Abstract
Objectives
The aim of this study was to compare the sedative effects in cats administered acepromazine–nalbuphine and acepromazine–butorphanol, intramuscularly (IM) and intravenously (IV), and the occurrence of adverse cardiorespiratory effects.
Methods
Forty-six cats were randomly divided into four groups and administered acepromazine (0.05 mg/kg) combined with nalbuphine (0.5 mg/kg) or butorphanol (0.4 mg/kg), IV (ACP-NALIV and ACP-BUTIV groups, respectively) or IM (ACP-NALIM and ACP-BUTIM groups, respectively). Sedation scores, ease of intravenous catheter placement (simple descriptive scale [SDS] scores), physiologic variables, venous blood gases and the propofol dose required for anesthetic induction were recorded.
Results
Mild sedation was observed in all groups approximately 30 mins after treatment administration (timepoint T1, prior to propofol administration). Sedation scores at T1 increased above baseline in all groups (P <0.05), but no significant difference was observed among groups. Dynamic interactive visual analogue scale sedation scores (range 0–100 mm) recorded at T1 were (median [interquartile range]): ACP-NALIM, 12 (10–12); ACP-NALIV, 11 (6–16); ACP-BUTIM, 11 (7–14); and ACP-BUTIV, 12 (7–19). Overall, SDS scores did not change from baseline at T1 and there was no significant difference among groups. The propofol dose did not differ among groups. Blood gases remained within the reference intervals for cats. Significant decreases from baseline were detected for all groups in systolic arterial pressure (SAP). Mean ± SD values at T1 were (mmHg): ACP-NALIM, 108 ± 13; ACP-NALIV, 102 ± 10; ACP-BUTIM, 97 ± 13; and ACP-BUTIV, 98 ± 21. Arterial hypotension (SAP <90 mmHg) was recorded at T1 in 0/11, 1/13, 4/11 and 5/11 cats in groups ACP-NALIM, ACP-NALIV, ACP-BUTIM and ACP-BUTIV, respectively, and was further exacerbated after the induction of anesthesia with propofol.
Conclusions and relevance
In healthy cats administered acepromazine–nalbuphine and acepromazine–butorphanol, IM and IV, the degree of sedation was mild regardless of the protocol and the route of administration. The main adverse effect observed was a reduction in arterial blood pressure.
Keywords: Opioids, phenothiazine derivatives, premedication, tranquilization
Introduction
Neuroleptanalgesia consists of the combination of a tranquilizer with an analgesic. 1 Neuroleptanalgesia protocols have been used in cats to provide a calming effect and analgesia, and to facilitate the handling of animals during preparation for surgical or diagnostic procedures. One neuroleptanalgesia protocol frequently used in cats is the combination of acepromazine with an opioid analgesic. In a recent study conducted with New Zealand veterinarians, 4/5 of the most commonly reported drug combinations used in cats undergoing ovariohysterectomy included acepromazine and an opioid. 2
The use of acepromazine in cats has been reported in combination with several opioids, such as methadone,3,4 buprenorphine,3,5 butorphanol3,6,7 and morphine. 6 The administration of these combinations in cats resulted in mild sedation, which was sufficient for intravenous catheter placement3,8 and echocardiographic evaluation. 7
Nalbuphine is a mu (μ) antagonist and kappa (κ) agonist opioid, characteristics that make it similar to butorphanol. 9 Owing to these pharmacological similarities between the drugs, nalbuphine might be used as a potential substitute for butorphanol in cats. This could be useful because there may be differences in drug availability and drug control in different countries. In a search of the current literature, only one published study on the use of nalbuphine in cats was found. 10 However, in that previous study, nalbuphine was administered alone. No studies were found on the use of nalbuphine in combination with acepromazine in cats. Despite the use of acepromazine–butorphanol in this species,3,6,7 the results cannot be extrapolated for acepromazine–nalbuphine.
In previous studies, where the sedative efficacy of acepromazine–opioid combinations was assessed, the drugs were injected intramuscularly (IM).3 –7 However, intravenous administration results in a rapid increase in plasma concentrations of the drug, which could, presumably, result in faster and more pronounced effects than intramuscular administration.
The aims of this study were to compare the degree of sedation in cats administered acepromazine–nalbuphine (ACP-NAL) and acepromazine–butorphanol (ACP-BUT), IM and intravenously (IV), and the occurrence of adverse cardiorespiratory effects. Our hypothesis was that there would be no difference in the degree of sedation between combinations with nalbuphine and butorphanol or between administration IV and IM.
Materials and methods
Animals
The study was approved by the Institutional Animal Care Committee of the Federal University of Rio Grande do Sul, Brazil (approval number 36482). Written informed consent was obtained from the owner of each animal.
Healthy male and female cats scheduled for elective surgical procedures requiring general anesthesia were included. Health status was based on clinical examination, a complete blood count and serum chemistry analyses. All findings were within the reference intervals for cats. Exclusion criteria included: age <4 months or >10 years, body condition score <3/9 or >7/9, and aggressive or uncooperative behavior.
