Highlights
-
•
PPDF significantly reduced weight gain and visceral fat accumulation of obese mice.
-
•
PPDF significantly alleviated HFD-induced dyslipidemia.
-
•
PPDF ameliorated HFD-induced gut microbiota dysbiosis.
-
•
PPDF prevented mice obesity via gut microbiota regulation.
Keywords: Pomelo peel, Dietary fiber, Antiobesity, Hyperlipidemia, Gut microbiota
Abstract
Pomelo peel has abundance of dietary fiber and various biological activities but is often discarded as waste. This study evaluated the biological activities of pomelo peel dietary fiber (PPDF) in preventing obesity and regulating intestinal microbiota in obese mouse model induced using a high-fat diet (HFD). As for the composition, the prepared PPDF contained 89.64% of total dietary fiber, 53.27% of insoluble dietary fiber, and 36.37% of soluble dietary fiber. PPDF treatment significantly reduced weight gain and fat accumulation in the liver and epididymal tissues of obese mice; significantly alleviated HFD-induced dyslipidemia; and restored the levels of triglycerides, low-density lipoprotein–cholesterol, and high-density lipoprotein–-cholesterol to control levels, and the PPDF 5% dose restored total cholesterol to the control level. Furthermore, PPDF ameliorated HFD-induced gut microbiota dysbiosis by increasing intestinal microbial diversity, decreasing the Firmicutes/Bacteroidetes ratio, increasing beneficial bacteria (Bifidobacterium, Alloprevotella, and Lactobacillus), and decreasing harmful bacteria (Staphylococcus and Corynebacterium_1). This study provided a new idea to use PPDF as functional food to prevent obesity, alleviate dyslipidemia, or a potential probiotic to ameliorate gut microbiota dysbiosis.
1. Introduction
Obesity prevalence has increased dramatically owing to unhealthy eating habits and lifestyle modification. Currently, one-third of the global population is either overweight or obese, which has made obesity a public health concern worldwide (Popkin, Adair, & Ng, 2012). Obesity-related metabolic diseases such as hypertension, diabetes, and hyperlipidemia have become common, thereby imposing a notable medical and social burden (Navar-Boggan et al., 2015). Orlistat, a lipase inhibitor that reduces fat absorption, and sibutramine, a drug that suppresses hunger. Both of them are used to treat obesity but cause adverse effects on the stomach and liver (Balaji et al., 2016, Yun, 2010). Therefore, a greater emphasis is placed on obesity prevention and intervention through alternative dietary solutions (Liu, He, Ma, & Chen, 2019).
Dietary fiber (DF) is a macromolecule that cannot be digested by intestinal digestive enzymes in humans. DF can reduce fat absorption from the diet, body fat percentage, and levels of blood lipid profile and can keep the balance and stability of gut microbiota. It also plays a remarkable role in weight control and is regarded as an important dietary supplement for obesity prevention (Makki et al., 2018, Mateos-Aparicio et al., 2020). DF can be divided into soluble DF (SDF) and insoluble DF (IDF) based on water solubility. SDF includes pectin and hemicellulose, which are beneficial for gut microbiota (Huang, Ye, Chen, & Xu, 2013). Examples of IDF are cellulose, hemicellulose, and lignin, all of which can stimulate intestinal peristalsis and promote defecation (Yang et al., 2020). Although SDF and IDF have different structural characteristics, both are crucial for lowering obesity risk (Papathanasopoulos & Camilleri, 2010).
Honey pomelo (Citrus grandis (L.) Osbeck), which belongs to the family Rutaceae, is one of the world’s most consumed but under-exploited fruits (FAO, 2008). Consumption of a large amount of pomelo leads to the accumulation of perishable pomelo peel, and causes environmental pollution. Pomelo peel, which accounts for approximately 30 %-50 % (w/w) of the total weight of the pomelo fruit, has abundance of essential oil, pectin, DF, polyphenols, and many other functional components (Liew et al., 2018, Yang et al., 2019). These components have shown various health-promoting effects such as anti-inflammatory, antitumor, anticoagulant, antibacterial, and antioxidant activities (Lan-Phi and Vy, 2015, Wang et al., 2017). Pomelo peels are directly used to prepare sugar-pickled pomelo peels, tea, jams, etc. The DF content in pomelo peels accounts for up to 85 % (Yan, Liao, Mao, & He, 2017), and the white capsule present in the inner layer of the pomelo peel is considered a main source of DF. Currently, the research on pomelo peel dietary fiber (PPDF) is limited on the evaluation of in vitro antioxidant activity (Czech, Malik, Sosnowska, & Domaradzki, 2021), and the effect of dietary supplementation of PPDF in preventing obesity, and the effect of PPDF on gut microbiota have not been reported before. This study investigated the effects of PPDF supplementation on changes in body weight and blood lipid profile in a high-fat diet (HFD)–induced obese mouse model and analyzed the biological activities of PPDF in preventing obesity and regulation intestinal microbiota.
2. Materials and methods
2.1. Extraction and determination of pomelo peel dietary fiber
Fresh Guanximiyu (Citrus grandis) was collected from Pinghe country of Fujian, China, in October 2022. After removing the yellow skin, the white capsule was obtained, which was dried at 60℃ for 12 h and then crushed. The dry powder of the pomelo peel was mixed with 85 % ethanol at a ratio of 1:20 (power: ethanol), and the mixture was extracted in a water bath at 25℃ for 4 h. The extract was then filtered to obtain the residue. The residue was subjected to extraction once again, after which the residue was dried, crushed, and passed through an 80-mesh (178 μm) sieve to obtain PPDF powder.
The components present in PPDF were determined according to the Chinese national standards testing, including water content (GB 5009.3–2016), ash content (GB 5009.4–2016), protein content (GB 5009.5–2016), and DF content (GB 5009.88–2014), in food. All assays were repeated three times. Particle size of the PPDF powder (0.1 g) was measured using a laser diffraction particle size analyzer (MS-2000, Malvern, United Kingdom), and the surface structure of the particles was observed under a scanning electron microscope (SEM; S-4800; Hitachi, Tokyo, Japan).
