Abstract
The mechanisms by which gene products inhibit the conjugal transfer of IncP plasmids (e.g., RP1) have been little studied. We have isolated and characterized one such gene, fipA (624 nucleotides), from the SmaI (14.8 kb)-AatII (15.6 kb) region of pKM101(IncN). This gene, which is also conserved in other IncN plasmids, is transcribed in an anticlockwise direction, probably as part of a transfer operon that includes traHI. The FipA protein (24 kDa) appears to be cytoplasmic and, when expressed from a multicopy plasmid, retards the growth of Escherichia coli WP2. The mode of action of fipA was compared with that of the apparently unrelated pifC gene from F(IncFI). Both genes inhibit the transfer of IncPα and IncPβ plasmids but to different degrees. They also inhibit the mobilization of RSF1010 (which requires the RP1 pilus genes and traG) but not of CloDF13 (which encodes a traG homolog). Evidence that traG was the specific target of inhibition was obtained in an artificial system in which cloned traG was used to enhance RSF1010 mobilization via the N pilus system. Such enhancement did not occur in the presence of fipA or pifC. The availability of an in vivo assay of PifC enabled us to show that F pif operon expression increased in cells carrying F′lac and traG, but only if the traG coding sequence was intact. This finding suggested that conjugal inhibition of RP1 was most likely due to a PifC-TraG protein interaction. On phenotypic grounds inhibition of traG by fipA is also likely to occur posttranscriptionally. Whether or not the selection of traG as the inhibition target is an evolutionary tactic to limit the spread of P plasmids, we anticipate that fipA and pifC will prove useful in further investigation of the conjugal roles of traG and its homologs.
Conjugative transfer by prototypes of the IncPα and IncPβ plasmid subgroups has been the subject of considerable study (23, 38, 50, 71). This has included comparisons with the F(IncFI) paradigm system (16, 19) and those determined by IncW (6, 42) and IncN plasmids (26, 52, 53, 75), as well as studies of the interplay between P conjugal activity and the replication and promiscuous transfer of these plasmids (27, 28). More recently, sequence comparisons have suggested a common ancestry for conjugal DNA transfer and that of oncogenic T-DNA transfer from Agrobacterium tumefaciens to plant cells and the Bordetella pertussis toxin export system (30, 41).
The conjugal transfer functions control mating pair formation (the Mpf or PIL system) and DNA transfer and replication (the Dtr or MOB system) (9, 50). In the case of RP1(IncPα), the Mpf genes are located in the Tra2 region except for traF, located in Tra1 (18, 24, 39, 40). The Mpf system is required for P-pilus formation and, in the form of a membrane-associated Mpf complex, promotes DNA export and the adsorption of donor-specific phages (13, 35). The Dtr genes are located in the Tra1 region, and their products, TraI, TraJ, and TraK, interact at oriT to form the relaxosome and initiate transfer of the single DNA strand (38, 49, 51). An additional Tra1 gene, traG, that is not part of the Mpf or Dtr systems is essential for DNA transfer, possibly serving to link the relaxosome to the DNA transport apparatus (38, 70).
Homologs of TraG occur in various conjugal systems (30, 41) and are essential both for transfer of the conjugative plasmid and for mobilization of nonconjugative plasmids (23). However, the frequency of mobilization can vary widely depending on the conjugal system being used, even when transfer of the conjugative plasmid is highly efficient. The finding that RP1 TraG can replace the TrwB homolog of R388 for the purposes of mobilization but not self-transfer raises the possibility that different domains of these homologs are involved in interactions with the relaxosome and the DNA transport complex (8). The degree to which these homologs can be interchanged has been the subject of a recent study (9).
In this report, we describe genes that inhibit the conjugal transfer of RP1 and which may prove useful in elucidating the roles of the transfer genes. Inhibition genes are common in plasmids (27), and those in pKM101(IncN) (76) and F(IncFI) (68) inhibit RP1 transfer without apparently affecting pilus function. The gene in F, pifC, has roles in the regulation of the F pif operon (11, 31, 44) and the initiation of F DNA replication (33, 44, 69), but neither pifC nor the gene in pKM101 (75) is an essential tra gene. We characterize the gene in pKM101, here named fipA, and show that both fipA and pifC exert their inhibition effects via traG. We also present evidence that the interaction between pifC and traG occurs at the protein level and not, as previously suggested (43), by binding to RP1 DNA.
(A preliminary report of this work was presented at Plasmid Biology 96: International Symposium on Plasmid Molecular Biology [59].)
MATERIALS AND METHODS
Nomenclature.
Restriction cleavage sites and their locations are presented in the format KpnI (24.1 kb). The designations MOB and PIL are used as phenotypic descriptors of the groups of genes from different plasmid type required, respectively, for conjugal DNA metabolism and for pilus synthesis and function (MOBP, PILN, etc.).
Strains, plasmids, and media.
The Escherichia coli K-12 derivatives used were HB101 (recA13 Strr) (7), a rifampin-resistant mutant of HB101 (LT101 [48]), UB1301 (Rifr [21]), and UB5201 (recA56 Nalr [58]). WP2 (25), a tryptophan auxotroph of E. coli B/r, and Pseudomonas aeruginosa PAO2637(RP1) (18) were also used.
The plasmids used are listed in Table 1 except for the IncN plasmids pCU1, N3, R199 (34), R390 (27), pVS101 (10), pVS144 (Smr Tcr; from the same source as pVS101), and pVS151 (Apr Smr Tcr; from Klebsiella aerogenes isolated from Melbourne sewage effluent) and IncPβ plasmids pJP4, R772, and R751 (64). Additional plasmids used were mutants of pVS659 and pVS520 (described in Tables 4 and 6, respectively) and plasmids that carried parts of the RP1 region (from position 48.869 kb [PtraG] through traG [48.495 to 46.588 kb] to position 46.573 kb) cloned directly from a 2.3-kb PCR product (see below) into pUC19 by utilizing internal SphI or terminal EcoRI sites. These plasmids, and the regions cloned in the sense orientation with respect to the Plac of the vector, were pVS1160 (48.869 to 48.707), pVS1161 (48.707 to 47.826), pVS1162 (47.826 to 47.561), pVS1163 (47.561 to 46.798), and pVS1164 (46.798 to 46.573).
TABLE 1.