Study design and treatments
This was a prospective, randomized, blinded study. Each cat underwent one of four treatments. A random distribution plan was generated using an open-access website (http://www.randomization.com). The treatments consisted of combinations of acepromazine (0.05 mg/kg [Acepran 0.2%; Vetnil]) with nalbuphine (0.5 mg/kg [Nubain; Cristália]) or with butorphanol (0.4 mg/kg [Torbugesic; Zoetis]). In the ACP-NALIV and ACP-BUTIV groups, acepromazine–nalbuphine and acepromazine–butorphanol, respectively, were administered IV through a cephalic catheter over 60 s. In the ACP-NALIM and ACP-BUTIM groups, acepromazine–nalbuphine and acepromazine–butorphanol, respectively, were injected IM into the semitendinosus muscle. On all occasions, the drugs were mixed in a single syringe immediately before administration.
Experimental protocol
Food, but not water, was withheld for 8 h. The cats were admitted to the University Veterinary Hospital on the day of the experiment and were housed in individual cages. The hair around the cephalic veins and palmar digital arteries was clipped and the cat was left undisturbed for 30 mins to acclimatize. Baseline values (timepoint T0) were measured after the acclimatization period.
Sedation was scored by a numeric descriptive scale (NDS) and a dynamic interactive visual analog scale (DIVAS). The NDS consists of five categories with scores ranging from 0 to 14 (Table 1). 11 The DIVAS consists of a 100 mm horizontal line where the left end represents no sedation (score 0) and the right end (score 100) represents the maximum sedation possible. Sedation was scored in the acclimatization room. First, the assessor observed the cat from outside the cage to evaluate its posture. The assessor then opened the door of the cage to interact with the cat and to evaluate the remaining categories of the NDS (eye position, palpebral reflex, degree of jaw relaxation and auditory response). The assessor, who was blinded to the treatment administered to each cat, assigned the DIVAS score first and then the NDS score.
Table 1.
Categories of the numeric descriptive scale used for evaluation of sedation in cats 11
| Category | Description |
|---|---|
| 1 Posture score | 0 = Standing |
| 1 = Sitting or ataxic | |
| 2 = Sternal recumbency | |
| 3 = Lateral recumbency | |
| 2 Eye position | 0 = Normal eye position |
| 1 = Partial ventromedial eye rotation | |
| 2 = Complete ventromedial eye rotation | |
| 3 Palpebral reflex | 0 = Strong palpebral reflex |
| 1 = Moderate palpebral reflex | |
| 2 = Mild palpebral reflex but still present | |
| 3 = Palpebral reflex absent | |
| 4 Degree of jaw relaxation | 0 = Normal tonus |
| 1 = Slightly weakened tonus | |
| 2 = Moderately weakened tonus | |
| 3 = No resistance to mouth opening | |
| 5 Auditory response (reaction to sound as produced by handclap) | 0 = Normal response 1 = Mild decrease in response (some eye movement with body movement) 2 = Moderate decrease in response (some eye movement without body movement) |
| 3 = Profound decrease in response (no movement) |
Sedation scores range from 0 (no sedation) to 14 (maximum sedation)
Heart rate (HR) was measured by auscultation and respiratory rate (RR) was counted by observation of chest wall movements. Systolic arterial pressure (SAP) was measured using a Doppler flow probe (model 811-B; Parks Medical Electronics) placed over the palmar digital artery. A sphygmomanometer and cuff was placed proximal to the carpus. Cuff width was 30–40% of limb circumference. A minimum of three consecutive measurements of SAP with ⩽5 mmHg variation within each other were recorded and the arithmetic mean was considered for analysis. Prior to the beginning of the study, the accuracy of the sphygmomanometer was checked with a mercury manometer. HR, RR and SAP were measured in the acclimatization room after sedation scores.
After NDS, DIVAS, HR, SAP and RR measurements, the cat was taken to the assessment room and was swaddled in a towel according to feline-friendly handling guidelines. 12 The person who held the cat was not involved in the measurement of variables. Both the acclimatization and the assessment room were quiet and the cat being assessed was the only animal inside it. Rectal temperature (RT) was measured with a digital thermometer. A 22 G catheter was percutaneously introduced into a cephalic vein. Ease of catheter placement IV was evaluated using a simple descriptive scale (SDS) modified from a previous study (Table 2). 3 The original scale was modified in an attempt to provide a more detailed description of each SDS level. If more than one attempt was necessary for intravenous catheter placement, the SDS score for that timepoint was based on all attempts. A single observer, unaware of the treatment administered, was responsible for assessing DIVAS, NDS and SDS scores throughout the study. The same person assessing the scores was responsible for intravenous catheter placement. Blood samples (0.5 ml) were collected from the cephalic catheter for blood gas analysis (pH, venous partial pressure of carbon dioxide [PvCO2] and bicarbonate concentration [HCO3]). The samples were collected into commercially available syringes with heparin (A-Line; BD Biosciences) and analyzed immediately (Cobas b121; Roche Diagnostics).
Table 2.
