Abstract
Background:
Design of disease modification (DM) trials for Parkinson’s disease (PD) is challenging. Successful delivery requires a shared understanding of priorities and practicalities.
Objective:
To seek stakeholder consensus on phase 3 trials’ overall goals and structure, inclusion criteria, outcome measures, and trial delivery and understand where perspectives differ.
Methods:
An international expert panel comprising people with Parkinson’s (PwP), care partners (CP), clinical scientists, representatives from industry, funders and regulators participated in a survey-based Delphi study. Survey items were informed by a scoping review of DM trials and PwP input. Respondents scored item agreement over 3 rounds. Scores and reasoning were summarized by participant group each round until consensus, defined as≥70% of at least 3 participant groups falling within the same 3-point region of a 9-point Likert scale.
Results:
92/121 individuals from 13 countries (46/69 PwP, 13/18 CP, 20/20 clinical scientists, representatives from 8/8 companies, 4/5 funders, and 1/1 regulator) completed the study. Consensus was reached on 14/31 survey items: 5/8 overall goals and structure, 1/8 Eligibility criteria, 7/13 outcome measures, and 1/2 trial delivery items. Extent of stakeholder endorsement for 428 reasons for scores was collated across items.
Conclusions:
This is the first systematic multi-stakeholder consultation generating a unique repository of perspectives on pivotal aspects of DM trial design including those of PwP and CP. The panel endorsed outcomes that holistically measure PD and the importance of inclusive trials with hybrid delivery models. Areas of disagreement will inform mitigating strategies of researchers to ensure successful delivery of future trials.
Keywords: Parkinson’s disease, disease modification, clinical trial, Delphi survey
INTRODUCTION
The incidence and prevalence of Parkinson’s disease (PD) are increasing, adding significantly to the global burden of neurological disorders [1, 2] creating an urgent, unmet need to identify disease modifying therapies (DMTs). In 2021, DMT trials represented 41.5% of 142 active clinical trials in PD [3]. Historically, despite DMTs showing efficacy in phase 2, phase 3 trials have been negative [4–13].
DMT trials for PD pose unique challenges [14]: there are no accepted biomarkers of progression [15]; PD is heterogeneous with phenotypic and genetic subtypes exhibiting different rates of progression [16–18]; and effective symptomatic therapies potentially confound routinely used clinical outcome measures. These factors have contributed to diverse approaches to DMT clinical trial design with regard to participant selection, trial duration, and outcome measures [19–21].
Considering patient and care partner priorities is vital to the design and conduct of trials to ensure effective recruitment and retention, both being major factors in the success and cost of trials [22–24]. As many as 45% of trials do not reach their pre-specified recruitment target [25]. Involving people with Parkinson’s (PwP) and their care partners in the design of trials is critical to ensure trials answer patient relevant questions and measure meaningful outcomes in ways that are acceptable to participants. Thus, the interaction of researchers conducting clinical trials with potential trial participants (including those with no previous trial experience) as well as the care-partners that support their participation in trials is vitally important.
This Delphi study facilitated international multistakeholder interaction with a view to seeking consensus on the design of PD trials that test protective treatments aiming to slow, halt, or reverse the progression of PD in a phase-3 setting. Acknowledging that DMT trials can be carried out in many different ways, we aimed to determine aspects of overall goals, eligibility criteria, outcome measures, and trial delivery, importantly including the voice of PwP and their care partners. We present a Delphi methodology that allowed the collection and unbiased presentation of stakeholder reasoning to support informed choice.
MATERIALS AND METHODS
Survey development
A Delphi questionnaire was developed based on a rapid scoping review of DMT trials in PD and PwP input. The survey was piloted on PwP and care partners and iteratively adjusted to ensure questions were suitable for lay participants. Further adjustment to question text was made after the first Delphi round where comments indicated misinterpretation by participants.
The questionnaire contained 31 items covering four domains: trial goals and structure; inclusion criteria; outcome measures; and delivery.
Question text and information displayed to participants as well as the questionnaire piloting process can be found in Supplementary Material 1.
Ethics
The study protocol was approved by the University of Plymouth Faculty of Health Research Ethics and Integrity Committee (Ref. 19/20-1307).
Delphi Panel composition and recruitment
We aimed to recruit 100 international expert panelists representing stakeholders involved in PD DMT clinical trials with the following recruitment targets and eligibility criteria:
-
•20 Clinical scientists
-
∘First or last author or named chief investigator on a publication/registry entry of a trial for a DMT in PD; or a clinical scientist identified as having extensive knowledge in DMT trials.
-
∘
-
•10 Pharmaceutical industry representatives (1 per organization)
-
∘From companies with a pipeline of DMTs for PD.
-
∘
-
•5 Funding agency representatives (charities) (1 per organization)
-
∘From an organization that actively supports clinical PD research.
-
∘
-
•40 PwP
-
∘Diagnosis of PD and the ability to give informed consent.
-
∘
-
•20 Care partners
-
∘Main carer of a PwP and the ability to give informed consent.
-
∘
-
•3 Regulator representatives (1 per organization)
-
∘Relevant knowledge of PD trials.
-
∘
PwP and their care partners were recruited via UK and international Parkinson’s charities, Facebook
Parkinson’s research interest groups and care partner forums. PwPs were purposively selected to create an international panel equally balanced for clinical trial experience. We ensured that trial experienced and trial naïve subgroups included equal proportions of age (40–50, 50–60, 70+), gender and disease duration (<5 years,>5 years). We aimed to recruit between 40 and 80 PwP to ensure appropriate representation of purposive sampling categories.
Clinical trial registry entries and publication databases were systematically searched for PD DMT trials to identify professional panelists that met eligibility criteria. Additional professional panelists were identified through targeted internet searching, including membership of professional bodies and consortia.
Delphi study
Figure 1 summarizes the Delphi study which consisted of four online surveys built and disseminated through the Jisc online surveys platform.
Fig. 1.
