Abstract
Purpose
Visual snow syndrome—characterized by flickering specks throughout the visual field and accompanied by other symptoms—can disrupt daily life and affects roughly 2% of the population. However, its neural bases remain mysterious, and treatments are lacking. Here, we report the first intervention that can temporarily eliminate the visual snow symptom, allowing many observers to see the world without snow for the first time since symptom onset. Prolonged viewing of a visual stimulus strongly reduces the responsiveness of the visual pathways to subsequent stimuli, and we tested whether such adaptation could affect visual snow.
Methods
Participants with visual snow (total n = 27) viewed high-contrast dynamic noise patterns, resembling television static, and then judged the strength of the symptom.
Results
Visual snow was temporarily reduced in strength to the point that it was invisible at longer adaptation durations for most observers. The effect followed typical trends of adaptation for physical stimuli in normally sighted observers: Effect duration increased monotonically with duration of exposure to the adapter and was specific to dynamic noise.
Conclusions
These results establish that spontaneous neural activity in the visual system is causally related to the visual snow percept. Because they perceive this activity, people with visual snow may provide a unique window into the generation and suppression of noise in the visual system. Adaptation allows reliable experimental control over visual snow, and so is a strong candidate for diagnostic testing and a promising tool for further understanding its neural origins, which could in turn aid the development of treatments.
Keywords: dynamic noise, visual adaptation, visual snow, psychophysics, persistent positive visual phenomena
Visual snow is the continuous perception of small elements flickering across the visual field, similar in appearance to television static or dynamic noise. Visual snow is a surprisingly common symptom, with population estimates ranging from 2.7 to 5.5%.1 In visual snow syndrome (VSS), it is associated with additional visual symptoms, including prominent afterimages, trails behind moving objects, excessive floaters, poor night vision, and light sensitivity, which can collectively present difficulties with everyday tasks.2,3 The full syndrome has been estimated to affect 1.4% to 3.3% of the population.1 Effective treatments are currently lacking,4–8 in part because little is known about the mechanisms producing VSS, other than it originates beyond the retina, which appears normal9–11 (but see Ref. 12). Accordingly, visual cortex is a likely origin.13–20
Is there a way to reduce the strength of the visual snow symptom? Besides the obvious potential clinical benefit, reducing the severity of the snow symptom should allow additional study of the mechanisms that produce it, for example, by measuring and comparing neural activity within an individual when snow is present and absent. Experimental control of symptom strength is particularly important for VSS and related disorders of perception, such as tinnitus or chronic pain, because comorbidities confound comparisons with control participants.
The visual system adjusts its function following prolonged stimulation, a process called adaptation, which can reduce the perceived intensity of many external stimuli.21,22 For example, viewing a continuously moving pattern reduces sensitivity to motion in the adapted direction, causing a static stimulus to appear to move in the opposite direction, as in the waterfall illusion.23 The response to online “visual snow relief videos” suggest that visual snow may also be susceptible to adaptation (e.g. https://www.youtube.com/watch?v=800f9UNiF4Y), but this possibility has not been examined scientifically.
We tested whether adaptation could affect visual snow. Participants with VSS viewed high contrast dynamic noise, resembling a very intense version of the symptom, for varying amounts of time, and reported the effect on their visual snow (Fig. 1). For most observers, adaptation substantially reduced the strength of their snow, often rendering it invisible.
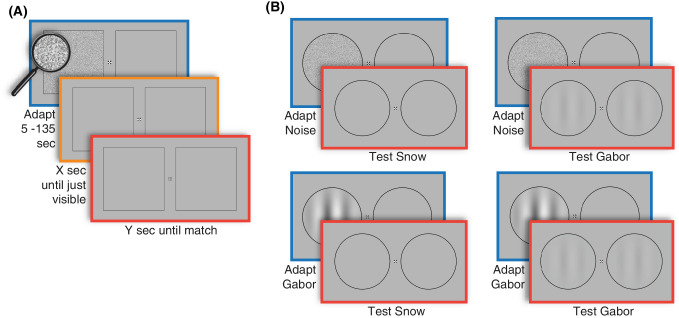
Figure 1.
Experimental methods. (A) In experiment 1, observers viewed high contrast dynamic visual noise for varying durations and pressed one button when their visual snow reappeared on the adapted side, and pressed another button when the snow on the two sides appeared the same. (B) In experiment 2, observers also adapted to a pattern of stripes (defined by a Gabor function) and were tested either with visual snow or a low contrast Gabor pattern, again pressing a button when both sides of the display appeared the same.
Materials and Methods
Participants
Three groups of participants were recruited—a small group of 5 participants with VSS to complete in-depth psychophysical experiments (VSS psychophysical), a group of 5 control participants who did not have visual snow, and a larger group of 25 participants who had VSS (VSS prospective) to test the generality of findings. Both the control and VSS psychophysical groups consisted of a mix of individuals with substantial psychophysics experience and naive observers. Three participants were in both psychophysical and prospective visual snow syndrome groups, for a total of 27 individuals with VSS.
