Abstract
Objectives
This study aimed to identify indicators of the risk of progression of preclinical hypertrophic cardiomyopathy (HCM).
Methods
This was a prospective cohort study following a population of cats with preclinical HCM. Cats serially underwent physical examination, blood pressure measurement, blood sampling and echocardiography. Development of congestive heart failure (CHF), arterial thromboembolism (ATE) or sudden death (SD) were considered cardiac-related events. Associations between factors recorded at baseline, and on revisit examinations, and the development of a cardiac-related event were explored using receiver operator characteristic (ROC) analysis.
Results
Forty-seven cats were recruited to the study and were followed for a median period of 1135 days. Fifteen cats (31.9%) experienced at least one cardiac-related event; six CHF, five ATE and five SD. One cat experienced a cardiac-related event per 10.3 years of patient follow-up. Cats with increased left atrial (LA) size and higher concentrations of N-terminal pro B-type natriuretic peptide (NTproBNP) at baseline were more likely to experience an event. Cats with a greater rate of enlargement of LA size between examinations were also more likely to experience an event.
Conclusions and relevance
Factors easily measured, either once or serially, in cats with preclinical HCM can help to identify those at greater risk of going on to develop clinical signs.
Keywords: Hypertrophic cardiomyopathy, HCM, preclinical, prognosis, biomarker
Introduction
Hypertrophic cardiomyopathy (HCM) is the most prevalent cardiac disease in cats. 1 It is estimated to affect as many as 16% of domestic cats.1–3 With the UK population of pet cats thought to number approximately 11.1 million 4 there could be over a million affected cats in the UK.
Although in some cats HCM is a progressive disease, many cats remain free from clinical signs for years.5,6 Some cats do develop serious clinical complications, including congestive heart failure, thromboembolism and sudden death.6–9 The challenge for veterinary surgeons is to distinguish those cats at greater risk of having progressive disease from those more likely to remain stable.
Information of prognostic value can be gained from signalment and clinical examination. Cats younger at the time of diagnosis have been shown to have a longer survival time.5,9 The presence of an arrhythmia and an audible gallop have been associated with a worse outcome 10 and a detectable arrhythmia has been associated with a greater risk of sudden death. 11
Echocardiography has consistently been shown to provide information of prognostic value in cats with HCM. Increased left atrial (LA) size has been associated with a shorter survival time and a higher likelihood of developing congestive heart failure, thromboembolism or experiencing sudden cardiac death.5,9,11–13 Greater left ventricular wall thickness has also been associated with a greater risk of death.8,10
More recently, two studies have demonstrated that measurement of cardiac biomarker concentrations may be of prognostic value.14,15 Both studies showed that higher concentrations of circulating troponin were associated with a worse outcome. The study by Borgeat et al 14 also demonstrated that an N-terminal pro B-type natriuretic peptide (NTproBNP) concentration of greater than 250 pmol/l was associated with an increased risk of cardiac death; however, this did not remain independently associated with a worse outcome when the presence of clinical signs and LA size were accounted for.
The majority of studies of feline HCM in the literature have been retrospective studies and have described populations of cats seen at referral centres by specialists. There is limited information about risk indicators in cats with preclinical HCM seen by non-specialists, outside the setting of a referral hospital. The aim of the current study was to prospectively follow a population of cats diagnosed with preclinical HCM, repeating clinical examination, systolic blood pressure measurement, blood tests (including cardiac biomarkers) and echocardiography approximately annually.
We hypothesised that in a population of cats with preclinical HCM, some of these measurements would be of prognostic value and serial measurements would give additional valuable information regarding outcome.
Materials and methods
Setting
Cats with HCM were identified from among cats with heart murmurs that were referred for further investigation from first-opinion veterinarians in northern England to one of two investigators, both RCVS cardiology certificate holders (VI and PT), between July 2010 and November 2015. Cats underwent examination either in the practice in which they would normally be examined or in another primary care practice near to their usual practice. Owners gave informed consent for their cats to be enrolled in the study. All the procedures undertaken as part of the study were standard diagnostic and monitoring procedures appropriate for patients with preclinical HCM and therefore the protocol did not undergo an ethical review. The study underwent review, and was funded, by PetSavers.
Cats underwent a full clinical examination, systolic blood pressure measurement, blood tests and echocardiography.
Physical examination
A complete physical examination was performed at each point of contact with each patient. Body weight, body condition score and pulse quality were recorded. Murmur intensity, the presence or absence of gallop sounds, the presence or absence of arrhythmias, heart rate and lung sounds were noted after thoracic auscultation.
Blood pressure measurement
Systolic blood pressure was measured non-invasively by Doppler sphygmomanometry (Ultrasonic Doppler flow detector, Model 811-B; Parks Medical Electronics) prior to collection of blood. Cuffs were placed on the right or left forelimb, and a mean of three consecutive readings was recorded.
Blood tests
Blood was collected by jugular venepuncture into plain, serum gel and EDTA tubes. After clotting, plain tube samples were centrifuged and the serum transferred to a clean plain tube. The serum gel and an EDTA tube were centrifuged within 5 mins of collection. EDTA plasma was then separated into a plain tube. A separate EDTA tube was submitted to the laboratory with a freshly prepared blood film. Samples were refrigerated and then sent via courier to a commercial laboratory (IDEXX Laboratories) for the following tests: urea, creatinine, glucose, alanine aminotransferase (ALT), sodium, potassium, total thyroxine, cardiac troponin I (cTnI; Beckman Coulter high sensitivity TnI), NTproBNP (first-generation Cardiopet proBNP assay until August 2013; second-generation Cardiopet proBNP assay thereafter) and a complete blood count.
