Abstract
Objectives
The objectives of this study included utilising a large database from a diagnostic laboratory to identify any breed, sex or age predilections for cutaneous and subcutaneous soft tissue sarcomas (STSs), and the most common anatomical locations. The second aim was to obtain clinical outcomes and to assess histological features of those tumours to identify any potentially useful prognostic indicators and propose a grading system.
Methods
Records from the laboratory were searched for feline submissions received from January 2012 to December 2013 diagnosed with STSs; the breed, age, sex and neuter status of the cat and anatomical location of the tumour were recorded. Clinical outcomes were acquired using a questionnaire to submitting practices, and histological features of tumours from patients with known outcomes were assessed.
Results
No sex, neuter status or breed predispositions were found. Most STSs arise in middle-aged and older cats, and the most common anatomical location was the trunk. Forty-seven cases had a known clinical outcome and archived tissues allowing for histological assessment of the tumour. Significant differences in median survival time (MST), mitotic index and histological score were detected between those cats that died of tumour-related disease and those that did not. A novel grading system applied to these tumours produced significant differences in MST between cats with low (MST = 900.5 days), intermediate (MST = 514 days) and high grade tumours (MST = 283 days).
Conclusions and relevance
This is the first study applying a histological grading system to these common tumours. Local recurrence is often the cause of a poor outcome, with metastatic disease apparently rare. The proposed grading system incorporates features that can be assessed on routine haematoxylin and eosin-stained sections; in this small study, the histological grade of the tumour appears to be associated with survival time.
Keywords: Neoplasia, sarcoma, grade, soft tissue
Introduction
Soft tissue sarcomas (STSs) are neoplasms arising from mesenchymal cells within the soft connective tissues of the body. While such tumours may potentially arise at any anatomical location, those arising from the cutaneous and subcutaneous tissues are most common. The STS group incorporates a number of neoplasms with different cell origins, but which are generally considered together due to their similar histological features and biological behaviours; these typically include fibrosarcoma, myxosarcoma, peripheral nerve sheath tumours (non-brachial plexus), perivascular wall tumours, undifferentiated sarcomas and sometimes also liposarcomas. 1 The existence of feline injection site sarcomas (FISSs) further complicates the clinical picture for cats; these are tumours that commonly arise at previous sites of vaccination (historically known as vaccine-associated fibrosarcoma) or other focal trauma.2,3
STSs tend to be locally infiltrative, often extending along fascial planes, demonstrating a ‘pseudocapsule’ and a relatively slow growth rate, but a low to moderate incidence of metastasis. 2 However their clinical behaviour can vary and post-surgical recurrence is a relatively common occurrence, due to their often poorly-defined peritumoural margins. 4 The tumours can be superficial and mobile, or may infiltrate deeper tissues, and may persist for weeks to months with only minor changes before undergoing rapid growth. 5
STSs are one of the most common forms of cutaneous neoplasia diagnosed in the UK cat population, with one retrospective study 6 identifying fibrosarcomas as the most common malignant neoplasm, a finding consistent with previous studies.7–10 One study has identified potential prognostic factors specifically for FISS, 11 but otherwise there are currently no published grading systems for feline STSs. In dogs, there is a well-established grading system and the prognostic factors, including completeness of surgical margins, have been studied.1,12 This canine grading system, originally a human grading scheme, 13 is based on the mitotic rate, presence and extent of tumour necrosis and a subjective score for tumour differentiation and, together with completeness of surgical margins, has been shown in canine STSs to be predictive for local recurrence.1,12
The aim of this study was to utilise the large number of feline STSs submitted to a commercial diagnostic laboratory to examine the signalment of affected cats and the anatomical location of their tumours. A retrospective study of the histological features of tumours with known clinical outcomes was then undertaken to identify potentially useful prognostic indicators.
Materials and methods
Records from a large commercial diagnostic laboratory, Finn Pathologists (Diss, UK), were searched for all feline STSs diagnosed based on fixed tissue samples submitted to the laboratory during the period of January 2012 to December 2013. These dates were selected based on the availability of archived material and to allow for a period of up to 6 years’ clinical follow-up for any given case. Clinical details including sex and neuter status, age, breed and anatomical location were recorded for all submissions diagnosed with STSs (n = 723 tumours).
Domestic shorthair (DSH), domestic longhair (DLH), ‘domestic cat’ and ‘cross-breed’ were amalgamated under the term ‘non-pedigree’; all others were considered pedigree, with 12 different breeds represented. The breed of cats in the study population was compared with the breed prevalence of a sample from the background population (n = 3771); this sample from the background population was based on the stated breed on submission forms accompanying fixed tissue samples received by the laboratory throughout the period April 2006 to May 2011 and with any diagnosis. 14 Anatomical location was categorised into seven anatomical sites including the head, pinnae, neck, trunk, limbs, digits or tail. Ambiguous locations such as ‘dermis’ or ‘injection site’ were excluded from this part of the study.