Bacterial plasmids used
| Plasmida | Relevant features and/or derivation | Reference or source |
|---|---|---|
| Conjugative plasmids and mutants | ||
| F′lac | lac pifO+C+A+B+ Tra+ IncFI | A. J. Pittard |
| pKM101 | AprfipA+ Tra+ IncN | 75 |
| pUB307 | RP1 derivative; Tcr Kmr Tra+ IncPα | 5 |
| pVS520 | RP1 derivative; Tcr Tra+ IncPα | 48 |
| R388 | Tpr Sur Tra+ IncW | 12a |
| pVS766 | traF mutant of pVS520; Tra− Dps− | 18 |
| pVS793 (pKM101fipA) | fipA::Tn1725 (Cmr) mutant of pKM101 | 18 |
| Nonconjugative plasmids | ||
| pBluescriptII SK+ and KS+ | Plasmid vectors; Apr | Stratagene |
| pBBR1MCS-2 | Plasmid vector; Kmr | 36 |
| pGEM7Zf+ | Plasmid vector; Apr | Promega |
| pGP1-2 | Carries the T7 RNA polymerase gene regulated by the λcIts repressor; Kmr | 67 |
| pUC18, pUC19 | Plasmid vectors; Apr | 77 |
| pRS3412 | pACYC184 carrying the BamHI (45.45 kb)-HindIII (47.4 kb)bpifC region of the pif operon; CmrpifC+ | 12 |
| pSU4628 (CloDF13a) | CloDF13::TnAΔEcoRV; Apr Mob+ | 9 |
| pVS514 (RSF1010t) | RSF1010 carrying the 2.0-kb EcoRI-PvuII Tcr fragment of pBR322; Tcr Mob+ | S. T. Fong |
| pVS657 (pSC101c) | pSC101 carrying the 2.1-kb HindIII-HincII Cmr fragment of pBR325; Tcr Mob+ | 18 |
| pVS658 | pUC19 carrying the HindIII (38.9 kb)-EcoRI (60.0 kb)d region of RP1; Apr KmroriT+ traI+J+K+L+M+ | 18 |
| pVS659 | pBR322 derivative carrying the ClaI (37.2 kb)-EcoRI (60.0 kb)d region of RP1; Apr Kmr Tra1+oriT+ | 18 |
| pVS660 (ColE1k) | ColE1 carrying the 1.3-kb SmaI-HindIII Kmr fragment of Tn5; Mob+ | 17 |
| pVS729 | pBR322 derivative carrying the 43.5–49.5-kbd region of RP1; AprtraE+F+G+H+ | 18 |
| pVS915 | pT7-4 carrying the 4.4-kb PstI-PvuII region of pBR325 (cloned into the MCS PstI/SmaI sites); Apr Cmr Tcr | S. T. Fong |
| pVS1107 | pUC18 carrying the SmaI (14.8 kb)-KpnI (17.0 kb)cfipA region of pKM101; AprfipA+ | This study |
| pVS1125 | pGEM7Zf+ carrying the SmaI (14.8 kb)-AatII (15.6 kb)cfipA region of pVS1107; AprfipA+ | This study |
| pVS1126/1127 | pBluescriptII KS+ and SK+ carrying the SmaI (14.8 kb)-AatII (15.6 kb)cfipA region of pVS1125 (cloned into the MCS SmaI-ApaI sites); AprfipA+ | This study |
| pVS1128/1129 | pBluescriptII KS+ and SK+ carrying the BglII (15.0 kb)-AatII (15.6 kb)cfipA region of pVS1125 (cloned into the MCS BamHI-ApaI sites); AprfipA | This study |
| pVS1136 | pBBR1MCS-2 carrying the SmaI (14.8 kb)-AatII (15.6 kb)cfipA region of pVS1127 (cloned into the MCS SmaI-ApaI sites); KmrfipA+ | This study |
| pVS1137 | pBBR1MCS-2 carrying the BamHI (45.45 kb)-HindIII (47.4 kb)bpifC region of pRS3412; KmrpifC+ | This study |
| pVS1140g | pBluescriptII SK+ carrying the 46.573–48.504-kbe region of RP1; AprtraG+ | This study |
| pVS1141g | pBluescriptII SK+ carrying the 46.573–48.869-kbf region of RP1; Apr PtraG traG+ | This study |
| pVS1142g | pBluescriptII KS+ carrying the 46.573–48.504-kb region of RP1 from pVS1140 (cloned into the MCS SacI/HindIII sites); Apr Plac traG+ | This study |
| pVS1150 | pBluescriptII SK+ carrying the 72–1162h-nt region of pVS1137; AprpifC+ | This study |
| RSF1010 | Sur Smr IncQ Mob+ | 22 |
Synonyms (shown in parentheses) are listed for some plasmids and are used in the text for the purpose of simplicity (e.g., RSF1010t versus pVS514).
F coordinates are those of Cram et al. (12).
pKM101 coordinates are those of Winans and Walker (75).
RP1 coordinates are those of Pansegrau et al. (50).
Primers used for PCR amplification of a region from pVS729 were GCGGAATTCGGATCCTAGGAGTAGATGAAGAACCGA and GCGGAATTCGGATCCAGGCGCTGGAAGCGGCTCATAT, which contain, at the ends, both EcoRI and BamHI restriction sites; the EcoRI sites were used for cloning purposes. Plasmid pVS1140 contains traG without PtraG in the antisense orientation in relation to Plac of the vector.
Primers used for PCR amplification of a region from pVS729 were GCCGAATTCGGATCCCTGGTCACGTGGAGCAGCAAGGAG and GCGGAATTCGGATCCAGGCGCTGGAAGCGGCTCATAT, which contain, at the ends, both EcoRI and BamHI restriction sites; the EcoRI sites were used for cloning purposes. Plasmid pVS1141 contains traG with PtraG in the antisense orientation with respect to Plac of the vector.
Plasmids pVS1140, pVS1141, and pVS1142 were all found to complement an RP1 traG mutant to wild-type levels (data not shown).
Primers used for PCR amplification of a region of pVS1137 were GCGGAATTCGGATCCATGCTAAGCCAGCTTAACCTG and GCGGAATTCGGATCCTATTACAGATCTCCGTACAGGCA, which contain, at the ends, both EcoRI and BamHI restriction sites; the BamHI sites were used for cloning purposes. Plasmid pVS1150 contains pifC in the sense orientation with respect to Plac of the vector. The coordinates given are those of Caughey et al. (11).
TABLE 4.
Involvement of the RP1 Tra1 genes in IncN-mediated mobilization of RSF1010
| Plasmid(s) in donor together with pKM101fipAb | Mobilization frequencya of:
|
|
|---|---|---|
| RSF1010t | pVS659 | |
| RSF1010t | 2.6 × 10−6 | |
| pVS659 (Tra1+) | 2.5 × 10−3 | |
| RSF1010t, pVS659 (Tra1+) | 1.1 × 10−2 | 2.9 × 10−3 |
| RSF1010t, pVS659 (Tra1+)c | 2.5 × 10−6 | 3.1 × 10−6 |
| RSF1010t, pVS659traI or traJd | 4.8 × 10−3 | 3.4 × 10−6 |
| RSF1010t, pVS659traF | 8.4 × 10−3 | 2.1 × 10−3 |
| RSF1010t, pVS659traG | 1.5 × 10−6 | 3.5 × 10−6 |
| RSF1010, pVS766 (traF)e | 2.1 × 10−6 | |
| RSF1010, pVS766 (traF), pVS659 (Tra1+)e | 1.0 × 10−2 | 2.2 × 10−3 |
Number of transconjugants per donor cell after a 2-h mating of HB101 donors and LT101 (Rifr) as the recipient. The mobilization frequencies of RSF1010t and pVS659 (or derivatives) were determined on NA-rifampin-tetracycline and NA-rifampin-kanamycin, respectively.
The transfer frequency of pKM101fipA (ca. 3.5 × 10−1 per donor in all matings) was determined on NA-rifampin-chloramphenicol. The transfer of pKM101 (fipA+) was not determined due to the absence of a selectable marker; however, its presence was monitored by Dps+.
In this donor, pKM101 (fipA+) replaced pKM101fipA.
Similar results were obtained when the donor carried pVS659 traI or pVS659 traJ. The various pVS659 mutants were traI (pVS769), traJ (pVS768), traF (pVS775), and traG (pVS773), described previously (18).
The transfer of pVS766 (2.5 × 10−7 per donor) was determined on NA-rifampin-tetracycline. The mobilization of RSF1010 (used in place of RSF1010t) was determined on DST-rifampin-sulfathiazole.
TABLE 6.
Effects of traG on F′lac Pif activity
| Plasmid(s) in donora
|
T7 EOPb | Pif phenotypec | |
|---|---|---|---|
| F factor | Other | ||
| None | None | 1.0 | − |
| F′lac | None | 9.1 × 10−2 | + |
| None | pVS520 | 1.0 | − |
| F′lac | pVS520 | <10−4 | ++ |
| None | pBluescriptII SK+ | 1.0 | − |
| F′lac | pBluescriptII SK+ | 6.4 × 10−2 | + |
| pVS1141 (PtraG traG+) | <10−4 | ++ | |
| pVS1140 (traG+) | <10−4 | ++ | |
| pVS580 (traG::Tn5)d | 6.2 × 10−2 | + | |
| pVS1161 (traGΔ)e | 6.1 × 10−2 | + | |
| pVS658 (traGΔH+I+) | 5.2 × 10−2 | + | |
All plasmids were carried by HB101.
T7 efficiency of plating (EOP) on HB101 was assigned a value of 1.0, and all other determinations were normalized to this value.
Pif−, large plaque with halo; Pif+, pinpoint plaque; Pif++, individual plaque not visible, but lysis evident at high PFU.
Similar results were obtained with pVS582, pVS583, and pVS587, which, like pVS580, are pVS520 derivatives with Tn5 inserted at different locations within traG (18).
Similar results were obtained when a number of other traG subclones (pVS1160, pVS1162, pVS1163, and pVS1164) were used (see Materials and Methods).