Echocardiography
For each echocardiographic examination, cats were clipped and placed in right and then left lateral recumbency on an ultrasound examination table. An ultrasound unit equipped with a 3.5–8 MHz probe and electrocardiogram (ECG) monitoring (Vivid-I, 7S-RS probe; GE Medical Systems) was used for all examinations. Each cat had all echocardiographic examinations performed by the same observer.
A standard echocardiographic examination was performed 16 using the 7S-RS probe at an appropriate frequency setting to optimise image quality. Simultan-eous ECG monitoring was achieved for all cats except those intolerant of the ECG leads. Images were digitally stored, and measurements were obtained from still or looped images. All reported linear measurements were obtained from two-dimensional images. Echocardiographic variables obtained were the average of at least three measurements from discrete cardiac cycles. The following parameters were measured at each echocardiographic examination. The LA long axis measurement, that is, the maximal dimension parallel to the plane of the mitral annulus measured at end-ventricular systole, was measured in the frame just before the mitral valve opening. 17 The LA and aortic dimensions were obtained from short axis images. They were measured on the first diastolic frame obtained after closure of the aortic valve. The aorta was measured parallel to the commissure of the right and non-coronary aortic valve cusps. The LA dimension was measured parallel to the commissure of the left and non-coronary aortic valve cusps. The left atrium to aortic ratio (LA:Ao) was then calculated. 18
The thickness of the left ventricular free wall and interventricular septum were both measured in diastole using the leading edge method. 19 Focal or generalised hypertrophy was characterised by a thickness of ⩾6 mm. 8 The left ventricular outflow tract maximal velocity was recorded from the left parasternal long axis view. In addition, the presence or absence of the following were noted: systolic anterior motion of the mitral valve (SAM); chordal anterior motion (CAM); and whether a dynamic left ventricular outflow velocity profile was observed. 20
If sedation was needed in order to complete the examinations, 2.5 mg/kg ketamine (Anaestamine; Animalcare) and 0.25 mg/kg midazolam (Hypnovel; Roche) were given intravenously via an IV cannula.
Enrolment criteria
Cats were recruited to the study during the period from July 2010 to November 2015.
Cats were considered eligible for inclusion in the study if diagnosed with preclinical HCM. HCM was diagnosed if evidence was found of left ventricular segmental or diffuse hypertrophy of unknown origin (interventricular septum [IVS] and/or left ventricular free wall [LVFW] thickness in diastole was ⩾6 mm). 8
Cats were excluded if they were found to have a cardiac disease other than HCM, clinical signs associated with HCM or other clinically relevant disease including hypertension, hyperthyroidism, diabetes mellitus, anaemia (a red blood cell count below the reference interval of the laboratory) and azotaemia.
After the initial diagnosis, owners of cats with an aortic velocity of ⩾4 m/s were offered the option of using atenolol at a dose rate of 6.25 mg q24h or q12h. No other cardiac medication was offered at this stage.
Follow-up
Re-examinations were scheduled at approximately yearly intervals for 2 years after the baseline visit. At re-examination, cats underwent the same tests as were performed at baseline. Examinations performed on individual cats were always repeated by the same investigator.
If follow-up echocardiography showed LA enlargement, clopidogrel for prevention of thromboembolism was discussed with the owners. If used, the dose was 18.75 mg q24h. Atenolol treatment was stopped if atrial dilation was noted.
Cats were followed until they experienced a cardiac-related event, were lost to follow-up, died (of any cause) or the study was concluded. A cat was considered to have experienced a cardiac-related event if any of the following occurred: the cat experienced a thromboembolic event, developed signs consistent with congestive heart failure (CHF) that required treatment or experienced sudden death assumed to be cardiac in origin.
Diagnosis of arterial thromboembolism (ATE) was made on the basis of characteristic clinical signs of the occlusion of arterial blood supply to at least one limb. A diagnosis of CHF was made on the basis of a cat developing clinical signs of tachypnoea and dyspnoea in the absence of another cause. The presence of the following was considered to corroborate a clinical diagnosis of heart failure: audible pulmonary crackles; a response to diuretic therapy; pulmonary infiltrates on a thoracic radiograph; and/or a pleural effusion on thoracic ultrasound. A cat was considered to have experienced sudden death as its first cardiac-related event if it was found dead by the owner having been known to be normal less than 24 h prior to being found dead in the absence of an alternative explanation for the death.
The study was concluded in March 2018. The primary outcome of interest was whether or not a cat experienced a cardiac-related event during the period of follow-up.
The following variables were recorded at baseline: sex (male/female); age (years); and breed (pedigree/not). The following variables were recorded at baseline and at each re-examination: body weight (kg); heart rate (beats per minute [bpm]); murmur intensity (/6); systolic blood pressure (mmHg); blood urea nitrogen (mg/dl); NTproBNP (pmol/l); cTnI (ng/ml); maximum left ventricular wall thickness in diastole (mm); LA:Ao; LA diameter in long axis; maximum aortic velocity (m/s); treated with atenolol (yes/no); and the presence of an arrhythmia (yes/no).