Further information was sought from these cases and was acquired for 51 cases using a questionnaire to the submitting practices, approved by the Royal Veterinary College Clinical Research Ethical Review Board. Data requested included whether the cat had succumbed to STS-related disease, non-STS-related disease or was still alive, together with the date of death where relevant, as well as questions regarding the site and appearance of the mass, and whether there had been any evidence of local recurrence at the sample site and/or of metastatic disease. Forty-nine of these cases had archived tissues available for histological assessment, and haematoxylin and eosin (HE)-stained sections of each tumour were blindly reviewed by a pathologist (MJD), with some cases additionally reviewed by a second pathologist (KCS). Two of these cases were excluded on assessment of the HE-stained sections as the tissue affected was not haired skin and/or subcutis.
None of the cats in this part of the study had concurrent tumours of other histological types. The cats typically presented owing to the mass itself or associated clinical signs (eg, lameness), or rarely for other reasons including vaccination (n = 3), flea-allergic dermatitis (n = 1) or a corneal ulcer (n = 1).
Each of the remaining 47 individual tumours was assessed for mitotic index (calculated as the number of mitotic figures per 10 high power fields [× 400; field area of 0.237 mm2]), degree of inflammation, presence and extent of necrosis (none; equal to or less than 50%; more than 50% of tumour present in the section), presence of multinucleate cells, ulceration and presence or absence of material resembling adjuvant within macrophages. Margins, where present in the HE-stained sections, were also measured to the closest 0.5 mm, and the size of the tumour (excisional biopsy samples only) to the nearest 1 mm. Margins were considered ‘incomplete’ if neoplastic cells were contiguous with at least one margin, ‘close’ if neoplastic cells were closer than 3 mm from the margin or if only a pseudocapsule was present and ‘complete’ if neoplastic cells were at least 3 mm from the margin (including amputations of limbs and tails). The number of inflammatory cells were subjectively categorised as none, mild, moderate or severe, while multinucleate cells were subjectively categorised as none, rare, occasional or prominent. A score for each of the following was assigned; mitotic index, tumour necrosis and degree of inflammation present within the tumour. The sum of these scores provides the histological grade for the tumour (Table 1). Survival time was assessed as time from first diagnosis until either the date the questionnaire was returned by the submitting practice or the date that death/euthanasia occurred, whether due to STS-related disease or other causes.
Table 1.
Grading system for feline cutaneous and subcutaneous soft tissue sarcomas
| Mitotic score | Number of mitoses per 10 HPFs; × 400 |
|---|---|
| 1 | 0–9 |
| 2 | 10–19 |
| 3 | >19 |
| Tumour necrosis score | Necrosis as % of tumour area |
| 0 | No necrosis |
| 1 | Equal to or less than 50% |
| 2 | More than 50% |
| Inflammation score | |
| 1 | None, minimal or very mild |
| 2 | Mild to moderate |
| 3 | Severe |
| Histological grade | Total score* |
| I (low grade) | Equal to or less than 3 |
| II (intermediate grade) | 4 or 5 |
| III (high grade) | 6 or more |
HPF = high power field (× 400; field area of 0.237 mm2)
Combined mitotic, tumour necrosis and inflammation scores
Data were analysed using IBM SPSS Statistics for Windows v19.0 and GraphPad Prism 8.3.0. Categorical variables were analysed using a χ2 or Fisher’s exact test if two binary variables were to be tested. The Shapiro–Wilk’s test was used to identify any normal distributions (P >0.05). The t-test was used to test association between two groups. For more than two groups, a one-way ANOVA was used. For non-normally distributed data, the Mann–Whitney U was used for testing of two groups or Kruskal–Wallis for three or more groups. Kaplan–Meier survival plots and corresponding non-parametric log-rank tests were examined for the three histological grades. A P value <0.05 was considered significant.
Results
The total number of feline STSs submitted to the laboratory over the time period of this study (January 2012 to December 2013) was 723; of these, 685 (94.7%) were submitted from cats classified as non-pedigree breeds (DSH, DLH, ‘domestic cat’ and ‘cross-breed’). Pedigree breeds accounted for 38 cases (5.3%), with eight tumours submitted from Maine Coon cats (1.1%), six each from British Shorthair and Persian cats (0.8%), four each from Burmese and Bengal cats (0.6%), three from Norwegian Forest Cats (0.4%), two cases from Ragdoll cats (0.3%) and one each from Siamese, Sphynx, Russian Blue, Scottish Fold and British Silver Tabby breeds (0.1%). When compared with the background population, no significant differences were found between pedigree and non-pedigree cats, and no breed predispositions were observed.
