Abstract
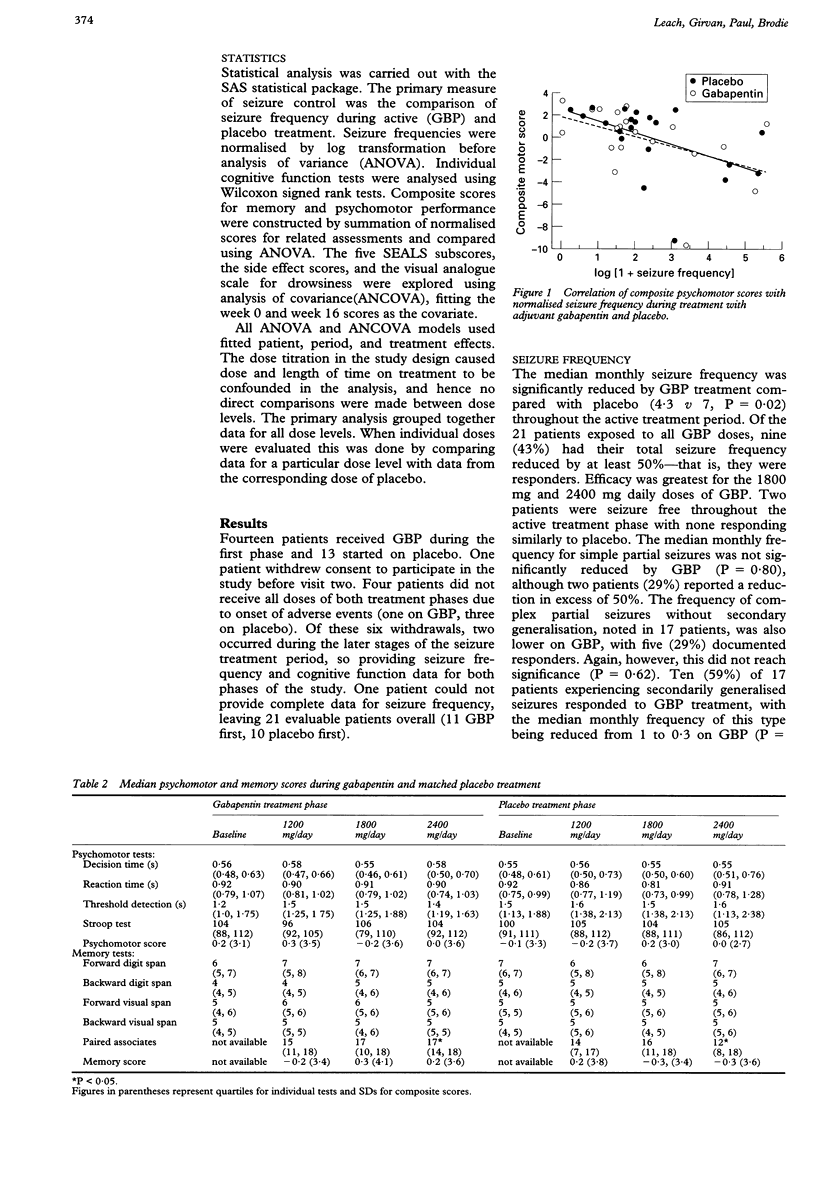
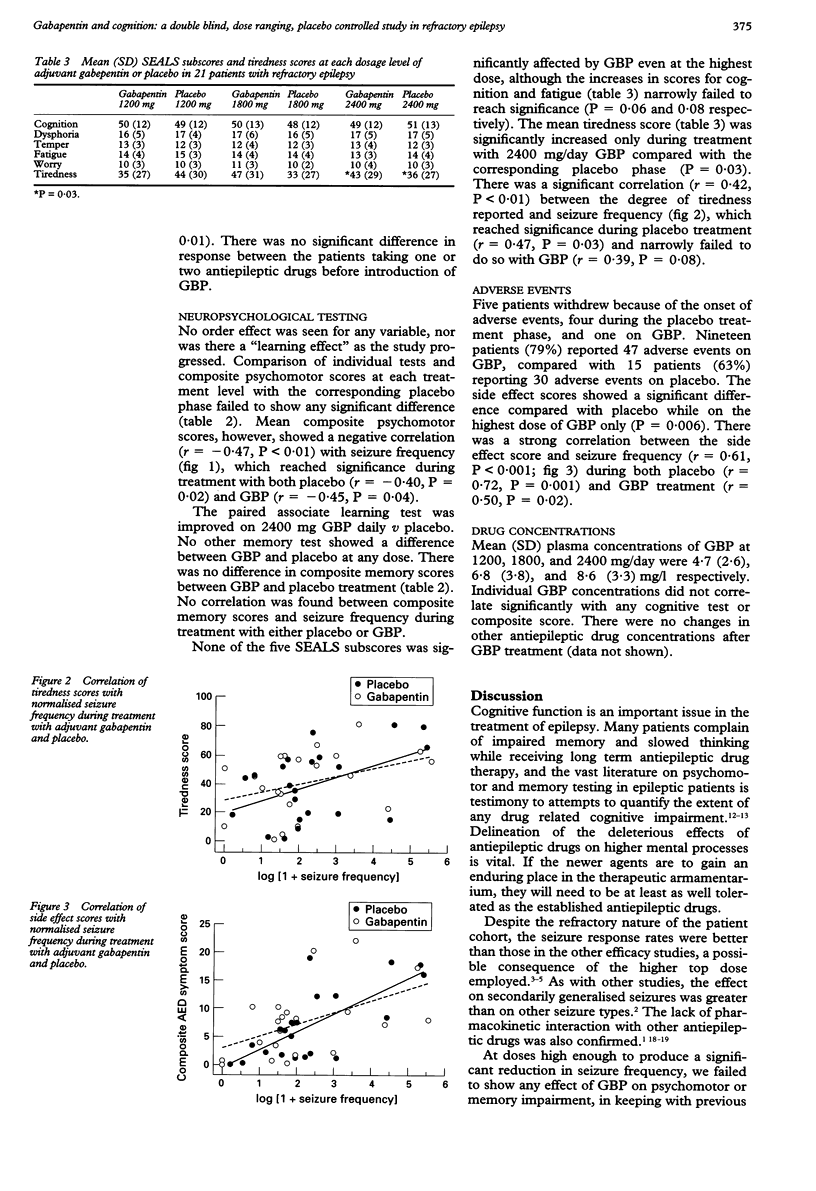
OBJECTIVE: To assess the effect of different doses of gabapentin (GBP) on cognitive function in treated epileptic patients. METHODS: Twenty seven patients with refractory partial seizures commenced a double blind, dose ranging, placebo controlled, crossover study of adjuvant GBP. Each treatment phase lasted three months, during which the dose of GBP or matched placebo was increased stepwise at intervals of four weeks (1200 mg/day, 1800 mg/day, and 2400 mg/day in three daily doses). Psychomotor and memory testing was carried out at the end of each four week period, at which time the patient also completed subjective measures of cognition, fatigue, worry, temper, and dysphoria. A visual analogue scale was used to assess drowsiness and a questionnaire was employed to gauge the severity of side effects. RESULTS: In the 21 patients completing the study, GBP produced a significant reduction in median monthly seizure frequency from 7 to 4.3 (P = 0.02), the decrease being most pronounced for secondarily generalised seizures (from 1.0 to 0.3, P = 0.01). Forty three per cent of patients reported a reduction in seizure frequency of at least 50% throughout all GBP doses. Mean (SD) plasma concentrations of GBP at 1200, 1800, and 2400 mg/day were 4.7 (2.6), 6.8 (3.8), and 8.6 (3.3) mg/l respectively. The drug had no effect on composite psychomotor and memory scores; nor was there alteration in any self assessment subscore. The mean drowsiness (P = 0.03) score was higher during treatment with 2400 mg GBP daily compared with matched placebo. Composite psychomotor (r = -0.47, P < 0.01), tiredness (r = 0.42, P < 0.01), and side effect (r = 0.61, P < 0.001) scores correlated significantly with