The upper limit of detection of the NTproBNP assay was 1500 pmol/l; cats with values above the upper limit were ascribed a concentration of 1500 pmol/l.
An a priori power analysis was not conducted but the study planned to recruit 50 cats.
Statistical analysis
Descriptive statistics for continuous variables are reported as median values and range; for categorical and ordinal variables, they are reported as frequency and proportions.
Variables at baseline were compared between cats that went on to experience an event and those that did not. Continuous variables were compared using an independent samples Mann–Whitney U test. Categorical variables were compared with a χ2 or Fisher’s exact test as appropriate.
Those variables where the distribution differed significantly between cats that experienced an event and those that did not were evaluated further for their ability to discriminate between the two groups using receiver operator characteristic (ROC) analysis. A cut-off value was calculated with the best discriminatory ability on the basis of the co-ordinate points of the ROC curve and commonly used cut-off values.
For the two variables shown by the ROC analysis to best discriminate between cats experiencing an event and those that did not, the predictive ability of combining these variables was examined. Cats were classified as having none, one or both values of these variables above the cut-offs determined by the ROC analysis and the proportions of cats in each group were compared for likelihood of experiencing an event.
Time to event analyses were undertaken comparing cats with neither, one or both values of variables above the chosen cut-offs using Kaplan–Meier and log-rank analysis. Cats that were lost to follow-up, died of a non-cardiac cause or survived until the end of the study were right-censored in the analysis at the point of their last known contact with investigators or the time of death.
Finally, a graph was plotted with values of the two variables on the axes illustrating those cats that did and did not experience an event.
To determine whether cats at risk of an event could be identified by repeated measurement of characteristics identified to be associated with the likelihood of an event at baseline, the following analyses were undertaken. For those variables that differed significantly between cats that subsequently experienced an event and those that did not at the baseline visit, values for the absolute change in the variable ([measurement at visit n+1] – [measurement at visit n]) and the percentage change in the variable between visits were calculated (100*[(measurement at visit n+1) – (measurement at visit n)]/[measurement at visit n]). The absolute and percentage change of each variable from the previous visit were compared between cats that subsequently experienced an event and those that did not. For those variables that demonstrated a significant difference between groups, an ROC curve was plotted using the absolute or percentage change in the variable as the discriminator and subsequent event yes/no as the outcome.
Results
Forty-seven cats were diagnosed with preclinical HCM and recruited to the study. Baseline characteristics of the cats are summarised in Table 1.
Table 1.
Baseline characteristics of cats recruited to the study
| Characteristic | All cats | Cats that experienced an event related to HCM | Cats that remained healthy or experienced signs related to other disease | P value for comparison |
|---|---|---|---|---|
| n | 47 (unless otherwise specified) | 15 (unless otherwise specified) | 32 (unless otherwise specified) | |
| Sex (male) (%) | 35 (74) | 12 (80) | 23 (71.9) | 0.73 |
| Age (years) | 4.0 (1.0–12.0) | 6.0 (1.0–12.0) | 4.0 (1.0–12.0) | 0.65 |
| Breed (pedigree/not) (% pedigree) | 7/40 (14.8) | 2/13 (13.3) | 5/27 (15.6) | >0.99 |
| Body weight (kg) | 4.4 (3.1–9.2) (n = 45) |
4.3 (3.1–8.2) (n = 14) |
4.7 (3.5–9.2) (n = 31) |
0.19 |
| Heart rate (beats/min) | 200 (130–250) | 180 (130–240) | 200 (140–250) | 0.19 |
| Murmur intensity grade 2/3/4 (out of 6) (n [%] for each
grade) |
3/21/23 (6/45/49) |
0/6/9 (0/40/60) |
3/15/14 (9/47/44) |
0.26 |
| Systolic blood pressure (mmHg) | 128 (80–180) (n = 42) |
120 (95–160) (n = 13) |
130 (80–180) (n = 29) |
0.50 |
| Creatinine (µmol/l) | 137 (82–213) | 137.0 (103.9–193.8) | 137.25 (82–213) | 0.64 |
| BUN (mg/dl) | 10.0 (5.7–16.7) | 11 (6.9–16.7) | 9.85 (5.7–13.6) | 0.33 |
| NTproBNP (pmol/l) | 515 (24–1500) (n = 46) |
851 (138–1500) (n = 14) |
321 (24–1500) | 0.001 |
| cTnI (ng/ml) | 0.23 (0.01–23.7) (n = 46) |
0.38 (0.04–0.82) (n = 14) |
0.22 (0.01–23.7) | 0.53 |
| Maximum left ventricular wall thickness in diastole (mm) | 7.0 (6.0–11.8) | 8.0 (6.0–11.8) | 7.0 (6.0–9.5) | 0.076 |
| LA:Ao | 1.33 (0.97–2.19) | 1.59 (1.08–2.19) | 1.28 (0.97–1.73) | <0.001 |
| Maximum aortic velocity (m/s) | 3.0 (1.1–6.6) | 3.0 (1.1–6.6) | 3.1 (1.1–6.6) | 0.57 |
| LA long (mm) | 15.8 (11.0–22.8) | 19.0 (13.4–22.8) | 15.5 (11.0–18.3) | 0.025 |
