Abstract
Objectives
Meloxicam therapy may benefit cats with degenerative joint disease, and retrospective studies suggest it could slow kidney disease progression and increase survival. This study aimed to prospectively evaluate the renal effects of low-dose meloxicam treatment (0.02 mg/kg/day) over 6 months in cats with chronic kidney disease (CKD).
Methods
Twenty-one cats with stable International Renal Interest Society stage 2 or 3 CKD were recruited and randomized to placebo or meloxicam groups. Cats were evaluated at baseline and at 1, 3 and 6 months, including blood pressure, chemistry, symmetric dimethylarginine (SDMA), glomerular filtration rate (GFR), urinalysis, urine protein:creatinine ratio (UPC), urine transforming growth factor-beta (β):creatinine ratio, urine clusterin, urine cystatin B and serum inosine.
Results
No statistical difference was observed in systolic blood pressure, blood urea nitrogen, creatinine, SDMA, GFR, urine transforming growth factor-β:creatinine ratio, urine clusterin, urine cystatin B or serum inosine in cats receiving meloxicam vs placebo. Mean UPC was greater in the meloxicam group (0.33) than the placebo group (0.1) at 6 months (P = 0.006). Four cats had meloxicam discontinued owing to potential (mainly gastrointestinal) adverse effects.
Conclusions and relevance
No decline in renal excretory function was observed when meloxicam was administered to cats with CKD. However, gastrointestinal adverse effects were observed, and cats that received meloxicam had greater proteinuria at 6 months than cats that received placebo. As proteinuria is associated with negative outcomes (progression of azotemia and hypertension) in cats with CKD, this finding suggests that meloxicam should be used with caution in cats with CKD and UPC monitored. Until further research is available, clinicians should weigh the risk of potential increased proteinuria against quality of life benefits when considering meloxicam for analgesia in cats with renal disease.
Keywords: Glomerular filtration rate, azotemia, chronic kidney disease, meloxicam, safety
Introduction
Chronic kidney disease (CKD) in cats increases with age, with 30–80% of cats aged >15 years having CKD.1–3 Nephron damage associated with CKD is irreversible and often progressive, resulting in tubulointerstitial fibrosis; however, the rate of progression in cats can be variable.4,5 Although antihypertensive and antiproteinuric treatments have shown promise in treating feline CKD, only dietary treatment has increased survival.6,7 Renoprotective treatments targeting additional pathways are needed.
Degenerative joint disease (DJD) causes morbidity in cats, with the risk of DJD increasing as cats age. 8 Concurrent CKD and DJD is common, with 68% of cats diagnosed with DJD having concurrent CKD. 3 Analgesia is often required to improve quality of life (QoL) in cats with DJD. While non-steroidal anti-inflammatory drugs (NSAIDs) can provide effective analgesia, the potential for adverse effects on renal function has led to cautious NSAID use in older cats.
Two retrospective studies evaluating the use of meloxicam in cats with both DJD and CKD concluded that meloxicam might slow progression of CKD and improve survival.9,10 Thirty-eight cats with DJD were reviewed: 22 with and 16 without CKD when starting meloxicam. 9 Meloxicam was tapered to the lowest effective dose (median 0.02 mg/kg/day) for a median duration of 467 days (CKD) and 327 days (no CKD). Meloxicam treatment had no detectable effect on serum creatinine in treated cats, and creatinine increased more slowly in meloxicam-treated CKD cats than in control CKD cats. 9 A follow-up study with 47 CKD cats receiving meloxicam (0.02 mg/kg/day) found a favorable survival time (1608 days; 95% confidence interval 1344–1919 days) vs previously published survival times for feline CKD.10–12 Meloxicam treatment could have resulted in better mobility and overall QoL from analgesia, improved appetite and/or water consumption, or those studies could represent a skewed population of robust CKD cats. Alternatively, meloxicam might have reduced tubulointerstitial inflammation or attenuated glomerular hyperfiltration caused, in part, by prostaglandins and thromboxanes. 10
In people with proteinuric renal disease, rofecoxib (a selective cyclooxygenase [COX]-2 inhibitor) decreases proteinuria without reducing systolic blood pressure. 13 Proteinuria is a negative predictor of progression of creatinine, hypertension and survival in cats with CKD, thus a similar protein-reducing effect of NSAIDs in proteinuric CKD cats would be beneficial.11,14,15 While the effects of meloxicam and robenacoxib on renal function have been evaluated in cats with CKD, their effects on proteinuria have not yet been reported.9,10,16
A variety of biomarkers have been evaluated to detect renal tubular damage and inflammation in cats and show promise for monitoring the effects of NSAID therapy on the kidney.17–20 Transforming growth factor-beta (TGF-β) is released from inflammatory cells and renal parenchymal cells, and urine TGF-β:creatinine ratio (uTGFβ) has been correlated to serum creatinine in cats with CKD.18,19,21 Measurements of uTGFβ, clusterin, cystatin B and inosine after meloxicam therapy are non-invasive and complement traditional laboratory parameters to assess the renal efficacy and safety of anti-inflammatory therapy in cats with CKD.17,19,20
While the World Small Animal Veterinary Association Global Pain Council supports long-term administration of the lowest effective dose of meloxicam to provide analgesia for cats with concurrent CKD and arthritis, a prospective randomized clinical trial evaluating meloxicam in cats with CKD had not yet been performed. 22 The purpose of this prospective study was to determine the renal effects of low dose (0.02 mg/kg/day) meloxicam treatment in client-owned cats with naturally occurring CKD over 6 months. It was hypothesized that meloxicam would decrease renal excretion of protein and TGF-β without decreasing renal excretory function. A secondary objective was to provide a preliminary assessment of the safety of low dose meloxicam in a population of cats with naturally occurring CKD, and it was hypothesized that cats would tolerate this dose of meloxicam well.
