatin (28.7 %), gum locust (54.2 %), maltodextrin (63.0 %), and unencapsulated anthocyanins (70.9 %), that proclaim minimized the loss of anthocyanins in simulated gastrointestinal digestion in vitro after encapsulation treatment (Mansour et al., 2020, Wu et al., 2020). The results of in vivo experiments showed that the modified β-glucan microcapsules could prolong the weight-bearing swimming time and increase the liver glycogen reserve of mice, the modified β-glucan microcapsule could decrease the level of blood urea nitrogen and blood lactic acid, and improve the anti-fatigue effect of Lycium barbarum anthocyanin (Gao, Shu, Chang, Cen, Cao, & Wang, 2019). Furthermore, in order to reduce the defects of single wall materials, many mixed wall materials have been explored to microencapsulated anthocyanins. Li et al. (2022) reported that composite wall which combined β‐cyclodextrin with proteins materials could offer higher encapsulation rates and thermal properties than single wall materials, achieving encapsulation rates up to 99 %. Another study further reported that after microencapsulation, the stability of anthocyanins increased clearly with the retention rate of microencapsulated anthocyanins with composite wall materials was still above 80 % after 5 weeks of storage at condition of room temperature and light exposure, while unencapsulated anthocyanins had already oxidized after 2 weeks (Liu, Cao, & Liu, 2004). Moreover, composite enclosing wall material also could well protect the core material from adverse stomach and small intestine conditions. For example, encapsulation rate of concentrated anthocyanins microencapsulated of black rice bran was 83.65 % by using gelatin, acacia gum, which has a low solubility to provide gradual, slow and controlled release of the targeted core active substance, potentially prolonging the intestinal absorption of the target substance that also acts as the drug ingredient (Devi, Das, & Badwaik, 2023). Additionally, when compared to blueberry anthocyanin powder microcapsules made with 4 % soy isolate protein alone, those with 2 % hypermethyl pectin and 4 % soy isolate protein combined had the longest half-life and the lowest rate of blueberry anthocyanins degradation during storage at 25 °C and 35 °C for 6 months. The blueberry anthocyanins release performance could also be improved by blending soy isolate protein/high methyl pectin. Although microcapsules exhibit sustained release of blueberry anthocyanins during simulated gastrointestinal digestion, the release stability of microcapsules prepared with composite wall materials was higher than that of single wall materials and free blueberry anthocyanins (Pan et al., 2022). Similarly, the Aronia melanocarpa anthocyanins release rate of the microcapsules with chitosan and β-whey protein as composite wall material was lower than that of the free anthocyanins in gastric digestive fluid, and the free anthocyanins release rate significantly decreased after 0–30 min of intestinal digestion, while the microencapsulated anthocyanin release rate was significantly increased. Microencapsulation of Aronia melanocarpa anthocyanins reduced the loss of anthocyanins in gastric juice and showed good sustained-release properties (Chen et al., 2023). On the other hand, the anthocyanins prepared by microencapsulation have higher biological activities than unencapsulated anthocyanins, which has been well demonstrated in vivo. For instance, microcapsules of anthocyanins with pectin and chitosan at concentrations of 5, 10, 20, and 40 µg/mL showed greater potential to protect normal rat kidney cells from acrylamide-induced damage and allowed their controlled release through the gastrointestinal tract compared to unencapsulated anthocyanins. They reduced reactive oxygen species, matrix metalloproteinase and glutathione levels, and the damage of normal human hepatocytes in the palmitic acid-induced damage model (Zhao et al., 2020). Moreover, the antioxidant activity of microcapsules prepared with ferulic acid grafted maltodextrin (0.0625 mg/mL) was higher than that of single microcapsules of anthocyanins. After 24 h culture in human colon cancer cells (HT-29), compared with free anthocyanins, complex microcapsules (0–60 mg/mL) reduced the content of reactive oxygen species, significantly upregulated the expression of glutamate-cysteine ligase catalytic subunit (GCLC) and quinone oxidoreductase 1(NQO1) of HT-29, and enhanced the antioxidant effect. It also has the ability to regulate the catalytic subunit of glutamate-cysteine ligase and quinone oxidoreductase 1 (Ma, Feng, Zeng, & Luo, 2019).
3.2. Emulsion
Emulsion-based encapsulation systems are getting popularity as a solution to overcome low stability and bioavailability of bioactive substances. Emulsion-based systems can be applied in food or pharmaceutical sectors to encapsulate both hydrophilic and hydro-phobic compounds.
3.2.1. Nanoemulsions
Nanoemulsions are colloidal dispersions with particle sizes of 20–500 nm that form by droplets of one liquid dispersed in another immiscible liquid, which can be stabilized by a surfactant layer as a system (Seibert et al., 2019, Mushtaq et al., 2023, Lan et al., 2023). Such a system is usually composed of an aqueous phase, an oil phase, and a stabilizer. Meanwhile, their small droplet sizes allow the nanoemulsions system to remain dispersed, thus preventing emulsification, flocculation, or precipitation during storage (Wulansari, Jufri, & Budianti, 2017). In recent years, nanoemulsions have attracted more and more attention due to their excellent properties. These properties include solving the problems of poor stability, oxidation, and absorption of active substances. Simultaneously, the physical and chemical stability in the gastrointestinal tract are improved, intestinal permeability is increased, release rate is controlled, and oxidation of encapsulated substances is prevented, all of these advantages are conducive to the improvement of encapsulated substances bioavailability (Oehlke et al., 2014, Rabelo et al., 2018, Wang et al., 2020). Therefore, nanoemulsions have received widespread attention in the fields of food and medicine.
The application of emulsion-based delivery systems to improve anthocyanins stability and bioavailability was studied both in vitro and in vivo. For example, açai berry anthocyanins loaded in water-in-oil (W/O) nanoemulsion had the highest retention rate (94.6 %) after 30 days of storage (Rabelo, Taarji, Khalid, Kobayashi, Nakajima, & Neves, 2018). Similarly, different concentrations of Brazil berry extract were successfully used to prepare a W/O nanoemulsion with relatively stable properties and showed no phase separation after 30 days. After 30 days of storage, all nanoemulsions retained their antioxidant activity and showed a high polyphenol retention rate. These results also indicated that a stable nanoemulsion can used as an anthocyanins protection system (Kenari & Razavi, 2022). Another research showed that the best hydrophilic and lipophilic balance index for prepared a stable Luoshenhua anthocyanins nanoemulsion was 6.7, and its subsequently in vitro release rate in buffer (pH 6.5–7.4) was 44.7–40.7 %. This nanoemulsion resulted in a relatively moderate anthocyanins release rate and significantly improved anthocyanins retention during long-term storage (Baba Shekh, Abdul Wahab, & Yahya, 2022). Besides, a water-in-oil (W/O) nanoemulsion from anthocyanins-rich mangosteen rind extract use virgin coconut oil, Tween 80, and polyethylene glycol 400 as oil phase could reached the loading rate of 125 mg/5 mg. At the cellular level, when Franz cells were cultured with anthocyanin nanoemulsion for 8 h, the diffusivity of anthocyanin was up to 97 %, which was 78 % higher than that of Franz cells cultured with free anthocyanins, indicating that W/O nanoemulsion improved the permeability and stability of anthocyanin through the cuticle (Pratiwi, Fudholi, Martien, & Pramono, 2017).
Beside the W/O emulsions, double emulsions (W1/O/W2) also have been developed as an effective carrier for both fat-soluble and water-soluble bioactive substances with relative stable properties because of the presence of multiple amphiphilic molecules with different hydrophilic-lipophilic balance values (Yin, Zheng, Ho, Huang, Wu, & Zhang, 2022). Moreover, double emulsion was able to control anthocyanins release in the stomach, and slower the release in simulated gastrointestinal fluid. This is due to the fact that the W1/O droplets broke during intestinal digestion, producing empty oil droplets and releasing anthocyanins in the internal water phase of the double emulsion (Xu et al., 2018). For instance, the addition of black rice anthocyanins into double emulsions presented a high encapsulation efficiency (99.45 ± 0.24 %) and the anthocyanins release rate was only 3.66 ± 2.32 % after incubation in simulated gastrointestinal fluid for 2 h (Huang & Zhou, 2019). Besides, the microencapsulation of anthocyanins rich black rice extract using double emulsion and compounded with gelatin arabic gum and chitosan carboxymethyl cellulose different hydrophilic emulsifiers showed the highest stability of anthocyanins, such as the highest half-life value (150 days), the highest thermal resistance temperature (78.1℃), the lowest reaction rate constant (4.8 × 10-3/day) and minimum activation energy (23.7 kJ/mol) (Kanha, Surawang, Pitchakarn, & Laokuldilok, 2020). These results indicated that compared with other emulsions, W1/O/W2 emulsion may be a suitable anthocyanins encapsulation system, which could trap anthocyanins in the emulsion system internal water droplets, protect anthocyanins from adverse conditions, and thus increase the biological efficacy of anthocyanins (Oehlke et al., 2014).