Participants with VSS were required to have experienced visual snow continually for at least the last 3 months, and endorsed at least 2 additional visual symptoms, including afterimages, trails behind moving objects, entoptic phenomena (floaters), poor night vision, or light sensitivity.3 To avoid a potential confound with hallucinogen persisting perception disorder, participants were excluded for use of hallucinogenic/dissociative substances 12 months prior to the onset of visual snow or within 12 months of their first study visit (Supplementary Table). We recruited comparable numbers of participants with (n = 3 in the VSS psychophysical group and n = 12 in the VSS prospective group) and without migraine (n = 2 in the VSS psychophysical and n = 13 in the VSS prospective) to avoid a possible confound of migraine status and analyzed both groups separately. See the supplementary materials for a demographic table and complete inclusion/exclusion criteria. All participants had at least 20/25 visual acuity with both eyes open.
All procedures were reviewed and approved by the University of Minnesota Internal Review Board and conformed to the Declarations of Helsinki. All participants provided written informed consent prior to participation and were compensated for their time.
Experiment 1
The purpose of experiment 1 was to determine whether visual adaptation to high contrast noise could affect visual snow and, if so, quantify the strength of the effect. Only participants from the VSS psychophysical (n = 5) completed experiment 1.
Displays
Stimuli were presented in a dark room using MATLAB and PsychToolbox-324 on an Apple iMac desktop and an Eizo FlexScan SX2462W monitor with a 60 Hz refresh rate and resolution of 1920 × 1200 with a screen size of 53 × 33 cm at a viewing distance of 70 cm. During adaptation, participants viewed an achromatic dynamic noise pattern (see Fig. 1A; square of 10 degrees visual angle centered at 6 degrees eccentricity, 60 Hz, pixel luminances drawn randomly from a uniform distribution between 0 and the maximum luminance, and bounded by a 2-pixel black border). This adapter was displayed on either the right or left side (counterbalanced across participants) of a fixation mark25 on a uniform gray mean field (80.8 cd/m2). The other side of the display contained an empty (mean gray) square with an identical border to the adapter. Following adaptation, empty squares were shown on both sides of the display (see Fig. 1A).
Conditions
To determine whether adaptation increased in strength with increasing adapter duration, we used 4 adapter durations: 5, 15, 45, and 135 seconds.
Procedure
Participants were instructed to maintain fixation during testing. During each trial, the adapter was displayed on one side of the screen for a specified duration, and then participants judged their visual snow within the outlined square regions on both sides of the fixation mark. If their visual snow disappeared on the adapted side, participants pressed one button as soon as it became visible again (if their snow did not disappear, they did not press the button). Observers then pressed a different button when their visual snow looked equally strong on both sides. The time between the end of the adapter display and the button presses was recorded. Participants completed initial practice trials (with adapter duration of 5 seconds) until they were comfortable with the task.
One trial of each adapter duration, in random order, was run within a block of four consecutive trials. Participants took self-timed breaks between trials, and completed 4 blocks, for a total of 16 trials. In all experiments, participants were allowed to redo a trial if they reported accidentally pressing a button at the wrong time.
Experiment 2
Displays
To determine whether adaptation of visual snow showed specificity to the adapting pattern, we tested whether visual dynamic noise was a stronger adapter for snow and low contrast visual dynamic noise than a stripe pattern adapter with dissimilar spatial and temporal frequencies, and vice versa. Participants from the VSS psychophysical (n = 5) and control group (n = 5) completed experiment 2.
Two types of adapter stimuli were presented—high contrast noise and a high contrast stripe pattern (see Fig. 1B). The noise adapter was the same as in experiment 1, but it was circular in shape rather than square (with 10 degrees visual angle diameter). The stripe adapter was a vertical Gabor pattern of the same size (0.3 cycles/degree, contrast reversing at 1 Hz, 100% contrast, Gaussian envelope with standard deviation of 2.5 degrees).
Control participants viewed simulated visual snow as a test stimulus. Participants with visual snow did not view the simulated snow in this experiment, and instead judged their symptom within blank circles. For controls, the simulated snow was based on visual snow symptom estimates from our five participants in the visual snow syndrome psychophysical group using a snow matching task reported elsewhere.26 The snow matching task involved setting three parameters: contrast, density, and speed. The simulated snow was generated by random independent draws from a binary distribution controlled by the contrast parameter. The density of the snow was adjusted by setting a proportion of pixels equal to the background luminance. The speed parameter determined the lifetime of each snow element, after which it was replaced with a new random draw. Each control participant was randomly matched with a visual snow participant whose mean simulated snow settings were displayed in the two circles (see Fig. 1B).