Materials and methods
Animals
The study was reviewed by the Institutional Animal Care and Use Committee of Kansas State University (protocol #3572), and owner written consent was obtained before patient enrollment. Client-owned cats with short-term stable International Renal Interest Society (IRIS) stage 2 or 3 CKD, as diagnosed by a serum creatinine of 1.6–3.5 mg/dl in a euhydrated state, were recruited from the Kansas State University Veterinary Health Center into the study. Cats were considered for enrollment if they had stable concurrent disease, but cats were excluded if they had evidence of unstable CKD, uncontrolled non-renal disease or known current infection.
Recruitment of cats was primarily opportunistic through routine telephone consultations between general practitioners and the Kansas State University Veterinary Health Center Internal Medicine Service, although study recruitment information was also posted online at the university hospital’s website and a flyer sent to referring veterinarians. Initial prescreening involved a case review with the primary veterinarian by phone to be certain potential study cats had evidence of stable CKD and no other concerning complications. Although cats with a creatinine concentration of 1.6–1.9 mg/dl remained within the laboratory’s reference interval (RI), they could be eligible for enrollment in accordance with IRIS guidelines if they were unable to adequately concentrate urine, had abnormal renal palpation or renal imaging findings, had renal proteinuria or had persistent increases in symmetric dimethylarginine (SDMA). 23
The owners of cats meeting the study criteria were contacted by the researchers to discuss the study goals and involvement; cat owners interested in participation had a pre-baseline screening appointment scheduled, with examination and testing performed, as listed in Table 1. Cats were fasted from food (not water) for 12 h prior to each visit. In every cat at each time point, lactated Ringer fluid (100 ml) was administered subcutaneously 2 h prior to the iohexol clearance test to correct potential subclinical dehydration. Iohexol was administered (300 mg/kg IV), and blood samples were obtained at 2, 3 and 4 h post-iohexol. All blood was collected using either the jugular vein (22 G needle) or medial saphenous vein (23 G butterfly catheter), and urine was collected via cystocentesis (22 G needle). Serum iohexol concentrations were measured and plasma clearance calculated using a 3-point curve as a representation of glomerular filtration rate (GFR). 24 Urine supernatant and serum were stored at −80ºC and shipped in batch for biomarker analysis. Urine TGF-β concentrations were measured using a commercially available ELISA (TGF-β1 multispecies kit; Invitrogen) and expressed as a ratio with urine creatinine; neither urine clusterin nor urine cystatin B were indexed to urine creatinine.19,25
Table 1.
Timing of diagnostic testing performed on enrolled cats throughout the study
| Pre-baseline | Baseline (t = 0) | 1 Month | 3 Months | 6 Months | |
|---|---|---|---|---|---|
| Physical examination* | x | x | x | x | x |
| Doppler blood pressure* | x | x | x | x | x |
| CBC † | x | x | x | x | |
| Biochemistry profile † | x | x | x | x | x |
| GFR by iohexol clearance ‡ | x | x | x | x | x |
| SDMA § | x | x | x | x | x |
| Serum inosine § | x | x | x | x | x |
| Total T4 concentration ‡ | x | ||||
| Urinalysis † | x | x | x | x | x |
| Aerobic urine culture † | x | ||||
| UPC † | x | x | x | x | x |
| Urine TGF-β1:creatinine ratio § | x | x | x | x | |
| Urine clusterin § | x | x | x | x | |
| Urine cystatin B § | x | x | x | x | |
| Abdominal radiographs and ultrasound* | x | ||||
| Thoracic radiographs* | x |
Examination or procedure was performed at the Kansas State University Veterinary Health Center
Testing was performed by the Kansas State Veterinary Diagnostic Laboratory
Testing was performed by the Diagnostic Center for Population and Animal Health, Michigan State University
Testing was performed by IDEXX Laboratories
CBC = complete blood count; GFR = glomerular filtration rate; SDMA = symmetric dimethylarginine; T4 = thyroxine; UPC = urinary protein:creatinine ratio; TGF-β = transforming growth factor-beta
To help confirm short-term stability of renal disease, cats were evaluated for a second baseline visit, 3–4 weeks after the first visit, with testing as listed in Table 1. Stability was defined as having <25% change in serum creatinine and plasma clearance of iohexol between baseline visits. Cats were excluded if they had suspected or confirmed comorbidities identified during screening such as pyelonephritis, neoplasia, uroliths, uncontrolled hypertension or uncontrolled hyperthyroidism. The need for concurrent medications was not a reason for exclusion unless there was known drug interaction with meloxicam. Proteinuria did not exclude a cat from enrollment.
Study design
This was a prospective, double-blinded, controlled study conducted at the Kansas State University Veterinary Health Center. A pre-study power calculation indicated that a sample size of 11 meloxicam and five placebo cats would be adequate to determine a statistical difference between groups using a two-sided test with alpha = 0.05 and β = 0.80, with effect sizes of 1 (uTGFβ), 1 (blood urea nitrogen [BUN]), and 0.8 (SDMA); five additional cats were enrolled to minimize the effect of cat or sample attrition.11,18,26 Cats were stratified by hypertension requiring amlodipine and then randomized in a 3:1 ratio into treatment (n = 15, meloxicam) or control (n = 6, placebo) groups by use of a random numbers table.