Based on stability, the application of nanoemulsion delivery systems to improve anthocyanins bioavailability was studied bothin vitroand in vivo. For example, different concentrations of yogurt were mixed with anthocyanins-rich Malva parviflora leaves extract nanoemulsion to study the inhibitory effect on acetic acid-induced ulcerative colitis in rats. Compared with control rats (colon thiobarbituric acid reactive substances level increased, colon glutathione and total antioxidant capacity decreased), malva parviflora leaves extract nanoemulsion combined with yogurt could inhibit the production of reactive oxygen species in rats, scavenged free radicals and improved the defense of antioxidant defense enzymes against acetic acid-induced ulcerative colitis. In addition, this nanoemulsion could also interfere with the transmission of inflammatory signals by inhibiting the binding of pro-inflammatory cytokines (such as TNF-α) to cell receptors, which may reduce inflammation and combat oxidative stress (El-Naggar et al., 2020). Another study fabricated nanoemulsion of anthocyanins rich leaf flower extract with encapsulation efficiency of 83.4 ± 0.8 % showed the average minimum inhibitory concentrations (MIC) for against Staphylococcus aureus, Escherichia coli, Listeria monocytogenes and Salmonella were 112.5, 87.5, 112.5 and 87.5 µg/mL, respectively, stronger than that of unencapsulated anthocyanin extract (Kenari & Razavi, 2022). Moreover, encapsulation of anthocyanins nanoemulsion have been developed to novel multifunctional food packaging. For example, anthocyanins-thyme oil nanoemulsion was mixed into the chitosan arabic gum film matrix, not only could be used as a plasticizer to improve the mechanical properties of the membrane, increase the elongation at break of the membrane to 76.1 %, when enhance the maximum degradation temperature to 305℃ and enhanced the UV visible light barrier which almost blocked all the ultraviolet rays. Amazingly, this membrane also had a certain effect in effectively extending the shelf life of milk, mainly by inhibiting the proliferation of spoilage bacteria and slowing down milk rancidity (Zhao et al., 2023). Furthermore, the anthocyanin double emulsion system has also shown great application potential for delivering water-soluble bioactive ingredients in 3D printing (Li, Guo, Cai, Yi, & Zhou, 2023).
3.2.2. Microemulsion
Microemulsions are mainly composed of water, oil, surfactant, and cosurfactant, considered as one of the most successful encapsulation systems for delivering bioactive materials. Compared with other emulsions, microemulsions have better fluidity, more uniform particle dispersion, and stronger stability due to the oil and water in the microemulsion (or other substances in the microemulsion) are linked and separated by the amphiphilic genes of the surfactant (Yang and Zhang, 2018, Gao et al., 2020). Microemulsions are typically 10–100 nm in size, which can provide a relatively stable and uniformly dispersed system (Chen et al., 2018, Liović et al., 2019, Garavand et al., 2021), these properties are help for improvement of absorption and bioavailability of bioactive ingredients, offer the advantages of multiple delivery routes, targeted delivery, ease of manufacture (Gorantla et al., 2021, Nazareth et al., 2021). Furthermore, microemulsions have able to provide new technologies to overcome the limitations of low water solubility and low permeability of drugs, provide new technologies for achieving high bioavailability of bioactive ingredients and regularity of absorption processes, contribute to the development of new plant therapies (Egito, Amaral-Machado, Alencar, & Oliveira, 2021).
Since anthocyanins are water-soluble (hydrophilic compounds), therefore, it can encapsulate in the corresponding aqueous core of the microemulsions system. The microemulsion system contained 425.54 ± 1.58 μg/g strawberry anthocyanins prepared with the ratio of oil phase to emulsifier 7:3. The addition of anthocyanins into the microemulsions exhibited a significantly increased retention rate from 43.1 % to 62.4 % at 60℃ for 10 days after microemulsion embedding and high storage stability over 25 days in presence of light (Chen et al., 2018). Furthermore, the microemulsion encapsulated anthocyanins also possess higher antioxidant and antibacterial activities than unencapsulated anthocyanins (Ma, 2017). Another study reported that anthocyanins microemulsion prepared with monoglyceride as surfactant and ethanol as co-surfactant showed a better preservation effect in fresh cut strawberries. After 14 days of storage, the quality loss and fungal deterioration of control strawberries were 4.35 % and 3 %, respectively, while those of microemulsion-treated strawberries were only 2.83 % and 1.3 %. Informed by the color change of strawberries, the anthocyanins content of microemulsion-coated strawberries decreased slowly, indicating that microemulsion had a certain potential be used in food preservation applications (Mendonça, Borges, Kringel, da Silveira, da Silva, & Schulz, 2020).
3.2.3. Pickering emulsion
The Pickering emulsions have been demonstrated as good carriers of anthocyanins in current studies. Pickering emulsion system has been prepared by adhering functional molecules onto the surface of the emulsion. Their droplet size is usually in the micron range and much larger that of microemulsion and nanoemulsions (Shao, Zhang, Niu, & Jin, 2018). Compared with a traditional molecular surfactant stabilized emulsion, the desorption energy of the particles at the interface of the Pickering emulsion (which is completely stabilized by colloidal particles) is very high because they are stabilized by colloidal surfactants (i.e., solid particles) (Matos et al., 2013, Ju et al., 2020). Once these solid particles are absorbed at the interface of Pickering emulsion, they are difficult to separate. Thus, this system not only has the advantages of strong anti-coalescence ability, good long-term stability, good biocompatibility, and adjustable performance, but also avoids the use of surfactants and reduces damage to the environment (Xiao et al., 2017, Shao et al., 2018, Sun et al., 2021). Therefore, a growing number of studies have been conducted to improve the stability of anthocyanins by combining anthocyanins with Pickering emulsions over recent years. On the one hand, a Pickering emulsion has a higher internal phase content, which is beneficial for increasing the anthocyanins loading. On the other hand, protein/carbohydrate solid particles can also be used as stabilizers to produce surfactant-free emulsions (Yin et al., 2022).
Lin et al. (2020) prepared an anthocyanins-loaded double Pickering emulsions (W1/O/W2) via adding anthocyanins loaded W1/O emulsions to W2 with two-step homogenization method. The encapsulation rate could be as high as 95.8 %, and the emulsion maintained the structural integrity and high encapsulation stability of anthocyanins in gastric digestion (86.6 %), as well as the whole system showed high stability to the change of pH and ionic strength for 7 days. Another study reported an double Pickering emulsions loaded anthocyanins and kafirin pellets with an encapsulation rate of 85.1 % could remained at 70–80 % after storage for up to 14 days (Xiao et al., 2017). Anthocyanins combined with protein-like substances for Pickering emulsions showed better physical and oxidative stability over the free anthocyanins. For example, Ju et al. (2020) developed an anthocyanins (0.15 %) loaded W/O Pickering emulsion using soy protein isolates as stabilizer displayed relative free radical scavenging capacities of DPPH (65 %) and ABTS (62 %) over free anthocyanins (25 % and 20 %, respectively), and the lipid hydrogen peroxide and malondialdehyde (MDA) contents decreased significantly from 63 % and 40 % to 10 % and 7 %, respectively. Similar results were also found in Pickering emulsions with black rice anthocyanins as stabilizers, as the anthocyanins could inhibit the lipid hydroperoxide content with little increase, whereas the lipid hydroperoxide content of Pickering emulsion with Tween 80 had significantly increased inhibitory effect which could reached 1.811 ± 0.006 μmol/g (Lu et al., 2022). All these results demonstrated that the Pickering emulsion had excellent oxidation stability probably due to their structure helped to prevent oxidant transfer from the continuous aqueous phase to the interfacial region (Zhou et al., 2018, Shen et al., 2021).
3.3. Hydrogels
Hydrogels are defined as cross-linked networks of hydrophilic polymers with three-dimensional shapes (Hoare & Kohane, 2008). Hydrogels can be formed from water-soluble polymers that include a wide range of chemical compounds and bulk physical properties that can absorb large amounts of water and swell and shrink appropriately to promote water release, while maintaining their three-dimensional network structure (Hu, Wang, Zhang, & Xu, 2020). Due to the high water content and physical and chemical similarity of these hydrogels to the natural extracellular matrix, the hydrogels have the advantages of high safety, reproducibility, high biocompatibility, and biodegradability when used as delivery systems for embedded nutrients or drugs (Park et al., 2011, Raak et al., 2017, Syed Azhar et al., 2022). Furthermore, hydrogels also have excellent controlled and sustained release characteristics which similar to soft tissues, and can be used for embedding and delivering bioactive substances (Liu et al., 2022, Ming et al., 2018).
Anthocyanins that are made into hydrogels tend to have better stability than those that are not. For example, purple corn anthocyanins-loaded alginate-pectin hydrogel showed significantly reduced fluorescence-induced photodegradation of anthocyanins during storage in both light and dark conditions, And the half-life values in purple corn anthocyanins encapsulated (630 h) were remarkable high than that of nonencapsulated treat (58 h) (Guo, Giusti, & Kaletunç, 2018). Liu et al. (2022) reported that the incorporation of gellan gum can improve the stability and bioavailability of gelatin anthocyanins hydrogels. In vitro release studies showed cumulative release of anthocyanins was 42.4 %, and 27.2 % from anthocyanins and anthocyanins-loaded hydrogels at gastric digestion, while 81.8 % and 30.1 % at intestinal digestion respectively. Moreover, complex wall material consisted hydrogels shown better protective effect than single one. Like encapsulation of blueberry anthocyanins in complex hydrogels system (Chondroitin sulfate and carrageenan) increased the anthocyanins stability to 94.40 % at 25 °C after 5 h of pH 6.0 storage than that of prepared by single wall material (88.14 %). This is because its high charge density and more smoother, regular structure, that could provide good protection against the degradation of blueberry anthocyanins at lower pH conditions (Xie, Wang, Ying, Wang, Wang, & Huang, 2020).
Based on this high stability property in the hydrogel system, anthocyanins hydrogels have shown good bioactivity in cell or animal models. Gelatin-entrapped black carrot anthocyanins showed high ability to maintain cell vitality than free anthocyanins in mouse fibroblasts L929. After the treatment with 0.01 % of hydrogel–loaded anthocyanins for 3 days, the cell density could be increased from 96 % to 99 % Hence, anthocyanins hydrogel could help with wound healing. Meanwhile, due to anthocyanins could eliminate residual glutaraldehyde radicals, the dark carrot anthocyanins-hydrogels had able to identify infections (Shineh, Kordestani, Tahriri, & Tayebi, 2019). Another study researched the injectable blueberry anthocyanins-loaded hydrogel significantly accelerated the wound healing process, promoted epithelial and tissue regeneration, exerted anti-inflammatory effects, and promoted both collagen deposition and angiogenesis in a full-thickness rat skin wound model. And had a ability to upregulate the expression of VEGF (vascular endothelial growth factor) and IL-10 proteins (interleukin-10), downregulate the NF-κB level (nuclear factor kappa-B), and promote the transformation of macrophages from M1 to M2 phenotypes. These effects showed that blueberry anthocyanins-hydrogel exerted a synergistic effect in promoting wound healing (Zhang et al., 2020). Biologically, for animals with heart failure, anthocyanins-loaded corn starch hydrogels could restore the glycogen content in liver and heart tissue in the fibrotic group (13 ± 1.4 and 5 ± 0.7 μmol glucose/g tissue) and slightly controlled the glycogen content in liver and heart tissue in the non-encapsulated anthocyanins-treated fibrotic group (10 ± 1.4 and 7 ± 0.8 μmol glucose/g tissue). In addition, malondialdehyde (MDA) and hydroxy proline (HYP) were also significantly reduced, indicating that anthocyanins loaded on starch-based hydrogels could improve histological cardiac function after injury (Hanafy, 2021).