Participants in both the control and visual snow syndrome psychophysical groups also viewed a low contrast Gabor pattern as a test stimulus. This test pattern was the same as the adapter Gabor pattern except that its contrast was 5%. The temporal phase of the contrast reversal in the left and right Gabor patterns were independently randomized across trials to discourage participants from using phase synchrony as a cue to recovery from adaptation.
Conditions and Procedure
Across trials, both test types were paired with both adapters, yielding four conditions. In the first condition, participants adapted to dynamic noise and then judged their visual snow (or simulated snow for controls). In the second condition, observers adapted to the high contrast Gabor pattern and then judged the visual snow (or simulated snow). In the third condition, participants adapted to the Gabor pattern and judged the strength of low contrast Gabor test. In the fourth condition, participants adapted to the dynamic noise, then judged the strength of the Gabor test. After adapting to either the dynamic noise or Gabor pattern for 30 seconds, the test patterns were viewed until the participants pressed a button, indicating that both sides appeared equal in strength. Physical tests (simulated snow and Gabor patterns) were presented on both sides of fixation, whereas for internal visual snow, empty circles were presented. Each block contained one trial of each condition—the order of which was randomized within blocks—with a total of six blocks comprising the experiment.
Prior to the start of each trial, participants were informed whether they would be asked to pay attention to snow or Gabors to make their judgment. Advancement through the task was self-timed, and practice trials were given as in experiment 1.
Experiment 3
The goal of the third adaptation experiment was to determine whether our adaptation results generalized to a larger sample of participants with VSS (n = 25). We used the same dynamic noise adapter as in experiment 1, but the durations were 1.6, 5, 15, and 45 seconds to reduce the total experiment time and avoid visual strain, given that this population might be more susceptible to discomfort from extended periods of visual stimulus presentation.27 After adapting, observers again judged when the visual snow inside the test squares appeared equally strong. There were four blocks of trials, each of which had one trial of every adapter duration in a randomized order.
Participants were also asked to verbally report their subjective experience during practice trials and if any test trials produced a different experience. Experimenters took care when explaining the task not to provide information that would bias participants regarding the expected results; informed consent did not mention internet videos, and although we did not explicitly ask, no participant in the VSS large group mentioned being aware of them. Prior to the test trials, participants completed practice trials (adapter duration = 5 seconds) until they were comfortable with the task.
Analysis
All analyses were performed using custom code in MATLAB (Mathworks, Inc., Natick, MA, USA). For all experiments, repeated measures ANOVAs determined whether the recovery time was related to the adapter conditions. In these analyses, duration of adapter was a continuous variable and participants were a random variable. For experiment 1, a MANOVA was used to test for linear effects of adapter duration and determine whether recovery time and disappearance duration were related with the duration of exposure to the adapter. For experiment 2, an ANOVA tested for differences in recovery time between the adapter and test conditions. In experiment 3, an ANOVA tested for linear effect of adaptation duration where block order was included as a categorical variable. Effect sizes were calculated as Cohen's d values. For the categorical subjective reports of adaptation effects in experiment 3, chi-squared tests for uniformity determined whether the number of reports per category were different from what would be expected by chance. We also calculated 95% confidence intervals for the percent of participants whose visual snow was alleviated by adaptation (disappeared or weakened).
Results
Adaptation Reduces the Strength of Visual Snow
We first measured effects of prolonged viewing of high contrast dynamic noise on the strength of the visual snow percept in five people with visual snow. Observers viewed the noise presented on one side of a fixation mark (see Fig. 1A). It was replaced with a uniform gray screen following a variable duration, and observers then judged the strength of their snow percept within a square region on that side of the display and indicated when it regained visibility by pressing a button. Continuing to monitor their snow percept, observers compared its strength to the snow in a square region on the other side of fixation, and pressed a second button when the two sides appeared the same.
All observers reported that their snow disappeared after viewing the adapter (Fig. 2, gray response curves; for one observer, this only occurred for the longer adapter durations). On average, even the 5 second adapter caused a brief disappearance of observers' snow (2.5 seconds mean), and the 135 second exposure eliminated the snow for 14.1 seconds on average.
Figure 2.
