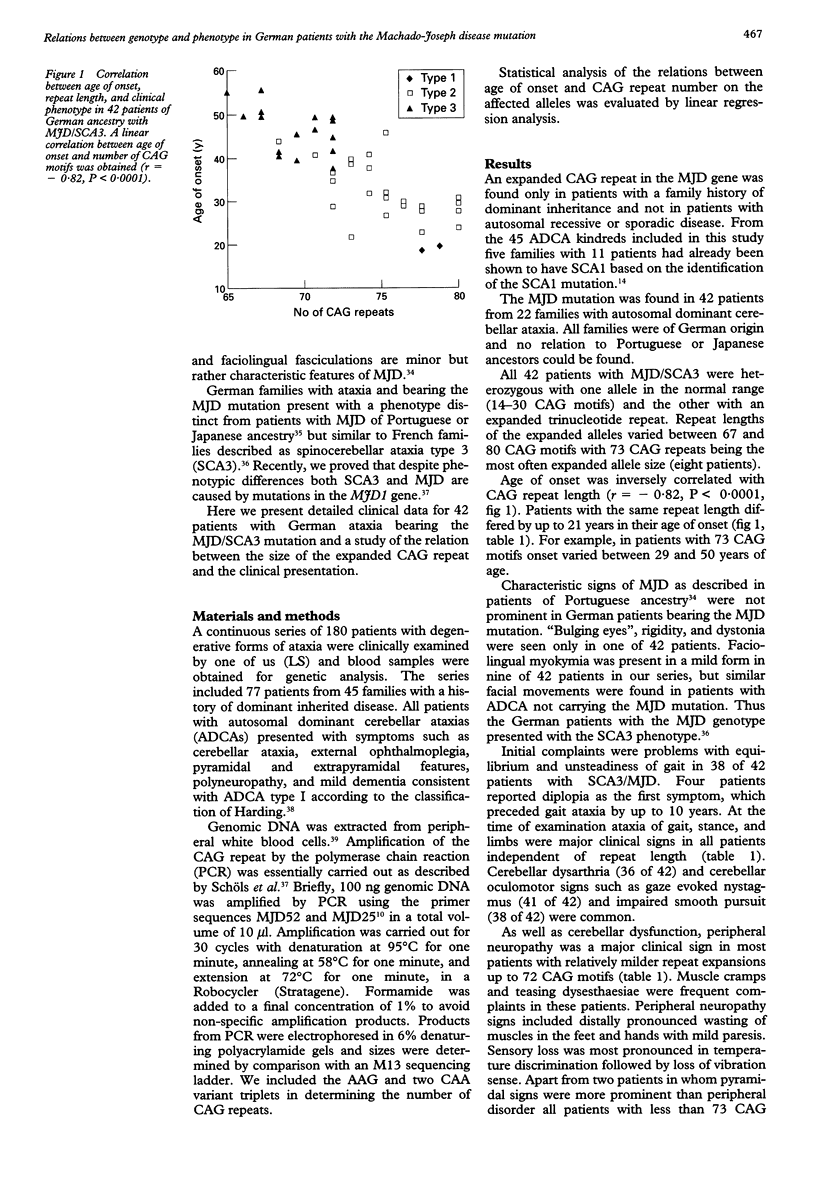
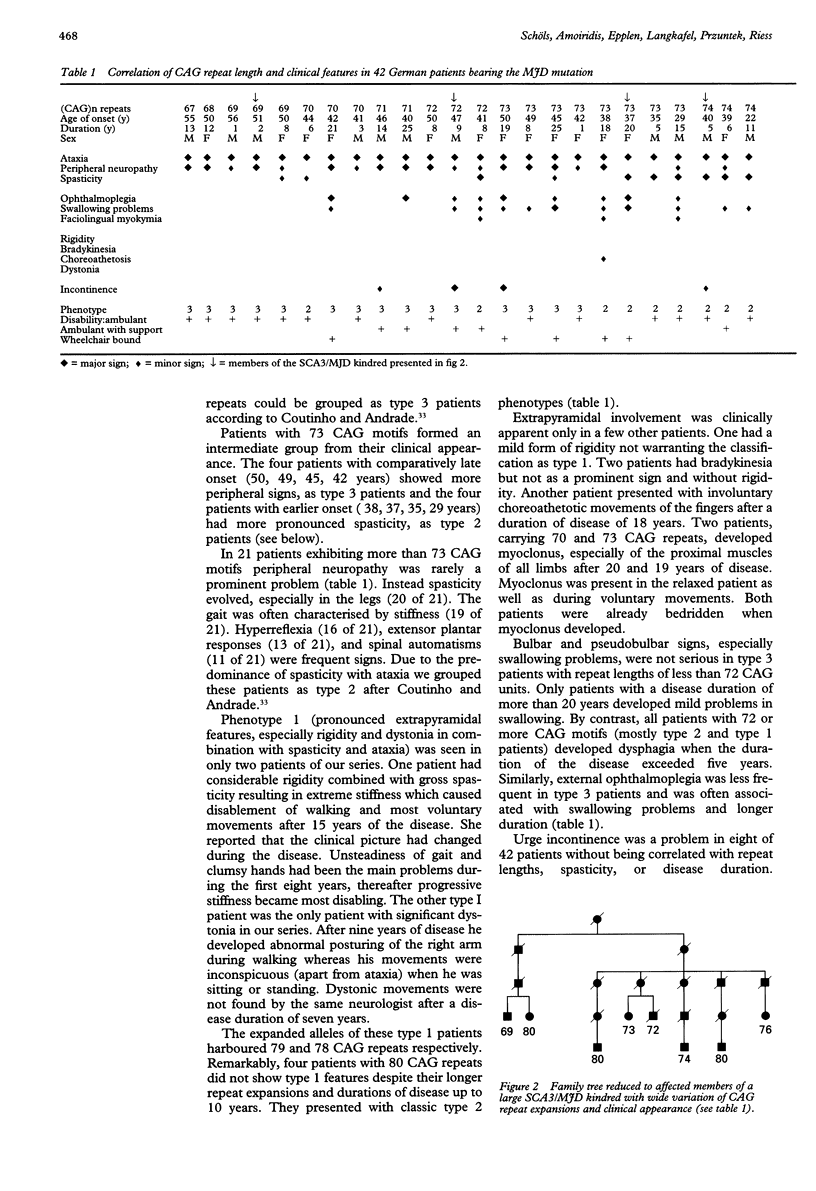
Abstract
OBJECTIVE: Machado-Joseph disease (MJD) is an autosomal dominant cerebellar ataxia with extensive phenotypic variability originally described in families of Portuguese ancestry. Recently, the mutation causing the disease has been identified as an expanded CAG trinucleotide repeat. In this study relations between genotype and phenotype were investigated. METHODS: A series of 180 German patients with degenerative forms of ataxia were clinically and genetically examined. Patients bearing the MJD mutation were assigned to three phenotypes: phenotype 1 characterised by early onset and dystonia or pronounced rigidity associated with ataxia and spasticity. Main symptoms in phenotype 2 were ataxia and spasticity. In phenotype 3 onset was relatively late and peripheral neuropathy accompanied ataxia. Clinical and molecular data were correlated. RESULTS: An expanded CAG array was found in 42 patients from 22 families. Repeat length of CAG varied between 67 and 80 CAG motifs and showed an inverse correlation with the age of onset. For the development of phenotype 1 early onset (< 20 years) seemed more decisive than extensive repeat length. Phenotype 2 was present in all patients with more than 73 CAG motifs and onset between 20 and 40. Phenotype 3 developed in most patients with less than 73 CAG motifs and onset was regularly beyond the age of 40. Intrafamilial variability of both repeat length and phenotype was large reflecting meiotic instability of the expanded CAG repeat. CONCLUSIONS: The MJD mutation is the most frequent cause of dominantly inherited ataxia in Germany. Variations in repeat lengths substantially influence age of onset as well as phenotype but cannot explain why MJD characteristics of Portuguese families such as "bulging eyes", dystonia, and rigidity are essentially missing in German families. Despite the genotypic and phenotypic relations found in this study a reliable individual prognosis of the course of the disease is not possible at a presymptomatic stage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M. A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993 Aug;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Barbeau A., Roy M., Cunha L., de Vincente A. N., Rosenberg R. N., Nyhan W. L., MacLeod P. L., Chazot G., Langston L. B., Dawson D. M. The natural history of Machado-Joseph disease. An analysis of 138 personally examined cases. Can J Neurol Sci. 1984 Nov;11(4 Suppl):510–525. doi: 10.1017/s0317167100034983. [DOI] [PubMed] [Google Scholar]