Simple descriptive scale used for evaluation of ease of catheter placement intravenously (IV) in the cephalic vein of cats (modified from Bortolami et al 3 )
| Score | Classification | Description |
|---|---|---|
| 0 | Very difficult | Impossible to perform intravenous catheterization because of one or more of the following: cat requires a lot of restraint; multiple attempts required and continuous withdrawal of the limb; extreme vocalization and/or aggressive behavior in response to intravenous catheter placement |
| 1 | Difficult | Cat requires good restraint; some attempts to withdraw the limb; may show moderate vocalization |
| 2 | Easy | Mild restraint required; mild or no vocalization; minimal attempts to withdraw the limb |
| 3 | Very easy | Minimal restraint required; no vocalization and no attempt to withdraw the limb during intravenous catheter placement |
The order of assessments was DIVAS, NDS, HR, RR, SAP (in the acclimatization room), RT, SDS scores and blood sampling (in the assessment room). After completion of the procedures at T0, the experimental treatment was administered and the cat was returned to its cage, in the acclimatization room, where it remained for 30 mins without any manipulation. Subsequently, variables were reassessed (timepoint T1). Another 22 G catheter was placed into the contralateral cephalic vein for scoring SDS at T1. Finally, a new sample of venous blood was collected for blood gas analysis.
After completion of assessments at T1, anesthesia was induced with propofol (Propovan; Cristália). Propofol was administered IV at a rate of 5.0 mg/kg/min by a syringe pump (RS700 Vet; RZVet) until the following endpoints were achieved: eyeball rotation, loss of medial palpebral reflex and relaxation of jaw tone. Thereafter, 0.1 ml of 2% lidocaine (Xylestesin; Cristália) was instilled over the larynx and orotracheal intubation was performed. The tracheal tube was connected to a double T-piece non-rebreathing system to deliver 100% oxygen (300 ml/kg/min) and HR, RR, SAP and RT were then reassessed (timepoint T2). The propofol dose required for induction of anesthesia and the time elapsed from administration of the experimental treatment to anesthetic induction were recorded. After assessments at T2 were completed, isoflurane was delivered in order to maintain adequate depth of anesthesia to perform the surgical procedure. Other drugs were administered at the discretion of the anesthesiologist. All cats were discharged on the same day of procedures without any systemic problems.
Statistical analysis
Sample size calculation was performed using G*Power for Windows Version 3.1.6 (Heinrich Heine Universität, Düsseldorf, Germany). An a priori analysis revealed that 10 cats per group would be required to detect a difference of 10 mm between group means of DIVAS sedation scores, with SDs of 35% of means, an alpha error of 0.05 and 80% power (1 – beta [β]). One additional animal was included in each group and the inclusion of animals was interrupted when all groups completed 11 cats.
Data analyses were performed using a statistical software for Windows platform (Graphpad Prism 8.0.1; GraphPad Software). Normality of data was assessed by the Shapiro–Wilk test.
Weight, age, time elapsed from treatment administration to anesthetic induction and propofol dose were analyzed by a one-way ANOVA followed by a Tukey multiple comparisons test or a Kruskal–Wallis test followed by a Dunn test, where appropriate.
For variables with a normal distribution (HR, RR, SAP, RT and blood gases), comparisons among groups and over time were performed using a mixed-effects model, considering time and treatment as fixed effects and individual as a random effect. Post-hoc group comparisons were performed by a Tukey multiple comparisons test, whereas post-hoc time comparisons were performed by a Dunnett test to identify differences between T1 and T2 from T0.
For non-parametric variables (sedation and SDS scores), comparisons among groups at each timepoint were performed using the Kruskal–Wallis test followed by Dunn multiple comparisons test. Differences between T1 and T0 for sedation and SDS scores were analyzed by a Wilcoxon signed rank test. The number of cats presenting hypotension (SAP <90 mmHg) in each group was compared by a χ2 test. For all analyses, differences were considered significant when P <0.05.
Results
Forty-six mixed-breed cats were included in the study (ACP-NALIM, n = 11; ACP-NALIV, n = 13; ACP-BUTIM, n = 11; ACP-BUTIV, n = 11). There was no significant difference among groups for weight, age, propofol dose and the time elapsed from administration of the experimental treatment to anesthetic induction (Table 3).
Table 3.
