Abstract
Background
The number of robotic surgical procedures performed yearly is constantly rising, due to improved dexterity and visualization capabilities compared with conventional methods. We hypothesized that outcomes after robotic-assisted inguinal hernia repair would not be significantly different from outcomes after laparoscopic or open repair.
Methods
All patients undergoing inguinal hernia repair between 2012 and 2016 were identified using institutional American College of Surgeons National Surgical Quality Improvement Program data. Demographics; preoperative, intraoperative, and postoperative characteristics; and outcomes were evaluated based on method of repair (Robot, Lap, or Open). Categorical variables were analyzed by Chi-square test and continuous variables using Mann–Whitney U.
Results
A total of 510 patients were identified who underwent unilateral inguinal hernia repair (Robot: 13.8% [n = 69], Lap: 48.1% [n = 241], Open: 38.1% [n = 191]). There were no demographic differences between groups other than age (Robot: 52 [39–62], Lap: 57 [45–67], and Open: 56 [48–67] years, p = 0.03). Operative duration was also different (Robot: 105 [76–146] vs. Lap: 81 [61–103] vs. Open: 71 [56–88] min, p < 0.001). There were no operative mortalities and all patients except one were discharged home the same day. Postoperative occurrences (adverse events, readmissions, and death) were similar between groups (Robot: 2.9% [2], Lap: 3.3% [8], Open: 5.2% [10], p = 0.53). Although rare, there was a significant difference in rate of postoperative skin and soft tissue infection (Robot: 2.9% [2] vs. Lap: 0% [0] vs. Open: 0.5% [1], p = 0.02). Cost was significantly different between groups (Robot: $7162 [$5942–8375] vs. Lap: $4527 [$2310–6003] vs. Open: $4264 [$3277–5143], p < 0.001).
Conclusions
Outcomes after robotic-assisted inguinal hernia repair were similar to outcomes after laparoscopic or open repair. Longer operative duration during robotic repair may contribute to higher rates of skin and soft tissue infection. Higher cost should be considered, along with surgeon comfort level and patient preference when deciding whether inguinal hernia repair is approached robotically.
Keywords: Robotics, Inguinal hernia repair, Laparoscopic inguinal hernia repair, Open inguinal hernia repair
Inguinal hernia repair is one of the most common general surgery procedures performed yearly in the United States and is associated with low rates of postoperative complications and recurrences [1, 2]. The operative treatment of inguinal hernias has followed the natural progression of surgical advancement over time. Although open mesh repairs using the Lichtenstein tension-free technique yield exceedingly low rates of morbidity and mortality, a minimally invasive approach to inguinal hernia repair using laparoscopy is a popular alternative and is associated with less postoperative pain [1]. Laparoscopy provides the surgeon with the option of completing the hernia repair via a transabdominal preperitoneal (TAPP) approach or a totally extraperitoneal (TEP) approach, either of which may be more appropriate depending on hernia size, initial or recurrent hernia status, previous approaches used, body habitus of the patient, and surgeon preference.
In addition to open and conventional laparoscopic approaches, surgeons in certain hospitals now have the ability to perform minimally invasive inguinal hernia repairs with robotic-assist. Successful use of the robot has been described for gynecologic and urologic procedures, removal of gastric and rectal cancers, and mitral valve operations [3–6]. Advantages of performing surgical procedures with robotic-assist include superior dexterity and improved visualization capabilities, while the disadvantages include increased cost and potentially longer operative times [7]. While well described for the repair of ventral hernias, authors have begun to describe the use of robotic-assist for inguinal hernia repairs [8, 9].
Although open and laparoscopic inguinal hernia repairs are rather short operations with exceptionally good outcomes, the benefits of performing inguinal hernia repairs with robotic-assist are not fully defined yet. The objective of this study was to compare outcomes after open, laparoscopic, and robotic-assisted inguinal hernia repairs. We hypothesized that outcomes after robotic-assisted inguinal hernia repair would not be significantly different from outcomes after laparoscopic or open repair.