2.2. Animal experiment
Fifty male Kunming mice (4-week-old, specific pathogen-free) were purchased from Beijing Huafukang Company (Certificate Number: SCXK 2019–0008) and reared in a specific pathogen-free (SPF) laboratory. The experimental conditions included a temperature of 22-26℃, relative humidity of 50 %-60 %, and interrupted day and night for 12 h:12 h. After 1 week of adaptation, all mice with a similar average body weight (26–28 g) were divided into five groups of 10 mice. Each group was housed in two cages of 5 mice. Mice in the normal diet (ND) group were allowed to feed on a basic diet (energy 3.42 kcal/g), whereas mice in the HFD group were fed on a high energy diet (energy 5.24 kcal/g). According to the standards of the European Food Science Commission and the Chinese Nutrition Society (European Commission, 2012, Chinese Nutrition Society, 2016), three different dosages of PPDF supplementation were designed: low-dose PPDF (LPPDF + HFD group): 2.5 % PPDF + 97.5 % HFD; medium-dose PPDF (MPPDF + HFD group): 5 %+95 % HFD; and high-dose PPDF (HPPDF + HFD group): 10 %+90 % HFD. The basic diet and the feed ingredients were purchased from Beijing Huafukang Company (Beijing, China, Certificate Number: 2019–06076). The composition of all the experimental diets are shown in Table S2 and S3. Each group of mice is given the same amount of food every day and the daily food intake is recorded. During the 6-week experiment, all mice had free access to water. The body weight of mice was recorded once a week, and the average daily food intake per mouse was calculated. All animal protocols in this study were approved by the Institutional Animal Care and Use Committee of Jimei University (Xiamen, China; No. SCXK 2016–0006), and were performed in accordance with the institutional ethical guidelines for experimental animals.
2.3. Sample collection
After 6 weeks of the experiment, 4 mice were sampled from each group for gut microbiota analysis. Fresh fecal pellets of each mouse were collected in sterile Eppendorf (EP) tubes and stored at − 80℃ for subsequent DNA extraction. The other 6 mice in each group were used for biochemical and histological analysis. Mice were fasted overnight (12 h), and blood samples were collected from the eyeball, using disposable vacuum sodium heparin collection tubes. Serum was separated from blood samples by centrifuge at 4℃, 606 × g for 10 min, and stored at − 80℃ for subsequent biochemical analysis. Next, the mice were sacrificed by cervical dislocation. Epididymal adipose tissue and liver were dissected, weighed, and then fixed with 4 % neutral paraformaldehyde for subsequent histological analysis.
2.4. Detection of serum biochemical indexes
Serum biochemical indicators, including total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C), were determined using an Automatic Biochemical Analyzer (BS-240VET; Mindray, China), according to the manufacturer’s instructions of the assay kits (Nanjing Jiancheng Bioengineering Institute).
2.5. Histological analysis
The fixed liver and epididymal adipose tissues were subjected to paraffin-embedding, sectioning, and dehydration using gradient alcohol. The liver sections were stained with hematoxylin and eosin (H&E), and the epididymal adipose tissue section was stained with Oil Red O. Histological observation was performed using an inverted microscope (Nikon, Japan).
2.6. Gut microbiota analysis
The FastDNA™ Fecal Genomic DNA Extraction Kit (MP Biomedicals, CA, USA) was used to extract total DNA from mouse fecal samples (n = 4 mice per group). The quality and concentration of the extracted DNA were assessed using NanoDrop. The V3-V4 hypervariable regions of the 16S rRNA gene were amplified using a polymerase chain reaction system. Subsequently, sequencing and bioinformatics analyses were performed by Beijing Ovison Gene Technology Co., Ltd. (Beijing, China), using the Illumina MiSeq platform. Paired-end reads were double-ended spliced using FLASH software (version 1.2.7), and quality control and sequence filtering were performed using USEARCH software (version 7.0). Optimized sequences with a 97 % sequence similarity were clustered into operational taxonomic units (OTUs) using the Ribosomal Database Project (RDP) classifier Bayesian algorithm in UPARSE software (version 7.0), and the representative sequences of each OTU were annotated according to the SILVA database (Release 132; https://www.arb-silva.de) to count the species composition of each sample. Mothur software (version 1.30) was used to determine alpha diversity, and R software was used to perform principal coordinate analysis (PCoA) based on Bray-Curtis distances. Sobs box plots were constructed to compare the bacterial communities between samples and groups.
2.7. Statistical analysis
All data were expressed as mean ± standard deviation, and statistical analysis was conducted using one-way analysis of variance and, subsequently, using Duncan test. Statistical analysis was performed using SPSS 18.0 software (IBM Corporation, NY, USA). The difference was considered statistically significant at P < 0.05.
3. Results
3.1. Conventional composition and structure analysis of PPDF
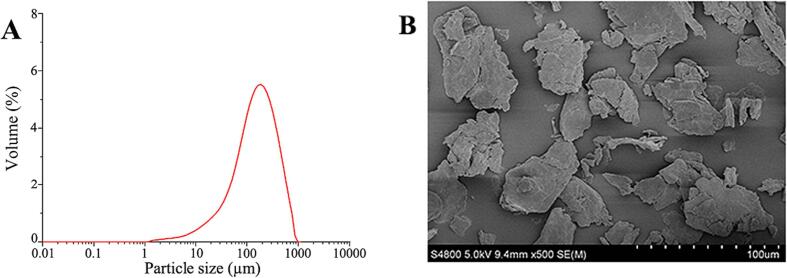
The total DF content in PPDF was about 90 %, wherein IDF and SDF accounted for 53 % and 36 %, respectively. The contents of water, ash, and protein in PPDF were about 13 %, 3 %, and 4 %, respectively (Supplementary Table S1). Particle size distribution of PPDF ranged from 1.5 to 632.456 µm, with an average size of 154.64 µm (Fig. 1A). SEM observations showed that the surface of PPDF particles appeared fragmented, with the particles being large and having an irregular shape (Fig. 1B).
Fig. 1.
Particle size distribution (A) and scanning electron microscopy images (500 × magnification) of pomelo peel dietary fiber (B).
