Abstract
INTRODUCTION
Blood biomarkers showed values for predicting future cognitive impairment. Evidence from the community‐based cohort was limited only in high‐income countries.
METHODS
This study included 1857 dementia‐free community residents recruited in 2009–2011 and followed up in waves 2014–2016 and 2019–2023 in the Shanghai Aging Study. We intended to explore the relationships of baseline plasma ALZpath phosphorylated tau 217 (p‐tau217), p‐tau181, neurofilament light chain (NfL) with follow‐up incident dementia, Alzheimer's disease (AD), and amyloidosis.
RESULTS
Higher concentrations of plasma p‐tau217, p‐tau181, and NfL were correlated to higher decline speed of Mini‐Mental State Examination score, and higher risk of incident dementia and AD. The p‐tau217 demonstrated a significant correlation with longitudinal neocortical amyloid‐beta (Aβ) deposition (r = 0.57 [0.30, 0.76]) and a high accuracy differentiating Aβ+ from Aβ‐ at follow‐ups (area under the receiver operating characteristic curve = 0.821 [0.703, 0.940]).
DISCUSSION
Plasma p‐tau217 may be an early predictive marker of AD and Aβ pathology in older community‐dwelling individuals.
Highlights
Plasma p‐tau217, p‐tau181, and NfL were positively associated with long‐term cognitive decline and risk of incident dementia.
Plasma p‐tau217 showed a better performance distinguishing Aβ+ individuals from Aβ‐ individuals at follow‐ups.
Plasma NfL may be a suitable predictor of general cognitive decline in older community‐dwelling individuals.
Keywords: Alzheimer's disease, Aβ, biomarker, cohort, community, dementia
1. BACKGROUND
It was estimated by the Global Burden of Disease Study that the worldwide number of people with dementia would surge to 153 million in 2050. 1 It is imperative to identify people who are at high risk of developing dementia or Alzheimer's disease (AD) in the general population because modifying risk factors may prevent or delay dementia. 2 Blood‐based biomarker may be a promising way to screen high‐risk populations, owing to its preferable accessibility and less invasiveness. 3
Recent studies demonstrated that plasma phosphorylated tau proteins (e.g., p‐tau217 and p‐tau181) as AD‐specific biomarkers, were associated with longitudinal cognitive decline 4 , 5 , 6 , 7 , 8 and clinical progression to dementia/AD. 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Of note, p‐tau217 exhibited a better performance than other p‐tau biomarkers in predicting the progression from mild cognitive impairment (MCI) to AD dementia. 17 Blood neurofilament light chain (NfL), a non‐specific biomarker reflecting the neurodegeneration, also showed value in predicting cognitive deterioration 18 , 19 , 20 and clinical progression. 20 , 21 In the ATN framework, T (as indicated by p‐tau) and N (as indicated by NfL) were more related to clinical performance than amyloid markers. 20 , 22 However, most participants of previous studies were recruited from hospitals, and representative community‐based cohorts are needed to verify the generalizability of these markers in the real‐world situation. In addition, previous assays for plasma p‐tau217 measurements were not commercially available, which hindered the verification of this marker in cohorts and countries from diverse populations.
To date, only a few community‐based cohort studies tried to verify the diagnostic performance of the plasma p‐tau217. The Mayo Clinic Study of Aging (MCSA) study found plasma p‐tau217 and p‐tau181 could predict amyloid‐beta (Aβ) positron emission tomography (PET) positive (area under the receiver operating characteristic curve [AUC] = 0.81–0.86) and tau PET positive in entorhinal cortex (AUC > 0.80). 23 Another study targeting the non‐Hispanic White, Hispanic, and African American populations from the Washington Heights‐Inwood Columbia Aging Project (WHICAP) demonstrated that increased plasma p‐tau217 and p‐tau181 were associated with future AD diagnosis. 16 However, the evidence from low‐ and middle‐income countries (LMICs) is lacking, especially for the Chinese population, which accounts for the one‐fifth of the global population.
The Shanghai Aging Study (SAS) is an ongoing prospective community‐based cohort study conducted in the Chinese population with a comparable study design and procedures to most epidemiological cohort studies in western countries. 8 By using the 10‐year longitudinal data from SAS, we intended to demonstrate the distribution of baseline plasma p‐tau217, p‐tau181, and NfL in diverse clinical diagnostic groups and their correlations with baseline cognition and longitudinal cognitive decline and incident dementia/AD. We also tried to explore whether these blood‐biomarkers could differentiate Aβ status after a decade in individuals remaining dementia‐free, using a subgroup with Aβ PET scans at follow‐up.
2. METHODS
2.1. Study participants
SAS is a community‐based longitudinal cohort initiated from 2009 in Shanghai, China. The original inclusion and exclusion criteria included (1) aged ≥ 60 years old; (2) permanent registered residents in the community; (3) without schizophrenia or mental retardation; (4) cooperated to complete the physical and neuropsychological examinations. 8 Demographics, lifestyles, and medical histories were collected by questionnaires. Apolipoprotein E (APOE) genotyping was achieved by the TaqmanSNP method. The detailed description of the study design and recruitment procedures have been reported elsewhere. 8
At baseline, 3141 older community residents were interviewed at the Department of Neurology, Huashan Hospital, Fudan University, among which, 2985 were diagnosed with dementia‐free, and were eligible for the follow‐up. In the first follow‐up wave from March 2014 to December 2016, 1659 participants have been successfully followed up. 24 For the second follow‐up wave from January 2019 to June 2023, 668 participants were interviewed, including 198 participants who had not been interviewed in the first wave. Thus, a total of 1857 participants with at least one follow‐up interview were included in the current study.
This study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University. All participants and/or their legal representatives signed the written informed consents at baseline and each follow‐up wave.
2.2. Blood biomarkers assays
Overnight fasting blood samples were collected, centrifuged, and stored at −80°C at baseline, and were used for biomarker assays in August 2023 (p‐tau217), November 2020 to January 2023 (p‐tau181), and November 2019 to January 2023 (NfL). EDTA plasma p‐tau217, p‐tau181, and NfL were quantified using an ultra‐sensitive single‐molecule array (Simoa) technology (Quanterix, MA, USA) on the automated Simoa HD‐X platform (GBIO, Hangzhou, China). The ALZpath Simoa p‐tau217 v2 (Cat No: 104371), 25 , 26 p‐tau181 v2 (Cat No: 103714), 27 and NfL (Cat No: 103186) 27 assay kits were used according to the technical notes. Samples were diluted at a 1:3 ratio for the assay of p‐tau217, and 1:4 ratio for the assays of p‐tau181 and NfL. For all the measurements, calibrators and quality controls were measured in duplicate. Sample measurement was performed on a single run basis using kits with the same lot numbers. Technicians were blind to participants’ information.
