Abstract
For patients with abdominal aortic aneurysm, randomized trials have found endovascular AAA repair (EVAR) is associated with lower perioperative morbidity and mortality than open surgical repair (OSR). However, OSR has fewer long-term aneurysm-related complications such as endoleak or late rupture. Patients treated with EVAR and OSR have similar survival rates within two years following surgery, and OSR does not require intensive surveillance. Few have examined if patient preferences are aligned with the type of treatment they receive for their AAA. While many assume that patients may universally prefer the less invasive nature of EVAR, our preliminary work suggests that patients who value the lower risk of late complications may prefer OSR.
In this study, called The PReferences for Open Versus Endovascular Repair of Abdominal Aortic Aneurysm (PROVE-AAA) trial, we describe a cluster-randomized trial to test if a decision aid can better align patients’ preferences and their treatment type for AAA. Patients enrolled in the study are candidates for either endovascular or open repair, and are followed at VA hospitals by vascular surgery teams who regularly perform both types of repair. In Aim 1, we will determine patients’ preferences for endovascular or open repair and identify domains associated with each repair type. In Aim 2, we will assess alignment between patients’ preferences and the repair type elected, and then compare the impact of a decision aid on this alignment between the intervention and control groups. This study will help us to accomplish two goals. First, we will better understand the factors that affect patient preference when choosing between EVAR and OSR. Second, we will better understand if a decision aid can help patients be more likely to receive the treatment strategy they prefer for their AAA. Study enrollment began on June 1, 2017. Between June 1, 2017 and November 1, 2018, we have enrolled 178 of a total goal of 240 Veterans from 20 VA Medical Centers and their vascular surgery teams across the country. We anticipate completing enrollment in PROVE-AAA in June, 2019, and study analyses will be performed thereafter.
Introduction
For patients with abdominal aortic aneurysm (AAA), endovascular repair (EVAR) and open surgical repair (OSR) have been compared extensively in a variety of settings, including in large randomized trials1–4. In these studies, the less invasive nature of EVAR repeatedly demonstrated several advantages over OSR in terms of short-term morbidity and mortality5. However, EVARs benefits came with tradeoffs. Mandatory surveillance imaging is required for the patients’ lifetime, and endoleaks requiring re-intervention occur at a rate of nearly 20% at four years6–8. These reinterventions often are endoleaks which, in many cases, can be treated using endovascular means.. Finally, late rupture is more common in patients treated with EVAR, and some randomized trials show a detriment in survival with patients over the long term for patients treated with EVAR 6–8.
These tradeoffs have made it difficult to find a clear “winner” between EVAR and OSR. Randomized trials gave surgeons and patients important information about the short and long-term outcomes of each approach, but have failed to identify a single AAA repair type that would be best for all patients. This is a setting wherein shared decision-making and patient decision aids may hold promise. It is our first hypothesis that patients, if cogent in their decision-making about the risks and benefits both EVAR and OSR, would choose either EVAR or OSR for specific, measurable reasons. For example, patients may choose open repair if they prioritized durability or if they wished to avoid the need for long-term surveillance. Similarly, patients may choose EVAR if they prioritized a brief recovery. Our second hypothesis is that patients who are aware of these tradeoffs – the “informed consumer” – may be more likely to receive the type of repair which aligns with their preferences.
In this report, we describe a 20-site, cluster randomized trial funded by VA Health Services Research and Delivery (VA HSR&D) designed to test this hypothesis. This study has two aims. First, we will use validated survey instruments to determine repair type preferences between EVAR versus OSR and identify domains in our survey associated with each repair type. Second, we will determine the effect of a validated decision aid on the agreement between patient preference for AAA repair type and the repair type the patient ultimately receives.
Design of the Intervention
Poor alignment between treatment preferences can result in poor patient satisfaction and outcomes, especially in surgical decisions9,10. For example, patients treated with open repair may have a longer hospital stay, more time lost from employment, greater rates of depression, and more social isolation because of the longer recovery time4,11–13. Similarly, for patients treated with endovascular repair, the need for continued surveillance with radiation-based CT scans, worries about complications, and the need for family support can have deleterious effects as well2,3,14.