Of the 723 cases, 697 had the gender specified on the submission form. Of these, 356 were male (51.1%; 282 neutered, 74 entire) and 341 were female (48.9%; 272 neutered, 69 entire). The age of 663 cats was specified on the submission form, giving a median age of 11 years (range 1–21 years). For non-pedigree breeds (n = 630), the median age was 11 years (range 1–21 years), whereas pedigree cats (n = 33) had a median age of 10 years (range 2–16 years); this difference did not reach statistical significance.
The anatomical location of the sample submitted was recorded on the submission form for 686 cases. The most common anatomical region affected was the trunk (34.5%), followed by the limbs (excluding digits; 28.3%), head (17.8%) and the neck (8.9%).
Forty-seven cases with known clinical outcome and tissue available for histopathological assessment were included in the second part of the study. Of these, 22 were from cases where the cat had died of tumour-related disease (TRD), although for one case the date of death was not recorded. Of the remaining 21 cases in this group, the median survival time (MST) was 205 days. There were 25 cats that either died of non-tumour-related disease (NTRD) or were still alive at the time of the study, with an MST of 854 days (four were lost to follow-up; those still alive at the time of the study were given an arbitrary date of 01/01/2018). The difference between these two MSTs is statistically significant (Mann–Whitney U test, U = 70; P <0.00001). For the 22 cases that died from TRD, the median mitotic index (MI) was 21 per 10 high power fields (× 400; range 5–52), while for the 25 cases that died of NTRD or were still alive at the time of the study, the median MI was 9 per 10 high power fields (× 400; range 1–70); this difference is statistically significant (Mann–Whitney U test, U value = 138; P = 0.00362).
Grading was applied to 47 cases (22 with death from TRD, 25 that died of NTRD or that were still alive at the time of the study). Of these, 16 cases were considered grade I (low grade), of which three died from TRD. Of those three, in two cases treatment was not attempted, while in the third there was local recurrence with a survival time of 802 days. The MST for cats with grade I tumours was 900.5 days. Fifteen cases were considered grade II (intermediate grade), and of these eight were reported to have died of TRD. Of those eight, six had reported local recurrence, one developed further soft tissue masses elsewhere suspected to be metastatic disease (although histopathological confirmation was not attempted) and in one case the reason was not reported other than ‘tumour-related’. The MST for cats with grade II tumours was 514 days. The remaining 16 cases were considered grade III (high grade), and of these 11 had died of TRD; in four cases there was local recurrence, for four cases treatment was not attempted and in three cases the primary veterinarian reported suspected metastatic disease, although histopathological confirmation was not attempted (two intrathoracic, one renal). The MST for cats with grade III tumours was 283 days. Kaplan–Meier analysis (Figure 1) showed that differences between the three survival curves was significant (Log-rank [Mantel–Cox] P = 0.0274).
Figure 1.
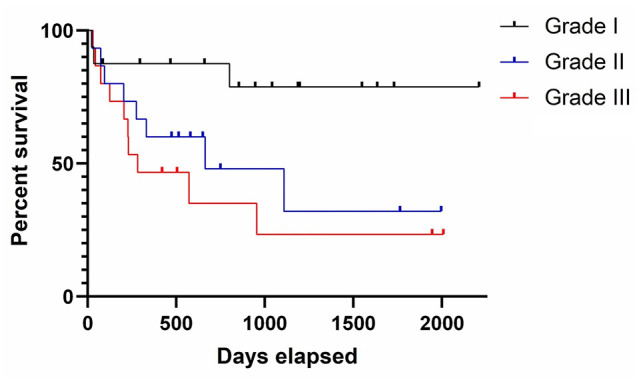
Kaplan–Meier survival curve for histological grade. The black line represents cats with grade I (low grade) soft tissue sarcomas (STSs), the blue line represents cats with grade II (intermediate grade) STSs and the red line represents cats with grade III (high grade) STSs. Tick lines represent censored cases (lost to follow-up or died of non-tumour-related causes)
The total score given as part of the grading process was compared between those cases with TRD (n = 22; median score = 5.5; range 3–8) with those with NTRD or that were still alive (n = 25; median score = 3; range 2–6); the difference was statistically significant (Mann–Whitney U test, U = 130; P = 0.00208).
Other histological features assessed included the degree of inflammation and the presence of multinucleate giant cells (MNGCs) and potential adjuvant within macrophages. There was a statistically significant association between the degree of inflammation and survival time (P = 0.015). Seventeen of the 47 tumours had MNGCs present (36%); of those, 10 were tumours from cats that died of TRD, and seven were from those that died of NTRD or that were still alive at the time of the study. Ten of the 17 tumours with MNGCs present were grade III (high grade), six were grade II (intermediate grade) and one was grade I (low grade). The presence of adjuvant within macrophages was noted in eight cases (17.0%), with only three of those cases from cats that died of TRD. Of those eight cases, six were grade III (high grade), two were grade II (intermediate grade) and none were grade I (low grade).