3.2. Effects of PPDF on changes in body weight, organ weight, and blood lipid profile in HFD-fed mice
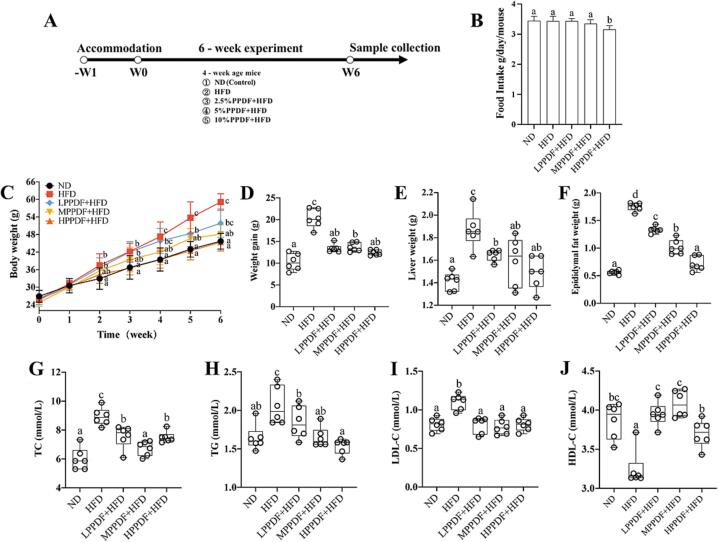
During the experiment, the mice showed normal diet and activity, their fur color remained unchanged and no obvious abnormalities were observed among all groups. The experiment procedure and the effects of PPDF on the HFD-fed mice are shown in Fig. 2. There was no difference in the food intake among the groups ND, HFD, LPPDF + HFD and MPPDF + HFD, however in the HPPDF + HFD group, the food intake was lower than those of others (P < 0.05) (Fig. 2B). From weeks 2–6, the body weight of mice in the HFD-fed group was higher when compared with that of the ND group (P < 0.05). The body weight gain, liver weight, and epididymal adipose tissue weight in the HFD group were also higher than those in the ND group (P < 0.05) (Fig. 2C-2F). PPDF supplementation reversed the modifications caused by HFD. The body weight of mice showed less increase, and the weight gain slowed down with increasing dosages of PPDF (Fig. 2C-2D). At week 6 of the experiment, PPDF supplementation also reduced the increase in liver and epididymal fat weight (P < 0.05). Notably in the HPPDF group, the weight levels were comparable to those of the ND group (Fig. 2E-2F). The serum levels of TC, TG, and LDL-C were higher and the HDL-C levels were lower in the HFD group vs the ND group (P < 0.05). On the contrary, the levels of TC, TG and LDL-C were lower, and HDL-C were higher in all three PPDF + HFD groups vs HFD group (P < 0.05), and the levels of TG, LDL-C and HDL-C in PPDF supplemented groups were comparable to those of the ND group. Furthermore, the TC concentration in the MPPDF + HFD group was not significantly different from that of the ND group (P > 0.05; Fig. 2G-2J).
Fig. 2.
PPDF supplementation alleviated the features of adiposity in HFD-fed mice. (A) Schematic diagram of experimental procedure. (B) Mean daily food intake. (C) Body weight. (D) Weight gain. (E) Liver weight. (F) Epididymal fat weight. (G) TC. (H) TG. (I) LDL-C. (J) HDL-C. Data are expressed as mean ± s. d. (n = 6). Different letters represent the significant difference between different groups (P < 0.05). PPDF: pomelo peel dietary fiber; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein–cholesterol; HDL-C: high-density lipoprotein–cholesterol; ND: normal diet; HFD: high-fat diet; LPPDF: low-dose pomelo peel dietary fiber; MPPDF: medium-dose pomelo peel dietary fiber; HPPDF: high-dose pomelo peel dietary fiber.
3.3. Histological analysis
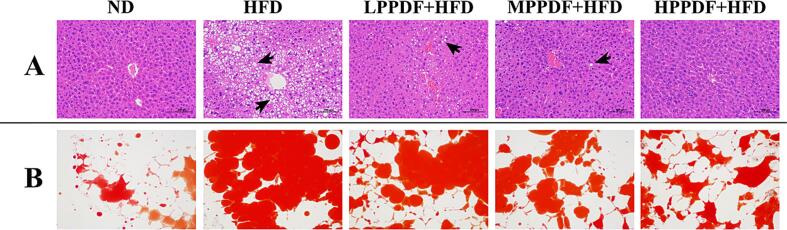
Images of H&E staining of the liver tissue section and Oil Red O staining of the epididymal adipose tissue section of mice are shown in Fig. 3. There are many round vacuoles of varying sizes in the cytoplasm, hepatic steatosis (shown by black arrows), and disorganized liver lobules in HFD group. PPDF supplementation reduced the degree of vacuolization of the envelope, especially in the medium- and high-dose groups, which showed intact cytoplasm and clear nuclei (Fig. 3A). As shown in Fig. 3B, the Oil Red–stained area in the HFD group was significantly larger than that in the ND group, indicating higher lipid accumulation. On the contrary, in the PPDF supplementation groups, the Oil Red–stained areas were reduced to varying degrees. The higher the amount of PPDF supplemented, the more obvious the reduction effect was.
Fig. 3.