Materials and methods
Patient population
All patients undergoing primary unilateral inguinal hernia repair between 2012 and 2016 at a single academic medical center were captured using institutional American College of Surgeons National Surgical Quality Improvement Program data. Financial data were obtained from the institutional Clinical Data Repository. The Institutional Review Board at the University of Virginia approved waiver of consent for this study. Preoperative patient characteristics (age, sex, body mass index [BMI], and comorbid conditions), perioperative elements (American Society of Anesthesiologists [ASA] classification, total operating time, concomitant procedures, and surgical approach), as well as 30-day postoperative outcomes, physician and hospital charges, and total cost were analyzed. Patients undergoing robotic-assisted repair (Robot), laparoscopic repair (Lap), and open repair (Open) were compared. A total of ten surgeons performed all operations, with all robotic procedures performed by two surgeons (who previously performed both open and laparoscopic repairs), all laparoscopic repairs performed by eight surgeons, and all open repairs performed by four surgeons. Repair approach was at the discretion of the operating surgeon based on surgeon preference and preoperative characteristics of the patient. All robotic-assisted and laparoscopic repairs were completed using mesh and the TAPP approach.
Data analysis
The primary outcome for this study was a composite endpoint of any postoperative occurrence. The secondary outcomes were operative time and specific postoperative occurrences. We report preoperative factors between the groups in addition to intraoperative variables and postoperative outcomes. Data were compared using Chi-square (χ2) test for categorical variables and appropriate parametric and non-parametric tests for continuous variables. A p value < 0.05 was used for statistical significance. SAS version 9.4 (SAS Company, Cary NC) was used for all analyses.
Results
A total of 510 patients were identified who underwent inguinal hernia repair between 2012 and 2016. Robotic-assist was used in 13.8% (n = 69) of repairs, laparoscopic approach in 48.1% (n = 241), and open approach in 38.1% (n = 191). There were no demographic differences between the groups in terms of sex, race, BMI, hospital status (inpatient/outpatient), or functional health status (Table 1). Age in years [median (interquartile range)] was significantly different between the three groups (Robot: 52 [39–62], Lap: 57 [45–67], Open: 56 [48–67], p = 0.03). The most common comorbidities in the entire cohort were hypertension, tobacco use, chronic obstructive pulmonary disease, and steroid use. There were no significant differences between the groups in prevalence of preoperative comorbidities, which also included insulin-dependent diabetes mellitus, heart failure, and kidney failure (Table 2).
Table 1.
Demographics
| Robot (n = 69) | Lap (n = 241) | Open (n = 191) | p value | |
|---|---|---|---|---|
| Age (years) | 52 [39–62]a | 57 [45–67] | 56 [48–67] | 0.03 |
| Female | 14.5 (10)b | 11.2 (27) | 8.4 (16) | 0.33 |
| White | 87 (60) | 88.4 (213) | 85.9 (164) | 0.74 |
| BMI (kg/m2) | 24.9 [22.9–28.7] | 25.8 [23.1–28.4] | 25.1 [23.2–27.8] | 0.70 |
| Outpatient status | 98.6 (68) | 94.6 (228) | 97.4 (186) | 0.18 |
| Functionally independent | 100 (69) | 100 (241) | 98.9 (189) | 0.50 |
Median [interquartile range], all such values
Percent (n), all such values
Table 2.