seizure frequency but not with GBP dose. CONCLUSION: GBP is a well tolerated and effective antiepileptic drug which had no measurable effect on cognition but did produce sedation at the highest dose. This study also supports the suggestion that seizures can cause cognitive impairment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anhut H., Ashman P., Feuerstein T. J., Sauermann W., Saunders M., Schmidt B. Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. The International Gabapentin Study Group. Epilepsia. 1994 Jul-Aug;35(4):795–801. doi: 10.1111/j.1528-1157.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., McPhail E., Macphee G. J., Larkin J. G., Gray J. M. Psychomotor impairment and anticonvulsant therapy in adult epileptic patients. Eur J Clin Pharmacol. 1987;31(6):655–660. doi: 10.1007/BF00541291. [DOI] [PubMed] [Google Scholar]
- Chadwick D. Gabapentin. Lancet. 1994 Jan 8;343(8889):89–91. doi: 10.1016/s0140-6736(94)90820-6. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Brodie M. J. New antiepileptic drugs. N Engl J Med. 1996 Jun 13;334(24):1583–1590. doi: 10.1056/NEJM199606133342407. [DOI] [PubMed] [Google Scholar]
- Forrest G., Sills G. J., Leach J. P., Brodie M. J. Determination of gabapentin in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996 Jun 7;681(2):421–425. doi: 10.1016/0378-4347(96)00074-6. [DOI] [PubMed] [Google Scholar]
- Gillham R. A., Blacklaw J., McKee P. J., Brodie M. J. Effect of vigabatrin on sedation and cognitive function in patients with refractory epilepsy. J Neurol Neurosurg Psychiatry. 1993 Dec;56(12):1271–1275. doi: 10.1136/jnnp.56.12.