Demographic data, time elapsed from administration of the treatment until anesthetic induction and propofol dose in 46 cats administered acepromazine (ACP; 0.05 mg/kg) combined with nalbuphine (NAL; 0.5 mg/kg) or butorphanol (BUT; 0.4 mg/kg), intravenously (ACP-NALIV and ACP-BUTIV groups) or intramuscularly (ACP-NALIM and ACP-BUTIM groups)
| ACP-NALIM(n = 11) | ACP-NALIV (n = 13) |
ACP-BUTIM (n = 11) |
ACP-BUTIV (n = 11) |
|
|---|---|---|---|---|
| Sex | ||||
| Male | 4 | 6 | 8 | 6 |
| Female | 7 | 7 | 3 | 5 |
| Surgical procedure | ||||
| Ovariohysterectomy | 5 | 5 | 1 | 4 |
| Orchiectomy | 2 | 2 | 3 | 3 |
| Dental procedure | 4 | 5 | 7 | 4 |
| Umbilical herniorrhaphy | 0 | 1 | 0 | 0 |
| Weight (kg) | 3.8 ± 1.2 | 4.1 ± 1.5 | 3.6 ± 0.9 | 3.7 ± 1.2 |
| Age (months) | 18 (7–36) | 24 (9–78) | 50 (10–60) | 24 (6–36) |
| Time from treatment to anesthetic induction (mins) | 65 (60–83) | 64 (53–68) | 63 (55–103) | 61 (55–78) |
| Propofol dose (mg/kg) | 6.3 ± 1.8 | 6.1 ± 2.4 | 7.2 ± 1.6 | 7.3 ± 2.1 |
Data are presented as mean ± SD or median (interquartile range)
All cats were assigned a sedation score of zero at T0 for both the NDS and the DIVAS. There was no significant difference among the groups regarding NDS and DIVAS scores at T0 and T1. Compared with T0, sedation scores increased at T1 in all groups (Table 4). P values for NDS scores were: ACP-NALIM, P = 0.001; ACP-NALIV, P = 0.002; ACP-BUTIM, P = 0.001; and ACP-BUTIV, P = 0.002. P values for DIVAS scores were: ACP-NALIM, P = 0.001; ACP-NALIV, P = 0.002; ACP-BUTIM, P = 0.001; and ACP-BUTIV, P = 0.001.
Table 4.
Median (interquartile range) numeric descriptive scale (NDS; range 0–14) and dynamic interactive visual analogue scale (DIVAS; range 0–100) sedation scores and ease of intravenous catheter placement score (simple descriptive scale [SDS]; range 0–3) in 46 cats administered acepromazine (ACP; 0.05 mg/kg) combined with nalbuphine (NAL; 0.5 mg/kg) or butorphanol (BUT; 0.4 mg/kg), IV (ACP-NALIV and ACP-BUTIV groups, respectively) or intramuscularly (IM; ACP-NALIM and ACP-BUTIM groups, respectively)
| ACP-NALIM(n = 11) | ACP-NALIV (n = 13) |
ACP-BUTIM (n = 11) |
ACP-BUTIV (n = 11) |
||
|---|---|---|---|---|---|
| NDS | T0 | 0 | 0 | 0 | 0 |
| T1 | 3 (1–3)* | 2 (1–3)* | 2 (2–2)* | 2 (1–3)* | |
| DIVAS (mm) | T0 | 0 | 0 | 0 | 0 |
| T1 | 12 (10–12)* | 11 (6–16)* | 11 (7–14)* | 12 (7–19)* | |
| SDS | T0 | 2 (1–2) | 2 (2–3) | 2 (2–2) | 2 (2–2) |
| T1 | 3 (2–3)* | 2 (2–3) | 2 (2–3) | 2 (2–3) |
Significant difference from T0 within a group (P <0.05)
T0 = baseline; T1 = 30 mins after administration of the treatment
There was no significant difference among the groups for SDS scores at any time point. A significant increase in SDS score above T0 was detected for ACP-NALIM (P = 0.008) but not for other groups (Table 4). A stratification of SDS scores at T0 and T1 is presented in Figure 1.
Figure 1.
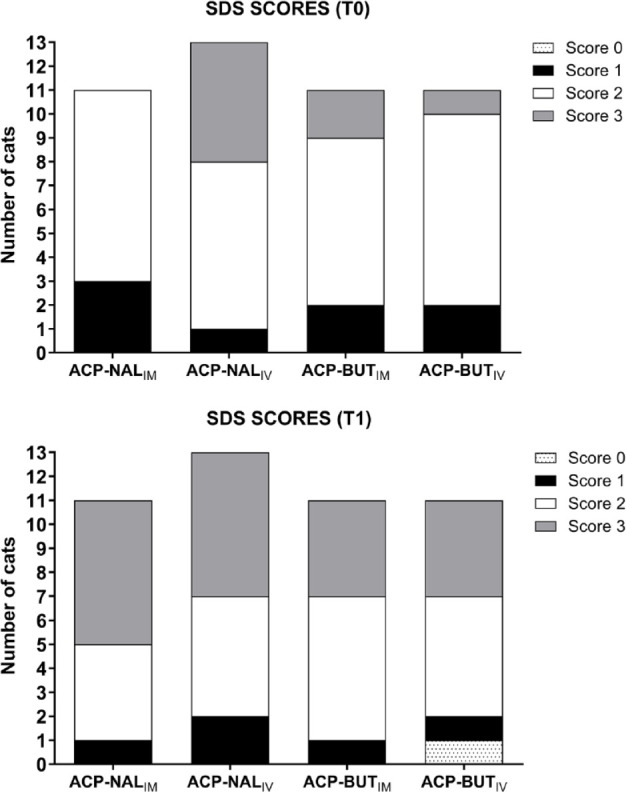
Distribution of simple descriptive scale (SDS) scores in 46 cats administered acepromazine (ACP; 0.05 mg/kg) combined with nalbuphine (NAL; 0.5 mg/kg) or butorphanol (BUT; 0.4 mg/kg) intravenously (IV; ACP-NALIV and ACP-BUTIV groups, respectively) or intramuscularly (IM; ACP-NALIM and ACP-BUTIM, respectively). The SDS was used for evaluation of ease of catheter placement IV in the cephalic vein of cats, as follows: score 0, very difficult; score 1, difficult; score 2, easy; score 3, very easy. T0 = baseline; T1 = 30 mins after administration of the treatment
There was no significant difference among the groups for HR and SAP. In the ACP-NALIM group, HR increased above T0 at T1 (P = 0.0006), whereas in the ACP-BUTIV group HR decreased below T0 at T2 (P = 0.0497). Compared with T0, SAP decreased in all groups at T1 (ACP-NALIM, P <0.0001; ACP-NALIV, P = 0.0004; ACP-BUTIM, P = 0.0002; ACP-BUTIV, P = 0.003) and T2 (ACP-NALIM, P <0.0001; ACP-NALIV, P <0.0001; ACP-BUTIM, P <0.0001; ACP-BUTIV, P = 0.0001) (Table 5). Arterial hypotension was recorded in 0/11, 1/13, 4/11 and 5/11 cats at T1 and in 7/11, 9/13, 7/11 and 9/11 cats at T2, respectively, in the ACP-NALIM, ACP-NALIV, ACP-BUTIM and ACP-BUTIV groups. An overall difference among groups in the prevalence of hypotension was detected at T1 (P = 0.023) but not at T2.