Nutrient agar (NA), nutrient broth (NB), and diagnostic sensitivity agar (DST) have been described previously (48). Minimal E medium was used for the growth of WP2 and its derivatives (25). Concentrations (in micrograms/milliliter) of antimicrobial supplements to NA were as follows: ampicillin (sodium salt), 50 (for the selection of pKM101) or 100 (for other plasmids); chloramphenicol, 10; mercuric chloride, 10; kanamycin sulfate, 10; nalidixic acid (sodium salt), 10; rifampin, 100; streptomycin, 100; and tetracycline, 5. Sulfathiazole (80 μg/ml) and trimethoprim (1 μg/ml) were used in DST.
Conjugation procedures and growth experiments.
Late-exponential-phase cultures in NB were used for conjugation experiments conducted by the quantitative filter method described previously (48). Experiments were performed three times, and the average transfer frequency (transconjugants per donor cell) was calculated.
For growth experiments, WP2 and its plasmid-carrying derivatives were each grown overnight in 10 ml of NB, and then the cells from a 2-ml aliquot were harvested, washed twice in minimal E medium, and suspended in 200 ml of the same medium. The 500-ml culture flasks were incubated, with shaking, at 37°C, and the absorbance of the culture at 560 nm was measured each half hour until stationary phase was reached. The set of growth experiments were performed in parallel on three occasions, and the results were subjected to multiple regression analysis (46).
Methods using phages.
The virulent phage T7 (obtained from R. A. Skurray via H. Dean, Monash University, Melbourne, Australia) and the donor-specific phage PR4 (66) were propagated on bacteria [HB101 and PAO2637(RP1), respectively] grown in soft-agar overlays on NA (1). The efficiency of plating of T7 was determined by spot phage assay (66) except that flood lawns were prepared with diluted (ca. 5 × 107 cells/ml) exponential-phase cultures. Sensitivity (Dps+) or resistance (Dps−) of bacteria to PR4 was determined by the same method but using a single drop of phage (ca. 109 PFU).
DNA techniques.
Standard molecular cloning techniques were performed as described previously (57). The fipA-containing SmaI (14.8 kb)-AatII (15.6 kb) region of pKM101 was sequenced on both strands by using overlapping subclones prepared from pVS1127. The sequencing reactions were performed with T7 DNA polymerase, universal or reverse primers, and protocols supplied with the Autoread sequencing kit (Pharmacia). The DNA sequence was determined with a Pharmacia LKB A.L.F. DNA sequencer and was analyzed by using software provided by the Australian National Genome Information Service (University of Sydney).
PCR amplification was carried out with the primers listed in Table 1 and reaction mixtures that contained 10% (vol/vol) dimethyl sulfoxide. The mixtures, overlaid with sterile mineral oil, were placed in a thermal cycler for 36 cycles under the following conditions: 92°C, 3 min (first cycle only); 92°C, 1 min; 60°C, 1 min; 72°C, 2 min; and 72°C, 5 min (final cycle only). The PCR product obtained (2.3, 1.9, or 1.1 kb) was cloned into pBluescriptII SK+ to create pVS1141, pVS1140, or pVS1150, respectively (Table 1).
DNA of various IncN-group plasmids was isolated by cesium chloride-ethidium bromide density gradient centrifugation and digested with a combination of SmaI and AatII. The DNA fragments, separated by electrophoresis, were transferred to a Hybond(N+) nylon filter (Amersham) and hybridized at 60°C to a probe prepared from the 0.8-kb fipA-containing AatII-SmaI fragment from pVS1127. Labeling of the probe and detection of hybridization were performed with DIG-DNA Labeling and DIG-Luminescent Detection kits (Boehringer Mannheim), respectively.
Expression of FipA protein.
Detection and molecular weight determination of FipA and truncated FipA (FipAΔ) were performed by the T7 RNA polymerase-promoter system (67) as previously described (3). The bacteria (HB101 carrying pGP1-2 and either a vector or recombinant) were exposed to 10 μCi of [35S]methionine-cysteine mix (New England Nuclear). In some cases, the labeled cells were fractionated (57) first by treatment with lysozyme (1 mg/ml) to release periplasmic proteins and then by four cycles of freezing (1.5 min at −70°C) and thawing (1.5 min at 37°C) to release cytoplasmic proteins. The remaining cell debris, resuspended, constituted the crude membrane fraction. Cell samples or fractions were denatured in sample buffer (3% sodium dodecyl sulfate [SDS], 5% β-mercaptoethanol) at 100°C for 3 min (3) prior to electrophoresis on denaturing polyacrylamide gels (12%) containing 0.1% SDS. The reference proteins used were Sigma Dalton Mark VII-L molecular weight standards. After polyacrylamide gel electrophoresis (PAGE), proteins were stained in 0.25% Coomassie brilliant blue (Sigma)–50% (vol/vol) methanol–9% (vol/vol) acetic acid; after destaining, autoradiographs of the dried gels were prepared.
Nucleotide sequence accession number.
The sequence reported here has been entered in GenBank under accession no. U42978.
RESULTS
Cloning and characterization of fipA.
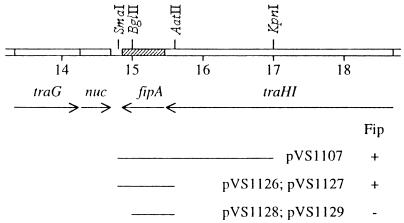
The fip region of pKM101 was localized between the endonuclease (nuc) and transfer (traHI) genes, in the vicinity of map position 15.0 kb (Fig. 1) (73, 76). To isolate the fip gene(s), parts of this region were cloned into pUC18 or pBluescriptII SK+, and the resulting plasmids were tested for the ability to inhibit the conjugal transfer of pUB307, a derivative of RP1. Plasmids pVS1107 [with the pKM101 SmaI (14.8 kb)-KpnI (17.0 kb) fragment] and the pair pVS1126 and pVS1127 [both with the SmaI (14.8 kb)-AatII (15.6 kb) fragment] were Fip+ and inhibited pUB307 even more strongly than did pKM101 (Table 2), probably reflecting the higher copy number of the vector replicons. Plasmids pVS1128 and pVS1129 [both with the BglII (15.0 kb)-AatII (15.6 kb) fragment] were Fip−. These data suggest that the fip gene(s) occurs in the pKM101 SmaI (14.8 kb)-AatII (15.6 kb) fragment and that the BglII (15.0 kb) site is necessary for its expression (Fig. 1).
FIG. 1.
Physical and genetic map of part of the transfer region of pKM101 from coordinates 13.2 to 18.8 kb. The pilus gene, traG (53, 75), and the mobilization gene, traHI (73, 75), are in separate gene clusters and are transcribed in a convergent manner (indicated by the arrows). The nuc (nuclease) gene is probably part of the pilus gene cluster (55), whereas fipA, the gene that is characterized in this work, is probably part of the mobilization gene cluster (see text). The lines below the map indicate clones of the transfer region that occur in the named pVS plasmids which are either able (+) or unable (−) to inhibit the conjugal transfer of a coresident IncP plasmid.
TABLE 2.
Effects of fipA and pifC on the conjugal transfer of IncPα and IncPβ plasmids
| Plasmid(s) in donora | Transfer frequency of IncP plasmidb |
|---|---|
| pUB307 | 4.0 × 10−1 |
| pUB307, pKM101c (fipA+) | 1.2 × 10−6 |
| pUB307, pVS1107 (fipA+) | <4.0 × 10−8 |
| pUB307, pVS1126 (fipA+) | <4.0 × 10−8 |
| pUB307, pVS1127 (fipA+) | <4.0 × 10−8 |
| pUB307, pVS1128 (fipA) | 5.5 × 10−1 |
| pUB307, pVS1129 (fipA) | 3.6 × 10−1 |
| R751 | 6.4 × 10−1 |
| R751, pVS1127 (fipA+) | <4.0 × 10−8 |
| R751, pVS1150 (pifC+) | 1.6 × 10−5 |
| R772 | 2.7 × 10−1 |
| R772, pVS1127 (fipA+) | 8.4 × 10−7 |
| R772, pVS1150 (pifC+) | 1.4 × 10−2 |
| pJP4 | 1.2 |
| pJP4, pVS1127 (fipA+) | 4.7 × 10−5 |
| pJP4, pVS1150 (pifC+) | 1.5 × 10−4 |
| pUB307, F′ lacc (pifC+) | 1.5 × 10−3 |
| pUB307, pVS1150 (pifC+) | 6.2 × 10−5 |
Plasmid pUB307 is an IncPα plasmid, whereas plasmids R751, R772, and pJP4 are IncPβ plasmids (64).