Preoperative comorbidities and perioperative characteristics
| Robot (n = 69) | Lap (n = 241) | Open (n = 191) | p value | |
|---|---|---|---|---|
| Comorbidities | ||||
| Hypertension | 37.7 (26)a | 30.7 (74) | 37.7 (72) | 0.26 |
| COPDb | 1.5 (1) | 0.4 (1) | 2.6 (5) | 0.15 |
| Insulin-dependent diabetes mellitus | 1.5 (1) | 0.4 (1) | 1.6 (3) | 0.72 |
| Dialysis-dependent | 1.5 (1) | 0 (0) | 1.6 (3) | 0.15 |
| Current tobacco use | 23.2 (16) | 18.3 (44) | 28.3 (54) | 0.05 |
| Heart failure | 0 (0) | 0 (0) | 0.5 (1) | 0.44 |
| Bleeding disorder | 1.5 (1) | 0.4 (1) | 1.1 (2) | 0.62 |
| Ascites | 0 (0) | 0 (0) | 2.6 (5) | 0.02 |
| Steroid use | 2.9 (2) | 0.8 (2) | 1.6 (3) | 0.42 |
| Perioperative characteristics Concurrent operation | 1.5 (1) | 0.8 (2) | 1.6 (3) | 0.76 |
| ASAc | ||||
| Class I | 13.0 (9) | 17.4 (42) | 16.2 (31) | 0.003 |
| Class II | 72.5 (50) | 67.2 (162) | 55.0 (105) | |
| Class III | 14.5 (10) | 15.4 (37) | 26.2 (50) | |
| Class IV | 0 (0) | 0 (0) | 2.6 (5) |
Percent (n), all such values
Chronic obstructive pulmonary disease
American Society of Anesthesiologists
The majority of patients received only an inguinal hernia repair and no other concurrent operation (Robot: 98.6% [68] vs. Lap: 99.2% [239] vs. Open: 98.4% [188], p = 0.76). There was a significant difference in ASA classification, with 55% of open repair patients being categorized as Class 3 or 4, compared with 10% for Robot, and 37% for Lap (Table 2). Operative duration (total time in the operating room) was significantly longer for patients undergoing robotic-assisted inguinal hernia repair (Robot: 105 [76–146] vs. Lap: 81 [61–103] vs. Open: 71 [56–88] min, p < 0.001, Fig. 1). Physician charges were not significantly different between groups (Robot: $2663 [$1350–3376] vs. Lap: $2239 [$2046–2824] vs. Open: $2186 [$1852–2871], p = 0.82), but total hospital charges (Robot: $27,017 [$20,993–34,443] vs. Lap: $16,016 [$11,444–21,761] vs. Open: $14,190 [$11,305–16,889], p < 0.001) and hospital cost (Robot: $7162 [$5942–8375] vs. Lap: $4527 [$2310–6003] vs. Open: $4264 [$3277–5,143], p < 0.001) were significantly different, with robotic-assisted inguinal hernia repairs being the most expensive (Fig. 2).
