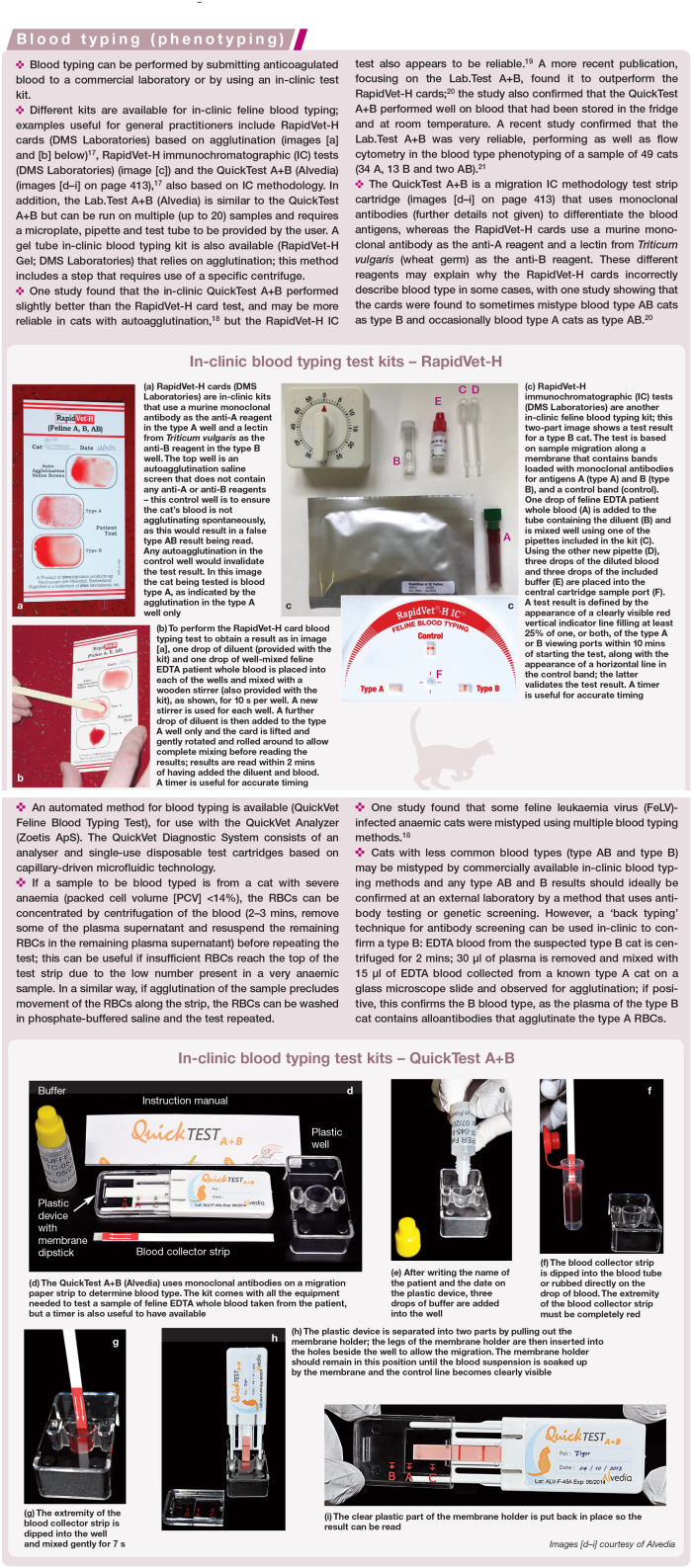
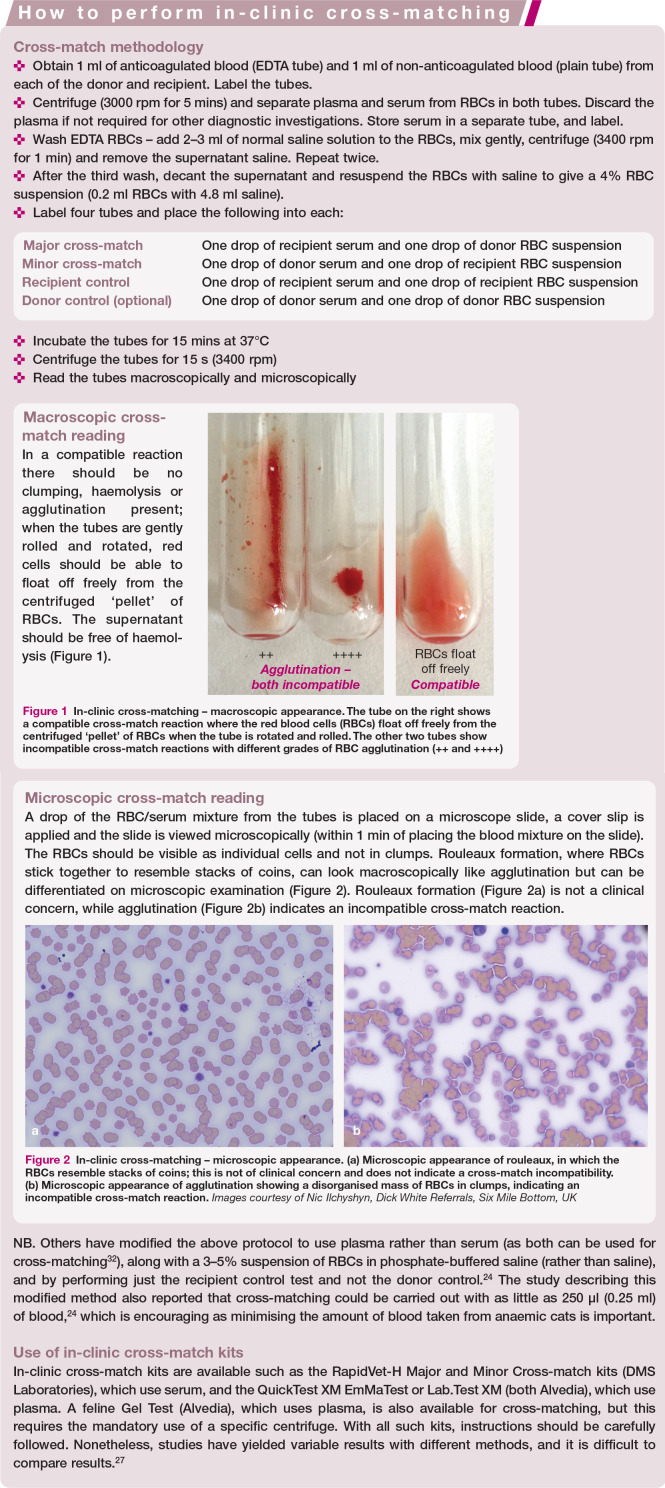
Abstract
Practical relevance:
Blood and blood products are increasingly available for practitioners to use in the management of haematological conditions, and can be lifesaving and therapeutically useful for patients with anaemia and/or coagulopathies. It is important for feline healthcare that donors are selected appropriately, and transfusions of blood or blood products are given to recipients that will benefit from them. Complications can occur, but can be largely avoided with careful donor management and recipient selection, understanding of blood type compatibility, and transfusion monitoring.
Clinical challenges:
Feline blood transfusion, while potentially a lifesaving procedure, can also be detrimental to donor and recipient without precautions. Cats have naturally occurring alloantibodies to red cell antigens and severe reactions can occur with type-mismatched transfusions. Blood transfusions can also transmit infectious agents to the recipient, so donor testing is essential. Finally, donors must be in good health, and sedated as appropriate, with blood collected in a safe and sterile fashion to optimise the benefit to recipients. Transfusion reactions are possible and can be mild to severe in nature. Autologous blood transfusions and xenotransfusions may be considered in certain situations.
Evidence base:
These Guidelines have been created by a panel of authors convened by the International Society of Feline Medicine (ISFM), based on available literature. They are aimed at general practitioners to provide a practical guide to blood typing, cross-matching, and blood collection and administration.
Keywords: Transfusion, plasma, xenotransfusion, transfusion reaction, blood type, cross-match
Introduction
Although feline blood transfusions are infrequently performed in primary care veterinary practice, they can be lifesaving.1,2 Availability of donors has limited the utility of this technique, but with the growth of blood banks providing access to feline blood, the procedure may become more routine. It is important that veterinary practitioners select appropriate recipients and donors (or stored blood) and administer blood correctly and with monitoring to mitigate the risks.
These Guidelines are written to provide information for practitioners on blood types and cross-matching, indications for transfusion, donor management, recipient preparation, blood/blood product administration, and monitoring and potential complications.

Feline blood types
Alloantigens
Blood types arise due to genetically determined antigenic markers present on the surface of red blood cells (RBCs). Blood type antigens are alloantigens, as they exist in alternative (allelic) forms in different cats, and can induce an immune response when RBCs of one blood type are transferred to a cat with a different blood type.
One blood group system, the AB system, has been extensively defined in cats. Within the AB blood group system there are three blood type phenotypes, namely type A, type B and type AB:
✜ Blood type A is common. N-glycolyl-neuraminic acid is the alloantigen on the RBC surface;
✜ Blood type B is less common overall, but common in some pedigree breeds (eg, British Shorthair, Birman, Devon Rex). N-acetyl-neuraminic acid is the alloantigen on the RBC surface;
✜ Blood type AB is rare. N-glycolyl-neuraminic acid and N-acetylneuraminic acid are the alloantigens on the RBC surface.
Blood type prevalence varies geographically (see Table 1). Type A is the most common worldwide and in some breeds 100% of cats are believed to be type A (eg, Siamese 4 ). The prevalence of type B is much lower than type A but it has been reported to be as high as 36% in non-pedigree cats in Australia, 7 and some breeds can contain high numbers of type B cats (especially the British Shorthair3,6). Type AB is much less common. Blood typing is essential to avoid potentially fatal transfusion reactions.
Table 1.
Blood types reported in different geographical locations in different breeds of cat in published studies
| Country/regional source of data (reference) | Breed | Number of cats | Type A % | Type B % | Type AB % |
|---|---|---|---|---|---|
| UK 3 | Non-pedigree | 139 | 87.1 | 7.9 | 5.0 |
| British Shorthair | 121 | 39.7 | 58.7 | 1.6 | |
| Birman | 24 | 62.5 | 29.2 | 8.3 | |
| Persian | 17 | 88.2 | 11.8 | 0 | |
| Other pedigrees | 45 | 77.8 | 6.7 | 15.5 | |
| UK 4 | Non-pedigree | 105 | 67.6 | 30.5 | 1.9 |
| Siamese | 13 | 100.0 | 0 | 0 | |
| Other pedigrees | 38 | 76.3 | 21.1 | 2.6 | |
| UK 5 | Bengal | 100 | 100.0 | 0 | 0 |
| Denmark 6 | Non-pedigree | 105 | 98.1 | 1.9 | 0 |
| Persian | 56 | 96.4 | 3.6 | 0 | |
| British Shorthair | 30 | 66.7 | 33.3 | 0 | |
| Abyssinian | 20 | 100 | 0 | 0 | |
| Other pedigrees | 33 | 90.9 | 9.1 | 0 | |
| Australia 7 | Non-pedigree | 355 | 62.0 | 36.0 | 1.6 |
| Siamese | 12 | 100.0 | 0 | 0 | |
| Devon Rex | 70 | 45.0 | 54.0 | 1.4 | |
| British Shorthair | 8 | 38.0 | 62.0 | 0 | |
| New Zealand 8 | Non-pedigree | 245 | 70.6 | 13.9 | 0.8 |
| France 9 | Non-pedigree | 320 | 83.8 | 14.4 | |
| Pedigree | 37 | 89.2 | 10.8 | 0 | |
| Central Italy 10 | Non-pedigree | 483 | 89.8 | 7.0 | 3.1 |
| North Italy 11 | Non-pedigree | 233 | 91.0 | 5.2 | 3.8 |
| South Italy 11 | Non-pedigree | 215 | 77.2 | 12.1 | 10.7 |
| Italy 12 | Ragdoll | 61 | 77.1 | 4.9 | 18.0 |
Alloantibodies
In contrast to dogs, cats can possess naturally occurring alloantibodies against the ‘foreign’ (non-self) alloantigen that they are lacking. These alloantibodies will recognise the alloantigens of another cat. Kittens develop these antibodies at 6–8 weeks of age. In the UK, for example, over 70% of type A cats have anti-B alloantibodies, 13 which are mostly present at low concentrations, while all type B cats have anti-A alloantibodies, often present at high concentrations. In a report from the USA, all type A cats had anti-B alloantibod-ies. 14 Type AB cats never have alloantibodies to either type A or type B antigens. The reaction between the blood type alloantigens and any existing alloantibodies is observed during cross-matching of donor and recipient blood.