Histological analysis of hematoxylin-eosin–stained liver tissue (A) and Oil Red O–stained epididymal fat tissue (B). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Effects of PPDF on alpha diversity of gut microbiota in HFD-fed mice
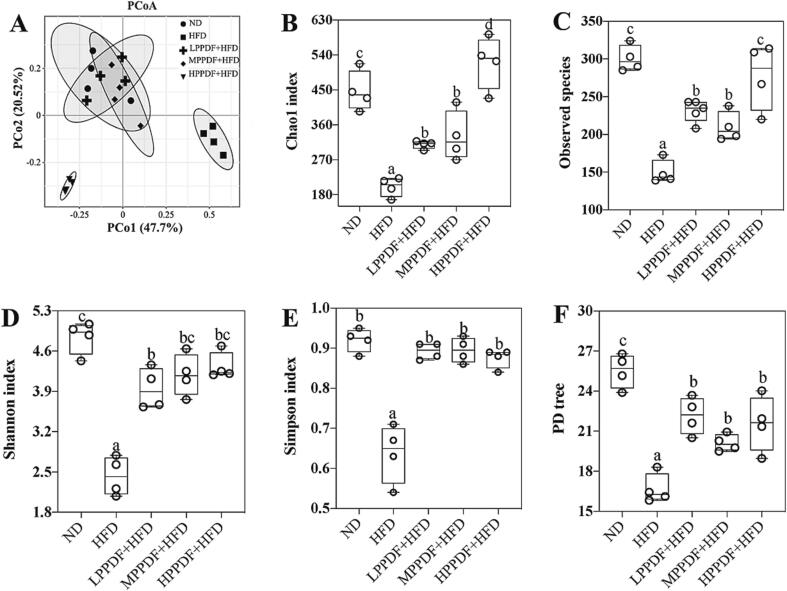
The results of PCoA revealed that samples from the HFD groups were clearly separated from those of the other groups, indicating that the intestinal flora of mice in the HFD group was significantly different from those in the other groups. However, samples from the LPPDF + HFD, MPPDF + HFD, and ND groups overlapped, indicating that low and medium dosages of PPDF restored the intestinal microflora of mice to levels comparable to those in the ND group. Nevertheless, samples from the high-dosage PPDF (HPPDF + HFD) group were clearly distinctive of the other groups, indicating a significantly different gut microbiota in mice of the HPPDF + HFD group (Fig. 4A). Alpha diversity refers to community richness (Chao index and observed species) and species diversity (Shannon index, Simpson index, and phylogenetic diversity). As shown in Fig. 4B-4F, the alpha diversity indexes in the HFD group were considerably lower (P < 0.05) than those of the ND group, whereas all the indexes increased after PPDF supplementation when compared with those of the HFD group (P < 0.05), wherein the Simpson index in all three PPDF supplementation groups was restored to the levels of the ND group, and the Shannon and Sob indexes in the high-dose PPDF (HPPDF + HFD) group were restored to the level comparable to those in the ND group, and the Chao1 index in the HPPDF + HFD group was higher than that in the ND group (P < 0.05) (Fig. 4B-4F).
Fig. 4.
Effects of pomelo peel dietary fiber on gut microbial diversity of HFD-fed mice. (A) Principal coordinates analysis (PCoA); (B) Chao index; (C) observed species; (D) phylogenetic diversity whole tree; (C) Shannon index; and (F) Simpson index. Data are expressed as mean ± s. d. (n = 4). Bars with different letters indicate significant differences (P < 0.05). ND: normal diet; HFD: high-fat diet; LPPDF: low-dose pomelo peel dietary fiber; MPPDF: medium-dose pomelo peel dietary fiber; HPPDF: high-dose pomelo peel dietary fiber.
3.5. Effects of PPDF on gut microbial composition in HFD-fed mice
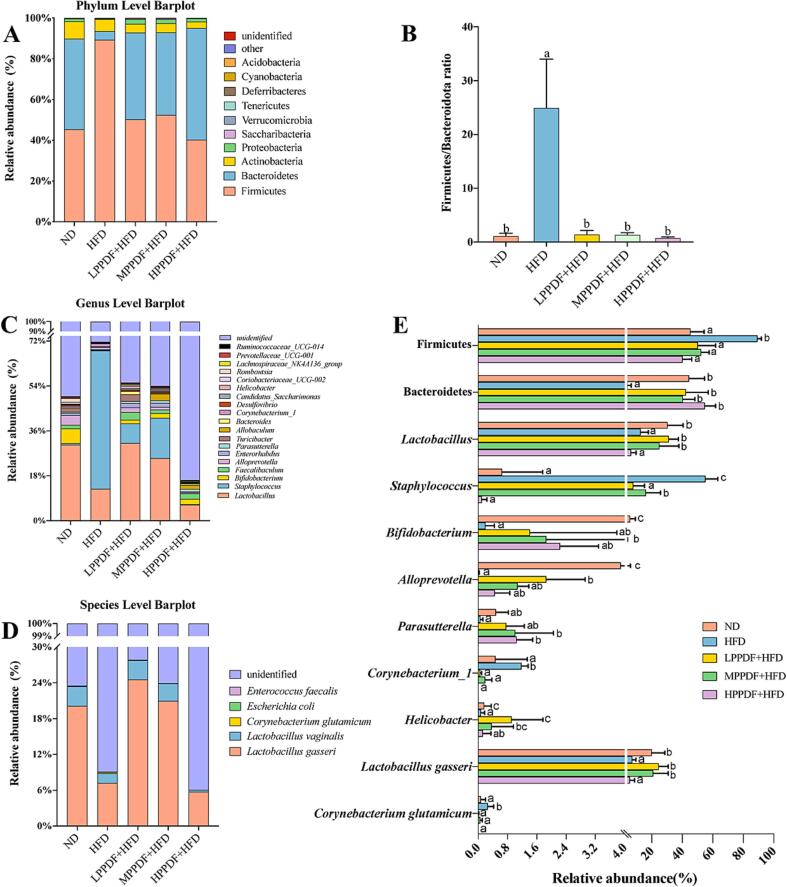
At the phylum level, the most prevalent phyla included Firmicutes, Bacteroidetes and Actinobacteria. Compared with the ND group, the HFD group showed a increase in the abundance of Firmicutes bacteria and a decrease in the abundance of Bacteroidetes bacteria, resulting in a increase in the Firmicutes/Bacteroidetes (F/B) ratio (P < 0.05; Fig. 5A-5B). PPDF supplementation decreased the abundance of Firmicutes bacteria by 37 %-49 % and increased the relative abundance of Bacteroidetes by 36 %-51 % in all three dose groups (P < 0.05; Fig. 5E). Therefore, the F/B ratio in PPDF supplemented groups decreased to the level of the ND group (Fig. 5B).
Fig. 5.
Gut microbial composition at the phylum level (A), ratio of Firmicutes to Bacteroidetes (B), gut microbial composition at the genus level (C), gut microbial composition at the species level (D), relative abundance of the identified bacteria with significant changes among the five groups (E). Significant differences (P < 0.05) are indicated with different letters (a, b, c).