Fig. 1.
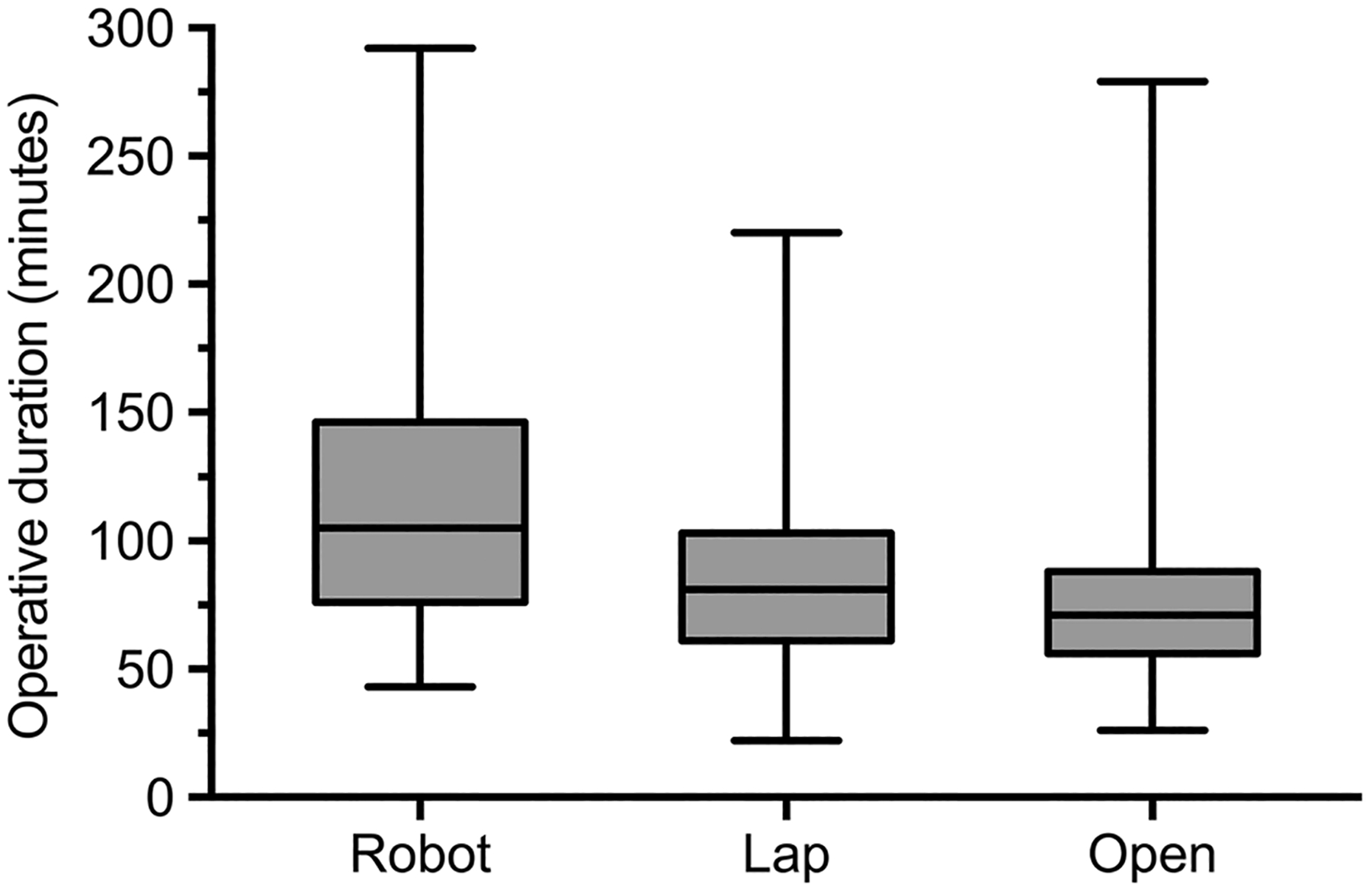
Total time in the operating room for robotic, laparoscopic, and open inguinal hernia repairs (Robot: 105 [76–146] vs. Lap: 81 [61–103] vs. Open: 71 [56–88] min, p < 0.001). Median [interquartile range], all such values
Fig. 2.
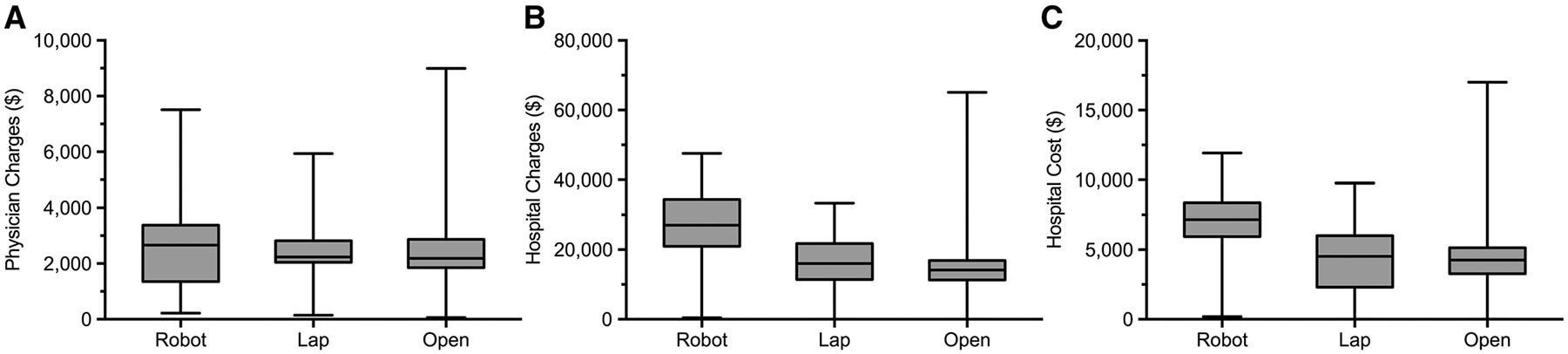
A Physician charges per case for robotic, laparoscopic, and open inguinal hernia repairs. (Robot: $2663 [$1350–3376] vs. Lap: $2239 [$2046–2824] vs. Open: $2186 [$1852–2871], p = 0.82). B Total hospital charges per case for robotic, laparoscopic, and open inguinal hernia repairs (Robot: $27,017 [$20,993–34,443] vs. Lap: $16,016 [$11,444–21,761] vs. Open: $14,190 [$11,305–16,889], p < 0.001). C Hospital cost per case for robotic, laparoscopic, and open inguinal hernia repairs (Robot: $7162 [$5942–8375] vs. Lap: $4527 [$2310–6003] vs. Open: $4264 [$3277–5143], p < 0.001). Median [interquartile range], all such values
Rates of any postoperative occurrence (adverse events, readmissions, and death) were similar between the groups (Robot: 2.9% [2], Lap: 3.3% [8], Open: 5.2% [10], p = 0.53), as seen in Table 3. There were no 30-day mortalities and all patients except one who underwent laparoscopic repair were discharged home the same day. Although rare, there was a significant difference in rate of postoperative skin and soft tissue infection (Robot: 2.9% [2] vs. Lap: 0% [0] vs. Open: 0.5% [1], p = 0.02). There were no hernia recurrences within one month after repair and the rate of related readmissions within 30-days was not significantly different between groups (Robot: 0% [0], Lap: 2.1% [5], Open: 3.7% [7], p = 0.21, Table 3).