2.3. Neuropsychological assessments and clinical diagnosis
At baseline, the Mini‐Mental State Examination (MMSE) and domain‐specific tests were administered by certified psychometrists. Neurologists and neuropsychologists reviewed neuropsychological results, medical histories, and other relevant information to reach a consensus clinical diagnosis of cognitively unimpaired (CU), MCI, and dementia. Dementia was diagnosed based on the DSV‐IV criteria. 28 Among participants with dementia, those who met the 1984 NINCDS‐ADRDA criteria were diagnosed as AD, 29 and the others were ascertained as non‐AD dementia. MCI was diagnosed according to the Petersen's criteria, and subtypes were classified (i.e., amnestic MCI single domain [aMCI‐SD], amnestic MCI multiple domains [aMCI‐MD], non‐amnestic MCI single domain [naMCI‐SD], and non‐amnestic MCI multiple domains [naMCI‐MD]). 30 , 31
RESEARCH IN CONTEXT
Systematic review: Literature was searched in PubMed using the following terms, “((phosphorylated tau) OR (p‐tau217) OR (p‐tau181) OR (p‐tau) OR (neurofilament light chain) OR (NfL)) AND ((blood) OR (plasma) OR (serum)) AND ((dementia) OR (Alzheimer*))”. Previous studies demonstrated that plasma p‐tau species and NfL were associated with the cognitive decline and the progression to dementia and Alzheimer's dementia (AD). Specifically, plasma p‐tau217 may be a better indicator of brain amyloid pathology and AD. However, the evidence from the community‐based cohorts was limited only in high‐income countries.
Interpretation: Our results indicated that plasma p‐tau217, p‐tau181, and NfL were associated with long‐term cognitive decline and incident dementia/AD in older community‐dwelling individuals. Plasma p‐tau217 showed the best performance distinguishing Aβ+ individuals from Aβ‐ individuals at follow‐ups among the three markers.
Future directions: The relationships between plasma p‐tau217, p‐tau181, NfL, and tau pathology deserves further exploring. Multi‐ethnic cohort studies should be conducted to verify whether plasma p‐tau217 could be used as a stand‐alone marker predicting AD.
At each wave of follow‐up, the same neuropsychological tests, diagnostic protocols and methods for incident dementia, AD, and non‐AD dementia were used. Those who did not meet the diagnosis criteria of dementia were regarded as non‐dementia (CU and MCI).
2.4. Aβ‐PET imaging
In the second wave of follow‐up, a subset of participants underwent Aβ‐PET using the tracer 18F‐florbetapir (18F‐AV45) at the PET center in Huashan hospital, Fudan University.
Aβ‐PET imaging was carried out 50 min after the intravenous injection of 7.4 MBq/kg (0.2 mCi/kg). It was reconstructed by means of filtered back projection algorithm with corrections for decay, normalization, dead time, photon attenuation, scatter, and random coincidences. Images were processed by Statistical Parametric Mapping 12 (SPM12) implemented in MATLAB (R2013b [8.2.0.701]). Briefly, raw PET images were co‐registered to the structural MRI images and corrected the partial volume effects. Images were spatially normalized to the Montreal Neurological Institute standard space using the transformation matrices of segmented individual structural MRI images. The normalized images underwent smoothing (full‐width at half‐maximum: 8 mm). 32
Aβ‐positive was visually determined by two independent neuroradiologists using the software (Siemens syngo.via). 32 , 33 The standardized uptake value ratio (SUVR) was calculated using the whole cerebellum gray matter as the reference region. A composite neocortical region of interest (ROI) amyloid SUVR for each participant was calculated by averaging SUVRs from the following regions based on the Automated Anatomical Labelling Atlas 3 34 : precuneus, prefrontal, orbitofrontal, parietal, temporal, and cingulate cortices. 35
2.5. Statistical analysis
To compare the difference of continuous variables, Student's t‐test was used for two groups, while the analysis of covariance (ANCOVA) and post hoc tests based on estimated marginal means (Bonferroni method for adjusting multiple comparisons) were used for more than three groups after adjusting for age, sex, years of education, and APOE ε4. Chi‐squared and post hoc z test (Bonferroni method) were adopted to compare the difference of categorical variables. For the analyzing purpose, original concentrations of plasma p‐tau217, p‐tau181, and NfL were log10 transformed to ensure the normal distribution. 36 Each transformed value was classified into quartile groups (Q1, Q2, Q3, and Q4). Univariate linear regression model was used to estimate the association of baseline biomarkers and baseline MMSE scores, while multivariate linear regression model with adjusted β was used to assess the correlation of baseline biomarkers’ concentrations with the annual decline of the MMSE score (i.e., [baseline MMSE score—follow‐up MMSE score]/follow‐up years), adjusting for age, sex, years of education, and APOE ε4. Linear mixed‐effects model was used to fit the association between follow‐up time and MMSE with the interaction item of follow‐up time and biomarker levels, including random intercepts but fixed slopes for participants, adjusting for the same covariates mentioned above. The cumulative incidence of dementia and AD was estimated using the Kaplan‐Meier method and compared using the log‐rank test. Cox proportional hazards regression model was used to estimate the adjusted hazard ratios (HRs) of dementia and AD in four quartile concentrations of biomarkers, using the lowest quartile as the reference group. The first time when incident dementia was diagnosed was regarded as the time to event. The linear trend was tested by entering the median value of each quartile of biomarker concentration as a continuous variable in the Cox regression model. 37
We used Pearson's correlation coefficient r to evaluate the relationship between the baseline biomarker and follow‐up neocortical Aβ SUVR. The AUC, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used to assess the predictive ability of baseline plasma biomarkers for Aβ positive at follow‐up. Voxel‐wise linear model was performed to estimate the association of levels of baseline biomarkers with follow‐up amyloid SUVR adjusting for age and sex. Statistical significance was set at p < 0.001 with a cluster size of k > 100 voxels. 36
All tests were set at a significance level of 0.05 with two tails unless otherwise noted. Statistical analyses were conducted in R (version 4.3.1), IBM SPSS v.25.0., and MATLAB (R2013b [8.2.0.701]). Graphical representations were achieved by R, MATLAB, and GraphPad Prism (version 8.0.0).
