Abstract
Objectives
Glucose monitoring is an integral part of diabetes management. Interstitial glucose monitoring systems are increasingly commonly being used for this purpose in dogs and cats, including the use of a flash glucose monitoring system (FGMS). The aim of this study was to describe the incidence and nature of complications associated with the use of an FGMS in diabetic cats.
Methods
The medical records of all cats that had placement of a 14-day FGMS during a 1-year period were retrospectively reviewed. Data retrieved included the number of days the sensor remained attached and functional, location of sensor placement and complications associated with the sensor. Complications were defined as early sensor detachment, sensor failure prior to the end of the 14-day monitoring period and dermatologic changes at the sensor site. Descriptive statistics were used to characterize the data.
Results
Twenty cats had a total of 33 FGMSs placed. The majority (30/33 [91%]) of sensors were placed over the dorsolateral aspect of the thorax just caudal to the scapula. Twenty (61%) FGMSs remained attached and functional for the full 14 days. The overall incidence of complications associated with FGMS use was 10/33 (30%). The most frequent complication was early sensor detachment (n = 5/33 [15%]). Mild dermatologic changes (erythema, crusts) were noted with 4/33 (12%) FGMSs. More serious complications (skin erosions, abscess formation) were noted with 2/33 (6%) FGMSs.
Conclusions and relevance
The use of the FGMS is relatively safe in cats, although there are potential complications that owners should be made aware of.
Keywords: Continuous, glucose, monitoring, complications
Introduction
Most diabetic cats require intermittent blood glucose monitoring so that their insulin dose can be adjusted in order to establish glycemic control. The goals of therapy in the diabetic cat are to control clinical signs, to avoid hypoglycemia and, ideally, to achieve diabetic remission. Though at times challenging to establish, good glycemic control helps achieve these goals.
Effective glucose monitoring enables the clinician to safely and adequately adjust the patient’s insulin dose. Traditional methods for glucose monitoring include in-hospital blood glucose curves, spot blood glucose measurements, at-home blood glucose curves, urine glucose measurement and the measurement of fructosamine concentrations. In-hospital curves lack convenience, are expensive and are difficult to interpret/poorly repeatable. 1 Spot blood glucose measurements may not provide enough information to permit safe dosing changes. Many cat owners are reluctant to draw blood to perform at-home curves. Urine glucose can be monitored using dipsticks; 2 however, dipstick results are poorly indicative of glycemic status, except as an indication of whether the blood glucose was above or below the renal threshold while the urinary bladder was filling. Fructosamine measurement is easy and provides insight into the mean glucose concentration over the preceding 1–2 weeks.3,4 However, for cats with glycemic variability, fructosamine values can be increased, decreased or normal depending on relative time spent in hypoglycemic vs hyperglycemic states. 5 Additionally, the fructosamine concentration should be interpreted cautiously in patients that are hypoproteinemic or that have comorbidities such as hyperthyroidism.6,7
Continuous glucose monitoring systems (CGMSs) utilize a small filament inserted underneath the skin. This filament contains glucose oxidase enzyme that is immobilized on an electrochemical sensor. 8 The CGMS measures the interstitial glucose (IG) at closely spaced intervals to provide nearly continuous information on glucose concentrations, and can be set to emit an alarm during periods of hypoglycemia. These devices are calibrated based on peripheral blood glucose measurements performed at least twice daily. CGMSs are commonly used in human diabetic patients, and their potential use in veterinary medicine has previously been described.9–15 A flash glucose monitoring system (FGMS; FreeStyle Libre [Abbott]) has been developed for humans. This is a factory calibrated device with the capability of providing IG readings over a 14-day period. The FGMS requires no blood sampling. Instead, it can be ‘flashed’ with a reader to display the IG concentration. The FGMS must be ‘flashed’ with the reader at least every 8 h so that its continuous data can be downloaded and stored. This FGMS was studied in stable diabetic dogs with good correlation between IG and peripheral blood glucose concentrations. Accuracy was 93%, 99% and 99% at low, normal and high blood glucose concentrations, respectively, and the sensor was easy to place and well tolerated. 16 Another study demonstrated good accuracy of the FGMS in dogs with diabetic ketoacidosis. 17 There are currently no published studies reporting accuracy of the same FGMS in cats, though this device is now more readily available in both general and specialty practices for glucose monitoring in cats and dogs, both in hospitalized patients and at home. Studies on complication rates and safety of the devices in dogs and cats do not exist.