Alloantibodies are responsible for potentially fatal blood transfusion reactions that can arise even when cats undergo their first blood transfusion, as they are already present in the cat’s circulation, ready to destroy RBCs of a different blood type phenotype. These allo-antibodies are also responsible for neonatal isoerythrolysis,15,16 a cause of neonatal death. The severity of a blood transfusion reaction depends on the quantity (ie, higher titres or concentrations are worse) and nature (eg, strongly agglutinating) of any alloantibodies present in the recipient or donor.
Non-AB feline blood groups
Evidence published in 2007 22 suggested that other (non-AB) blood group systems existed in cats because transfusion reactions have occurred in cats given AB-matched blood transfusions. The study from the USA reported the absence of a novel feline RBC antigen named Mik in three of 65 type A cats tested, in association with the presence of naturally occurring anti-Mik alloantibodies, which mediated a clinically significant transfusion reaction despite the blood donor and recipient cat being AB-matched. 22 However, one study in UK cats, 23 and another in German cats, 24 found no evidence of anti-Mik alloantibodies in the cats sampled, as no positive cross-matches between AB-matched blood samples were found in transfusion-naive cats.
Other studies have, however, documented the presence of positive cross-matches between AB-matched blood samples,21,25,26 suggesting the presence of non-AB blood group systems, although the clinical significance of these is not always clear and tests for Mik and other RBC antigens are not available commercially. In the most recent study, 27 type A cats were evaluated for naturally occurring non-AB alloantibodies by cross-matching and at least 7% of the cats had incompatible cross-matching, documenting the presence of naturally occurring alloantibodies. Five distinct RBC antigens were hypothesised to be present outside of the AB blood group system and one of these was thought to correspond to the previously described Mik antigen. 27

Cross-matching
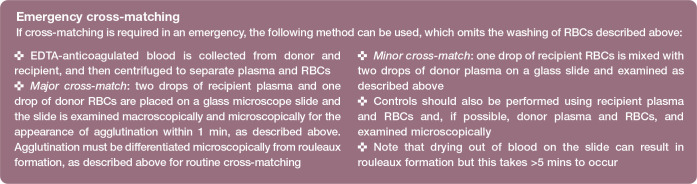
Cross-matching can be performed in-clinic or at an external laboratory; the latter is ideal as the test is complex and takes time but obviously this results in a delay in obtaining results.
Figure 1.
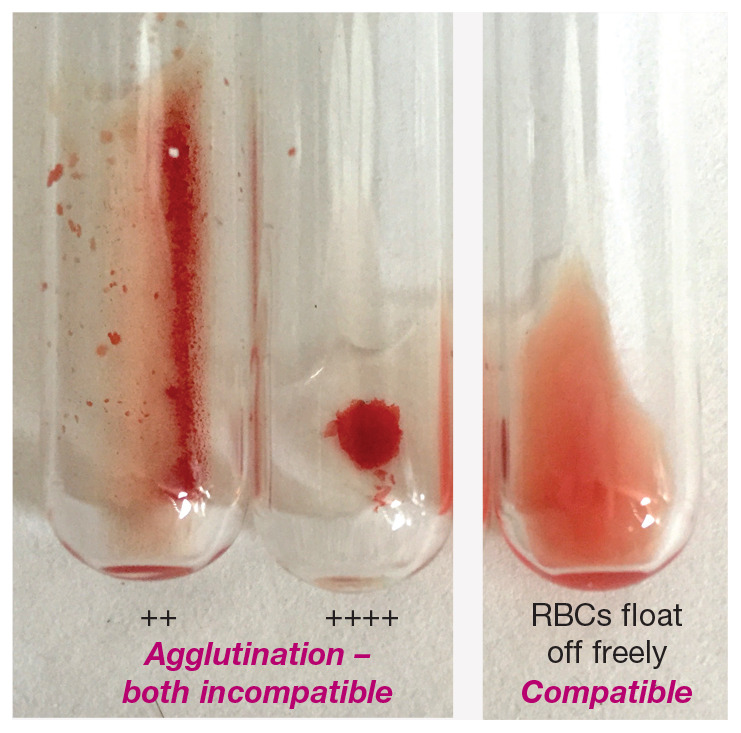
In-clinic cross-matching – macroscopic appearance. The tube on the right shows a compatible cross-match reaction where the red blood cells (RBCs) float off freely from the centrifuged ‘pellet’ of RBCs when the tube is rotated and rolled. The other two tubes show incompatible cross-match reactions with different grades of RBC agglutination (++ and ++++)
In-clinic cross-matching kits are available. Based on all existing studies, the clinical effectiveness and need for cross-matching before a first transfusion remains controversial. However, given that transfusion-naive cats may have incompatible major cross-matches, cross-matching, as well as blood typing, is recommended by some before each transfusion where possible in cats,27–29 although others have acknowledged that the strength of evidence for this is weak. 30 A recent Australian study, surveying primarily general veterinary practitioners, reported that compatibility testing, including cross-matching, before feline blood transfusions was commonly performed; cross-matching alone by 26% of respondents, blood typing alone by 27.6%, and both by 34.1% of respondents. 31
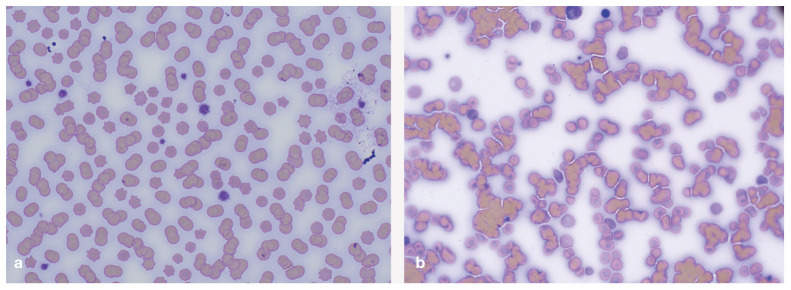
Figure 2.
In-clinic cross-matching – microscopic appearance. (a) Microscopic appearance of rouleaux, in which the RBCs resemble stacks of coins; this is not of clinical concern and does not indicate a cross-match incompatibility. (b) Microscopic appearance of agglutination showing a disorganised mass of RBCs in clumps, indicating an incompatible cross-match reaction. Images courtesy of Nic llchyshyn, Dick White Referrals, Six Mile Bottom, UK
Selecting a donor
Infectious disease screening
Risks from transfusion include the trans mission of infectious agents from donor to recipient, which can be largely avoided through donor selection and screening. Such a process must inevitably vary between countries/regions and practices, and will depend on locally endemic diseases, the practicalities of selecting donors that do not carry them and the cost/availability of screening compared with the risk of not having any available blood.

In addition to the considerations above, individual donor factors such as indoor/outdoor status, ectoparasite control and time of last testing will influence the likelihood of infectious agent presence. These factors will in turn determine which agents should be screened for, the most appropriate methodology and also the required frequency, which may be deemed to be annually, or at the time of every blood donation if there is a high risk of novel exposure or intermittent circulation of a pathogen.33,34
Core infectious agents to test donor cats for Haemotropic mycoplasmas
The pathogenic epierythrocyte parasitic bacterium Mycoplasma haemofelis can be transmitted by blood products, although it appears to be inactivated during storage of whole blood for 1 week. 35 Blood smear evaluation is insensitive for diagnosis and also lacks specificity, and thus the diagnostic test of choice for screening blood donors is PCR. PCR testing for ‘Candidatus Mycoplasma haemominutum’ (which may survive for a week in stored blood 35 ) and ‘Candidatus Mycoplasma turicensis’ can also be considered; however, as these agents are of lower pathogenicity, 36 donors may not be excluded if positive for these organisms if the donor pool is very limited. Ideally, however, cats should be negative for these agents, too.
Bartonella species
Numerous Bartonella species can be present in the blood of cats and have been associated with several clinical conditions. 37 Bartonella henselae has been shown to survive in stored blood. 38 Donors should ideally be serology and PCR negative for Bartonella species but seropositivity may be common in endemic areas and sensitive testing methods are not always readily available. Seropositive cats may have intermittent bacteraemia, 39 but can be considered for donation if PCR negative.
Feline leukaemia virus/feline immunodeficiency virus
Both FeLV and feline immunodeficiency virus (FIV) can be transmitted by blood transfusion and thus donor cats need to be negative for these agents. 40 Antigen tests for FeLV are commonly available, such as ELISA or IC in-clinic tests, but proviral DNA testing (by PCR) should be performed, if at all possible, as transmission of FeLV infection through transfusion of FeLV provirus positive, antigen negative blood (eg, PCR positive, ELISA negative) has been documented. 41
Antibody tests for FIV are commonly available as ELISA or IC in-clinic tests, too, and cats should be negative for FIV antibody before being used as donors. Although certain FIV antibody tests may be able to differentiate true FIV infected cats from those cats that have been vaccinated for FIV (in countries where FIV vaccination is, or has been, available for use in cats), it is recommended that only FIV antibody negative cats are used as blood donors due to the potential for confusion in interpretation of test results. 42
Additional agents to consider testing donor cats for Anaplasma species
Anaplasma phagocytophilum can cause illness in cats, and can be transmitted by blood inoculation and exist as a persistent infection.43,44 Donor cats with potential tick exposure (particularly Ixodes ricinus) from endemic areas should ideally be screened by serology and PCR, if available. Seropositive, PCR negative cats may be used in endemic regions if no other suitable donor can be identified. Infection with Anaplasma platys has been documented in cats, 43 and so cats living in areas endemic for Rhipicephalus species ticks should be screened for this agent by PCR.
Cytauxzoon felis, Babesia felis, Ehrlichia canis, Leishmania infantum and Neorickettsia risticii
These are all vector-borne agents,43–49 and may be transmissible by blood products. Although pre-donation physical examination and blood smear examination should minimise transmission risk, the optimal standard would be for donor cats to be negative by PCR, if available, for these agents if living in endemic areas.
Other infectious agents
Screening for coronavirus, Rickettsia felis and Toxoplasma gondii is not recommended for donor cats. Transmission of these agents by blood products has not been documented.
Donor characteristics
Donors should be healthy, and between 1 and 8 years old, with a lean body weight above 4.5 kg. They should be of calm temperament and easy to handle to reduce sedation requirements. All applicable vaccinations and parasite control should be current and donors should ideally live indoors without recent introduction of other cats to the household, to reduce their exposure to infections. No other recent medications should have been given and they should never have received a transfusion nor be currently pregnant. Cats that have previously had a litter may still be donors.