Table 5.
Heart rate (HR), systolic arterial pressure (SAP), respiratory rate (RR), venous pH, venous partial pressure of carbon dioxide (PvCO2), venous bicarbonate (HCO3) and rectal temperature (RT) in 46 cats administered acepromazine (ACP; 0.05 mg/kg) combined with nalbuphine (NAL; 0.5 mg/kg) or butorphanol (BUT; 0.4 mg/kg), intravenously (IV; ACP-NALIV and ACP-BUTIV groups, respectively) or intramuscularly (IM; ACP-NALIM and ACP-BUTIM, respectively)
| ACP-NALIM (n = 11) |
ACP-NALIV (n = 13) |
ACP-BUTIM (n = 11) |
ACP-BUTIV (n = 11) |
||
|---|---|---|---|---|---|
| HR (bpm) | T0 | 170 ± 26 | 182 ± 36 | 200 ± 49 | 204 ± 40 |
| T1 | 199 ± 38* | 209 ± 39 | 231 ± 46 | 211 ± 42 | |
| T2 | 181 ± 30 | 164 ± 23 | 169 ± 34 | 167 ± 24* | |
| SAP (mmHg) | T0 | 138 ± 17 | 133 ± 17 | 141 ± 23 | 127 ± 18 |
| T1 | 108 ± 13* | 102 ± 10* | 97 ± 13* | 98 ± 21* | |
| T2 | 81 ± 14* | 78 ± 14* | 80 ± 13* | 74 ± 13* | |
| RR (breaths/min) | T0 | 47 ± 15 | 52 ± 10 | 45 ± 14 | 57 ± 19 |
| T1 | 46 ± 14 | 43 ± 18 | 39 ± 11 | 52 ± 27 | |
| T2 | 29 ± 6* | 28 ± 7* | 25 ± 6* | 26 ± 5* | |
| pH ‡ | T0 | 7.34 ± 0.04 | 7.34 ± 0.03 | 7.33 ± 0.05 | 7.36 ± 0.02 |
| T1 | 7.36 ± 0.04* † | 7.36 ± 0.03* † | 7.31 ± 0.06 | 7.37 ± 0.03 † | |
| PvCO2 ‡ (mmHg) | T0 | 35.2 ± 4.5 | 37.6 ± 3.2 | 38.3 ± 5.4 | 34.3 ± 2.3 |
| T1 | 33.8 ± 3.3 † | 36.1 ± 4.4* | 40.6 ± 7.2 | 33.6 ± 2.9 † | |
| HCO3 ‡ (mmol/l) | T0 | 18.6 ± 1.4 | 20.1 ± 1.0 | 19.7 ± 1.1 | 19.2 ± 1.6 |
| T1 | 18.9 ± 1.4 | 19.9 ± 1.5 | 19.8 ± 1.5 | 19.3 ± 1.4 | |
| RT (ºC) | T0 | 39.1 ± 0.7 | 38.8 ± 0.8 | 39.0 ± 0.9 | 39.3 ± 0.6 |
| T1 | 38.9 ± 1.0 | 38.6 ± 0.9 | 38.6 ± 1.0 | 39.0 ± 1.0 | |
| T2 | 38.7 ± 0.5 | 38.6 ± 0.9 | 38.2 ± 1.1 | 38.8 ± 1.1 |
Data are mean ± SD
Significant difference from T0 within a group
Significant difference from ACP-BUTIM (P <0.05)
Blood gases were available from 9/11, 11/13, 10/11 and 10/11 cats in the ACP-NALIM, ACP-NALIV, ACP-BUTIM and ACP-BUTIV groups, respectively
T0 = baseline; T1 = 30 mins after administration of the treatment; T2 = immediately after anesthetic induction with propofol
No significant difference in RR was detected among the groups. Compared with T0, RR decreased in all groups at T2 (ACP-NALIM, P = 0.002; ACP-NALIV, P <0.0001; ACP-BUTIM, P = 0.0002; ACP-BUTIV, P = 0.0002). Apnea and cyanosis were not detected in any cat at any time point (Table 5).
