Abstract
The high-molecular-weight secondary cell wall polymer (SCWP) from Bacillus stearothermophilus PV72/p2 is mainly composed of N-acetylglucosamine (GlcNAc) and N-acetylmannosamine (ManNAc) and is involved in anchoring the S-layer protein via its N-terminal region to the rigid cell wall layer. In addition to this binding function, the SCWP was found to inhibit the formation of self-assembly products during dialysis of the guanidine hydrochloride (GHCl)-extracted S-layer protein. The degree of assembly (DA; percent assembled from total S-layer protein) that could be achieved strongly depended on the amount of SCWP added to the GHCl-extracted S-layer protein and decreased from 90 to 10% when the concentration of the SCWP was increased from 10 to 120 μg/mg of S-layer protein. The SCWP kept the S-layer protein in the water-soluble state and favored its recrystallization on solid supports such as poly-l-lysine-coated electron microscopy grids. Derived from the orientation of the base vectors of the oblique S-layer lattice, the subunits had bound with their charge-neutral outer face, leaving the N-terminal region with the polymer binding domain exposed to the ambient environment. From cell wall fragments about half of the S-layer protein could be extracted with 1 M GlcNAc, indicating that the linkage type between the S-layer protein and the SCWP could be related to that of the lectin-polysaccharide type. Interestingly, GlcNAc had an effect on the in vitro self-assembly and recrystallization properties of the S-layer protein that was similar to that of the isolated SCWP. The SCWP generally enhanced the stability of the S-layer protein against endoproteinase Glu-C attack and specifically protected a potential cleavage site in position 138 of the mature S-layer protein.
Many bacteria and archaea possess crystalline bacterial cell surface layers (S-layers) as their outermost cell envelope component (3, 36, 38). S-layers are composed of identical protein or glycoprotein subunits which assemble into two-dimensional crystalline arrays showing oblique, square, or hexagonal lattice symmetry. S-layer subunits from bacteria are linked to each other and to the underlying cell envelope layer by noncovalent interactions and may therefore be isolated from whole cells or cell wall fragments by different procedures involving chaotropic agents, detergents, chelating agents, or high salt concentrations or by alkaline or acidic pH conditions. During removal of the disrupting agents, e.g., by dialysis, the S-layer subunits frequently reassemble into flat sheets or open-ended cylinders (in vitro self-assembly in suspension; for reviews, see references 37 and 38).
Studies regarding the binding mechanism between the S-layer protein and the underlying cell envelope layer have shown that in gram-negative bacteria, the N-terminal region of the S-layer subunits recognizes specific lipopolysaccharides in the outer membrane (9, 29, 41). For Aeromonas hydrophila it was found, however, that the C-terminal part of the S-layer protein is essential for interaction with the outer membrane (40). A similar observation was reported for the S-layer protein from the gram-positive Corynebacterium glutamicum. A hydrophobic stretch of 21 amino acids located at the C-terminal end of the S-layer protein was found to interact with a hydrophobic layer in the cell wall proper that most probably consisted of mycolic acid (8). In earlier studies it was suggested that secondary cell wall polymers could represent the binding sites for the S-layer proteins from Bacillus sphaericus (15, 16) and Lactobacillus buchneri (24).
Recently, a high-molecular-weight secondary cell wall polymer (SCWP) containing glucose and N-acetylglucosamine (GlcNAc) was extracted from peptidoglycan-containing sacculi of two Bacillus stearothermophilus wild-type strains (PV72/p6 and ATCC 12980 [10]). An SCWP of different chemical composition could be isolated from peptidoglycan-containing sacculi of an oxygen-induced variant strain from B. stearothermophilus PV72/p6 (35). The SCWP produced by this variant strain (B. stearothermophilus PV72/p2) is mainly composed of GlcNAc and N-acetylmannosamine (ManNAc) and shows a molecular weight of about 24,000 (33). Binding studies with proteolytic cleavage fragments and native peptidoglycan-containing sacculi revealed that the N-terminal region is involved in anchoring the S-layer subunits to the rigid cell wall layer (10, 11, 33). Several observations have supported the notion that a specific recognition and binding mechanism exists between the SCWP and the N-terminal region of the S-layer proteins from B. stearothermophilus strains. (i) Despite the overall heterogeneity, S-layer proteins from B. stearothermophilus wild-type strains possess an identical N-terminal region and are capable of binding to an SCWP of identical chemical composition. (ii) B. stearothermophilus PV72/p6 and the oxygen-induced p2 variant produce an SCWP of different chemical composition and structure. (iii) The S-layer protein from B. stearothermophilus PV72/p2 did not recognize native peptidoglycan-containing sacculi from B. stearothermophilus wild-type strains as binding sites (35). (iv) The S-layer protein from B. stearothermophilus PV72/p6 (SbsA) and the oxygen-induced p2 variant (SbsB) are encoded by different genes which show little overall identity (19, 20), and only SbsB possesses a typical S-layer homologous (SLH) domain (23) at the N-terminal part.
By sequence comparison, SLH domains (23) were identified on the N-terminal part of several S-layer proteins (6, 13, 23, 27, 30) or at the very C-terminal end of cell-associated exoenzymes and exoproteins (21, 22, 25, 26). SLH domains were suggested to anchor these proteins permanently or transiently to the cell surface. So far, evidence for a binding function of an SLH domain was provided for the S-layer protein of Thermus thermophilus (30) and for the outer-layer proteins of the cellulosome complex from Clostridium thermocellum (21, 22).
In the present study, the influence of the SCWP on the formation of self-assembly products in suspension and on the recrystallization properties of the S-layer protein from B. stearothermophilus PV72/p2 on solid supports such as poly-l-lysine-coated electron microscopy (EM) grids was investigated. Moreover, studies on the stability of the S-layer protein against endoproteinase Glu-C attack in the presence and the absence of the SCWP were carried out.
MATERIALS AND METHODS
Organism, growth conditions, and preparation of cell wall fragments.