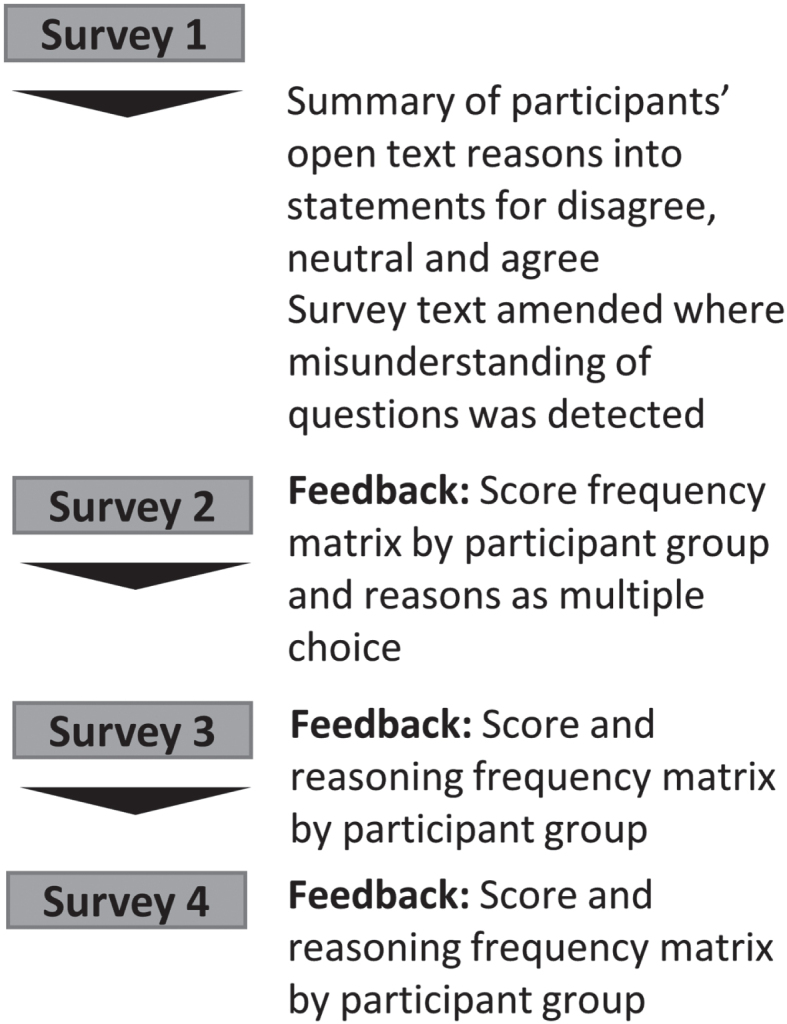
Study overview. A summary of survey edits and type of feedback supplied to participant within each survey.
Non-respondents were sent weekly completion reminders and received a text message or call once before survey closure.
Participants were asked to rate agreement with statements on a 9-point Likert scale from 1 (strongly disagree) to 9 (strongly agree) or indicate that they did not know how to answer. Participants were encouraged to share reasoning for their choices (survey 1 only), suggest further survey topics (survey 1 only) and overall feedback.
For each survey statement, participants were provided with the percentage of votes per participant group falling into the categories 1–3 (disagree), 4–6 (neutral), and 7–9 (agree) (See example in Supplementary Material 1) from preceding surveys as well as their own previous score. Feedback on participants’ reasoning was prepared and presented as follows: two reviewers (MLZ and CBC) independently summarized participant reasons into summary statements. Conceptual overlap of statements was assessed and a maximum of 20 statements/question categorized into agree, neutral and disagree. From survey 2, participants were encouraged to select up to five statements most reflecting reasons for their rating or to suggest additional reasons. This was optional to reduce participant burden.
From survey 3 onwards, participant feedback included percentage of votes per participant group for summary statements (Supplementary Material 1).
A fourth survey was conducted for items where the question text or information was changed resulting from survey 1 feedback. Thus, each finalized item underwent a maximum of 3 rounds or until consensus was reached.
Consensus definition
Consensus for an item was reached when≥70% of panelists’ ratings of at least 3 groups fell within the same 3-point region (that is 1–3, 4–6, or 7–9) [26, 27].
Analysis
IBM SPSS statistics 25 software was used for all statistical analyses. Where there were three or less participants in a group, percentages and reasoning were not provided in the feedback to participants to protect participant anonymity as well as ensuring individual views did not disproportionately affect other participants’ votes. This was clearly communicated to participants.
Data sharing
Qualified external researchers can request access to anonymized participant-level survey responses, respecting patient informed consent, from the corresponding author on reasonable request, and on execution of an appropriate data sharing agreement.
RESULTS
Delphi participants
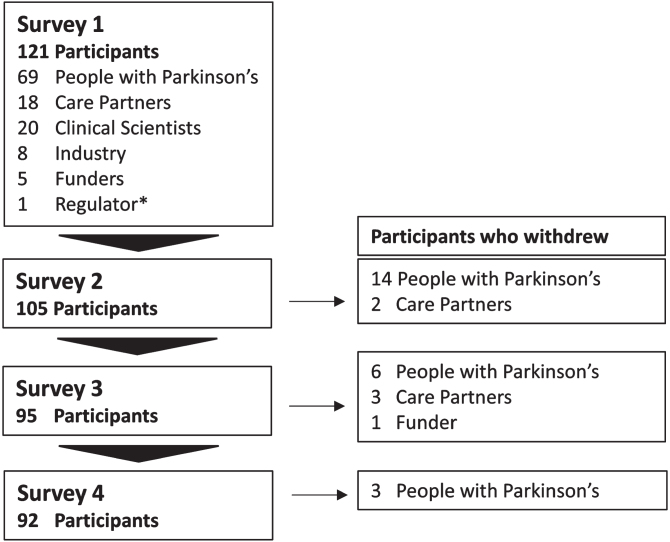
121 participants from 13 different countries participated in survey 1 including 69 PwP, 18 care partners, 20 clinical scientists, a representative from 8 pharmaceutical companies, 5 funders, and 1 regulatory agency (Fig. 2). Industry representatives were in diverse roles within their organizations with a median of 7.5 years (5.5 IQR) of experience in DMT trials for PD. Their job titles included: Chief Scientific Officer, Clinical Programme Director, Vice President, Medical Advisor, Global Medical Affairs Manager, Lead Medical Specialist, and Pharmaceutical Physician. Details of participant demographics are shown in Table 1.