The aim of this study was to identify the incidence and nature of complications associated with the use of the FGMS in cats. Our hypothesis was that the FGMS would be safe and well tolerated, without any major adverse effects.
Materials and methods
Medical records of cats that had the 14-day FGMS placed between March 2019 and March 2020 at the Foster Hospital for Small Animals at the Cummings School of Veterinary Medicine at Tufts University were reviewed. The following terms were used to search the electronic medical record: ‘Freestyle’, ‘Libre’ and ‘blood glucose monitoring system’.
All sensors were placed by trained veterinary technicians. The desired site for placement was shaved and gently cleaned with alcohol. The site was allowed to air dry for several minutes. An adhesive wipe (SkinTac Adhesive Barrier Wipes) was used on the cleaned site. The FGMS was placed using the device supplied by the manufacturer and following the manufacturer’s instructions. For some cats a small additional amount of cyanoacrylate tissue glue (Vet Close Cyanoacrylate Surgical Glue) was placed on the contact surface of the sensor prior to placement. Following placement, the device was scanned immediately to link it to the reader, though a 1 h warm-up period was required before data collection could be initiated.
Cases were included if the FGMS was placed between March 2019 and March 2020 for the purpose of interstitial glucose monitoring, if the date of sensor placement was recorded and if the sensor remained attached for at least 24 h. Cases were excluded if the date of placement was not recorded or if the sensor fell off within 24 h after placement.
Medical records were reviewed for patient and FGMS placement data, as well as complications documented in hospital, at home or during follow-up visits. Patient data included signalment, body condition score (BCS; 1–9 scale), primary disease process and comorbidities. 18 FGMS placement data included site of sensor placement, technique for placement (ie, whether or not additional tissue glue was used), duration that the sensor remained attached and functional and complications. Some cases were excluded for having incomplete FGMS placement data. The types of complications are defined in Table 1. For patients with complications the medical records were reviewed further to determine if additional treatment was necessary.
Table 1.
Types of complications associated with flash glucose monitoring system (FGMS) use
| Complication | Definition |
|---|---|
| None | Sensor remains attached and functional for 14 days, or is removed early for owner convenience or due to euthanasia. Patient is tolerant of the sensor and no adverse effects are observed |
| Early detachment | Sensor inadvertently detaches before the 14-day monitoring period is complete |
| Mild dermatologic changes | Erythema, mild crusting, abrasions, mild pruritus/discomfort noted upon removal/detachment of the FGMS. Additional intervention not required |
| Major dermatologic changes | Changes include erosions, ulceration, abscessation and severe pruritus noted upon removal/detachment of the FGMS. Additional intervention required |
| Dysfunctional sensor | Sensor remains attached but error message is given suggesting sensor is no longer operational |
Descriptive statistical analysis was performed for applicable variables (age, weight, BCS). Data were analyzed for normality using Microsoft Excel formulae for kurtosis and skewness, and were expressed as median and range (non-parametric) or mean ± SD (parametric).
Results
A total of 38 cats underwent FGMS placement between March 2019 and March 2020. Eighteen of these cats were excluded from the study, including four whose FGMS detached within 24 h of placement, and 14 with incomplete FGMS placement information in their medical records. Twenty cats met the criteria for enrollment in the study; 13 were castrated males and seven were spayed females. Domestic shorthair was the most common breed (n = 10 [50%]), followed by domestic longhair (n = 5 [25%]), domestic mediumhair (n = 2 [10%]) and one each of Siberian, Siamese and Maine Coon. The mean age of the included cats was 12.35 ± 2.96 years. Median weight was 5.3 kg (range 2.6–10.7 kg). Median BCS was 5/9 (range 2–9).
A total number of 33 FGMSs were placed in the included cats, all for the purpose of diabetes monitoring. Thirty were used for routine diabetes monitoring. The other three were placed in cats hospitalized for management of diabetic ketoacidosis. Comorbidities were present in 13 cats and included neoplasia (n = 5 [25%]), pancreatitis (n = 5 [25%]), liver disease (n = 2 [10%]) and one cat each with hyperthyroidism, chronic kidney disease, feline lower urinary tract disease and hypersomatotropism. Several cats had more than one comorbidity (n = 4 [20%]).