Annual health screening of potential blood donors, including haematology and serum biochemistry profiles, is recommended. In addition, a complete history and physical examination, as well as determination of packed cell volume (PCV) or haemoglobin concentration, should be completed before each blood collection.
Occult cardiomyopathy is excluded with echocardiography screening by some clinicians prior to allowing cats to join a donor programme, given that up to 30% of cats with cardiac disease will not have a murmur. 50 However, others would omit echocardiogra-phy and exclude cats with murmurs, gallop rhythms or arrythmias from donation, or perform quantitative NT-proBNP serum testing, which has been shown to reliably discriminate normal cats from those with occult cardiomyopathy. 51
Indications for blood transfusion
Due to restrictions on storage of animal blood, most cats in the UK and Europe in need of a blood transfusion will receive fresh whole blood (FWB), which contains all of the blood components: RBCs, platelets, coagulation factors and plasma proteins. However, in countries where blood storage is available, feline FWB donations are processed into packed red blood cells (pRBCs) and fresh-frozen plasma (FFP) components. The use of these blood components has many advantages including extending resources, allowing specific replacement therapy, and potentially reducing the number of transfusion reactions.

RBC products
RBC products, namely FWB and pRBCs, increase the oxygen-carrying capacity of the blood and thereby improve oxygen delivery to tissues. While FWB and pRBC transfusions can be used interchangeably in most anaemic cats, administration of pRBCs would be preferable to FWB for those, for example, with underlying cardiac disease to help avoid circulatory overload, as well as for those, for example, with anaemia due to haemolysis rather than blood loss.
The decision to administer an RBC product transfusion is frequently based on measurement of the cat’s PCV, haematocrit or haemoglobin concentration. However, a ‘transfusion trigger’ or threshold PCV below which an RBC transfusion is administered has not been clearly defined in human or veterinary medicine, and accompanying clinical signs are very important to consider in deciding if a transfusion is required. In two studies involving RBC transfusions in more than 265 cats, the pre-transfusion PCV was 15% (median value 25 ) and 17% (mean value 26 ), with a range of 5–40%. In some cats with peracute blood loss and hypovolaemia, RBC transfusions may be indicated even though their PCV is normal. These patients will predictably develop a low PCV following fluid resuscitation with asanguineous fluids.
Ineffective erythropoiesis and blood loss are the most common general causes of anaemia reported in cats receiving RBC transfusions, with haemolysis noted less frequently in approximately 5–25% of cats.25,26,29 Underlying conditions frequently associated with development of non-regenerative anaemia in cats, and the potential need for an RBC transfusion, include chronic kidney disease, lymphoma, systemic inflammatory disease, infectious diseases, bone marrow disorders 52 and chronic unspecified diseases. 29 An often-overlooked factor contributing to development of anaemia in hospitalised critically ill cats is repeated phlebotomy for blood sampling, with 74% of non-anaemic cats in one intensive care unit population going on to develop anaemia during the hospitalisa-tion period. 53 In this study, cats that required a pRBC transfusion had significantly more daily blood samples taken (median 3 blood samples, range 1–6) than cats that did not require a transfusion (median 2 blood samples, range 1–4). 53
Plasma products
Plasma separated from RBCs within 8 h of blood collection is referred to as fresh plasma, but in countries where stored veterinary blood products are available, fresh plasma is more often frozen after preparation and stored (at -20° to -30°C) for up to 1 year; this type of plasma is referred to as fresh-frozen plasma (FFP). Fresh plasma and FFP contain haemostatic proteins (coagulation factors, von Willebrand factor, anticoagulant proteins and fibrinolysins), albumin and immunoglobulins.
The main indication for use of fresh plasma or FFP is bleeding due to inherited or acquired coagulopathies, but use of these products has also been reported in cats with hypotension, liver disease, neoplasia and sepsis. 54 Although the benefit of prophylactic administration of plasma to cats with a coagulopathy (but not showing clinical signs of bleeding) undergoing an invasive procedure is unclear, it was reported to be the main reason for FFP transfusions in cats in another study. 55 Anticoagulant rodenticide toxicity is uncommon in cats compared with dogs, but may occur after consumption of poisoned prey, and FFP as well as FWB may be included in the treatment protocol.55–57
Hereditary haemostatic disorders are diagnosed infrequently in cats. There are two case reports of type 3 von Willebrand disease (VWD)58,59 and sporadic reports of haemophilia A and B55,60,61 causing bleeding in cats. Plasma would be appropriate to provide replacement of von Willebrand factor or factor VIII or IX in cats with VMD, or haemophilia A or B, respectively, that are experiencing bleeding, though FWB would be an alternative if fresh plasma or FFP was not available or the cat was also anaemic.
The effect of plasma on colloid osmotic pressure is less than that of synthetic colloids; therefore, plasma is less effective for volume expansion. 62
Platelet products
Owing to technical challenges associated with preparing platelet-rich plasma from a small volume feline FWB unit, cats in need of a platelet transfusion generally are administered FWB, although this will not provide adequate platelets to correct thrombocytopenia.
There are few indications for platelet transfusions in cats, but they include uncontrolled or life-threatening haemorrhage (eg, pulmonary haemorrhage) with thrombocytopenia or thrombopathia, and possibly massive transfusion (rare, but is when a high number of pRBCs have been given, which can cause a dilutional effect on the recipient’s clotting factors and platelets). While platelet disorders are uncommon in cats, primary immune thrombocytope-nia can lead to severe blood loss anaemia, which can be managed with FWB transfu-sions. 63 Cats with bleeding secondary to a thrombopathia typically require administration of functional platelets (for practical reasons in the form of FWB) to control bleeding. 64
Chemical restraint of the donor for blood transfusion
It is possible to perform blood collection in conscious cats with a skilled veterinary care team. Patients must be cooperative but, even still, blood donation may be a negative experience for donors. Movement during donation and signs of anxiety have been reported in conscious cats much more often than in sedated cats. 71 Additionally, stress produced by handling may affect the cellular and chemical composition of the blood (eg, hyperglycaemia). 72 Therefore, sedation or general anaesthesia is now commonly used for feline donors. The choice of a short-term (30 mins) protocol for chemical restraint will avoid an uncomfortable experience for the cat and failed, repeated interventions that could produce injuries to the veterinary care team. It will also influence owner satisfaction with the donor experience. 73
Chemical restraint for feline blood donors is no different to any other anaesthetic procedure in the sense that pre-operative examination and appropriate fasting (6 h) are mandatory. An anaesthetic plan, including monitoring and careful choice of dosage regimens, is required. The use of local anaesthetic creams (Figure 3) and pheromones may be part of the overall management of the patient to help reduce stress.
Figure 3.
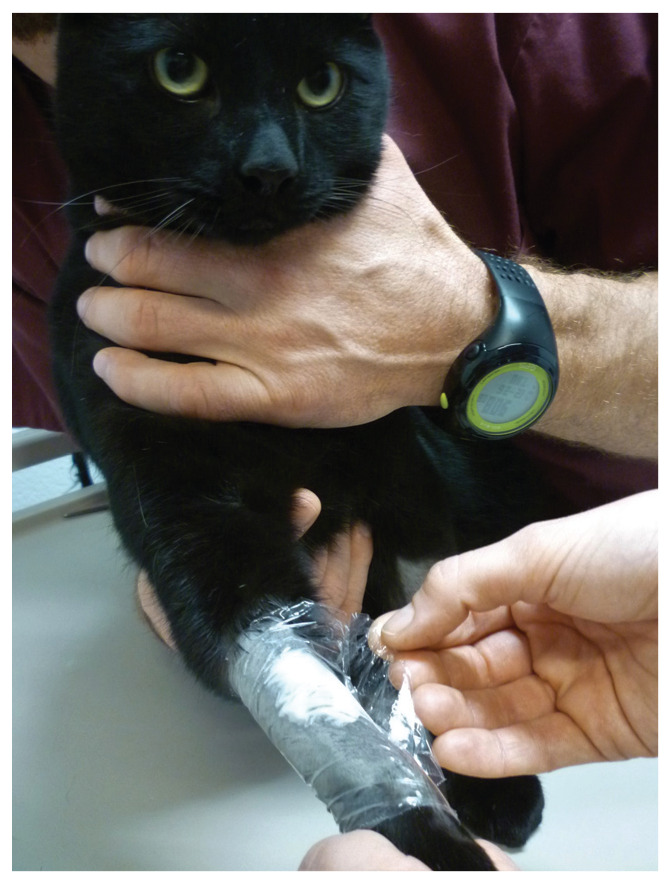
Local anaesthetic cream can be applied over the cephalic and jugular veins of the blood donor, after clipping the hair, for desensitisation of the skin before chemical restraint. A light occluding bandage (cellophane is used here) protects the area where the cream has been applied. Approximately 20-40 mins later an intravenous catheter is placed in the cephalic vein for drug administration (low doses of ketamine and diazepam or midazolam, for example). The cat can be placed in its transport carrier or a cosy cat kennel in a quiet room or ward during these 20-40 mins. The jugular vein site will be used for blood withdrawal
The intravenous route of administration is often preferred due to the rapid onset of action and the use of lower doses of anaesthetic agents when compared with the intramuscular and subcutaneous routes. Several studies have reported the feasibility of different drug combinations, as well as effects on major blood analytes, in cat donors (see Table 2). Overall, each protocol has its unique advantages and disadvantages. Ideally, the drug combination should have a short onset with adequate depth and duration of action, and include a smooth and rapid anaesthetic recovery for the donor, with minimal cardio-respiratory depression. The choice will be also dependent on drug availability and the familiarity of the veterinary care team with the protocol. Eye lubricant should be applied to all cats regularly (every 35–45 mins) to avoid eye ulcers and lesions. Ideally, the cat should resume its normal behaviour, and eat and drink, shortly after the end of sedation/ anaesthesia.
Table 2.