Fig. 2.
Study participation. The number of participants and those who withdrew by participant group over 4 surveys.
Table 1.
Participant characteristics
| People with Parkinson’s | Care partner | Clinical Scientists | Industry | Funders | ||||
| Survey 1 | Survey 4 | Survey 1 | Survey 4 | Survey 1 | Survey 1 | Survey 1 | Survey 4 | |
| (n = 69) | (n = 46) | (n = 18) | (n = 13) | (n = 20) | (n = 8) | (n = 5) | (n = 4) | |
| Median (IQR) or N (% per participant group) | ||||||||
| Age | 65 (13) | 63 (11) | 68 (6.5) | 68 (5) | 50 (14.25) | 43 (18.25) | 54 (8) | 56 (7.25) |
| ≤5 y disease duration | 37 (53.6%) | 27 (58.7%) | – | – | – | – | – | – |
| Male | 45 (65.2%) | 32 (69.6%) | 2 (11.1%) | 1 (7.7%) | 15 (75%) | 6 (75%) | 2 (40%) | 2 (50%) |
| Female | 24 (34.8%) | 14 (30.4%) | 16 (88.9%) | 12 (92.3%) | 5 (25%) | 2 (25%) | 3 (60%) | 2 (50%) |
| Have clinical trial experience | 32 (46.4%) | 24 (52.2%) | 8 (44.4%) | 6 (46.1%) | – | – | – | – |
| Years of experience with DMT trials | – | – | – | – | 8.5 (12) | 7.5 (5.5) | – | – |
| Non-UK | 21 (30.4%) | 12 (26.1%) | 2 (11.1%) | 2 (15.4%) | 8 (40%) | 5 (62.5%) | 3 (60%) | 2 (50%) |
| Ethnicity not declared | 3 (4.3%) | 0 | 0 | 0 | – | – | – | – |
| White | 64 (92.7%) | 45 (97.8%) | 18 (100%) | 13 (100%) | – | – | – | – |
| Help provided by care partner | ||||||||
| None | – | – | 2 (11.1%) | 2 (15.4%) | – | – | – | – |
| Little | – | – | 12 (66.7%) | 7 (53.8%) | – | – | – | – |
| Lots | – | – | 3 (16.7%) | 3 (23.1%) | – | – | – | – |
| Constant | – | – | 1 (5.5%) | 1 (7.7%) | – | – | – | – |
| Highest level of experience | ||||||||
| Principal Investigator | – | – | – | – | 8 (40%) | – | – | – |
| Chief Investigator | – | – | – | – | 8 (40%) | – | – | – |
| First or last author | – | – | – | – | 1 (5%) | – | – | – |
| Other | – | – | – | – | 3 (15%) | – | – | – |
The regulatory representative participated in an advisory capacity only: scores did not contribute towards consensus and are not included in this publication. Participants were informed throughout that regulator scores did not represent the official views, rather the personal opinion and experience of the contributor.
Analysis of withdrawals and attrition bias
Thirty participants withdrew over the course of the study, including 23 PwP, 5 care partners, and 1 funder (Fig. 2). Unsolicited reasons for withdrawal included the repetitive nature and difficulty of the questionnaire, time taken to complete the survey, bereavement and a worsening of participants’ PD over the course of the study.
There were no major differences in participant demographics between the first and last survey for PwP or care partners (Table 1). Understanding of questions may have contributed to attrition and there was no statistical evidence of attrition bias (Supplementary Material 2) [26, 28].
Feedback engagement
Every question across all participants was counted as an opportunity to leave feedback, creating 9682 opportunities throughout the study. Feedback was provided on 93.7% of occasions.
Delphi Consensus
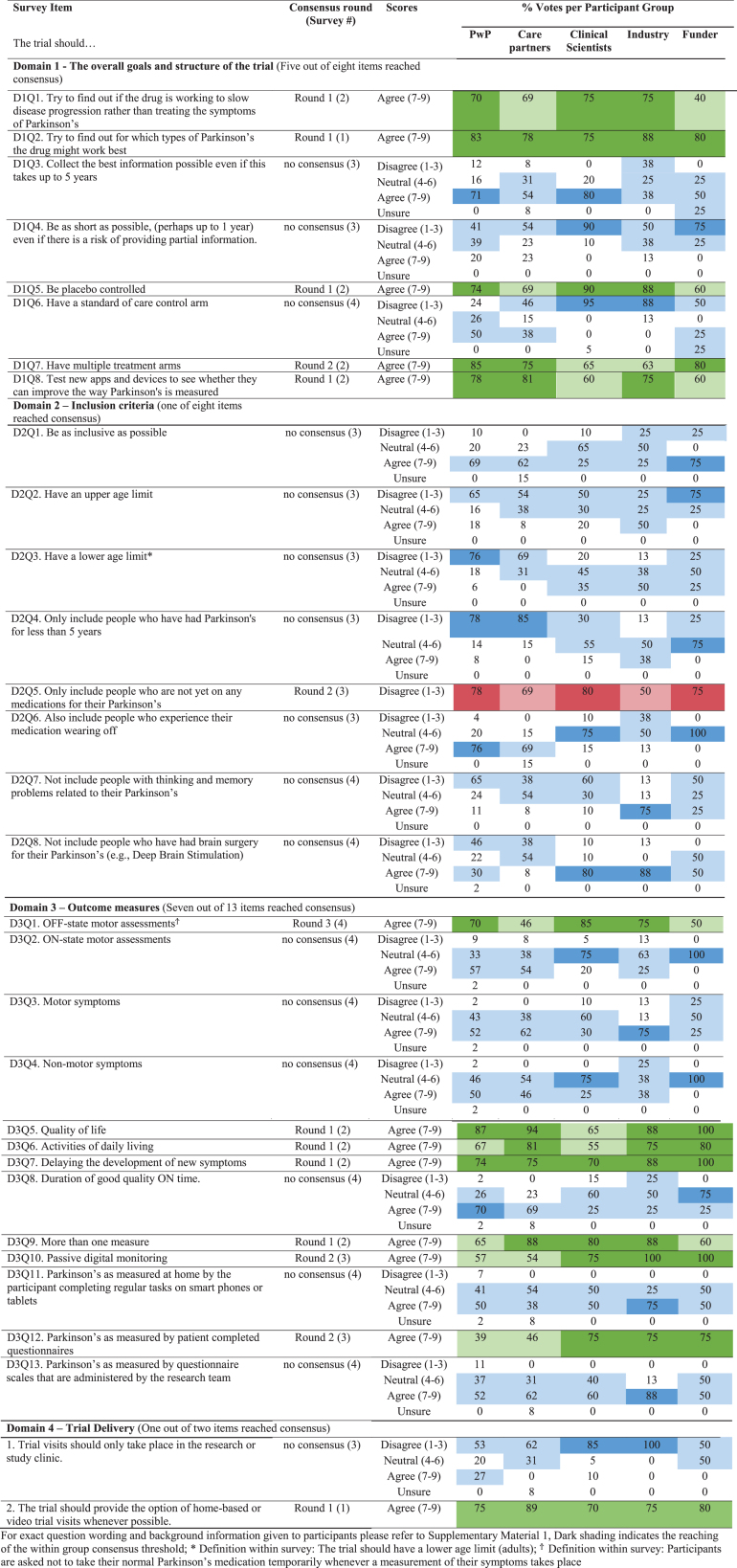
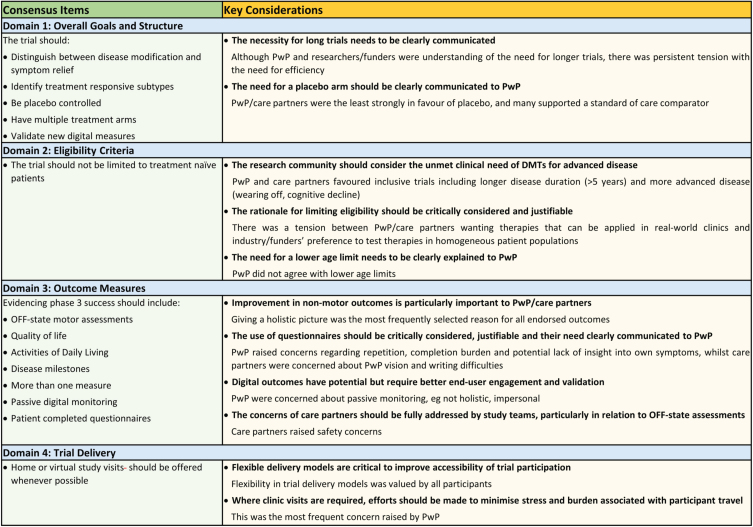
For each domain (trial goals and structure, eligibility criteria, outcome measures, trial delivery) details of score distributions across participant groups are given in Table 2 and details of reasoning for scores can be found in Supplementary Material 3. In total, 14 out of 31 (45%) items reached consensus (1 in survey 1, 8 in survey 2, 3 in survey 3, and 1 in survey 4) with core recommendations highlighted in Fig. 3. A total of 428 reasons for scores with extent of stakeholder endorsement was collated across items. These can be found in Supplementary Material 3.
Table 2.
Summary of item scores across 4 survey domains
Fig. 3.
Consensus Items and Key Considerations by survey domain.