Shared decision-making is an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options to achieve informed preferences15,16. Poor decision satisfaction and limited shared decision-making are likely to result when treatment decisions are made without considering patient preferences17,18. We hypothesize that overestimating or underestimating risk may result in poor alignment between Veterans’ preferences and the repair type they receive for treatment of their AAA.
Our study aims to accomplish two integrated goals. First, we hope to perform qualitative studies to better delineate the factors that determine preferences for AAA repair type. Second, we aim to perform a quantitative assessment of how commonly preferences agree with AAA treatments. These two goals integrate a theoretical framework for shared decision making19–21 shown in our conceptual model (Figure 1). In this model, the first step is to introduce the decision, using a validated AAA decision aid and survey that will describe the risks and choices with AAA. The decision aid and survey have been validated in prior studies in the National Health System22, and have been adapted and tested for use in VA hospitals (Appendix). We added two elements to this instrument: a simple pre-survey to determine the Veteran’s initial preference (if he or she has one) before the study begins, and a validated instrument which measures the surgeon’s preference for repair type as well as the influence of the Veteran’s preference on the surgeon’s preference. We tested our version in several cognitive interviews and piloted these instruments with surgeon Site Investigators in our study (Appendix). The second step is to describe the options for AAA repair. We accomplish this in our proposal using our survey instrument during the context of the patient visit. The third step involves helping the patient reach his or her decision for a preferred strategy for AAA repair. We will measure the agreement between Veterans’ treatment preferences and their actual AAA repair type, as well as decision satisfaction and shared decision making. The effect of open and endovascular repair on quality of life will vary over time. While ideally, we would measure these effects over time for many years, early follow-up is our focus in this study given the increasing costs of evaluating this measure over time.
Figure 1:
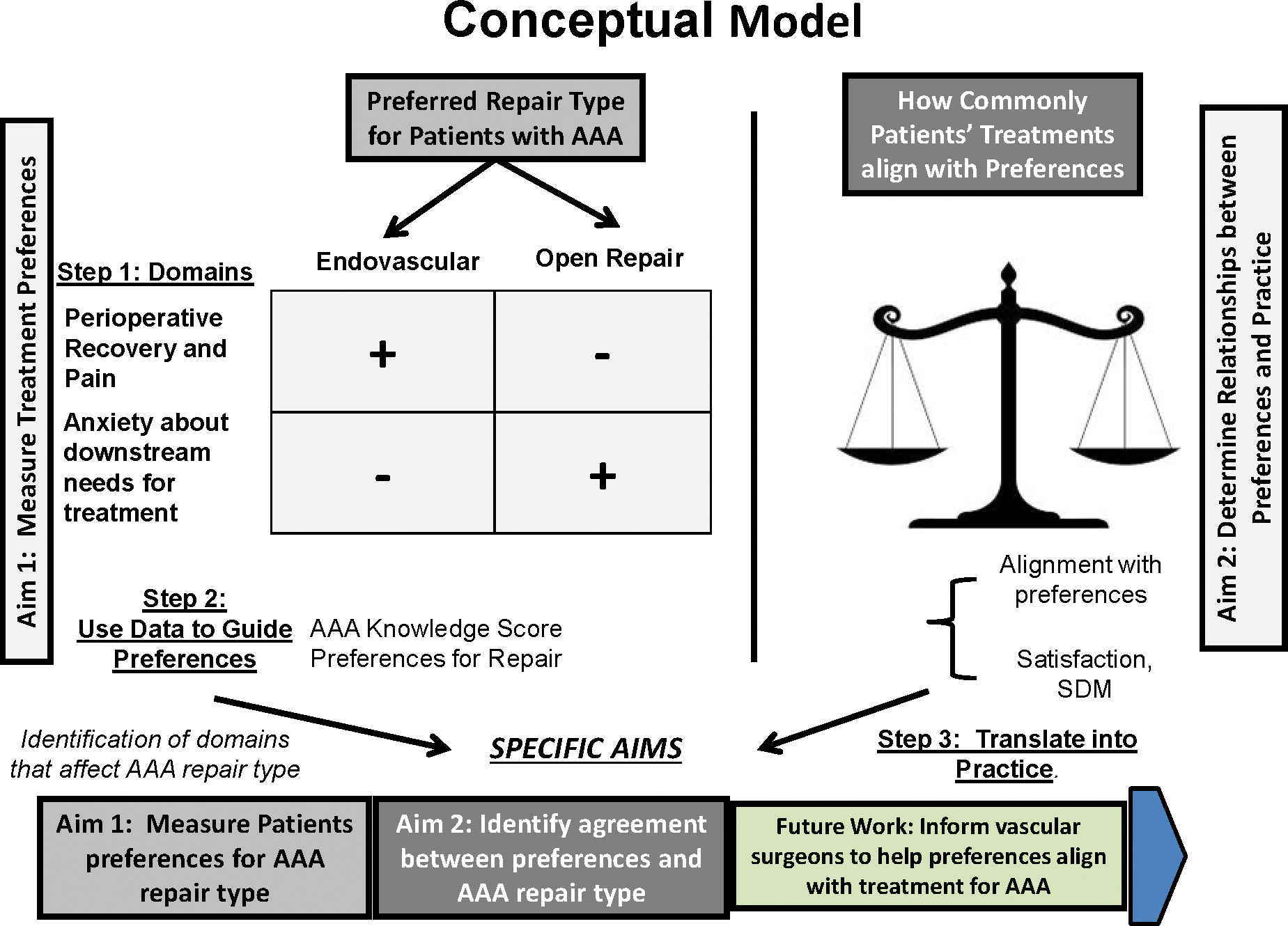
Conceptual model for our study of preferences for abdominal aortic aneurysm repair type
Study Design
PROVE-AAA is a cluster-randomized trial which compares the effect of two different mechanisms of patient education (a decision aid versus usual care) on the alignment between preferences and repair type for Veterans facing repair of their AAA. We will compare these outcomes between our intervention groups, who receive the decision aid, and our control groups, who receive usual care, as shown in Figure 2. This approach allows us to (1) determine what Veterans and surgeons prefer for repair type, and (2) study the efficacy of our decision aid as a tool to enhance agreement between Veterans’ preferences for repair type and repair type they received. Our trial design is a cluster-randomized trial comparing two ways to better align Veterans’ preferences and treatments for AAA: a validated decision aid describing AAA repair types with a survey measuring Veterans’ preference for repair type – versus the survey alone.