To obtain biomass with defined properties, B. stearothermophilus PV72/p2 was grown in continuous culture on complex SVIII medium in a 5-liter bioreactor (Biostat E; Braun, Melsungen, Germany) at 57°C with 1.2 g of glucose/liter as the major carbon source (35). The dilution rate was kept either at 0.1 h−1 (low specific growth rate) or at 0.4 h−1 (high specific growth rate). The rate of aeration was adjusted to 5 liters of air/min, leading to oxygen-limited growth during continuous cultivation. The pH value was maintained at 7.2 by adding either 1 N NaOH or 2 M H2SO4. Cells were separated from spent medium by continuous centrifugation at 16,000 × g at 4°C. The preparation of cell wall fragments was carried out as described previously (33).
Isolation of the SCWP from peptidoglycan-containing sacculi.
After extraction of the S-layer protein from cell wall fragments with 5 M guanidine hydrochloride (GHCl) in 50 mM Tris-HCl buffer (pH 7.2) for 20 min at 4°C, the suspension was centrifuged at 40,000 × g for 20 min at 4°C. Subsequently, the pellet consisting of peptidoglycan-containing sacculi was washed at least three times with 50 mM Tris-HCl buffer (pH 7.2) at 4°C. In order to inactivate the autolysins, peptidoglycan-containing sacculi were incubated in sodium dodecyl sulfate (SDS) solution (1% in distilled water) for 30 min at 100°C (24). After being cooled to 10°C, peptidoglycan-containing sacculi were sedimented at 40,000 × g for 20 min at 10°C. The pellet was washed six times with distilled water, frozen at −20°C, and lyophilized. The SCWP was subsequently extracted from lyophilized peptidoglycan-containing sacculi with 48% hydrofluoric acid (HF) for 96 h at 4°C (33), and peptidoglycan was separated by centrifugation at 40,000 × g for 20 min at 4°C. The clear supernatant was carefully removed, and the SCWP was precipitated with chilled ethanol (12) and incubated for 24 h at −20°C. After centrifugation at 40,000 × g for 20 min at −10°C, the pellet was washed twice with chilled ethanol (−20°C) and finally dissolved in distilled water. The solution was dialyzed against distilled water at 4°C for 48 h (Biomol membrane type 8; molecular weight cutoff, 12,000 to 16,000).
Chemical analyses of the SCWP.
To obtain information on the molecular weight of the HF-extracted SCWP, 5-mg portions of lyophilized samples were dissolved in 1 ml of 150 mM NaCl in 50 mM Tris-HCl buffer (pH 7.2), and the solutions were applied to a calibrated Sephadex G-150 column (Pharmacia, Uppsala, Sweden). Elution of the SCWP was monitored with a refraction index detector. The homogeneity of the SCWP was examined by reversed-phase high-pressure liquid chromatography (RP-HPLC) according to the method of Bock et al. (5). For amino sugar and neutral sugar analyses, 0.5-mg samples of lyophilized SCWP were hydrolyzed with 2.2 N trifluoroacetic acid for 4 h, 2 N HCl for 2 h, 4 N HCl for 4 h, 4 N HCl for 6 h, 6 N HCl for 2 h, 6 N HCl for 4 h, and 6 N HCl for 6 h, all at 110°C. Hydrolyzed samples were subjected to amino acid HPLC according to the method of Altmann (1), to a DIONEX DX-300 sugar gradient chromatography system (5), and to sugar polyacrylamide gel electrophoresis (PAGE) (17).
Studies on the in vitro self-assembly of the S-layer protein extracted from biomass cultivated at a low or a high specific growth rate.
For extraction of the S-layer protein, 0.5-g wet pellets of cell wall fragments (obtained by centrifugation at 20,000 × g for 20 min at 4°C) were suspended in 10 ml of 5 M GHCl in 50 mM Tris-HCl buffer (pH 7.2) and stirred for 20 min at 4°C. After centrifugation of the suspension at 40,000 × g for 20 min at 4°C, the clear supernatant was carefully removed, centrifuged twice under the same conditions, and finally dialyzed against 10 mM CaCl2 at 20°C (Biomol membrane type 8; molecular weight cutoff, 12,000 to 16,000). Samples were taken 1, 2, 3, 4, and 18 h after starting the dialysis procedure. Self-assembly products were separated from soluble (monomeric and/or oligomeric) S-layer protein (18, 32) by centrifugation at 40,000 × g for 10 min at 4°C. For determination of the degree of assembly (DA; percentage of assembled from total S-layer protein), the protein content of the suspension, the resuspended pellet, and the clear supernatant was determined by the biocinchoninic acid protein assay (39). The clear supernatants from samples taken 2 h after starting the dialysis procedure were subsequently dialyzed against distilled water for 18 h at 4°C. To determine the amount of associated SCWP, 0.5 mg of lyophilized samples were hydrolyzed with 4 N HCl for 6 h and finally subjected to sugar HPLC. S-layer self-assembly products obtained 18 h after the dialysis procedure was started were washed once with distilled water and then lyophilized, and the amount of associated SCWP was determined as described above.
Recrystallization of the S-layer protein on poly-l-lysine-coated EM grids.
To investigate the capability of the soluble (monomeric and/or oligomeric) S-layer protein (18, 32) to recrystallize on solid supports, 30-μl portions of the clear supernatants from samples taken 2 h after the dialysis procedure was started were incubated with poly-l-lysine (Sigma P2636; 1 mg/ml of distilled water)-coated EM grids for 1 h at 20°C (32). After the grids were washed with distilled water, the S-layer protein was fixed with glutaraldehyde (2.5% in 0.1 M sodium cacodylate buffer [pH 7.0]) and negatively stained with uranyl acetate (1% in distilled water) as previously described (32).
Investigation of the binding capacity of S-layer self-assembly products and the soluble S-layer protein for the SCWP.