Summary of different drug combinations that can be used in blood donors and their effect on major blood analytes
| Drugs (reference[s]) | Dose /dosage or inhalant concentration in common usage | Comments | Changes in blood analytes reported |
|---|---|---|---|
| Ketamine and diazepam 74 | 10 mg ketamine + 0.5 mg diazepam per cat, both IV | Protocol for short-term venepuncture (5
mins). Short onset and duration of action with excellent chemical restraint but may not be enough to complete phlebotomy. Note diazepam should not be administered IM |
Minimal decreases in plasma triglycerides and albumin, and minimal increases in activated partial thromboplastin and prothrombin times, likely without clinical relevance |
| Ketamine and midazolam75–77 |
4-6 mg/kg ketamine + 0.4 mg/kg midazolam, both IM or IV | Mixed in the same syringe for IM injection. Prolonged anaesthetic effects, with ataxia and recumbency for up to 4–6 h after phlebotomy. Alternative protocols include the addition of butorphanol (0.3 mg/kg) or buprenorphine (0.01 mg/kg) IM. Hyperthermia may occur with ketamine-based protocols | Decreases of around 24-25% in RBC count, Hb concentration and PCV after higher doses of ketamine (10 mg/kg) and midazolam (0.5 mg/kg) IV. Based on these PCV changes, some donors may be falsely diagnosed with anaemia |
| Dexmedetomidine and butorphanol73,75,78 | 0.01 mg/kg dexmedetomidine + 0.2 mg/kg butorphanol, both IM | Ease of administration with short onset of action and possibility of dexmedetomidine reversal with atipamezole (0.1 mg/kg IM) are benefits. Also good muscle relaxation. Adverse effects include emesis, bradycardia, increased systemic vascular resistance and decreases in cardiac output. Peripheral vasoconstriction poses an additional challenge with respect to venous catheterisation and blood collection; several donations were aborted due to this. Higher doses of dexmedetomidine might be required in some cats. Overall, best avoided due to above-mentioned adverse effects | Decreases in RBC count, Hb concentration and HCT value (ie, sequestration of erythrocytes by the spleen induced by reduced sympathetic activity) |
| Alfaxalone and butorphanol73,79,80 | 2 mg/kg alfaxalone + 0.2–0.4 mg/kg butorphanol, both IM | Minimal cardiorespiratory changes. Large volume of IM injection. Rapid recovery from anaesthesia (just over 30 mins). Additional sedation or gentle physical restraint might be required in some cats; further administration of alfaxalone (0.1 mg/kg IV) can be used but will prolong duration and recovery of anaesthesia. Twitching has been observed by one of the Panel authors (PS) | No changes in complete blood count or serum biochemical values in experimental cats after doses of 5 mg/kg and 15 mg/kg IV of alfaxalone |
| Tiletamine and zolazepam 81 | 2.5 mg/kg of each agent, both IM | Short onset of action. Hypothermia can be observed. Increases in heart rate and blood pressure due to hypovolaemia and drug-induced sympathetic stimulation | RBC, WBC, platelet, neutrophil and monocyte counts, HCT value and Hb concentration decreased, and lymphocyte, eosinophil and basophil counts increased after blood collection (not statistically significant) |
| Sevoflurane 76 | Mask or ‘box’ induction with sevoflurane (4–5% for induction followed by 2–3% for maintenance using 2 l/min of oxygen) | Potentially stressful to the cat to be restrained for mask or box induction, plus risk of environmental exposure of the veterinary care team to the inhalant anaesthetic. Hence not recommended for collection of blood from donor cats. Higher prevalence of hypotension when compared with ketamine combinations | Not reported |
RBC = red blood cell; HCT = haematocrit; Hb = haemoglobin; WBC = white blood cell; PCV = packed cell volume; IV = intravenous; IM = intramuscular
Alpha-2-adrenergic receptor agonists (xylazine, medetomidine and dexmedetomi-dine) are to be avoided for several reasons (see Table 2). Propofol produces significant cardiorespiratory depression and may lead to the formation of Heinz bodies, so is also best avoided. Ketamine is often used as a component of drug protocols; however, it should not be administered alone since muscle jerks, hallucinogenic behaviour, hyperaesthesia and emergence delirium (growling, biting, scratching, lunging at the cage) have been observed. Sevoflurane has been used for feline blood donation since induction of, and recovery from, anaesthesia is rapid and predictable, but there are significant concerns regarding its use (see Table 2).
Monitoring of mucous membrane colour, temperature, pulse and respiratory rate of the donor cat should be performed throughout the sedation/anaesthetic procedure and blood collection. Pulse oximetry can be used as a non-invasive method to determine the percentage of arterial haemoglobin saturated with oxygen (SpO2). The device can be placed over the plantar digit of a pelvic limb during collection. Hypothermia is prevented by positioning the cat over a circulating warm water blanket or other warming device (with appropriate safety measures). Blood donation implies controlled losses of up to 20% (40–60 ml) of a cat’s blood volume over a short period of time. As hypotension (systolic blood pressure <80–90 mmHg, mean blood pressure <60–70 mmHg, diastolic blood pressure <40 mmHg) is commonly observed with both injectable and inhalant anaesthetic proto-cols,76,82 blood pressure monitoring is recommended due to the potential for hypovolaemia and anaesthetic complications. Depending on the donor protocol, balanced crystalloid solutions may be provided intravenously or via the subcutaneous route immediately after donation. Arterial partial pressure of oxygen can decrease during chemical restraint and oxygenation via a tight facemask is recommended. Desaturation (SpO2 <90%) indicates hypoxaemia and oxygen therapy must be administered, especially with protocols using a combination of opioid–dexmedetomidine-ketamine or alfax-alone. 83 Other measures may additionally be required including drug reversal and termination of the procedure.
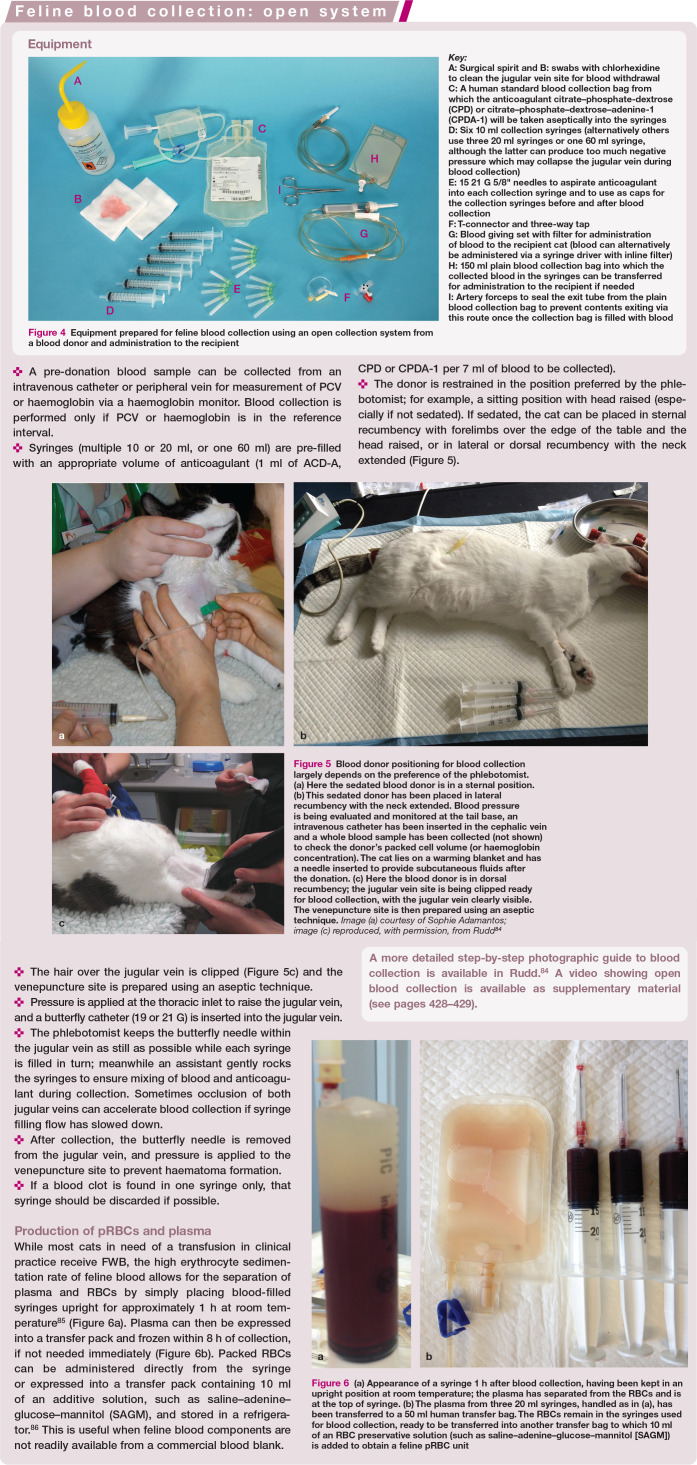
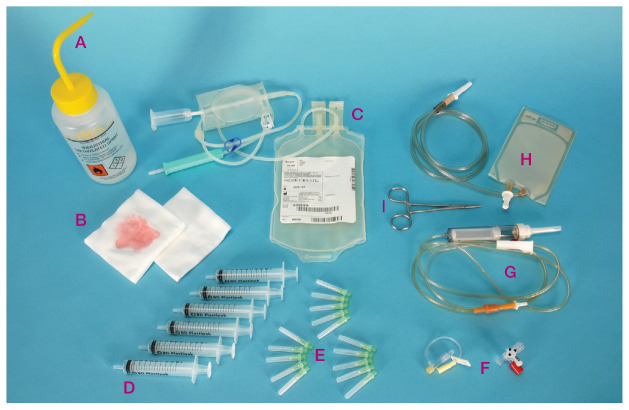
Figure 4.
Equipment prepared for feline blood collection using an open collection system from a blood donor and administration to the recipient
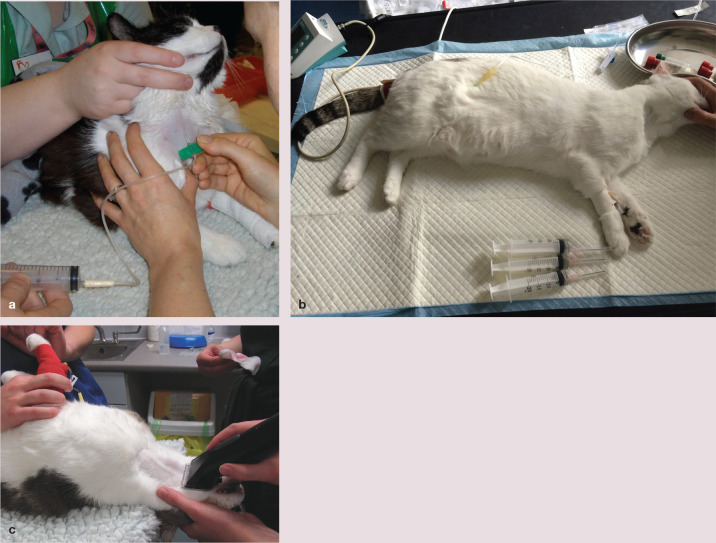
Figure 5.
Blood donor positioning for blood collection largely depends on the preference of the phlebotomist. (a) Here the sedated blood donor is in a sternal position. (b) This sedated donor has been placed in lateral recumbency with the neck extended. Blood pressure is being evaluated and monitored at the tail base, an intravenous catheter has been inserted in the cephalic vein and a whole blood sample has been collected (not shown) to check the donor’s packed cell volume (or haemoglobin concentration). The cat lies on a warming blanket and has a needle inserted to provide subcutaneous fluids after the donation. (c) Here the blood donor is in dorsal recumbency; the jugular vein site is being clipped ready for blood collection, with the jugular vein clearly visible. The venepuncture site is then prepared using an aseptic technique. Image (a) courtesy of Sophie Adamantos; image (c) reproduced, with permission, from Rudd 84
Figure 6.