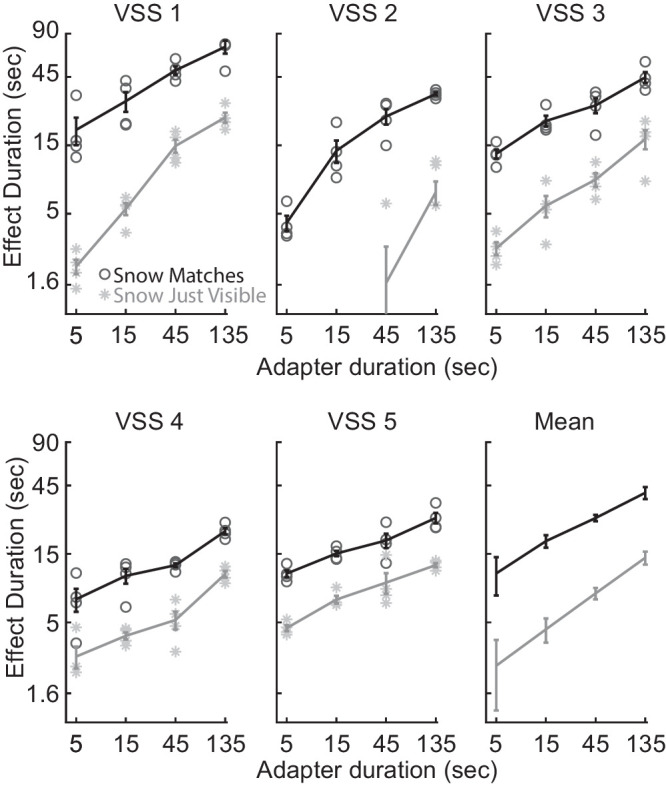
Results of experiment 1 for 5 observers with VSS and means. Time until the snow re-appeared (gray) and regained full strength (black) is plotted as a function of adapter duration. Error bars are plus or minus one standard error of the mean for each condition within participants. Individual trials are shown in light markers and means are shown with darker markers. For plot of the mean data in this figure and Figures 3 and 4, error bars are corrected for consistent across participant differences as is appropriate for a within-subjects design.28
The snow increased in visibility over time, and it took many additional seconds for the snow to regain full strength (see Fig. 2, black curves). On average, it took 40 seconds for the snow to appear the same as the unadapted side following 135 seconds of adaptation.
These effects increased monotonically with adapter duration (see Fig. 2, slopes of gray and black curves) and were statistically reliable within all our observers, as well as in the group average (ANOVA with linear effect of adapter duration: F(1, 4) = 351.7, P = 5.1 × 10−40; and interaction between adapter duration and disappear versus match: F(1, 4) = 64.2, P = 4.0 × 10−13). The effect size was large, with a Cohen's d of 13.5 for adapting for 135 seconds versus 5 seconds. The curves were linear in shape on log-log axes following a power law that is characteristic of visual adaptation processes in general.21,29
Specificity of Adaptation
Another hallmark of adaptation in control observers is its specificity; adapters that are more similar to a test pattern have greater effects on appearance of the test than adapters that are less similar.21,22 We tested whether adaptation affecting visual snow showed this specificity by repeating the experiment with a different adapter, a pattern of stripes whose luminance followed a Gabor function (see Fig. 1B).
We first confirmed in control observers that adaptation to these patterns showed specificity. We used simulated snow and a low contrast Gabor as test patterns. The simulated snow was low-contrast noise with parameters (contrast, temporal frequency, and density) set using a snow appearance matching task reported elsewhere.26 Observers adapted for 30 seconds, either to the noise or to the Gabor pattern. Then, they viewed either the simulated snow or the Gabor test pattern on both sides of fixation. Observers responded when the two test patterns appeared the same.
Adapting to noise caused the simulated snow to appear weaker (disappearance was not measured in this experiment) for 10.9 seconds on average. Adapting to the Gabor pattern had a much smaller effect on simulated snow, with an average recovery time of only 4.1 seconds, and this difference was reliable in all 5 observers as well as the mean (Fig. 3A). The low contrast Gabor test pattern showed a similar trend; adapting to a high contrast Gabor pattern reduced its visibility for a longer period of time (21.5 seconds on average) than adapting to the high contrast noise did (9.6 seconds on average). This double dissociation, where the noise adapter primarily affected the simulated snow test and the Gabor pattern adapter primarily affected the Gabor pattern test, was highly reliable (ANOVA: interaction between adapter and test F(1, 4) = 230.2, P = 1.1 × 10−27).
Figure 3.
Results of experiment 2. (A) Time until the test stimuli regained full strength is plotted for each of the four stimulus conditions (adapt to noise [AN] and test snow [TS], adapt to Gabor [AG] and test snow, adapt to Gabor and test Gabor [TG], and adapt to noise and test Gabor) for each of the five control observers and their means. Individual trials are shown in light markers and means are shown with darker markers. Error bars are as in Figure 2. (B) Results from five participants with VSS and their means.
Critically, people with visual snow showed the same specificity of adaptation when judging the strength of their snow percepts (Fig. 3B, ANOVA: interaction between adapter and test: F(1, 4) = 553.2, P = 1.5 × 10−42, Cohen's d = 5.3 for adapt noise test snow vs. adapt Gabor test snow). Adapting to noise again caused the snow to appear weaker in strength, with a mean recovery time of 18.9 seconds; adapting to the Gabor had a much smaller effect on snow, with a recovery time of only 5.8 seconds on average. The visibility of the low contrast Gabor test pattern showed a similar trend as in control observers, with the Gabor pattern adapter reducing its perceived strength for a much longer period of time than the noise adapter.