One milligram of lyophilized S-layer self-assembly products was resuspended in 1 ml of distilled water or 10 mM CaCl2. Then 0.5 mg of SCWP was added, and the suspension was stirred for 1 h at 20°C. After centrifugation at 40,000 × g for 10 min at 4°C, the pellet was washed once with distilled water, lyophilized, and subjected to chemical analysis. To prepare “blank” samples, S-layer self-assembly products from biomass cultivated at a low specific growth rate were suspended in distilled water or 10 mM CaCl2, and the suspension was treated as described above. To determine the binding capacity of the S-layer protein that was kept in the water-soluble state by the SCWP, 1 mg of S-layer self-assembly products was dissolved per ml of 5 M GHCl in 50 mM Tris-HCl buffer (pH 7.2), 0.5 mg of lyophilized SCWP was added, and the solution was dialyzed against 10 mM CaCl2 for 18 h at 20°C. After centrifugation at 40,000 × g for 10 min at 4°C, 2 ml of the clear supernatant containing the soluble S-layer protein and the SCWP was applied to a Sephacryl S-200-HR column (Pharmacia) with 150 mM NaCl in 50 mM Tris-HCl buffer (pH 7.2) for elution. Fractions containing the S-layer protein were pooled, dialyzed against distilled water, lyophilized, hydrolyzed with 4 N HCl for 6 h, and subjected to chemical analysis.
Influence of the SCWP on the formation of self-assembly products and studies on the recrystallization properties of the soluble S-layer protein with poly-l-lysine-coated EM grids as solid supports.
One milligram of lyophilized S-layer self-assembly products (from biomass cultivated at a low specific growth rate and pretreated as described above to remove the associated SCWP) was dissolved per ml of 5 M GHCl in 50 mM Tris-HCl buffer (pH 7.2). Different amounts of SCWP (10 to 600 μg/mg of S-layer protein) were directly added either to the GHCl-extracted S-layer protein or to the clear supernatants from samples taken 2 h after the dialysis procedure was started and containing the soluble (monomeric and/or oligomeric) S-layer protein (18, 32). After the addition of the SCWP, the solutions were dialyzed for 18 h against 10 mM CaCl2 at 20°C, the samples were centrifuged at 40,000 × g for 20 min at 20°C, and the DA was determined. The clear supernatants were used for recrystallization experiments on poly-l-lysine-coated EM grids.
Extraction of the S-layer protein from cell wall fragments with GlcNAc.
Ten milligrams of lyophilized cell wall fragments was incubated with 10 ml of GlcNAc solution (1 M in distilled water) for 2 h at 4°C, and the S-layer protein content of the cell wall fragments was determined before and after the GlcNAc extraction procedure by the biocinchoninic acid protein assay (39). After the insoluble material was removed by centrifugation, the clear supernatant was subsequently dialyzed against 10 mM CaCl2 or distilled water for 48 h at 4°C. Dialyzed samples were incubated with poly-l-lysine-coated EM grids and subjected to SDS-PAGE, and the S-layer protein content was assayed as described before (39). To determine the amount of GlcNAc that remained associated with the S-layer protein, 5 mg of lyophilized samples was dissolved in 2 ml of 150 mM NaCl in 50 mM Tris-HCl buffer (pH 7.2) and applied to a Sephacryl S-200-HR column with the same buffer for elution. Fractions containing the S-layer protein were collected, dialyzed against distilled water, lyophilized, and subjected to chemical analysis.
Proteolytic degradation of the S-layer protein in the absence or in the presence of the SCWP.
For proteolytic degradation of the S-layer protein, 1 mg of washed, lyophilized S-layer self-assembly products was dissolved in 1 ml of 2 M GHCl in 50 mM Tris-HCl buffer (pH 7.8); 40 μg of endoproteinase Glu-C (Staphylococcus aureus V8 protease) was then added, and proteolysis was performed either before or after addition of 250 μg of SCWP/mg of S-layer protein for 1 h at 37°C. After the reaction was stopped by heating the samples for 10 min at 100°C, the solutions were dialyzed against distilled water overnight at 4°C and then subjected to SDS-PAGE. Furthermore, proteolytic degradation of the S-layer protein was performed in presence of the SCWP after the GHCl was removed by dialysis against 50 mM Tris-HCl buffer (pH 7.8) for 24 h at 4°C. Edman degradation of blotted protein bands was carried out as described previously (10).
RESULTS
Comparison of the SCWP from biomass grown in continuous culture at a low or a high specific growth rate.
The SCWP was extracted from peptidoglycan-containing sacculi from B. stearothermophilus PV72/p2 with 48% HF, which cleaves phosphodiester linkages between the polymer chains and the hydroxyl groups from C-6 of N-acetylmuramic acid (2, 12). After precipitation with chilled ethanol, the HF-extracted SCWP was purified by gel permeation chromatography (GPC). As shown by sugar HPLC, the maximum amount of glucosamine and mannosamine was liberated when hydrolysis of the SCWP was performed with 4 N HCl for 6 h or with 6 N HCl for 2 h, while up to 50% of the mannosamine was destroyed when hydrolysis was carried out with 6 N HCl for 6 h. To calculate the molar ratios between the different sugars, the maximum amount of each was used. Since preliminary studies have shown that glucosamine and mannosamine are N acetylated (33), the following composition is suggested for the SCWP: a GlcNAc/ManNAc ratio of 2:1. Modification of monosaccharides in hydrolyzed samples (4 N HCl, 6 h) with 2-aminoacridone and separation by PAGE (17) confirmed that the SCWPs obtained from both biomasses had identical chemical compositions (not shown).
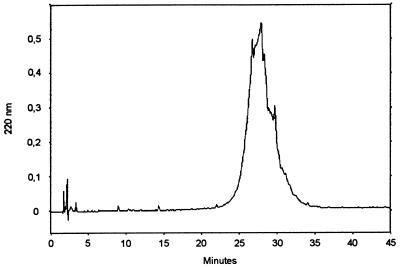
As determined by GPC with a calibrated Sephadex G-150 column, the molecular weight of the SCWP was independent of the specific growth rate of the biomass and was in the range of 24,000. After application of RP-HPLC according to the method of Bock et al. (5), the SCWP isolated from both biomasses eluted as a single peak, indicating that the material was homogeneous in length and structure (Fig. 1). The amount of SCWP which was covalently bound to the peptidoglycan did not depend of the specific growth rate and represented about 20% of the peptidoglycan-containing sacculus dry weight. As determined from the molecular weight and the chemical composition, a single polymer chain must be composed of about 120 monosaccharide residues. Chemical analysis further showed that one phosphate group was available per 100 sugar residues, confirming that the polymer chains are covalently linked via phosphodiester bonds to the peptidoglycan backbone (2).
FIG. 1.
Elution profile of the SCWP from B. stearothermophilus PV72/p2 after RP-HPLC as described by Bock et al. (5).