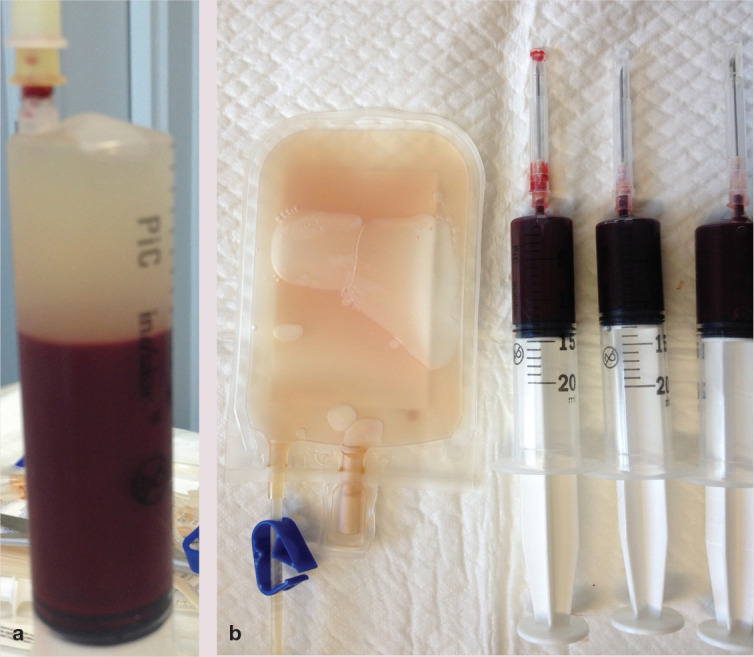
(a) Appearance of a syringe 1 h after blood collection, having been kept in an upright position at room temperature; the plasma has separated from the RBCs and is at the top of syringe. (b) The plasma from three 20 ml syringes, handled as in (a), has been transferred to a 50 ml human transfer bag. The RBCs remain in the syringes used for blood collection, ready to be transferred into another transfer bag to which 10 ml of an RBC preservative solution (such as saline–adenine–glucose–mannitol [SAGM]) is added to obtain a feline pRBC unit
Practical blood collection
The anticoagulant–preservative solutions most often used for collection of blood for transfusion purposes are ACD-A (anticoagulant citrate-dextrose solution), CPD (citrate-phosphate-dextrose) or CPDA-1 (citrate-phosphate-dextrose-adenine-1). The volume of anticoagulant used and the duration of time for which the blood product can be stored vary depending on the anticoagulant-preservative solution and the collection method. ACD-A, CPD and CPDA-1 typically are used in a ratio of 1 ml anticoagulant to 7 ml of blood. Sodium citrate (3.2% or 3.8%) alone (without RBC preservatives) may be used at a ratio of 1 ml anticoagulant to 9 ml of blood if the blood is to be administered within 24 h of collection. Heparin is not recommended as an anticoagulant for blood collected for transfusion.
Blood collection systems are described as ‘open’ or ‘closed’. A closed system is one in which the only exposure of the collection bag or its contents to air prior to administration is when the needle is uncapped to perform venepuncture during collection. An open system, as is frequently used in cats, is one in which there is one or more additional sites of potential bacterial contamination during blood collection or processing, with examples being the use of syringes or empty bags with added anticoagulant to collect blood (see box on pages 421-422). Blood products collected in an open system should ideally be administered within 4 h, or within 24 h if stored in a refrigerator (1-6°C).
Figure 7.
Closed blood collection system (TEC 724 Kit; Futurlab) licensed for use in cats and in small animals. This is a medical device for veterinary use for collection of known volumes of blood from cats (in three steps of 20 ml each, for example). It is possible to use the device with donor cats of any size by connecting it to a butterfly needle of an appropriate gauge. (a) Equipment set-up before use. (b) Section of the equipment during collection, showing the 20 ml syringe (D) containing donor blood
A commercially available feline closed collection system (see box below) has been evaluated for storage of feline blood for 35 days. The investigators found that one of eight blood units showed bacterial growth (Serratia marcescens) on day 35 but not day zero, 87 highlighting the fact that bacterial contamination of a blood unit during collection is an issue regardless of whether an open or closed collection system is used. Another closed feline blood collection system was recently evaluated that permits blood collection by suction using a vacuum chamber; this accelerated the process without being detrimental to the blood donor or collected blood, therefore optimising collection. 89 In addition, the study directly compared this closed system with an open system for evidence of bacterial contamination of the units, and did not observe any difference between the two collection systems. 89 In a separate study, blood units and blood products collected using open systems were stored successfully for 35 days without microbial growth, although all blood banking was performed by experienced staff and blood was collected with appropriate aseptic collection methods, processing and careful storage to prevent contamination, 90 which may have contributed to this result.
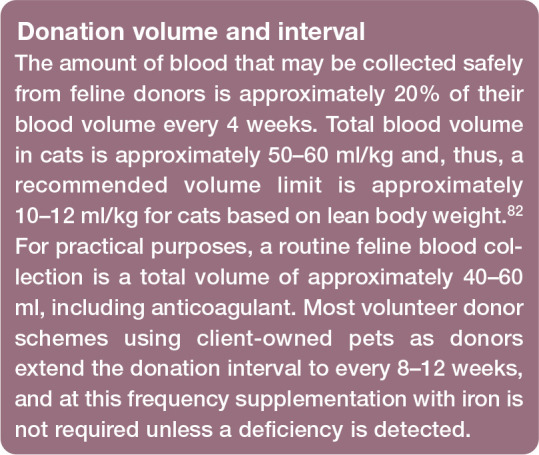
The jugular vein is the recommended venepuncture site in cats because of its size and accessibility. Strict aseptic technique minimises the risk of bacterial cont amination. In a retrospective observational study involving 115 feline blood donations (70 non-sedated and 45 sedated donors), evidence of cardiovascular or respiratory distress was noted in three non-sedated cats after donation; panting, tachypnoea and collapse were each observed in one cat, with all three donor cats determined to be normotensive within minutes of the untoward event and recovering fully. 71 Another large study of 3690 donations (81% performed under sedation) from 1792 feline donors revealed 1.14% suffered post-donation reactions, of which 0.22% were acute (weakness, pallor, tachypnoea and open-mouth breathing) and 0.92% were delayed (haematomas and skin rashes, negative behavioural reactions at home and gastrointestinal signs). All cats recovered fully. 92 Further study is required to assess the complication rate in sedated and non-sedated donors but, in most cats, sedation is preferred to reduce anxiety, and potential movement and trauma to the jugular vein, during donation. Evaluation of donor temperament should form part of the pre-donation assessment and examination.
After blood collection and during recovery from chemical restraint donors should continue to be monitored as discussed above (mucous membrane colour, temperature, pulse and respiratory rate, and systolic blood pressure if indicated) and optionally provided with subcutaneous or intravenous fluids. The cat may be discharged once vital parameters are in the normal range, it is able to ambulate normally and ideally after food is eaten.
Practical blood administration
Blood products are usually given intravenously via a peripheral vein, but occasionally via a central vein or via an intraosseous route in small cats and kittens. A dedicated intravenous line should be used.
Blood products should not be administered with intravenous fluids supplemented with calcium or glucose (eg, Hartmann’s, lactated Ringer’s). Calcium overwhelms the chelating ability of the citrate anticoagulant in stored blood and increases the risk of clot formation. Appropriate fluid choices would be 0.9% saline or Plasmalyte (Baxter International).
Blood products must be administered using an appropriate filter to reduce the numbers of red cell aggregates and microthrombi entering the recipient’s circulation. Filters in standard fluid therapy administration sets are too small and blood will clot if administered through them. Use of a syringe and microaggre-gate filter system does not appear to damage transfused RBCs. 93 Use of a standard blood administration set that includes a filter by gravity flow is possible, but control of the administration rate is more difficult. Ideally, a syringe and syringe driver with an inline microaggregate filter system (eg, Hemo-Nate 18 Lim filter; IMS) is best. In most centres, the filter is placed in the administration line as close to the patient as possible (Figure 8). Some Panel authors will filter the blood as it is aspirated from the bag into a syringe, prior to administration to the patient. Either is an appropriate method for removing microthrombi from the FWB or pRBCs.
Figure 8.
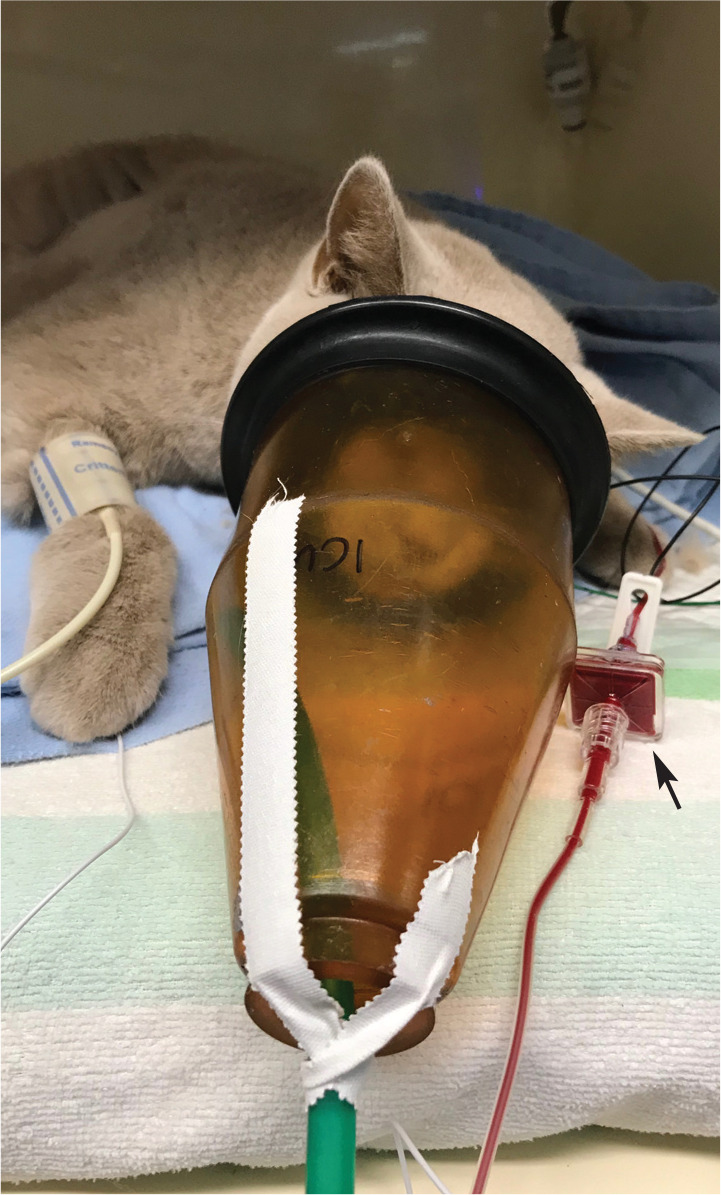
An inline microaggregate filter (arrow) (Hemo-Nate 18 |jm filter; IMS) has been placed in the administration line from the unit of blood, as close to the patient as possible. A video showing blood collected via an open system being attached to a haemofilter in preparation for transfusion to a recipient is available as supplementary material (see pages 428–429)
Infusion pumps have been theorised to damage RBCs during administration but their use allows precision around low rates of transfusion administration and they help to avoid inadvertent rapid delivery or volume overload. A recent study showed that the use of two linear peristaltic infusion pumps (NIKI V4 [Everest] and Infusomat FmS [B Braun]) did not result in more haemolysis than gravity alone. 94
Transfusion volume and rate
The volume of blood product administered to a patient can be calculated with various formulae, with the following formula performing best in one study, although it was noted that formulae frequently fail to accurately predict recipient PCV post-transfusion. 95
In practical terms, the administration volume is rounded to the nearest unit (40–60 ml of whole blood) unless the recipient is very small, in which case a half unit or 10 ml/kg may be administered.
The rate of administration of the transfusion is determined by the condition of the animal. Patients with severe clinical signs associated with anaemia (eg, weakness, tachycardia, tachypnoea, hyperdynamic or weak pulses, hypotension, dull mentation) may require blood products faster (ie, as a bolus or over 1–2 h). Alternatively, as cats with severe or chronic anaemia may have signs of left heart overload, 96 transfusions may need to be given over a longer period (eg, 4–6 h) to reduce the risk of transfusion-associated circulatory overload. However, recent work described a lack of transfusion-associated circulatory overload in anaemic cats and dogs receiving transfu-sions, 97 suggesting that such an adjustment may not be required routinely; it nonetheless remains a risk in patients with underlying cardiac disease, for example, and so close monitoring is always indicated. If blood products are kept at room temperature for more than 4 h there is a greater risk of bacterial contamination, which should also be considered when calculating administration rates.