Adaptation Effects Generalize to a Larger Sample
We also tested adaptation to noise in a larger population of 25 people with visual snow. Observers again adapted for different durations and reported when the visual snow appeared the same on the adapted and unadapted sides. As in our smaller sample, adaptation had a large effect which increased monotonically with adapter duration (Fig. 4A, ANOVA with linear effect of adapter duration: F(1, 25) = 27.34, P = 2.1 × 10−5, Cohen's d for 45 seconds versus 1.6 seconds of adaptation = 5.5). The curves were again linear in shape on log-log axes. We also asked observers to verbally report how adaptation affected their snow. Sixty percent of observers reported that their snow disappeared or weakened following adaptation; others reported no visible effect, and a few reported strengthening (Fig. 4B). Nonetheless, all but one participant showed a positive slope for effect duration versus adapter duration. The number of participants in each category (snow disappeared, weakened, no difference, strengthened, and other) was reliably nonuniform (χ2 [4, n = 25] = 20.0, P = 5 × 10−4). The 95% confidence interval for the proportion of participants for whom adaptation was effective (i.e. disappeared or weakened) spanned 36% to 76%. This proportion was reliably larger than the proportion of participants giving each of the other responses (all pairwise χ2 > 14.1, all P < 1.7 × 10−4).
Figure 4.
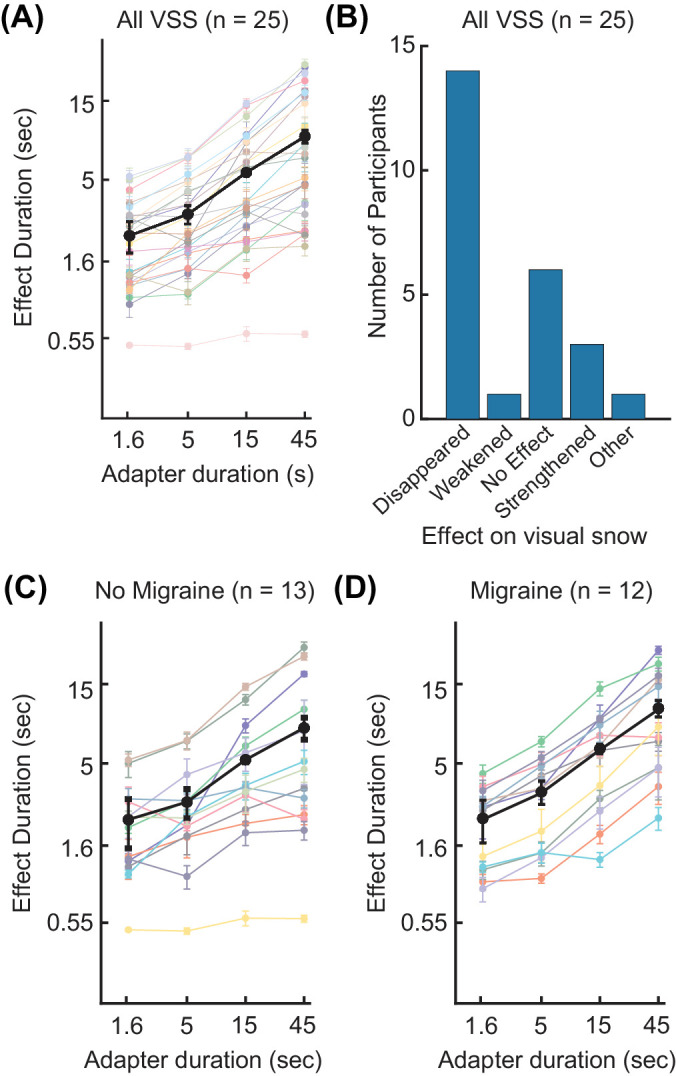
Results of experiment 3. (A) The average time in seconds until visual snow matched on the adapted and unadapted sides is plotted as a function of adapter duration for 25 participants with visual snow syndrome (color) with the mean across participants (black). Error bars are As in Figure 2. (B) A bar graph shows the number of participants who reported various perceptual effects of adaptation on their visual snow. Visual snow effect categories included disappearance, weakening, no effect, strengthening, and expanding outward from the center of the square. (C) The same as panel A, but showing participants with visual snow but without migraine (n = 13). (D) The same as panels A and C, but showing only participants with migraine and visual snow (n = 12).
To investigate whether migraine might affect adaptation durations, we analyzed our VSS participants with (n = 12) and without (n = 13) migraine separately (Figs. 4C, 4D). In both groups, effect duration increased with adapter duration (no migraine: ANOVA, F(1, 12) = 9.14, P = 0.0106; migraine: ANOVA, F(1, 11) = 21.19, P = 0.0008). We did not find evidence suggesting a difference in adaptation effects between participants with and without migraine (ANOVA, F1,23 = 0.95, p = 0.34).
