Abstract
Lactobacillus sake can use arginine via the arginine deiminase (ADI) pathway. We designed degenerate primers based on an alignment of known sequences of ornithine transcarbamoylase (OTC)-encoding genes in order to amplify the L. sake counterpart sequences by PCR. Screening a genomic library of L. sake in λEMBL3 allowed us to isolate a clone containing a 10-kb L. sake genomic DNA insert. Sequence analysis revealed that the genes involved in arginine catabolism were clustered and encoded ADI (arcA), OTC (arcB), carbamate kinase (arcC), and a putative carrier with high similarity to the arginine/ornithine antiporter of Pseudomonas aeruginosa (arcD). Additionally, a putative transaminase-encoding gene (arcT) was located in this region. The genes followed the order arcA arcB arcC arcT arcD, which differs from that found in other microorganisms. arcA, arcB, arcC, and arcD mutants were constructed, and the ADI pathway was impaired in all of them. Transcriptional studies indicated that arcA gene is subject to catabolite repression, and under the conditions used, several transcripts could be detected, suggesting the existence of different initiation sites or processing of a larger mRNA.
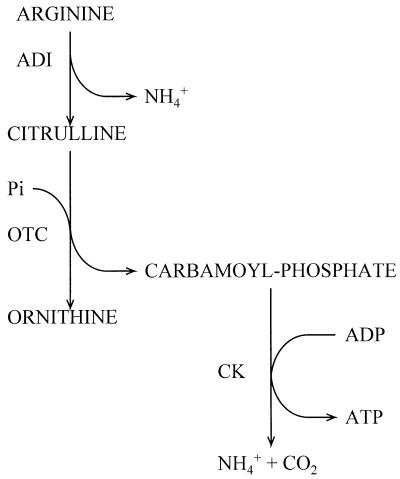
The arginine deiminase (ADI) pathway catalyzes the conversion of arginine to ornithine, ammonia, and carbon dioxide and concomitantly generates 1 mol of ATP per mol of arginine consumed (Fig. 1). A variety of bacteria, both gram positive and gram negative, can catabolize arginine through this pathway. ADI activity has been detected in several lactic acid bacteria (LAB), bacilli, clostridia, pseudomonads, aeromonads, mycoplasmas, halobacteria, and cyanobacteria (2). This pathway basically includes three enzymes: ADI, ornithine transcarbamoylase (OTC), and carbamate kinase (CK). Moreover, in Pseudomonas aeruginosa, a fourth gene that encodes a transport protein catalyzing an electroneutral exchange between arginine and ornithine has been identified (25). A functionally similar system had been previously described for Lactococcus lactis, some heterofermentative lactobacilli, and several enterococci and streptococci (19).
FIG. 1.
ADI pathway.
Lactobacillus sake is a facultative heterofermentative LAB and one of the most commonly found species in meat and fermented meat products (9). The existence of the ADI pathway in this species represents a characteristic taxonomic feature. Moreover, the ADI pathway is a likely energy source and a mechanism for survival in acidic environments (16).
However, the ADI pathway has not been thoroughly studied in LAB at the molecular level. In most LAB studied, the ADI pathway is repressed by sugars and induced by arginine (10, 15, 19, 24). In L. sake, glucose repression on the ADI pathway has also been observed, but induction by arginine had not been previously reported (17). Others considered the possibility that energy depletion was the triggering signal for induction of the ADI pathway (2). However, results reported showed that repression is to some extent dependent on the sugar used as the energy source; for instance, stronger repression by galactose than by glucose or lactose was reported for Lactobacillus leichmannii (15), but in L. lactis, weaker repression by galactose than glucose was reported (19), suggesting that carbon catabolite repression could account for the regulation of the pathway.
To gain deeper insight into the regulation of arginine catabolism, we have cloned and characterized the genes involved in the ADI pathway of L. sake. The genes encoding the enzymes involved are clustered, as in other microorganisms, but are arranged in a different order. We have constructed mutants by genetic disruption in all of the encoded enzymes and found that all of these enzymes are necessary for correct functioning of the pathway.