| Respiratory rate | 40 (24–90) (n = 20) |
45 (28–80) (n = 6) |
34 (24–90) (n = 14) |
0.49 |
| Received atenolol at anytime (yes) (%) |
15 (32) | 8 (53) | 7 (22) | 0.046 |
Values in bold indicate P <0.05. Continuous and ordinal data are reported as median (range). Nominal data are reported as n (%)
HCM = hypertrophic cardiomyopathy; BUN = blood urea nitrogen; NTproBNP = N-terminal pro B-type natriuretic peptide; cTnI = cardiac Troponin I; LA:Ao = left atrium to aortic ratio; LA long = left atrial long axis measurement
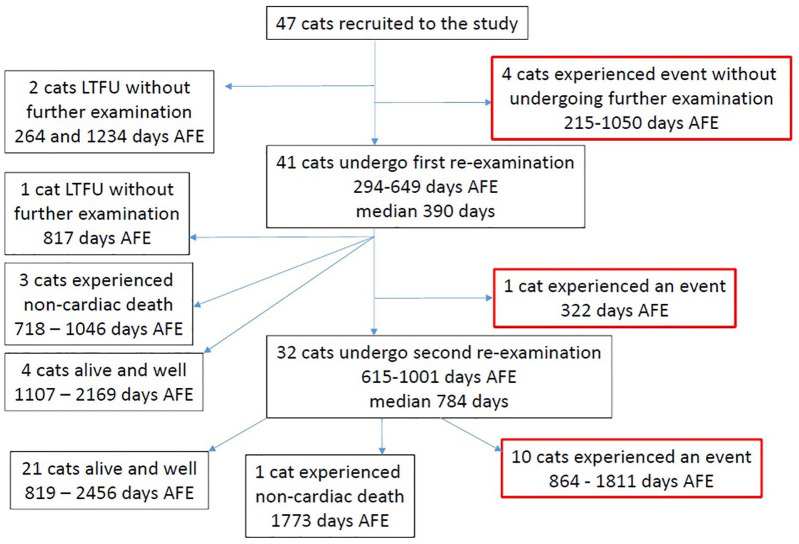
Of the 47 cats, 15 experienced at least one cardiac-related event (32%); six developed signs consistent with CHF (13%), five experienced sudden death (11%) and five experienced ATE (9%). One cat experienced CHF and ATE concurrently. Four cats experienced death owing to non-cardiac causes (9%). Twenty-eight cats (60%) were alive and known not to have experienced a cardiac-related event at the time of last contact with the investigators. Figure 1 is a flow chart illustrating the outcome for all 47 cats recruited to the study.
Figure 1.
Flow diagram indicating the outcome and time to outcome for the 47 cats recruited to the study.
AFE = after first examination; LTFU = lost to follow-up
The median time of follow-up for all cats in the study was 1135 days (range 215–2456 days); that is, greater than 3 years. The median time in the study for those cats that experienced an event was 1016 days (range 215–1811 days). The median time in the study for those cats that did not experience an event was 1210.5 days (range 264–2456 days). In total there were 56,444 days (154.6 years) of patient follow-up, meaning that there was one event per 10.3 years of patient follow-up, giving an incidence rate of 9.7% (95% confidence interval [CI] 5.4–16%) per year.
Eight cats required sedation in order to perform at least one of their echocardiographic examinations. Seven cats required sedation at the first examination, of which five were subsequently examined (once, n = 2; or twice, n = 3) without the need for sedation. One cat sedated at the initial examination required sedation at both subsequent examinations and one cat required sedation at the second re-examination only. One cat that did not require sedation at the baseline visit and first re-examination required sedation for the second re-examination.
Baseline variables that differed significantly between cats going on to experience an event and those that did not were as follows: LA:Ao (P <0.001), NTproBNP concentration (P = 0.001) and LA long axis measurement (P = 0.025). For all three variables, values were higher in the group of cats that went on to experience an event.
Results of the ROC analysis testing the ability of these three variables to discriminate between those cats that went on to experience an event and those cats that did not are illustrated in Table 2. Cut-off values are derived from the co-ordinate points of the ROC curves for the two most promising discriminators and the sensitivity, specificity and positive and negative likelihood ratios calculated on the basis of these cut-offs.
Table 2.
Area under the receiver operator characteristic (ROC) curves and their 95% confidence intervals (CIs) for the ability of the listed variables to discriminate cats that will go on to experience an event from those that will not
| NTproBNP concentration | LA:Ao | LA long | |
|---|---|---|---|
| AUC for ROC curve (95% CIs) |
0.821 (0.689–0.954) |
0.852 (0.721–0.983) |
0.704 (0.522–0.877) |
| P value | 0.001 | <0.001 | 0.025 |
| Proposed cut-off value | 700 pmol/l | 1.5 | |
| Sensitivity (95% CIs) |
0.786 (0.524–0.924) |
0.733 (0.48–0.891) |
|
| Specificity (95% CIs) |
0.813 (0.647–0.911) |
0.813 (0.647–0.911) |
|
| Positive likelihood ratio (95% CIs) |
4.203 (1.938–9.063) |
3.92 (1.787–8.559) |
|
| Negative likelihood ratio (95% CIs) |
0.263 (0.095–0.729) |
0.328 (0.14–0.772) |
AUC = area under the curve; LA:Ao = left atrium to aortic ratio; LA long = left atrial long axis measurement; NTproBNP = N-terminal pro B-type natriuretic peptide
The numbers of cats experiencing an event (and not experiencing an event) according to whether they had none, one or both risk indicators above the proposed cut-offs are reported in Tables 3 and 4. A Kaplan–Meier graph illustrating the proportion of cats remaining free of an event against time for the three groups of cats (neither risk indicator elevated, one risk indicator elevated and both risk indicators elevated) is shown in Figure 2. Cats without either risk factor were significantly less likely to experience an event compared with those with one factor (P = 0.018) and cats with both risk factors (P <0.001). The median time to event was not reached in the group with neither risk factor. The median time to event was 1693 days (95% CI 665–2720 days) for cats with one risk factor and 1016 days (95% CI 647–1384 days) for cats with both risk factors; however, the difference between these groups was not significant (P = 0.124).