Table 3.
Postoperative outcomes
| Robot (n = 69) | Lap (n = 241) | Open (n = 191) | p value | |
|---|---|---|---|---|
| Discharged home same day | 100 (69)a | 99.6 (240) | 100 (191) | 0.58 |
| Mortality with 30-days | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Any postoperative occurrence | 2.9 (2) | 3.3 (8) | 5.2 (10) | 0.53 |
| Adverse events | ||||
| Hernia recurrence | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Acute renal failure | 0 (0) | 0.4 (1) | 0 (0) | 0.58 |
| Stroke | 0 (0) | 0.4 (1) | 0.5 (1) | 0.84 |
| Myocardial Infarction | 0 (0) | 0 (0) | 0.5 (1) | 0.44 |
| Urinary tract infection | 0 (0) | 0.4 (1) | 0 (0) | 0.58 |
| Red blood cell transfusion | 0 (0) | 0.4 (1) | 0 (0) | 0.58 |
| Skin and soft tissue infection | 2.9 (2) | 0 (0) | 0.5 (1) | 0.02 |
| Sepsis | 0 (0) | 0.4 (1) | 0.5 (1) | 0.84 |
| Pulmonary embolism | 0 (0) | 0 (0) | 0.5 (1) | 0.44 |
| Other | 0 (0) | 0 (0) | 0.5 (1) | 0.44 |
| Readmission | ||||
| Related | 0 (0) | 2.1 (5) | 3.7 (7) | 0.21 |
| Unrelated | 0 (0) | 0.4 (1) | 0 (0) | 0.58 |
Percent (n), all such values
Discussion
The present study compared outcomes after robotic-assisted, laparoscopic, and open inguinal hernia repairs to determine if there were benefits to performing this operation with robotic-assist compared to more conventional methods of repair. When evaluating outcomes based on the composite endpoint of any postoperative occurrence (adverse events, readmissions, recurrences, and death), there were no significant differences between all three surgical approaches. Operative duration was significantly longer in robotic-assisted procedures and the rate of postoperative skin and soft tissue infections, although low overall, was higher in cases performed with the robot. Hospital charges and cost were higher in robotic-assisted cases. Hernia recurrences, readmissions, and number of deaths were similar between all three groups.
Robotic-assisted operations have become very common in certain surgical specialties, such as gynecology and urology. Pelvic operations are notoriously challenging due to anatomic and visual constraints, making the robot the ideal solution to provide the surgeon with improved dexterity and visualization. Urologists performing robotic-assisted radical prostatectomies have described concurrent inguinal hernia repair as a safe and effective method to mitigate the high rate of postoperative hernia formation [10–12]. Subsequently, surgeons have begun to use the robot to perform primary inguinal hernia repairs, but there is little published data identifying what benefits the robot may provide in this population.