MATERIALS AND METHODS
Bacterial strains and vectors.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and vectors used
| Strain or vector | Genotype or description | Reference or source |
|---|---|---|
| L. sake strains | ||
| 23K | Wild-type isolate | 1 |
| RV4000 | 23K, arcA::pRV300, Erir | This work |
| RV4010 | 23K, arcB::pRV300, Erir | This work |
| RV4020 | 23K, arcC::pRV300, Erir | This work |
| RV4030 | 23K, arcD::pRV300, Erir | This work |
| BL13 | Type strain | CECT 906 |
| E. coli strains | ||
| XL1Blue | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 (F′ proAB lacIqlacZΔM15 Tn10) | Stratagene |
| LE392 | F−hsdR574 (rK− mK−) supE44 supF58 lacY1 galK2 galT22 metB1 trpR55 | Promega |
| Cloning vectors | ||
| pACYC184 | Tcr Cmr | New England Biolabs |
| pBluescriptII SK+ | Amr | Stratagene |
| pT7Blue T-vector | Amr | Novagen |
| pRV300 | Erir from pAMβ1 | 13 |
| λEMBL3 | BamHI arms | Promega |
| λ-arc | λ arms ligated to the L. sake BL13 chromosomal fragment containing the arc genes | This work |
| pACYC-ER1 | pACYC184 containing EcoRI fragment 1 (see Fig. 2) | This work |
| pBS-H5 | pBluescript containing HindIII fragment 5 (see Fig. 2) | This work |
| pBS-H8 | pBluescript containing HindIII fragment 8 (see Fig. 2) | This work |
| pBS-N1 | pBluescript containing NheI fragment 1 (see Fig. 2) | This work |
| pBS-N2 | pBluescript containing NheI fragment 2 (see Fig. 2) | This work |
Media, growth conditions, and transformation.
L. sake strains were routinely grown in MRS medium. For determination of arginine degradation or gene expression, MAM medium (Tryptone, 10 g; yeast extract, 5 g; arginine, 3 g; cysteine, 0.5 g; KH2PO4, 0.5 g; MgSO4, 0.2 g; MnSO4, 0.05 g; Tween 80, 1 ml; H2O, 1,000 ml; pH 6.0) was used. After autoclaving, sugars were added at various concentrations from 20% (wt/vol) filter-sterilized stock solutions. All incubations were carried out at 30°C. Escherichia coli was grown in Luria-Bertani medium at 37°C with vigorous shaking, and 2% agar was added for solid media. Ampicillin (50 μg/ml), chloramphenicol (25 μg/ml), tetracycline (15 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 80 μg/ml), and isopropyl-β-d-thiogalactopyranoside (20 mM) were added when required. Transformation of E. coli was performed by electroporation using a Bio-Rad Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) as recommended by the manufacturer. Electroporation and selection conditions for L. sake were as described before (1, 13).
Enzyme assays.
Cell extracts for enzyme assays were obtained from an exponentially growing culture. Cells were harvested by centrifugation at 8,500 rpm for 15 min at 5°C (JA14 rotor; Beckman model J2MC centrifuge), washed twice, and resuspended in ice-cold Tris buffer (0.1 M; pH 7.5) to a final optical density of 25 ml−1. Cells were disrupted in a bead beater with glass beads (three pulses of 3 min each) and then centrifuged at 15,000 rpm for 15 min at 5°C (12053 rotor; Sigma model 3MK centrifuge). The supernatant was used for enzymatic assays; protein concentrations were determined with the Coomassie protein assay reagent (Pierce, Rockford, Ill.).
The standard assay to determine ADI activity was performed with 3 mM arginine and 100 μg protein of the cell extract in 0.01 M phosphate buffer (pH 7). After incubation of 1 h at 37°C, the reaction was stopped with 2 N HCl, the cell extract was centrifuged at 100,000 rpm (12053 rotor; Sigma model 3MK centrifuge) to remove proteins, and the amount of citrulline in the supernatant was determined as described previously (17). One activity unit was the amount of enzyme needed to produce 1 μmol of citrulline per h per mg of protein.
OTC activity was measured by a method modified from that of Bringel (1a). A solution containing cell extracts (75 μg of protein), 10 mM ornithine, and 0.05 M EDTA (pH 8) was prewarmed for 5 min at 37°C, and then the reaction was initiated by the addition of 10 mM carbamyl phosphate (final concentration). After 10 min at 37°C, the reaction was stopped with 2 N HCl and the amount of citrulline formed was determined as described above. CK activity was determined as described by Liu et al. (14).
From DNA isolation to sequence analysis.