3.4. Nanoliposomes
Nanoliposomes are one of the most commonly used colloidal drug delivery systems in food and nutrition research (Ghorbanzade, Jafari, Akhavan, & Hadavi, 2017). Nanoliposomes are mainly made from surfactants (phospholipids), which forms an aqueous core and an amphiphilic lipid bilayer by embedding water-soluble substances in the water phase and fat-soluble substances in the oil phase (Chi et al., 2019, Zhang et al., 2022). The unique bilayer structure of nanoliposomes has the ability to equip them with strong biocompatibility, enabling them to easily fuse with bacterial membranes and enter microorganisms. Hence, bioactive substances could be transported into cells, that promoted drugs distributed in tissues and organs, and drugs are protected from enzymatic degradation. In addition, nanoliposome features such as low toxicity, flexibility, complete biodegradability, non-immunogenicity, self-assembly ability, and modification acceptability are all desirable (Cai et al., 2022, Yin et al., 2022). Anthocyanins are water-soluble which can be loaded in the internal water phase of nanoliposome. Encapsulation of anthocyanins in nanoliposomes can avoid environmental degradation such as light, pH, and temperture.
Preparation of anthocyanins-loaded nanoliposomes by ultrasonic film dispersion using soybean lecithin as carrier, which enhanced the stability under environment of light and temperture still exceeded higher retention rate of anthocyanins at 7d was 80 % (Zhang et al., 2022). According to research by Chi et al. (2019) at a lecithin concentration of 10.75 g/L, anthocyanins-loaded nanoliposomes that maintained a retention rate of 85.60 % at 25 °C for 16 days, which considerably increased the stability of anthocyanins. Another study showed that the encapsulation efficiency was higher than 75 % when the ratio of phosphatidylcholine base loaded with anthocyanins extracted from red onions was 1:6. Moreover, from the cumulative release of red onion anthocyanins concluded as a successful nanostructure with a two-fold increase in intestinal medium (Sahin, Dundar, Uzuner, Parlak, Dagdelen, & Saricaoglu, 2023). This is because of anthocyanins in the gut could interact electrostatic with phospholipid polar groups and hydrophobic with alkyl chains that not only improved the physicochemical stability of anthocyanins both in vitro and in vivo, but also promoted their bioavailability at target organs (Bonarska-Kujawa & Kleszczyńska, 2012). The inhibitory ability of anthocyanins to inhibit cancer cells can be enhanced via nanoliposome encapsulation. Used phosphatidylcholine as polar lipid to fabricate cyanidin-3-O-glucosid-loaded nanoliposomes with an encapsulation efficiency of 77.5 %, which could reach an inhibitory effect of 90–100 % on cancer cells (GES-1) (Liang, Guan, Quan, Tao, Liu, & Hu, 2019). Similarly, encapsulation of cyanidin-3-O-glucoside (C3G) (0.25 mg/mL) in nanoliposomes outperformed the inhibition of Caco-2 in cancer cells compared with free C3G (the inhibition was about 15 % higher) and reduced the mitochondrial activity of cells, which promoted the ability of anthonyanins to inhibit the proliferation of human tumor cells (Liang, Guan, Wang, Shen, Xia, & Liu, 2017). Nanoliposomes encapsulation of anthocyanins mays significantly improved concentration of anthocyanins in the blood, particularly at 3 h after rats that were fed anthocyanins nanoliposomes and black rice anthocyanins by gavage. The area under curve of black rice anthocyanins nanoliposomes was 6.634 μg/mL·h, which was about 1.46 times higher than black rice anthocyanins (4.553 μg/mL·h), T peak (which represents the time to reach area under curve after administration) in nanoliposomes dose group (2.117 h) was significantly longer than that in free anthocyanins group (1.959 h), and nanoliposomes also prolonged the half-life of anthocyanins in the gastrointestinal tract, delayed their release. These results showed phospholipid complex enhances the in vivo bioavailability of black rice anthocyanins (Wang, Lv, Zhao, & Li, 2017). Similarly, in vivo studies showed that the glycosylation rates of albumin and glycated hemoglobin in diabetic mice were reduced to 46.35 ± 1.20 % and 3.60 ± 0.25 % respectively when 100 mg/kg of delphinium pigment liposomes were administered daily, which was 9.21 % and 1.35 % higher than that of free anthocyanins, effectively improving diabetes in mice (Gharib, Faezizadeh, & Godarzee, 2013). In addition, Homayoonfal, Mousavi, Kiani, Askari, Desobry and Arab-Tehrany (2021) confirmed that liposomes enhanced the antioxidant capacity of anthocyanins in a highly significant and effective manner after 1 week of treatment. Their administration also increased metabolite activity and replication of human mesenchymal and fibroblast stem cells at concentrations of 0.5 mg/mL and 10.4 µg/mL, respectively.
After modification of nanoliposomes showed better functional properties over the uncoated liposomes due to the improvement of the stability of liposomes, enable controlled release, and increase the encapsulation efficiency of liposomes (Yin et al., 2022). For example, after chitosan modification, the encapsulation rate of anthocyanins can be increased by about 10 %, and its release rate after 24 h is 48 %, which is much lower than that without chitosan modification (64 %), indicating that chitosan coating reduced the release of bioactive compounds from liposomes (Gibis, Ruedt, & Weiss, 2016). Wang, Wang, Wang, Lu and Zhang (2022) also showed that chitosan could inhibit the release of anthocyanins from blueberry anthocyanins liposomes, which is beneficial to prolong the release application. These effects may be attributed to biocompatibility, biodegradability, non-immunogenicity, low toxicity, and adhesion of chitosan (Buschmann et al., 2013, Peng et al., 2014, Kumar et al., 2020). Besides, compared with unmodified liposomes, N-trimethyl chitosan coated anthocyanins liposomes, which could alleviate oxidative stress of selenite induced cataract in rats, enhance the transepithelial transport of liposomes in the cornea of rats to a depth of 40 mm, extend the residence time 3.3 times, increased corneal epithelial permeability, improved the activities of superoxide dismutase and catalase in the lens, as well as increased glutathione activity and decreased lipid peroxidation (Zhang et al., 2016). Another study showed that pectin/chitosan polymer-coated pelargonidin-3-O-glycoside functionalized nanoliposomes increased the thermal stability and food simulation stability of pelargonidin-3-O-glycoside. The retention rates of pelargonidin-3-O-glucoside in nanoliposomes were 47.5 % and 57.5 % respectively, which were higher than that of free anthocyanins during in vitro digestion (Shishir et al., 2020). Furthermore, the nanoliposomes could be used as a co-delivery system for anthocyanins and other nutraceuticals by appropriate preparation methods. The bilayer membrane of novel docosahexaenoic acid − anthocyanidin-codelivery nanoliposomes remained intact in simulated gastric fluid. And novel docosahexaenoic acid nanoliposomes were not toxic to Caco-2 cells and significantly increased the uptake of novel docosahexaenoic acid and anthocyanins by cells (Xu et al., 2021). All these results indicate that nanoliposome encapsulation is a promising method that could protect the degradation of anthocyanins in food matrix, and enhancing their bioavailability, which could be widely used as a delivery vehicle for functional foods.
3.5. Nanoparticles
Nanoparticles have proven to be excellent materials for encapsulating phenolic compounds and improving their bioavailability that generally defined as “solid colloidal particles ranging in size from 10 to 1000 nm.” (McNamara & Tofail, 2017). The loading of nanoparticles increases the water solubility of polyphenols, which can be absorbed by cells through endocytosis, trapping biomolecules into their internal structures, or absorbing molecules onto their external surfaces, thereby significantly increasing the absorption rate of polyphenols (Liu, Jiao, Wang, Zhou, & Zhang, 2008). Through this encapsulation, the solubility and stability of the bioactive substance can be improved. In addition, nanoparticles play an important role in drug delivery, nanoparticle carrier materials are usually biodegradable or ion sensitive that have low toxic side effects,and have controllable release properties. On the other hand, nanoparticles can also penetrate cell and tissue gaps to reach target sites to improve the bioavailability of bioactive substances (Jung, Kamm, Breitenbach, Kaiserling, Xiao, & Kissel, 2000).
The application of nanoparticles systems to improve anthocyanins stability and bioavailability was studied both in vitro and in vivo. For instance, red raspberry pomace anthocyanins-loaded in β-lactoglobulin nanoparticles, with encapsulation efficiency of 77 %, showed high bioavailability of 19.23 % than that of unencapsulated anthocyanins free extract (11.25 %) (Salah, Mansour, Zogona, & Xu, 2020). He et al. (2017) designed an anthocyanin-loaded nanoparticles to improve the anthocyanins stability, thereby finding that nanoparticles had high storage stability of 30.8 % over 35 days at 40℃, while that of the free anthocyanins was only 6.3 % for the same time periods. Ma et al. (2020) prepared a gelatin/chitosan-coated blueberry anthocyanins-loaded nanoparticles also exhibited a loading efficiency of 83.81 %, and suitable long-term storage capacity at room temperature with a retention rate of 50 % after 15 d. Besides, under the optimum conditions with ratio of bovine serum albumin to anthocyanin was 1:10, blueberry anthocyanins-loaded bovine serum albumin self-assembled nanoparticles was almost unchanged after 6 h in the simulated intestinal system, yet decreased by 70 % of free anthocyanins (Chen, Tao, Zhang, Sun, & Zhao, 2015). Moreover, Anthocyanins-ceria nanoparticles stabilized can substantially slow down the oxidation of anthocyanins of grapes caused by hydrogen peroxide at pH ≥ 7.0 (Ivanov, Usatenko and Shcherbakov, 2009).