Fig. 5C shows the top 20 genera in the abundance of each group. Compared with ND HFD not only decreased the abundance of the beneficial bacteria Lactobacillus, Bifidobacterium, Alloprevotella, and Helicobacter (P < 0.05) but also increased the abundance of the pathogenic bacteria Staphylococcus and Corynebacterium_1 (P < 0.05; Fig. 5E). By contrast, after PPDF supplementation, the abundances of Corynebacterium_1 in all three PPDF dosage groups and Staphylococcus in the LPPDF and HPPDF groups decreased to the level that was not significantly different from those of the ND group (P > 0.05). Furthermore, the abundances of Lactobacillus, Bifidobacterium, Alloprevotella, and Helicobacter were recovered to varying degrees in the PPDF supplementation groups. Among them, the abundances of Lactobacillus and Helicobacter in the LPPDF + HFD and MPPDF + HFD groups were restored to levels of the ND group. In addition, the abundances of Parasutterella in the MPPDF + HFD and HPPDF + HFD groups were higher than those in the HFD group (P < 0.05; Fig. 5E).
At the species level, a total of 16 identified species were detected in all the five experimental groups (Fig. 5D). Among the identified species with abundance higher than 0.01 %, the abundance of Lactobacillus gasseri decreased and that of Corynebacterium glutamicum increased in the HFD group (P < 0.05). After PPDF supplementation, the abundance of Corynebacterium glutamicum decreased to the ND level, whereas that of Lactobacillus gasseri in the LPPDF and MPPDF groups increased to the ND levels (P > 0.05; Fig. 5E).
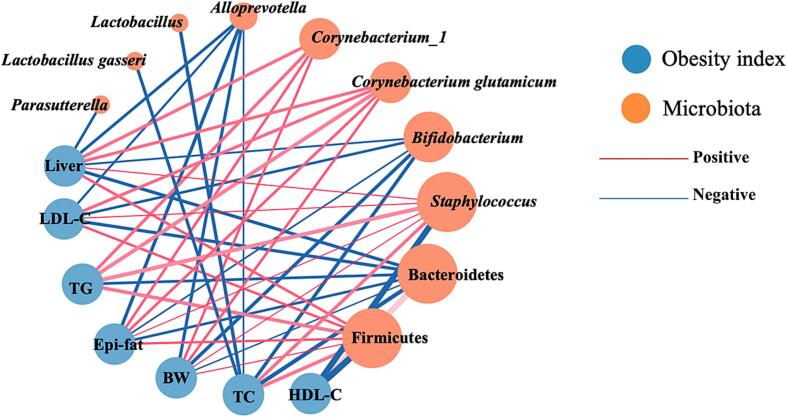
3.6. Correlation between gut microbiota and obesity-related indicators
The correlations between the significantly changed bacterial taxa (indicated in Fig. 5) and six obesity-related indicators (body weight, liver weight, epididymal fat weight, TC, TG, and LDL-C) and HDL-C levels (the beneficial cholesterol) are shown in Fig. 6. Firmicutes bacteria and Staphylococcus showed strong positive correlations with the six obesity-related indices and a strong negative correlation with HDL-C levels (P < 0.01). By contrast, Bacteroidetes, Bifidobacterium, and Alloprevotella were negatively correlated with the obesity-related indexes (except TG), wherein Bacteroidetes showed a highly positive correlation with HDL-C levels (P < 0.01). Corynebacterium_1 and Corynebacterium glutamicum were positively correlated with body weight, liver weight, epididymal fat weight, and TG level (P < 0.05), and Corynebacterium glutamicum was positively correlated with LDL-C levels (P < 0.05). In addition, Parasutterella was negatively correlated with liver weight, and Lactobacillus and Lactobacillus gasseri were negatively correlated with TC levels (P < 0.05). As shown in Fig. 6, Firmicutes, Bacteroidetes, and Staphylococcus had the greatest number of correlated obesity indicators (largest circles), and Firmicutes, Bacteroidetes, Staphylococcus, and Alloprevotella had stronger correlations with obesity-related indicators (thicker lines) than other bacterial taxa.
Fig. 6.
Correlation network diagram showing obesity indexes and significant changes in bacterial taxa. Blue and orange dots represent obesity indexes and bacterial taxa, respectively. Edges between nodes indicate Spearman’s positive (red) and negative (blue) correlations; edge thickness indicates the strength of the correlations, and node size indicates the number of related obesity indicators. TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein–cholesterol; HDL-C: high-density lipoprotein–cholesterol; BW: body weight. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
DF has various beneficial physiological characteristics, such as good water-holding, oil-holding, and adsorption power abilities. DF promotes satiety and satiation while decreasing meal intake by causing bulking and intragastric gelation (Chew & Brownlee, 2018; Kikuchi et al., 2018). In the present study, HFD successfully induced obesity in mice, and different doses of PPDF supplementation alleviated HFD-induced body weight gain and dyslipidemia and reduced visceral fat accumulation in mice. However, only in the HPPDF + HFD group, the food intake of mice decreased significantly, no significant decrease was present in the low or medium dose of PPDF group. This indicated that, in addition to reducing food intake, there are other weight loss mechanisms of PPDF, PPDF’s adsorption and viscosity hindered the absorption of fatty acids and cholesterol from the diet, resulting in reduced fat absorption (Giacco et al., 2014, Ozyurt and Ötles, 2016). Moreover, PPDF has a higher IDF content (53.27 %), with a large particle size (154.64 µm) and a rough, porous surface. IDF can enhance stool weight and volume by binding to water molecules, thus speeding intestinal motility and facilitating defecation (Kaczmarczyk, Miller, & Freund, 2012; Li et al., 2020).
The HFD-induced increase in serum TC and TG levels is the main cause for hyperlipidemia in mice. Excessive concentrations of LDL-C can lead to the accumulation and oxidation of blood vessels, possibly leading to atherosclerosis (Liu, Wang, Xu, & Wang, 2022). The active groups on the DF surface have good ability to bind to cholesterol and bile acids, which helps prevent hyperlipidemia by reducing cholesterol absorption from the diet and increasing the excretion of fecal bile acids and cholesterol (Nie and Luo, 2021, van Bennekum et al., 2005). IDF may have a stronger ability to absorb cholesterol (Rodriguez-Gutierrez, Rubio-Senent, Lama-Munoz, Garcia, & Fernandez-Bolanos, 2014). In this experiment, PPDF supplementation significantly decreased the levels of TC, TG, and LDL-C in HFD-fed mice and reduced the risk of hyperlipidemia. A low level of HDL-C is an important risk factor for cardiovascular disease (Fang et al., 2021, Liu et al., 2022). PPDF supplementation also restored HDL-C levels in the HFD group consistent with the level of the ND group.