Total DNA was isolated from L. sake by the method described by Posno et al. (20). Plasmid DNA isolation from E. coli (2), restriction analysis, and ligations were performed by standard procedures. Sequencing was performed by the dideoxy-chain termination method (23).
The University of Wisconsin Genetics Computer Group (GCG) software package (version 8.0) was used for computer-assisted sequence analysis. Database searches were performed at the National Center for Biotechnology Information (NCBI) by using the BLAST network service and the FASTA and TFASTA programs included in the GCG package. Protein sequence alignments were performed by using the Pileup program included in the GCG package.
Amplification by PCR.
To amplify by PCR the OTC-encoding gene, various synthetic primers were designed from conserved regions deduced from an alignment of known OTC amino acid sequences. The nucleotide sequences of the primers used were as follows: OTC-for2, GTWGCWGATACWGCWAARGTN; OTC-rev1, DCCCATWSWDRYCCDACATC; and OTC-rev2, WACRTGYARYCKRTTYTCNGC (Y = C or T; R = A or G; K = T or G; S = C or G; W = A or T; D = A, T, or G; N = A, T, C, or G). The amplification reaction mixture contained 0.1 μg of L. sake total DNA as template, 100 pmol of each primer, and 1 U of Taq DNA polymerase (Boehringer Mannheim GmbH). The reaction conditions were 30 cycles of 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C. The amplified DNA fragments of the expected sizes were cloned into pT7Blue T-vector (Novagen Inc., Madison, Wis.) as specified by the manufacturer and checked by DNA sequencing.
Construction of mutations of the ADI pathway.
Mutations were obtained by chromosomal integration in the arcA, arcB, arcC, and arcD genes by the use of the integrative vector pRV300 (13). Primers were designed from the sequence and used to amplify internal fragments of the different genes. These primers were modified at the 5′ end by addition of restriction sites (underlined sequences) for KpnI and AvaI (arcA and arcB genes), EcoRI (arcC gene), and EcoRI-ClaI (arcD gene). The primers used for the amplification were 5′-GGGGTACCACAATCCAAAAAGAA-3′ plus 5′-CCCYCGRGAACGTCATTGCATTG-3′ (arcA), 5′-GGGGTACCTGGTAAACAGTGCATG-3′ plus 5′-CCCYCGRGGAAAAAAATTCACC-3′ (arcB), 5′-CGGAATTCAACAATTASGTTGCA-3′ plus 5′-CGGAATTCCATGCTGCCTTAGC-3′ (arcC), and 5′-CGGAATTCCCAGTGTTAATCGCA-3′ plus 5′-CCATCGATAAACGTCGGTGCTTT-3′ (arcD) (variable nucleotides [Y, R, and S] are as defined above). Hence, four internal fragments of 915, 660, 711, and 875 bases were amplified from genes arcA, arcB, arcC, and arcD, respectively. These fragments were cloned at the corresponding sites of the multicloning site of the vector pRV300, leading to plasmids pRV401, pRV402, pRV403, and pRV404. They were then used to transform L. sake 23K for erythromycin resistance as previously described (1, 13). Integration of the pRV300 derivatives was checked in the corresponding mutants by Southern analysis of the chromosomal DNA, and the phenotypic behavior of these mutants was analyzed.
Construction and screening of a genomic library of L. sake.
To obtain a genomic library of L. sake, total DNA of this microorganism was subject to partial digestion with Sau3AI and sucrose gradient centrifugation. Reaction conditions were optimized for DNA fragments in the size range of 10 to 20 kb. Fragments were purified and ligated to λEMBL3 BamHI arms (Promega, Madison, Wis.) as instructed by the manufacturer. Ligated DNA was packaged by using the Gigapack II Plus packaging extract (Stratagene, La Jolla, Calif.). Standard methods were used for titrating and phage propagation using as a host E. coli LE392. Screening was performed by using the method of Griffin et al. (8).
Southern blot analysis.
Standard procedures were used for the transfer of DNA from agarose gels to Hybond-N membranes (Amersham International plc.). Probes were labeled with digoxigenin-dUTP by using a Boehringer nonradioactive DNA labeling and detection kit. Hybridization, washing, and staining were done as instructed by the supplier.
RNA isolation and labeling.