Some in vivo studies have also shown that the nanoparticle system could enhance the bioactivities of anthocyanins. Liang, Zhang and Jing (2019) reported that the self-assembled nanoparticles made of the biopolymers chitosan, chondroitin sulfate significant and black rice anthocyanins could be increased the apoptosis of HCT-116 cellline by 35.1 % whereas only 12.1 % of free anthocyanins treatment. Perumcherry et al. (2022) reported that feeding anthocyanin-loaded-chitosan nanoparticles to gastric ulcer in male Wistar rats could significantly alleviate inflammation of induced-gastric ulcer, affected expression of anti-inflammatory cytokines (Interleukin 4, IL-4) and inhibition of pro-inflammatory cytokines (Interferon gamma, IFN-c). Besides, Sreerekha et al. (2021) researched showed that feeding anthocyanin-loaded-chitosan nanoparticles (ACNPs) to male hyperlipidemic Wistar rats significantly reduced the total cholesterol and triglycerides levels in serum, decreased the lipid-mediated oxidative stress and hepatic lipid levels, and also normalized the protein expression of fatty acid synthase and hydroxymethylglutaryl Co-A reductase activities. This is probably due to the anthocyanins contained hydroxyl groups that can crosslink through nanoparticles bonds, thereby to exert its biological activity better. In terms of biological effects, a study showed that anthocyanins conjugated with PEG-AuNPs (AnPEG-AuNPs) could reduce A 1–42-induced neuroinflammatory and neurophotonic markers via inhibiting the p-JNK/NF-κB/p-GSK3β pathway more effective compared to anthocyanins alone (Kim, Rehman, Amin, & Kim, 2017). Similarly, Ali, Kim, Rehman, Ahmad and Kim (2017) also reported the anthocyanins nanoparticles–loaded PEG-AuNPs and anthocyanins treatment could ameliorated memory impairments in the Aβ1-42-injected mice with a dose of 12 μg/g for 14 days,while the anthocyanin–loaded PEG-AuNPs were more effective than free anthocyanins. Meanwhile, nanoparticles also regulated the p-PI3K/p-Akt/p-GSK3β pathway, which could prevent the hyperphosphorylation of tau protein at serine 413 and 404 in the Aβ1-42-injected mice.
3.6. Co-assembly with amphiphilic peptides
Molecular self-assembly is one of the most important strategies for the preparation of nanomaterials, which refers to the spontaneous aggregation of molecules into ordered nanostructures. This process is usually driven by non-covalent bonds such as ionic bonds, hydrophobic interactions, van der Waals interactions, and hydrogen bonds (Qiu, Chen, Tang, & Zhao, 2018). A class of amphiphilic peptides with a hydrophobic tail and a hydrophilic head are widely used in molecular self-assembly. These amphiphilic peptides have good biocompatibility, biodegradable self-assembly and chemical variability which can form a variety of nanostructures with certain application value (Zhao et al., 2021, Qiao et al., 2022).
The simplest way to improve the color stability of anthocyanins is the addition of peptides and amino acids to foods containing anthocyanins. Such as e-poly-l-lysine, l-phenylalanine, l-tyrosine, and l-tryptophan) to a purple carrot anthocyanins drink (Chung, Rojanasasithara, Mutilangi, & McClements, 2017). The results found under the high temperature storage conditions (40 °C for 7 d), the addition of amino acids or peptides (0.1 %) improved the color stability of anthocyanins compared with that without peptide addition, especially for l-tryptophan showing the most significant improvement, and the half-life of anthocyanin color increased from 2 to 6 days that maintained and extended good organoleptic quality of the beverages. Li et al. (2021) reported that the combination with silk fibroin peptide could improve the physical and chemical stability of C3G to a certain extent. After Co-assembly with fibroin peptide, its tolerance to alkaline environments and high temperatures (80 °C), as well as the retention rate of metal ions (Cu2+) at different concentrations, could be improved significantly. Meanwhile, the DPPH radical scavenging ability in the nanostructure of C3G was also significantly increased by about 5 %. Similaly, co-assembled a tryptophan-containing amphiphilic peptide C6 with anthocyanins, which significantly improved the stability of anthocyanins under different alkaline conditions, high temperature (80 °C), long-term storage, and exposure to different concentrations of Cu2+ ions. Moreover, the excellent inherent abilities of anthocyanins to scavenge free radicals was also retained (Yao, Xu, Zhang, Zheng, Liu, & Zhang, 2021). In subsequent vivo studies, C3G was self-assembled with low-viscosity sodium alginate, which the encapsulation efficiency of 84.2 %. During simulated gastrointestinal digestion, C3G retention rate reached 77.6 %, which was far higher than the rate of 60.1 % of free C3G. Interestingly, this composite structure increased the C3G reaction concentration in mouse serum by 27.4 %, and the C3G content in feces was significantly lower than that of free anthocyanins by about 20 %. These results proved that the controlled release of complex structure could significantly improve the bioavailability of C3G in the intestinal digestion process (Zou et al., 2021). Amphiphilic Peptides, as a new type of material, bind to anthocyanins through self-assembly, reducing the preparation difficulties and loss of anthocyanins caused by complex processes and equipment. They can significantly improve the controlled release of anthocyanins, which have great researching space in the future of food and medicine.
4. Conclusion and outlook
Anthocyanins are the most widely used polyphenols in plants, with many beneficial effects such as antioxidant, anti-inflammatory, and prevention of chronic diseases. Because of the exponential growth in consumer demand for cognition-enhancing foods, healthy foods, and organic foods, natural food colors such as anthocyanins and their medical functions have received increasing interest for many years. However, because of their poor stability and low bioavailability, the application of anthocyanins under different processes and storage conditions remains limited. Therefore, through the above review, it could be found that the stability, biological activity, and bioavailability of anthocyanins could be significantly improved by designing the encapsulation process reasonably. Although the available data on the transfer and enhancement of anthocyanins in functional foods are encouraging, several problems persist. For example, in certain studies, crude extracts of anthocyanins from different foods were selected instead of pure compounds and their biological activities were studied before and after encapsulation. While crude extracts are certainly easier to prepare in large quantities on an experimental scale or by the food industry, such studies cannot disclose the true biological activity of bioactive compounds. In some cases, because of the synergistic effect of different components, the activity of the whole extract may be higher than that of pure compounds. Therefore, more research attention should be directed at the mechanism of use of pure anthocyanins. Moreover, both the toxicity and safety of encapsulated anthocyanins need to be further assessed. Finally, more in vivo studies are needed to compare free and formulated anthocyanins. At present, in vitro studies have mainly shown that the stability and bioavailability of anthocyanins can be improved by encapsulation, but these results cannot be directly extrapolated to the human body. Hence, more in vivo studies need to be studied that promote the application of anthocyanins encapsulation in the food and medical industries.
Funding
This work was supported by the Science and Technology Innovation Platform and Talent Project of Hunan Province [ grant numbers 2019TP1029]; the Natural Science Foundation of Hunan Province [grant numbers 2022JJ50325]; and Key Project of State Key R & D Program, China [grant numbers 2022YFF1100200].
CRediT authorship contribution statement
Yingying Cheng: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Jiayi Liu: Conceptualization, Resources. Ling Li: Conceptualization, Resources. Jiali Ren: Conceptualization, Resources. Jun Lu: Supervision, Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Feijun Luo: Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yingying Cheng, Email: 20211200592@csuft.edu.cn.
Jiayi Liu, Email: 20221100439@csuft.edu.cn.
Ling Li, Email: 20221100448@csuft.edu.cn.
Jiali Ren, Email: Jialiren_zhen@hotmail.com.
Jun Lu, Email: lujun925@csuft.edu.cn.
Feijun Luo, Email: T20121480@csuft.edu.cn.
Data availability
No data was used for the research described in the article.