Medications
Meloxicam (Metacam; Boehringer Ingelheim) 0.5 mg/ml oral suspension was dispensed from the Kansas State University Dispensary with a target dose of 0.02 mg/kg by mouth once daily. Cats in the placebo group were prescribed a similar volume oral starch-based suspension (SyrSpend; Fagron) to be administered by mouth once daily. Cats were dispensed an unmarked study drug (either meloxicam or placebo) at the baseline visit, to be continued for 6 months. All cats were exclusively fed a commercially available prescription renal-specific diet throughout the study. Clinical management of each case was at the discretion of the attending clinician.
Follow-up examinations
Each cat was examined at 1, 3 and 6 months after the initiation of meloxicam or placebo therapy, following the testing protocol in Table 1. A urine culture was performed at the discretion of the clinician as indicated by clinical signs and urinalysis. Clients were asked at each visit if they had any difficulty administering the study medication, if they were administering the medication as directed, and if they perceived any adverse effects from the medication.
Statistical analysis
GFR results were standardized to body weight and corrected as previously described; the corrected GFR was reported and used for statistical analysis for each cat. 27 Variables of body weight, blood pressure, packed cell volume (PCV), BUN, creatinine, phosphorus, potassium, GFR, SDMA, urine protein:creatinine ratio (UPC), uTGFβ, clusterin, cystatin B and inosine were compared over time within the meloxicam and placebo groups by repeated measure ANOVA followed by Newman–Keuls multiple comparisons post-hoc. Both baselines were used as two separate time points for multiple comparisons over time. An independent group t-test was used to compare variables between meloxicam and placebo treatment groups at each time point, including age, body weight, blood pressure, PCV, BUN, creatinine, phosphorus, potassium, GFR, SDMA, UPC, uTGFβ, clusterin, cystatin B and inosine. Percent change in blood pressure vs pre-baseline (excluding cats receiving amlodipine) was also compared between meloxicam and placebo-treated cats at each time point using an independent group t-test. For individual visits, UPC and uTGFβ results were not included in the statistical analysis if any of the following were observed: (1) >5 white blood cells per high-power field; (2) >200 red blood cells per high-power field; or (3) urinary tract infection (UTI) based on culture and/or bacteriuria with pyuria. P ⩽0.05 was considered significant for all comparisons. Potential adverse effects were described.
Results
Figure 1 details the flow of cats screened and enrolled in this study. Twenty-one cats were enrolled, 15 receiving meloxicam and six receiving placebo. The median age was 14 years (range 4.8–18 years), with no difference between groups (P = 0.704). Eight were female spayed and 13 were male castrated cats. Thirteen cats were domestic shorthairs, four were domestic longhairs, one was a Siamese mix, one was a Ragdoll, one was an Ocicat and one was a Maine Coon. At baseline, there were minor statistical differences in phosphorus and blood pressure between groups, but no difference in other values including creatinine, GFR, uTGFβ or other biomarkers (Table 2).
Figure 1.
CONSORT diagram detailing patient recruitment, enrollment and flow through a clinical trial evaluating low dose oral meloxicam vs placebo in cats with chronic kidney disease (CKD). IRIS = International Renal Interest Society
Table 2.
Measured variables in chronic kidney disease cats receiving meloxicam or placebo therapy over the 6-month study period
| RI | Pre-baseline | Baseline (t = 0) | 1 month | 3 months | 6 months |
|
|---|---|---|---|---|---|---|
| Meloxicam (n = 15); placebo (n = 6) | Meloxicam (n = 15); placebo (n = 6) | Meloxicam (n = 15); placebo (n = 6) | Meloxicam (n = 14*);placebo (n = 5*) | Meloxicam (n = 13*);placebo (n = 5*) | ||
| Body weight (kg) | NA | |||||
| Meloxicam | 4.2 ± 1.4 | 4.2 ± 1.4 | 4.2 ± 1.5 | 4.3 ± 1.5 | 4.3 ± 1.5 | |
| Placebo | 5.2 ± 1.6 | 5.2 ± 1.5 | 5.2 ± 1.5 | 5.4 ± 1.7 | 5.4 ± 1.8 | |
| P value | 0.146 | 0.148 | 0.144 | 0.175 | 0.184 | |
| Blood pressure (mmHg) | NA | |||||
| Meloxicam | 140.7 ± 9.2 | 142.3 ± 12.1 | 141.3 ± 12.0 | 138.6 ± 15.0 | 142.7 ± 17.6 | |
| Placebo | 128.3 ± 18.6 | 128.3 ± 13.3 | 130.0 ± 21.2 | 135.0 ± 22.4 | 130.0 ± 25.7 | |
| P value | 0.172 | 0.031 † | 0.134 | 0.692 | 0.245 | |
| Percent change in systolic blood pressure from pre-baseline (%) | NA | |||||