Investigation of the in vitro self-assembly process and studies on the recrystallization properties on poly-l-lysine-coated EM grids by using the S-layer protein from biomass grown at a low (0.1 h−1) or a high (0.4 h−1) specific growth rate.
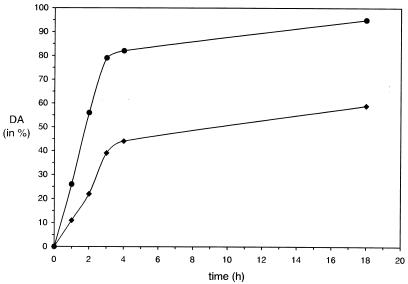
To investigate the in vitro self-assembly in suspension, dialysis of the GHCl-extracted S-layer protein was stopped 1, 2, 3, 4, and 18 h, and the DA was determined. As shown in Fig. 2, the S-layer proteins extracted from the different biomasses had quite different in vitro self-assembly properties. In general, the self-assembly process was more rapid in the case of the S-layer protein isolated from biomass grown at a low specific growth rate. After complete removal of GHCl by dialysis against 10 mM CaCl2, at least 90% of the S-layer protein had assembled into flat sheets or open-ended cylinders (data not shown), while under identical conditions only about half (59%) of the S-layer protein from the high-specific-growth-rate group was incorporated into self-assembly products (Fig. 2).
FIG. 2.
Curves demonstrating the in vitro self-assembly of the GHCl-extracted S-layer protein from B. stearothermophilus PV72/p2 in suspension. The S-layer protein was isolated from biomass grown in continuous culture at a low (•) or a high (⧫) specific growth rate. The DA that could be achieved strongly depended on the amount of SCWP associated with the S-layer protein, which correlated with the specific growth rate in continuous culture: 10 μg of SCWP/mg of S-layer protein at a low (0.1 h−1) specific growth rate or 30 μg of SCWP/mg of S-layer protein at a high (0.4 h−1) specific growth rate.
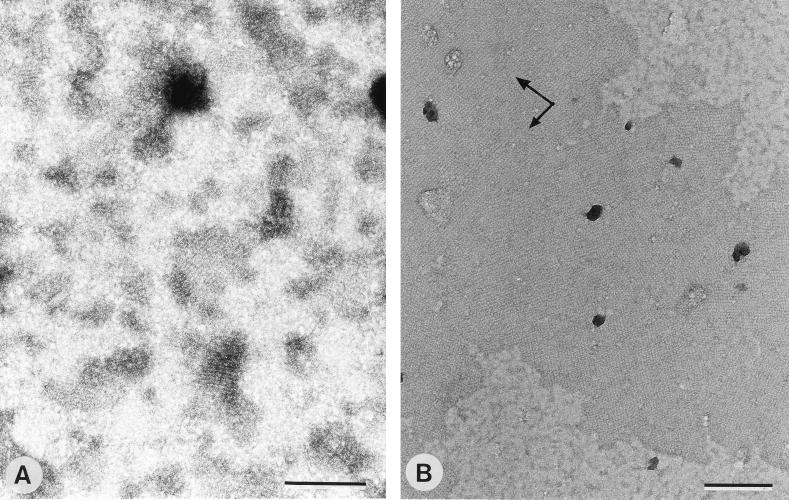
After 2 h of dialysis, the recrystallization properties of the soluble (monomeric and/or oligomeric) S-layer protein (18, 32) were investigated by using poly-l-lysine-coated EM grids as solid supports. As shown in Fig. 3A, only small crystallites were observed in negatively stained preparations when S-layer protein from biomass cultivated at a low specific growth rate was used. The S-layer protein from biomass grown at a high specific growth rate recrystallized into significantly larger patches (Fig. 3B). As derived from the orientation of the base vectors of the oblique lattice, the subunits had bound with their charge-neutral outer face. Depending on whether the organism was cultivated at a low or a high specific growth rate, the SCWP represented either 1 or 3% from the dry weight of the fraction containing the soluble (monomeric and/or oligomeric) S-layer protein. This was identical to the amount of SCWP which was associated with S-layer self-assembly products obtained at the end of the dialysis procedure (Fig. 2).
FIG. 3.
Negatively stained preparations of monolayer crystallites formed by recrystallization of the soluble (monomeric and/or oligomeric) S-layer protein from B. stearothermophilus PV72/p2 on poly-l-lysine-coated EM grids. (A) Small crystallites were formed by the S-layer protein isolated from biomass grown at a low specific growth rate (10 μg of SCWP/mg of S-layer protein). (B) Significantly larger monolayer patches were obtained with the S-layer protein from biomass cultivated at a high specific growth rate (30 μg of SCWP/mg of S-layer protein). In both cases, the S-layer subunits had bound with their charge-neutral outer face. Arrows indicate base vectors. Bars, 200 nm.
Investigation of the binding capacity of S-layer self-assembly products for the SCWP.
During removal of GHCl by dialysis, the S-layer protein reassembled into flat sheets or open-ended cylinders which were either monolayers or double layers (not shown). In double-layer self-assembly products the S-layer subunits are bound to each other with their charge-neutral outer face (33, 35), leaving the N-terminal region with the polymer binding domain exposed to the ambient environment. Under the applied experimental conditions, S-layer self-assembly products were capable of binding 30 μg of SCWP/mg of S-layer protein, which corresponded to one polymer chain per eight S-layer subunits. No SCWP could be detected in S-layer self-assembly products which were incubated and washed under the same conditions as the samples but without added SCWP.
Addition of the SCWP to the GHCl-extracted S-layer protein and to the fraction containing the soluble (monomeric and/or oligomeric) S-layer protein.