References
- Ab Rashid S., Tong W.Y., Leong C.R., Abdul Ghazali N.M., Taher M.A., Ahmad N., Teo S.H. Anthocyanin microcapsule from Clitoria ternatea: Potential bio-preservative and blue colorant for baked food products. Arabian Journal for Science and Engineering. 2021;46(1):65–72. doi: 10.1007/s13369-020-04716-y. [DOI] [Google Scholar]
- Agulló V., Villaño D., García-Viguera C., Domínguez-Perles R. Anthocyanin metabolites in human urine after the intake of new functional beverages. Molecules. 2020;25(2):371. doi: 10.3390/molecules25020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahammed G.J., Yang Y. Anthocyanin-mediated arsenic tolerance in plants. Environmental Pollution. 2022;292 doi: 10.1016/j.envpol.2021.118475. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Ashraf B., Gani A., Gani A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. International Journal of Biological Macromolecules. 2018;109:435–442. doi: 10.1016/j.ijbiomac.2017.11.122. [DOI] [PubMed] [Google Scholar]
- Ali T., Kim M.J., Rehman S.U., Ahmad A., Kim M.O. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ 1–42 mouse model of Alzheimer’s disease. Molecular Neurobiology. 2017;54:6490–6506. doi: 10.1007/s12035-016-0136-4. [DOI] [PubMed] [Google Scholar]
- Alu'datt M.H., Alrosan M., Gammoh S., Tranchant C.C., Alhamad M.N., Rababah T.…Ghozlan K. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and health-promoting ingredients. Food Bioscience. 2022;50 doi: 10.1016/j.fbio.2022.101971. [DOI] [Google Scholar]
- Alvarez-Suarez J.M., Cuadrado C., Redondo I.B., Giampieri F., González-Paramás A.M., Santos-Buelga C. Novel approaches in anthocyanin research-Plant fortification and bioavailability issues. Trends in Food Science & Technology. 2021;117:92–105. doi: 10.1016/j.tifs.2021.01.049. [DOI] [Google Scholar]
- Baba Shekh A.O., Abdul Wahab R., Yahya N.A. Formulation of roselle extract water-in-oil nanoemulsion for controlled pulmonary delivery. Journal of Dispersion Science and Technology. 2022;1–12 doi: 10.1080/01932691.2022.2046044. [DOI] [Google Scholar]
- Barbosa M.P., Rigolon T.C.B., Borges L.L.R., Queiroz V.A.V., Stringheta P.C., de Barros F.A.R. Effect of light, food additives and heat on the stability of sorghum 3-deoxyanthocyanins in model beverages. International Journal of Food Science & Technology. 2021;56(9):4746–4755. doi: 10.1111/ijfs.15123. [DOI] [Google Scholar]
- Bonarska-Kujawa D., Kleszczyńska P. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cellular & Molecular Biology Letters. 2012;17(2):289–308. doi: 10.2478/s11658-012-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann M.D., Merzouki A., Lavertu M., Thibault M., Jean M., Darras V. Chitosans for delivery of nucleic acids. Advanced Drug Delivery Reviews. 2013;65(9):1234–1270. doi: 10.1016/j.addr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Li X., Chen J., Jiang X., Ma X., Sun J.…Pan Z. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130611. [DOI] [PubMed] [Google Scholar]
- Chai J., Jiang P., Wang P., Jiang Y., Li D., Bao W.…Norde W. The intelligent delivery systems for bioactive compounds in foods: Physicochemical and physiological conditions, absorption mechanisms, obstacles and responsive strategies. Trends in Food Science & Technology. 2018;78:144–154. doi: 10.1016/j.tifs.2018.06.003. [DOI] [Google Scholar]
- Chen C., Li Z., Wang C., Liu S., Wang Y., Zhang M.…Xia G. Stability and antioxidant activity of chitosan/β-Lactoglobulin on anthocyanins from Aronia melanocarpa. LWT. 2023;173 doi: 10.1016/j.lwt.2022.114335. [DOI] [Google Scholar]
- Chen F., Du X., Zu Y., Yang L. A new approach for preparation of essential oil, followed by chlorogenic acid and hyperoside with microwave-assisted simultaneous distillation and dual extraction (MSDDE) from Vaccinium uliginosum leaves. Industrial Crops and Products. 2015;77:809–826. doi: 10.1016/j.indcrop.2015.09.058. [DOI] [Google Scholar]
- Chen J., Ma X.H., Yao G.L., Zhang W.T., Zhao Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. International Journal of Food Properties. 2018;21(1):1152–1165. doi: 10.1080/10942912.2018.1485032. [DOI] [Google Scholar]
- Chen J., Tao X., Zhang M., Sun A., Zhao L. Properties and stability of blueberry anthocyanin-bovine serum albumin nanoparticles. Journal of Agricultural and Food Chemistry. 2015;94:1781–1786. doi: 10.1002/jsfa.7170. [DOI] [PubMed] [Google Scholar]
- Chi J., Ge J., Yue X., Liang J., Sun Y., Gao X., Yue P. Preparation of nanoliposomal carriers to improve the stability of anthocyanins. LWT. 2019;109:101–107. doi: 10.1016/j.lwt.2019.03.070. [DOI] [Google Scholar]
- Chung C., Rojanasasithara T., Mutilangi W., McClements D.J. Stability improvement of natural food colors: Impact of amino acid and peptide addition on anthocyanin stability in model beverages. Food Chemistry. 2017;218:277–284. doi: 10.1016/j.foodchem.2016.09.087. [DOI] [PubMed] [Google Scholar]
- Davies A., Mazza G. Copigmentation of simple and acylated anthocyanins with colorless phenolic compounds. Journal of Agricultural and Food Chemistry. 1993;41(5):716–720. doi: 10.1021/jf00029a007. [DOI] [Google Scholar]
- Delgado-Vargas F., Paredes-Lopez O. CRC Press; 2002. Natural colorants for food and nutraceutical uses. [Google Scholar]
- Devi L.M., Das A.B., Badwaik L.S. Effect of gelatin and acacia gum on anthocyanin coacervated microcapsules using double emulsion and its characterization. International Journal of Biological Macromolecules. 2023;235 doi: 10.1016/j.ijbiomac.2023.123896. [DOI] [PubMed] [Google Scholar]
- Eker M.E., Aaby K., Budic-Leto I., Rimac Brnčić S., El S.N., Karakaya S.…de Pascual-Teresa S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods. 2019;9(1):2. doi: 10.3390/foods9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar M.E., Hussein J., El-sayed S.M., Youssef A.M., El Bana M., Latif Y.A., Medhat D. Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. Journal of Materials Research and Technology. 2020;9(6):14500–14508. doi: 10.1016/j.jmrt.2020.10.047. [DOI] [Google Scholar]
- Egito E., Amaral-Machado L., Alencar E.N., Oliveira A.G. Microemulsion systems: From the design and architecture to the building of a new delivery system for multiple-route drug delivery. Drug Delivery and Translational Research. 2021;11(4):2108–2133. doi: 10.1007/s13346-020-00872-8. [DOI] [PubMed] [Google Scholar]
- Escalante-Aburto A., Mendoza-Córdova M.Y., Mahady G.B., Luna-Vital D.A., Gutierrez-Uribe J.A., Chuck-Hernandez C. Consumption of dietary anthocyanins and their association with a reduction in obesity biomarkers and the prevention of obesity. Trends in Food Science & Technology. 2023;140 doi: 10.1016/j.tifs.2023.104140. [DOI] [Google Scholar]
- Fang J. Bioavailability of anthocyanins. Drug Metabolism Reviews. 2014;46(4):508–520. doi: 10.3109/03602532.2014.978080. [DOI] [PubMed] [Google Scholar]
- Feitosa B.F., Decker B.L.A., de Brito E.S., Rodrigues S., Mariutti L.R.B. Microencapsulation of anthocyanins as natural dye extracted from fruits–A systematic review. Food Chemistry. 2023;424 doi: 10.1016/j.foodchem.2023.136361. [DOI] [PubMed] [Google Scholar]
- Felgines C., Texier O., Besson C., Fraisse D., Lamaison J.-L., Remesy C. Blackberry anthocyanins are slightly bioavailable in rats. The Journal of Nutrition. 2002;132(6):1249–1253. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- Fernandes, I., Marques, C., Évora, A., Faria, A., Calhau, C., Mateus, N., & de Freitas, V. (2019). Anthocyanins: Nutrition and health. In Bioactive Molecules in Food. Spinger Cham, 1097-1133.
- Gao, Q. C., Shu, T., Chang, Y. J., Chen, H., Cao, X. H., & Wang, S. H. (2019). The evaluation of anti-fatigue function of the solution of microencapsulation loading anthocyanidins of Lycium ruthenicum Murr by modified β-glucan from highland barley. Food Science and Technology, 44(1), 316-320. 10.13684/j.cnki.spkj.2019.01.054.