HFD-induced gut microbiota dysbiosis is one of the important causes for host obesity and related metabolic diseases, and an important indicator of a gut microbiota disorder is a significant decrease in the abundance and diversity of the gut microbiota (Liu, He, Ma, & Chen, 2019). In the present study, HFD significantly reduced the diversity of the gut microbiota in mice, whereas PPDF supplementation reversed these changes, consistent with the result of a previous study.
HFD also caused changes in the abundance of key bacteria in the gut. At the phylum level, HFD led to a significant increase in the F/B ratio, and a high F/B ratio in the gut is considered a biomarker of obesity in both humans and animals (Dao & Clément, 2018). In the present study, the abundance of Firmicutes was positively correlated to the obesity indicators body weight, liver weight, and epididymal fat weight, whereas the abundance of Bacteroides bacteria was negatively correlated with these indicators. PPDF supplementation restored the F/B ratio to normal levels and significantly inhibited obesity. PPDF supplementation also restored the decline in the abundance of important beneficial bacteria caused by HFD, such as Bifidobacterium, Alloprevotella, Parasutterella, Lactobacillus, and Lactobacillus gasseri. These bacteria can produce short-chain fatty acids (SCFAs) (Hossain et al., 2022, Sa'ad et al., 2010), which enhance satiety and maintenance of glucose homeostasis through the production of enterokinin YY and glucagon-like peptide 1 (Fukumoto et al., 2003, Neuman et al., 2015). SCFAs can also enhance adipose tissue thermogenesis and reduce fat accumulation (Blaak, et al., 2020). Lactobacillus gasseri is thought to have a certain immunoregulatory effect, and it can act against Helicobacter pylori by producing dl-lactic acid and, consequently, reducing the risk of enteritis (Kadooka et al., 2010). Spearman correlation analysis indicated that the abundance of these bacterial taxa decreased significantly in the HFD group, whereas it was recovered in the PPDF + HFD group, which was significantly negatively correlated with the obesity indicators.
PPDF supplementation significantly reduced the increase in the abundance of Staphylococcus, Corynebacterium-1, and Corynebacterium glutamicum caused by HFD. Staphylococcus causes chronic inflammation and insulin resistance (Santacruz et al., 2010). Corynebacterium-1 causes lower respiratory tract infections, lung abscesses, and lymphadenitis (Wu et al., 2021). Corynebacterium glutamicum, a functional amino acid–producing bacterium, can increase glucose uptake (Ruan, Yu, & Xu, 2020). These bacteria significantly increased in the HFD group but significantly decreased in the PPDF + HFD group and were significantly positively correlated with obesity indicators (Kalliomäki, Carmen Collado, Salminen, & Isolauri, 2008), indicating that PPDF supplementation can prevent obesity by regulating the intestinal flora to prevent inflammation, reduce insulin resistance, and decrease glucose uptake.
Due to the high cost of in vivo experiments in animals and humans, ethical constraints and difficulty in sampling from the gut, the TNO in vitro model of the colon (TIM-2) is a good alternative for investigating the effects of potential prebiotics on gut microbiota (Venema & Van den Abbeele, 2013). Several studies have reported the effects of fruit by-products (peels, seeds and bagasses) on gut microbiota in the TIM-2 model. The results showed that the fermented Mango peel could promote the growth of Bifidobacterium and Lactobacillus (Sáyago-Ayerdi, Zamora-Gasga, & Venema, 2019), and the fermented Orange bagasses could produce more SCFAs (acetate, propionate and butyrate), and stimulated the growth of Ruminococcus, Lachnospira, Roseburia, Dialister and Bacteroides. The passion fruit peels were tested in the TIM-2 model to produce succinate and lactate, and could promote the growth of Bacteroides, Lachnospira and Ruminococcus (De Souza, Jonathan, Saad, Schols, & Venema, 2019). In the mouse obesity model of this study, PPDF also restored the abundance of Bacteroidetes, Bifidobacterium, and Lactobacillus, and other bacteria (Alloprevotella, Helicobacter and Parasutterella) which could produce SCFAs and prevent obesity (Hossain, Ranadheera, Fang, Masum, & Ajlouni, 2022).
Previous studies have shown a well-established inverse association between fiber intake and Metabolic syndrome (MetS) (Chen, Chen, Wang, Qin, & Bai, 2017), and the positive effect of fiber intake in preventing dyslipidemia has been demonstrated in several interventional human studies (Zhou, Wu, Tang, Wang, Lu, & Wang, 2015). This effect was primarily characterized by a rise in HDL levels and a decrease in TC, which is also consistent with the effect of PPDF in the present study. PPDF was derived from pomelo peels, which have been consumed by humans for many years. The probiotic effects of PPDF on humans can be verified by using TIM-2 model or by directly eating PPDF functional foods. In view of the positive activities in alleviating weight gain, visceral fat accumulation and dyslipidemia, and in regulating gut microbiota in obese mice, PPDF has the potential to be developed into functional foods and pharmacological alternatives for preventing obesity.
PPDF is present in abundance in natural products, and the processing method is simple and easy. The present study demonstrated the potential of PPDF to be developed into functional foods and pharmacological alternatives for treating obesity and regulating dyslipidemia and gut microflora dysbiosis. The development of PPDF will not only realize high-value utilization of pomelo peels but also reduce environmental pollution. This study may also help explain the mechanism of the beneficial effects of PPDF on obesity and hyperlipidemia. However, studies on intestinal metabolomics should be conducted in the future to unravel the underlying mechanism, which was not covered in the present study.
5. Conclusion
In this study, PPDF with fiber content of about 90 % was extracted from pomelo peel wastes. Dietary supplementation with PPDF for 6 weeks significantly reduced the weight gain of body, liver and epididymal fat accumulation of obese mice, and restored lipid indices TG, LDL-C, HDL-C, and TC to normal levels in a dose-dependent pattern. PPDF supplementation restored the decrease in gut microbiota diversity and the increase in F/B ratio caused by HFD, restored the abundance of key anti-obesity bacteria (such as Bacteroidetes, Bifidobacterium, and Lactobacillus, etc), and inhibited the growth of bacteria significantly associated with obesity and inflammation (Firmicutes, Staphylococcus, and Corynebacterium-1, etc). Therefore, PPDF alleviated HFD-induced body weight gain and dyslipidemia and alleviated visceral fat accumulation in mice. These positive benefits were associated with the regulation of gut microbiota. PPDF has the potential to be developed into functional foods and prebiotics for preventing obesity, regulating lipid and intestinal flora.