3. RESULTS
3.1. Characteristics of study participants
As shown in Table 1, 1857 dementia‐free participants were included in this study at baseline, among which, 1540 (82.9%) individuals were CU and the other 317 (17.1%) individuals were MCI. During the median 5.6 (range, 0.9–13.5) years of follow‐up, 207 (11.1%) participants progressed to dementia (including 145 AD), and 1650 (88.9%) participants remained dementia‐free. Participants with incident AD were significantly older (79.1 [6.1] vs. 69.8 [7.2] years), less educated (8.7 [5.1] vs. 12.2 [3.7] years), and more likely carrying APOE ε4 allele (27.0% vs. 15.8%) than non‐dementia participants. Participants who progressed to non‐AD dementias were significantly older (77.8 [5.9] vs. 69.8 [7.2] years), less likely to be male (29.0% vs. 45.6%), and less educated (9.3 [5.2] vs. 12.2 [3.7] years) than non‐demented participants. Plasma p‐tau217, p‐tau181, and NfL were measured in 1708 (92.0%), 1713 (92.2%), and 1707 (91.9%) participants, respectively. Participants with incident AD had the highest baseline concentrations of p‐tau217 (0.71 [0.6] pg/mL), p‐tau181 (3.12 [2.3] pg/mL), and NfL (28.39 [19.5] pg/mL) (all p < 0.001).
TABLE 1.
Baseline and follow‐up characteristics of study participants.
| Follow‐up diagnosis | |||||
|---|---|---|---|---|---|
|
All (N = 1857) |
Non‐dementia (N = 1650) |
AD dementia (N = 145) |
Non‐AD dementias (N = 62) |
p‐Value † | |
| Baseline | |||||
| Age, years, mean (SD) | 70.8 (7.6) | 69.8 (7.2) | 79.1 (6.1) | 77.8 (5.9) | <0.001a,c |
| Sex, male, n (%) | 826 (44.5) | 753 (45.6) | 55 (37.9) | 18 (29.0) | 0.009c |
| Education, years, mean (SD) | 11.8 (4.0) | 12.2 (3.7) | 8.7 (5.1) | 9.3 (5.2) | <0.001a,c |
| APOE ε4 allele, positive, n (%) | 288/1743 (16.5) | 248/1569 (15.8) | 33/122 (27.0) | 7/52 (13.5) | 0.005a |
| MMSE, mean (SD) | 28.2 (2.0) | 28.6 (1.6) | 25.8 (3.2) | 25.6 (3.0) | <0.001a,c |
| Clinical diagnosis, n (%) | <0.001a,c | ||||
| Cognitively unimpaired | 1540 (82.9) | 1461 (88.5) | 50 (34.5) | 29 (46.8) | |
| Mild cognitive impairment | 317 (17.1) | 189 (11.5) | 95 (65.5) | 33 (53.2) | |
| Plasma p‐tau217, pg/mL, mean (SD) * | 0.39 (0.3) | 0.36 (0.2) | 0.71 (0.6) | 0.43 (0.3) | <0.001a,b |
| Plasma p‐tau181, pg/mL, mean (SD) * | 2.26 (1.7) | 2.20 (1.6) | 3.12 (2.3) | 2.22 (0.8) | <0.001a,b |
| Plasma NfL, pg/mL, mean (SD) * | 17.97 (11.6) | 17.01 (10.2) | 28.39 (19.5) | 23.67 (13.4) | <0.001a,c |
| Follow‐up | |||||
| Follow‐up years, median (range) | 5.6 (0.9, 13.5) | 5.7 (2.7, 13.5) | 4.7 (0.9, 13.3) | 5.2 (1.1, 13.1) | <0.001a,b |
| MMSE, mean (SD) | 26.6 (4.4) | 27.8 (2.1) | 18.8 (4.2) | 13.6 (8.7) | <0.001a,b,c |
Note: The numbers of unavailable data in five variables were: APOE (n = 114), p‐tau217 (n = 149), p‐tau181 (n = 144), NfL (n = 150), and follow‐up MMSE (n = 5).
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; SD, standard deviation.
Original value.
Welch and post hoc Games‐Howell test were used to test the difference of continuous variables, while chi‐square and post hoc z test (Bonferroni method for adjusting multiple comparisons) were adopted to compare the difference of categorical variables among participants of non‐dementia, AD dementia, and non‐AD dementias. The superscripts a, b, and c indicated the significant difference of post hoc analyses between AD dementia and non‐dementia (a), AD dementia and non‐AD dementias (b), and non‐AD dementias and non‐dementia (c).
3.2. Correlations of plasma p‐tau217, p‐tau181, and NfL with clinical diagnosis and MMSE at baseline
Characteristics of participants with different baseline clinical diagnoses were presented in Table S1. As depicted in Figure 1A‐C, participants with aMCI‐MD at baseline had significantly higher p‐tau217 concentrations than those with CU and other MCI subtypes (all p < 0.05). The p‐tau217 concentrations increased stepwise from CU, aMCI‐SD to aMCI‐MD. For p‐tau181, CU participants had a significantly lower concentration than those with aMCI‐SD and aMCI‐MD (both p < 0.05). However, we only found a significantly lower NfL concentration in CU participants, comparing to aMCI‐SD (p < 0.001). Baseline p‐tau217 (β [95% confidence interval, CI] = −0.94 [−1.36, −0.51], p < 0.001), p‐tau181 (β = −0.79 [−1.23, −0.35], p < 0.001), and NfL (β = −1.47 [−1.90, ‐ 1.04], p < 0.001) were negatively correlated with MMSE score (Figure 1D‐F).
FIGURE 1.
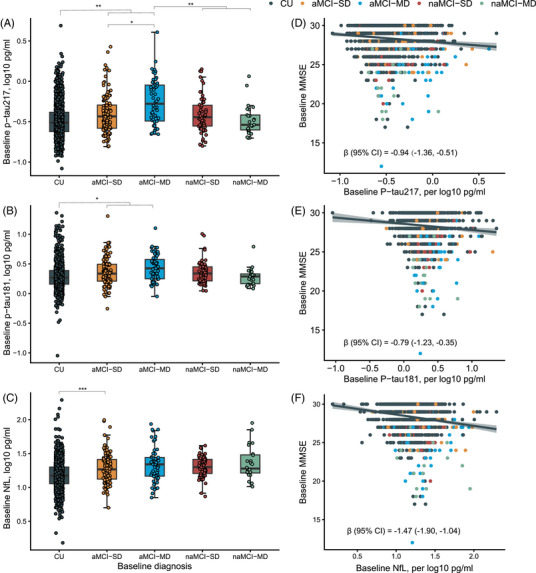
Plasma p‐tau217, p‐tau181, and NfL in different diagnostic groups at baseline, and the correlations with baseline MMSE. In (A–C), ANCOVA and post hoc tests based on estimated marginal means (Bonferroni method for adjusting multiple comparisons) were used to test the difference of plasma biomarkers among baseline diagnostic groups after adjusting for age, sex, years of education, and APOE ε4. *p < 0.05, **p < 0.01, and ***p < 0.001. In (D–F), the β with the 95% confidence interval from the univariate linear regression model indicated the association between baseline biomarkers and baseline MMSE. aMCI‐MD, amnestic mild cognitive impairment‐multiple domains; aMCI‐SD, amnestic mild cognitive impairment‐single domain; ANCOVA, analysis of covariance; APOE, apolipoprotein E; CI, confidence interval; CU, cognitively unimpaired; MMSE, Mini‐Mental State Examination; naMCI‐MD, non‐amnestic mild cognitive impairment‐multiple domains; naMCI‐SD, non‐amnestic mild cognitive impairment‐single domain; NfL, neurofilament light chain.