Figure 2:
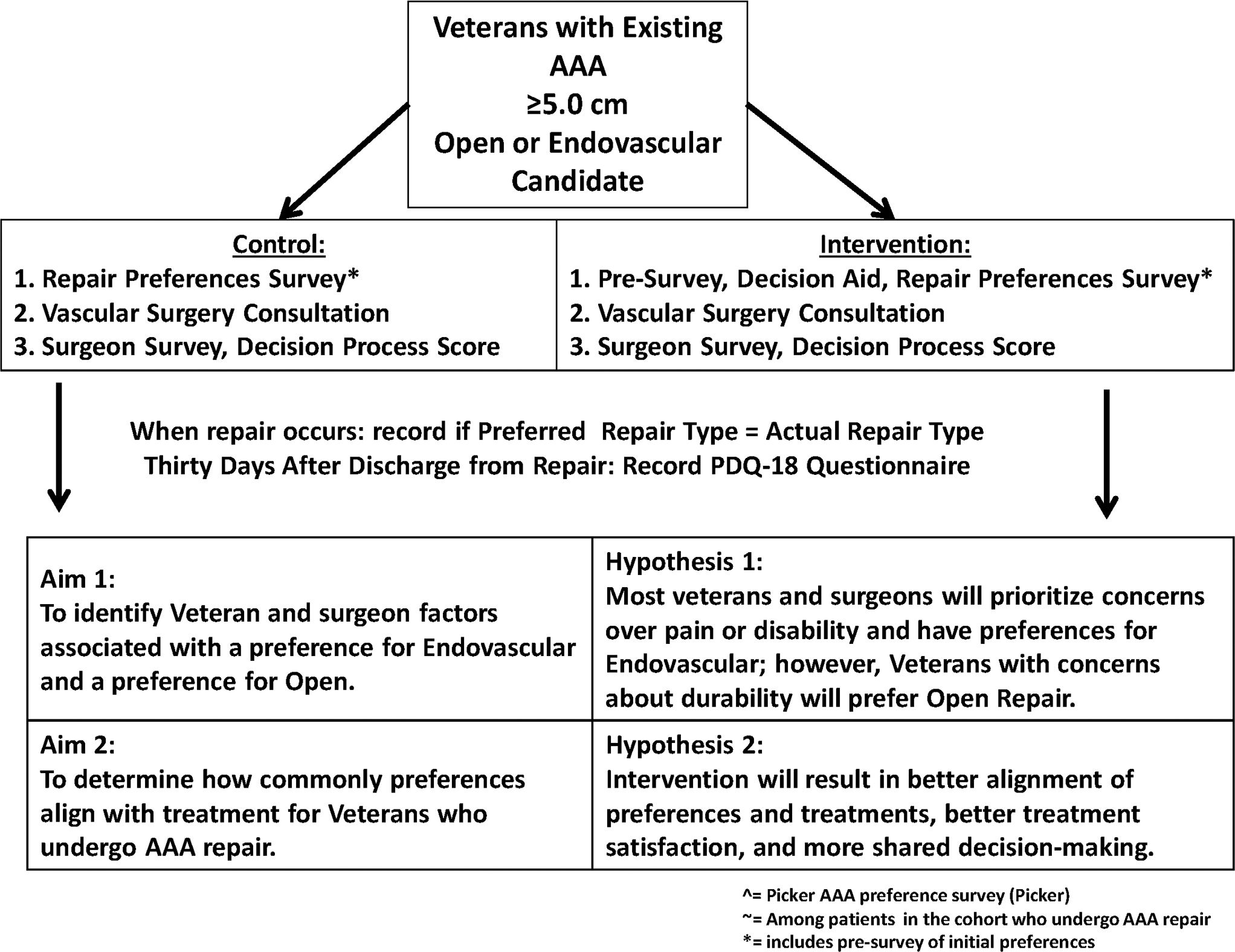
Study design for our study of preferences for abdominal aortic aneurysm repair type.
Enrolled Veterans will be candidates for either endovascular or open repair and be followed at VA hospitals by vascular surgery teams who regularly perform both types of repair. In Aim 1, using validated surveys of Veterans’ and surgeons’ preferences, we will determine which domains are associated with preference for endovascular or open repair. In Aim 2, we will compare our primary outcome, agreement between Veterans’ preferences and their actual repair type, between the decision aid / survey and survey-alone groups. Secondary outcomes will be post-operative satisfaction with their decision and extent of shared decision-making.
Study Sites and Institutional Review
Our study sites consist of twenty VA Medical Centers and their vascular surgery programs (Figure 3). At each site, enrollment will consist of twelve Veterans referred to vascular clinic for an AAA at least 5.0 cm in diameter that can be treated by either endovascular or open repair, based upon a preliminary review by a Site Principal Investigator. Clinic schedules are reviewed by study coordinators prior to the Veterans appointment to determine those who will present with AAA within the size threshold for repair. Our study was granted Central Institutional Review Board (CIRB) approval by the Veteran’s Health Administration and registered at ClinicalTrials.gov (www.clinicaltrials.gov NCT02220686). We included VA medical centers which had performed at least 20 aortic aneurysm repairs annually in the year prior to the study period, based on an audit using data from the VA’s Corporate Data Warehouse (CDW).