Locations for FGMS attachment included overlying the dorsolateral aspect of the thorax just caudal to the scapula (n = 30 [91%]) and between the shoulders on the dorsum (n = 3 [9%]) (Figure 1). Twenty of 33 (61%) FGMSs remained attached and functional for the full 14 days, with two of those remaining attached longer (16 and 28 days, respectively). The sensors that remained attached longer only provided 14 days of data. Three FGMSs were removed or replaced early (despite ongoing functionality) because it was convenient for the owners. One FGMS was removed just 4 days after placement because the patient was euthanized. The use of cyanoacrylate tissue glue was recorded for 6/33 FGMS placements. Cyanoacrylate tissue glue was avoided for 13/33 FGMS placements. The remaining 14 FGMSs lacked information on whether or not additional tissue glue was used.
Figure 1.
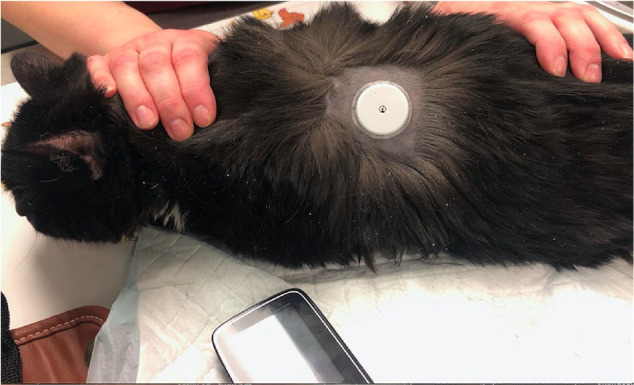
Placement of the flash glucose monitoring system over the left dorsolateral aspect of the thorax of a domestic cat
A summary of complications is provided in Table 2. The overall incidence of complications associated with FGMS use was 10/33 (30%).
Table 2.
Incidence of complications associated with flash glucose monitoring system use in cats
| Complication type | Number | Percentage |
|---|---|---|
| None | 23 | 70 |
| Early detachment | 5 | 15 |
| Mild dermatologic changes | 4 | 12 |
| Major dermatologic changes | 2 | 6 |
| Dysfunctional sensor | 1 | 3 |
Eight of 30 (27%) FGMSs applied over the dorsolateral aspect of the thorax and 2/3 (67%) FGMSs applied on the dorsum had associated complications. The most common complication recorded was the FGMS falling off before 14 days of data had been collected (n = 5/33 [15%]). One FGMS remained attached appropriately but stopped working after 3 days. Upon sensor detachment/removal, 4/33 (12%) FGMSs were associated with mild skin erythema, superficial abrasions and/or crusts (Figure 2). One of the four cats with mild dermatologic complications had the sensor placed with additional tissue glue. Three cats with mild complications had the FGMS replaced without additional complication on the dorsolateral thorax on the opposite side. Two of 33 (6%) FGMSs were associated with serious dermatologic complications. One cat experienced severe skin erosions following early removal of a questionably functional FGMS (Figure 3). Another developed a serious abscess at the location of the sensor that required debridement and antibiotics. This was appreciated during routine sensor replacement. Pasteurella was cultured from the abscess. The FGMSs associated with the more serious dermatologic complications were both placed using small amounts of cyanoacrylate tissue glue, and were both positioned on the dorsum between the shoulders. Neither of these cats had obvious underlying disease processes that would have predisposed them to major complications. The majority of sensors (n = 23/33 [70%]) were placed and utilized without reported or observed complications. Three of the 10 cats that experienced complications had undergone previous, routine FGMS placement at the same location (two over the dorsolateral thorax and one over the dorsum). None of these FGMSs were placed with tissue glue.
Figure 2.