- Gao W., Bai X.P., Liu Y.W., Shi Z.Z., He K.M., Wang G.D., Sun G.Y. Optimization of Special Oil Nanoemulsion Prepared using Ultrasonic by Response Surface Methodology and Its Characteristics Analysis. Science and Technology of Food Industry. 2020;41(16):131–139. doi: 10.13386/j.issn1002-0306.2020.16.022. [DOI] [Google Scholar]
- Garavand F., Jalai-Jivan M., Assadpour E., Jafari S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chemistry. 2021;364 doi: 10.1016/j.foodchem.2021.130376. [DOI] [PubMed] [Google Scholar]
- Gharib A., Faezizadeh Z., Godarzee M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Medica. 2013;79(17):1599–1604. doi: 10.1055/s-0033-1350908. [DOI] [PubMed] [Google Scholar]
- Ghorbanzade T., Jafari S.M., Akhavan S., Hadavi R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry. 2017;216:146–152. doi: 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Sarkar T., Das A., Chakraborty R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112527. [DOI] [Google Scholar]
- Gibis M., Ruedt C., Weiss J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Research International. 2016;88:105–113. doi: 10.1016/j.foodres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Gorantla S., Wadhwa G., Jain S., Sankar S., Nuwal K., Mahmood A.…Singhvi G. Recent advances in nanocarriers for nutrient delivery. Drug Delivery and Translational Research. 2021;1–26 doi: 10.1007/s13346-021-01097-z. [DOI] [PubMed] [Google Scholar]
- Guo J., Giusti M.M., Kaletunç G. Encapsulation of purple corn and blueberry extracts in alginate-pectin hydrogel particles: Impact of processing and storage parameters on encapsulation efficiency. Food Research International. 2018;107:414–422. doi: 10.1016/j.foodres.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Hanafy N.A. Starch based hydrogel NPs loaded by anthocyanins might treat glycogen storage at cardiomyopathy in animal fibrotic model. International Journal of Biological Macromolecules. 2021;183:171–181. doi: 10.1016/j.ijbiomac.2021.04.131. [DOI] [PubMed] [Google Scholar]
- He B., Liang J., Wu L., Gao X. Research progress on stability and microencapsulation anthocyanins in blueberry. Chinese Agricultural Science Bulletin. 2015;31(05):127–131. [Google Scholar]
- He B., Ge J., Yue P., Yue X., Fu R., Liang J., Gao X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chemistry. 2017;221:1671–1677. doi: 10.1016/j.foodchem.2016.10.120. [DOI] [PubMed] [Google Scholar]
- He J., Wallace T.C., Keatley K.E., Failla M.L., Giusti M.M. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. Journal of Agricultural and Food Chemistry. 2009;57(8):3141–3148. doi: 10.1021/jf900567t. [DOI] [PubMed] [Google Scholar]
- Hoare T.R., Kohane D.S. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- Homayoonfal M., Mousavi S.M., Kiani H., Askari G., Desobry S., Arab-Tehrany E. Encapsulation of berberis vulgaris anthocyanins into nanoliposome composed of rapeseed lecithin: A comprehensive study on physicochemical characteristics and biocompatibility. Foods. 2021;10(3):492. doi: 10.3390/foods10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Wang Y., Zhang L., Xu M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125632. [DOI] [PubMed] [Google Scholar]
- Huang Y., Zhou W. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chemistry. 2019;278:357–363. doi: 10.1016/j.foodchem.2018.11.073. [DOI] [PubMed] [Google Scholar]
- Ivanov V., Usatenko A., Shcherbakov A. Antioxidant activity of nanocrystalline ceria to anthocyanins. Russian Journal of Inorganic Chemistry. 2009;54(10):1522–1527. doi: 10.1134/S0036023609100039. [DOI] [Google Scholar]
- Jafari S.M., Assadpoor E., He Y., Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Drying Technology. 2008;26(7):816–835. doi: 10.1080/07373930802135972. [DOI] [Google Scholar]
- Jezek M., Allan A.C., Jones J.J., Geilfus C.M. Why do plants blush when they are hungry? New Phytologist. 2023;239:494–505. doi: 10.1111/nph.18833. [DOI] [PubMed] [Google Scholar]
- Ju M., Zhu G., Huang G., Shen X., Zhang Y., Jiang L., Sui X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocolloids. 2020;99 doi: 10.1016/j.foodhyd.2019.105329. [DOI] [Google Scholar]
- Jung T., Kamm W., Breitenbach A., Kaiserling E., Xiao J., Kissel T. Biodegradable nanoparticles for oral delivery of peptides: Is there a role for polymers to affect mucosal uptake? European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):147–160. doi: 10.1016/S0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- Kalt W., Cassidy A., Howard L., Krikorian R., Stull A., Tremblay F., Zamora-Ros R. Recent research on the health benefits of blueberries and their anthocyanins. Advances in Nutrition. 2020;11:224–236. doi: 10.1093/advances/nmz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanha N., Surawang S., Pitchakarn P., Laokuldilok T. Microencapsulation of copigmented anthocyanins using double emulsion followed by complex coacervation: Preparation, characterization and stability. LWT. 2020;133 doi: 10.1016/j.lwt.2020.110154. [DOI] [Google Scholar]
- Kenari R.E., Razavi R. Encapsulation of bougainvillea (Bougainvillea spectabilis) flower extract in Urtica dioica L. seed gum: Characterization, antioxidant/antimicrobial properties, and in vitro digestion. Food Science & Nutrition. 2022;10(10):3436–3443. doi: 10.1002/fsn3.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research. 2017;61(1):1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Rehman S.U., Amin F.U., Kim M.O. Enhanced neuroprotection of anthocyanins-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomedicine: Nanotechnology, Biology and Medicine. 2017;13(8):2533–2544. doi: 10.1016/j.nano.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Kumar S., Dutta J., Dutta P.K., Koh J. A systematic study on chitosan-liposome based systems for biomedical applications. International Journal of Biological Macromolecules. 2020;160:470–481. doi: 10.1016/j.ijbiomac.2020.05.192. [DOI] [PubMed] [Google Scholar]
- Kuntz S., Asseburg H., Dold S., Römpp A., Fröhling B., Kunz C., Rudloff S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food & Function. 2015;6(4):1136–1149. doi: 10.1039/c4fo00755g. [DOI] [PubMed] [Google Scholar]
- Lan X., Liu Y., Wang L., Wang H., Hu Z., Dong H., Yu Z.W., Yuan Y.K. A review of curcumin in food preservation: Delivery system and photosensitization. Food Chemistry. 2023;424 doi: 10.1016/j.foodchem.2023.136464. [DOI] [PubMed] [Google Scholar]
- Li B., Zhao Y., Wang M., Guan W., Liu J., Zhao H., Brennan C.S. Microencapsulation of roselle anthocyanins with β-cyclodextrin and proteins enhances the thermal stability of anthocyanins. Journal of Food Processing and Preservation. 2022;46(5):16612. doi: 10.1111/jfpp.16612. [DOI] [Google Scholar]
- Li D., Wang P., Luo Y., Zhao M., Chen F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition. 2017;57(8):1729–1741. doi: 10.1080/10408398.2015.1030064. [DOI] [PubMed] [Google Scholar]
- Li J., Guo C., Cai S., Yi J., Zhou L. Fabrication of anthocyanin–rich W1/O/W2 emulsion gels based on pectin–GDL complexes: 3D printing performance. Food Research International. 2023;168 doi: 10.1016/j.foodres.2023.112782. [DOI] [PubMed] [Google Scholar]
- Li W., Peng C., Li Z.J., Wu W. Chemopreventive and therapeutic properties of anthocyanins in breast cancer: A comprehensive review. Nutrition Research. 2022;107:48–64. doi: 10.1016/j.nutres.2022.08.005. [DOI] [PubMed] [Google Scholar]
- Li Y., Meng X., Zhou Y. Effects of metal ions and food additives on the stability of blueberry anthocyanins. Food Science. 2009;30(9):80–84. doi: 10.7506/spkx1002-6630-200909019. [DOI] [Google Scholar]
- Li Y., Yao L., Zhang L., Zhang Y., Zheng T., Liu L., Zhang L. Enhanced physicochemical stabilities of cyanidin-3-O-glucoside via combination with silk fibroin peptide. Food Chemistry. 2021;355 doi: 10.1016/j.foodchem.2021.129479. [DOI] [PubMed] [Google Scholar]
- Liang T., Guan R., Quan Z., Tao Q., Liu Z., Hu Q. Cyanidin-3-o-glucoside liposome: Preparation via a green method and antioxidant activity in GES-1 cells. Food Research International. 2019;125 doi: 10.1016/j.foodres.2019.108648. [DOI] [PubMed] [Google Scholar]
- Liang T., Guan R., Wang Z., Shen H., Xia Q., Liu M. Comparison of anticancer activity and antioxidant activity between cyanidin-3-O-glucoside liposomes and cyanidin-3-O-glucoside in Caco-2 cells in vitro. RSC Advances. 2017;7(59):37359–37368. doi: 10.1039/C7RA06387C. [DOI] [Google Scholar]
- Liang T., Zhang Z., Jing P. Black rice anthocyanins embedded in self-assembled chitosan/chondroitin sulfate nanoparticles enhance apoptosis in HCT-116 cells. Food Chemistry. 2019;301 doi: 10.1016/j.foodchem.2019.125280. [DOI] [PubMed] [Google Scholar]
- Lila M.A., Burton-Freeman B., Grace M., Kalt W. Unraveling anthocyanin bioavailability for human health. Annual Review of Food Science and Technology. 2016;7:375–393. doi: 10.1146/annurev-food-041715-033346. [DOI] [PubMed] [Google Scholar]
- Lin X., Li S., Yin J., Chang F., Wang C., He X.…Zhang B. Anthocyanin-loaded double Pickering emulsion stabilized by octenylsuccinate quinoa starch: Preparation, stability and in vitro gastrointestinal digestion. International Journal of Biological Macromolecules. 2020;152:1233–1241. doi: 10.1016/j.ijbiomac.2019.10.220. [DOI] [PubMed] [Google Scholar]
- Liović N., Bošković P., Drvenica I., Jambrak A.R., Dropulić A.M., Krešić C., Nedović V., Bugarski B., Zorić Z., Pedisić S., Bilušić T. Phenolic extracts from Vaccinium corymbosum L. loaded in microemulsions and liposomes as enhancers of olive oil oxidative stability. Polish Journal of Food & Nutrition Sciences. 2019;69(1):23–33. doi: 10.31883/pjfns-2019-0003. [DOI] [Google Scholar]
- Liu J.G., Si X., Tian J.L., Xue B., Zhang Y., Cui H.J., Xie X., Li B. Advances in Nutritional Absorption and Stabilization of Anthocyanins. China Fruit & Vegetable. 2020;40(5):7–13. doi: 10.19590/j.cnki.1008-1038.2020.05.002. [DOI] [Google Scholar]