- Benomar A., Krols L., Stevanin G., Cancel G., LeGuern E., David G., Ouhabi H., Martin J. J., Dürr A., Zaim A. The gene for autosomal dominant cerebellar ataxia with pigmentary macular dystrophy maps to chromosome 3p12-p21.1. Nat Genet. 1995 May;10(1):84–88. doi: 10.1038/ng0595-84. [DOI] [PubMed] [Google Scholar]
- Doyu M., Sobue G., Mukai E., Kachi T., Yasuda T., Mitsuma T., Takahashi A. Severity of X-linked recessive bulbospinal neuronopathy correlates with size of the tandem CAG repeat in androgen receptor gene. Ann Neurol. 1992 Nov;32(5):707–710. doi: 10.1002/ana.410320517. [DOI] [PubMed] [Google Scholar]
- Dubourg O., Dürr A., Cancel G., Stevanin G., Chneiweiss H., Penet C., Agid Y., Brice A. Analysis of the SCA1 CAG repeat in a large number of families with dominant ataxia: clinical and molecular correlations. Ann Neurol. 1995 Feb;37(2):176–180. doi: 10.1002/ana.410370207. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Gouw L. G., Kaplan C. D., Haines J. H., Digre K. B., Rutledge S. L., Matilla A., Leppert M., Zoghbi H. Y., Ptácek L. J. Retinal degeneration characterizes a spinocerebellar ataxia mapping to chromosome 3p. Nat Genet. 1995 May;10(1):89–93. doi: 10.1038/ng0595-89. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Koide R., Tanaka H., Onodera O., Igarashi S., Takahashi H., Kondo R., Ishikawa A., Tomoda A., Miike T. Dentatorubral-pallidoluysian atrophy: clinical features are closely related to unstable expansions of trinucleotide (CAG) repeat. Ann Neurol. 1995 Jun;37(6):769–775. doi: 10.1002/ana.410370610. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- Komure O., Sano A., Nishino N., Yamauchi N., Ueno S., Kondoh K., Sano N., Takahashi M., Murayama N., Kondo I. DNA analysis in hereditary dentatorubral-pallidoluysian atrophy: correlation between CAG repeat length and phenotypic variation and the molecular basis of anticipation. Neurology. 1995 Jan;45(1):143–149. doi: 10.1212/wnl.45.1.143. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Roling D. B., Harding A. E., Warner C. L., Spiegel R., Hausmanowa-Petrusewicz I., Yee W. C., Fischbeck K. H. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992 Dec;2(4):301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Lima L., Coutinho P. Clinical criteria for diagnosis of Machado-Joseph disease: report of a non-Azorena Portuguese family. Neurology. 1980 Mar;30(3):319–322. doi: 10.1212/wnl.30.3.319. [DOI] [PubMed] [Google Scholar]
- Maciel P., Gaspar C., DeStefano A. L., Silveira I., Coutinho P., Radvany J., Dawson D. M., Sudarsky L., Guimarães J., Loureiro J. E. Correlation between CAG repeat length and clinical features in Machado-Joseph disease. Am J Hum Genet. 1995 Jul;57(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Nakamura S., Matsuyama Z., Sakai T., Doyu M., Sobue G., Seto M., Tsujihata M., Oh-i T., Nishio T. Molecular features of the CAG repeats and clinical manifestation of Machado-Joseph disease. Hum Mol Genet. 1995 May;4(5):807–812. doi: 10.1093/hmg/4.5.807. [DOI] [PubMed] [Google Scholar]
- Matilla T., McCall A., Subramony S. H., Zoghbi H. Y. Molecular and clinical correlations in spinocerebellar ataxia type 3 and Machado-Joseph disease. Ann Neurol. 1995 Jul;38(1):68–72. doi: 10.1002/ana.410380113. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Sato K., Shirayama T., Ohsaki E., Bundo M., Takeda T., Tadokoro K., Kondo I., Murayama N. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994 Jan;6(1):14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- Nakano K. K., Dawson D. M., Spence A. Machado disease. A hereditary ataxia in Portuguese emigrants to Massachusetts. Neurology. 1972 Jan;22(1):49–55. doi: 10.1212/wnl.22.1.49. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Schut L. J., Lundgren J. K., Orr H. T., Livingston D. M. Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet. 1994 Nov;8(3):280–284. doi: 10.1038/ng1194-280. [DOI] [PubMed] [Google Scholar]
- Romanul F. C., Fowler H. L., Radvany J., Feldman R. G., Feingold M. Azorean disease of the nervous system. N Engl J Med. 1977 Jun 30;296(26):1505–1508. doi: 10.1056/NEJM197706302962606. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N. Machado-Joseph disease: an autosomal dominant motor system degeneration. Mov Disord. 1992;7(3):193–203. doi: 10.1002/mds.870070302. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N., Nyhan W. L., Bay C., Shore P. Autosomal dominant striatonigral degeneration. A clinical, pathologic, and biochemical study of a new genetic disorder. Neurology. 1976 Aug;26(8):703–714. doi: 10.1212/wnl.26.8.703. [DOI] [PubMed] [Google Scholar]