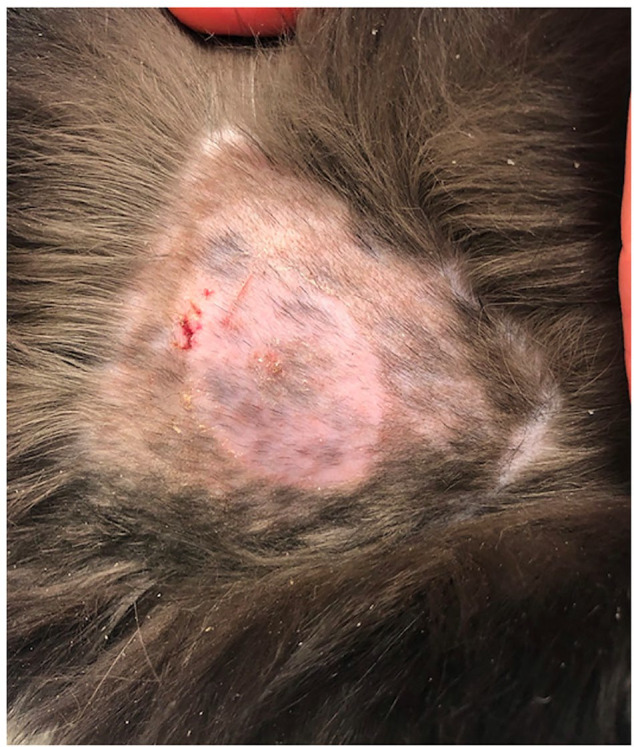
Mild erythema, abrasion and crusting noted after early detachment of the flash glucose monitoring system
Figure 3.
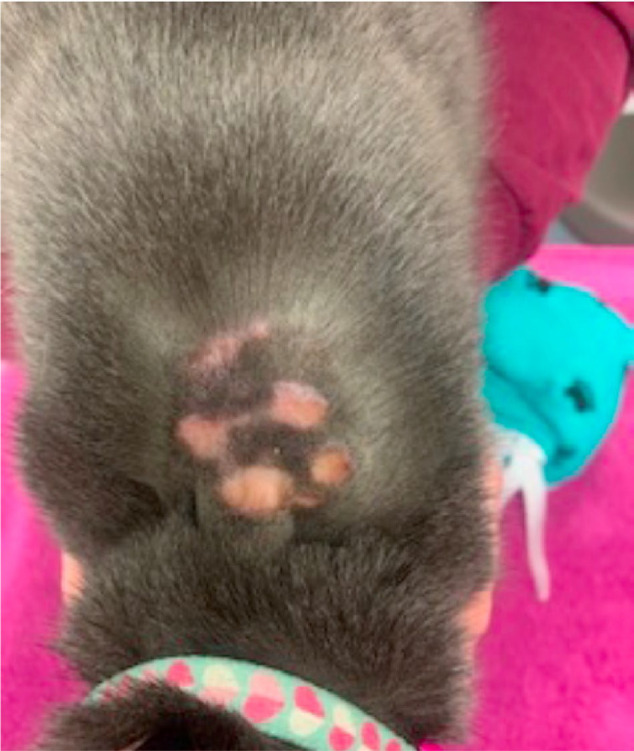
Skin erosions following early removal of a flash glucose monitoring system placed with additional cyanoacrylate tissue glue
Discussion
Our study provides a descriptive review of complications associated with the use of an FGMS in cats. This device is being used more frequently by both primary care veterinarians and specialists because it is easy to use and can provide consecutive days of monitoring in the home environment. The accuracy of the FGMS has been established in diabetic dogs.16,17 Neither the accuracy nor the safety of the FGMS has been evaluated in the feline population.
Most of the complications reported were mild and required no additional treatment. Erythema and crusts at the FGMS placement site were relatively common, and could be related to contact between the skin and the device or secondary to skin preparation prior to placement. Contact dermatitis is an increasingly prevalent complication associated with multiple brands of glucose sensors in people, and may be related to an allergy to plastic and adhesive materials in the sensors. 19 The FGMS examined in this study has been shown to contain adhesive chemicals (isobornyl acrylate and N,N-dimethylacrylamide), which can cause contact allergy in people.20,21 Studies in people suggest that anywhere from 5% to 46% of glucose sensor users have skin problems related to their sensors.19,22 A small percentage of these individuals will need to stop using the sensor or use skin barrier films or barrier creams under the sensor. A similar contact dermatitis is a possibility in cats, and should be considered with the development of erythema, pruritus or crusts. Our results indicate that mild dermatologic adverse effects are usually not bothersome and may not preclude placement of subsequent devices if the site of placement can be altered and if additional adhesives can be avoided.