Discussion
Visual snow was strongly affected by adaptation, being reduced in strength to the point that it was rendered invisible in many conditions for most observers. This adaptation closely paralleled trends found for physical stimuli in normally sighted observers; it was linear on a log-log axis, obeying the duration scaling law, and showed the same pattern of adapter specificity as physical snow did in controls. One might be concerned about the validity of these results given that our measures of visual snow are subjective, depending on observers' report of its appearance. However, there is a long history of using subjective (i.e. Brindley's class B) data throughout vision science with great value, from color matching to binocular rivalry judgments. Our data are consistent, replicate objective effects, and are not attributable to confabulation, and so comprise a valid methodology. The results indicate that the visual snow percept is not an observer bias or malingering: If either were the case, it would be highly unlikely that observers' responses would so systematically resemble the complex pattern of results obtained in normally sighted observers.
Instead, the simplest account of our results is that adaptation affects the neural activity producing visual snow in the same way it affects stimulus-driven activity in controls. Prolonged exposure to dynamic noise reduces responsiveness of visual neurons beginning in the retina30 and effects of adaptation are also visible through primary visual cortex and into middle and later areas along the visual processing pathways.31 The fact that such adaptation can affect visual snow strongly suggests that spontaneous (i.e. non-stimulus driven) neural activity in the visual pathways is causally necessary to produce the visual snow percept.
People with visual snow are able to perceive this noise in their visual systems (it is noise at least as in relation to the signals useful for most typical every day or laboratory visual tasks). This may provide a unique window available to psychophysics and neuroimaging for studying the processes by which noise is suppressed and generated in neural systems generally.
Which specific regions give rise to the visual snow percept remains unclear. Visual cortex in people with VSS appears to be more active at rest,18,20 to be sending stronger signals to other parts of the brain at rest13,14,16 and to have increased grey matter volume14,15,18,20 compared to normally sighted controls. The lingual gyrus, found on the ventral surface of the occipital lobe, shows these differences most consistently,13–15,18,20 and probably supports mid-level visual processing. However, comorbid symptoms associated with visual snow (e.g. photophobia, palinopsia, tinnitus, and migraine) make it difficult to determine whether reported differences are due to visual snow specifically. Using adaptation will allow future imaging studies to compare activation with and without visual snow within observers, and so better isolate activity underlying the visual snow symptom.
Adaptation represents one of the first reliable perceptual effects of visual snow (other than symptom reporting). Past psychophysical results on visual snow are sparse and paint a mixed picture. Tests of visual acuity, color vision, and perimetry are all normal in people with visual snow.9,11,13,27,32–34 Contrast sensitivity, a standard visual test measuring the ability to detect faint differences in intensity, has been found to be better,35 worse,36 or to show no difference11 in VSS. The only other reported perceptual difference is a smaller contrast illusion in people with visual snow.37 We found that adapting to noise produced a strong perceptual effect on the appearance of visual snow, strong enough to be measured highly reliably within individual participants (Cohen's d values >5).
Given this reliability, adaptation may be able to form the basis of an objective test for visual snow, particularly when coupled with known methods for reducing effects of bias on appearance judgments.38,39 For example, we have begun piloting a procedure in which aftereffects of adaptation are visible to people with visual snow, but not to normally sighted controls. People with visual snow may be objectively better at identifying the location of an adapting stimulus shown during a task that directs attention elsewhere in space, that is, a demanding fixation task. Specifically, when the fixation task stops, observers are asked to identify which of the many locations on the screen that contained noise adapters were the last to be stimulated. The strong aftereffect in those last-stimulated locations may allow people with visual snow to identify them better than control participants, who have minimal aftereffects and hence less information on which to base their judgment.
Not every high contrast pattern can provide relief from visual snow; viewing a pattern of stripes had only a negligible effect on the snow symptom. Our paradigm also demonstrates that the location of the adapting pattern matters. If adaptation did not show this specificity for retinal location, then both sides would have appeared the same immediately following adaptation. Adaptation to physical stimuli in normally sighted observers also shows retinotopic specificity.22
Such specificity of adaptation has proven to be a useful tool, “the psychophysicist's electrode,”40 for determining properties of neurons responsible for adaptation effects. Neurons in different visual areas vary in their preferred stimuli. Future behavioral studies could provide evidence about which areas contribute to the snow by determining in more detail what stimuli may or may not affect it. For example, some visual areas contain neurons that respond selectively to motion in a particular direction. If adapting to motion affects the appearance of visual snow, causing it to appear to move in the opposite direction, then the activity producing visual snow must arise at or before the location of direction selective neurons in the visual pathways.
Our results from the larger sample likely underestimate the potential effectiveness of adaptation in the general population. Because of adaptation's specificity to location, our paradigm requires stable fixation to produce differences in appearance on the two sides of the display. Although we encouraged participants to fixate, inexperienced observers are often unaware of their eye movements, and it is especially likely that some shifted their gaze given that individuals with VSS show reduced inhibition of eye movements compared to controls.34,41,42 Enforcing fixation by monitoring gaze position should increase the number of participants showing adaptation effects. Additionally, in prior work, we have demonstrated that individuals differ in the properties of their visual snow, such as its size, density, and flicker rate.26 Given the specificity of adaptation, tailoring an adapter to an individual's snow percept should increase its effectiveness. Finally, the 45 second duration used for our larger sample is short for adaptation paradigms, and effects would increase with longer durations. Thus, it remains possible that adaptation could reduce the strength of snow in virtually every participant.