Overall goals of the trial (Domain 1)
Participants reached consensus that a trial aiming to investigate DMTs should try to identify treatment responsive subtypes, gather evidence that supports the drug working to slow disease progression, be placebo controlled and have multiple treatment arms, as well as being used to validate new digital outcome measures (Fig. 3).
There was no consensus on the two questions pertaining to trial length (Table 2, Domain 1, D1Q3, whether trials should collect the best information possible even if this takes up to five years; D1Q4, whether trials should be as short as possible even if there is a risk of providing partial information).
Items where consensus was reached but within-group consensus within at least one participant group lay below 60% are described below:
In contrast to the remaining panel only 40% of funders agreed that trials should be aiming to gather evidence that supports the drug working to slow disease progression.
Inclusion criteria (Domain 2)
The Delphi panel reached consensus on one out of 8 inclusion criteria disagreeing with the statement that the trial should only include patients who do not yet require PD medication (Fig. 3) with 78% of PwP, 80% of clinical scientists and 75% of funders disagreeing with a restriction of trials to drug naïve PwP.
No consensus was reached on whether trials should be as inclusive as possible, have an upper age limit, have a lower age limit, only include those with a disease duration of less than 5 years, include those experiencing wearing off, not include those with cognitive impairment, not include those who have had brain surgery for their PD (Table 2, Domain 2).
Although the panel did not reach consensus on any other inclusion criteria, the majority of PwP were in favor of inclusivity on all items with the exception of eligibility restrictions based on participants having undergone brain surgery for their PD (Table 2, Domain 2).
Items where consensus was reached but within-group consensus within at least one participant group lay below 60% are described below:
In contrast to the rest of the panel, only 50% of industry respondents disagreed with the statement that the trial should only include those who are not yet on any medications for their PD.
Outcome measures (Domain 3)
Consensus was achieved on the importance of considering seven of 13 items proposed within the survey for phase 3 outcome assessment: OFF-state motor assessments, quality of life, activities of daily living, delaying the development of new symptoms, passive digital measures, patient completed questionnaires, and utilizing more than one measure (Fig. 3).
No consensus was achieved regarding ON-state motor assessments, motor symptoms, non-motor symptoms, duration of good quality ON time, digital measures requiring completion of regular tasks or questionnaire scales administered by the research team (Table 2, Domain 3).
Items where consensus was reached but within-group consensus within at least one participant group lay below 60% are described below:
care partners (46%) and funders (50%) were supportive OFF-state motor assessments (Table 2, D3Q1); clinical scientists were least supportive of activities of daily living (ADL) measures (55%) (Table 2, D3Q6); passive digital monitoring was highly supported by professional participant groups (75%, 100%, 100% of clinical scientist, industry and funder respondents respectively), while PwP and care partners were less supportive (57% and 54% of votes respectively) (Table 2 D3Q10,); Patient completed questionnaires were viewed as important measures by 75% of respondents within all professional participant groups whilst PwP and care partners were less supportive with 39% and 46% rating within the agreement region of the scale respectively (Table 2, D3Q12).