Total RNA was isolated as described by Obst et al. (18) for transcript analysis in regulation assays. However, for mRNA size determination by Northern blotting, the method of Greenberg and Bender (7) was used. Sample preparation, denaturing agarose gel electrophoresis, and RNA transfer were performed according to standard protocols (23).
RNA probes for arcA, arcB, and arcD were synthesized from derivatives of pBlueScriptII SK+, where we had subcloned a HindIII-BamHI fragment from pBS-H5 (arcA probe), an XbaI-HindII fragment from pBS-H5 (arcB probe), and an NheI-HindIII fragment from pBS-H8 (arcD probe). Antisense RNAs were synthesized in vitro from linearized plasmids with T3 RNA polymerase, using the reagents from the Boehringer digoxigenin-RNA labeling kit as instructed by the manufacturer.
In the primer extension assay, the oligonucleotide prom2 (5′-CTTTACCTGGCCGTTTTAGTAAGACCG-3′) was labeled at the 5′ end and used for reverse transcription. Then, reverse transcriptase extension products were resolved in a denaturing polyacrylamide gel, together with DNA sequencing reactions performed with the same primer according to standard procedures (23).
Nucleotide sequence accession number.
The sequence reported has been submitted to the EMBL nucleotide sequence database. The accession number is AJ001330.
RESULTS
Amplification of the arcB gene and screening of the genomic library.
Amino acid sequences of OTC proteins show several conserved domains (data not shown). Four of these domains were selected, taking into account their sizes and distance in sequence. Codon preference was not considered for the primer design, since there are only a few known sequences from L. sake, but the overall G+C content was taken into account. Among the different primer pairs tested, we obtained two overlapping fragments when using the pairs OTC-for2–OTC-rev1 and OTC-for2–OTC-rev2 (fragments PCR3 and PCR4, 0.5 and 0.6 kb, respectively), whose sequences shared high similarity at the amino acid level with the other OTC sequences.
Next, two specific primers based on the sequence of the amplified DNA fragment PCR3 were designed and used for PCR screening of the λ library. A positive plaque (λ-arc) was isolated, and phages from this plaque were purified and confirmed by Southern blot analysis using PCR3 as a probe.
Structure of the ADI gene cluster of L. sake.
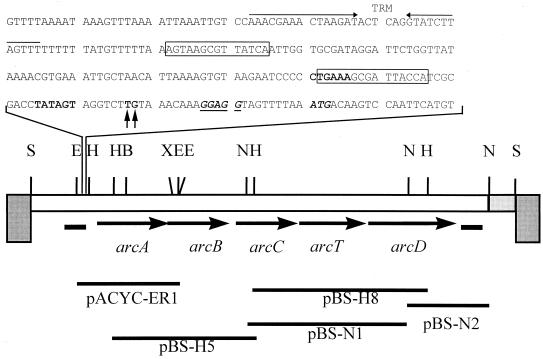
To sequence the OTC gene and surrounding regions, several fragments encompassing the complete operon were subcloned in pBlueScriptII or pACYC184 (Fig. 2) and sequenced. Sequence analysis revealed five open reading frames (ORFs); all of them were preceded by a putative Shine-Dalgarno box and started with an ATG codon, suggesting that they were translated. Part of an ORF with no significant homology to available sequences was detected upstream from ORF1 (arcA), and it was followed by a putative rho-independent terminator. Thus, we suggest that ORF1 is the first gene of the cluster. Sequence comparisons revealed significant similarity at the amino acid level to the gene products of the arc operon of P. aeruginosa: ORF1, 33% identity to the P. aeruginosa arcA product; ORF2, 49% to that of arcB; ORF3, 40% to that of arcC; and ORF5, 46% to that of arcD. According to these data, the ORFs could encode the following putative proteins: ORF1, ADI (409 amino acids [aa], predicted molecular weight [MW], 45,999.42); ORF2, OTC (337 aa; MW, 37,773.84); ORF3, CK (312 aa; MW, 33,223.66); ORF4, a noncharacterized transaminase (371 aa; MW, 41,376.83); and ORF5, an arginine-ornithine antiporter (475 aa; MW, 51,881.00). Therefore, we named these genes arcA, arcB, arcC, arcT, and arcD, after the designations proposed for the genes of the arc operon of P. aeruginosa.
FIG. 2.