To investigate the influence of the SCWP on the formation of self-assembly products in suspension and on the recrystallization properties of the soluble S-layer protein when using poly-l-lysine-coated EM grids as solid supports, different amounts of SCWP were added to the GHCl-extracted S-layer protein or to the fraction containing the soluble S-layer protein, and dialysis was continued for 18 h. In comparison to the “blank” samples (i.e., S-layer self-assembly products with no detectable SCWP), which achieved a DA of >90%, the samples containing 120 to 600 μg of SCWP/mg of S-layer protein achieved a DA only in the range of 10%; this DA was independent of the amount of added SCWP within this relatively high concentration range (Table 1). On the other hand, the DA clearly correlated with the amount of added SCWP within the lower concentration range. Samples containing 20 μg of SCWP/mg of S-layer protein achieved a DA of 80%, which was very close to the value determined for the blank samples. When the concentration of the SCWP was increased to 30 μg/mg of S-layer protein, the DA was 60% and decreased to 20% at a concentration of 60 μg of SCWP/mg of S-layer protein (Table 1). Thus, the SCWP inhibited the formation of self-assembly products in suspension, most probably by acting as a spacer between the individual S-layer subunits and thereby masking the intersubunit bonding sites.
TABLE 1.
Influence of the SCWP on in vitro self-assembly of the S-layer protein from B. stearothermophilus PV72/p2
| Amt of SCWP (μg/mg of S-layer protein)a | Molar ratio (SCWP/S-layer subunits) | DA (%)b | % Coverage of poly-l-lysinecoated EM grids with monolayer patches |
|---|---|---|---|
| 10 | 1:24 | 90 | 0 |
| 20 | 1:12 | 80 | 0 |
| 30 | 1:8 | 60 | NDc |
| 60 | 1:4 | 20 | 30–50 |
| 100 | 1:2.4 | 15 | 50–70 |
| 120 | 1:2 | 10 | ND |
| 150 | 1:1.6 | 10 | >70 |
| 250 | 1:1 | 5 | >90 |
| 600 | 1:0.4 | 5 | <90 |
Different amounts of SCWP were added to the GHCl-extracted S-layer protein or to the fraction containing the soluble (monomeric and/or oligomeric) S-layer protein.
The DA was determined after 18 h of dialysis against 10 mM CaCl2 at 20°C as described in the text.
ND, not determined.
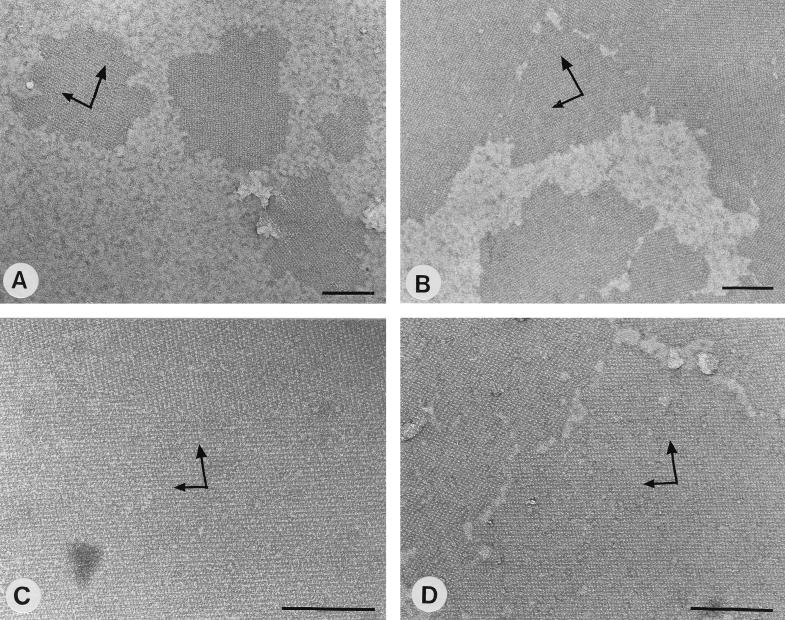
As shown by negative staining, samples containing 10 μg of SCWP/mg of S-layer protein did not recrystallize into monolayers on poly-l-lysine-coated EM grids. When the concentration of the SCWP was increased to 60 μg/mg of S-layer protein, large (average, 0.5 μm) crystallites showing the oblique lattice structure and covering up to 50% of the surface from poly-l-lysine-coated EM grids were formed (Fig. 4A). About 70% of the poly-l-lysine-coated EM grids were covered with 1- to 1.5-μm crystallites when the concentration of the SCWP was increased to 150 μg/mg of S-layer protein (Fig. 4B), whereas a completely closed monolayer was obtained at a concentration of 250 μg of SCWP/mg of S-layer protein (Fig. 4C). As determined from the molecular weight of the SCWP and that of the S-layer protein, this concentration corresponded to one polymer chain per S-layer subunit. In general, the optimal concentration of the SCWP for monolayer formation was in the range of 250 to 500 μg/mg of S-layer protein. The orientation of the base vectors of the oblique S-layer lattice confirmed that the subunits had bound with their outer charge-neutral face to the poly-l-lysine-coated EM grids.
FIG. 4.
Negatively stained preparations of monolayer crystallites obtained by using the fraction of the water-soluble (monomeric and/or oligomeric) (A to C) or the GlcNAc-extracted and dialyzed (D) S-layer protein from B. stearothermophilus PV72/p2 for recrystallization on poly-l-lysine-coated EM grids. (A to C) To keep the S-layer protein in the water-soluble state, different amounts of SCWP were added to the GHCl-extracted S-layer protein, and the solutions were dialyzed against 10 mM CaCl2. The size of the individual crystallites correlated with the amount of added SCWP: 60 (A), 150 (B), and 250 (C) μg of SCWP/mg of S-layer protein. (D) Monolayer crystallites were also formed by the GlcNAc-extracted dialyzed S-layer protein. In both cases, the S-layer subunits had bound with their charge-neutral outer face. Arrows indicate base vectors. Bars, 200 nm.
To determine the binding capacity of the soluble S-layer protein for the SCWP, S-layer self-assembly products were dissolved in 5 M GHCl, isolated SCWP was added, and the samples were dialyzed against 10 mM CaCl2 and then subjected to GPC. Fractions containing the S-layer protein were pooled and dialyzed against distilled water. Chemical analysis showed that after separation by GPC under nondenaturing conditions, 30 μg of SCWP/mg of S-layer protein remained associated, which was identical to the maximum binding capacity determined for the S-layer self-assembly products.
Extraction of the S-layer protein from cell wall fragments with GlcNAc and investigation of the self-assembly and the recrystallization properties.