As with any new technology, learning to efficiently operate with robotic-assist is associated with a significant learning curve [13]. Considering the time and monetary investment required to become proficient operating with the robot, determining whether or not there are benefits to using the technology is an important first step. The present study found that robotic-assisted inguinal hernia operations take longer and are associated with higher hospital charges and cost, but had similar outcomes to laparoscopic and open repairs. In a cohort of one hundred consecutive robotic-assisted inguinal hernia repairs, Arcerito M et al. report minimal postoperative pain, resumption of normal activity within 4 days, and only 1 recurrence in 12 months, but did not compare robotic-assisted patients with laparoscopic or open repair patients [14]. In a case report, Cetrulo and colleagues describe using the robot to perform repair of a large, incarcerated recurrent inguinal hernia and credit the robot with allowing for fine dissection and appropriate visualization of structures [15]. In select patients, use of the robot appears to provide benefits to the surgeon that he or she would not otherwise have with the laparoscopic or open approach but whether these benefits persist for all patients needing inguinal herniorrhaphy is yet to be determined.
In the present study, the rate of postoperative occurrences after robotic-assisted, laparoscopic, and open inguinal hernia repairs was similar, with no identifiable advantage to any one surgical approach. Postoperative pain scores were not obtained, but in a single-surgeon experience comparing 24 laparoscopic repairs to 39 robotic-assisted repairs, postoperative pain in recovery was significantly less for the robotic-assisted repairs [16]. The authors also note that operative duration was significantly longer for cases completed with the robot compared to laparoscopic repairs. These findings support the results of the present study, which found that operative duration was longest for robotic-assisted repairs, with open repairs having the shortest total time in the operating room. Long operative durations for some robot cases can be attributed to the inevitable learning curve. All operations had resident involvement, which likely contributed to longer operating times for all repairs on average. Of note, robotic-assisted cases also had the highest prevalence, albeit rare, of postoperative skin and soft tissue infections, which have been associated with longer operative times [17]. Additional reasons to explain the higher infection rate include familiarity with draping and docking the robot, different operating surgeons, and patient demographics.
Hospital charges and total cost were highest for robotic-assisted inguinal hernia repairs. As is expected with new technology, cost will remain high until the technology is widely accepted and adopted into routine clinical practice. Determining whether or not to pursue the use of expensive technology, in this case the surgical robot, is challenging considering that an improvement in patient outcomes may not be apparent for quite some time.
The present study and its findings are limited by the retrospective nature of the study, the single-institution design, and the lack of long-term follow up data, quality-of-life data, and postoperative pain scores. Considering that postoperative morbidities and mortalities after inguinal hernia repair are exceedingly rare, detecting any significant differences would be difficult with a randomized controlled trial, let alone the retrospective, single-institution experience described here. Additionally, mesh type was not standardized and the benefit of minimally invasive surgery to repair bilateral hernias was not captured in these data. That being said, we present a fairly large cohort of 510 inguinal hernia repairs over a 4-year period and compare three surgical approaches. As is evident by the difference in age and ASA classification between groups, selection bias is present as well. In light of robotic-assisted repair patients being younger and having lower average ASA scores, outcomes were still similar between all three groups.
Outcomes after robotic-assisted inguinal hernia repair were similar to laparoscopic and open repairs in this single-institution cohort analysis. The frequency of postoperative adverse events, recurrences, readmissions, and deaths was not different regardless of surgical approach. Although the robot provides improved dexterity and visualization, whether these advantages benefit patients undergoing inguinal hernia repair is yet to be determined as no clinical benefit was identified. Longer operative duration during robotic repair may contribute to higher rates of skin and soft tissue infection but surgeon and resident efficiency with robotic-assisted cases is likely to continue to increase as use of the robot becomes more routine and robot access becomes more ubiquitous. Total cost was highest for robotic-assisted inguinal hernia repairs, with no apparent return on investment in terms of improved patient outcomes. Until large dataset analyses or randomized controlled trials can be completed, hospital costs, surgeon comfort level, and patient preference should dictate whether inguinal hernia repair is approached robotically.
Acknowledgements
The authors acknowledge Kathleen B. Meneses for her assistance maintaining the robotic surgery database.
Funding
The National Heart, Lung, and Blood Institute under award numbers T32 HL007849 (JHM) and UM1 HL088925 (EJC) supported research reported in this publication.