Number of transconjugants per donor cell after a 2-h mating of HB101 donors and LT101 (Rifr) as the recipient or, for experiments involving IncPβ plasmids, of UB5201 donors and UB1301 (Rifr) as the recipient. The transfer frequencies of pUB307, R751, R772, and pJP4 were determined on NA-rifampin-tetracycline, DST-rifampin-tetracycline, NA-rifampin-kanamycin, and NA-rifampin-mercuric chloride, respectively.
The transfer frequency of pKM101 (2.2 × 10−1 per donor) was determined on NA-rifampin-ampicillin. The transfer of F′lac was not determined.
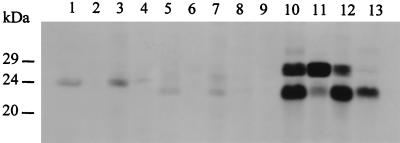
Proteins encoded by the SmaI-AatII fragment in pVS1126 and pVS1127 (both Fip+) and the BglII-AatII fragment in pVS1128 and pVS1129 (both Fip−) were expressed in vivo from the T7 φ10 promoter of the vector (67) and analyzed by SDS-PAGE (Fig. 2). A single protein was expressed from pVS1127 (24 kDa; lane 1) and pVS1129 (22 kDa; lane 5) but not from the corresponding plasmids with the insert in the opposite orientation (pVS1126 and pVS1128) (data not shown) or from the plasmid vector (lane 9). These findings suggest that the pKM101 SmaI (14.8 kb)-AatII (15.6 kb) fragment encodes a single intact gene, here named fipA, that is transcribed in an anticlockwise (AatII→SmaI) direction (Fig. 1), yielding a protein of 24 kDa. The BglII site near the 3′ end of fipA accounts for the expression of a truncated FipA from pVS1129. Cells expressing FipA and FipAΔ were also fractionated prior to SDS-PAGE, and the distribution of the proteins was compared with that of chloramphenicol acetyltransferase (Cat; a cytoplasmic protein [62]) and β-lactamase (Bla; a periplasmic protein [15]) expressed in a parallel experiment from cells carrying pVS915 (Fig. 2). Although the expression of the two FipA proteins was relatively poor (lanes 1 and 5), each occurred predominantly in the cytoplasmic fraction (lanes 3 and 7), as did Cat (lane 12).
FIG. 2.
Autoradiogram of radioactively labeled polypeptides separated on denaturing 12.5% polyacrylamide gels containing 0.1% (wt/vol) SDS. Locations of the protein standards, carbonic anhydrase (29 kDa), trypsiongen (24 kDa), and trypsin inhibitor (20 kDa), are shown. Lanes 1, 5, and 10 show whole-cell extracts of HB101(pGP1-2) carrying pVS1127 (FipA [24 kDa]), pVS1129 (FipAΔ [22 kDa]), and pVS915 (Bla [29 kDa] and Cat [23 kDa]), respectively. Corresponding periplasmic (lanes 2, 6, and 11), cytoplasmic (lanes 3, 7, and 12) and crude membrane (lanes 4, 8, and 13) fractions are also shown. Lane 9 shows whole-cell extract of HB101(pGP1-2) carrying the vector, pBluescriptII SK+.
The DNA sequence of the SmaI (14.8 kb)-AatII (15.6 kb) region of pKM101 was determined, defining fipA as an open reading frame (ORF) of 624 nucleotides (nt) commencing with an ATG start codon 82 nt from the AatII site and ending with a TGA stop codon 116 nt from the SmaI site. A putative ribosome-binding site (AGAAGGG) (63) occurs 12 nt upstream of the start codon, but no consensus −10 and −35 E. coli-like promoter sequences (45, 65) were detected in the upstream sequence. Hydropathy analysis (37) of the deduced 23.8-kDa FipA protein (208 amino acid residues) revealed a hydrophobic region at the amino terminus (residues 32 to 48) predicted by the ALOM program (32) to be a transmembrane domain. No probable signal sequence was detected. Despite these predictions that FipA may be membrane associated, the experimental findings are more suggestive that it is cytoplasmic. No significant similarities were detected between fipA and sequences in the GenBank/EMBL databases when the nucleotide or translated sequences were compared.
Conservation of fipA-like sequences and the effect of fipA on the growth of E. coli WP2.
Members of the IncN plasmid group share a range of phenotypic characteristics, only one of which is the conjugal inhibition of IncP plasmids (10, 26, 27, 74). When seven IncN plasmids from diverse sources and with different resistance profiles were tested, all inhibited the conjugal transfer of pUB307 (103- to 105-fold [data not shown]). These plasmids had distinguishable SmaI/AatII restriction profiles, but six (pCU1, pVS101, pVS144, pVS151, R199, and R309) carried a 0.8-kb SmaI-AatII fragment that hybridized with the corresponding fipA-carrying fragment from pKM101 (data not shown). The seventh plasmid (N3) lacked this fragment, but hybridization occurred with a fragment of >9 kb. These data suggest that fipA-like sequences are conserved in IncN plasmids.
The ability of fipA to retard the growth rate of E. coli WP2 was also tested, as such an effect has been associated with the pKM101 BalI (14.6 kb)-KpnI (17.0 kb) region (25), shown here to include fipA. The doubling time of WP2 (54 min) was not significantly affected by the carriage of pKM101 (52 min), pBluescriptII SK+ (59 min), or pVS1129 (Fip−) (60 min) but was increased by the carriage of pVS1127 (Fip+) (69 min; significant at P < 0.05). These data demonstrate that fipA can retard the growth of WP2, but in our hands, this occurred only when the gene was cloned (i.e., multicopy) and not, as observed by Hall (25), when present in the native pKM101.
Characteristics of fipA and pifC and the ability of these genes to inhibit RP1-mediated mobilization of nonconjugative plasmids.
The pifC gene of plasmid F, like fipA (76), inhibits the conjugal transfer of RP1 without affecting surface exclusion (Sfx+) or sensitivity to donor-specific phages (Dps+) (43, 68). Inhibition by pifC is, however, at least 100-fold weaker than that by fipA. This is the case whether the genes occur on the native plasmid (F′lac versus pKM101) or have been cloned (pVS1150 versus pVS1127) and whether the target is an IncPα plasmid (the RP1 derivative pUB307) or an IncPβ plasmid (e.g., R751) (Table 2).
The presence of fipA or pifC was also found to inhibit RP1-mediated mobilization of RSF1010, ColElk, and pSC101c (using the RP1 derivative pVS520 [Table 3]), a process that requires the MOB system of the nonconjugative plasmid together with the pilus genes (PILP) and traG of RP1 (18, 40). This finding implicates traG as the specific target of inhibition by fipA and pifC with the proviso that the Dps+ phenotype of the various donors (Table 3) reflects both the presence of P pili and their ability to function normally in conjugation. The possibility that fipA and pifC directly inhibit the MOB systems of the nonconjugative plasmids was eliminated in the case of RSF1010 by studying its mobilization by R388 (IncW). The transfer of this conjugative plasmid (ca. 1.2 per donor) was not affected by fipA (in pVS1136) or pifC (in pVS1137) nor was the mobilization of RSF1010, although this was inefficient (ca. 10−4), as has been observed previously (8). Evidence supporting traG as the inhibition target, and also demonstrating that pilus function is normal in the presence of fipA or pifC, was obtained in experiments using CloDF13a, a nonconjugative plasmid that encodes a traG homolog (9). In this case, the presence of fipA or pifC inhibited conjugal transfer of RP1 but had no effect on the ability of RP1 to mobilize CloDF13a (Table 3).
TABLE 3.