More serious dermatologic complications (erosions and abscess formation) were noted in two cats. It is possible that these complications represented more severe cases of contact dermatitis or hypersensitivity. Interestingly, additional cyanoacrylate tissue glue was applied to the skin-facing surface of the FGMS in each of these cats. Cyanoacrylate adhesives, though they may induce mild acute and chronic inflammation on a microscopic level, are considered to be safe and effective when used on their own for superficial skin closure in people, dogs and cats.23–25 The effect of combining cyano-acrylate with the adhesives already existing on the FGMS is unknown but could have contributed to a more robust and irritating inflammatory response in these two cats. The cat with erosions had the FGMS removed only 4 days after it was placed. It is also possible that early removal of a still tightly adhered sensor resulted in direct trauma to the skin. Each of the more serious complications occurred with FGMSs that were applied to the dorsum rather than overlying the dorsolateral thorax. Subsequent FGMS placement was avoided in these two cats. Though larger studies are needed to determine if additional adhesives, timing of sensor removal and sensor location have an influence on the incidence or type of complications, we are currently avoiding the use of cyanoacrylate tissue glue and favor placement over the dorsolateral thorax with alternating of sides if sensor placement is to be repeated.
The most common complication in the present study was that the FGMS frequently fell off early. In addition, four cats excluded from the study had sensor detachment within 24 h of placement. Possible causes for early detachment include patient factors (self-removal secondary to scratching, rubbing against furniture, high activity level, housemate interactions) and ineffective placement (site not shaved or cleaned effectively, patient movement during placement). A study in diabetic children being monitored with the same FGMS demonstrated early detachment in 43.3% of the study participants. 26 Though early detachment of the sensor generally will not be detrimental to patient care, it is important to specify to cat owners that it may occur so that expectations are appropriately set.
There are a number of limitations to this study. The main limitation was the retrospective nature of the data and small sample size. Larger studies are needed to determine if there are risk factors for the development of complications. In addition, the data for the study was collected over a year-long period during which there was variation in the involved personnel. All technicians placing the sensors were trained to do so but may have used slightly different techniques or varied in their tendency to use cyanoacrylate tissue glue during placement. Because some complications were reported by the owner in follow-up telephone calls or emails, very minor complications such as temporary sensor error might not have been deemed concerning enough for owners to mention.
Conclusions
The examined FGMS appears to be relatively safe and well tolerated. The majority of the devices remained functional for a full 14 days and were not associated with complications. Sensor detachment and mild dermatologic adverse effects (erythema, crusts) are relatively common complications that should be discussed with cat owners prior to placement.
Footnotes
Accepted: 16 September 2020
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). For any animals or humans individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
ORCID iD: Adam M Shoelson  https://orcid.org/0000-0002-3910-1496
https://orcid.org/0000-0002-3910-1496
References
- 1. Alt N, Kley S, Haessig M, et al. Day-to-day variability of blood glucose concentration curves generated at home in cats with diabetes mellitus. J Am Vet Med Assoc 2007; 230: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 2. Behrend EN, Tapia J, Welles EG, et al. Evaluation of a conventional urine glucose test strip method for detection of glucosuria in dogs and cats [abstract]. J Vet Intern Med 2008; 22: 790a. [Google Scholar]