Figure 3:
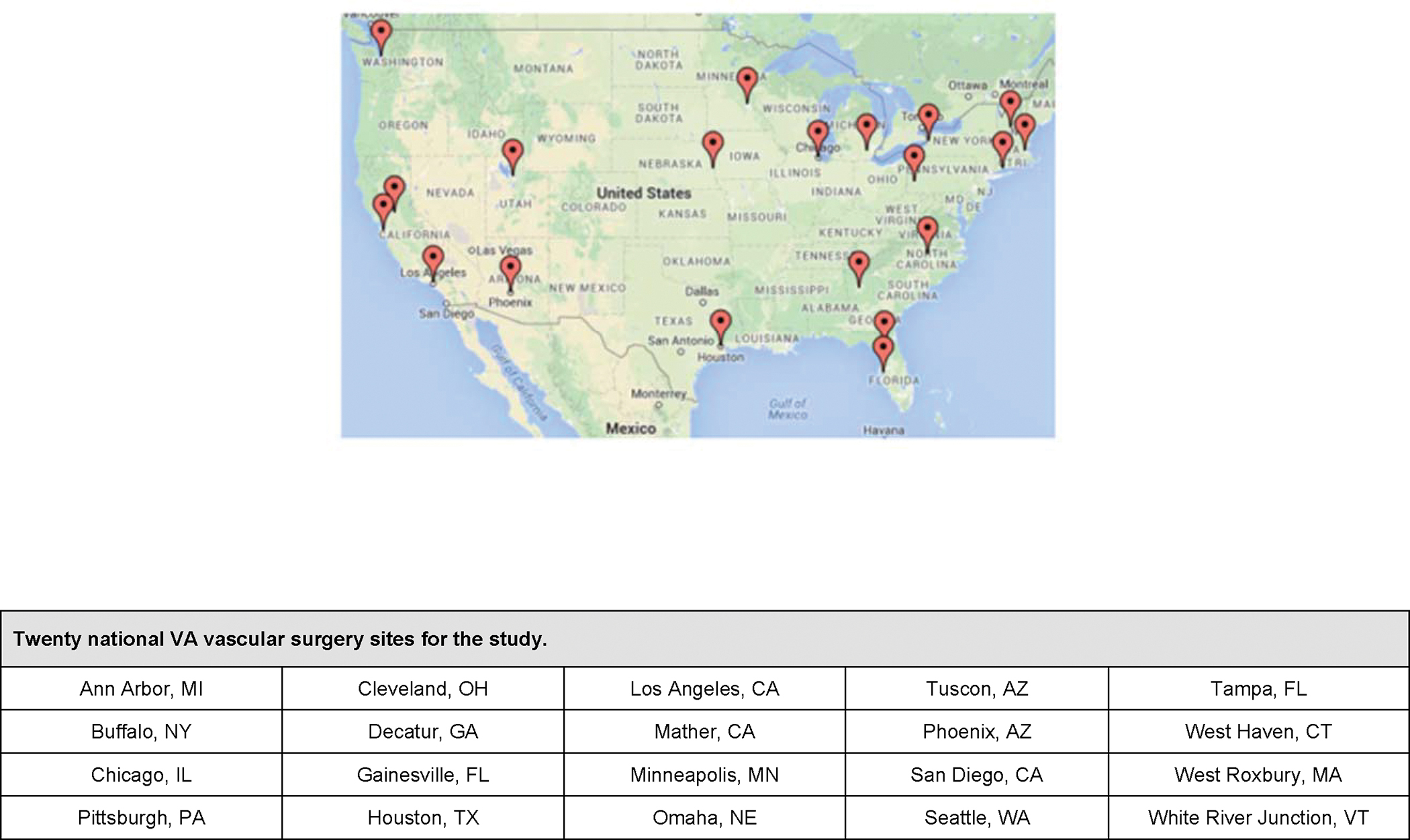
Study sites for the PROVE-AAA study.
Screening Processes and Enrollment Criterial
Before the Veteran is seen by the surgeon, the study-related screening, consent, and enrollment will be performed by a Site Study Coordinator. At ten intervention sites, the Site Study Coordinator will administer a pre-survey, a decision aid and survey. At ten control sites, the Site Study Coordinator will administer the survey alone. In both groups, the Veteran will proceed with a vascular surgery clinic visit. We will follow all patients for two years. When Veterans undergo repair, our primary outcome will be the agreement between Veterans’ preference for endovascular or open repair (as indicated by their survey) and the repair type they actually receive. Surgeon preference will also be measured using an instrument which asks the surgeon which repair type he or she recommended for the patient, and why this recommendation was put forth.
Neither the intervention nor the control group will have counseling from the surgeon before completing the survey. This approach allows direct measurement of the effect of the decision aid, as this is the only variable that will vary between the intervention and control groups. This design also avoids “contamination” by surgeon preferences. This model – testing a decision aid plus survey versus a naïve survey alone – has been described as an effective way to gain insights into how patients make treatment choices23, and has been successfully used in prior studies, albeit none related to surgical interventions 24,25.