Passive digital monitoring was highly supported by professional participant groups, with PwP and care partners being less supportive (Table 2).
Patient completed questionnaires were viewed as important measures by 75% of professional participants. PwP and care partners were less supportive with 39% and 46% rating within the agreement region of the scale respectively (Table 2).
Trial Delivery (Domain 4)
More than 70% of all participant groups agreed that the trial should provide the option of home based or video trial visits whenever possible (Table 2, D4Q2).
No consensus was reached on trial visits taking place only within the research or study clinic (Table 2, D4Q1).
DISCUSSION
This international Delphi study engaged multiple stakeholders with an interest in DMT trials for PD, capturing stakeholder perspectives, facilitating exchange of viewpoints, and reaching consensus on 14 out of 31 items covering trial goals and structure, eligibility criteria, outcome measures and trial delivery. A succinct but comprehensive summary of arguments for and against aspects of trial design was generated through the process, highlighting synergistic as well as contrasting views of stakeholders (Supplementary Material 3).
An important aspect of this study was the inclusion of both professional and lay participant groups, in particular both trial naïve and experienced PwP and care partners. Trial naïve PwP are an important cohort for future participation in disease modification studies [14, 29] thus their inclusion in discussions pertaining to the conduct of trials is essential, especially considering that many trials fail due to an inability to meet recruitment targets [30].
Non-numerical feedback was a key feature of this study: it enabled lay participants to gain an understanding of advantages and disadvantages of survey items, allowing meaningful participation, and highlighted areas of contention between participant groups. Although this increased participant burden, adherence with provision of feedback across the survey was high (94%) and non-adherence was not associated with attrition.
In contrast to conventional Delphi methodology [26, 31], the anonymous online survey format, consensus definition and feedback representation by participant group, granted each group equal weight, prevented individuals from disproportionately influencing the panel and protected against bias through unequal group attrition [32].
The distribution of items reaching consensus across survey rounds (1 in survey 1, 8 in survey 2, 3 in survey 3, and 1 in survey 4) highlights 3 surveys as optimum number of Delphi rounds.
There were several limitations to the study. As lay panel members, PwP and care partners may not have fully understood all implications of their preferences despite being able to review reasoning and scores provided by other participant groups. Thus, the outcomes of this Delphi process may support researchers in considering PwP and care partner as well as other stakeholders’ preferences sensitively and serve as a starting point to explore these within the context of their own trial as part of their patient engagement activities to support trial design decisions and communications strategies.
Efforts were made to balance PwP for age, disease duration range and trial experience. Nevertheless, the nature of the study precluded the recruitment of a sample representative of the PwP population. English language requirements and online recruitment and conduct meant that the sample was younger, digitally literate, and not ethnically diverse, and therefore further studies will be needed to explore the translatability of findings to more diverse PwP and care partner groups. Our selection methodology resulted in a median age of 65 (13 IQR) which although younger than incident PD population [33], was slightly older than the average participant age in DMT trials (62 years) [21]. Finally, no information was collected on PwP/Care partners’ experience with medical devices or digital outcomes for PD which could have aided in the interpretation of PwP/care partners’ views.
There was higher attrition from lay participants than professional groups and some participant group sizes were small, although representation of collected demographics remained similar between rounds and no attrition bias was identified.
We included a question on the necessity of distinguishing DMT versus symptomatic effects in DMT trials although this was implicit in the setup of the study. The question was included because this distinction remains a methodological challenge in DMT trials, and there is increasing discussion over its need. We were interested to understand the perspectives of the different stakeholder groups regarding the importance of making this distinction. Some survey participants felt that the answers to questions covered within the study would depend on the DM agent being trialed or the aim of the study. Although the weight and prioritization of factors influencing trial design decisions will be determined by the therapy being tested and its stage of development, future researchers will be able to draw on this panel’s perspectives and develop strategies to mitigate general concerns raised by stakeholders. The propensity of neutral views is clearly represented and frequency of reasons reflecting a neutral attitude by the panel were captured and displayed for the reader (Supplementary Material 3).
In terms of overall DMT trial aims and design, the panel favored designs that maximize efficiency and learnings such as having multiple treatment arms, trying to identify treatment responsive subtypes, validating new digital outcome measures, evidencing disease modification, and being placebo controlled. No consensus was reached on trial length. Interestingly, all stakeholders understood the necessity for long term trials to measure real, long-term impacts of DMTs on patients and ensure potential benefits are not missed, although only clinical scientists and PwP voted in favor of long trials (Supplementary Material 3, D1Q3). This demonstrates that PwP understand the complexity of their disease warranting the need for longer trials and echoes regulatory guidance which recommends DMT trial durations of between 2–5 years [34]. This highlights the need for stakeholder engagement with funders, industry, and care-partners to address practical challenges of supporting long term trials.
The eligibility section of the survey was the most contentious yielding least consensus. Overall, industry participants favored less inclusive trials reflecting a need to reduce the potential impact of disease heterogeneity on trial findings.
The only point on which the panel agreed concerning eligibility was that the trial should not be restricted to drug naïve patients. This contrasts common practice of restricting recruitment to early, untreated PwP in DMT trials, which is argued to reduce the confounding effect of symptomatic therapy and increase the window for meaningful intervention in pathological pathways [14, 29].
PwP were in favor of inclusivity for almost all eligibility aspects presented in the study, including the inclusion of participants with more advanced disease highlighting an unmet need to address disease modification in this population. Narrow inclusion criteria make positive results less generalizable and are not representative of real world situations which can raise important safety and efficacy concerns [35], especially since participant demographics for PD trials often misalign with the final user population [30]. In addition, variability in PD progression is poorly understood with proposed subtypes often yielding unreproducible results [36, 37] and it is therefore questionable whether attempts at cohort homogenization through eligibility criteria is sufficiently justified especially in a phase 3 setting. The importance of ensuring trial participants are representative of the intended target population is increasingly recognized, has led to national efforts such as the INCLUDE project in the UK and is embedded within regulatory guidance [35, 38]. The FDA specifically urges the critical review of common eligibility criteria to ensure a strong scientific or clinical rationale [39]. Thus, eligibility criteria need to be aligned with the trial aim, with advantages and disadvantages carefully weighed by investigators and decision making clearly communicated with stakeholders, especially the patient community.