As shown by SDS-PAGE and protein determination, about half of the S-layer protein from cell wall fragments could be extracted with 1 M GlcNAc. During removal of the GlcNAc by dialysis, the S-layer protein did not form self-assembly products (DA < 5%) and stayed in the water-soluble state. However, the GlcNAc-extracted dialyzed S-layer protein recrystallized into 1- to 2-μm monolayer patches on poly-l-lysine-coated EM grids (Fig. 4D), which covered at least 70% of the surface available for recrystallization. The orientation of the oblique S-layer lattice confirmed that the subunits had bound with their charge-neutral outer face. Chemical analysis showed that the GlcNAc-extracted, dialyzed S-layer protein still contained 100 μg of GlcNAc/mg of S-layer protein, which was comparable to the amount of SCWP significantly inhibiting the in vitro self-assembly in suspension. After purification by GPC, only traces of the amino sugar (<3 μg/mg of S-layer protein) remained associated with the S-layer protein.
Proteolytic degradation of the S-layer protein with the endoproteinase Glu-C in the absence or in the presence of the SCWP.
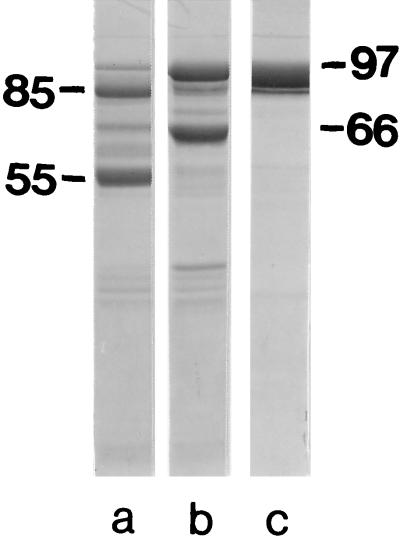
When proteolysis was performed in 2 M GHCl in the absence of the SCWP, most of the S-layer protein was attacked by endoproteinase Glu-C, leading to two major cleavage fragments with apparent molecular weights of 85,000 and 55,000 on SDS gels. In addition, a minor proteolytic cleavage fragment with an apparent molecular weight of 66,000 was formed (Fig. 5a). As shown by Edman degradation, the 85,000- and 55,000-molecular-weight cleavage fragments had identical N-terminal regions, V-T-K-G-K-T-P-T-S-F, starting with valine in position 139 of the mature S-layer protein (19). The 66,000-molecular-weight cleavage fragment carried the N terminus of the mature S-layer protein. When proteolysis was carried out in presence of the SCWP, only about half of the S-layer protein was attacked by endoproteinase Glu-C, and the N-terminal 66,000-molecular-weight proteolytic cleavage fragment represented the major one (Fig. 5b). These results clearly showed that the SCWP could protect the endoproteinase Glu-C cleavage site in position 138 of the mature S-layer protein. After the GHCl was removed by dialysis, the S-layer protein stayed in the water-soluble state when sufficiently large amounts of SCWP were available (Table 1). As shown in Fig. 5c, this water-soluble S-layer protein was not attacked by endoproteinase Glu-C, in contrast to the results obtained with the S-layer self-assembly products solubilized in 0.1% SDS (33).
FIG. 5.
SDS-PAGE patterns of the S-layer protein from B. stearothermophilus PV72/p2 degraded with endoproteinase Glu-C in 2 M GHCl in the absence (a) and in the presence (b) of the SCWP. (c) The S-layer protein kept in the water-soluble state after GHCl was removed by dialysis due to the presence of SCWP (250 μg/mg of S-layer protein). Molecular size is indicated in kilodaltons.
DISCUSSION
In a previous study, evidence was provided that a high-molecular-weight SCWP composed mainly of GlcNAc and ManNAc is involved in anchoring the S-layer protein from B. stearothermophilus PV72/p2 via its N-terminal region to the rigid cell wall layer (33). In addition to this binding function, the SCWP was found to influence the in vitro self-assembly and the recrystallization properties of the isolated S-layer protein (33). In the present study it could be demonstrated that the SCWP inhibits the formation of the cylindrical and sheet-like self-assembly products and keeps the S-layer protein in the water-soluble state, most probably by acting as a spacer between the individual S-layer subunits. To confirm this hypothesis, the SCWP was added to the GHCl-extracted S-layer protein and to the fraction containing the monomers and/or oligomers.
The critical concentration of the SCWP for the in vitro self-assembly was found to be in the range of 20 to 60 μg/mg of S-layer protein. As shown in Table 1, the DA decreased from 80 to 20% when the concentration of the SCWP was increased from 20 to 60 μg/mg of S-layer protein, which corresponded to an average decrease of 15% per 10 μg of additional SCWP. For comparison, in the next concentration step, ranging from 60 to 100 μg of SCWP/mg of S-layer protein, the DA decreased from 20 to 15%, which corresponded to only 1% per additional 10 μg of SCWP. Thus, the most significant influence of the SCWP on the formation of self-assembly products was observed when the molar ratio between the SCWP and the S-layer subunits was increased from 1:12 to 1:4 (Table 1). Interestingly, the maximum binding capacity of S-layer self-assembly products for the SCWP was determined to be 30 μg/mg of S-layer protein, which corresponded to one polymer chain per 8 S-layer subunits. If added to the self-assembly products, the SCWP can only attach to appropriate surface-located binding sites, but it cannot function as spacer between the individual S-layer subunits or even disintegrate the S-layer lattice. An identical amount of SCWP remained associated with the water-soluble S-layer protein, which was purified by GPC under nondenaturing conditions.
The S-layer protein that was kept in the water-soluble state by the SCWP showed a high tendency to recrystallize into monolayers on poly-l-lysine-coated EM grids. The size of the individual crystallites and the extent of coverage of the poly-l-lysine-coated EM grids increased with increasing amounts of added SCWP. The orientation of the oblique S-layer lattice confirmed that the subunits had bound with their charge-neutral outer face, leaving the N-terminal region with the polymer binding domain exposed to the ambient environment. A closed monolayer consisting of 1- to 2-μm crystallites was obtained when 250 μg of SCWP was available per mg of S-layer protein. The formation of self-assembly products in suspension was completely inhibited at this concentration, but the fact that closed monolayers of recrystallized S-layer protein were formed on poly-l-lysine-coated EM grids strongly indicated that the interactions between the outer face of the S-layer subunits and the net positively charged EM grids are stronger than those between the SCWP and the polymer binding domain. It cannot even be excluded that binding of the S-layer subunits to the poly-l-lysine-coated EM grids led to the dissociation of the SCWP from the S-layer protein.