Patients must meet two key enrollment criteria for inclusion in the study. First, the Veteran’s AAA must measure at least 5.0 cm in diameter. For enrollment in the study, patients had to have an abdominal aortic aneurysm where the maximal anterior-posterior diameter needed to be a minimum of 5.0 centimeters. We chose this size because our preliminary discussions with surgeons suggested this was the point in time when the goals of the visits shift from surveillance of small aneurysms to consideration and eventual planning for repair. Second, the Veteran must be a candidate, both anatomically and physiologically, for both endovascular and open surgical repair. The surgeon may have a preference for one repair type or another for any reason; for example, preferring endovascular repair in a patient with obesity and pulmonary disease. However, the surgeon must at least consider the patient to be a candidate for either type of repair. Characteristics that may influence surgeon decisions, such as the anatomic features of the aneurysm and its endograft placement, will also be examined.
Cluster randomized study design
Notable in our study design is the use of a cluster-randomized intervention rather than a simple randomization scheme. A cluster-randomized design means that the randomized aspects of the study – whether or not the patient receives the decision aid – occurs at the level of the study site, or cluster, rather than at the patient level. In other words, each site, rather than each patient, is randomized to one treatment arm or the other.
Why choose this approach? We did so because this approach avoids “contamination” of our surgeons by questions from Veterans who have received the decision aid. For example, in a simple randomization scheme, at any individual site, some Veterans would receive the decision aid, and some would not. After hearing questions from a Veteran who received the decision aid, a surgeon might change his or her advice when counseling all subsequent Veterans. The surgeon has thus become “contaminated” by the decision aid, which may lessen the treatment effect of the decision aid itself.
A cluster randomization scheme, while slightly more expensive, obviates this potential disadvantage by allowing the site to be the unit of randomization. This prevents the “control” surgeons from ever becoming exposed to the decision aid, and thereby prevents potential contamination from this type of unintended interaction. This study design adds approximately 20% more patients than if we performed a simple randomization scheme alone, and added approximately $75,000 in study expense. While more costly, it allows a more optimal examination of the effect of the decision aid itself, and will ensure no “contamination” effects occur.
Study intervention, and power
This is a clinic-based intervention, and will occur in vascular surgeon clinics at the VA hospitals in our study. After either receiving the decision aid or usual advice, the Veteran then proceeds through their clinic appointment, and then has their remaining interaction with the study team. For some patients, repair will ensue soon thereafter, whereas others will undergo repair. Patients receive a follow-up survey instrument aimed at measuring their satisfaction with their decision.
We hypothesize that this study may cause a small proportion of patients to choose open repair over EVAR especially those who are young and may prefer to avoid the need for long-term surveillance. Our study has an 80% power to detect a 15% difference in the proportion of patients who prefer endovascular repair, and sensitivity analyses will be performed as well. We designed our 240 patient study across 20 sites, anticipating that 8 of the 12 patients enrolled at each site will complete all steps of the study (initial enrollment, repair, and completion of follow-up study instruments). Study analyses will use multivariable models to determine factors associated with each repair type, and kappa statistics will be used to determine agreement of preferences and repair type in both the intervention and control groups.
Importance and health relevance of study
The importance and significance of our proposal lies in our structured approach towards studying preference-based AAA repair. While survival at two years following endovascular and open repair is similar, tradeoffs between the short-term benefits and long-term risks of endovascular and open repair can make it difficult for patients to make the best choices. Our study will directly address these challenges.
New methodologies to be used in our study
First, our study design involves the use of shared decision-making strategies in determining treatment preferences for patients with AAA. While shared decision-making has been used extensively in helping patients make choices about long-term care options and other difficult health-related decisions26, it has been largely unexplored in deciding on vascular surgery treatment. This approach has been used primarily in orthopedic surgery and cancer surgery decisions9,10,27–29 – but rarely vascular care. These strategies can help patients take an active role in their health care decisions, and result in vascular treatments that align with their preferences.