Many participants in our study were excited and surprised to be able to view part of the world for the first time without a veil of dots, even if the experience was temporary. The short-term control over the symptom given by adaptation should provide an important tool for advancing understanding of visual snow, which could inform the development of treatments.
Supplementary Material
Acknowledgments
The authors would like to recognize Karly Allison for assisting with data collection. We also thank our participants for sharing their thoughtful insights into visual snow.
Supported by the National Eye Institute of the National Institutes of Health under Award Number F31 EY034016, a T32 EY025187 vision training grant, the Diversifying the Community of Neuroscience Program award number R25 NS117356, UL1 TR002494, the National Science Foundation Research Training Grant in translational and sensory science award number DGE 1734815 at the University of Minnesota, and a UMN OVPR Grant-in-Aid (#574483). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or The University of Minnesota.
Data Availability: Data and code are available upon request and will be posted on a public archive with links on the Engel and Schallmo lab websites.
Disclosure: S.A. Montoya, None; C.B. Mulder, None; M.S. Lee, None; M.-P. Schallmo, None; S.A. Engel, None
References
- 1. Kondziella D, Olsen MH, Dreier JP. Prevalence of visual snow syndrome in the UK. Eur J Neurol. 2020; 27: 764–772. [DOI] [PubMed] [Google Scholar]
- 2. Solly EJ, Clough M, Foletta P, White OB, Fielding J. The psychiatric symptomology of visual snow syndrome. Front Neurol. 2021; 12: 703006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 4. Eren O, Schankin CJ. Mirtazapine for treatment of visual snow syndrome: a case series with insights into pathophysiology and therapy. Clin Transl Neurosci. 2020; 4: 2514183X2092569. [Google Scholar]
- 5. Puledda F, Vandenbussche N, Moreno-Ajona D, Eren O, Schankin C, Goadsby PJ. Evaluation of treatment response and symptom progression in 400 patients with visual snow syndrome. Br J Ophthalmol. 2022; 106(9): 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Dongen RM, Waaijer LC, Onderwater GLJ, Ferrari MD, Terwindt GM. Treatment effects and comorbid diseases in 58 patients with visual snow. Neurology. 2019; 93: e398–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lukáčová V, Mastík J, Minks E. Could repetitive transcranial magnetic stimulation (rTMS) help patients with visual snow? Act Nerv Super (Praha). 2018; 60: 27–31. [Google Scholar]
- 8. Mehta DG, Garza I, Robertson CE. Two hundred and forty-eight cases of visual snow: a review of potential inciting events and contributing comorbidities. Cephalalgia. 2021; 41: 1015–1026. [DOI] [PubMed] [Google Scholar]
- 9. Tegetmeyer H. Visual snow syndrome: symptoms and ophthalmological findings. Thieme. 2017; 05: 713–718. [DOI] [PubMed] [Google Scholar]
- 10. Vaphiades MS, Grondines B, Cooper K, Gratton S, Doyle J. Diagnostic evaluation of visual snow. Front Neurol. 2021; 12: 743608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoo Y-J, Yang HK, Choi J-Y, Kim J-S, Hwang J-M. Neuro-ophthalmologic findings in visual snow syndrome. J Clin Neurol. 2020; 16: 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaroban N, Kedar S, Anderson D, Vuppala A-A. Analysis of retinal structure and electrophysiological function in visual snow syndrome: an exploratory case series. J Neuroophthalmol. 2022; 43(2): 227–231. [DOI] [PubMed] [Google Scholar]
- 13. Aldusary N, Traber GL, Freund P, et al.. Abnormal connectivity and brain structure in patients with visual snow. Front Hum Neurosci. 2020; 14: 582031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Traber GL, Aldusary N, Freund P, et al.. Visual snow patients show functional hyperconnectivity and structural abnormalities of brain regions involved in visual processing. Invest Ophthalmol Vis Sci. 2020; 61: 3387–3387. [Google Scholar]
- 15. Puledda F, Bruchhage M, O'Daly O, Ffytche D, Williams SCR, Goadsby PJ. Occipital cortex and cerebellum gray matter changes in visual snow syndrome. Neurology. 2020; 95: e1792–e1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puledda F, O'Daly O, Schankin C, Ffytche D, Williams SC, Goadsby PJ. Disrupted connectivity within visual, attentional and salience networks in the visual snow syndrome. Hum Brain Mapp . 2021; 42: 2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puledda F, Ffytche D, Lythgoe DJ, et al.. Insular and occipital changes in visual snow syndrome: a BOLD fMRI and MRS study. Ann Clin Transl Neurol. 2020; 7: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schankin CJ, Maniyar FH, Chou DE, Eller M, Sprenger T, Goadsby PJ. Structural and functional footprint of visual snow syndrome. Brain. 2020; 143: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Unal-Cevik I. The role of occipital cortex hyperexcitability in visual snow syndrome. Neurol Sci Neurophysiol. 2022; 39: 61. [Google Scholar]