- 3. Sparkes AH, Cannon M, Church D, et al. ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J Feline Med Surg 2015; 17: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Behrend EN, Holford A, Lathan P, et al. 2018 AAHA diabetes management guidelines for dogs and cats. J Am Anim Hosp Assoc 2018; 54: 1–21. [DOI] [PubMed] [Google Scholar]
- 5. Cook AK. Monitoring methods for dogs and cats with diabetes mellitus. J Diabetes Sci Technol 2012; 6: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reusch CE, Haberer B. Evaluation of fructosamine in dogs and cats with hypo- or hyperproteinaemia, azotaemia, hyperlipidaemia and hyperbilirubinaemia. Vet Rec 2001; 148: 370–376. [DOI] [PubMed] [Google Scholar]
- 7. Reusch CE, Tomsa K. Serum fructosamine concentration in cats with overt hyperthyroidism. J Am Vet Med Assoc 1999; 215: 1297–1300. [PubMed] [Google Scholar]
- 8. Mancini G, Berioli MG, Santi E, et al. Flash glucose monitoring: a review of the literature with a special focus on type 1 diabetes. Nutrients 2018; 10: 992. DOI: 10.3390/nu10080992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davison LJ, Slater LA, Herrtage ME, et al. Evaluation of a continuous glucose monitoring system in diabetic dogs. J Small Anim Pract 2003; 44: 435–442. [DOI] [PubMed] [Google Scholar]
- 10. DeClue AE, Cohn LA, Kerl ME, et al. Use of continuous blood glucose monitoring for animals with diabetes mellitus. J Am Anim Hosp Assoc 2004; 40: 171–173. [DOI] [PubMed] [Google Scholar]
- 11. Wiedmeyer CE, Johnson PJ, Cohn LA, et al. Evaluation of a continuous glucose monitoring system for use in veterinary medicine. Diabetes Technol Ther 2005; 7: 885–895. [DOI] [PubMed] [Google Scholar]
- 12. Wiedmeyer CE, Declue AE. Continuous glucose monitoring in dogs and cats. J Vet Intern Med 2008; 22: 2–8. [DOI] [PubMed] [Google Scholar]
- 13. Affenzeller N, Benesch T, Thalmhammer JG, et al. A pilot study to evaluate a novel subcutaneous continuous glucose monitoring system in healthy Beagle dogs. Vet J 2010; 184: 105–110. [DOI] [PubMed] [Google Scholar]
- 14. Moretti S, Tschuor F, Osto M, et al. Evaluation of a novel real-time continuous glucose-monitoring system for use in cats. J Vet Intern Med 2010; 24: 120–126. [DOI] [PubMed] [Google Scholar]
- 15. Reineke EL, Fletcher DJ, Lesley GK, et al. Accuracy of a continuous glucose monitoring system in dogs and cats with diabetic ketoacidosis. J Vet Emerg Crit Care 2010; 20: 303–312. [DOI] [PubMed] [Google Scholar]
- 16. Corradini S, Pilosio B, Dondi F, et al. Accuracy of a flash glucose monitoring system in diabetic dogs. J Vet Intern Med 2016; 30: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malerba E, Cattani C, Del Baldo F, et al. Accuracy of a flash glucose monitoring system in dogs with diabetic ketoacidosis. J Vet Intern Med 2020; 34: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laflamme DP. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 19. Hyry H, Liippo J, Virtanen H. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis 2019; 81: 161–166. [DOI] [PubMed] [Google Scholar]
- 20. Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle Libre®, a newly introduced glucose sensor. Contact Dermatitis 2017; 77: 367–373. [DOI] [PubMed] [Google Scholar]
- 21. Mowitz M, Herman A, Baeck M, et al. N,N-dimethylacrylamide – a new sensitizer in the FreeStyle Libre glucose sensor. Contact Dermatitis 2019; 81: 27–31. [DOI] [PubMed] [Google Scholar]
- 22. Berg AK, Nørgaard K, Thyssen JP, et al. Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross sectional study. Diabetes Technol Ther 2018; 20: 475–482. [DOI] [PubMed] [Google Scholar]
- 23. Penoff J. Skin closures using cyanoacrylate tissue adhesives. Plastic Reconstruct Surg 1999; 103: 730–731. [DOI] [PubMed] [Google Scholar]
- 24. Faria M, de Almeida F, Serrão M, et al. Use of cyanoacrylate in skin closure for ovariohysterectomy in a population control programme. J Feline Med Surg 2005; 7: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pope J, Knowles T. The efficacy of n-butyl-cyanoacrylate tissue adhesive for closure of canine laparoscopic ovariectomy port site incisions. J Small Anim Pract 2013; 54: 190–194. [DOI] [PubMed] [Google Scholar]
- 26. Massa G, Gys I, Op’t Eyndt A, et al. Evaluation of the FreeStyle® Libre flash glucose monitoring system in children and adolescents with type 1 diabetes. Horm Res Paediatr 2018; 89: 189–199. [DOI] [PubMed] [Google Scholar]