| Meloxicam | NA | 2.3 ± 5.7 | 0.8 ± 9.7 | −3.8 ± 8.0 | 1.5 ± 12.3 | |
| Placebo | NA | 1.8 ± 6.1 | 1.3 ± 8.8 | 3.8 ± 4.4 | −0.5 ± 6.6 | |
| P value | NA | 0.871 | 0.923 | 0.066 | 0.737 | |
| PCV (%) | 35–50 | |||||
| Meloxicam | 31.9 ± 4.9 | NA | 31.3 ± 4.0 | 32.9 ± 4.0 | 32.6 ± 3.5 | |
| Placebo | 33.8 ± 8.9 | NA | 31.7 ± 7.9 | 34.1 ± 9.6 | 34.0 ± 8.1 | |
| P value | 0.522 | NA | 0.905 | 0.799 | 0.719 | |
| BUN (mg/dl) | 15–31 | |||||
| Meloxicam | 50.0 ± 15.7 | 47.7 ± 16.1 | 48.6 ± 26.1 | 44.2 ± 14.3 | 46.6 ± 14.1 | |
| Placebo | 38.8 ± 9.5 | 37.8 ± 9.6 | 38.8 ± 13.1 | 36.2 ± 8.1 | 34.8 ± 7.2 | |
| P value | 0.123 | 0.178 | 0.398 | 0.257 | 0.096 | |
| Creatinine (mg/dl) | 0.9–1.9 | |||||
| Meloxicam | 2.77 ± 0.58 | 2.74 ± 0.56 | 2.75 ± 1.05 | 2.5 ± 0.59 | 2.59 ± 0.70 | |
| Placebo | 2.50 ± 0.33 | 2.60 ± 0.35 | 2.65 ± 0.54 | 2.42 ± 0.38 | 2.42 ± 0.49 | |
| P value | 0.293 | 0.576 | 0.834 | 0.783 | 0.625 | |
| Phosphorus (mg/dl) | 2.6–5.3 | |||||
| Meloxicam | 4.05 ± 0.78 | 4.06 ± 0.64 | 4.17 ± 1.38 | 3.80 ± 0.81 | 4.27 ± 0.63 | |
| Placebo | 3.22 ± 0.74 | 3.47 ± 0.68 | 3.45 ± 0.46 | 3.56 ± 0.36 | 3.70 ± 0.34 | |
| P value | 0.037 † | 0.074 | 0.092 | 0.534 | 0.077 | |
| Potassium (mmol/l) | 3.4–4.9 | |||||
| Meloxicam | 4.06 ± 0.44 | 4.42 ± 0.63 | 4.29 ± 0.67 | 4.44 ± 0.39 | 4.45 ± 0.50 | |
| Placebo | 4.25 ± 0.73 | 4.31 ± 0.31 | 4.27 ± 0.27 | 4.36 ± 0.46 | 4.18 ± 0.28 | |
| P value | 0.471 | 0.707 | 0.945 | 0.703 | 0.266 | |
| Corrected GFR (ml/min/kg) | 1.11–2.37 | |||||
| Meloxicam | 0.99 ± 0.30 | 0.98 ± 0.26 | 1.02 ± 0.31 | 1.03 ± 0.31 | 0.96 ± 0.34 | |
| Placebo | 1.01 ± 0.09 | 0.93 ± 0.14 | 1.28 ± 0.78 | 0.95 ± 0.29 | 0.98 ± 0.22 | |
| P value | 0.751 | 0.673 | 0.456 | 0.665 | 0.921 | |
| SDMA (µg/dl) | 0–14 | |||||
| Meloxicam | 22.9 ± 8.0 | 22.5 ± 9.5 | 22.5 ± 10.6 | 21.9 ± 6.9 | 22.8 ± 9.7 | |
| Placebo | 21.8 ± 7.2 | 22.0 ± 4.0 | 22.3 ± 7.7 | 21.0 ± 6.0 | 19.2 ± 6.3 | |
| P value | 0.773 | 0.910 | 0.975 | 0.796 | 0.455 | |
| Serum inosine (µg/dl) | NA | |||||
| Meloxicam | 525.6 ± 163.1 | 478.1 ± 167.8 | 451.9 ± 132.6 | 416.1 ± 138.0 | 464.5 ± 116.9 | |
| Placebo | 528.3 ± 319.2 | 475.2 ± 234.4 | 394.0 ± 243.8 | 481.3 ± 171.0 | 553.0 ± 239.4 | |
| P value | 0.982 | 0.976 | 0.512 | 0.445 | 0.366 | |
| Urine protein:creatinine | NA | |||||
| Meloxicam ‡ | 0.59 ± 1.36 (n = 12) | 0.33 ± 0.44 (n = 13) | 0.30 ± 0.26 (n = 12) | 0.37 ± 0.37 (n = 10) | 0.33 ± 0.21 (n = 11) | |
| Placebo ‡ | 0.18 ± 0.10 | 0.15 ± 0.08 | 0.16 ± 0.09 (n = 5) | 0.14 ± 0.05 (n = 5) | 0.1 ± 0.0 (n = 4) | |
| P value | 0.323 | 0.178 | 0.264 | 0.087 | 0.006 † | |
| Urine TGF-β1:creatinine | NA | |||||
| Meloxicam ‡ | 517.8 ± 941.7 (n = 12) | NA | 851.7 ± 1886.6 (n = 12) | 1236.8 ± 2009.5 (n = 10) | 893.3 ± 1828.8 (n = 11) | |
| Placebo ‡ | 1006.3 ± 1491.5 | NA | 281.6 ± 148.5 (n = 5) | 389.4 ± 282.3 (n = 5) | 267.0 ± 96.7 (n = 4) | |
| P value | 0.405 | NA | 0.518 | 0.221 | 0.284 | |
| Urine clusterin (ng/ml) | NA | |||||
| Meloxicam | 688.5 ± 762.1 | NA | 482.6 ± 550.5 | 450.6 ± 477.2 | 281.8 ± 218.7 | |
| Placebo | 988.8 ± 1264.8 | NA | 370.3 ± 415.7 | 246.8 ± 200.9 | 423.2 ± 676.9 | |
| P value | 0.508 | NA | 0.659 | 0.374 | 0.670 | |
| Urine cystatin B (ng/ml) | NA | |||||
| Meloxicam | 116.7 ± 227.7 | NA | 107.7 ± 293.1 | 49.3 ± 50.2 | 70.1 ± 95.1 | |
| Placebo | 65.0 ± 60.7 | NA | 40.5 ± 19.5 | 34.2 ± 18.7 | 67.4 ± 65.9 | |
| P value | 0.428 | NA | 0.392 | 0.527 | 0.955 |
Data are mean ± SD
Decreased number of cats at 3 and 6 months
P <0.05
Decreased sample size for urine protein:creatinine ratio and urine transforming growth factor-beta (TGF-β)1:creatinine ratio due to exclusion from hematuria or pyuria
RI = reference interval; NA = not available; PCV = packed cell volume; BUN = blood urea nitrogen; GFR = glomerular filtration rate; SDMA = symmetric dimethylarginine
Treatment group cats were prescribed a mean of 0.02 mg/kg/day of meloxicam (range 0.02–0.03 mg/kg). Table 3 shows concurrent stable conditions and therapy for cats in both groups. No cats received angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, but two cats received amlodipine. Three cats received subcutaneous fluids to maintain euhydration, two received famotidine, one received oral potassium supplementation and one received polyethylene glycol (MiraLAX; Bayer). Six UTIs were diagnosed during the study in meloxicam group cats (one each in two cats, two each in two cats) and treated with antimicrobials based on culture and susceptibility; no UTIs were diagnosed in placebo cats. Ten additional urine samples were excluded from UPC and uTGFβ analysis due to hematuria.