3.3. Associations of plasma p‐tau217, p‐tau181, NfL with cognitive decline and incident dementia
As shown in Figure 2, baseline p‐tau217 (β = 0.54 [0.34, 0.75], p < 0.001), p‐tau181 (β = 0.33 [0.12, 0.53], p < 0.01), and NfL (β = 0.54 [0.31, 0.77], p < 0.001) showed positive correlations with annual MMSE decline (Figure 2A‐C). Participants with the highest log10 quartile concentration (Q4) of plasma p‐tau217 (≥ 0.43 pg/mL), p‐tau181 (≥ 2.49 pg/mL), and NfL (≥ 20.89 pg/mL) presented significantly steeper cognitive trajectories than those with Q1, Q2, and Q3 levels (all p < 0.01). Participants with the Q3 concentration of p‐tau217 (≥ 0.31 and < 0.43 pg/mL), p‐tau181 (≥ 1.90 and < 2.49 pg/mL), and NfL (≥ 15.49 and < 20.89 pg/mL) also demonstrated faster cognitive deterioration than those with Q1 levels (all p < 0.01). Significant differences of the MMSE declining rate were also found between participants with Q3 and Q2 (≥ 11.75 and < 15.49 pg/mL) concentrations of NfL (p < 0.05), and those with Q2 and Q1 (< 11.75 pg/mL) (p < 0.01) (Figure 2D‐F).
FIGURE 2.
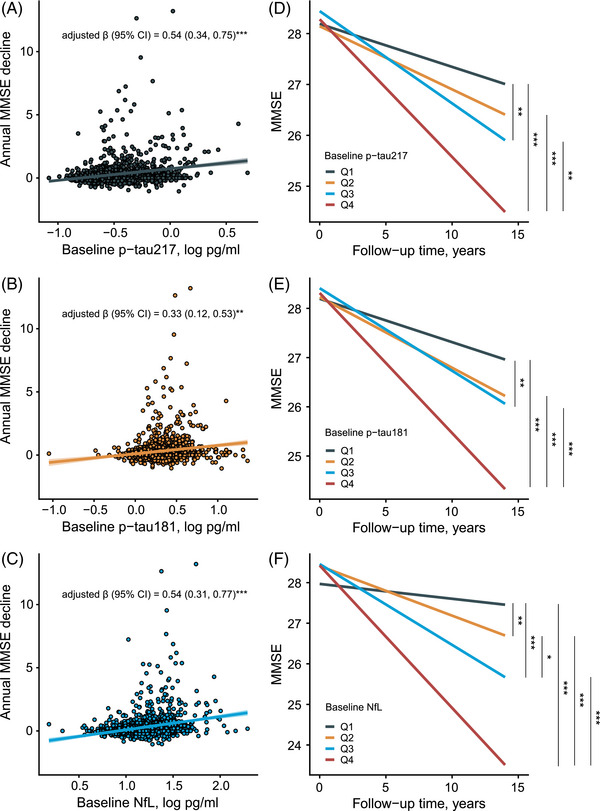
Correlations of baseline plasma biomarkers and longitudinal MMSE trajectory. In (A–C), multivariate linear regression model with β was used to assess the association between baseline plasma biomarkers and MMSE declining rate adjusting for age, sex, years of education, and APOE ε4. In (D–F), linear mixed‐effects model was used to fit the association between follow‐up time×quartered biomarker levels and MMSE with individualized random intercept and fixed slope, adjusting for age, sex, year of education, and APOE ε4. The slopes of MMSE decline among four levels were compared. *p < 0.05, **p < 0.01, and ***p < 0.001. The p‐tau217 Q1, < 0.24 pg/mL; Q2, ≥ 0.24 and < 0.31 pg/mL; Q3, ≥ 0.31 and < 0.43 pg/mL; Q4, ≥ 0.43 pg/mL. The p‐tau181 Q1, < 1.47 pg/mL; Q2, ≥ 1.47, and < 1.90 pg/mL; Q3, ≥ 1.90 and < 2.49 pg/mL; Q4, ≥ 2.49 pg/mL. NfL Q1, < 11.75 pg/mL; Q2, ≥ 11.75 and < 15.49 pg/mL; Q3, ≥ 15.49 and < 20.89 pg/mL; Q4, ≥ 20.89 pg/mL. APOE, apolipoprotein E; CI, confidence interval; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain.
Significant differences of cumulative incident dementia/AD rates were observed among four quartiles of concentrations in baseline p‐tau217, p‐tau181, and NfL (log‐rank tests, all p < 0.001). Multivariate Cox regression analyses (adjusted for age, sex, education year, and APOE ε4) showed that participants with Q4 concentrations of baseline p‐tau217 (HR [95% CI] = 2.91 [1.60, 5.28], p < 0.001), p‐tau181 (HR = 2.50 [1.46, 4.30], p < 0.001), and NfL (HR = 2.51 [1.26, 4.99], p < 0.01) had significantly higher risks of incident dementia than those with Q1 concentrations of the three biomarkers (Figure 3A‐C). The p‐tau217 at Q3 and Q4 levels showed three‐ and six‐times higher risks of incident AD than the Q1 level (Q3, HR = 3.03 [1.15, 7.98], p < 0.05; Q4, HR = 6.03 [2.36, 15.4], p < 0.001). However, p‐tau181 (HR = 2.66 [1.38, 5.11], p < 0.01) and NfL (HR = 2.50 [1.07, 5.84], p < 0.05) exhibited significantly higher risks of incident AD only at the Q4 level (Figure 3D‐F).
FIGURE 3.