Structure and restriction map of λ-arc and sequence of the promoter region. Grey boxes indicate λ arms; the lightly shaded box indicates an uncharacterized region. Arrows indicate putative ORFs; thick lines indicate partial ORFs; thin lines indicate the subcloned fragments for sequencing. B, BamHI; E, EcoRI; H, HindIII; N, NheI; S, SalI; X, XbaI. The sequence spanning the promoter region upstream of arcA is shown in detail (positions 20 to 259 in EMBL accession no. AJ001330). Arrows at top indicate a putative rho-independent terminator (TRM); line frames contain the putative cre sequences matching the consensus; bold letters highlight the −35 and −10 promoter regions; vertical arrows indicate the mapped transcription initiation points; the ribosomal binding site is indicated in bold letters and underlined; the translation start site of arcA is shown in bold italics.
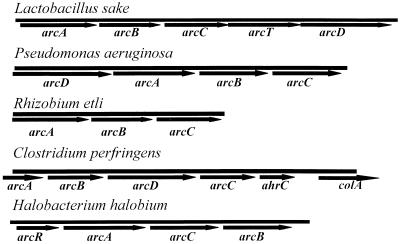
Gene organization is thus different from that of the other arc clusters described so far (Fig. 3). Moreover, an extra gene (arcT) has been found in L. sake. Analysis of the amino acid sequence with Motifs software revealed a putative pyridoxal-phosphate binding site (SLSKTYSVPGIRVG, aa 219 to 232). The sequence of this motif indicates that the arcT gene may encode a transaminase of class I. Similarity searches also showed a greater similarity to proteins belonging to this family, such as aspartate and tyrosine transaminases and 1-aminocyclopropane-1-carboxylate synthases.
FIG. 3.
Comparison of structures of the known arc operons from L. sake, P. aeruginosa (4, 5), R. etli (3), C. perfringens (EMBL accession no. X97768), and H. halobium (22).
Determination of the transcription initiation site of arcA and analysis of the 5′ region.
We isolated total RNA from cultures of BL13 grown in MAM medium with, per liter, 3 g of arginine plus 0.1 g of glucose or 1 g of galactose. The oligonucleotide prom2, which was complementary to the 5′ end of the arcA gene, was used for the primer extension reaction (data not shown). Two adjacent start sites were detected at T216 and G217 (Fig. 4), and two regions that matched Lactobacillus consensus promoter sites (21) were located at positions −7 (TATAGT) and −31 (CTGAAA) from the transcription initiation site (Fig. 2). Putative regulatory regions were also found in the promoter area. Two catabolite repression elements (cre), matching the consensus sequence described for gram-positive bacteria (26), were located upstream of the arcA gene (Fig. 2 and Table 2).
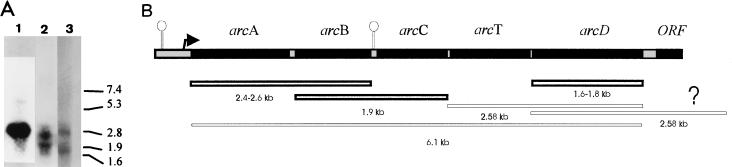
FIG. 4.
Northern blot analysis performed with total RNA extracted from fully induced cells of wild-type L. sake. (A) Three blots hybridized with probes for arcA (lane 1), arcB (lane 2), and arcD (lane 3). Migration of RNA molecular size standards is marked on the right in kilobases. (B) Proposed structures and lengths of transcripts found in wild-type L. sake.
TABLE 2.
Alignment of some selected cre sequences
| Organism | Gene | Sequencea |
|---|---|---|
| Bacillus subtilis | amyE | TTAAA TGTAAGCG TTAACA AAATT |
| xylA | TATTT TGGAAGCG TAAACA AAGTG | |
| gntR | CTGAT TGAAAGCG GTACCA TTTTA | |
| B. megaterium | xylA | CATTT TGAAAGCG CAAACA AAGTT |
| Lactobacillus pentosus | xylA | GTTCT AGAAAGCG TTTACA AAAAT |
| L. caseib | lacT | ATAAA ATAAAACG TTTACA TTCTG |
| L. sakec | arcA | TTTAA AGTAAGCG TTATCA ATTGG |
| CCCCC TGAAAGCGATTACCA TCGCG | ||
| Lactococcus lactis | sacB | ACTTC TGTAAGCG AAATCA TTTTA |
| Staphylococcus xylosus | xylA | TTTTA AGTAAGCG TTTACA AAAAA |
| Streptococcus mutans | fruA | GGATA AGATAGCG CTTACA ACATT |
| Consensus | WGNAASCG NWWNCA |
Construction of mutations in the ADI pathway.