Blood gases were available for 9/11, 11/13, 10/11 and 10/11 cats in the ACP-NALIM, ACP-NALIV, ACP-BUTIM and ACP-BUTIV groups, respectively. Data for the remaining cats were unavailable because of failure in the gas analyzer. Venous pH was lower in ACP-BUTIM than in other groups at T1 (P = 0.022 vs ACP-NALIM; P = 0.027 vs ACP-NALIV; P = 0.005 vs ACP-BUTIV). At the same timepoint, PvCO2 was higher in ACP-BUTIM than in ACP-NALIM (P = 0.006) and ACP-BUTIV (P = 0.0042). Compared with T0, venous pH increased at T1 in ACP-NALIM (P = 0.004) and ACP-NALIV (P = 0.013), whereas PvCO2 decreased in ACP-NALIV (P = 0.031). There was no significant difference among the groups or within groups for venous HCO3 and RT (Table 5).
Discussion
The main findings of the present study were that neither the sedation protocol (acepromazine–nalbuphine or acepromazine–butorphanol) nor the route of administration significantly influenced the degree of sedation observed in cats.
In the present study, the main purposes of premedication, which are to calm the animal and facilitate its handling, were not achieved with any of the protocols. The degree of sedation was mild in all experimental groups and there was no clear improvement in SDS scores. Mild sedation has also been reported in previous studies in cats administered acepromazine IM in combination with methadone,3,4,13 butorphanol3,7 and buprenorphine.3,5 In the present study, there was no significant difference between the effectiveness of sedation induced by acepromazine–butorphanol or acepromazine–nalbuphine. In a previous study, administration of the opioids buprenorphine, methadone and butorphanol, combined with acepromazine, resulted in similar sedation scores. 3 Similar results were observed in another study with cats administered combinations of acepromazine, methadone and butorphanol. 8 The results of this study and previous studies reinforce that combinations of acepromazine with opioids cause low levels of sedation in cats and that the choice of opioid in the combination does not influence the effectiveness of the protocol.
The finding that SDS scores at T1 increased above T0 in ACP-NALIM, but not in other groups, might suggest that this protocol was more effective than the others in reducing the response to intravenous catheter placement in cats. However, caution must be taken with this interpretation for the following reasons. First, SDS scores did not differ significantly among the groups at T1. Second, 54–73% of cats in all groups had an SDS score of 2 at T0, which represents easy intravenous catheterization in cats that were not administered any sedative or analgesic drug. It is possible that the high SDS scores at T0 resulted, at least in part, from the exclusion of aggressive and/or uncooperative cats from the study. Third, it is possible that cats acquired some memory after the first intravenous catheterization, which could influence their response to a second catheterization and explain why SDS scores did not increase in most groups at T1 compared with T0. Finally, to our knowledge, there is no validated scale to evaluate ease of intravenous catheter placement in cats. Therefore, the SDS may not have been sensitive to detect differences in the ease of intravenous catheter placement between timepoints T0 and T1.
The absorption of drugs after administration IM can be influenced by factors such as drug solubility, blood flow to the absorption site and the available surface area. 14 Intravenous administration circumvents factors that limit absorption resulting in complete and rapid bioavailability, with potentially immediate effects. 15 In a previous study, cats administered 5 mg/kg of alfaxalone IV had sedation scores indicative of general anesthesia immediately after injection. By contrast, cats that received the same dose IM had a longer latency period and scores compatible with deep sedation. 16 Despite the bioavailability after intramuscular administration usually being high and the absorption being fast, 15 higher peak drug concentrations are expected after intravenous than intramuscular administration, 16 which might explain the differences in effect often observed. Nevertheless, in the present study no significant difference was detected in sedation scores between administration IV and IM.
The dose of butorphanol used in the present study was based on previous studies in cats. In one study, administration of butorphanol (0.4 mg/kg IM) combined with dexmedetomidine (10 µg/kg), resulted in effective sedation. 17 In a pharmacokinetic study, the same dose of butorphanol (0.4 mg/kg IM) maintained plasma concentrations indicative of analgesia for 2.7 h. 18
The nalbuphine dose used in the present study was extrapolated from a previous study in dogs. 19 In that previous study, the combination of acepromazine (0.05 mg/kg) with nalbuphine, administered IV, resulted in moderate sedation in most dogs. 19 However, this study should not encourage extrapolation of drug doses between dogs and cats because large differences in the pharmacokinetics of drugs may exist. Further studies are warranted to determine the optimal dose of nalbuphine in cats.