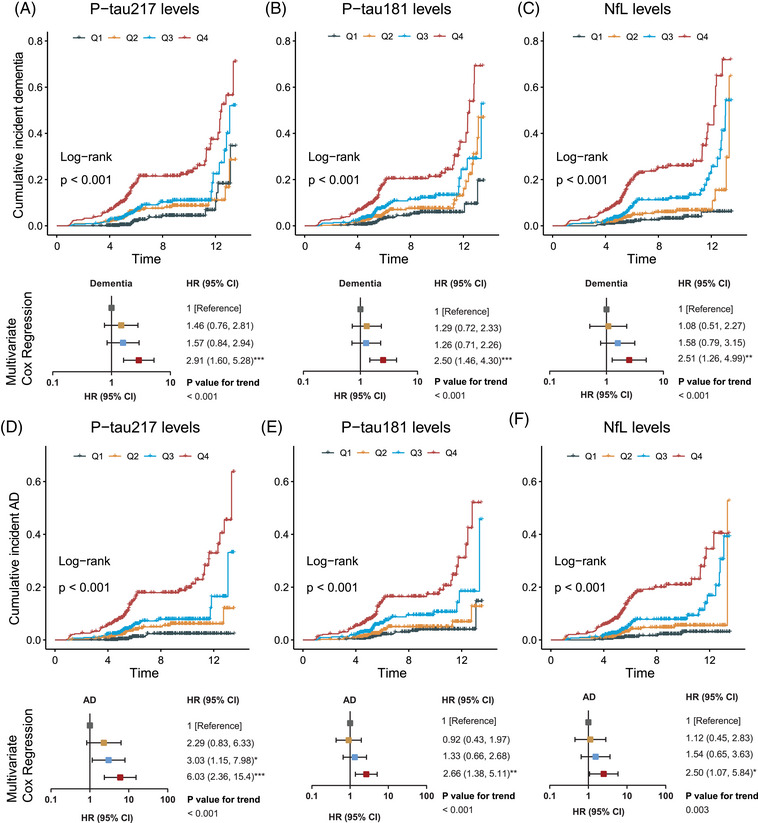
Associations of plasma p‐tau217, p‐tau181, NfL, and incident dementia/AD. In the multivariate Cox regression model, age, sex, education year, and APOE ε4 were adjusted. The linear trend was tested by entering the median value of each quartile of biomarker concentration as a continuous variable in the Cox regression model. *p < 0.05, **p < 0.01, and ***p < 0.001. The p‐tau217 Q1, < 0.24 pg/mL; Q2, ≥ 0.24 and < 0.31 pg/mL; Q3, ≥ 0.31 and < 0.43 pg/mL; Q4, ≥ 0.43 pg/mL. The p‐tau181 Q1, < 1.47 pg/mL; Q2, ≥ 1.47, and < 1.90 pg/mL; Q3, ≥ 1.90 and < 2.49 pg/mL; Q4, ≥ 2.49 pg/mL. NfL Q1, < 11.75 pg/mL; Q2, ≥ 11.75 and < 15.49 pg/mL; Q3, ≥ 15.49 and < 20.89 pg/mL; Q4, ≥ 20.89 pg/mL. AD, Alzheimer's disease; CI, confidence interval; HR, hazard ratio; NfL, neurofilament light chain.
3.4. Values of plasma p‐tau217, p‐tau181, NfL in differentiating Aβ pathology at follow‐up
At the follow‐up, 71 participants volunteered to the Aβ PET scanning. Although their clinical diagnoses were all dementia‐free, Aβ+ were found in 10 (14.1%) participants. The characteristics of these participants were presented at Table S2. Notably, Aβ+ participants had significantly higher p‐tau217 and p‐tau181 concentrations at baseline, comparing to Aβ‐ participants (Figure 4A‐C).
FIGURE 4.
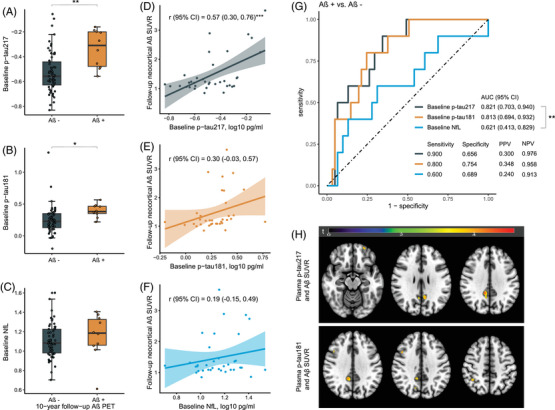
Plasma p‐tau217, p‐tau181, NfL, and follow‐up Aβ‐PET. (A)–(C) showed the concentrations of 10‐year‐ago baseline plasma biomarkers in Aβ negative vs. Aβ positive. (D)‐(F) demonstrated the association between baseline plasma biomarkers and neocortical Aβ SUVR. Pearson's correlation (r) with 95% CI was indicated accordingly. (G) Presented the predictive value of baseline biomarkers for follow‐up Aβ positive. (H) Illustrated the voxel‐wise association of baseline plasma biomarkers with Aβ PET. Colored regions were statistically significant with uncorrected p < 0.001 with a cluster size of k > 100 voxels. *p < 0.05, **p < 0.01, and ***p < 0.001. Aβ, amyloid‐beta; AUC, area under the curve; CI, confidence interval; NfL, neurofilament light chain; PET, positron emission tomography; PPV, positive predictive value; NPV, negative predictive value; SUVR, standardized uptake value ratio.
As shown in Figure 4, only baseline p‐tau217 showed a strong correlation with follow‐up neocortical Aβ SUVR (r [95% CI] = 0.57 [0.30, 0.76], p < 0.001) (Figure 4D‐F). Both baseline p‐tau217 (AUC [95% CI] = 0.821 [0.703, 0.940]) and p‐tau181 (AUC = 0.813 [0.694, 0.932]) presented high values distinguishing Aβ+ individuals from Aβ‐ individuals at follow‐ups (p‐tau217 vs. p‐tau181, p comparison > 0.05). The p‐tau217 performed significantly better than NfL (AUC = 0.621 [0.413, 0.829]) in differentiating Aβ+ from Aβ‐ at follow‐ups (p comparison < 0.01) (Figure 4G). Voxel‐wise association analysis demonstrated that baseline p‐tau217 was associated with Aβ deposition in clusters located in the right median cingulate and paracingulate gyri, left precuneus, and left orbital middle frontal gyrus (Figure 4H, Table S3), while p‐tau181 was associated with Aβ deposition in clusters located in the right median cingulate and paracingulate gyri, right middle frontal gyrus, right inferior parietal gyrus, and right precuneus (Figure 4H, Table 4). No statistically significant association was found between baseline plasma NfL and Aβ deposition at the voxel scale.