We constructed mutations in the four main genes of the ADI pathway, arcA, arcB, arcC, and arcD, and tested enzymatic activities of the mutant strains under optimal conditions. The levels of activity were as follows: ADI, 0.10 ± 0.01 U in the arcA mutant and 3.19 ± 0.03 U in the wild-type parental strain; OTC, 0.05 ± 0.01 U in the arcB mutant and 3.22 ± 0.54 U in the wild type; CK, 0.05 U in the arcC mutant and 0.57 U in the wild type. The arcD mutant was tested for arginine transport and found to be affected in ornithine-arginine exchange (27). All of these results indicate that arcA, arcB, arcC, and arcD encode an ADI, a catabolic OTC, a CK, and an arginine-ornithine antiport system, respectively.
Transcription analysis.
Total RNA extracted from the wild type was used in Northern blot analysis performed with different probes. With the arcA probe, we observed a predominant mRNA species of about 2.4 kb that could span arcA and arcB (arcAB) (minimal theoretical size of 2,273 bases). We also detected a larger (6.1-kb) but fainter band (Fig. 4A), which could correspond to the estimated size of an mRNA spanning all five genes. Both mRNAs would start at the mapped transcription initiation site at position 216 or 217, and the arcAB transcript might end at position 2570, coinciding with a palindromic structure resembling a rho-independent terminator found donstream of the arcB gene (data not shown). An arcAB transcript could also be found with the arcB probe (2.6 kb) (Fig. 4). With this probe, a smaller (1.9-kb) mRNA whose size matched that expected for an arcBC transcript (theoretically 2,053 bases) was displayed. Transcription analysis of arcD showed two species of 1.8 and 2.58 kb that matched the expected sizes of arcD mRNA (theoretically at least 1,425 bases) and another arcD-containing mRNA, possibly arcTD (theoretically 2,573 bases). However, no terminator-like structure was found at the 3′ end of arcD, meaning that the 2.58-kb mRNA could also involve a downstream gene. Also, the size of the largest transcript (6.1 kb) could be greater than estimated (Fig. 4B).
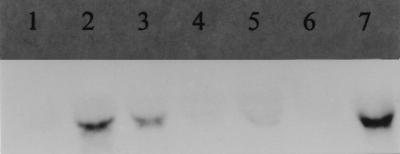
To study the regulation of the arc genes of L. sake, we isolated total RNA from L. sake BL13 cells grown with and without arginine and different amounts of glucose or 0.1 g of galactose per liter and analyzed it by Northern blotting with the arcA probe. Comparison of lanes 1 and 2 in Fig. 5 shows that arginine induced transcription of arcA. Furthermore, in the presence of arginine, glucose repressed transcription proportionally to the amount added, while galactose had no repressive effect (Fig. 5, lanes 3 to 7).
FIG. 5.
Northern blot with the arcA probe of RNA extracts of L. sake BL13 grown on glucose or galactose with or without 3 g of arginine per liter as follows: lane 1, 0.1 g of glucose per liter alone; lane 2, arginine alone; lane 3, 0.1 g of glucose per liter and arginine; lane 4, 0.5 g of glucose per liter and arginine; lane 5, 1 g of glucose per liter and arginine; lane 6, 5 g of glucose per liter and arginine; lane 7, 1 g of galactose per liter and arginine.
DISCUSSION
L. sake being a saprophyte, the existence of the ADI pathway for arginine catabolism in this organism could be important for its survival in meat environments. Through the alignment of OTC sequences and PCR amplification, we have isolated a λEMBL3 clone with a fragment of L. sake DNA that showed homology to other arc operons described in the literature and databases. This comparison shows that the gene arrangement is specific for each microorganism (Fig. 3). The major differences in the structural genes involved the absence of an antiporter gene, arcD, in Halobacterium halobium (salinarum) (22) and the presence in L. sake of arcT, possibly encoding a transaminase. With the available data, we could not deduce its likely role, since sequence analysis of the putative pyridoxal-phosphate binding site shows that the arcT gene could belong to a different family of transaminases (class I) than known ornithine transaminases (class III). However, it could not be assigned to any of the existing subclasses of class I transaminases.