The infusion rate used for anesthetic induction in the present study (5.0 mg/kg/min) was based on a previous study where two propofol infusion rates were compared (1.5 mg/kg/min and 5.0 mg/kg/min) for induction of anesthesia in cats premedicated with methadone. 13 In this previous study, the propofol dose required for anesthetic induction and the occurrence of excitement were lower after the higher rate of infusion. 13
The doses of propofol required for anesthetic induction in the present study did not differ significantly among the groups and ranged from 6.1 to 7.3 mg/kg. In a previous study, the median dose of propofol in cats premedicated with acepromazine (0.02 mg/kg SC) combined with methadone (0.6 mg/kg SC) was 8.9 mg/kg, 8 which is higher than the propofol doses in the present study. Conversely, a lower dose of propofol (5.3 mg/kg) than in the present study was reported in cats premedicated with acepromazine (0.05 mg/kg IM) combined with methadone (0.3 mg/kg IM). 13 Differences in the propofol induction doses observed between the studies may be due to variations in drug doses, routes of administration used in premedication, in the rate of injection of propofol and in endpoints considered during anesthetic induction.
The most relevant adverse effect in the present study was arterial hypotension. This effect was observed in 0–45% of cats after administration of each treatment (T1), and its occurrence was increased after anesthetic induction with propofol (T2) when it was observed in 64–82% of cats in each group. The reduction in blood pressure in cats after administration of combinations of acepromazine with opioids has been attributed to vasodilation induced by phenothiazine. 7 However, in a recent study in awake dogs, the reduction in blood pressure after administration of acepromazine occurred owing to a decrease in stroke volume and cardiac output and not as a result of vasodilation. 20 An unexpected finding in our study was that a greater number of cats presented hypotension at T1 in acepromazine–butorphanol groups than in acepromazine–nalbuphine groups (36–45% vs 0–8%, respectively), and an overall significant difference between groups was detected. This might indicate that acepromazine–butorphanol results in greater prevalence of hypotension than acepromazine–nalbuphine. Further studies are required to evaluate if butorphanol can enhance the hypotensive effects of acepromazine. The increase in the occurrence of hypotension at T2 was likely due to the negative inotropic action of propofol, which results in decreased cardiac output and, consequently, a reduction in blood pressure. 21
Although the present study revealed a high frequency of arterial hypotension, these results should be interpreted carefully. SAP was measured by a non-invasive method, which is not the gold standard. Previous studies have suggested that blood pressure measured in cats with a Doppler flow probe results in underestimated SAP values compared with the invasive method.22,23 Therefore, it is possible that if SAP had been measured invasively in the present study, a considerably smaller number of cats would have SAP values <90 mmHg.
The administration of all treatments did not result in significant changes in RR. Although minor, significant changes in blood gases were recorded at T1, values remained within the reference intervals for venous blood gases in cats. 24 After anesthetic induction with propofol (T2), there was a significant reduction in RR of all groups. The reduction in RR after propofol administration occurs owing to a central effect resulting in respiratory depression. 25 Although apnea and cyanosis were not observed at T2, it is not possible to rule out the occurrence of respiratory depression and hypoventilation because blood gases were not measured at this timepoint.
This study has limitations. First, the study was not completely blinded, which may have biased the assessment of sedation and SDS scores. The assessor did not know which treatment had been assigned, but it was known that all cats had received a combination of acepromazine–nalbuphine or acepromazine–butorphanol. Second, the power analysis was conducted based on DIVAS scores that are usually not normally distributed. Therefore, the sample size estimation based on a variable with unknown distribution may be inaccurate. Third, venous instead of arterial blood samples were used for gas analysis, which does not allow the assessment of arterial oxygenation. Finally, SAP was measured non-invasively because the cats were awake, making arterial catheterization unfeasible.
Conclusions
Healthy cats were administered acepromazine–nalbuphine and acepromazine–butorphanol, IM and IV. The degree of sedation was mild, regardless of the protocol and the route of administration. The main adverse effect observed was a reduction in arterial blood pressure.