With the exception of passive digital measures and OFF-state assessments, the Delphi panel favored outcome measures that give a holistic, clinically relevant picture of PD progression such as quality of life, activities of daily living, delaying the development of new symptoms, showing an effect on more than one measure, and patient completed questionnaire scales. This is appropriate for phase 3 trial outcomes where regulatory guidance for evidencing disease-modification claims requires collected evidence to reflect meaningful and persistent changes in clinical function [34].
However, there are clear challenges as PD is a slowly progressing condition with a heterogeneous disease course and fluctuating symptom severity that can mask small signals as well as the detection of persistent changes. Current initiatives are exploring our understanding of PD experience to develop novel clinical outcome assessments, including milestone-based approaches [40–43].
A patient centric approach is crucial to reduce the burden of study participation, to maximize data quality and completeness. As well as being less supportive of patient completed questionnaires than professional participant groups, patients and care partners both expressed concern regarding the potential of patient and researcher completed questionnaires to increase the physical, emotional and cognitive strain on participants (Supplementary Material 3, D3Q12/D3Q13).
OFF-state motor assessments were also endorsed by the panel as a phase 3 outcome, albeit not until the fourth survey, with both funders and care partners taking a more neutral stance. Care partners in particular raised safety concerns (Supplementary Material 3, D3Q1). A recent qualitative sub-study of the PD-STAT trial, a multi-center trial with 235 participants at baseline, also highlighted OFF assessments as one of the most prominent challenges reported by both patients and care partners and accounted for 37% of 51 withdrawals from the study [44]. From a regulatory standpoint, an OFF-state motor examination is not an adequate primary outcome for phase 3 trials on its own [34].
Digital outcomes are becoming increasingly important, with the COVID-19 pandemic accelerating their development [45–47]. This Delphi study facilitated the discussion of multiple aspects of digital outcomes and remote methods of trial delivery (D1Q8, D3Q10, D3Q11). Digital measures were viewed to be an important area to develop, having the potential to improve the way PD is measured by capturing more continuous, objective data, reduce trial cost and be easier, time saving solutions for participants (Supplementary Material 3, D3Q10, D3Q11). As with clinical outcome measures, digital outcome development is a vibrant, fast changing field with an increasing need to close the gap between digital innovation and validation of measures to clinical trial standards [46, 48]. Initiatives such as WATCH-PD as well as incorporation of digital measures as exploratory endpoints in trials are important to drive digital outcome development forward [49]. Whilst passive digital measures were endorsed by the panel, no consensus was reached on active measures. Furthermore, patients and care partners in particular held a more neutral position towards digital outcomes and despite the majority voting for positive statements, a wide range of limitations/concerns regarding digital measures were raised with the most frequent being concerns around diminishing emotional support through the trial and the inability of passive measures to capture all aspects of PD. Notably, although not receiving significant traction within the panel, concerns around active measures were centered around user ability, participant burden, compliance and retention while concerns raised by the panel around passive measures focused around data integrity and privacy (Supplementary Material 3, D3Q10, D3Q11). Thus, in addition to careful validation, development and use of digital outcomes requires engagement with user groups ensuring user-friendly, engaging design with appropriate consideration of the provision of participant support.
There was unanimous agreement of the Delphi panel that trial delivery should have a homebased or video trial visit component whenever possible, which would help support geographical inclusivity and retention. Stress and anxiety around travel were the main considerations for PwP rather than the burden of clinic visits themselves (Supplementary Material 3, D4Q1). Travel burden, particularly in the OFF state, has been identified as a significant barrier to participation [44]. Frequency of remote versus in person assessments was not considered within this study and this could have significantly influenced the panel’s decision. Research accessibility and flexibility tailored to participants’ needs is critically important for retention.
Conclusion
This Delphi study has generated a unique repository of stakeholder perspectives regarding pivotal aspects of DMT trial design in PD, importantly including those of patients and care partners. It provides an understanding of where consensus exists and a basis for further exploration of views where it does not. Despite patient and care partner opinion often not being pivotal in trial design decisions, due to the need for considering rigor and practicalities, reservations need to be addressed with a shared understanding critical to build trust and for patient-centered trial design and delivery.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all participants for their time and effort in completing the survey. We are especially grateful to Helen Matthews for her enthusiasm and support throughout.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230109.
FUNDING
The study was funded by Cure Parkinson’s (Grant Reference CC02).
CONFLICT OF INTEREST
Prof. Camille B. Carroll is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
None of the other authors have any conflict of interest to report.