By using 1 M GlcNAc, which is one of the N-acetylated amino sugars occurring in the SCWP, about half of the S-layer protein could be extracted from cell wall fragments, most probably by splitting the bonds between the S-layer subunits and the SCWP and by acting as a spacer between the individual S-layer subunits. In accordance with this assumption, GlcNAc was found to have an effect similar to that of the SCWP on the in vitro self-assembly in suspension and on the recrystallization properties of the S-layer protein on poly-l-lysine-coated EM grids. After purification of the GlcNAc-extracted S-layer protein by GPC, less than 3 μg/mg of S-layer protein remained associated. These results clearly showed that the interactions between the S-layer protein and GlcNAc are relatively weak, as is generally described for cell surface located carbohydrate-binding proteins and the respective monosaccharides (34, 42).
From the results obtained in the in vitro experiments it can be concluded that in the bacterial cell wall fabric, the SCWP functions both as an S-layer-specific anchor and as a spacer. Polymer chains which are exposed on the surface of the peptidoglycan-containing layer anchor the S-layer subunits in defined orientation with respect to the rigid cell wall layer, while polymer chains being integrated into the peptidoglycan sacculus act as spacers between the S-layer subunits and prevent the self-assembly of the S-layer protein pool entrapped within the rigid cell wall layer (7, 31). Moreover, the polymer chains must be presented on the cell surface in a way that they can function as anchoring structures only. Otherwise, dissociation of the S-layer lattice and release of the S-layer subunits into the culture fluid would occur.
The S-layer protein that was kept in the water-soluble state by the SCWP was highly resistant to endoproteinase Glu-C attack and, even in the presence of 2 M GHCl, a potential endoproteinase Glu-C cleavage site in position 138 of the mature S-layer protein was protected by the SCWP. Chemical analysis of S-layer carrying cell wall fragments revealed that the molar ratio between the SCWP and the S-layer subunits was approximately 1:1 (33). Thus, it seems that the SCWP can protect the S-layer subunits stored as an S-layer protein pool within the rigid cell wall layer from proteases that occur in the periplasmic space of gram-positive organisms (4, 14).
To summarize the findings presented here, the S-layer protein from B. stearothermophilus PV72/p2 can be considered a carbohydrate-binding cell surface protein that recognizes specific oligosaccharide structures on the bacterial cell surface. According to this definition, the linkage type between the S-layer subunits and the SCWP would correspond to that of the polysaccharide-lectin type (34, 42), which was supported by the GlcNAc extraction experiments. Studies regarding the definition of the binding domain on the S-layer protein and determination of the binding constants are in progress.
ACKNOWLEDGMENTS
This work was supported by the Austrian Science Foundation, projects P12938-MOB and S72/02 and by the Ministry of Science and Transportation.
We thank Sonja Zayni for sugar and amino acid analysis and Christoph Hotzy for technical assistance.
REFERENCES
- 1.Altmann F. Determination of amino sugars and amino acids in glycoconjugates using precolumn derivatization with o-phthalaldehyde. Anal Biochem. 1992;204:215–219. doi: 10.1016/0003-2697(92)90164-3. [DOI] [PubMed] [Google Scholar]
- 2.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 3.Beveridge T J. Bacterial S-layers. Curr Opin Struct Biol. 1994;4:204–212. [Google Scholar]
- 4.Beveridge T J. The periplasmic space and the periplasm in gram-positive and gram-negative bacteria. ASM News. 1995;61:125–127. [Google Scholar]
- 5.Bock K, Schuster-Kolbe J, Altman E, Stahl B, Christian R, Sleytr U B, Messner P. Primary structure of the O-glycosidically linked glycan chain of the crystalline surface layer glycoprotein of Thermoanaerobacter thermohydrosulfuricus L111-69. Galactosyl tyrosine as a novel linage unit. J Biol Chem. 1994;269:7137–7144. [PubMed] [Google Scholar]
- 6.Bowditch R D, Baumann P, Yousten A A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989;171:4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitwieser A, Gruber K, Sleytr U B. Evidence for an S-layer protein pool in the peptidoglycan of Bacillus stearothermophilus. J Bacteriol. 1992;174:8008–8015. doi: 10.1128/jb.174.24.8008-8015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chami M, Bayan N, Peyret J L, Guli-Krzywicki T, Leblon G, Shechter E. The S-layer protein of Corynebacterium glutamicum is anchored to the cell wall by its C-terminal hydrophobic domain. Mol Microbiol. 1997;23:483–492. doi: 10.1046/j.1365-2958.1997.d01-1868.x. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin J, Tummuru M K R, Blaser M J. A lipopolysaccharide-binding domain of the Campylobacter fetus S-layer protein resides within the conserved N terminus of a family of silent and divergent homologs. J Bacteriol. 1995;177:1734–1741. doi: 10.1128/jb.177.7.1734-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egelseer E M, Leitner K, Jarosch M, Hotzy C, Zayni S, Sleytr U B, Sára M. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J Bacteriol. 1998;180:1488–1495. doi: 10.1128/jb.180.6.1488-1495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egelseer E M, Schocher I, Sleytr U B, Sára M. Evidence that an N-terminal S-layer protein fragment triggers the release of a cell-associated high-molecular-weight amylase in Bacillus stearothermophilus ATCC 12980. J Bacteriol. 1996;178:5602–5609. doi: 10.1128/jb.178.19.5602-5609.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekwunife F S, Singh J, Taylor K G, Doyle R J. Isolation and purification of a cell wall polysaccharide of Bacillus anthracis (Sterne) FEMS Microbiol Lett. 1991;82:257–262. doi: 10.1016/0378-1097(91)90270-k. [DOI] [PubMed] [Google Scholar]