The different gene clusters have different putative regulatory genes, i.e., arcR in H. halobium (salinarum) and ahrC in Clostridium perfringens (EMBL accession no. X97768); in contrast, the P. aeruginosa arc operon contains no regulatory gene (4, 5). Future research will be needed to determine if L. sake contains a specific regulatory gene for the ADI pathway.
Functionality of the L. sake genes was also studied in this work. Recently developed gene inactivation techniques for this microorganism (13) were used to obtain mutations in all of the ORFs essential in the ADI pathway, i.e., arcA, arcB, arcC, and arcD. Each of the mutant strains was impaired in catabolism of arginine through the ADI pathway.
In the wild-type strain L. sake 23K, a long mRNA of about 6.1 kb that spans at least arcABCTD is present in a low concentration, and the predominant transcripts found corresponded to the predicted lengths of arcAB, arcBC, arcD, and possibly arcTD. These shorter transcripts might result from processing of the 6.1-kb mRNA, from downstream promoters or early transcription stop sites. A palindromic structure was observed downstream from arcB. Thus, the arcAB transcript might be initiated at the promoter determined by primer extension and stopped at this terminator-like structure. However, no other promoter sequences were observed, and no terminator structures were detected downstream of arcC or arcD. It is thus possible that the other transcripts are derived from a longer messenger.
With regard to regulation of the pathway, Northern blot analysis showed arginine induction of the operon, since higher levels of mRNA were observed when arginine was added to the growth medium.
It has been reported that glucose exerts a repressive effect on ADI activity in L. sake (17). Similar results have been found for other LAB such as L. leichmannii (15) and L. lactis (19). However, these results were based only on quantification of enzyme activities. Only the regulation of the arc operon of P. aeruginosa has been thoroughly analyzed, and its transcription was shown to be controlled by the Anr protein, which activates transcription under oxygen limitation (4). Northern blot analysis showed that in L. sake, transcription of the arcA gene was clearly repressed by glucose, which explains the repressive effect found for glucose on ADI activity (17).
In L. sake, glucose is translocated through both a phosphotransferase system (PTS) and a non-PTS permease (12). It is possible that, as for other Lactobacillus genes (6), the PTS-CcpA signal transduction system is involved in regulation of the transcription of arc genes. This suggestion is further supported by the presence of two putative cre sequences upstream of the arcA gene homologous to the target for the transcriptional regulatory factor CcpA.
In summary, we have cloned and sequenced a gene cluster of L. sake similar to other known operons encoding the enzymes of the ADI pathway and have demonstrated that they encode functional enzymes of this pathway. Regarding its regulation, this operon is possibly induced by arginine and strongly repressed by glucose, probably through the PTS-CcpA signal transduction pathway.
ACKNOWLEDGMENTS
This work was financed by EU projects BIO2-CT92-0137 and AIR2-CT94-1517 and the Spanish Commission for Science and Technology through project ALI95-0038. Cooperation between IATA-CSIC and INRA-Jouy was promoted by a bilateral Picasso Action (Spanish reference HF95-0255B).
REFERENCES
- 1.Berthier F, Zagorec M, Champomier-Vergès M C, Morel-Deville F. High frequency transformation of Lactobacillus sake by electroporation. Microbiology. 1996;142:1273–1279. doi: 10.1099/13500872-142-5-1273. [DOI] [PubMed] [Google Scholar]
- 1a.Bringel, F. Personal communication.