DATA AVAILABILITY
The raw data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- [1]. Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic.} }, . J Parkinsons Dis 8, s3–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, Bassetti CL, Vos T, Feigin VL (2020) The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 5, e551–e567. [DOI] [PubMed] [Google Scholar]
- [3]. McFarthing K, Rafaloff G, Baptista MAS, Wyse RK, Stott SRW (2021) Parkinson’s disease drug therapies in the clinical trial pipeline: 2021 update. J Parkinsons Dis 11, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Zeissler M-L, Li V, Parmar MKB, Carroll CB (2020) is it possible to conduct a multi-arm multi-stage platform trial in Parkinson’s disease: Lessons learned from other neurodegenerative disorders and cancer. J Parkinsons Dis 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Parkinson Study Group SURE-PD Investigators; Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K (2014) Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: A randomized clinical trial. Jama Neurol 71, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Schwarzschild MA, Ascherio A, Casaceli C, Curhan GC, Fitzgerald R, Kamp C, Lungu C, Macklin EA, Marek K, Mozaffarian D, Oakes D, Rudolph A, Shoulson I, Videnovic A, Scott B, Gauger L, Aldred J, Bixby M, Ciccarello J, Gunzler SA, Henchcliffe C, Brodsky M, Keith K, Hauser RA, Goetz C, LeDoux MS, Hinson V, Kumar R, Espay AJ, Jimenez-Shahed J, Hunter C, Christine C, Daley A, Leehey M, de Marcaida JA, Friedman JH, Hung A, Bwala G, Litvan I, Simon DK, Simuni T, Poon C, Schiess MC, Chou K, Park A, Bhatti D, Peterson C, Criswell SR, Rosenthal L, Durphy J, Shill HA, Mehta SH, Ahmed A, Deik AF, Fang JY, Stover N, Zhang L, Dewey RB Jr., Gerald A, Boyd JT, Houston E, Suski V, Mosovsky S, Cloud L, Shah BB, Saint-Hilaire M, James R, Zauber SE, Reich S, Shprecher D, Pahwa R, Langhammer A, LaFaver K, LeWitt PA, Kaminski P, Goudreau J, Russell D, Houghton DJ, Laroche A, Thomas K, McGraw M, Mari Z, Serrano C, Blindauer K, Rabin M, Kurlan R, Morgan JC, Soileau M, Ainslie M, Bodis-Wollner I, Schneider RB, Waters C, Ratel AS, Beck CA, Bolger P, Callahan KF, Crotty GF, Klements D, Kostrzebski M, McMahon GM, Pothier L, Waikar SS, Lang A, Mestre T (2021) Effect of urate-elevating inosine on early Parkinson disease progression: The SURE-PD3 Randomized Clinical Trial. JAMA 326, 926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Schapira AH, McDermott MP, Barone P, Comella CL, Albrecht S, Hsu HH, Massey DH, Mizuno Y, Poewe W, Rascol O, Marek K (2013) Pramipexole in patients with early Parkinson’s disease (PROUD): A randomised delayed-start trial. Lancet Neurol 12, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Olanow CW, Hauser RA, Jankovic J, Langston W, Lang A, Poewe W, Tolosa E, Stocchi F, Melamed E, Eyal E, Rascol O (2008) A randomized, double-blind, placebo-controlled, delayed start study to assess rasagiline as a disease modifying therapy in Parkinson’s disease (the ADAGIO study): Rationale, design, and baseline characteristics. Mov Disord 23, 2194–2201. [DOI] [PubMed] [Google Scholar]
- [9]. Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators; Kieburtz K Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Pérez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM (2015) Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 313, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. NINDS NET-PD Investigators (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66, 664–671. [DOI] [PubMed] [Google Scholar]
- [11]. Parkinson Study Group STEADY-PD III Investigators (2020) Isradipine versus placebo in early Parkinson disease: A randomized trial. Ann Intern Med 172, 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Parkinson Study Group (2013) Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord 28, 1823–1831. [DOI] [PubMed] [Google Scholar]
- [13]. Verschuur CV, Suwijn SR, Post B, Dijkgraaf M, Bloem BR, van Hilten JJ, van Laar T, Tissingh G, Deuschl G, Lang AE, de Haan RJ, de Bie RM (2015) Protocol of a randomised delayed-start double-blind placebo-controlled multi-centre trial for Levodopa in EArly Parkinson’s disease: The LEAP-study. BMC Neurol 15, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Athauda D, Foltynie T (2016) Challenges in detecting disease modification in Parkinson’s disease clinical trials. Parkinsonism Relat Disord 32, 1–11. [DOI] [PubMed] [Google Scholar]
- [15]. Li T, Le W (2020) Biomarkers for Parkinson’s disease: How good are they? . Neurosci Bulletin 36, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Lawton M, Baig F, Rolinski M, Ruffman C, Nithi K, May MT, Ben-Shlomo Y, Hu MT (2015) Parkinson’s disease subtypes in the Oxford Parkinson Disease Centre (OPDC) Discovery Cohort. J Parkinsons Dis 5, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 140, 1959–1976. [DOI] [PubMed] [Google Scholar]
- [18]. Latourelle JC, Beste MT, Hadzi TC, Miller RE, Oppenheim JN, Valko MP, Wuest DM, Church BW, Khalil IG, Hayete B, Venuto CS (2017) Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson’s disease: A longitudinal cohort study and validation. Lancet Neurol 16, 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Hart RG, Pearce LA, Ravina BM, Yaltho TC, Marler JR (2009) Neuroprotection trials in Parkinson’s disease: Systematic review. Mov Disord 24, 647–654. [DOI] [PubMed] [Google Scholar]
- [20]. Lang AE, Melamed E, Poewe W, Rascol O (2013) Trial designs used to study neuroprotective therapy in Parkinson’s disease. Mov Disord 28, 86–95. [DOI] [PubMed] [Google Scholar]
- [21]. McGhee DJM, Royle PL, Thompson PA, Wright DE, Zajicek JP, Counsell CE (2013) A systematic review of biomarkers for disease progression in Parkinson’s disease. BMC Neurol 13, 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Moore TJ, Heyward J, Anderson G, Alexander GC (2020) Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015–2017: A cross-sectional study. BMJ Open 10, e038863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, Knox C, Nadin B, Rothwell J, Surtees M, Julious SA (2017) Recruitment and retention of participants in randomised controlled trials: A review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 7, e015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, Elbourne DR, Francis D, Garcia J, Roberts I, Snowdon C (2006) What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Sully BGO, Julious SA, Nicholl J (2013) A reinvestigation of recruitment to randomised, controlled, multicenter trials: A review of trials funded by two UK funding agencies. Trials 14, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Whitham D, Turzanski J, Bradshaw L, Clarke M, Culliford L, Duley L, Shaw L, Skea Z, Treweek SP, Walker K, Williamson PR, Montgomery AA; Site Performance Metrics for Multicentre Randomised Trials Collaboration (2018) Development of a standardised set of metrics for monitoring site performance in multicentre randomised trials: A Delphi study. Trials 19, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Valentijn PP, Vrijhoef HJM, Ruwaard D, Boesveld I, Arends RY, Bruijnzeels MA (2015) Towards an international taxonomy of integrated primary care: A Delphi consensus approach. BMC Family Pract 16, 64–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, Clarke M, Gargon E, Gorst S, Harman N, Kirkham JJ, McNair A, Prinsen CAC, Schmitt J, Terwee CB, Young B (2017) The COMET Handbook: Version 1.0. Trials 18, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Devos D, Hirsch E, Wyse R (2021) Seven solutions for neuroprotection in Parkinson’s disease. Mov Disord 36, 306–316. [DOI] [PubMed] [Google Scholar]
- [30]. Vaswani PA, Tropea TF, Dahodwala N (2020) Overcoming barriers to Parkinson disease trial participation: Increasing diversity and novel designs for recruitment and retention. Neurotherapeutics 17, 1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Keeney S, Hasson F, McKenna H(2011) The Delphi Technique in Nursing and Health. In The Delphi Technique in Nursing and Health Research, pp. i-x.