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham R. A., Williams N., Wiedmann K. D., Butler E., Larkin J. G., Brodie M. J. Cognitive function in adult epileptic patients established on anticonvulsant monotherapy. Epilepsy Res. 1990 Dec;7(3):219–225. doi: 10.1016/0920-1211(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Gillham R. A., Williams N., Wiedmann K., Butler E., Larkin J. G., Brodie M. J. Concentration-effect relationships with carbamazepine and its epoxide on psychomotor and cognitive function in epileptic patients. J Neurol Neurosurg Psychiatry. 1988 Jul;51(7):929–933. doi: 10.1136/jnnp.51.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick E. S., Forrest G., Brodie M. J. Concentration--effect and concentration--toxicity relations with lamotrigine: a prospective study. Epilepsia. 1996 Jun;37(6):534–538. doi: 10.1111/j.1528-1157.1996.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Larkin J. G., McKee P. J., Brodie M. J. Rapid tolerance to acute psychomotor impairment with carbamazepine in epileptic patients. Br J Clin Pharmacol. 1992 Jan;33(1):111–114. doi: 10.1111/j.1365-2125.1992.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee P. J., Blacklaw J., Friel E., Thompson G. G., Gillham R. A., Brodie M. J. Adjuvant vigabatrin in refractory epilepsy: a ceiling to effective dosage in individual patients? Epilepsia. 1993 Sep-Oct;34(5):937–943. doi: 10.1111/j.1528-1157.1993.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Radulovic L. L., Wilder B. J., Leppik I. E., Bockbrader H. N., Chang T., Posvar E. L., Sedman A. J., Uthman B. M., Erdman G. R. Lack of interaction of gabapentin with carbamazepine or valproate. Epilepsia. 1994 Jan-Feb;35(1):155–161. doi: 10.1111/j.1528-1157.1994.tb02926.x. [DOI] [PubMed] [Google Scholar]
- Vermeulen J., Aldenkamp A. P. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res. 1995 Oct;22(2):65–95. doi: 10.1016/0920-1211(95)00047-x. [DOI] [PubMed] [Google Scholar]