Table 3.
Concurrent stable conditions and therapy of cats with chronic kidney disease in both the meloxicam and placebo groups
| Condition | Meloxicam group | Placebo group |
|---|---|---|
| Hyperthyroidism (the cat received methimazole throughout the study) | 1 | 0 |
| Hypertension (both cats received amlodipine throughout the study) | 2 | 0 |
| Hypercalcemia (one cat received alendronate throughout study) | 2 | 0 |
| Heart murmur | 10 | 2 |
| Osteoarthritis (two cats received monthly polysulfated glycosaminoglycan injections) | 3 | 0 |
Calculated UPCs for meloxicam and placebo-treated cats are available in the supplementary material. One meloxicam cat had a UPC of 4.9 at pre-baseline with no active urine sediment, negative urine culture, no concurrent illness and improved UPC at subsequent visits; this cat was considered an outlier. Although there was not a significant difference between groups (P = 0.323), this outlier value resulted in an increased mean UPC in the meloxicam group (0.59) vs the placebo group (0.18) (Table 2). If this cat was removed from UPC analysis, the mean UPC of the meloxicam group at the pre-baseline visit was 0.20 and not significantly different from the placebo group (P = 0.773) (Figure 2).
Figure 2.
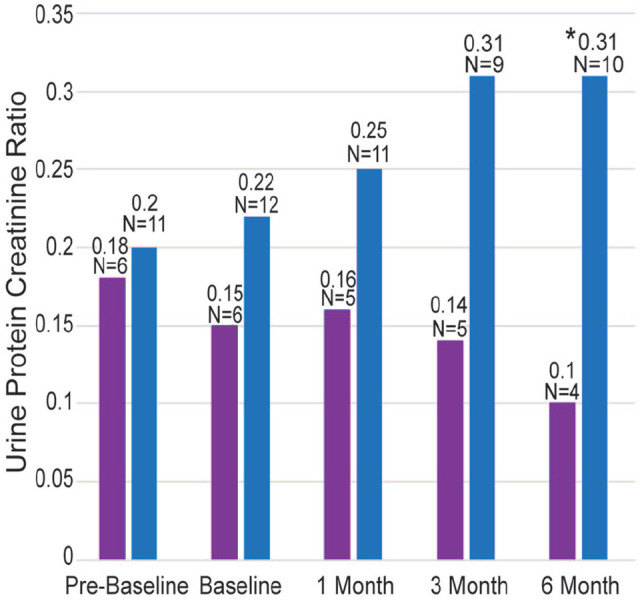
Mean calculated urine protein:creatinine ratios (UPC) for cats receiving meloxicam (blue) and placebo (purple) treatments, excluding one meloxicam-treated cat that had an outlier UPC at pre-baseline. N signifies the number of samples included at each time period; some samples were excluded owing to hematuria or pyuria. The asterisk denotes that the mean UPC was significantly higher in the meloxicam group than the placebo group at 6 months (P = 0.014)
When comparing the meloxicam and placebo groups, UPC was greater in the meloxicam group than the placebo group at 6 months (0.33 vs 0.1, P = 0.006) (Table 2) but was not significantly different at other time points. If the outlier cat was removed from the UPC analysis, the mean UPC for the meloxicam group remained 0.31 at 6 months, and meloxicam cats continued to have a higher mean UPC than placebo cats (P = 0.014) (Figure 2). There were no differences in creatinine, GFR, uTGFβ or other biomarkers between treated and placebo cats.
In meloxicam-treated cats, there were no significant changes over time in body weight, blood pressure, PCV, BUN, creatinine, phosphorus, GFR, UPC or any biomarker. However, for meloxicam-treated cats that completed the study (n = 13), mean serum potassium was lower at pre-baseline (4.1 ± 0.4 mmol/l) vs each other time point, where it remained at 4.4–4.5 mmol/l (P = 0.018).
In placebo-treated cats, there were no significant changes over time in body weight, blood pressure, PCV, BUN, creatinine, phosphorus, potassium, SDMA, GFR, UPC, clusterin, cystatin B or inosine. Of placebo cats, uTGFβ was greater at pre-baseline than at 1 or 6 months (P = 0.041).