Summary
With the introduction of EVAR more than twenty years ago, patients with AAA were presented with a new option for a treatment wherein they left the hospital in days rather than weeks, and recovered back to their usual activities in weeks rather than months. However, with these new opportunities came new challenges, such as the concept of an intraluminally repaired aortic aneurysm as a “chronic disease” which requires surveillance, and potentially have complications from endoleaks, limb thrombosis, or delayed rupture requiring further testing and costly interventions. With these choices emerges an opportunity to engage patients and their preferences into the decision-making process. We anticipate that the PROVE-AAA trial will help us to better understand patient preferences related to the treatment of their AAA, whether decision making tools in AAA repair help to inform and educate the patient for the most suitable and preferred treatment options, as well as improve our ability to align their treatments with their preferences.
Supplementary Material
Appendix: Control and Intervention Site Study instruments for the PROVE-AAA trial.
Acknowledgements
The authors also wish to acknowledge our Study Site Coordinators for their outstanding work in assembling our national team in PROVE-AAA, including by not limited to: Cory Gaudette, Francisco Grippa, Amy Voorhees, Kayla Moore (White River Junction / PROVE Coordinating Center), Catherine Dowse (Minneapolis, MN), Sarah Barbey (Gainesville, FL), Ann Galla and Lori Grove (Buffalo, NY), Karen Belanger (Ann Arbor, MI), Angela Karamoto (Phoenix, AZ), Veep Patel (Houston, TX), Susan Bigda (Seattle, WA), Sinan Jabori (Los Angeles, CA), Kevin Chun (Sacramento, CA), Julie Beckstrom and Maria Maloney (Salt Lake City, UT), Molly Schieber (Omaha, NE), Adam Zoble (Tampa, FL), Stephanie Anderson (West Haven, CT), and Michael Morrison (West Roxbury, MA).
Funding / Disclosures:
This study was funded by a Multicenter Clinical Trials Pilot grant from the Society for Vascular Surgery, as well as MERIT Review Grant (015-85) from VA HSR&D.
Footnotes
Clinical Trial Registration:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jesse A. Columbo, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Ravinder Kang, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Emily L. Spangler, The Birmingham VA.
Karina Newhall, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Benjamin S. Brooke, Salt Lake City VA.
Hasan Dosluoglu, Buffalo VAMC.
Eugene S. Lee, Sacramento VAMC.
Joseph D. Raffetto, West Roxbury VA.
Peter K. Henke, Ann Arbor VAMC.
Gale S. Tang, Seattle VAMC.
Leila Mureebe, Durham VAMC.
Panagoitis Kougias, Houston VAMC.
Jason Johanning, Omaha VAMC.
Shipra Arya, Atlanta VAMC.
Salvatore T. Scali, Gainesville VAMC.
David H. Stone, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Bjoern D. Suckow, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Kristine Orion, West Haven VAMC.
Vivienne Halpern, Phoenix VAMC.
Jessica O’Connell, Los Angeles VAMC.
Daniel Inhat, Minneapolis VAMC.
Peter Nelson, Tampa VAMC.
Edith Tzeng, Pittsburgh VAMC.
Wei Zhou, Tuscon VAMC.
Michael Barry, Massachusetts General Hospital Center for Shared Decision Making.
Brenda Sirovich, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
Philip P. Goodney, Department of Surgery and VA Outcomes Group, White River Junction VA Medical Center.