- Liu W.L., Chen H.X., He L.Q. Research Progress on Encapsulation and Controlled Release Technologies for Flavour and Fragrance. Flavour Fragrance Cosmetics. 2019;4:82–88. [Google Scholar]
- Liu L., Zhang D., Song X., Guo M., Wang Z., Geng F.…Nie S. Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion. Food Hydrocolloids. 2022;127 doi: 10.1016/j.foodhyd.2022.107487. [DOI] [Google Scholar]
- Liu Y.H., Cao X.H., Liu Y. Study on microencapsulation of anthocyanins. Food Science and Technology. 2004;11:18–20. doi: 10.3969/j.issn.1005-9989.2004.11.006. [DOI] [Google Scholar]
- Liu Z., Jiao Y., Wang Y., Zhou C., Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lu X., Huang Q., Xiao J., Wang Y. Milled miscellaneous black rice particles stabilized Pickering emulsions with enhanced antioxidation activity. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132639. [DOI] [PubMed] [Google Scholar]
- Ma X.H. Hainan University; Haikou: 2017. The preparation of anthocyanins micro emulsion and its antioxidant activity in vitro. [Google Scholar]
- Ma Y., Feng Y., Zeng W., Luo H. Anthocyanin encapsulated by ferulic acid-grafted-maltodextrin (FA-g-MD) microcapsules potentially improved its free radical scavenging capabilities against H2O2-induced oxidative stress. Molecules. 2019;24(8):1596. doi: 10.3390/molecules24081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Li S., Ji T., Wu W., Sameen D.E., Ahmed S.…Liu Y. Development and optimization of dynamic gelatin/chitosan nanoparticles incorporated with blueberry anthocyanins for milk freshness monitoring. Carbohydrate Polymers. 2020;247 doi: 10.1016/j.carbpol.2020.116738. [DOI] [PubMed] [Google Scholar]
- Mansour M., Salah M., Xu X. Effect of microencapsulation using soy protein isolate and gum arabic as wall material on red raspberry anthocyanin stability, characterization, and simulated gastrointestinal conditions. Ultrasonics Sonochemistry. 2020;63 doi: 10.1016/j.ultsonch.2019.104927. [DOI] [PubMed] [Google Scholar]
- Matos M., Timgren A., Sjöö M., Dejmek P., Rayner M. Preparation and encapsulation properties of double Pickering emulsions stabilized by quinoa starch granules. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2013;423:147–153. doi: 10.1016/j.colsurfa.2013.01.060. [DOI] [Google Scholar]
- McGhie T.K., Walton M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Molecular Nutrition & Food Research. 2007;51(6):702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- McNamara K., Tofail S.A. Nanoparticles in biomedical applications. Advances in Physics: X. 2017;2(1):54–88. doi: 10.1080/23746149.2016.1254570. [DOI] [Google Scholar]
- Mendonça C.R.B., Borges C.D., Kringel A.L., da Silveira R.P., da Silva F.A., Schulz G.A.S. Application of microemulsions as coating in fresh cut strawberries. Journal of Food Science and Technology. 2020;57(7):2764–2770. doi: 10.1007/s13197-020-04515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming L., Zhipeng Y., Fei Y., Feng R., Jian W., Baoguo J.…Peixun Z. Microfluidic-based screening of resveratrol and drug-loading PLA/Gelatine nano-scaffold for the repair of cartilage defect. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46:336–346. doi: 10.1080/21691401.2017.1423498. [DOI] [PubMed] [Google Scholar]
- Mushtaq A., Wani S.M., Malik A.R., Gull A., Ramniwas S., Nayik G.A., Ercisli S., Marc R.A., Ullah R., Bari A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth M.S., Shreelakshmi S., Rao P.J., Shetty N.P. Micro and nanoemulsions of Carissa spinarum fruit polyphenols, enhances anthocyanin stability and anti-quorum sensing activity: Comparison of degradation kinetics. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129876. [DOI] [PubMed] [Google Scholar]
- Neuenfeldt N.H., de Moraes D.P., de Deus C., Barcia M.T., de Menezes C.R. Blueberry Phenolic Composition and Improved Stability by Microencapsulation. Food and Bioprocess Technology. 2022;1–18 doi: 10.1007/s11947-021-02749-1. [DOI] [Google Scholar]
- Oehlke K., Adamiuk M., Behsnilian D., Mayer-Miebach E., Walz E., Greiner R. Potential bioavailability enhancement of bioactive compounds using food-grade engineered nanomaterials: A review of the existing evidence. Food & Function. 2014;5(7):1341–1359. doi: 10.1039/c3fo60067j. [DOI] [PubMed] [Google Scholar]
- Oliveira H., Correia P., Pereira A.R., Araújo P., Mateus N., de Freitas V.…Fernandes I. Exploring the applications of the photoprotective properties of anthocyanins in biological systems. International Journal of Molecular Sciences. 2020;21(20):7464. doi: 10.3390/ijms21207464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza P.I., Cremonini E., Fraga C.G. Anthocyanin actions at the gastrointestinal tract: Relevance to their health benefits. Molecular Aspects of Medicine. 2022;89 doi: 10.1016/j.mam.2022.101156. [DOI] [PubMed] [Google Scholar]
- Pan L.H., Chen L.P., Wu C.L., Wang J.F., Luo S.Z., Luo J.P., Zheng Z. Microencapsulation of blueberry anthocyanins by spray drying with soy protein isolates/high methyl pectin combination: Physicochemical properties, release behavior in vitro and storage stability. Food Chemistry. 2022;395 doi: 10.1016/j.foodchem.2022.133626. [DOI] [PubMed] [Google Scholar]
- Park S.A., Lee S.H., Kim W. Fabrication of hydrogel scaffolds using rapid prototyping for soft tissue engineering. Macromolecular Research. 2011;19(7):694–698. doi: 10.1007/s13233-011-0708-0. [DOI] [Google Scholar]
- Peng H., Li W., Ning F., Yao L., Luo M., Zhu X.…Xiong H. Amphiphilic chitosan derivatives-based liposomes: Synthesis, development, and properties as a carrier for sustained release of salidroside. Journal of Agricultural and Food Chemistry. 2014;62(3):626–633. doi: 10.1021/jf4039925. [DOI] [PubMed] [Google Scholar]
- Perumcherry Raman S., Dara P.K., Vijayan D.K., Chatterjee N.S., Raghavankutty M., Mathew S.…Anandan R. Anti-ulcerogenic potential of anthocyanin-loaded chitosan nanoparticles against alcohol-HCl induced gastric ulcer in rats. Natural Product Research. 2022;36(5):1306–1310. doi: 10.1080/14786419.2020.1860041. [DOI] [PubMed] [Google Scholar]
- Pratiwi L., Fudholi A., Martien R., Pramono S. Self-nanoemulsifying Drug Delivery System (Snedds) for topical delivery of mangosteen peels (Garcinia mangostana L.,): Formulation design and in vitro studies. Journal of Young Pharmacists. 2017;9(3):341. doi: 10.5530/jyp.2017.9.68. [DOI] [Google Scholar]
- Qiao L., Yang H., Gao S., Li L., Fu X., Wei Q. Research progress on self-assembled nanodrug delivery systems. Journal of Materials Chemistry B. 2022 doi: 10.1039/D1TB02470A. [DOI] [PubMed] [Google Scholar]
- Qiu F., Chen Y., Tang C., Zhao X. Amphiphilic peptides as novel nanomaterials: Design, self-assembly and application. International Journal of Nanomedicine. 2018;5003–5022 doi: 10.2147/IJN.S166403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raak N., Rohm H., Jaros D. Cross-linking with microbial transglutaminase: Isopeptide bonds and polymer size as drivers for acid casein gel stiffness. International Dairy Journal. 2017;66:49–55. doi: 10.1016/j.idairyj.2016.10.015. [DOI] [Google Scholar]
- Rabelo C.A., Taarji N., Khalid N., Kobayashi I., Nakajima M., Neves M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with açaí berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Research International. 2018;106:542–548. doi: 10.1016/j.foodres.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Rosales T.K.O., Fabi J.P. Nanoencapsulated anthocyanin as a functional ingredient: Technological application and future perspectives. Colloids and Surfaces B: Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112707. [DOI] [PubMed] [Google Scholar]
- Saha S., Singh J., Paul A., Sarkar R., Khan Z., Banerjee K. Anthocyanin profiling using UV-vis spectroscopy and Liquid Chromatography Mass Spectrometry. Journal AOAC International. 2020;103:23–39. doi: 10.5740/jaoacint.19-0201. [DOI] [PubMed] [Google Scholar]
- Sahin O.I., Dundar A.N., Uzuner K., Parlak M.E., Dagdelen A.F., Saricaoglu F.T. Lyophilized nano-liposomal system for red onion (Allium cepa L.) peel anthocyanin: Characterization, bioaccessibility and release kinetics. Food. Bioscience. 2023;102702 doi: 10.1016/j.fbio.2023.102702. [DOI] [Google Scholar]
- Salah M., Mansour M., Zogona D., Xu X. Nanoencapsulation of anthocyanins-loaded β-lactoglobulin nanoparticles: Characterization, stability, and bioavailability in vitro. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109635. [DOI] [PubMed] [Google Scholar]
- Seibert J.B., Bautista-Silva J.P., Amparo T.R., Petit A., Pervier P., dos Santos Almeida J.C.…de Souza G.H.B. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chemistry. 2019;287:61–67. doi: 10.1016/j.foodchem.2019.02.078. [DOI] [PubMed] [Google Scholar]
- Shao P., Zhang H., Niu B., Jin W. Physical stabilities of taro starch nanoparticles stabilized Pickering emulsions and the potential application of encapsulated tea polyphenols. International Journal of Biological Macromolecules. 2018;118:2032–2039. doi: 10.1016/j.ijbiomac.2018.07.076. [DOI] [PubMed] [Google Scholar]
- Shen R., Lin D., Liu Z., Zhai H., Yang X. Fabrication of Bacterial Cellulose Nanofibers/Soy Protein Isolate Colloidal Particles for the Stabilization of High Internal Phase Pickering Emulsions by Anti-solvent Precipitation and Their Application in the Delivery of Curcumin. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.734620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhang N., Tian J., Xin G., Liu L., Sun X., Li B. Advanced approaches for improving bioavailability and controlled release of anthocyanins. Journal of Controlled Release. 2022;341:285–299. doi: 10.1016/j.jconrel.2021.11.031. [DOI] [PubMed] [Google Scholar]
- Shineh G., Kordestani S.S., Tahriri M., Tayebi L. Evaluation of L929 cell morphology on anthocyanin-containing gelatin-based hydrogel for early detection of infection. Bio-Design and Manufacturing. 2019;2(3):181–186. doi: 10.1007/s42242-019-00047-6. [DOI] [Google Scholar]