Disclosures
Dr. Hallowell reports an educational grant and travel expenses for an educational course from Intuitive Surgical, Inc. Dr. Sawyer reports consulting fees from 3M, Merck & Co., Inc., Pfizer Inc., and GlaxoSmithKline. Drs. Charles, Mehaffey, Tache-Leon, and Yang have no conflicts of interest or financial ties to disclose. The National Heart, Lung, and Blood Institute under award numbers T32 HL007849 (JHM) and UM1 HL088925 (EJC) supported research reported in this publication.
Footnotes
Presented at the SAGES 2017 Annual Meeting, March 22–25, 2017, Houston, Texas.
References
- 1.Heikkinen T, Bringman S, Ohtonen P, Kunelius P, Haukipuro K, Hulkko A (2004) Five-year outcome of laparoscopic and Lichtenstein hernioplasties. Surg Endosc 18:518–522 [DOI] [PubMed] [Google Scholar]
- 2.Lau WY (2002) History of treatment of groin hernia. World J Surg 26:748–759 [DOI] [PubMed] [Google Scholar]
- 3.Alkatout I, Mettler L, Maass N, Ackermann J (2016) Robotic surgery in gynecology. J Turk Ger Gynecol Assoc 17:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitwood WR Jr (2016) Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 5:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SH, Lee HM, Son T, Hyung WJ, Kim HI (2016) Robotic surgery for gastric tumor: current status and new approaches. Transl Gastroenterol Hepatol 1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohd Azman ZA, Kim SH (2016) A review on robotic surgery in rectal cancer. Transl Gastroenterol Hepatol 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoikes N, Webb D, Voeller G (2016) Robotic hernia repair. Surg Technol Int 29:119–122 [PubMed] [Google Scholar]
- 8.Escobar Dominguez JE, Gonzalez A, Donkor C (2015) Robotic inguinal hernia repair. J Surg Oncol 112:310–314 [DOI] [PubMed] [Google Scholar]
- 9.Warren JA, Cobb WS, Ewing JA, Carbonell AM (2017) Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc 31:324–332 [DOI] [PubMed] [Google Scholar]
- 10.Collins JN, Britt RC, Britt LD (2011) Concomitant robotic repair of inguinal hernia with robotic prostatectomy. Am Surg 77:238–239 [PubMed] [Google Scholar]
- 11.Kyle CC, Hong MK, Challacombe BJ, Costello AJ (2010) Outcomes after concurrent inguinal hernia repair and robotic-assisted radical prostatectomy. J Robot Surg 4:217–220 [DOI] [PubMed] [Google Scholar]
- 12.Ito F, Jarrard D, Gould JC (2008) Transabdominal preperitoneal robotic inguinal hernia repair. J Laparoendosc Adv Surg Tech A 18:397–399 [DOI] [PubMed] [Google Scholar]
- 13.Andolfi C, Umanskiy K (2017) Mastering robotic surgery: where does the learning curve lead us? J Laparoendosc Adv Surg Tech A 27(5):470–474 [DOI] [PubMed] [Google Scholar]
- 14.Arcerito M, Changchien E, Bernal O, Konkoly-Thege A, Moon J (2016) Robotic inguinal hernia repair: technique and early experience. Am Surg 82:1014–1017 [PubMed] [Google Scholar]
- 15.Cetrulo LN, Harmon J, Ortiz J, Canter D, Joshi AR (2015) Case report of a robotic-assisted laparoscopic repair of a giant incarcerated recurrent inguinal hernia containing bladder and ureters. Int J Med Robot 11:15–17 [DOI] [PubMed] [Google Scholar]
- 16.Waite KE, Herman MA, Doyle PJ (2016) Comparison of robotic versus laparoscopic transabdominal preperitoneal (TAPP) inguinal hernia repair. J Robot Surg 10:239–244 [DOI] [PubMed] [Google Scholar]
- 17.Campbell DA Jr, Henderson WG, Englesbe MJ, Hall BL, O’Reilly M, Bratzler D, Dellinger EP, Neumayer L, Bass BL, Hutter MM, Schwartz J, Ko C, Itani K, Steinberg SM, Siperstein A, Sawyer RG, Turner DJ, Khuri SF (2008) Surgical site infection prevention: the importance of operative duration and blood transfusion–results of the first American College of Surgeons-National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg 207:810–820 [DOI] [PubMed] [Google Scholar]