Monitoring the recipient
Patients must be monitored very closely whenever receiving a blood transfusion, particularly within the first 30 mins of administration as this is the most common time for a severe transfusion reaction to develop. A baseline check of vital signs is performed before commencing the transfusion, including temperature, heart rate, pulse quality, blood pressure, mucous membrane colour, respiratory rate/effort, oxygen saturation and patient mentation.
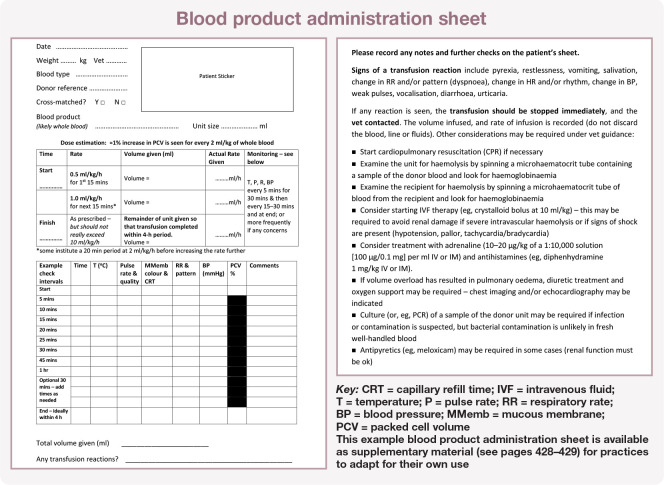
These parameters are checked very frequently (initially every 5 mins) for the first 30–60 mins (Figure 9). Depending on the clinical status of the patient, a multiparameter machine may be used to allow continuous monitoring of heart rate, electrocardiogram, SpO2, blood pressure and temperature throughout the transfusion. Assessment of vital parameter data trends (eg, gradual increase in heart rate, respiratory rate, temperature) is key to early identification of a transfusion reaction, and ensuring detailed records are kept throughout the transfusion is extremely important. An example blood product administration sheet used for monitoring cats to help allow for early detection of any problems is shown in the box below and available as supplementary material (see pages 428–429).
Figure 9.
(a) Monitoring of the recipient is performed frequently (every 5 mins) during the first 30–60 mins of a transfusion, as this is the most common time for a transfusion reaction to develop. (b) Later in the transfusion, monitoring frequency can be reduced based on the cat’s response and vital parameter data trends
Transfusion reactions
Despite appropriate screening, transfusion reactions in cats remain unpredictable and can vary in severity. As described in Table 4, they can be defined as acute, acute-to-delayed or delayed. The most common transfusion reactions seen in cats include febrile non-haemolytic transfusion reactions, allergic reactions and transfusion associated circulatory overload. Transfusion reactions may cause immune-mediated haemolysis, which can result in jaundice, pigmenturia and/or pyrexia, mainly due to anti-blood type reactions. Transfusion reactions can also cause non-haemolytic sequelae, such as transient increases in body temperature, facial pruritis, facial swelling (Figure 10), vomiting and salivation. Increased vocalisation or agitation can often be a preceding sign to a non-haemolytic reaction.
Table 4.
Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Consensus Working Group definitions of transfusion reactions
| Transfusion reaction | Definition |
|---|---|
| Acute transfusion reactions | |
| Acute haemolytic transfusion reaction (AHTR) | Acute, non-infectious, immunological or non-immunological reaction that occurs secondarily to accelerated destruction of transfused or recipient red blood cells (RBCs) and is characterised by acute haemolysis. AHTRs occur during or within 24 h of blood product administration. Causes of AHTRs can be divided into blood type incompatibilities and other causes of damage to transfused blood cells. Blood type incompatibilities are immunological acute haemolytic reactions (type II hypersensitivity reactions) due to major or minor incompatibilities between donor and recipient RBCs. A classic example would be in the case of a type A unit of blood given to a type B cat. Non-immunological causes of AHTRs may include thermal, osmotic, mechanical or chemical factors that damage transfused blood cells |
| Allergic reaction | Acute immunological reaction that is secondary to a type I hypersensitivity response to an antigen within a blood product. This reaction occurs during or within 4 h of transfusion. It is characterised by clinical signs varying from transient and self-limiting to life-threatening anaphylaxis. Feline type I hypersensitivity reactions are typically respiratory (due to upper respiratory tract oedema, bronchoconstriction and excessive mucus production), although gastrointestinal signs and severe pruritus can also occur |
| Febrile non-haemolytic transfusion
reaction (FNHTR) |
Acute non-immunological or immunological reaction characterised by a temperature >39°C (102.5°F) and an increase in temperature of >1°C (1.8°F) from the pre-transfusion body temperature, during or within 4 h of a transfusion, where external warming, underlying patient infection, AHTR, TRALI (see below) and TTI (see below) have been ruled out |
| Transfusion associated circulatory overload (TACO) | Acute, non-immunological reaction that is secondary to an increase in blood volume mediated by blood transfusion, characterised by acute respiratory distress and hydrostatic pulmonary oedema. This reaction occurs during or within 6 h of transfusion. It is associated with clinical, echocardiographic, radiographic or laboratory evidence of left atrial hypertension or volume overload. These patients typically have a positive response to diuretic therapy |
| Transfusion associated lung injury (TRALI) | Acute, immunological reaction that is secondary to antigen–antibody interactions in the lungs. TRALI is characterised by acute hypoxaemia with evidence of non-cardiogenic pulmonary oedema on thoracic radiographs, during or within 6 h of allogenic blood transfusion. Patients diagnosed with TRALI have no prior lung injury, no evidence of left atrial hypertension and no temporal relationship to an alternative risk factor for acute respiratory distress syndrome (ARDS) |
| Transfusion associated dyspnoea (TAD) | Acute transfusion reaction characterised by the development of acute respiratory distress during or within 24 h of the end of a transfusion where TACO, TRALI, allergic reaction and underlying pulmonary disease have been ruled out |
| Hypotensive transfusion reaction | Acute, non-immunological reaction that is secondary to the infusion of stimulators of vasodilation and hypotension. It is characterised by the rapid onset of significant hypotension during or shortly after the completion of a transfusion, in the absence of other causes of hypotension, and improvement with cessation of the infusion. There is usually a decrease in systolic blood pressure of at least 30 mmHg from baseline |
| Citrate toxicity | Acute, non-immunological reaction that is secondary to the transfusion of a large volume of blood with citrate as the anticoagulant, and is characterised by a significant systemic hypocalcaemia within hours of initiating the transfusion |
| Hyperammonaemia | Acute, non-immunological reaction that is secondary to hyperammonaemia and characterised by signs of development of encephalopathy (neurological signs such as ataxia, head pressing, circling, seizures and vomiting), during or immediately after (minutes to a few hours) blood transfusion of stored blood or stored blood components. It is a potentially life-threatening reaction in patients with liver disease (liver failure, portosystemic shunt) or in premature neonates with an immature functioning liver, which are unable to metabolise and excrete ammonia properly |
| Acute-to-delayed transfusion reactions | |
| Transfusion transmitted infection (TTI) | Acute or delayed, non-immunological reaction secondary to the transfusion of pathogen-contaminated blood or blood components. TTI can occur hours to years after the transfusion due to the presence of the infectious agent in the blood/blood component unit collected from an infected donor, or from pathogen contamination of blood/blood component units during processing, storage or transfusion. Clinical signs are highly dependent on the pathogen transmitted and its pathogenicity for cats, and the clinical status of the recipient |
| Transfusion-associated graft vs host disease (TAGVHD) | Acute to delayed, immunological reaction that is secondary to donor lymphocytes engrafting on and eventually attacking host tissue. TAGVHD occurs 48 h to 6 weeks following transfusion and has a high mortality rate in human patients (>90%). The reaction is characterised by a skin rash, diarrhoea, fever, hepatic dysfunction and bone marrow hypoplasia. Liver and skin histopathology have a characteristic appearance. In humans, it is most common in immunocompromised individuals or when special circumstances cause transient immunosuppression |
| Delayed transfusion reactions | |
| Delayed haemolytic transfusion reaction (DHTR) | Delayed, non-infectious, immunological or non-immunological reaction that occurs secondarily to lysis or accelerated clearance of transfused RBCs. Delayed haemolytic transfusion reactions occur 24 h to 28 days after blood product administration. Immunological DHTRs are typically caused by a secondary immune response to the donor’s RBCs. Non-immunological DHTRs occur due to thermal, osmotic, mechanical or chemical factors that damage transfused blood cells, causing delayed haemolysis |
| Delayed serological transfusion reaction (DSTR) | Delayed, immunological reaction that is secondary to the development of new, clinically significant antibodies against the transfused product without evidence of haemolysis. DSTRs occur 24 h to 28 days after a transfusion 24 |
| Post-transfusion purpura (PTP) | Delayed, immunological reaction that is secondary to alloimmunisation against platelet antigens. PTP is characterised by thrombocytopenia arising 5–12 days following transfusion of any platelet-containing blood product |
Reproduced with permission from the AVHTM 98
Figure 10.

This cat demonstrates facial swelling, which can occur as a transfusion reaction. However, more common reactions include vocalisation, an increase in body temperature, vomiting and salivation
In a study of 126 cats receiving blood transfusions, non-haemolytic reactions (7.9%) were more common than haemolytic reactions (0.8%). 99 In another study of 91 cats, a transfusion reaction was noted in only 1.2% of the cats. 57
The risk of a transfusion reaction increases with subsequent transfusions (typically from 2 days after an initial transfusion). 24 However, in 27 cats that received multiple blood transfusions, transfusion reactions remained uncommon. 100 Appropriate record-keeping is nonetheless essential so that all treating veterinarians are aware that the patient has received a blood transfusion.
Should the patient develop mild signs of a transfusion reaction (eg, mild, 1-2°C increase in temperature or one episode of vomiting) then the transfusion rate should be reduced. If marked clinical signs develop the transfusion should be stopped and blood replaced with a crystalloid solution (see box below). Monitoring of the patient (temperature, pulse rate, mucous membrane colour and systolic blood pressure) should be continued for evidence of shock. Serum and urine should be assessed for haemolysis and haemoglobinuria with sample centrifugation to look for evidence of haemolysis of the transfused red cells (eg, red discolouration of serum or urine).

Cats may develop signs of volume overload following a transfusion. This is of particular concern in patients with cardiac disease, normo-volaemic to hypervolaemic anaemic patients (eg, immune-mediated haemolytic anaemia), and chronically or severely anaemic patients, although, as mentioned above, transfusion-associated volume overload in chronic anaemia may be less of a clinical concern. 95 If patients become tachypnoeic following a transfusion, or develop a serous nasal discharge or conjunctival oedema, thoracic radiography or thoracic ultrasound should be performed to evaluate for pleu-ral effusion, pulmonary oedema and to measure a left atrial:aorta ratio. If pleural effusion is present, thoracocentesis is indicated. If pulmonary oedema is present, furosemide 1-2 mg/kg IV is given every 2 h as required (based on respiratory auscultation, respiratory rate and response), and oxygen therapy should also be instigated.
Summary Points
Feline blood donation and transfusion can be performed safely and effectively in veterinary practice, but the decision to undertake these procedures must be made carefully.
Donor and recipient cats should be blood typed, and ideally cross-matched if possible, prior to transfusion to avoid severe transfusion reactions.
The decision to administer a transfusion is based on the potential recipient’s clinical condition and cause of anaemia, rather than PCV alone.
Donors should be assessed for health, temperament and infectious agents and, in most cases, sedated appropriately for blood collection.