Table 3.
Number of cats above and below the cut-off values for the two risk indicators identified
| Risk indicator | LA:Ao | ||
|---|---|---|---|
| <1.5 | ⩾1.5 | ||
| NTproBNP concentration | <700 pmol/l | 22 cats 1/21 (4.5%) |
7 cats 2/5 (28.6%) |
| ⩾700 pmol/l | 8 cats 3/5 (37.5%) |
9 cats 8/1 (88.9%) |
|
Data are presented for the 46 cats for which the values of both variables were known at baseline. The number of cats in each cell is presented as the total number, followed by the number of cats that experienced the event in bold/the number of cats that did not experience an event (percentage of cats that experienced an event)LA:Ao = left atrium to aortic ratio; NTproBNP = N-terminal pro B-type natriuretic peptide
Table 4.
Number of cats (percentage) experiencing an event and not experiencing an event according to whether they had none, one or two of the identified risk indicators above the proposed cut-off
| Number of risk indicators above the proposed cut-off | |||
|---|---|---|---|
| None | One | Two | |
| Number (proportion) of cats not experiencing an event | 21 (95.5%) | 10 (66.7%) | 1 (11.1%) |
| Number (proportion) of cats experiencing an event | 1 (4.5%) | 5 (33.3%) | 8 (88.9%) |
Figure 2.
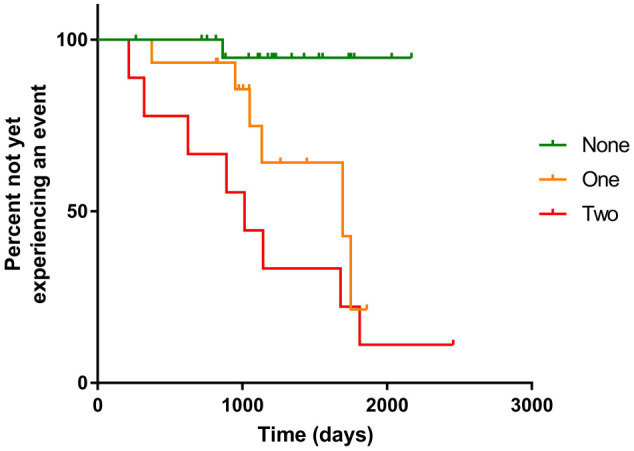
Kaplan–Meier plot indicating the proportion of cats that had not yet experienced an event against time according to whether they had none, one or two of the identified risk indicators above the proposed cut-off values
A graph illustrating the concentrations of NTproBNP and LA:Ao of individual cats measured at baseline is illustrated in Figure 3.
Figure 3.
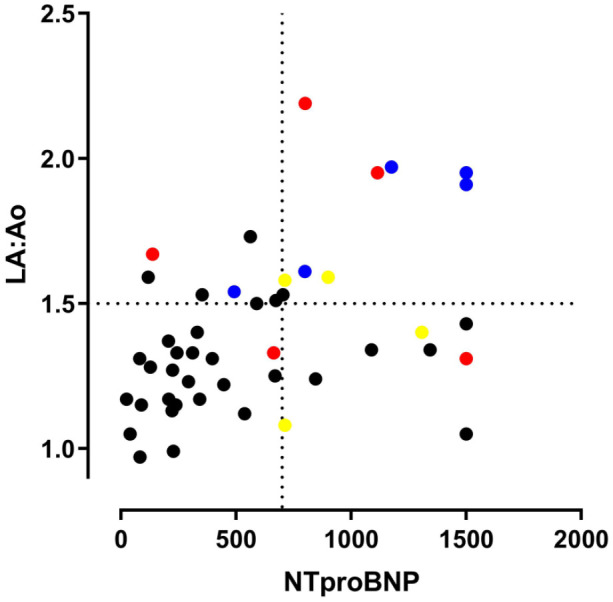
Dot-plot showing the baseline values of N-terminal pro B-type natriuretic peptide (NTproBNP) concentration and left atrium to aortic ratio (LA:Ao) for all cats in the study for which both measurements were available. Cats indicated by coloured dots went on to experience an event, cats indicated by black dots did not experience an event in the period of follow-up. Cats indicated by a blue dot developed congestive heart failure. Cats indicated by a red dot experienced sudden death. Cats indicated by a yellow dot experienced thromboembolism
A significantly greater proportion of cats that experienced an event received atenolol at some point during their follow-up (P = 0.046).