- Shishir M.R.I., Karim N., Xie J., Rashwan A.K., Chen W. Colonic delivery of pelargonidin-3-O-glucoside using pectin-chitosan-nanoliposome: Transport mechanism and bioactivity retention. International Journal of Biological Macromolecules. 2020;159:341–355. doi: 10.1016/j.ijbiomac.2020.05.076. [DOI] [PubMed] [Google Scholar]
- Sigurdson G.T., Tang P., Giusti M.M. Natural colorants: Food colorants from natural sources. Annual Review of Food Science and Technology. 2017;8:261–280. doi: 10.1146/annurev-food-030216-025923. [DOI] [PubMed] [Google Scholar]
- Sreerekha P., Dara P.K., Vijayan D.K., Chatterjee N.S., Raghavankutty M., Mathew S.…Anandan R. Dietary supplementation of encapsulated anthocyanin loaded-chitosan nanoparticles attenuates hyperlipidemic aberrations in male Wistar rats. Carbohydrate Polymer Technologies and Applications. 2021;2 doi: 10.1016/j.carpta.2021.100051. [DOI] [Google Scholar]
- Steinert R.E., Ditscheid B., Netzel M., Jahreis G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in ussing type chambers. Journal of Agricultural and Food Chemistry. 2008;56(13):4995–5001. doi: 10.1021/jf703670h. [DOI] [PubMed] [Google Scholar]
- Sun J., Cao X., Bai W., Liao X., Hu X. Comparative analyses of copigmentation of cyanidin 3-glucoside and cyanidin 3-sophoroside from red raspberry fruits. Food Chemistry. 2010;120(4):1131–1137. doi: 10.1016/j.foodchem.2009.11.031. [DOI] [Google Scholar]
- Sun Z., Yan X., Xiao Y., Hu L., Eggersdorfer M., Chen D.…Weitz D.A. Pickering emulsions stabilized by colloidal surfactants: Role of solid particles. Particuology. 2021;64:153–163. doi: 10.1016/j.partic.2021.06.004. [DOI] [Google Scholar]
- Syed Azhar S.N.A., Ashari S.E., Zainuddin N., Hassan M. Nanostructured lipid carriers-hydrogels system for drug delivery: Nanohybrid technology perspective. Molecules. 2022;27(1):289. doi: 10.3390/molecules27010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera S., Felgines C., Texier O., Besson C., Lamaison J.-L., Rémésy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. The Journal of Nutrition. 2003;133(12):4178–4182. doi: 10.1093/jn/133.12.4178. [DOI] [PubMed] [Google Scholar]
- Tan C., Dadmohammadi Y., Lee M.C., Abbaspourrad A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Comprehensive Reviews in Food Science and Food Safety. 2021;20(4):3164–3191. doi: 10.1111/1541-4337.12772. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sasaki N., Ohmiya A. BioNanostructured lipid carriers-hydrogels system for drug delivery: Nanohybrid technology perspective.synthesis of plant pigments: Anthocyanins, betalains and carotenoids. The Plant Journal. 2008;54(4):733–749. doi: 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- Tarone A.G., Cazarin C.B.B., Junior M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Research International. 2020;133 doi: 10.1016/j.foodres.2020.109092. [DOI] [PubMed] [Google Scholar]
- Tena N., Martín J., Asuero A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants. 2020;9(5):451. doi: 10.3390/antiox9050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T. Anthocyanins as Functional Food Factors—Chemistry, Nutrition and Health Promotion—. Food Science and Technology Research. 2012;18(3):315–324. doi: 10.3136/fstr.18.315. [DOI] [Google Scholar]
- Vidana Gamage G.C., Lim Y.Y., Choo W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. Journal of Food Science and Technology. 2021;1–15 doi: 10.1007/s13197-021-05054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.J., Su S., Wu J., Du H., Li S.S., Huo J.W.…Wang L.-S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chemistry. 2014;160:357–364. doi: 10.1016/j.foodchem.2014.03.081. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang L., Wang X., Lu B., Zhang J. Preparation of blueberry anthocyanin liposomes and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification. Food Science & Nutrition. 2022;10(1):75–87. doi: 10.1002/fsn3.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. S., Lv, X. T., Zhao, H. J., & Li, J. (2017). Preparation and bioavailability of black rice anthocyanin phospholipid complex. Food Science and Technology, 5, 242-245. 10.13684/j.cnki.spkj.2017.05.047.
- Wang S., Wang X., Liu M., Zhang L., Ge Z., Zhao G., Zong W. Preparation and characterization of Eucommia ulmoides seed oil O/W nanoemulsion by dynamic high-pressure microfluidization. LWT. 2020;121 doi: 10.1016/j.lwt.2019.108960. [DOI] [Google Scholar]
- Weng Y., Ranaweera S., Zou D., Cameron A.P., Chen X., Song H., Zhao C.-X. Improved enzyme thermal stability, loading and bioavailability using alginate encapsulation. Food Hydrocolloids. 2023;137 doi: 10.1016/j.foodhyd.2022.108385. [DOI] [Google Scholar]
- Wu Y., Han T., Lyu L., Li W.L., Wu W.L. Research progress in understanding the biosynthesis and regulation of plant anthocyanins. Scientia Horticulturae. 2023;321 doi: 10.1016/j.scienta.2023.112374. [DOI] [Google Scholar]
- Wu Y., Han Y., Tao Y., Li D., Xie G., Show P.L., Lee S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109098. [DOI] [PubMed] [Google Scholar]
- Wulansari A., Jufri M., Budianti A. Studies on the formulation, physical stability, and in vitro antibacterial activity of tea tree oil (Melaleuca alternifolia) nanoemulsion gel. International Journal of Applied Pharmaceutics. 2017;9(1):135–139. doi: 10.22159/ijap.2017.v9s1.73_80. [DOI] [Google Scholar]
- Xiao J., Lu X., Huang Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocolloids. 2017;62:230–238. doi: 10.1016/j.foodhyd.2016.08.014. [DOI] [Google Scholar]
- Xie C., Wang Q., Ying R., Wang Y., Wang Z., Huang M. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocolloids. 2020;100 doi: 10.1016/j.foodhyd.2019.105448. [DOI] [Google Scholar]
- Xu W., Yang Y., Xue S.J., Shi J., Lim L.-T., Forney C.…Bamba B.S.B. Effect of in vitro digestion on water-in-oil-in-water emulsions containing anthocyanins from grape skin powder. Molecules. 2018;23(11):2808. doi: 10.3390/molecules23112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhao W., Ye Y., Cui W., Dong L., Yao Y.…Liu W. Novel nanoliposome codelivered DHA and anthocyanidin: Characterization, in vitro infant digestibility, and improved cell uptake. Journal of Agricultural and Food Chemistry. 2021;69(32):9395–9406. doi: 10.1021/acs.jafc.1c02817. [DOI] [PubMed] [Google Scholar]
- Yang D., Zhang W.B. Study on the Preparation and Stability of O/W Microemulsion. China Cleaning Industry. 2018;02:47–52. [Google Scholar]
- Yao L., Xu J., Zhang L., Zheng T., Liu L., Zhang L. Physicochemical stability-increasing effects of anthocyanin via a co-assembly approach with an amphiphilic peptide. Food Chemistry. 2021;362 doi: 10.1016/j.foodchem.2021.130101. [DOI] [PubMed] [Google Scholar]
- Yin Z., Zheng T., Ho C., Huang Q., Wu Q., Zhang M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Science and Human Wellness. 2022;11(3):537–556. doi: 10.1016/j.fshw.2021.12.011. [DOI] [Google Scholar]
- Yuan Y., Tian Y., Gao S., Zhang X.M., Gao X.F., He J.P. Effects of environmental factors and fermentation on red raspberry anthocyanins stability. LWT. 2023;173 doi: 10.1016/j.lwt.2022.114252. [DOI] [Google Scholar]
- Zhang J., Liang X., Li X., Guan Z., Liao Z., Luo Y., Luo Y. Ocular delivery of cyanidin-3-glycoside in liposomes and its prevention of selenite-induced oxidative stress. Drug Development and Industrial Pharmacy. 2016;42(4):546–553. doi: 10.3109/03639045.2015.1088867. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhang Q., Oliveira H., Mateus N., Ye S., Jiang S.…Wu M. Preparation of nanoliposomes loaded with anthocyanins from grape skin extracts: Stability, gastric absorption and antiproliferative properties. Food & Function. 2022;13(21):10912–10922. doi: 10.1039/D2FO02008D. [DOI] [PubMed] [Google Scholar]
- Zhang S., Jiang Z., Wang X., Yang C., Shi J. Facile method to prepare microcapsules inspired by polyphenol chemistry for efficient enzyme immobilization. ACS Applied Materials & Interfaces. 2015;7(35):19570–19578. doi: 10.1021/acsami.5b03823. [DOI] [PubMed] [Google Scholar]
- Zhang W., Qi X., Zhao Y., Liu Y., Xu L., Song X.…Hou M. Study of injectable Blueberry anthocyanins-loaded hydrogel for promoting full-thickness wound healing. International Journal of Pharmaceutics. 2020;586 doi: 10.1016/j.ijpharm.2020.119543. [DOI] [PubMed] [Google Scholar]
- Zhao C., Chen H., Wang F., Zhang X. Amphiphilic self-assembly peptides: Rational strategies to design and delivery for drugs in biomedical applications. Colloids and Surfaces B: Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112040. [DOI] [PubMed] [Google Scholar]
- Zhao R., Chen J., Yu S., Niu R., Yang Z., Wang H.…Wang W. Active chitosan/gum Arabic-based emulsion films reinforced with thyme oil encapsulating blood orange anthocyanins: Improving multi-functionality. Food Hydrocolloids. 2023;134 doi: 10.1016/j.foodhyd.2022.108094. [DOI] [Google Scholar]
- Zhao X., Zhang X., Tie S., Hou S., Wang H., Song Y.…Tan M. Facile synthesis of nano-nanocarriers from chitosan and pectin with improved stability and biocompatibility for anthocyanins delivery: An in vitro and in vivo study. Food Hydrocolloids. 2020;109 doi: 10.1016/j.foodhyd.2020.106114. [DOI] [Google Scholar]
- Zhou F.Z., Yan L., Yin S.W., Tang C.H., Yang X.Q. Development of Pickering emulsions stabilized by gliadin/proanthocyanidins hybrid particles (GPHPs) and the fate of lipid oxidation and digestion. Journal of Agricultural and Food Chemistry. 2018;66(6):1461–1471. doi: 10.1021/acs.jafc.7b05261. [DOI] [PubMed] [Google Scholar]
- Zou C., Huang L., Li D., Ma Y., Liu Y., Wang Y., Sun L. Assembling cyanidin-3-O-glucoside by using low-viscosity alginate to improve its in vitro bioaccessibility and in vivo bioavailability. Food Chemistry. 2021;355 doi: 10.1016/j.foodchem.2021.129681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.