Effects of fipA and pifC on RP1-mediated mobilization of nonconjugative plasmids
| Plasmid(s) in donora together with pVS520 | Transfer frequencyb of IncP plasmid | Mobilization frequencyb of nonconjugative plasmid |
|---|---|---|
| RSF1010c | 6.0 × 10−1 | 5.6 × 10−1 |
| RSF1010, pVS1136 (fipA+) | <4.0 × 10−8 | <4.0 × 10−8 |
| RSF1010, pVS1137 (pifC+) | 2.2 × 10−3 | 1.8 × 10−3 |
| CloDF13a | 6.9 × 10−1 | 5.0 × 10−2 |
| CloDF13a, pVS1136 (fipA+) | <4.0 × 10−8 | 2.1 × 10−1 |
| CloDF13a, pVS1137 (pifC+) | 5.8 × 10−6 | 1.7 × 10−1 |
All donors were sensitive to phage PR4 (i.e., Dps+).
Number of transconjugants per donor after a 2-h mating of HB101 donors and LT101 (Rifr) as the recipient. The transfer of pVS520 and the mobilization of RSF1010 and CloDF13a were determined on NA-rifampin-tetracycline, DST-rifampin-sulfathiazole, and NA-rifampin-ampicillin, respectively.
Similar results were obtained when RSF1010 was replaced with either pSC101c or ColE1k (in the latter case, pVS1136 and pVS1137 were replaced with pVS1127 and pVS1150, respectively).
Development of a conjugal system to assay RP1 traG activity.
To further investigate the possibility that the RP1 traG gene is the target of inhibition by fipA and pifC, we developed an artificial conjugal assay of traG activity based on the finding that the weak mobilization of RSF1010 by IncN plasmids (9, 81) is greatly enhanced in the presence of the RP1 Tra1 genes (Table 4). In this assay, the donors carried three compatible plasmids: RSF1010t(MOBQ), a fipA mutant of pKM101(pVS793; MOBNPILN), and a clone of the entire RP1 Tra1 region (pVS659; MOBP traF+G+ oriT+). By substituting pVS659 with mutants of the Tra1 genes, the roles of the N and P transfer genes in the pKM101fipA-mediated mobilization of RSF1010t and pVS659 could be assessed.
The first observations related to pVS659 (Table 4), which was efficiently mobilized by pKM101fipA (2.5 × 10−3). This showed that the MOBP and PILN systems can function together in conjugation in the same way as has been found with MOBW and PILN (6). As expected, mutations in the MOBP genes of Tra1 (traI and traJ) or in traG abolished mobilization of the pVS659 mutant. This also confirmed that pKM101 genes cannot complement mutations in these Tra1 genes (18). In contrast, a mutation in the PILP gene, traF (70), had no effect on mobilization presumably because the need for this gene was obviated by the intact PILN system.
The second observation related to RSF1010t (Table 4), which was efficiently mobilized by pKM101fipA in the presence of pVS659 (1.1 × 10−2) but not in its absence (2.6 × 10−6). Such mobilization did not require traJ, traI, or traF, as RSF1010t encodes the requisite MOBQ system (14) and PILN, as described above, provides the contact system. The traG gene, however, was essential for mobilization, as is also the case when RSF1010 is mobilized by RP1 alone (40, 70). The significant feature of the conjugal assay was that efficient mobilization of RSF1010 by pKM101fipA occurred when traG was provided by pVS659 (1.1 × 10−2) but not when it was provided by RP1 (using pVS766) (Table 4). This finding suggested that the high copy number of pVS659, and hence hyperexpression of traG (and other Tra1 genes), was the basis for the unexpectedly efficient mobilization of RSF1010t by pKM101fipA. Such mobilization did not occur when pKM101fipA was replaced by pKM101 (fipA+) (Table 4, line 4), confirming that a Tra1 gene(s), namely, traG, is the target of fipA-mediated inhibition. Finally, a prediction from these findings was that hyperexpression of traG should also permit mobilization of an RP1pil mutant (pVS766). This was found to be the case but, as mentioned above, is an example not of complementation but of successful combination of the MOBP and PILN systems.
Hyperexpression of traG enhances pKM101-mediated mobilization of nonconjugative plasmids, but this effect is abolished in the presence of fipA or pifC.
To confirm that traG is the target of inhibition by fipA and pifC, traG was isolated alone and its effect was tested in the conjugal assay described above. This was done by using a 2.3-kb fragment containing traG and its promoter (PtraG), which was PCR amplified from pVS729 and then cloned into pBluescriptII SK+ (Table 1). The transformants recovered all carried the insert in the antisense orientation with respect to the Plac of the vector. One such derivative, pVS1141, when used in the conjugal assay promoted efficient mobilization of RSF1010t by pKM101fipA (5.1 × 10−2 [Table 5]). This showed that traG is the sole RP1 gene required for such mobilization. When fipA was also present in the donor, mobilization of RSF1010t was reduced to the control level (5.0 × 10−6). This confirmed that traG is the target of inhibition by fipA.
TABLE 5.
Involvement of the RP1 traG gene in IncN-mediated mobilization of RSF1010
| Plasmid(s) in donor together with RSF1010tb and pKM101fipAa | Mobilization frequency of RSF1010ta |
|---|---|
| None | 5.4 × 10−6 |
| pVS1141 (PtraG traG+) | 5.1 × 10−2 |
| pVS1141 (PtraG traG+), pVS1136 (fipA+) | 5.0 × 10−6 |
| pVS1141 (PtraG traG+), pVS1137 (pifC+) | 1.1 × 10−2 |
| pVS1142 (Plac traG+) | 7.4 × 10−3 |
| pVS1142 (Plac traG+), pVS1136 (fipA+) | 2.2 × 10−6 |
| pVS1142 (Plac traG+), pVS1137 (pifC+) | 6.4 × 10−6 |
| pVS1140 (traG+) | 4.3 × 10−5 |
| pVS1140 (traG+), pVS1136 (fipA+) | 4.8 × 10−6 |
| pVS1140 (traG+), pVS1137 (pifC+) | 2.6 × 10−6 |
Number of transconjugants per donor cell after a 2-h mating of HB101 donors and LT101 (Rifr) as the recipient. The transfer of pKM101fipA and mobilization of RSF1010t were determined on NA-rifampin-chloramphenicol and NA-rifampin-tetracycline, respectively. The transfer frequency of pKM101fipA from all donors was ca. 5.5 × 10−1 per donor.
Similar results were obtained when RSF1010t was replaced with pSC101c (data not shown), and the transfer of pKM101fipA and mobilization of pSC101c were determined on NA-rifampin-ampicillin and NA-rifampin-tetracycline, respectively.
In the corresponding experiment in which the donor carried pifC instead of fipA, mobilization of RSF1010t was reduced only about fivefold to 1.1 × 10−2 (Table 5). This suggested that if traG is also the target of inhibition by pifC, then the effect of pifC is almost negated by traG hyperexpression in pVS1141, a finding that accords with the weaker inhibition by pifC than fipA (Tables 2 and 3). Implicit in this explanation is the notion that pifC-mediated inhibition of traG may be a posttranscriptonal event. Data consistent with this view were obtained when plasmids with reduced traG expression were used. These plasmids carried a promoterless traG gene cloned in the sense (pVS1142) or antisense (pVS1140) orientation relative to the Plac of the vector (Table 1). Compared to pVS1141, the reduced expression of traG was clearly reflected in the intermediate level of RSF1010t mobilization effected by pVS1142 (7.4 × 10−3) and the very poor level of mobilization effected by pVS1140 (4.3 × 10−5). When the two comparable donors also carried pifC, the inhibitory effects of this gene were now discernible by the reduction in RSF1010t mobilization to the control level. This confirmed that traG is the target of inhibition by pifC. The behavior of fipA was different from that of pifC in that it abolished RSF1010t mobilization in combinations with all three traG clones. Whether this reflects differences in the mechanism of action of these genes is not known, but it seems likely that inhibition by pifC is exerted at the protein level.
Levels of transfer similar to those shown in Table 5 were also obtained in corresponding experiments in which pSC101c replaced RSF1010t (data not shown). This was not surprising, as the mobilization of these plasmids is dependent on traG (4, 18, 40).
Use of F′lac Pif activity to study pifC-mediated inhibition of traG.