At the first re-examination, the absolute and percentage change in LA:Ao, LA long axis measurement and NTproBNP concentration did not differ between cats that went on to experience an event and those that did not (Table 5). At the second re-examination, the absolute and percentage change in LA:Ao from the previous visit were greater in cats that went on to experience an event (Table 6). The absolute change in the NTproBNP concentration was lower in those cats that went on to experience an event compared with those that did not (Table 6). The absolute and percentage changes in LA:Ao were significantly associated with the likelihood of an event in the ROC analysis (Table 7). As can be seen from Figure 1, 10 events occurred after the second re-examination and data were only available for nine of those cats, representing only 60% of all cats that experienced an event.
Table 5.
Absolute and percentage change in left atrium to aortic ratio (LA:Ao), N-terminal pro B-type natriuretic peptide (NTproBNP) concentration and left atrial long axis measurement (LA long) compared between cats that went on to experience an event and those that did not at the first re-examination
| Change between baseline and first re-examination | ||||||
|---|---|---|---|---|---|---|
| Absolute change | Percentage change | |||||
| Cats that experienced an event | Cats that did not experience an event | P value | Cats that experienced an event | Cats that did not experience an event | P value | |
| n | 11 (unless otherwise specified) | 30 (unless otherwise specified) | 11 (unless otherwise specified) | 30 (unless otherwise specified) | ||
| LA:Ao | 0.045 (−0.24 to 0.91) (n = 10) |
0.05 (−0.18 to 0.55) |
0.842 | 2.20 (−15.1 to 56.5) (n = 10) |
4.03 (−13.4 to 49.11) |
0.701 |
| NTproBNP (pmol/l) |
24 (−503 to 836) (n = 10) |
157 (−1278 to 822) (n = 29) |
0.601 | 2.66 (−33.5 to 125.9) (n = 10) |
48.7 (−85.2 to 324.1) (n = 29) |
0.418 |
| LA long (mm) | 0.34 (−2.62 to 6.25) (n = 10) |
−0.07 (−1.89 to 1.36) |
0.379 | 2.56 (−13.3 to 34.5) (n = 10) |
−0.47 (−14.3 to 9.66) |
0.315 |
Owing to missing data for at least one visit: values for absolute and percentage change in the LA:Ao and LA long were only available for 10 cats in the event group; and values for absolute and percentage change in NTproBNP concentrations were only available for 10 cats in the event group and 29 cats in the group that did not experience events
Table 6.
Absolute and percentage change in left atrium to aortic ratio (LA:Ao), N-terminal pro B-type natriuretic peptide (NTproBNP) concentration and left atrial long axis measurement (LA long) compared between cats that went on to experience an event and those that did not between the first re-examination and the second re-examination
| Change between first re-examination and second re-examination | ||||||
|---|---|---|---|---|---|---|
| Absolute change | Percentage change | |||||
| Cats that experienced an event | Cats that did not experience an event | P value | Cats that experienced an event | Cats that did not experience an event | P value | |
| n | 10 (unless otherwise specified) | 22 (unless otherwise specified) | 10 (unless otherwise specified) | 22 (unless otherwise specified) | ||
| LA:Ao | 0.30 (−0.07 to 0.90) (n = 9) |
0.03 (−0.35 to 0.28) (n = 21) |
<0.001 | 22.1 (−4.83 to 76.3) (n = 9) |
2.03 (−20.2 to 26.2) (n = 21) |
0.003 |
| NTproBNP (pmol/l) | −42 (−400 to 551) |
95 (−229 to 1148) |
0.031 | −3.6 (−40.3 to 58.1) |
13.8 (−83.3 to 326.14) |
0.10 |
| LA long (mm) | 0.03 (−3.0 to 2.92) (n = 9) |
2.35 (−3.0 to 9.15) (n = 21) |
0.063 | 9.12 (−15.0 to 49.3) (n = 9) |
−0.19 (−17.7 to 26.2) (n = 21) |
0.056 |
Owing to missing data for at least one visit, values for absolute and percentage change in LA:Ao and LA long were only available for nine cats in the event group and 21 cats in the group that did not experience events. Values in bold indicate P <0.05
Table 7.
Area under the receiver operator characteristic (ROC) curves and their 95% confidence intervals (CIs) for the ability of the absolute change in left atrium to aortic ratio (LA:Ao) and percentage change in LA:Ao between the first and second re-examinations to discriminate cats that go on to experience an event from those that do not
| Variable | AUC for ROC curve | 95% CIs for AUC | P value | Proposed cut-off value | Sensitivity of proposed cut-off (95% CI) | Specificity of proposed cut-off (95% CI) |
|---|---|---|---|---|---|---|
| LA:Ao absolute change | 0.902 | 0.751–1.0 | <0.001 | 0.12 | 0.889 (0.565–0.98) | 0.905 (0.711–0.973) |
| LA:Ao percentage change | 0.836 | 0.665–1.0 | <0.001 | 8.04% | 0.778 (0.453–0.937) | 0.81 (0.6–0.923) |
AUC = area under the curve
Discussion
This is the first study to prospectively follow a cohort of cats with preclinical HCM managed in a primary care setting by non-specialists. It provides additional information about the natural history of this common disease, confirms the value of known echocardiographic risk indicators and demonstrates the value of measurement of circulating biomarkers in identifying cats at greater risk of going on to experience a cardiac-related event. It also provides information regarding the value of serial evaluation of risk indicators.