Potential adverse effects to medication
No owner reported difficulty administering the study medication nor a lack of compliance during the study. Owners reported potential adverse effects, which included decreased appetite (two meloxicam cats, one placebo cat), lethargy (two meloxicam cats), vomiting (one meloxicam cat), constipation (one meloxicam cat) and salivation (one placebo cat).
Four cats had meloxicam discontinued at some point during the study (Figure 1). Two meloxicam cats were euthanized during the study. The first cat had progressive CKD that was non-responsive to supportive care; additional signs included blindness and circling (without hypertension), and on necropsy chronic nephritis and perivascular lymphocytic encephalitis were documented. The second cat had intermittent gastrointestinal signs despite stable azotemia and a persistent UPC of 0.1, including inappetence, weight loss, constipation and lethargy, which were initially responsive to discontinuation of meloxicam and supportive care but eventually resulted in euthanasia. Necropsy showed a normal gastrointestinal tract, mild pancreatitis and moderate lymphoplasmacytic interstitial nephritis. Two other cats had temporary discontinuation of meloxicam (7 and 10 days, respectively) due to gastrointestinal signs, despite stable azotemia and resumed therapy with no further complications (Figure 1).
Discussion
This study documented that administration of oral meloxicam (0.02 mg/kg daily) to cats with stable IRIS stage 2 or 3 CKD was not associated with a statistical decline in renal excretory function, as measured by iohexol clearance, serum creatinine or SDMA when compared with CKD cats receiving placebo. Although the current study did not evaluate survival, the lack of an effect of meloxicam on creatinine in CKD cats over 6 months mirrored what was seen in the retrospective studies by Gowan et al,9,10 and was further supported by the concordant GFR results. Similarly, there were no differences in the biomarkers uTGFβ, clusterin, cystatin B or inosine between the treated and placebo cats, or within meloxicam-treated cats over time. Although it was hypothesized that meloxicam might decrease proteinuria in cats with CKD, this beneficial effect was not observed in this study population. Instead, after 6 months of therapy, meloxicam-treated cats had a statistically significant higher UPC than placebo-treated cats, suggesting that until further research can be performed, caution should be used with meloxicam in cats with CKD, especially proteinuric CKD.
NSAIDs inhibit COXs, interfering with prostaglandin synthesis, which results in beneficial effects, including a reduction in inflammation and pain. Meloxicam is a COX-2 preferential NSAID, blocking both the inflammatory effects of COX-2 and partially blocking the protective effects of COX-1 and COX-2. In human patients with dehydration or hypotension, COX-2 is upregulated to enhance prostaglandin-mediated vasodilation, and NSAID-mediated inhibition of prostaglandins can lead to decreased renal blood flow, decreased GFR, azotemia, sodium and water retention, and systemic hypertension.28,29 Patients with renal disease have been considered at risk for these adverse effects, resulting in cautious use of NSAIDs in cats with renal impairment. Renal toxicity from NSAIDs is likely dose-related in cats as in dogs and humans; thus, if a low dose of meloxicam can be helpful for arthritic analgesia, it may lower the risk of NSAID-induced renal injury as compared with traditional dosing.30,31 The retrospective study of Gowan et al 9 demonstrated that using a low dose of meloxicam could be safe in well-monitored stable CKD cats, as measured by longitudinal assessment of serum creatinine.
The present study expanded on the foundation of Gowan and colleagues with a prospective study that more thoroughly assessed renal function with iohexol clearance to measure GFR, as well as systolic blood pressure, UPC and various biomarkers. Similar to the studies of Gowan et al,9,10 the current study found no adverse effects on renal excretory function, as measured here by creatinine, SDMA or GFR. The present study found no adverse effect on systolic blood pressure from meloxicam, either with absolute blood pressure measurements or when assessing percent change from pre-baseline. A slight increase in potassium was seen in the meloxicam-treated group vs pre-baseline, and hyperkalemia is known to occur with NSAID therapy owing to a reduction in renin release, decreased angiotensin and inhibition of aldosterone production. 29 As the potassium remained within the RI, this increase was not considered to be of clinical importance. Gowan’s group also documented that CKD cats treated with meloxicam had slowed deterioration of renal function over time, and they theorized this could be due to a direct effect on the kidney from meloxicam causing decreased renal inflammation or a consequence of meloxicam causing reduced proteinuria, both of which the current study aimed to further explore. 9
The current study used a panel of biomarkers to evaluate the effect of meloxicam on the overall renal health and inflammation in CKD cats. Use of additional renal biomarkers could help identify renal effects of meloxicam not detected by traditional monitoring methods and be beneficial in assisting clinicians with the earlier detection of renal disease, differentiate types of renal disease, and both target and monitor therapy. 20 TGF-β1 is a profibrotic cytokine, and urinary TGF-β1 excretion as measured by uTGFβ correlates with the degree of renal inflammation in the cat. 32 In the current study, there was no statistical difference in uTGFβ at any time point between meloxicam- and placebo-treated cats, or a difference in the meloxicam group over time; thus, no renal anti-inflammatory benefit from meloxicam could be verified with this biomarker. While there were fluctuations in both groups, the mean uTGFβ levels in the current study were much lower than previously reported for cats with CKD, with medians of 6698 pg/mg (range 3601–9831 pg/mg) and 13,938 ± 13,311 pg/mg, respectively.18,19
Clusterin and cystatin B are both biomarkers for tubular damage, and serum inosine is a marker of acute kidney injury. No statistical differences were observed between or within groups when urine clusterin and cystatin B concentrations were measured from CKD cats in the present study; however, clusterin concentrations from cats with CKD were much higher than previously reported in healthy dogs and people (<100 ng/ml). 17 Decreased serum inosine concentration can be an indicator of ischemic or hypoxic renal injury, and although serum inosine concentrations have not been previously reported in healthy or CKD cats, no effect on serum inosine was documented in CKD cats receiving meloxicam therapy over time in the present study. 17