The findings described above show that traG is the target of inhibition by fipA and pifC, also raising the possibility that pifC may mediate its effect at the protein level. To investigate this further, we took advantage of the role of PifC to negatively regulate the F pif operon, the expression of which inhibits the propagation of phage T7 (denoted Pif+) (31, 56). Miller et al. (43) showed that F′lac Pif activity increased in cells that also carried RP4 (=RP1), reflecting the removal of PifC with consequent reduced plating and altered plaque morphology (denoted Pif++). The Pif phenotype thus provides an in vivo assay of the levels of PifC, and we used this phenotype to further study pifC-mediated inhibition of traG.
The results obtained (Table 6) showed that Pif activity of F′lac (Pif+) was indeed enhanced by the presence of the RP1 derivative pVS520 (Pif++). We have also shown that an equivalent effect was produced if the only RP1 gene present was traG, with (pVS1141) or without (pVS1140) its native promoter. This suggested that traG may be the sole RP1 gene required to enhance F′lac Pif activity. Significantly, enhanced Pif activity did not occur if traG was disrupted at different sites by a Tn5 insertion mutation (e.g., pVS580) or if only parts of the traG gene were present (e.g., pVS1161). The fact that the coding sequence per se was important and that this must be present intact implied that the removal of PifC is more likely to occur by an interaction with the TraG protein than by the binding of PifC to traG DNA. This conclusion is contrary to that drawn by Miller et al. (43), who favored PifC binding to RP4 DNA. Indeed, a possible PifC-binding sequence has been identified by computer analysis within the overlapping traI and traH genes of RP1 (50). When a plasmid which carries this sequence but not traG (i.e., pVS658 [Table 6]) was tested, it failed to enhance F′lac Pif activity. These combined data suggest that the conjugal inhibition of RP1 by pifC probably arises by a PifC-TraG protein interaction and that the same interaction may be responsible for the enhanced Pif activity of cells carrying both F′lac and RP1.
DISCUSSION
This study concerned the mode of action of two genes that can inhibit the conjugal transfer of the P-group plasmid RP1. One gene, fipA [from pKM101(IncN)], was isolated and characterized in this work. The other, pifC [from plasmid F(IncFI)], has been the subject of considerable study, including two reports concerning transfer inhibition (43, 68). We confirmed that the sole inhibitory effect of pKM101 and F was to depress transfer of RP1 (68, 76) and showed, in parallel experiments, that inhibition by pifC is weaker than by fipA even when their effects are enhanced by cloning (Table 2). These studies also showed that fipA is the sole gene in the pKM101 fip region (76) required for inhibition. The fipA gene, of 624 nt (pifC is 1,086 nt [11]), occurs in the pKM101 SmaI (14.8 kb)-AatII (15.6 kb) fragment and is transcribed anticlockwise, yielding a protein of 24 kDa (predicted mass, 23.8 kDa) (Fig. 2). Deletion of the SmaI (14.8 kb)-BglII (15.0 kb) portion resulted both in truncation of the protein (to 22 kDa) and abolition of its inhibition effect, showing that the 23 C-terminal amino acid residues are essential for FipA activity. Removal of the two C-terminal residues from the PifC protein (40 kDa [12]) significantly reduced its inhibition effect (from 4,000- to 70-fold) (43).
The FipA protein was detected predominantly in the cytoplasmic cell fraction but also occurred in the crude membrane fraction, a distribution like that of Cat (Fig. 2). On the basis of this comparison, we concluded that FipA, like Cat (62) (and also PifC, which is a repressor [31]), is a cytoplasmic protein. Additional confirmation of this is needed, as a possible transmembrane domain occurs in the FipA N terminus (residues 32 to 48) suggestive of membrane association. FipA was also expressed poorly compared to Cat and Bla (Fig. 2), and this occurred when other vectors carrying the T7 φ10 promoter were used (60). As the difference is not due to a paucity of Met or Cys residues (i.e., poor labeling of FipA), it suggests that efficient inhibition of RP1 (ca. 105-fold [Table 2]) is elicited even when fipA expression is at its lowest, in pKM101.
The fip region mapped in pKM101 (76) occurs between two transfer regions, one encoding surface exclusion (54) and pilus genes (53) and the other with oriT and four other tra genes (75). We found that fipA is transcribed anticlockwise in pKM101 but detected no associated promoter, suggesting that fipA may be part of an operon. This possibility seems likely from an inspection of the upstream DNA sequence which encodes the traHI ORF (73). This ORF is read in the same direction as fipA, and its stop codon partially overlaps the fipA start codon, indicating translational coupling of gene expression (Fig. 1). If this overlap is correct, then the pKM101Ω1246::Tn9 mutation (Tra+ Fip+) which demarked traH and fipA (75, 76) probably occurs within traHI. This indicates that neither the loss of C-terminal residues from TraHI nor the reduction in FipA levels expected from polarity is sufficient to alter the phenotypic effects of these proteins. The possibility that fipA may be part of a tra operon is also interesting in light of the association between nuc and traG (53, 55). These two operons are transcribed convergently with nuc and fipA (which are adjacent) as the last genes (Fig. 1). The linkage to tra genes may account for our finding that fipA-like sequences are common in IncN plasmids even though fipA is not required for pKM101 transfer (76). Instead, fipA can affect the growth of E. coli WP2 (25) but, in our hands, only when hyperexpressed. The significance of this finding is not known. The nuc gene, which encodes an EDTA-resistant nuclease (55), may also be conserved, as all IncN plasmids tested by Winans and Walker (74) elaborated such an activity.
The pifC gene, like fipA, does not play a role in the conjugal transfer of its native plasmid but, rather, is involved in its replication (69). The two genes have similar phenotypic effects, namely, inhibition of RP1 transfer but not of other conjugal functions (Sfx or Dps [68, 76]). From the known distribution and roles of the RP1 tra genes (50), this phenotype suggested interference with one or more of the Tra1 components, i.e., the mob genes (traIJK), oriT, or traG. Given these possible targets and the nonidentity of fipA and pifC, it was surprising to find that inhibition by both genes was directed to traG. This was suggested first (Table 3) by the ability of fipA and pifC to block RP1-mediated mobilization of nonconjugative plasmids reliant on traG (RSF1010, ColE1, and pSC101 [18, 40, 70]) but not of CloDF13, which encodes a traG homolog (9, 38). The demonstration that traG is indeed the inhibition target was obtained in an artificial system in which the N-conjugal functions were supplemented with traG (discussed further below). The mobilization of RSF1010 (or pSC101) from such donors could be blocked in the presence of fipA or pifC, confirming traG as the inhibition target (Table 5).
Significantly, fipA and pifC differed in that RSF1010 mobilization was totally blocked by fipA (a strong inhibition gene), but this only occurred with pifC (a weaker inhibition gene) when traG expression was low (Table 5). The fact that inhibition by pifC could be negated by high traG expression suggested titration of PifC, by binding to either traG DNA, its transcript, or TraG protein. Interaction with the DNA seemed likely as PifC is a DNA-binding protein (11) that negatively regulates the F pif operon (31). Indeed, this role of PifC permits its removal to be gauged by the enhanced inhibition of phage T7 (the Pif phenotype) (43) (see above). In our study, Pif activity increased in donors carrying F′lac and RP1 (or cloned traG) but only if the traG coding sequence was intact (Table 6). If the sequence was interrupted by various insertion mutations or only parts of traG or PtraG were present, Pif activity was normal. Hence, it is unlikely that PifC binds to traG DNA or mRNA unless, in the latter case, the 5′ terminus is involved. We conclude instead that PifC interacts with the TraG protein, causing enhanced Pif activity and inhibition of TraG-dependent RP1 conjugal functions. The mechanism of fipA-mediated inhibition of traG cannot be determined from this study, but it is unlikely to affect transcription. FipA has no detectable DNA-binding motif, and despite strong inhibition, there is no polar effect on the traF pilus gene downstream of traG (i.e., RP1 donors carrying fipA are Dps+ [Table 3]). The ability of fipA and pifC to inhibit transfer of IncPβ plasmids (Table 1) was not surprising, as the Tra1 regions of RP1 and R751, and particularly the traG gene sequences, are highly conserved (78). This may account for the similarity of the RP1 and R751 responses compared to those of R772 and pJP4, in which sequence divergence may be greater (Table 1).