- Schöls L., Riess O., Schöls S., Zeck S., Amoiridis G., Langkafel M., Epplen J. T., Przuntek H. Spinocerebellar ataxia type 1: Clinical and neurophysiological characteristics in German kindreds. Acta Neurol Scand. 1995 Dec;92(6):478–485. doi: 10.1111/j.1600-0404.1995.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Schöls L., Vieira-Saecker A. M., Schöls S., Przuntek H., Epplen J. T., Riess O. Trinucleotide expansion within the MJD1 gene presents clinically as spinocerebellar ataxia and occurs most frequently in German SCA patients. Hum Mol Genet. 1995 Jun;4(6):1001–1005. doi: 10.1093/hmg/4.6.1001. [DOI] [PubMed] [Google Scholar]
- Sequeiros J., Coutinho P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv Neurol. 1993;61:139–153. [PubMed] [Google Scholar]
- Stevanin G., Le Guern E., Ravisé N., Chneiweiss H., Dürr A., Cancel G., Vignal A., Boch A. L., Ruberg M., Penet C. A third locus for autosomal dominant cerebellar ataxia type I maps to chromosome 14q24.3-qter: evidence for the existence of a fourth locus. Am J Hum Genet. 1994 Jan;54(1):11–20. [PMC free article] [PubMed] [Google Scholar]
- Takiyama Y., Igarashi S., Rogaeva E. A., Endo K., Rogaev E. I., Tanaka H., Sherrington R., Sanpei K., Liang Y., Saito M. Evidence for inter-generational instability in the CAG repeat in the MJD1 gene and for conserved haplotypes at flanking markers amongst Japanese and Caucasian subjects with Machado-Joseph disease. Hum Mol Genet. 1995 Jul;4(7):1137–1146. doi: 10.1093/hmg/4.7.1137. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Woods B. T., Schaumburg H. H. Nigro-spino-dentatal degeneration with nuclear ophthalmoplegia. A unique and partially treatable clinico-pathological entity. J Neurol Sci. 1972 Oct;17(2):149–166. doi: 10.1016/0022-510x(72)90137-2. [DOI] [PubMed] [Google Scholar]
- Yakura H., Wakisaka A., Fujimoto S., Itakura K. Letter: Hereditary ataxia and HL-A. N Engl J Med. 1974 Jul 18;291(3):154–155. doi: 10.1056/NEJM197407182910314. [DOI] [PubMed] [Google Scholar]