References
- 1.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. The New England Journal of Medicine. Nov 22 2012;367(21):1988–1997. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA : the Journal of the American Medical Association. Oct 14 2009;302(14):1535–1542. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Archives of Internal Medicine. May 22 2000;160(10):1425–1430. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Wilson SE, Johnson GR, et al. Design of the abdominal aortic Aneurysm Detection and Management Study. ADAM VA Cooperative Study Group. Journal of Vascular Surgery. Aug 1994;20(2):296–303. [DOI] [PubMed] [Google Scholar]
- 5.Aljabri B, Al Wahaibi K, Abner D, et al. Patient-reported quality of life after abdominal aortic aneurysm surgery: a prospective comparison of endovascular and open repair. Journal of Vascular Surgery. Dec 2006;44(6):1182–1187. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA . Apr 18 2012;307(15):1621–1628. [DOI] [PubMed] [Google Scholar]
- 7.Schermerhorn ML, Giles KA, Sachs T, et al. Defining perioperative mortality after open and endovascular aortic aneurysm repair in the US Medicare population. Journal of the American College of Surgeons. Mar 2011;212(3):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. The New England Journal of Medicine. Jan 31 2008;358(5):464–474. [DOI] [PubMed] [Google Scholar]
- 9.Lantz PM, Janz NK, Fagerlin A, et al. Satisfaction with surgery outcomes and the decision process in a population-based sample of women with breast cancer. Health services research. Jun 2005;40(3):745–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Aug 20 2005;23(24):5526–5533. [DOI] [PubMed] [Google Scholar]
- 11.Malas MB, Freischlag JA. Interpretation of the results of OVER in the context of EVAR trial, DREAM, and the EUROSTAR registry. Seminars in vascular surgery. Sep 2010;23(3):165–169. [DOI] [PubMed] [Google Scholar]
- 12.Parodi JC. Endoluminal Stent Grafts: Overview. J Invasive Cardiol. Apr 1997;9(3):227–229. [PubMed] [Google Scholar]
- 13.Lederle FA, Stroupe KT, Open Versus Endovascular Repair Veterans Affairs Cooperative Study G. Cost-effectiveness at two years in the VA Open Versus Endovascular Repair Trial. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. Dec 2012;44(6):543–548. [DOI] [PubMed] [Google Scholar]
- 14.Ng TT, Mirocha J, Magner D, Gewertz BL. Variations in the utilization of endovascular aneurysm repair reflect population risk factors and disease prevalence. Journal of Vascular Surgery. Apr 2010;51(4):801–809, 809 e801. [DOI] [PubMed] [Google Scholar]
- 15.Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341:c5146. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor AM, Wennberg JE, Legare F, et al. Toward the ‘tipping point’: decision aids and informed patient choice. Health Affairs. May-Jun 2007;26(3):716–725. [DOI] [PubMed] [Google Scholar]
- 17.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Medical decision making : an international journal of the Society for Medical Decision Making. Jul-Aug 2003;23(4):281–292. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor AM. Validation of a decisional conflict scale. Medical decision making : an international journal of the Society for Medical Decision Making. Jan-Mar 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 19.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. Journal of General Internal Medicine. Oct 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. The New England Journal of Medicine. Mar 1 2012;366(9):780–781. [DOI] [PubMed] [Google Scholar]
- 21.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Annals of Internal Medicine. Jan 15 2002;136(2):127–135. [DOI] [PubMed] [Google Scholar]
- 22.Institute TP. Survey of Patient Preference for Method of Abdominal Aortic Aneurysm Repair. In: the Picker Institute Report, The Picker Institute, London, England: 2010. [Google Scholar]
- 23.Schnurr PP. The rocks and hard places in psychotherapy outcome research. Journal of traumatic stress. Oct 2007;20(5):779–792. [DOI] [PubMed] [Google Scholar]
- 24.Watts BV, Schnurr PP, Zayed M, Young-Xu Y, Stender P, Llewellyn-Thomas H. A Randomized Controlled Clinical Trial of a Patient Decision Aid for Posttraumatic Stress Disorder. Psychiatric services. Oct 15 2014. [DOI] [PubMed] [Google Scholar]
- 25.Ronconi JM, Shiner B, Watts BV. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. Journal of psychiatric practice. Jan 2014;20(1):25–37. [DOI] [PubMed] [Google Scholar]
- 26.The Veterans Administration Makes Shared Decision Making Resources Available to Help with Long Term Care Choices. http://www.informedmedicaldecisions.org/2013/03/28/the-veterans-administration-makes-shared-decision-making-resources-availableto-help-with-long-term-care-choices/, 2014.
- 27.Hoagland TV. The principles and problems in psychotherapy with veterans. Journal - Michigan State Medical Society. Jan 1952;51(1):62–64. [PubMed] [Google Scholar]
- 28.Littlewood C, Ashton J, Mawson S, May S, Walters S. A mixed methods study to evaluate the clinical and cost-effectiveness of a self-managed exercise programme versus usual physiotherapy for chronic rotator cuff disorders: protocol for the SELF study. BMC musculoskeletal disorders. 2012;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein JN, Clay K, Morgan TS. Informed patient choice: patient-centered valuing of surgical risks and benefits. Health Affairs. May-Jun 2007;26(3):726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Control and Intervention Site Study instruments for the PROVE-AAA trial.