- 20. Van Laere K, Ceccarini J, Gebruers J, Goffin K, Boon E. Simultaneous 18F-FDG PET/MR metabolic and structural changes in visual snow syndrome and diagnostic use. EJNMMI Res . 2022; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007; 97: 3155–3164. [DOI] [PubMed] [Google Scholar]
- 22. Webster MA. Visual adaptation. Annu Rev Vis Sci. 2015; 1: 547–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anstis S, Verstraten FAJ, Mather G. The motion aftereffect. Trends Cogn Sci. 1998; 2: 111–117. [DOI] [PubMed] [Google Scholar]
- 24. Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What's new in psychtoolbox-3. 2007; 36(14): 1–16. [Google Scholar]
- 25. Thaler L, Schütz AC, Goodale MA, Gegenfurtner KR. What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vision Res . 2013; 76: 31–42. [DOI] [PubMed] [Google Scholar]
- 26. Montoya SA, Mulder CB, Allison KD, Lee MS, Engel SA, Schallmo M. What does visual snow look like? Quantification by matching a simulation. PsyArXiv. Preprint at 10.31234/osf.io/9zmaf. 2023. [DOI] [PMC free article] [PubMed]
- 27. Lauschke JL, Plant GT, Fraser CL. Visual snow: a thalamocortical dysrhythmia of the visual pathway? J Clin Neurosci. 2016; 28: 123–127. [DOI] [PubMed] [Google Scholar]
- 28. Morey RD. Confidence intervals from normalized data: a correction to Cousineau (2005). Tutor Quant Methods Psychol. 2008; 4: 61–64. [Google Scholar]
- 29. Greenlee MW, Georgeson MA, Magnussen S, Harris JP. The time course of adaptation to spatial contrast. Vision Res. 1991; 31: 223–236. [DOI] [PubMed] [Google Scholar]
- 30. Demb JB. Functional circuitry of visual adaptation in the retina: retinal mechanisms for visual adaptation. J Physiol. 2008; 586: 4377–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasaki Y, Murakami I, Cavanagh P, Tootell RHB. Human brain activity during illusory visual jitter as revealed by functional magnetic resonance imaging. Neuron. 2002; 35: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 32. Bessero A-C, Plant GT. Should ‘visual snow’ and persistence of after-images be recognised as a new visual syndrome? J Neurol Neurosurg Psychiatry. 2014; 85: 1057–1058. [DOI] [PubMed] [Google Scholar]
- 33. Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ. ‘Visual snow’ – a disorder distinct from persistent migraine aura. Brain. 2014; 137: 1419–1428. [DOI] [PubMed] [Google Scholar]
- 34. Solly EJ, Clough M, McKendrick AM, Folette P, White OB, Fielding J. Eye movement characteristics provide an objective measure of visual processing changes in patients with visual snow syndrome. Sci Rep. 2021; 11: 9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brooks CJ, Chan YM, Fielding J, White OB, Badcock DR, McKendrick AM. Visual contrast perception in visual snow syndrome reveals abnormal neural gain but not neural noise. Brain. 2022; 145(4): 1486–1498. [DOI] [PubMed] [Google Scholar]
- 36. Eren OE, Straube A, Schöberl F, Ruscheweyh R, Eggert T, Schankin CJ. Age- and frequency-dependent changes in dynamic contrast perception in visual snow syndrome. J Headache Pain. 2021; 22: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McKendrick AM, Chan YM, Tien M, et al.. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurology. 2017; 88: 1243–1249. [DOI] [PubMed] [Google Scholar]
- 38. Morgan MJ. A bias-free measure of retinotopic tilt adaptation. J Vis. 2014; 14: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jogan M, Stocker AA. A new two-alternative forced choice method for the unbiased characterization of perceptual bias and discriminability. J Vis. 2014; 14: 20. [DOI] [PubMed] [Google Scholar]
- 40. Frisby JP. Seeing : illusion, brain, and mind/John P. Frisby. New York, NY: Oxford University Press; 1979. [Google Scholar]
- 41. Foletta PJ, Clough M, McKendrick AM, Solly EJ, White OB, Fielding J. Delayed onset of inhibition of return in visual snow syndrome. Front Neurol. 2021; 12: 738599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solly EJ, Clough M, McKendrick AM, Foletta P, White OB, Fielding J. Ocular motor measures of visual processing changes in visual snow syndrome. Neurology. 2020; 95: e1784–e1791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.