4. DISCUSSION
In this community‐based cohort study, we found higher baseline plasma p‐tau217, ptau181, and NfL were associated with longitudinal cognitive decline, progressing to incident dementia and AD during the 10‐year follow‐up. Comparing to p‐tau181 and NfL, p‐tau217 was associated with a significantly higher risk of incident AD at a relatively lower level (greater than or equal to 0.31 pg/mL). Dementia‐free participants with Aβ+ at follow‐up had significantly higher p‐tau217 and p‐tau181 levels as early as a decade ago compared to those with Aβ‐. In addition, baseline p‐tau217 showed a strong correlation with neocortical Aβ deposition in areas that were early affected and had a high distinguishable value of future amyloid status.
The SAS provided a unique opportunity to explore the predictive values of blood biomarkers for 10‐year‐later incident dementia/AD and brain amyloidosis in the community older adults without dementia. Another strength of this study is that we verified the predictive value of the up‐to‐date ALZpath p‐tau217, and had a head‐to‐head comparison with previously available Simoa p‐tau181 and NfL in a longitudinal cohort. Furthermore, all biomarkers were tested in fasting blood samples, which could eliminate the influence of the food intake. 38 Last, it was one of the few studies with a longitudinal study design exploring the predictive value of blood biomarkers among the Chinese population living in a LMIC.
In the current study, plasma p‐tau217 demonstrated a better discriminative ability among MCI subgroups and CU than p‐tau181 and NfL, which has seldomly been reported. Notably, participants with aMCI‐MD had higher p‐tau217 concentrations than aMCI‐SD. Since individuals with aMCI‐MD were more likely to progress to AD dementia than those with aMCI‐SD, 39 p‐tau217 might better reflect the insidious AD pathology than p‐tau181.
Previously, few population‐based cohort studies in high‐income countries reported the associations of blood biomarkers with cognitive decline and clinical progression. The Rotterdam study in the Netherland revealed that higher baseline plasma NfL at the Q4 concentration was associated with a higher risk of all‐cause dementia (HR = 2.70) and AD (HR = 3.28). 9 The multicenter BALTAZAR study in France found that the third tertile of plasma p‐tau181 was associated with a significantly higher risk of converting to AD dementia compared with the first tertile (HR = 3.8). 10 In another study targeting Aβ+ CU participants from the Swedish BioFINDER‐1 and the Wisconsin Registry for Alzheimer Prevention cohorts, plasma p‐tau217 showed the best value predicting cognitive decline and the conversion to AD, comparing to p‐tau181, NfL, p‐tau231, and GFAP. 6 Also, p‐tau217 and p‐tau181 were associated with follow‐up AD diagnosis in non‐demented participants selected from the WHICAP study in United States. 16 In our study, we found baseline p‐tau217, p‐tau181, and NfL were similarly associated with faster cognitive decline and higher risk of incident dementia. Specifically, we found NfL, a marker reflecting the general neurodegeneration, 40 showed a better value distinguishing diverse cognitive trajectories in four quartiles, which implied that the change of this marker may be more parallel with the change of cognition. However, when it comes to AD dementia, p‐tau217 outperformed the other two markers, as the HR (3.03) reached statistical significance at the Q3 level (≥ 0.31 and < 0.43 pg/mL) and achieved the highest (HR = 6.03) at the Q4 level (≥ 0.43 pg/mL).
Previous studies found plasma p‐tau217 and p‐tau181 were associated with amyloid PET, 16 , 36 , 41 , 42 , 43 , 44 and could distinguish Aβ+ from Aβ‐, 11 , 14 , 16 , 23 , 36 , 45 , 46 , 47 , 48 which implied that plasma p‐tau reflected the brain amyloid pathology. Comparing to plasma p‐tau181, p‐tau217 could more accurately distinguish Aβ+ from Aβ‐ in community residents in the MCSA. 23 Plasma p‐tau217, but not p‐tau181 or NfL, increased faster in Aβ+ versus Aβ‐ individuals in CU and MCI participants. 49 Also, plasma p‐tau217 increased in individuals with preclinical AD when the Aβ burden was low and had the strongest association with Aβ signals in regions with early accumulating compared with p‐tau181 and NfL. 36 Autopsy studies demonstrated that plasma p‐tau217 and p‐tau181 could predict post mortem AD, 16 and p‐tau181 has increased as early as ten years prior to the death in individuals with intermediate or severe AD pathology. 4 Our results also found that only plasma p‐tau217 had a significant correlation with early neocortical Aβ accumulation and could differentiate Aβ+ from Aβ‐ individuals at the dementia‐free stage with high precision and sensitivity, which is in favor p‐tau217 may be an early indicator of brain amyloidosis.
The National Institute on Aging and the Alzheimer's Association proposed the revised clinical guidelines for AD at the Alzheimer's Association International Conference 2023, and stated that the biological diagnosis of AD is projected to revolutionize the clinical care due to the generally accessible of blood biomarkers. It also highlighted that blood biomarkers need to be verified in diverse and more representative cohorts. 50 In the current study, we provided evidence that plasma p‐tau217 might be an early screening marker for future amyloid pathology and incident AD in community settings, while plasma NfL may be more suitable for predicting general cognitive decline and incident dementia. High‐risk individuals who were screened out by blood biomarkers in community settings may be transferred to secondary or tertiary hospitals for further consultations, neurological examinations, and disease‐modifying therapies. More population‐based cohort studies should be conducted to further verify the robustness of blood biomarkers, especially in LMICs with diverse ethnicities and social backgrounds. Recently, one study proposed a two‐step workflow incorporating plasma p‐tau217 to detect Aβ+ in memory‐clinic MCI participants, which showed great performance. 51 Whether it could be generalized to wider populations including CU individuals also deserves further validating.
One limitation of this study was that the diagnosis of AD was based on the clinical diagnostic criteria without the pathological confirmation. We can only infer which of the individuals who eventually developed dementia actually had underlying AD. Usually, it's very difficult for large‐sampled community‐based studies, such as the Rotterdam study 9 and the Monongahela‐Youghiogheny Healthy Aging Team (MYHAT) cohort study 5 to obtain CSF or Aβ PET due to the invasiveness or high costs, because most of the community‐dwelling individuals do not have cognitive concerns. Second, the number of participants who underwent Aβ PET was relatively small because of the low response rate from the community setting. Within such a small subgroup, we found that p‐tau217 could differentiate Aβ+ from Aβ‐ individuals at the dementia‐free stage. Studies with a larger sample size were needed to further verify the superiority of this marker. Third, individuals participated the follow‐up interviews were younger (70.8 [7.6] vs. 72.8 [8.3], p < 0.001), and had the better cognitive function at baseline (MMSE score: 28.2 [2.0] vs. 27.8 [2.4], p < 0.001; MCI prevalence: 17.1% vs. 24.8%, p < 0.001) comparing to those who were lost‐to follow‐up. This selection bias may lead to the underestimation of the incidence of dementia/AD. Fourth, the follow‐up interviews of SAS were not conducted regularly, thus the follow‐up time may not reflect the exact time of dementia/AD onset. Some incident cases before 10 years were detected later, which made a dramatic increase of dementia/AD incidence in the Kaplan‐Meier curves. Last, besides Aβ, tau protein is another core pathology of AD. Previous studies found that CSF and blood p‐tau species were more related to Aβ than tau accumulation. 22 , 41 , 43 However, among CU participants, plasma p‐tau217 measured by the Meso Scale Discovery method might more accurately reflect the entorhinal cortex tau PET than Simoa p‐tau181. 41 More studies are needed to verify whether Simoa p‐tau217 was a better indicator of tau pathology than other p‐tau species.