NSAIDs have been recognized for their ability to reduce proteinuria in people. An early NSAID, indomethacin, was found to slow progression of proteinuria and renal failure in people, yet adverse effects (mainly gastrointestinal) have prevented its widespread use. 33 Rofecoxib reduces proteinuria without a detrimental effect on systemic blood pressure. 13 Both indomethacin and rofecoxib therapy result in increased creatinine and corresponding decreased GFR, which has been used to explain some reduction in proteinuria, but a reduction in fractional proteinuria has also been documented to verify a true protein-lowering effect. 13 By inhibiting prostaglandins and reducing inflammation, NSAIDs might reduce intraglomerular pressure, thus potentially reducing proteinuria in patients with CKD. It is unclear why the proteinuria-reducing effects of NSAIDs were not translated to cats with CKD treated with meloxicam in the present study, and instead UPC was higher in meloxicam-treated than placebo-treated cats at 6 months. This could be due to species differences in prostaglandin expression, choice of NSAID or dose. Cats might have differing prostaglandin distribution than humans to explain the discrepancy. Although this study found that low dose meloxicam can result in increased proteinuria in cats with CKD, additional research could be performed to determine if alternative COX-2 inhibitor NSAIDs might have a beneficial or neutral effect on proteinuria in cats and would therefore be a safer analgesic option for cats with concurrent CKD and DJD.
This study was limited by not including information on the presence of DJD, pain status or QoL of enrolled cats; in retrospect, this information would have been valuable. With concurrent CKD and DJD being common, especially in older cats, many cats require analgesia and veterinarians must weigh the benefits vs known risks of each medication. Unfortunately, there are limited oral analgesic options available for cats, and QoL is of utmost concern. While previous retrospective studies have found meloxicam did not affect survival in CKD cats, the current study found meloxicam to increase proteinuria after 6 months of therapy.9,10 The clinical relevance of proteinuria has been well established, with even minor increases in UPC being associated with negative outcomes such as progressive creatinine, hypertension and decreased survival.11,14 New effects on proteinuria provided by the current study can be used in concurrence with the current literature and recommendations from the World Small Animal Veterinary Association Global Pain Council to give practitioners evidence-based support as they make difficult treatment decisions in the best interests of their patients’ overall welfare. 22 Additional research is needed to elucidate further analgesic options for cats with CKD.
This study was limited by a small number of cats and a short 6-month study duration. The cat with an initial UPC of 4.9 skewed the mean UPC for the meloxicam group. There was no evidence of an active urine sediment in this cat; thus, the UPC was not eliminated from statistical analysis. However, it was not clear if this high value was due to undiagnosed concurrent illness or laboratory error. For interpretation, we reviewed and presented UPC data both with and without this cat’s values, and the UPC difference at 6 months was statistically different, even when excluding this cat, confirming the validity of the observation.
A second meloxicam-treated cat appeared stable at both baseline visits but had a decline of renal function and development of neurologic disease after 1 month of therapy; it is unclear if this decline was related to meloxicam causing worsening renal function and uremic encephalopathy, natural progression of CKD, or unrelated neurologic and systemic disease that resulted in a decrease in thirst, dehydration and progression of renal disease. While every attempt was made to ensure that CKD patients had short-term stable renal disease prior to enrollment, additional baseline visits could be considered in future studies.
A larger sample size in a pivotal clinical trial would help more clearly define the influence of meloxicam on renal parameters and minimize the impact of outliers. Specifically, samples with hematuria or pyuria were excluded from UPC and uTGFβ calculation, and a larger sample size would ensure that we achieved adequate power to determine a significant difference between groups for these variables if it exists.
Additionally, temporary cessation of meloxicam in several cats owing to perceived adverse effects may have led to underestimation of the overall effects of meloxicam on renal parameters, especially for the cat that discontinued therapy for 26 days. A single low dose of meloxicam was tested, and it is possible that an alternative dose of meloxicam or alternative NSAID could be safe for renal function without the observed difference in proteinuria; however, thorough research into the ideal dose and safety of these protocols would be warranted prior to clinical implementation. Finally, owing to the short duration of this study, survival data could not be obtained.
Conclusions
Low dose meloxicam did not negatively affect the creatinine, SDMA or GFR of cats with CKD. However, rather than resulting in improved proteinuria, as hypothesized, meloxicam-treated cats had worsened proteinuria, with cats receiving meloxicam having a higher mean UPC after 6 months of therapy than cats receiving placebo. As proteinuria is associated with negative outcomes, such as progression of azotemia and hypertension for cats with CKD, meloxicam should be used with caution in cats with CKD and UPC should be monitored on individual cats with CKD if meloxicam is administered.11,14 Some cats with CKD receiving meloxicam had gastrointestinal adverse effects which may also limit its use in this population. Further research is warranted to better understand the safety of meloxicam in cats with CKD. Ultimately, clinicians should weigh the risks of potential increased proteinuria and adverse effects vs benefits of improved QoL when considering meloxicam as an analgesic therapy for cats with CKD.
Supplemental Material
Calculated urine protein creatinine ratios for cats treated with meloxicam (blue dots) and placebo (purple squares) over the 6-month study. The blue dots with black outline show values from the outlier cat over time.
Acknowledgments
The authors thank Mal Hoover for assistance with figure preparation.