The findings in the described population of cats confirm that many cats with preclinical HCM can live for long periods without experiencing a cardiac-related event. It has previously been reported that for many cats with HCM, particularly those that are free of clinical signs at the time of diagnosis, the disease can be a relatively benign and slowly progressive or non-progressive.5,6,12 In the current study, during more than 150 years of patient follow-up only 15 cats experienced cardiac-related events, occurring at a rate of one event per 10.3 years. The three individual events that were considered cardiac-related events, the onset of CHF, ATE and sudden or unexpected death, occurred with similar frequency. In the population as a whole, fewer than one third of the affected cats experienced an event in a period of follow-up of, on average, 3 years.
Many previous studies have demonstrated the value of LA:Ao as an indicator of cats at greater risk of an adverse outcome.5,9,11–13 In the current study, this result was confirmed with cats having an LA:Ao ⩾1.5 being approximately four times more likely to experience a cardiac-related event. The current study also demonstrated that NTproBNP concentrations, when considered in isolation, were of similar predictive value to LA:Ao. Cats with a concentration ⩾700 pmol/l were also approximately four times more likely to experience a cardiac-related event. These markers appeared to be complementary in their ability to identify cats at higher risk. Cats with values of both indicators above the cut-off were the most likely to experience an event and did so more quickly.
In contrast to previous studies,14,15 cTnI did not prove to be a useful indicator of the risk of a cardiac-related event in this population. One possible reason for this is that the cats recruited into this study were all at the preclinical stage of their disease. If the release of troponin from myocardium is a late event in the course of HCM then it may only be a good indicator of risk in populations including those in the later stages of their disease; that is, those not at the preclinical stage of the disease. Another possible reason for the lack of demonstrated association is that the population described in the current study is relatively small; however, this cohort is larger and was followed for longer than both of those previously described.14,15
Both of the identified risk indicators, LA:Ao and NTproBNP, were evaluated serially in this population. Cats that subsequently experienced a cardiac-related event appeared to have a greater absolute change and percentage change in LA:Ao in the time interval prior to their experiencing the event. This suggests there is value in serial monitoring of LA:Ao. However, it is worth noting that fewer than two thirds of the cats (30 in total) contributed data to this analysis. Some cats had already experienced the event before they were re-examined or their owners chose not to return for subsequent examinations. Clearly, serial measurements can only be of value in those patients in which they can be obtained. Methods of prognostication for cats that are only seen on a single occasion must also be used because cats may experience an event before they are re-examined and owners may not be willing to wait for a second examination before an opinion on their cat’s likelihood of experiencing an event is given.
Unexpectedly, those cats that experienced an event had a lower absolute change in NTproBNP concentration between their first and second re-examination. This may be a consequence of there being an upper limit for the highest concentration of NTproBNP that can be registered by the assay involved. Cats with concentrations above 1500 pmol/l that had an increase in concentration would not be correctly identified by this method of measurement. This would mean the analysis would only correctly recognise elevations in cats that initially had lower concentrations, but not in those that initially had high concentrations. It makes it doubtful, using the current assay, that there will be value in serial measurement of NTproBNP in cats despite the concentrations measured at the first visit being good indicators of risk.
It is interesting to note that the cut-off value in this study proposed to distinguish cats at greater risk was 700 pmol/l. This is considerably higher than cut-offs that were previously proposed to distinguish cats in heart failure from those with respiratory distress due to other causes, and higher than cut-offs proposed to distinguish cats with preclinical cardiomyopathy from cats without cardiomyopathy. 21 There may be several reasons for this. First, the feline NTproBNP assay has been through several iterations and it may be that absolute values obtained from earlier versions of the assay are not directly comparable with those from more recent iterations. Second, every cat in the current study was known to have heart disease and the differentiation being made is between those with ‘worse’ heart disease and milder heart disease. This may mean that a higher cut-off is required to distinguish those two groups compared with a cut-off being used to distinguish cats without heart disease from those with heart disease.
Treatment with atenolol was offered to cats in which an elevated left ventricular outflow tract velocity was found, because at the time our study was designed it was believed that beta-blockade may improve the outcome in cats with preclinical hypertrophic obstructive cardiomyopathy and such treatment was widely recommended by cardiologists. 22 Systematically withholding such treatment from cats in the study was considered unethical. However, as our study progressed a trial was published that failed to show a benefit of atenolol administration in cats with hypertrophic obstructive cardiomyopathy. 12 Treatment was not consistently administered in all cases in which it was prescribed. One clinical trial had suggested that once in heart failure, cats receiving atenolol did less well than those not receiving atenolol 23 and for that reason, treatment was withdrawn in cats where evidence of disease progression was found.
A significantly greater proportion of cats that experienced an event received atenolol at some point in the duration of the study, but it should not be concluded that this represents a detrimental effect of the treatment. Treatment was not randomly allocated, nor were investigators blinded to treatment allocation. It is possible that there was some degree of allocation bias, with cats administered treatment being somehow different to those to which treatment was not administered.