- 13.Etienne-Toumelin I, Sirard J-C, Duflot E, Mock M, Fouet A. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham L L, Beveridge T J, Nanninga N. Periplasmic space and the concept of periplasm. Trends Biochem Sci. 1991;16:328–329. doi: 10.1016/0968-0004(91)90135-i. [DOI] [PubMed] [Google Scholar]
- 15.Hastie A T, Brinton C C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979;138:1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastie A T, Brinton C C., Jr Isolation, characterization, and in vitro assembly of the tetragonally arrayed layer of Bacillus sphaericus. J Bacteriol. 1979;138:999–1009. doi: 10.1128/jb.138.3.999-1009.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson P. Polyacrylamide gel electrophoresis of reducing saccharides labeled with the fluorophore 2-aminoacridone: subpicomolar detection using an imaging system based on a cooled charge-coupled device. Anal Biochem. 1991;196:238–244. doi: 10.1016/0003-2697(91)90460-b. [DOI] [PubMed] [Google Scholar]
- 18.Jaenicke R, Welsch R, Sára M, Sleytr U B. Stability and self-assembly of the S-layer protein of the cell wall of Bacillus stearothermophilus. Biol Chem Hoppe-Seyler. 1985;366:663–670. doi: 10.1515/bchm3.1985.366.2.663. [DOI] [PubMed] [Google Scholar]
- 19.Kuen B, Koch A, Asenbauer E, Sára M, Lubitz W. Molecular characterization of the Bacillus stearothermophilus PV72 S-layer gene sbsB induced by oxidative stress. J Bacteriol. 1997;179:1664–1670. doi: 10.1128/jb.179.5.1664-1670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuen B, Sleytr U B, Lubitz W. Sequence analysis of the sbsA gene encoding the 130 kDa surface layer protein of Bacillus stearothermophilus PV72. Gene. 1994;145:115–120. doi: 10.1016/0378-1119(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 21.Leibovitz E, Lemaire M, Miras I, Salamitou S, Beguin P, Ohayon H, Gounon P, Matuschek M, Sahm K, Bahl H. Occurrence and function of a common domain in S-layer and other exocellular proteins. FEMS Microbiol Rev. 1997;20:127–133. [Google Scholar]
- 22.Lemaire M, Ohayon H, Gounon P, Fujino T, Beguin P. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J Bacteriol. 1995;177:2451–2459. doi: 10.1128/jb.177.9.2451-2459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J Bacteriol. 1994;176:1224–1233. doi: 10.1128/jb.176.5.1224-1233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda K, Kawata T. Reassembly of a regularly arranged protein in the cell wall of Lactobacillus buchneri and its reattachment to cell walls: chemical modification studies. Microbiol Immunol. 1985;29:927–938. [PubMed] [Google Scholar]
- 25.Matuschek M, Burchhardt G, Sahm K, Bahl H. Pullulanase of Thermoanaerobacterium thermosulfurigenes EM1 (Clostridium thermosulfurogenes): molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J Bacteriol. 1994;176:3295–3302. doi: 10.1128/jb.176.11.3295-3302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matuschek M, Sahm K, Zibat A, Bahl H. Characterization of genes from Thermoanaerobacterium thermosulfurigenes EM1 that encode two glycosyl hydrolases with conserved S-layer like domains. Mol Gen Genet. 1996;252:493–496. doi: 10.1007/BF02173016. [DOI] [PubMed] [Google Scholar]
- 27.Mesnage S, Tosi-Couture E, Mock M, Gounon P, Fouet A. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol Microbiol. 1997;23:1147–1155. doi: 10.1046/j.1365-2958.1997.2941659.x. [DOI] [PubMed] [Google Scholar]
- 28.Messner P, Sleytr U B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- 29.Nomellini J F, Küpcü S, Sleytr U B, Smit J. Factors controlling in vitro recrystallization of the Caulobacter crescentus paracrystalline S-layer. J Bacteriol. 1997;179:6349–6354. doi: 10.1128/jb.179.20.6349-6354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olabarría G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4722. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pink T, Langer K, Hotzy C, Sára M. Regulation of S-layer protein synthesis of Bacillus stearothermophilus PV72 through variation of continuous cultivation conditions. J Biotechnol. 1996;50:189–200. [Google Scholar]
- 32.Pum D, Sára M, Sleytr U B. Structure, surface charge, and self-assembly of the S-layer lattice from Bacillus coagulans E38-66. J Bacteriol. 1989;171:5296–5303. doi: 10.1128/jb.171.10.5296-5303.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ries W, Hotzy C, Schocher I, Sleytr U B, Sára M. Evidence that the N-terminal part of the S-layer protein of Bacillus stearothermophilus PV72/p2 recognizes a secondary cell wall polymer. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rini J M. Lectin structure. Annu Rev Biophys Biomol Struct. 1995;24:551–577. doi: 10.1146/annurev.bb.24.060195.003003. [DOI] [PubMed] [Google Scholar]
- 35.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sára M, Sleytr U B. Crystalline bacterial cell surface layers (S-layers): from cell structure to biomimetics. Prog Biophys Mol Biol. 1996;65:83–111. doi: 10.1016/s0079-6107(96)00007-7. [DOI] [PubMed] [Google Scholar]
- 37.Sleytr U B, Messner P. Self-assembly of crystalline bacterial cell surface layers, S-layers. In: Plattner H, editor. Electron microscopy of subcellular dynamics. Boca Raton, Fla: CRC Press; 1983. pp. 13–31. [Google Scholar]
- 38.Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface proteins. Austin, Tex: Landes Company/Academic Press; 1996. [Google Scholar]
- 39.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goerke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 40.Thomas S, Austin J W, McCubbin W D, Kay W D, Trust T J. Roles of structural domains in the morphology and surface anchoring of the tetragonal paracrystalline array of Aeromonas hydrophila. J Mol Biol. 1992;228:652–661. doi: 10.1016/0022-2836(92)90847-d. [DOI] [PubMed] [Google Scholar]
- 41.Walker S G, Karunaratne D N, Ravenscroft N, Smit J. Characterization of mutants of Caulobacter crescentus defective in surface attachment of the paracrystalline surface layer. J Bacteriol. 1994;176:5568–5572. doi: 10.1128/jb.176.20.6312-6323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weis W I. Cell-surface carbohydrate recognition by animal and viral lectins. Curr Opin Struct Biol. 1997;7:624–630. doi: 10.1016/s0959-440x(97)80070-x. [DOI] [PubMed] [Google Scholar]