The artificial conjugation system used in this study also provided information on the interplay of P, N, and other conjugal components. The PILN system (in pKM101fipA) can mobilize a MOBP plasmid (pVS659) (Table 4) under conditions where P-relaxosome formation is normal (requiring traI and traJ) but traG is hyperexpressed (using a cloned MOBP). The dependence on hyperexpression explains why the PILN system could not mobilize an RP1pil mutant (pVS766) (Table 4). In analogous experiments, the PILN (but not PILP) system has also been shown to mobilize a MOBW plasmid (6), and this too is probably due to hyperexpression of TrwB, a TraG homolog. In both cases, the homolog presumably interacted efficiently with its relaxosome, but not with the PILN DNA transport complex unless at elevated levels. It may be that the TraG homologs, in addition to a delivery/coupling role (9, 50), may also make a contribution to nucleic acid or pilin transport in the transport complex. This may be the reason why mutations affecting TraG and F TraD can alter Dps properties (18) or pilus number (2) even though these proteins, unlike the Ti VirD4 homolog (20), are dispensable for pilus synthesis (19, 24).
The PILN system with hyperexpressed traG also mobilized RSF1010 (IncQ) and pSC101 (Table 5). Thus, TraG can interact with different relaxosomes and efficiently couple these to the normal PILP or TraG-supplemented PILN systems. PILW with hyperexpressed traG can also mobilize RSF1010 (and ColE1), but the efficiencies are lower (ca. 10−3/donor), comparable to those obtained with R388 itself (using trwB function) (8). The limiting step in this case seems to be poor association of the TraG–Q-relaxosome with the PILW transport complex. Taken together, the data suggest that TraG and the equivalent N-system homolog, traJ (70a), are more similar to each other than to TrwB. Such similarities are likely to be regional, as studies of the functional relationships (9) and predicted topology of TraG-like proteins (9, 19, 47) suggest that interactions with relaxosomes and transport complexes may involve different domains. This would be consistent with the finding that some RP1pil mutants continue to mobilize nonconjugative plasmids, albeit at reduced levels (18).
We expect that the demonstration of genes that specifically inhibit traG, one targeting the TraG protein, will provide an additional tool for investigating the complex role of TraG and its various homologs.
ACKNOWLEDGMENTS
We thank P. R. Fisher for assistance with statistical and DNA sequence analysis and the following colleagues who generously provided strains or plasmids: F. de la Cruz, H. Dean, S. T. Fong, R. M. Hall, V. Iyer, M. E. Kovach, A. J. Pittard, and R. A. Skurray.
Financial support for this work was provided by the Australian Research Council. J.M.S. was the recipient of an Australian Postgraduate Research Award.
REFERENCES
- 1.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers, Inc.; 1959. [Google Scholar]
- 2.Armstrong G D, Frost L S, Sastry P A, Paranchych W. Comparative biochemical studies on F and EDP208 conjugative pili. J Bacteriol. 1980;141:333–341. doi: 10.1128/jb.141.1.333-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-International; 1987. [Google Scholar]
- 4.Balzer D, Pansegrau W, Lanka E. Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4. J Bacteriol. 1994;176:4285–4295. doi: 10.1128/jb.176.14.4285-4295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett P M, Grinsted J, Richmond M H. Transposition of TnA does not generate deletions. Mol Gen Genet. 1977;154:205–211. doi: 10.1007/BF00330839. [DOI] [PubMed] [Google Scholar]
- 6.Bolland S, Llosa M, Avila P, de la Cruz F. General organization of the conjugal transfer genes of the IncW plasmid R388 and interactions between R388 and IncN and IncP plasmids. J Bacteriol. 1990;172:5795–5802. doi: 10.1128/jb.172.10.5795-5802.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 8.Cabezón E, Lanka E, de la Cruz F. Requirements for mobilization of plasmids RSF1010 and ColE1 by the IncW plasmid R388: trwB and RP4 traG are interchangeable. J Bacteriol. 1994;176:4455–4458. doi: 10.1128/jb.176.14.4455-4458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabezón E, Sastre J S, de la Cruz F. Genetic evidence of a coupling role for the TraG protein-family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- 10.Caudry S D, Stanisich V A. Incidence of antibiotic-resistant Escherichia coli associated with frozen chicken carcasses and characterization of conjugative R plasmids derived from such strains. Antimicrob Agents Chemother. 1979;16:701–709. doi: 10.1128/aac.16.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey P A, de Feyter R, Lane H E D. The miniF plasmid C protein sequence, purification and DNA binding. Nucleic Acids Res. 1986;14:9699–9712. doi: 10.1093/nar/14.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cram D, Ray A, Skurray R. Molecular analysis of F plasmid pif region specifying abortive infection of T7 phage. Mol Gen Genet. 1984;197:137–142. doi: 10.1007/BF00327934. [DOI] [PubMed] [Google Scholar]
- 12a.Datta N, Hedges R W. Trimethoprim resistance conferred by W plasmids in Enterobacteriaceae. J Gen Microbiol. 1972;72:349–355. doi: 10.1099/00221287-72-2-349. [DOI] [PubMed] [Google Scholar]
- 13.Daugelavičius R, Bamford J K H, Grahn A M, Lanka E, Bamford D H. The IncP plasmid-encoded cell envelope-associated DNA transfer complex increases cell permeability. J Bacteriol. 1997;179:5195–5202. doi: 10.1128/jb.179.16.5195-5202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derbyshire K M, Willetts N. Mobilization of the non-conjugative plasmid RSF1010: a genetic and DNA sequence analysis of the mobilization region. Mol Gen Genet. 1987;206:154–160. doi: 10.1007/BF00326552. [DOI] [PubMed] [Google Scholar]
- 15.Dougan G, Saul M, Twigg A, Gill R, Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979;138:48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firth N, Ippen-Ihler K, Skurray R A. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 2377–2401. [Google Scholar]
- 17.Fong S T. Genetic and molecular analysis of the Tra1 and FiwA regions of plasmid RP1. Ph.D. thesis. Bundoora, Victoria, Australia: La Trobe University; 1992. [Google Scholar]
- 18.Fong S T, Stanisich V A. Identification and characterization of RP1 Tra1 cistrons involved in pilus function and plasmid mobilization. J Bacteriol. 1993;175:448–456. doi: 10.1128/jb.175.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost L S, Ippen-Ihler K, Skurray R S. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullner J K, Lara J C, Nester E W. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 21.Grinsted J, Saunders J R, Ingram L C, Sykes R B, Richmond M H. Properties of an R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972;110:529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerry P, Van Embden J, Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974;117:619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiney D G. Broad host range conjugative and mobilizable plasmids in Gram-negative bacteria. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Publishing Corp.; 1993. pp. 75–104. [Google Scholar]
- 24.Haase J, Lurz R, Grahn A M, Bamford D H, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall R M. Identification and mapping of regions of the plasmid pKM101 which influence the growth rate and resistance to phleomycin E of Escherichia coli WP2. J Bacteriol. 1985;163:1142–1146. doi: 10.1128/jb.163.3.1142-1146.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer V N. IncN group plasmids and their genetic systems. In: Thomas C M, editor. Promiscuous plasmids of Gram-negative bacteria. London, England: Academic Press; 1989. pp. 165–183. [Google Scholar]
- 27.Jacob A E, Shapiro J A, Yamamoto L, Smith D L, Cohen S N, Berg D. Plasmids studied in Escherichia coli and other enteric bacteria. In: Bukhari A I, Shapiro J A, Adhya S L, editors. DNA insertion elements, plasmids and episomes. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1977. pp. 607–638. [Google Scholar]
- 28.Jagura-Burdzy G, Khanim F, Smith C A, Thomas C M. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20:3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagura-Burdzy G, Thomas C M. Dissection of the switch between genes for replication and transfer of promiscuous plasmid RK2: basis of the dominance of trfAP over trbAP and specificity for KorA in controlling the switch. J Mol Biol. 1997;265:507–518. doi: 10.1006/jmbi.1996.0747. [DOI] [PubMed] [Google Scholar]