Blood can be collected using an open or closed system and recipients should be monitored for signs of a transfusion reaction.
If compatible feline RBCs are not available, xenotransfusion may be given only once, in certain situations, allowing for short-term stabilisation of the recipient, but destruction of donated RBCs occurs after a short time.
Supplemental Material
Supplemental material, sj-pdf-1-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery
Supplemental material, sj-docx-2-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-3-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery
Acknowledgments
ISFM are grateful to Andrea Harvey for highlighting some of the ethical and practical issues that drove the development of these Guidelines, and also to Karen Hiestand for input into the drafting of the ‘Ethical considerations’ Appendix.
Footnotes
Supplementary material: The following files are available online:
Video showing blood collection from a donor cat under sedation using an open collection system.
Video showing blood collected via an open system being attached to a haemofilter in preparation for transfusion to a recipient.
Séverine Tasker has received financial support for infectious disease research from BSAVA PetSavers, Journal of Comparative Pathology Educational Trust, Langford Trust, Langford Vets Clinical Research Fund, Morris Animal Foundation, NERC/BBSRC/MRC, PetPlan Charitable Trust, South West Biosciences DTP, Wellcome Trust and Zoetis Animal Health. Paulo Steagall has provided consultancy services to Boehringer Ingelheim, Dechra Pharmaceuticals, Elanco, Procyon and Zoetis; has acted as a key opinion leader to Boehringer Ingelheim, Dechra Pharmaceuticals, Elanco, Vetoquinol and Zoetis; and has received speaker honoraria from Boehringer Ingelheim, Dechra Pharmaceuticals, Elanco and Zoetis. The other members of the Panel have no conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and, therefore, ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and, therefore, informed consent was not required. For any animals or people individually identifiable within this publication, informed consent (verbal or written) for their use in the publication was obtained from the people involved.
Contributor Information
Samantha Taylor, International Society of Feline Medicine, Tisbury, UK.
Eva Spada, Veterinary Transfusion Research Laboratory (REVLab), Department of Veterinary Medicine (DIMEVET), University of Milan, Italy.
Mary Beth Callan, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, USA.
Rachel Korman, Cat Specialist Services, Underwood, Queensland, Australia.
Ellie Leister, Pet Intensive Care Unit, Underwood, Queensland, Australia.
Paulo Steagall, Department of Clinical Sciences, Faculty of Veterinary Medicine, Universite de Montréal, QC, Canada.
Remo Lobetti, Bryanston Veterinary Hospital, Johannesburg, South Africa.
Mayank Seth, Dick White Referrals, Six Mile Bottom, UK.
Séverine Tasker, Bristol Veterinary School, University of Bristol, Langford, UK; and Linnaeus Group, Shirley, UK.
References
- 1. Barfield D, Adamantos S. Feline blood transfusions: a pinker shade of pale. J Feline Med Surg 2011; 13: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davidow B. Transfusion medicine in small animals. Vet Clin North Am Small Anim Pract 2013; 43: 735–756. [DOI] [PubMed] [Google Scholar]
- 3. Knottenbelt CM, Addie DD, Day MJ, et al. Determination of the prevalence of feline blood types in the UK. J Small Anim Pract 1999; 40: 115–118. [DOI] [PubMed] [Google Scholar]
- 4. Forcada Y, Guitian J, Gibson G. Frequencies of feline blood types at a referral hospital in the south east of England. J Small Anim Pract 2007; 48: 570–573. [DOI] [PubMed] [Google Scholar]
- 5. Gunn-Moore DA, Simpson KE, Day MJ. Blood types in Bengal cats in the UK. J Feline Med Surg 2009; 11: 826–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen AL, Olesen AB, Arnbjerg J. Distribution of feline blood types detected in the Copenhagen area of Denmark. Acta Vet Scand 1994; 35: 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malik R, Griffin DL, White JD, et al. The prevalence of feline A/B blood types in the Sydney region. Aust Vet J 2005; 83: 38–44. [DOI] [PubMed] [Google Scholar]
- 8. Cattin RP. Distribution of blood types in a sample of 245 New Zealand non-purebred cats. N Z Vet J 2016; 64: 154–157. [DOI] [PubMed] [Google Scholar]
- 9. Nectoux A, Guidetti M, Barthelemy A, et al. Assessment of risks of feline mismatched transfusion and neonatal isoerythrolysis in the Lyon (France) area. JFMS Open Rep 2019; 5. DOI: 10.1177/2055116919863175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Tommaso M, Miglio A, Crisi PE, et al. Frequency of blood types A, B and AB in a population of non-pedigree domestic cats from Central Italy. Animals (Basel) 2020; 10: 1937. DOI: 10.3390/ani10101937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spada E, Perego R, Baggiani L, et al. Prevalence of blood types and alloantibodies of the AB blood group system in non-pedigree cats from Northern (Lombardy) and Southern (Sicily) Italy. Animals (Basel) 2020; 10: 1129. DOI: 10.3390/ani10071129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Proverbio D, Spada E, Perego R, et al. Assessment of blood types of Ragdoll cats for transfusion purposes. Vet Clin Pathol 2013; 42: 157–162. [DOI] [PubMed] [Google Scholar]
- 13. Knottenbelt CM, Day MJ, Cripps PJ, et al. Measurement of titres of naturally occurring alloantibodies against feline blood group antigens in the UK. J Small Anim Pract 1999; 40: 365–370. [DOI] [PubMed] [Google Scholar]
- 14. Bucheler J, Giger U. Alloantibodies against A and B blood types in cats. Vet Immunol Immunopathol 1993; 38: 283–295. [DOI] [PubMed] [Google Scholar]
- 15. Axner E. A questionnaire on survival of kittens depending on the blood groups of the parents. J Feline Med Surg 2014; 16: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cain GR, Suzuki Y. Presumptive neonatal isoerythrolysis in cats. J Am Vet Med Assoc 1985; 187: 46–48. [PubMed] [Google Scholar]
- 17. Rudd S. Feline blood types and blood typing methods. In: Harvey AM, Tasker S. (eds). BSAVA manual of feline practice: a foundation manual. Gloucester: British Small Animal Veterinary Association, 2013, pp 454–456. [Google Scholar]
- 18. Seth M, Jackson KV, Giger U. Comparison of five blood-typing methods for the feline AB blood group system. Am J Vet Res 2011; 72: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hourani L, Weingart C, Kohn B. Evaluation of a novel feline AB blood typing device. J Feline Med Surg 2014; 16: 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spada E, Proverbio D, Baggiani L, et al. Evaluation of an immunochromatographic test for feline AB system blood typing. J Vet Emerg Crit Care (San Antonio) 2016; 26: 137–141. [DOI] [PubMed] [Google Scholar]
- 21. Goy-Thollot I, Nectoux A, Guidetti M, et al. Detection of naturally occurring alloantibody by an in-clinic antiglobulin-enhanced and standard crossmatch gel column test in non-transfused domestic shorthair cats. J Vet Intern Med 2019; 33: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinstein NM, Blais MC, Harris K, et al. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med 2007; 21: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasker S, Barker EN, Day MJ, et al. Feline blood genotyping versus phenotyping, and detection of non-AB blood type incompatibilities in UK cats. J Small Anim Pract 2014; 55: 185–189. [DOI] [PubMed] [Google Scholar]
- 24. Hourani L, Weingart C, Kohn B. Alloimmunisation in transfused patients: serial cross-matching in a population of hospitalised cats. J Feline Med Surg 2017; 19: 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McClosky ME, Cimino Brown D, Weinstein NM, et al. Prevalence of naturally occurring non-AB blood type incompatibilities in cats and influence of crossmatch on transfusion outcomes. J Vet Intern Med 2018; 32: 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sylvane B, Prittie J, Hohenhaus AE, et al. Effect of cross-match on volume after transfusion of packed red blood cells in transfusion-naive anemic cats. J Vet Intern Med 2018; 32: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Binvel M, Arsenault J, Depre B, et al. Identification of 5 novel feline erythrocyte antigens based on the presence of naturally occurring alloantibodies. J Vet Intern Med 2021; 35: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Humm KR, Chan DL. Prospective evaluation of the utility of cross-matching prior to first transfusion in cats: 101 cases. J Small Anim Pract 2020; 61: 285–291. [DOI] [PubMed] [Google Scholar]
- 29. Martinez-Sogues L, Blois SL, Manzanilla EG, et al. Exploration of risk factors for non-survival and for transfusion-associated complications in cats receiving red cell transfusions: 450 cases (2009 to 2017). J Small Anim Pract 2020; 61: 177–184. [DOI] [PubMed] [Google Scholar]
- 30. Safrany B, Adamantos S. Is a cross-match necessary before a cat’s first blood transfusion? Vet Evidence 2020; 5. DOI: 10.18849/VE.V5I2.306. [DOI] [Google Scholar]
- 31. Poh D, Claus M, Smart L, et al. Transfusion practice in Australia: an internet-based survey. Aust Vet J 2021; DOI: 10.1111/avj.13049. [DOI] [PubMed] [Google Scholar]
- 32. Vap LM, Harr KE, Arnold JE, et al. ASVCP quality assurance guidelines: control of preanalytical and analytical factors for hematology for mammalian and nonmammalian species, hemostasis, and crossmatching in veterinary laboratories. Vet Clin Pathol 2012; 41: 8–17. [DOI] [PubMed] [Google Scholar]
- 33. Pennisi MG, et al. ; European Advisory Board on Cat Diseases. Blood transfusion in cats. ABCD guidelines. http://www.abcd-catsvets.org/blood-transfusion-in-cats/ (updated March 2020, accessed February 13, 2021).
- 34. Wardrop KJ, Birkenheuer A, Blais MC, et al. Update on canine and feline blood donor screening for blood-borne pathogens. J Vet Intern Med 2016; 30: 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gary AT, Richmond HL, Tasker S, et al. Survival of Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in blood of cats used for transfusions. J Feline Med Surg 2006; 8: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barker EN. Update on feline hemoplasmosis. Vet Clin North Am Small Anim Pract 2019; 49: 733–743. [DOI] [PubMed] [Google Scholar]
- 37. Alvarez-Fernandez A, Breitschwerdt EB, Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 2018; 11: 624. DOI: 10.1186/s13071-018-3152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bradbury CA, Green M, Brewer M, et al. Survival of Bartonella henselae in the blood of cats used for transfusion [abstract]. J Vet Intern Med 2010; 24: 759. [Google Scholar]
- 39. Kordick DL, Breitschwerdt EB. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res 1997; 58: 492–497. [PubMed] [Google Scholar]
- 40. Hartmann K. Clinical aspects of feline retroviruses: a review. Viruses 2012; 4: 2684–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nesina S, Helfer-Hungerbuehler AK, Riond B, et al. Retroviral DNA - the silent winner: blood transfusion containing latent feline leukemia provirus causes infection and disease in naïve recipient cats. Retrovirology 2015; 12: 105. DOI: 10.1186/s12977-015-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Little S, Levy J, Hartmann K, et al. 2020 AAFP feline retrovirus testing and management guidelines. J Feline Med Surg 2020; 22: 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lappin MR, Tasker S, Roura X. Role of vector-borne pathogens in the development of fever in cats: 2. Tick- and sandfly-associated diseases. J Feline Med Surg 2020; 22: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pennisi MG, Hofmann-Lehmann R, Radford AD, et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg 2017; 19: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lappin MR, Griffin B, Brunt J, et al. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2006; 8: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nentwig A, Meli ML, Schrack J, et al. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit Vectors 2018; 11: 292. DOI: 10.1186/s13071-018-2728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hartmann K, et al. ; European Advisory Board on Cat Diseases. Babesiosis. ABCD guidelines. http://www.abcdcatsvets.org/babesiosis/ (updated August 2020, accessed February 13, 2021).