Footnotes
Accepted: 6 September 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Ethical approval: This work involved the use of non-experimental animals (owned or unowned) and procedures that differed from established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Eduardo R Monteiro  https://orcid.org/0000-0001-8672-7830
https://orcid.org/0000-0001-8672-7830
Rafael C Beck  https://orcid.org/0000-0001-5684-0138
https://orcid.org/0000-0001-5684-0138
Fernanda VA da Costa  https://orcid.org/0000-0002-1031-7728
https://orcid.org/0000-0002-1031-7728
References
- 1. Hall LW, Clarke KW, Trim CM. Principles of sedation, analgesia and premedication. In: Hall LW, Clarke KW, Trim CM. (eds). Veterinary anaesthesia. London: WB Saunders, 2001, pp 75–112. [Google Scholar]
- 2. Gates MC, Littlewood KE, Kongara K, et al. Cross-sectional survey of anaesthesia and analgesia protocols used to perform routine canine and feline ovariohysterectomies. Vet Anaesth Analg 2020; 47: 38–46. [DOI] [PubMed] [Google Scholar]
- 3. Bortolami E, Murrell JC, Slingsby LS. Methadone in combination with acepromazine as premedication prior to neutering in the cat. Vet Anaesth Analg 2013; 40: 181–193. [DOI] [PubMed] [Google Scholar]
- 4. Mair A, Kloeppel H, Ticehurst K. A comparison of low dose tiletamine-zolazepam or acepromazine combined with methadone for pre-anaesthetic medication in cats. Vet Anaesth Analg 2014; 41: 630–635. [DOI] [PubMed] [Google Scholar]
- 5. Hunt JR, Grint NJ, Taylor PM, et al. Sedative and analgesic effects of buprenorphine, combined with either acepromazine or dexmedetomidine, for premedication prior to elective surgery in cats and dogs. Vet Anaesth Analg 2013; 40: 297–307. [DOI] [PubMed] [Google Scholar]
- 6. Hall TL, Duke T, Townsend HGC, et al. The effect of opioid and acepromazine premedication on the anesthetic induction dose of propofol in cats. Can Vet J 1999; 40: 867–870. [PMC free article] [PubMed] [Google Scholar]
- 7. Ward JL, Schober KE, Fuentes VL, et al. Effects of sedation on echocardiographic variables of left atrial and left ventricular function in healthy cats. J Feline Med Surg 2012; 14: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warne LN, Beths T, Holm M, et al. Comparison of perioperative analgesic efficacy between methadone and butorphanol in cats. J Am Vet Med Assoc 2013; 243: 844–850. [DOI] [PubMed] [Google Scholar]
- 9. Kukanich B, Wiese AJ. Opioids. In: Grimm KA, Lamont LA, Tranquilli WJ, et al. (eds). Lumb & Jones’ veterinary anesthesia and analgesia. Hoboken, NJ: Wiley Blackwell, 2015, pp 207–226. [Google Scholar]
- 10. Sawyer DC, Rech RH. Analgesia and behavioral effects of butorphanol, nalbuphine, and pentazocine in the cat. J Am Anim Hosp Assoc 1987; 23: 438–446. [Google Scholar]
- 11. Monteiro ER, Campagnol D, Parrilha LR, et al. Evaluation of cardiorespiratory effects of combinations of dexmedetomidine and atropine in cats. J Feline Med Surg 2009; 11: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliveira RLS, Moreira CMR, Barcellos MCB, et al. Effect of administration rate on propofol requirement in cats. J Feline Med Surg 2018; 20: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoelting RK, Hillier SC. Pharmacokinetics and pharmacodynamics of injected and inhaled drugs. In: Stoelting RK, Hillier SC. (eds). Pharmacology and physiology in anesthetic practice. Philadelphia, PA: Lippincott Williams & Wilkins, 2006, pp 3–41. [Google Scholar]
- 15. Buxton ILO, Benet LZ. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Chabner BA, Knollmann BC. (eds). Goodman & Gilman’s: the pharmacological basis of therapeutics. New York: McGraw-Hill, 2011, p 12. [Google Scholar]
- 16. Mocholí DR, Escudero E, Belda E, et al. Pharmacokinetics and effects of alfaxalone after intravenous and intramuscular administration to cats. N Z Vet J 2018; 66: 172–177. [DOI] [PubMed] [Google Scholar]
- 17. Bhalla RJ, Trimble TA, Leece EA, et al. Comparison of intramuscular butorphanol and buprenorphine combined with dexmedetomidine for sedation in cats. J Feline Med Surg 2018; 20: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells SM, Glerum LE, Papich MG. Pharmacokinetics of butorphanol in cats after intramuscular and bucal transmucosal administration. Am J Vet Res 2008; 69: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 19. Gomes VH, Oliveira RL, Marques JL, et al. Comparison of the sedative effects of nalbuphine and butorphanol, alone or in combination with acepromazine in dogs. Vet Anaesth Analg 2018; 45: 68–72. [DOI] [PubMed] [Google Scholar]
- 20. Rangel JPP, Monteiro ER, Bitti FS, et al. Hemodynamic, respiratory and sedative effects of progressively increasing doses of acepromazine in conscious dogs. Vet Anaesth Analg 2020; 47: 447–453. [DOI] [PubMed] [Google Scholar]
- 21. Pagel PS, Waltier DC. Negative inotropic effects of propofol as evaluated by the regional preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology 1993; 78: 100–108. [DOI] [PubMed] [Google Scholar]
- 22. Caulkett NA, Cantwell SL, Houston DM. A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Vet Surg 1998; 27: 370–377. [DOI] [PubMed] [Google Scholar]
- 23. Grandy JL, Dunlop CI, Hodgson DS, et al. Evaluation of the Doppler ultrasonic method of measuring systolic arterial blood pressure in cats. Am J Vet Res 1992; 53: 1166–1169. [PubMed] [Google Scholar]
- 24. Bachmann K, Kutter APN, Schefer RJ, et al. Determination of reference intervals and comparison of venous blood gas parameters using standard and non-standard collection methods in 24 cats. J Feline Med Surg 2017; 19: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kashiwagi M, Okada Y, Kuwana SI, et al. A neuronal mechanism of propofol-induced central respiratory depression in newborn rats. Anesth Analg 2004; 99: 49–55. [DOI] [PubMed] [Google Scholar]