CRediT authorship contribution statement
Jing Ni: Conceptualization, Data curation, Investigation, Methodology, Formal analysis, Writing – original draft. Yuchen Shangguan: Conceptualization, Data curation, Investigation, Methodology, Formal analysis, Writing – original draft. Lili Jiang: Conceptualization, Formal analysis. Chuanbo He: Investigation, Methodology, Data curation. Ying Ma: Conceptualization, Funding acquisition, Supervision, Methodology, Writing – review & editing. Hejian Xiong: Conceptualization, Funding acquisition, Supervision, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Science and Technology Project of Fujian Province (2023I0020), Xiamen Science and Technology Planning Project (2022CXY0307) and Natural Science Foundation of Fujian Province (2023J01779), China. The authors would like to thank MogoEdit (https://www.mogoedit.com) for providing assistance in English language editing during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100993.
Contributor Information
Ying Ma, Email: maying@jmu.edu.cn.
Hejian Xiong, Email: hjxiong@jmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Balaji M., Ganjayi M.S., Kumar G.E.H., Parim B.N., Mopuri R., Dasari S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obesity research & clinical practice. 2016;10(4):363–380. doi: 10.1016/j.orcp.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Blaak E., Canfora E., Theis S., Frost G., Groen A., Mithieux G.…Van Harsselaar J. Short chain fatty acids in human gut and metabolic health. Beneficial microbes. 2020;11(5):411–455. doi: 10.3920/B.2020.0057. [DOI] [PubMed] [Google Scholar]
- Chen J.P., Chen G.C., Wang X.P., Qin L., Bai Y. Dietary fiber and metabolic syndrome: A meta-analysis and review of related mechanisms. Nutrients. 2017;10(1):24. doi: 10.3390/nu10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, K. Y., & Brownlee, I. A. (2018). The impact of supplementation with dietary fibers on weight loss: A systematic review of randomised controlled trials. Bioactive Carbohydrates and Dietary Fibre, 14, 9-19. Doi.org/10.1016/j.bcdf.2017.07.010.
- Chinese Nutrition Society, 2016 Chinese Nutrition Society (CNS) Chinese Dietary Guidelines (2016) http://www.fao.org/nutrition/education/food-based-dietary-guidelines/regions/countries/china/en/.
- Czech A., Malik A., Sosnowska B., Domaradzki P. Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. International Journal of Food Science. 2021;2021:1–14. doi: 10.1155/2021/6662259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M.C., Clément K. Gut microbiota and obesity: Concepts relevant to clinical care. European journal of internal medicine. 2018;48:18–24. doi: 10.1016/j.ejim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- De Souza C.B., Jonathan M., Saad S.M.I., Schols H.A., Venema K. Degradation of fibres from fruit by-products allows selective modulation of the gut bacteria in an in vitro model of the proximal colon. Journal of Functional Foods. 2019;57:275–285. doi: 10.1016/j.jff.2019.04.026. [DOI] [Google Scholar]
- Commission E. Guidance document for competent authorities for the control of compliance with EU legislation On: Council Directive 90/496/EEC of 24 September 1990 on nutrition labelling of foodstuffs. 2012. https://ec.europa.eu/food/sites/food/files/safety/docs/labelling_legislation_guidance_methods_2012_en.pdf accessed October 2015.
- Fang D., Wang D., Ma G., Ji Y., Zheng H., Chen H.…Zhao L. Auricularia polytricha noodles prevent hyperlipemia and modulate gut microbiota in high-fat diet fed mice. Food Science and Human Wellness. 2021;10(4):431–441. doi: 10.1016/j.fshw.2021.04.005. [DOI] [Google Scholar]
- FAO, 2008. http://www.fao.org/faostat/en/#search/Grapefruit%20inc.%20pomelos. Accessed 21 September 2020.
- Fukumoto, S., Tatewaki, M., Yamada, T., Fujimiya, M., Mantyh, C., Voss, M., Eubanks, S., Harris, M., Pappas, T.N., & Takahashi, T. (2003). Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 284(5), R1269-R1276. Doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed]
- Giacco R., Costabile G., Della Pepa G., Anniballi G., Griffo E., Mangione A.…Landberg R. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(8):837–844. doi: 10.1016/j.numecd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Hossain M.N., Ranadheera C.S., Fang Z., Masum A., Ajlouni S. Viability of Lactobacillus delbrueckii in chocolates during storage and in-vitro bioaccessibility of polyphenols and SCFAs. Current Research in Food Science. 2022;5:1266–1275. doi: 10.1016/j.crfs.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Ye R., Chen J., Xu F. An improved method for rapid quantitative analysis of the insoluble dietary fiber in common cereals and some sorts of beans. Journal of cereal science. 2013;57(3):270–274. doi: 10.1016/j.jcs.2012.11.009. [DOI] [Google Scholar]
- Kaczmarczyk M.M., Miller M.J., Freund G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61(8):1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y.…Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. European journal of clinical nutrition. 2010;64(6):636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- Kalliomäki M., Carmen Collado M., Salminen S., Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. The American journal of clinical nutrition. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y., Nozaki, S., Makita, M., Yokozuka, S., Fukudome, S.i., Yanagisawa, T., & Aoe, S. (2018). Effects of whole grain wheat bread on visceral fat obesity in Japanese subjects: A randomized double-blind study. Plant Foods for Human Nutrition, 73, 161-165. Doi: 10.1007/s11130-018-0666-1. [DOI] [PubMed]
- Lan-Phi N., Vy T. Chemical composition, antioxidant and antibacterial activities of peels' essential oils of different pomelo varieties in the south of Vietnam. International Food Research Journal. 2015;22(6):2426. http://www.ifrj.upm.edu.my Journal homepage: [Google Scholar]
- Li L., Pan M., Pan S., Li W., Zhong Y., Hu J., Nie S. Effects of insoluble and soluble fibers isolated from barley on blood glucose, serum lipids, liver function and caecal short-chain fatty acids in type 2 diabetic and normal rats. Food and Chemical Toxicology. 2020;135 doi: 10.1016/j.fct.2019.110937. [DOI] [PubMed] [Google Scholar]