- 2.Cunin R, Glansdorff N, Piérard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Hooghe I, Vander Wauver C, Michiels J, Tricot C, de Wilde P, Vanderleyden J, Stalon V. The arginine deiminase pathway in Rhizobium etli: DNA sequence analysis and functional study of the arcABC genes. J Bacteriol. 1997;179:7403–7409. doi: 10.1128/jb.179.23.7403-7409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamper M, Zimmermann A, Haas D. Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J Bacteriol. 1991;173:4542–4550. doi: 10.1128/jb.173.15.4742-4750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamper M, Ganter B, Polito M R, Haas D. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J Mol Biol. 1992;226:943–957. doi: 10.1016/0022-2836(92)91044-p. [DOI] [PubMed] [Google Scholar]
- 6.Gosalbes M J, Monedero V, Alpert C-A, Perez-Martinez G. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol Lett. 1997;148:83–89. doi: 10.1111/j.1574-6968.1997.tb10271.x. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg M E, Bender T P. Preparation and analysis of RNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1993. pp. 4.1.1–4.2.8. [Google Scholar]
- 8.Griffin H G, Anson K J I, Gasson M J. Rapid isolation of genes from bacterial lambda libraries by direct polymerase chain reaction screening. FEMS Microbiol Lett. 1993;112:49–54. doi: 10.1111/j.1574-6968.1993.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 9.Hammes W P, Bantleon A, Min S. Lactic acid bacteria in meat fermentation. FEMS Microbiol Rev. 1990;87:165–174. [Google Scholar]
- 10.Hiraoka Y B, Mogi M, Fukasawa K, Harada M. Coordinate repression of arginine aminopeptidase and three enzymes of the arginine deiminase pathway in Streptococcus mitis. Biochem Int. 1986;12:881–887. [PubMed] [Google Scholar]
- 11.Hueck C J, Hillen W, Saier M H., Jr Analysis of a cis-active sequence mediating catabolite repression in Gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 12.Lauret R, Morel-Deville F, Berthier F, Champomier-Vergès M C, Postma P, Ehrlich S D, Zagorec M. Carbohydrate utilization in Lactobacillus sake. Appl Environ Microbiol. 1996;62:1922–1927. doi: 10.1128/aem.62.6.1922-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leloup L, Ehrlich S D, Zagorec M, Morel-Deville F. Single crossing-over integration in the Lactobacillus sake chromosome and insertional inactivation of the pts and lacI genes. Appl Environ Microbiol. 1997;63:2117–2123. doi: 10.1128/aem.63.6.2117-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S Q, Pritchard G, Hardman M J, Pilone G J. Occurrence of arginine deiminase pathway enzymes in arginine catabolism by wine lactic acid bacteria. Appl Environ Microbiol. 1995;61:310–316. doi: 10.1128/aem.61.1.310-316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manca de Nadra M C, Nadra C A, Pesce de Ruiz Holgado A. Arginine metabolism in Lactobacillus leichmannii. Curr Microbiol. 1986;13:155–158. [Google Scholar]
- 16.Marquis R E, Bender G R, Murray D E, Wong A. Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol. 1987;53:198–200. doi: 10.1128/aem.53.1.198-200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montel M C, Champomier M C. Arginine catabolism in Lactobacillus sake isolated from meat. Appl Environ Microbiol. 1987;53:2683–2685. doi: 10.1128/aem.53.11.2683-2685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obst M, Meding E R, Vogel R F, Hammes W P. Two genes encoding the β-galactosidase of Lactobacillus sake. Microbiology. 1995;141:3059–3066. doi: 10.1099/13500872-141-12-3059. [DOI] [PubMed] [Google Scholar]
- 19.Poolman B, Driessen A J M, Konings W N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987;169:5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posno M, Leer R J, van Luijk N, van Giezen M J F, Heuvelmans P T H M, Lokman B C, Pouwels P H. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl Environ Microbiol. 1991;57:1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pouwels P H, Leunissen J A. Divergence in codon usage of Lactobacillus species. Nucleic Acids Res. 1994;22:929–936. doi: 10.1093/nar/22.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruepp A, Soppa J. Fermentative arginine degradation in Halobacterium salinarum (formerly Halobacterium halobium): genes, gene products, and transcripts of the arcRABC gene cluster. J Bacteriol. 1996;178:4942–4947. doi: 10.1128/jb.178.16.4942-4947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.Simon J P, Wargnies B, Stalon V. Control of enzyme synthesis in the arginine deiminase pathway of Streptococcus faecalis. J Bacteriol. 1982;150:1085–1090. doi: 10.1128/jb.150.3.1085-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhoogt H J C, Smit H, Abee T, Gamper M, Driessen A J M, Haas D, Konings W N. arcD, the first gene of the arc operon for anaerobic arginine catabolism in Pseudomonas aeruginosa, encodes an arginine-ornithine exchanger. J Bacteriol. 1992;174:1568–1573. doi: 10.1128/jb.174.5.1568-1573.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weickert M J, Chambliss G H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zúñiga, M., and M. Champomier-Vergès. Unpublished data.