In conclusion, plasma p‐tau217, p‐tau181, and NfL may be accessible predictive biomarkers for incident dementia in general population. Specifically, plasma p‐tau217 may be an ultra‐early predictor of amyloid pathology and incident AD in older individuals in the real world.
AUTHOR CONTRIBUTIONS
Ding Ding had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ding Ding designed the original study. Wanqing Wu, Zhenxu Xiao, Xiaoniu Liang, and Xiaoxi Ma collected data. Zhenxu Xiao, Wanqing Wu, and Jie Wu collected and processed blood samples. Zhenxu Xiao analyzed the data and Yang Cao corroborated the data analysis. Zhenxu Xiao and Ding Ding drafted the manuscript. All co‐authors reviewed and revised the manuscript critically.
CONFLICT OF INTEREST STATEMENT
Dr Ding reported receiving grants from Shanghai Municipal Science and Technology Major Project and ZJ LAB, Shanghai Municipal Health Commission, National Natural Science Foundation of China, and Key Project of the Ministry of Science and Technology, China. Dr Zhao reported receiving grants from Shanghai Hospital Development Center, the National Natural Science Foundation of China, and MOE Frontiers Center for Brain Science. Dr Liang reported receiving grants from Shanghai Sailing Program. All payments were made to the institution. No other disclosures were reported. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants and/or their legal representatives signed the written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (82173599, 82071200, 82371429), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01) and ZJ LAB, Key Project of the Ministry of Science and Technology, China (2020YFC2005003, 2021YFE0111800), Shanghai Hospital Development Center (SHDC2020CR4007), MOE Frontiers Center for Brain Science (JIH2642001/028), Shanghai Sailing Program (20YF1404000), and Shanghai Municipal Health Commission Project (2020YJZX0101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Xiao Z, Wu W, Ma X, et al. Plasma p‐tau217, p‐tau181, and NfL as early indicators of dementia risk in a community cohort: The Shanghai Aging Study. Alzheimer's Dement. 2023;15:e12514. 10.1002/dad2.12514
REFERENCES
- 1. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. The Lancet Public Health. 2022;7(2):e105‐e125. doi: 10.1016/s2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. The Lancet. 2020;396(10248):413‐446. doi: 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. The Lancet Neurology. 2021;21(1):66‐77. doi: 10.1016/s1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 4. Smirnov DS, Ashton NJ, Blennow K, et al. Plasma biomarkers for Alzheimer's disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022;143(4):487‐503. doi: 10.1007/s00401-022-02408-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira PCL, Zhang Y, Snitz B, et al. Plasma biomarkers identify older adults at risk of Alzheimer's disease and related dementias in a real‐world population‐based cohort. Alzheimers Dement. [published online ahead of print, Mar 6 2023. doi: 10.1002/alz.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattsson‐Carlgren N, Salvadó G, Ashton NJ, et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 2023;80(4):360‐369. doi: 10.1001/jamaneurol.2022.5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422‐433. doi: 10.1016/s1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 8. Ding D, Zhao Q, Guo Q, et al. The Shanghai Aging Study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. 2014;43(2):114‐122. doi: 10.1159/000366163 [DOI] [PubMed] [Google Scholar]
- 9. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid‐β levels and risk of dementia; a population‐based cohort study. Brain. 2020;143(4):1220‐1232. doi: 10.1093/brain/awaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehmann S, Schraen‐Maschke S, Vidal JS, et al. Plasma phosphorylated tau 181 predicts amyloid status and conversion to dementia stage dependent on renal function. J Neurol Neurosurg Psychiatry. 2023;94(6):411‐419. doi: 10.1136/jnnp-2022-330540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cullen NC, Janelidze S, Mattsson‐Carlgren N, et al. Test‐retest variability of plasma biomarkers in Alzheimer's disease and its effects on clinical prediction models. Alzheimers Dement. [published online ahead of print, Jun 14 2022]. doi: 10.1002/alz.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palmqvist S, Stomrud E, Cullen N, et al. An accurate fully automated panel of plasma biomarkers for Alzheimer's disease. Alzheimers Dement. 2022;19(4):1204‐1215. doi: 10.1002/alz.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stocker H, Beyer L, Perna L, et al. Association of plasma biomarkers, p‐tau181, glial fibrillary acidic protein, and neurofilament light, with intermediate and long‐term clinical Alzheimer's disease risk: results from a prospective cohort followed over 17 years. Alzheimers Dement. 2022;19(1):25‐35. doi: 10.1002/alz.12614 [DOI] [PubMed] [Google Scholar]
- 14. Planche V, Bouteloup V, Pellegrin I, et al. Validity and performance of blood biomarkers for Alzheimer disease to predict dementia risk in a large clinic‐based cohort. Neurology. 2023;100(5):e473‐e484. doi: 10.1212/WNL.0000000000201479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379‐386. doi: 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 16. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17(8):1353‐1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janelidze S, Bali D, Ashton NJ, et al. Head‐to‐head comparison of 10 plasma phospho‐tau assays in prodromal Alzheimer's disease. Brain. 2023;146(4):1592‐1601. doi: 10.1093/brain/awac333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bangen KJ, Thomas KR, Weigand AJ, et al. Elevated plasma neurofilament light predicts a faster rate of cognitive decline over 5 years in participants with objectively‐defined subtle cognitive decline and MCI. Alzheimers Dement. 2021;17(10):1756‐1762. doi: 10.1002/alz.12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugarman MA, Zetterberg H, Blennow K, et al. A longitudinal examination of plasma neurofilament light and total tau for the clinical detection and monitoring of Alzheimer's disease. Neurobiol Aging. 2020;94:60‐70. doi: 10.1016/j.neurobiolaging.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cullen NC, Leuzy A, Palmqvist S, et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nature Aging. 2020;1(1):114‐123. doi: 10.1038/s43587-020-00003-5 [DOI] [PubMed] [Google Scholar]