- 30.Kado C I. Promiscuous DNA transfer system of Agrobacterium tumefaciens: role of the virB operon in sex pilus assembly and synthesis. Mol Microbiol. 1994;12:17–22. doi: 10.1111/j.1365-2958.1994.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy M, Chandler M, Lane D. Mapping and regulation of the pifC promoter of the F plasmid. Biochim Biophys Acta. 1988;950:75–80. doi: 10.1016/0167-4781(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 32.Klein P, Kanehisha M, DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985;815:468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- 33.Kline B C. Aspects of plasmid F maintenance in Escherichia coli. Can J Microbiol. 1988;34:526–535. doi: 10.1139/m88-090. [DOI] [PubMed] [Google Scholar]
- 34.Konarska-Kozlowska M, Iyer V N. Sequence homology between IncN group plasmids. Plasmid. 1983;10:211–223. doi: 10.1016/0147-619x(83)90035-5. [DOI] [PubMed] [Google Scholar]
- 35.Kotilainen M M, Grahn A M, Bamford J K H, Bamford D H. Binding of an Escherichia coli double-stranded DNA virus PRD1 to a receptor coded by an IncP-type plasmid. J Bacteriol. 1993;175:3089–3095. doi: 10.1128/jb.175.10.3089-3095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad host range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 37.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 38.Lanka E, Wilkins B M. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- 39.Lessl M, Balzer D, Lurz R, Waters V L, Guiney D G, Lanka E. Dissection of IncP conjugative plasmid transfer: definition of the transfer region Tra2 by mobilization of the Tra1 region in trans. J Bacteriol. 1992;174:2493–2500. doi: 10.1128/jb.174.8.2493-2500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lessl M, Balzer D, Weyrauch K, Lanka E. The mating pair formation system of plasmid RP4 defined by RSF1010 mobilization and donor-specific phage propagation. J Bacteriol. 1993;175:6415–6425. doi: 10.1128/jb.175.20.6415-6425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lessl M, Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 42.Llosa M, Grandoso G, de la Cruz F. Nicking activity of TrwC directed against the origin of transfer of the IncW plasmid R388. J Mol Biol. 1995;246:54–62. doi: 10.1006/jmbi.1994.0065. [DOI] [PubMed] [Google Scholar]
- 43.Miller J F, Lanka E, Malamy M H. F factor inhibition of conjugal transfer of broad-host-range plasmid RP4: requirement for the protein product of Pif operon regulatory gene pifC. J Bacteriol. 1985;163:1067–1073. doi: 10.1128/jb.163.3.1067-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller J F, Malamy M H. Mutational and in vivo methylation analysis of F-factor PifC protein binding to the pif operator and the region containing the primary origin of mini-F replication. Proc Natl Acad Sci USA. 1986;83:1433–1437. doi: 10.1073/pnas.83.5.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulligan M E, Hawley D K, Entriken R, McClure W R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984;12:789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neter J, Wasserman W. Applied linear statistical models: regression, analysis of variance and experimental designs. R. D. Homewood, Ill: Irwin, Inc.; 1974. [Google Scholar]
- 47.Okamoto S, Toyoda-Yamamoto A, Ito K, Takebe I, Machida Y. Localization and orientation of the VirD4 protein of Agrobacterium tumefaciens in the cell membrane. Mol Gen Genet. 1991;228:24–32. doi: 10.1007/BF00282443. [DOI] [PubMed] [Google Scholar]
- 48.Palombo E A, Yusoff K, Stanisich V A, Krishnapillai V, Willetts N S. Cloning and genetic analysis of tra cistrons of the Tra2/Tra3 region of RP1. Plasmid. 1989;22:59–69. doi: 10.1016/0147-619x(89)90036-x. [DOI] [PubMed] [Google Scholar]
- 49.Pansegrau W, Balzer D, Kruft V, Lurz R, Lanka E. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc Natl Acad Sci USA. 1990;87:6555–6559. doi: 10.1073/pnas.87.17.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPα plasmids: compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 51.Pansegrau W, Schröder W, Lanka E. Relaxase (TraI) of IncPα plasmid RP4 catalyzes a site- and strand-specific cleaving-joining reaction of single-stranded DNA. Proc Natl Acad Sci USA. 1993;90:2925–2929. doi: 10.1073/pnas.90.7.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson E S, Iyer V N. The oriT region of the conjugative transfer system of plasmid pCU1 and specificity between it and the mob region of other N tra plasmids. J Bacteriol. 1992;174:499–507. doi: 10.1128/jb.174.2.499-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pohlman R F, Genetti H D, Winans S C. Common ancestry between IncN conjugal transfer genes and macromolecular export systems of plant and animal pathogens. Mol Microbiol. 1994;14:655–668. doi: 10.1111/j.1365-2958.1994.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 54.Pohlman R F, Genetti H D, Winans S C. Entry exclusion of the IncN plasmid pKM101 is mediated by a small hydrophilic protein containing a lipid attachment motif. Plasmid. 1994;31:158–165. doi: 10.1006/plas.1994.1017. [DOI] [PubMed] [Google Scholar]
- 55.Pohlman R F, Liu F, Wang L, Moré M I, Winans S C. Genetic and biochemical analysis of an endonuclease encoded by the IncN plasmid pKM101. Nucleic Acids Res. 1993;21:4867–4872. doi: 10.1093/nar/21.21.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotman G S, Cooney R, Malamy M H. Cloning of the pif region of the F sex factor and identification of a pif protein product. J Bacteriol. 1983;155:254–264. doi: 10.1128/jb.155.1.254-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 58.Sanchez J, Bennett P M, Richmond M H. Expression of eltB, the gene encoding the β subunit of the heat-labile enterotoxin of Escherichia coli, when cloned in pACYC184. FEMS Microbiol Lett. 1982;14:1–5. [Google Scholar]
- 59.Santini J M, Stanisich V A. Characterization of plasmid-borne genes that inhibit the conjugal transfer of IncP plasmids. Plasmid. 1997;37:227–228. [Google Scholar]
- 60.Santini, J. M., and V. A. Stanisich. Unpublished data.
- 61.Scherzinger E, Lurz L, Otto S, Dobrinski B. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 1992;20:41–48. doi: 10.1093/nar/20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw W V. Chloramphenicol acetyltransferase from chloramphenicol resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 63.Shine J, Dalgarno L. The 3′-terminal sequence of E. coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1984;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith C A, Thomas C M. Relationships and evolution of IncP plasmids. In: Thomas C M, editor. Promiscuous plasmids of Gram-negative bacteria. London, England: Academic Press; 1989. pp. 57–77. [Google Scholar]
- 65.Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984;12:505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanisich V A. The properties and host range of male-specific bacteriophages of Pseudomonas aeruginosa. J Gen Microbiol. 1974;84:332–342. doi: 10.1099/00221287-84-2-332. [DOI] [PubMed] [Google Scholar]
- 67.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for the controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanimoto K, Iino T. Transfer inhibition of RP4 by F factor. Mol Gen Genet. 1983;192:104–109. doi: 10.1007/BF00327654. [DOI] [PubMed] [Google Scholar]
- 69.Tanimoto K, Iino T. An essential gene for replication of the mini-F plasmid from origin I. Mol Gen Genet. 1984;196:59–63. doi: 10.1007/BF00334092. [DOI] [PubMed] [Google Scholar]
- 70.Waters V L, Strack B, Pansegrau W, Lanka E, Guiney D G. Mutational analysis of essential IncPα plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992;174:6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkins B M, Lanka E. DNA processing and replication during plasmid transfer between Gram-negative bacteria. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Publishing Corp.; 1993. pp. 105–136. [Google Scholar]
- 72.Willetts N S, Crowther C C. Mobilization of the non-conjugative IncQ plasmid RSF1010. Genet Res. 1981;37:311–316. doi: 10.1017/s0016672300020310. [DOI] [PubMed] [Google Scholar]
- 73.Winans, S. C. Unpublished data (GenBank nucleotide accession no. U43676).
- 74.Winans S C, Walker G C. Genetic localization and characterization of a pKM101-coded endonuclease. J Bacteriol. 1983;154:1117–1125. doi: 10.1128/jb.154.3.1117-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winans S C, Walker G C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985;161:402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winans S C, Walker G C. Fertility inhibition of RP1 by IncN plasmid pKM101. J Bacteriol. 1985;161:425–427. doi: 10.1128/jb.161.1.425-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76a.Woodgate, R. Unpublished data (GenBank nucleotide accession no. AF000361).
- 77.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 78.Ziegelin G, Pansegrau W, Strack B, Balzer D, Kröger M, Kruft V, Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1:303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]