- Liew S.Q., Ngoh G.C., Yusoff R., Teoh W.H. Acid and Deep Eutectic Solvent (DES) extraction of pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Biocatalysis and agricultural biotechnology. 2018;13:1–11. doi: 10.1016/j.ijbiomac.2018.05.013. [DOI] [Google Scholar]
- Liu J., He Z., Ma N., Chen Z. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. Journal of Agricultural and Food Chemistry. 2019;68(1):33–47. doi: 10.1021/acs.jafc.9b06817. [DOI] [PubMed] [Google Scholar]
- Liu T., Wang N., Xu X., Wang D. Effect of high quality dietary fiber of Hericium erinaceus on lowering blood lipid in hyperlipidemia mice. Journal of Future Foods. 2022;2(1):69–76. doi: 10.1016/j.jfutfo.2022.03.018. [DOI] [Google Scholar]
- Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell host & microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Mateos-Aparicio I., De la Peña Armada R., Pérez-Cózar M.L., Rupérez P., Redondo-Cuenca A., Villanueva-Suárez M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioactive Carbohydrates and Dietary Fibre. 2020;23 doi: 10.1016/j.bcdf.2020.100219. [DOI] [Google Scholar]
- Navar-Boggan A.M., Peterson E.D., D’Agostino R.B., Sr, Neely B., Sniderman A.D., Pencina M.J. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131(5):451–458. doi: 10.1161/CIRCULATIONAHA.114.012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman H., Debelius J.W., Knight R., Koren O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS microbiology reviews. 2015;39(4):509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- Nie Y., Luo F. Dietary fiber: An opportunity for a global control of hyperlipidemia. Oxidative Medicine and Cellular Longevity. 2021;2021 doi: 10.1093/femsre/fuu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt V.H., Ötles S. Effect of food processing on the physicochemical properties of dietary fibre. Acta Scientiarum Polonorum Technologia Alimentaria. 2016;15(3):233–245. doi: 10.17306/J.AFS.2016.3.23. [DOI] [PubMed] [Google Scholar]
- Papathanasopoulos, A., & Camilleri, M. (2010). Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology, 138(1), 65-72. e62. Doi: 10.1053/j.gastro.2009.11.045. [DOI] [PMC free article] [PubMed]
- Popkin B.M., Adair L.S., Ng S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutrition reviews. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gutierrez, G., Rubio-Senent, F. t., Lama-Munoz, A., Garcia, A., & Fernandez-Bolanos, J. (2014). Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: binding of water, oil, bile acids, and glucose. Journal of Agricultural and Food Chemistry, 62(36), 8973-8981. Doi: 10.1021/jf502062b. [DOI] [PubMed]
- Ruan H., Yu H., Xu J. The glucose uptake systems in Corynebacterium glutamicum: A review. World Journal of Microbiology and Biotechnology. 2020;36:1–9. doi: 10.1007/s11274-020-02898-z. [DOI] [PubMed] [Google Scholar]
- Sa'ad H., Peppelenbosch M.P., Roelofsen H., Vonk R.J., Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of. Lipids. 2010;1801(11):1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Santacruz A., Collado M.C., Garcia-Valdes L., Segura M., Martin-Lagos J., Anjos T.…Campoy C. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. British Journal of Nutrition. 2010;104(1):83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- Sáyago-Ayerdi S.G., Zamora-Gasga V.M., Venema K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2) Food Research International. 2019;118:89–95. doi: 10.1016/j.foodres.2017.12.024. [DOI] [PubMed] [Google Scholar]
- van Bennekum A.M., Nguyen D.V., Schulthess G., Hauser H., Phillips M.C. Mechanisms of cholesterol-lowering effects of dietary insoluble fibres: Relationships with intestinal and hepatic cholesterol parameters. British Journal of Nutrition. 2005;94(3):331–337. doi: 10.1079/bjn20051498. [DOI] [PubMed] [Google Scholar]
- Venema K., Van den Abbeele P. Experimental models of the gut microbiome. Best Practice & Research Clinical Gastroenterology. 2013;27(1):115–126. doi: 10.1016/j.bpg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Wang H., Xu Y.S., Wang M.L., Cheng C., Bian R., Yuan H.…Zhou H. Protective effect of naringin against the LPS-induced apoptosis of PC12 cells: Implications for the treatment of neurodegenerative disorders. International Journal of Molecular Medicine. 2017;39(4):819–830. doi: 10.3892/ijmm.2017.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Chen Q., Liu J., Chen X., Luo H., Ye Z., Liu J. Microbiome analysis reveals gut microbiota alteration in mice with the effect of matrine. Microbial pathogenesis. 2021;156 doi: 10.1016/j.micpath.2021.104926. [DOI] [PubMed] [Google Scholar]
- Yan, R. l., Liao, Y., Mao, R. y., & He , F. l. (2017). Functional properties and optimization of microwave assisted alkaline extracton of dietary fiber from pomelo peel. Food & Machinery, 33(12), 143-147. Doi: 10.13652/j.issn.1003-5788.2017.12.029.
- Yang L., Zhao Y., Huang J., Zhang H., Lin Q., Han L.…Liu H. Insoluble dietary fiber from soy hulls regulates the gut microbiota in vitro and increases the abundance of bifidobacteriales and lactobacillales. Journal of food science and technology. 2020;57:152–162. doi: 10.1007/s13197-019-04041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Xu Y., Tuo J., Li A., Liu L., Shi H. Preparation of modified pomelo peel's pulp adsorbent and its adsorption to uranyl ions. Royal Society open science. 2019;6(3) doi: 10.1098/rsos.181986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.W. Possible anti-obesity therapeutics from nature–A review. phytochemistry. 2010;71(14–15):1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wu J., Tang J., Wang J.-J., Lu C.-H., Wang P.-X. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among Chinese rural-to-urban migrant workers. International journal of environmental research and public health. 2015;12(5):4726–4738. doi: 10.3390/ijerph120504726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.