- 48. Pennisi MG, et al. ; European Advisory Board on Cat Diseases. Cytauxzoonosis. http://www.abcdcatsvets.org/cytauxzoono-sis/ (updated January 2021, accessed February 13, 2021).
- 49. Meinkoth JH, Kocan AA. Feline cytauxzoonosis. Vet Clin North Am Small Anim Pract 2005; 35: 89–101, vi. [DOI] [PubMed] [Google Scholar]
- 50. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardio-myopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 51. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N-terminal probrain natriuretic peptide (NT-pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011; 25: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 52. Winzelberg Olson S, Hohenhaus AE. Feline non-regenerative anemia: diagnostic and treatment recommendations. J Feline Med Surg 2019; 21: 615–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balakrishnan A, Drobatz KJ, Reineke EL. Development of anemia, phlebotomy practices, and blood transfusion requirements in 45 critically ill cats (2009-2011). J Vet Emerg Crit Care 2016; 26: 406–411. [DOI] [PubMed] [Google Scholar]
- 54. Lane WG, Sinnott-Stutzman VB. Retrospective evaluation of fresh frozen plasma use in 121 cats: 2009-2016. J Vet Emerg Crit Care 2020; 30: 558–566. [DOI] [PubMed] [Google Scholar]
- 55. Mansi ET, Waldrop JE, Davidow EB. Retrospective evaluation of the indications, safety and effects of fresh frozen plasma transfusions in 36 cats (2014-2018). J Feline Med Surg 2020; 22: 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kohn B, Weingart C, Giger U. Haemorrhage in seven cats with suspected anticoagulant rodenticide intoxication. J Feline Med Surg 2003; 5: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg 2004; 6: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bebar KN, Sinnott V, Brooks MB. Recurrent hemorrhage caused by type 3 von Willebrand disease in a domestic longhaired cat. J Vet Emerg Crit Care 2014; 24: 326–331. [DOI] [PubMed] [Google Scholar]
- 59. French TW, Fox LE, Randolph JF, et al. A bleeding disorder (von Willebrand’s disease) in a Himalayan cat. J Am Vet Med Assoc 1987; 190: 437–439. [PubMed] [Google Scholar]
- 60. Maggio-Price L, Dodds WJ. Factor IX deficiency (hemophilia B) in a family of British shorthair cats. J Am Vet Med Assoc 1993; 203: 1702–1704. [PubMed] [Google Scholar]
- 61. Cotter SM, Brenner RM, Dodds WJ. Hemophilia A in three unrelated cats. J Am Vet Med Assoc 1978; 172: 166–168. [PubMed] [Google Scholar]
- 62. Snow SJ, Ari Jutkowitz L, Brown AJ. Trends in plasma transfusion at a veterinary teaching hospital: 308 patients (1996-1998 and 2006-2008). J Vet Emerg Crit Care 2010; 20: 441–445. [DOI] [PubMed] [Google Scholar]
- 63. Wondratschek C, Weingart C, Kohn B. Primary immune-mediated thrombocytopenia in cats. J Am Anim Hosp Assoc 2010; 46: 12–19. [DOI] [PubMed] [Google Scholar]
- 64. Callan MB, Griot-Wenk ME, Hackner SG, et al. Persistent throm-bopathy causing bleeding in 2 domestic shorthaired cats. J Vet Intern Med 2000; 14: 217–220. [PubMed] [Google Scholar]
- 65. Bovens C, Gruffydd-Jones T. Xenotransfusion with canine blood in the feline species: review of the literature. J Feline Med Surg 2013; 15: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oron L, Bruchim Y, Klainbart S, et al. Ultrasound-guided intra-cardiac xenotransfusion of canine packed red blood cells and epinephrine to the left ventricle of a severely anemic cat during cardiopulmonary resuscitation. J Vet Emerg Crit Care 2017; 27: 218–223. [DOI] [PubMed] [Google Scholar]
- 67. Euler CC, Raj K, Mizukami K, et al. Xenotransfusion of anemic cats with blood compatibility issues: pre- and posttransfusion laboratory diagnostic and crossmatching studies. Vet Clin Pathol 2016; 45: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Le Gal A, Thomas EK, Humm KR. Xenotransfusion of canine blood to cats: a review of 49 cases and their outcome. J Small Anim Pract 2020; 61: 156–162. DOI: 10.1111/jsap.13096. [DOI] [PubMed] [Google Scholar]
- 69. Marion RS, Smith JE. Survival of erythrocytes after autolo-gous and allogeneic transfusion in cats. J Am Vet Med Assoc 1983; 183: 1437–1439. [PubMed] [Google Scholar]
- 70. Cole LP, Humm K. Twelve autologous blood transfusions in eight cats with haemoperitoneum. J Feline Med Surg 2019; 21: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Doolin KS, Chan DL, Adamantos S, et al. Retrospective evaluation of unexpected events during collection of blood donations performed with and without sedation in cats (2010-2013). J Vet Emerg Crit Care 2017; 27: 555–560. [DOI] [PubMed] [Google Scholar]
- 72. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyper-glycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 73. Reader RC, Barton BA, Abelson AL. Comparison of two intramuscular sedation protocols on sedation, recovery and ease of venipuncture for cats undergoing blood donation. J Feline Med Surg 2019; 21: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reynolds BS, Geffre A, Bourges-Abella NH, et al. Effects of intravenous, low-dose ketamine-diazepam sedation on the results of hematologic, plasma biochemical, and coagulation analyses in cats. J Am Vet Med Assoc 2012; 240: 287–293. [DOI] [PubMed] [Google Scholar]
- 75. Troyer HL, Feeman WE, Gray TL, et al. Comparing chemical restraint and anesthetic protocols used for blood donation in cats: one teaching hospital’s experience. Vet Med 2005; 100: 652–658. [Google Scholar]
- 76. Killos MB, Graham LF, Lee J. Comparison of two anesthetic protocols for feline blood donation. Vet Anaesth Analg 2010; 37: 230–239. [DOI] [PubMed] [Google Scholar]
- 77. Dhumeaux MP, Snead EC, Epp TY, et al. Effects of a standardized anesthetic protocol on hematologic variables in healthy cats. J Feline Med Surg 2012; 14: 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Biermann K, Hungerbuhler S, Mischke R, et al. Sedative, cardiovascular, haematologic and biochemical effects of four different drug combinations administered intramuscularly in cats. Vet Anaesth Analg 2012; 39: 137–150. [DOI] [PubMed] [Google Scholar]
- 79. Granfone MC, Walker JM, Smith LJ. Evaluation of an intramuscular butorphanol and alfaxalone protocol for feline blood donation: a pilot study. J Feline Med Surg 2018; 20: 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Muir W, Lerche P, Wiese A, et al. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Vet Anaesth Analg 2009; 36: 42–54. [DOI] [PubMed] [Google Scholar]
- 81. Spada E, Proverbio D, Bagnagatti De, Giorgi G, et al. Clinical and haematological responses of feline blood donors anaesthetised with a tiletamine and zolazepam combination. J Feline Med Surg 2015; 17: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Iazbik MC, Gomez Ochoa P, Westendorf N, et al. Effects of blood collection for transfusion on arterial blood pressure, heart rate, and PCV in cats. J Vet Intern Med 2007; 21: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 83. Cremer J, Ricco CH. Cardiovascular, respiratory and sedative effects of intramuscular alfaxalone, butorphanol and dexmedetomidine compared with ketamine, butorphanol and dexmedetomidine in healthy cats. J Feline Med Surg 2018; 20: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rudd S. Blood transfusion. In: Harvey AM, Tasker S. (eds). BSAVA manual of feline practice: a foundation manual. Gloucester: British Small Animal Veterinary Association, 2013, pp 456–460. [Google Scholar]
- 85. Mansell CL, Boller M. Blood component processing and storage. I. In: Yagi K, Holowaychuk MK. (eds). Manual of veterinary transfusion medicine and blood banking. Ames, IA: Wiley Blackwell, 2016, pp 237–255. [Google Scholar]
- 86. Spada E, Perego R, Baggiani L, et al. Evaluation of feline packed red blood cell units obtained by blood sedimentation and stored for 42 days for transfusion purposes [abstract]. J Vet Intern Med 2020; 34: 418. [Google Scholar]
- 87. Crestani C, Stefani A, Carminato A, et al. In vitro assessment of quality of citrate-phosphate-dextrose-adenine-1 preserved feline blood collected by a commercial closed system. J Vet Intern Med 2018; 32: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. FuturLab. The first closed system for blood collection and storage for cats. www.youtube.com/watch?v=fPFg8oGSwRk (accessed February 6, 2021).
- 89. Binvel M, Fairbrother JH, Levesque V, et al. Comparison of a closed system and an open system for blood collection in feline donors. J Feline Med Surg 2020; 22: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Spada E, Perego R, Baggiani L, et al. Hematological, biochemical and microbiological evaluation of feline whole blood units collected using an open system and stored for 35 days. Vet J 2019; 254: 105396. [DOI] [PubMed] [Google Scholar]
- 91. Spada E, Proverbio D, Baggiani L, et al. Change in haematologi-cal and selected biochemical parameters measured in feline blood donors and feline whole blood donated units. J Feline Med Surg 2017; 19: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Abreu TAM, Oliveira AST, Ferreira RRF, et al. Feline blood donation adverse reactions: classification and description of acute and delayed reactions in a donor population. J Feline Med Surg. In press 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Heikes BW, Ruaux CG. Effect of syringe and aggregate filter administration on survival of transfused autologous fresh feline red blood cells. J Vet Emerg Crit Care 2014; 24: 162–167. [DOI] [PubMed] [Google Scholar]
- 94. Blasi-Brugé C, Sanchez IM, Ferreira RRF, et al. Quantitative assessment of infusion pump-mediated haemolysis in feline packed red blood cell transfusions. J Feline Med Surg. Epub ahead of print 15 March 2021. DOI: 10.1177/1098612X21999990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reed N, Espadas I, Lalor SM, et al. Assessment of five formulae to predict post-transfusion volume in cats. J Feline Med Surg 2014; 16: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wilson HE, Jasani S, Wagner TB, et al. Signs of left heart volume overload in severely anaemic cats. J Feline Med Surg 2010; 12: 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Donaldson RE, Seo J, Fuentes VL, et al. Left heart dimensions in anemic cats and dogs before and after blood transfusion. J Vet Intern Med 2021; 35: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Davidow B, Blois S, Goy-Thollot I, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part one: definitions and clinical signs. J Vet Emerg Crit Care. In press 2021. DOI: 10.1111/vec.13044.2021. [DOI] [PubMed] [Google Scholar]
- 99. Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusions in cats: 126 cases (1999). J Am Vet Med Assoc 2005; 226: 920–923. [DOI] [PubMed] [Google Scholar]
- 100. Roux FA, Deschamps JY, Blais MC, et al. Multiple red cell transfusions in 27 cats (2003-2006): indications, complications and outcomes. J Feline Med Surg 2008; 10: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery
Supplemental material, sj-docx-2-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-3-jfm-10.1177_1098612X211007071 for 2021 ISFM Consensus Guidelines on the Collection and Administration of Blood and Blood Products in Cats by Samantha Taylor, Eva Spada, Mary Beth Callan, Rachel Korman, Ellie Leister, Paulo Steagall, Remo Lobetti, Mayank Seth and Séverine Tasker in Journal of Feline Medicine and Surgery