The current study has several limitations. The number of cats followed in the study was relatively small; however, there are very few large prospective studies of cats with HCM in the literature and none conducted in a non-specialist setting. The low number of cats and the low event rate mean that the total number of cats experiencing events contributing to the analyses is only 15. The low number of events means that sub-analyses of the three separate cardiac-related events would not be worthwhile. It also means that multivariable analysis cannot be undertaken. The complementary value of measurement of NTproBNP and LA:Ao is, however, suggested by analyses including the Kaplan–Meier analysis and examination of the proportions of cats with none, one and two elevated risk indicators going on to experience an event. To conclusively demonstrate an independent and complementary value of the two tests, a larger study with a greater number of events would be required.
The diagnosis of HCM was made using published guidelines by cardiologists with an advanced postgraduate qualification in cardiology but was not confirmed by a specialist or by post-mortem examination. Two investigators made the diagnoses and carried out the follow-up examinations on the cases described; however, the agreement between the two investigators and the reproducibility of their findings was not evaluated.
The study was conducted over a long period of time, during which the assay for the measurement of feline NTproBNP was changed. This may have introduced a confounding factor, particularly in the evaluation of serial concentrations. The lengthy period over which the study was conducted also resulted in the recommendations for treatment of preclinical HCM and prevention of ATE changing during the period of the study. Treatment was therefore variable over the period of the study and conclusions regarding the efficacy of treatment cannot be made.
The diagnosis of ATE was made on the basis of clinical signs in the majority of cases and post-mortem examination or advanced imaging were not performed. A diagnosis of sudden death was made on the basis of the owner’s description and presumed to be cardiac in origin. The diagnosis of CHF was made on the basis of clinical presentation and response to therapy and was not confirmed by diagnostic imaging in every case. Confirmation of a diagnosis of CHF can be challenging in cats and the performance of diagnostic imaging is not possible in every case, especially at the time of an emergency presentation for breathlessness.
Conclusions
This study has demonstrated that a larger left atrium and/or higher concentrations of NTproBNP on initial examination of cats with preclinical HCM indicates those at higher risk of experiencing CHF, ATE or sudden cardiac death. In cats that underwent serial measurement of LA:Ao, those with increasing LA size had a greater risk of experiencing the same events compared with those in which LA size was static or reduced. Although the measurement of LA:Ao requires ultrasound equipment and expertise, the measurement of NTproBNP is widely available (through a diagnostic laboratory) and may help to identify patients with preclinical HCM at greater risk when echocardiography is not available.
Acknowledgments
The authors would like to thank the nursing staff and owners for their cooperation during this long study.
Footnotes
Accepted: 8 June 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by the BSAVA PetSavers charity.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Adrian Boswood  https://orcid.org/0000-0003-1795-4364
https://orcid.org/0000-0003-1795-4364
References
- 1. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015; 17 Suppl 1: S244–S257. [DOI] [PubMed] [Google Scholar]
- 2. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 3. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010; 12: 171–182. [DOI] [PubMed] [Google Scholar]
- 4. PDSA. PDSA Animal Wellbeing (PAW) Report. https://www.pdsa.org.uk/media/4371/paw-2018-full-webready.pdf (2018, accessed June 24, 2020). [Google Scholar]
- 5. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010; 51: 540–547. [DOI] [PubMed] [Google Scholar]
- 6. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL study. J Vet Intern Med 2018; 32: 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkins CE, Gallo AM, Kurzman ID, et al. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc 1992; 201: 613–618. [PubMed] [Google Scholar]
- 8. Fox PR, Lui S-K, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. Circulation 1995; 92: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 9. Rush JE, Freeman LM, Fenollosa NK, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002; 220: 202–207. [DOI] [PubMed] [Google Scholar]
- 10. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 11. Payne JR, Borgeat K, Brodbelt DC, et al. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2015; 17: S318–S328. [DOI] [PubMed] [Google Scholar]
- 12. Schober KE, Zientek J, Li X, et al. Effect of treatment with atenolol on 5-year survival in cats with preclinical (asymptomatic) hypertrophic cardiomyopathy. J Vet Cardiol 2013; 15: 93–104. [DOI] [PubMed] [Google Scholar]
- 13. Peterson EN, Moise NS, Brown CA, et al. Heterogeneity of hypertrophy in feline hypertrophic heart disease. J Vet Intern Med 1993; 7: 183–189. [DOI] [PubMed] [Google Scholar]
- 14. Borgeat K, Sherwood K, Payne JR, et al. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014; 28: 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014; 28: 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J Vet Intern Med 1993; 7: 247–252. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 18. Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000; 14: 429–435. [DOI] [PubMed] [Google Scholar]
- 19. Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 20. Schober K, Todd A. Echocardiographic assessment of left ventricular geometry and the mitral valve apparatus in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2010; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 21. Oyama MA, Boswood A, Connolly DJ, et al. Clinical usefulness of an assay for measurement of circulating N-terminal pro-B-type natriuretic peptide concentration in dogs and cats with heart disease. J Am Vet Med Assoc 2013; 243: 71–82. [DOI] [PubMed] [Google Scholar]
- 22. Rishniw M, Pion PD. Is treatment of feline hypertrophic cardiomyopathy based in science or faith? A survey of cardiologists and a literature search. J Feline Med Surg 2011; 13: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox PR. Prospective, double-blinded multicentre evaluation of chronic therapies for feline diastolic heart failure: interim analysis [abstract]. J Vet Intern Med 2003; 17: 398–399. [Google Scholar]