- 21. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(2):277‐283. doi: 10.1038/s41591-018-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horie K, Salvado G, Barthelemy NR, et al. CSF MTBR‐tau243 is a specific biomarker of tau tangle pathology in Alzheimer's disease. Nat Med. 2023;29(8):1954‐1963. doi: 10.1038/s41591-023-02443-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398‐1405. doi: 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiao Z, Wu W, Zhao Q, Liang X, Luo J, Ding D. Association of glaucoma and cataract with incident dementia: a 5‐Year follow‐up in the Shanghai Aging Study. J Alzheimers Dis. 2020;76(2):529‐537. doi: 10.3233/JAD-200295 [DOI] [PubMed] [Google Scholar]
- 25. Chenna A, Jeromin A, Yee B, et al. Analytical and clinical assessment of plasma phospho‐tau isoforms in Alzheimer's disease (AD) (S15.006). Neurology. 2023;100(17 Supplement 2):3239. doi: 10.1212/WNL.0000000000203126 [DOI] [Google Scholar]
- 26. Ashton NJ, Brum WS, Di Molfetta G, et al. Diagnostic accuracy of the plasma ALZpath ptau217 immunoassay to identify Alzheimer's disease pathology. medRxiv. 2023. doi: 10.1101/2023.07.11.23292493 [DOI] [Google Scholar]
- 27. Xiao Z, Wu X, Wu W, et al. Plasma biomarker profiles and the correlation with cognitive function across the clinical spectrum of Alzheimer's disease. Alzheimers Res Ther. 2021;13(1):123. doi: 10.1186/s13195-021-00864-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Psychiatric Association : Diagnostic and Statistical Manual of Mental Disorders, ed 4. Washington, American Psychiatric Association, 1994, pp 143‐147
- 29. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939‐944. doi: 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 30. Ding D, Zhao Q, Guo Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross‐sectional analysis of the Shanghai Aging Study. Alzheimers Dement. 2015;11(3):300‐309.e2. doi: 10.1016/j.jalz.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 31. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256(3):240‐246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 32. Lu J, Ma X, Zhang H, et al. Head‐to‐head comparison of plasma and PET imaging ATN markers in subjects with cognitive complaints. Transl Neurodegener. 2023;12(1):34. doi: 10.1186/s40035-023-00365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lundeen TF, Seibyl JP, Covington MF, Eshghi N, Kuo PH. Signs and artifacts in amyloid PET. Radiographics. 2018;38(7):2123‐2133. doi: 10.1148/rg.2018180160 [DOI] [PubMed] [Google Scholar]
- 34. Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. NeuroImage. 2020;206:116189. doi: 10.1016/j.neuroimage.2019.116189 [DOI] [PubMed] [Google Scholar]
- 35. Therriault J, Benedet AL, Pascoal TA, et al. Determining amyloid‐beta positivity using (18)F‐AZD4694 PET imaging. J Nucl Med. 2021;62(2):247‐252. doi: 10.2967/jnumed.120.245209 [DOI] [PubMed] [Google Scholar]
- 36. Milà‐Alomà M, Ashton NJ, Shekari M, et al. Plasma p‐tau231 and p‐tau217 as state markers of amyloid‐β pathology in preclinical Alzheimer's disease. Nat Med. 2022;28(9):1797‐1801. doi: 10.1038/s41591-022-01925-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause‐specific mortality among Nonwhite populations. Ann Intern Med. 2017;167(4):228‐235. doi: 10.7326/M16-2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huber H, Ashton NJ, Schieren A, et al. Levels of Alzheimer's disease blood biomarkers are altered after food intake‐A pilot intervention study in healthy adults. Alzheimer's & Dementia. [published online ahead of print, 2023] doi: 10.1002/alz.13163 [DOI] [PubMed]
- 39. Petersen RC. Clinical practice. Mild cognitive impairment. New Engl J Med. 2011;364(23):2227‐2234. doi: 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 40. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870‐881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 41. Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78(9):1108‐1117. doi: 10.1001/jamaneurol.2021.2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ni M, Zhu ZH, Gao F, et al. Plasma core Alzheimer's disease biomarkers predict amyloid deposition burden by positron emission tomography in chinese individuals with cognitive decline. ACS Chem Neurosci. 2023;14(1):170‐179. doi: 10.1021/acschemneuro.2c00636 [DOI] [PubMed] [Google Scholar]
- 43. Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs. tau positron emission tomography. JAMA Neurol. 2023;80(2):188‐199. doi: 10.1001/jamaneurol.2022.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mattsson‐Carlgren N, Janelidze S, Bateman RJ, et al. Soluble P‐tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021;13(6):e14022. doi: 10.15252/emmm.202114022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Therriault J, Servaes S, Tissot C, et al. Equivalence of plasma p‐tau217 with cerebrospinal fluid in the diagnosis of Alzheimer's disease. Alzheimers Dement. [published online ahead of print, Apr 20 2023] doi: 10.1002/alz.13026 [DOI] [PMC free article] [PubMed]
- 46. Keshavan A, Pannee J, Karikari TK, et al. Population‐based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021;144(2):434‐449. doi: 10.1093/brain/awaa403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bilgel M, An Y, Walker KA, et al. Longitudinal changes in Alzheimer's‐related plasma biomarkers and brain amyloid. Alzheimers Dement. [published online ahead of print, May 22 2023].doi: 10.1002/alz.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janelidze S, Palmqvist S, Leuzy A, et al. Detecting amyloid positivity in early Alzheimer's disease using combinations of plasma Aβ42/Aβ40 and p‐tau. Alzheimers Dement. 2022;18(2):283‐293. doi: 10.1002/alz.12395 [DOI] [PubMed] [Google Scholar]
- 49. Ashton NJ, Janelidze S, Mattsson‐Carlgren N, et al. Differential roles of Abeta42/40, p‐tau231 and p‐tau217 for Alzheimer's trial selection and disease monitoring. Nat Med. 2022;28(12):2555‐2562. doi: 10.1038/s41591-022-02074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. The National Institute on Aging and the Alzheimer's Association (NIA‐AA). NIA‐AA Revised Clinical Guidelines for Alzheimer's. Accessed August 23 2023. https://aaic.alz.org/nia‐aa.asp
- 51. Brum WS, Cullen NC, Janelidze S, et al. A two‐step workflow based on plasma p‐tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nature Aging. [published online ahead of print, 2023]. doi: 10.1038/s43587-023-00471-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