- [32]. Hsu C-C, Sandford BA (2007) The Delphi Technique: Making sense of consensus. Pract Assess Res Eval 12, 10. [Google Scholar]
- [33]. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T (2016) The incidence of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 46, 292–300. [DOI] [PubMed] [Google Scholar]
- [34]. European Medicines Agency, Guideline on clinical investigation of medicinal products in the treatment of Parkinson’s disease (Revision 2),https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-parkinsons-disease-scientific-guidelinelast updated July 6, 2012, Accessed on April 24, 2023.
- [35]. Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, Knee J, Maier RH, Mountain GA, Nestor G, Oliva L, Prowse SR, Tortice A, Wason J, Rochester L, INCLUDE writing group (2020) Developing a roadmap to improve trial delivery for under-served groups: Results from a UK multi-stakeholder process. Trials 21, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K (2016) How stable are Parkinson’s disease subtypes in de novo patients: Analysis of the PPMI cohort? . Parkinsonism Relat Disord 28, 62–67. [DOI] [PubMed] [Google Scholar]
- [37]. Mestre TA, Eberly S, Tanner C, Grimes D, Lang AE, Oakes D, Marras C (2018) Reproducibility of data-driven Parkinson’s disease subtypes for clinical research. Parkinsonism Relat Disord 56, 102–106. [DOI] [PubMed] [Google Scholar]
- [38]. Duggal M, Sacks L, Vasisht KP (2021) Eligibility criteria and clinical trials: An FDA perspective. Contemp Clin Trials 109, 106515. [DOI] [PubMed] [Google Scholar]
- [39]. American Food and Drug Administration –(CDER) USDoHaHSFaDACfDEaR, Enhancing the Diversity of Clinical Trial Populations –Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry,https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial, Last updated November, 2020, Accessed on April 24, 2023.
- [40]. Morel T, Cleanthous S, Andrejack J, Barker RA, Blavat G, Brooks W, Burns P, Cano S, Gallagher C, Gosden L, Siu C, Slagle AF, Trenam K, Boroojerdi B, Ratcliffe N, Schroeder K (2022) Patient experience in early-stage Parkinson’s disease: Using a mixed methods analysis to identify which concepts are cardinal for clinical trial outcome assessment. Neurol Ther 11, 1319–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Staunton H, Kelly K, Newton L, Leddin M, Rodriguez-Esteban R, Chaudhuri KR, Weintraub D, Postuma RB, Martinez-Martin P (2022) A patient-centered conceptual model of symptoms and their impact in early Parkinson’s disease: A qualitative study. J Parkinsons Dis 12, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Tosin MHS, Simuni T, Stebbins GT, Cedarbaum JM (2022) Tracking emergence of new motor and non-motor symptoms using the MDS-UPDRS: A novel outcome measure for early Parkinson’s disease? . J Parkinsons Dis 12, 1345–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Marsili L, Mahajan A (2022) Clinical milestones in Parkinson’s disease: Past, present, and future. J Neurol Sci 432, 120082. [DOI] [PubMed] [Google Scholar]
- [44]. Kehagia AA, North TK, Grose J, Jeffery AN, Cocking L, Chapman R, Carroll C (2022) Enhancing trial delivery in Parkinson’s disease: Qualitative insights from PD STAT. J Parkinsons Dis 12, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Valdovinos BY, Modica JS, Schneider RB (2022) Moving forward from the COVID-19 pandemic: Needed changes in movement disorders care and research. Curr Neurol Neurosci Rep 22, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Stephenson D, Badawy R, Mathur S, Tome M, Rochester L (2021) Digital progression biomarkers as novel endpoints in clinical trials: A multistakeholder perspective. J Parkinsons Dis 11, S103–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Masoli JAH, Down K, Nestor G, Hudson S, O’Brien JT, Williamson JD, Young CA, Carroll C (2021) A report from the NIHR UK working group on remote trial delivery for the COVID-19 pandemic and beyond. Trials 22, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Stephenson D, Alexander R, Aggarwal V, Badawy R, Bain L, Bhatnagar R, Bloem BR, Boroojerdi B, Burton J, Cedarbaum JM, Cosman J, Dexter DT, Dockendorf M, Dorsey ER, Dowling AV, Evers LJW, Fisher K, Frasier M, Garcia-Gancedo L, Goldsack JC, Hill D, Hitchcock J, Hu MT, Lawton MP, Lee SJ, Lindemann M, Marek K, Mehrotra N, Meinders MJ, Minchik M, Oliva L, Romero K, Roussos G, Rubens R, Sadar S, Scheeren J, Sengoku E, Simuni T, Stebbins G, Taylor KI, Yang B, Zach N (2020) Precompetitive consensus building to facilitate the use of digital health technologies to support Parkinson disease drug development through regulatory science. Digit Biomark 4(Suppl 1), 28–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Adams JL, Kangarloo T, Tracey B, O’Donnell P, Volfson D, Latzman RD, Zach N, Alexander R, Bergethon P, Cosman J, Anderson D, Best A, Severson J, Kostrzebski MA, Auinger P, Wilmot P, Pohlson Y, Waddell E, Jensen-Roberts S, Gong Y, Kilambi KP, Herrero TR, Ray Dorsey E; Parkinson Study Group Watch-PD Study Investigators and Collaborators (2023) Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. NPJ Parkinsons Dis 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.