Footnotes
Accepted: 26 May 2020
Supplementary material: The following file is available online:
Figure: calculated urine protein creatinine ratios for cats treated with meloxicam (blue dots) and placebo (purple squares) over the 6-month study. The blue dots with black outline show values from the outlier cat over time.
Dr Grauer is a consultant for Boehringer Ingelheim, IDEXX, Kindred Bio and Vetoquinol. Drs Farace, Yerramilli and Peterson are full-time employees of IDEXX and part of the team of scientists who have developed most of the biomarkers reported in the manuscript, and are inventors on the patent application for these biomarkers and the assays that measure them. There is no conflict of interest to report for Drs KuKanich, George or Roush, or Mrs Sharp.
Funding: Funding was provided by the ACVIM Foundation. Sample analysis was supported, in part, by IDEXX.
Ethical approval: This work involved the use of non-experimental animals (owned or unowned) and procedures that differed from established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Kate KuKanich  https://orcid.org/0000-0002-0215-3284
https://orcid.org/0000-0002-0215-3284
References
- 1. Brown SA. Linking treatment to staging in chronic kidney disease. In: August JR. (ed). Consultations in feline internal medicine. St Louis, MO: Elsevier Saunders, 2010, pp 475–482. [Google Scholar]
- 2. Lulich JP, Osborne CA, O’Brien TD, et al. Feline renal failure: questions, answers, questions. Compend Cont Educ Pract Vet 1992; 14: 127–153. [Google Scholar]
- 3. Marino CL, Lascelles BDX, Vaden SL, et al. The prevalence and classification of chronic kidney disease in cats randomly selected within four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 5. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 6. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–957. [DOI] [PubMed] [Google Scholar]
- 7. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 8. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 9. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gowan RA, Baral RM, Lingard AE, et al. A retrospective analysis of the effects of meloxicam on the longevity of aged cats with and without overt chronic kidney disease. J Feline Med Surg 2012; 14: 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. King JN, Tasker S, Gunn-Moore DA, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–916. [PubMed] [Google Scholar]
- 12. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 13. Vogt L, de Zeeuw D, Woittiez AJJ, et al. Selective cyclooxygenases-2 (COX-2) inhibition reduces proteinuria in renal patients. Nephrol Dial Transplant 2009; 24: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 14. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 15. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 16. King JN, King S, Budsberg SC, et al. Clinical safety of robenacoxib in feline osteoarthritis: results of a randomized, blinded, placebo-controlled clinical trial. J Feline Med Surg 2016; 18: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yerramilli M, Farace G, Quinn J, et al. Kidney disease and the nexus of chronic kidney disease and acute kidney injury; the role of novel biomarkers as early and accurate diagnostics. Vet Clin Small Anim 2016; 46: 961–993. [DOI] [PubMed] [Google Scholar]
- 18. Arata S, Ohmi A, Mizukoshi F, et al. Urinary transforming growth factor-β in feline chronic renal failure. J Vet Med Sci 2005; 67: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 19. Habenicht LM, Webb TL, Clauss LA, et al. Urinary cytokine levels in apparently healthy cats and cats with chronic kidney disease. J Feline Med Surg 2013; 15: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quimby JM. Searching for biomarkers in feline chronic kidney disease: a new frontier. Vet J 2015; 206: 3–4. [DOI] [PubMed] [Google Scholar]
- 21. Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect 1999; 1: 1349–1365. [DOI] [PubMed] [Google Scholar]
- 22. Monteiro B, Steagall PVM, Lascelles BDX, et al. Long-term use of non-steroidal anti-inflammatory drugs in cats with chronic kidney disease: from controversy to optimism. J Small Anim Pract 2019; 60: 459–462. [DOI] [PubMed] [Google Scholar]
- 23. International Renal Interest Society Staging of CKD Guidelines. Available at: http://www.iris-kidney.com/guidelines/staging.html (accessed December 11, 2019).
- 24. Innis CJ, Kennedy A, McGowan JP, et al. Glomerular filtration rates of naturally cold-stunned Kemp’s Ridley turtles (Lepidochelys kempii): comparison of initial vs. convalescent values. J Herp Med Surg 2016; 26: 100–103. [Google Scholar]
- 25. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 2010; 78: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall JE, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finch N. Measurement of glomerular filtration rate in cats: methods and advantages over routine markers of renal function. J Feline Med Surg 2014; 16: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelton A. Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDS and non-NSAID analgesics. Am J Therapeut 2000; 7: 63–74. [DOI] [PubMed] [Google Scholar]
- 29. Kim S, Joo KW. Electrolyte and acid-base disturbances associated with non-steroidal anti-inflammatory drugs. Electrolyte Blood Pressure 2007; 5: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. KuKanich B, Bidgood T, Knesl O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet Anesth Analg 2012; 39: 69–90. [DOI] [PubMed] [Google Scholar]
- 31. Matthews ML. The role of dose reduction with NSAID use. Am J Manag Care 2013; 19 Suppl 14: S273–S277. [PubMed] [Google Scholar]
- 32. Lawson JS, Syme HM, Wheeler-Jones CPD, et al. Urinary active transforming growth factor B in feline chronic kidney disease. Vet J 2016; 214: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vriesendorp R, Donker AJM, de Zeeuw D, et al. Antiproteinuric effect of naproxen and indomethacin. Am J Nephrol 1985; 5: 236–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculated urine protein creatinine ratios for cats treated with meloxicam (blue dots) and placebo (purple squares) over the 6-month study. The blue dots with black outline show values from the outlier cat over time.