In 22 cases, margins were available for histological assessment (excisional biopsy or amputation) from those cases with a known clinical outcome; of these, four cases reported local recurrence. Of those four cases, one had incomplete margins, two had close margins and one had histologically complete margins (suspected to be FISS). The remaining 18 cases reported no local recurrence; six had incomplete margins, 11 had close margins and one had complete margins on histological assessment.
Discussion
A recent study showed that neoplasia arising at any anatomical site is the fourth most common cause of death in cats presenting to primary care veterinary practices in the UK. 15 Other studies have demonstrated that tumours arising in the skin and subcutis are the most common form of neoplasia in cats7,8 and, of those, more than half are malignant.6,16 Fibrosarcomas, a common subtype of STS in cats, are reported to be the second most common skin tumour diagnosed in the UK cat population. 6
The current study found no significant sex, neuter status or breed predispositions in cats to developing STSs, despite the large study size. There was also no significant difference noted when pedigree breed cats were compared with non-pedigree. Previous studies7,8 also found no apparent breed predispositions, although Graf et al reported females to be at increased risk compared with males. 8 The large-scale study by Ho et al found certain breeds were at decreased risk of developing fibrosarcoma of the skin and soft tissues, including Persian, Siamese, Burmese and British Blue cats, and also reported no gender difference. 6 Ho et al utilised the same database as the present study, although their study spanned a greater time period (2006–2013) and encompassed all skin masses submitted for histopathological assessment. 6
The median age of the cats in this present study was 11 years, with a range from 1 to 21 years. This is largely in agreement with previous studies, with such tumours mostly arising in middle-aged to older cats, although with a wide age range represented.6–8 Two studies looking at FISS in particular reported median ages of 9.5 years 3 and 11 years. 11
In the current study, the most commonly reported anatomical locations for STSs to arise were the trunk, followed by the limbs, the head and the neck. Miller et al reported the most common sites as the limb and head, followed by the trunk and with none reported on the neck; this study specified fibrosarcomas, however, and was based on a North American feline population. 7 There may also have been differences in the way head, neck, trunk and limb were defined between the two studies, as anatomical regions such as ‘limb’ do not necessarily have clear boundaries.
Clinical outcome and archived tissues were available for 47 cases in the present study. The outcome for 22 of those cases was death related to the tumour; this included six cases where further treatment was not attempted after the diagnosis of STS was made (via incisional biopsy). Eleven cases reported local recurrence, and four cases had suspected metastatic disease, including to the kidneys, lungs and other soft tissue sites (although no attempt was made to confirm metastatic disease [or local recurrence] histologically in these cases). Poor quality of life was commonly reported as forming an important part of the decision to euthanase in these cases. Twenty-five cats did not succumb to TRD; some of these cats died from other non-tumour-related causes, a reflection of the generally older age of affected cats and the long follow-up period of this study. Two of these cases also reported local recurrence, not resulting in euthanasia but in repeat surgery. Some of these cats were still alive at the time of the study, up to 6 years following treatment by excisional biopsy or amputation of the affected limb (or tail). When comparing the group of cats that succumbed to TRD with the group of those that did not, there were statistically significant differences in MST, the mitotic count of their STSs and in the total score of the tumour (as calculated as part of the grading process). The study was designed to allow a prolonged period from time of diagnosis to the time the questionnaire was returned, with the presumption that given sufficient time cats could also potentially succumb to non-tumour-related causes as well as to TRD. These cases were included in the survival analysis, on the assumption that this was a sufficiently long time period to have lapsed without any obvious sign of local recurrence/metastatic disease leading to death owing to TRD.
Histological assessment of these 47 tumours followed by grading revealed that there was a statistically significant difference in the MST between tumours of differing grades; for grade I (low grade) tumours, the MST was 900.5 days, compared with 514 days for grade II (intermediate grade) and 283 days for grade III (high grade) tumours. A greater degree of inflammation was also found to be associated with shorter survival times and was thus incorporated into the grading system, replacing the subjective ‘degree of tumour cell differentiation’ component of the traditional canine STS grading scheme.
MNGCs and suspected adjuvant within macrophages were also more frequently noted within higher grade tumours, but not necessarily with TRD. All except one of the tumours with MNGCs were grade II or III, and macrophages containing adjuvant were noted in grade II (n = 2) and grade III (n = 6) tumours only. Both MNGCs and suspected adjuvant within macrophages are features noted in FISS, 17 although they may not always be present, meaning that differentiation of FISS from other forms of STSs can be challenging from a histological perspective. In human tumours, the presence of MNGCs is generally regarded as an expression of anaplasia and is assumed to indicate a more aggressive phenotype. Couto et al 3 reported that MNGCs were a common finding in FISS and were most often seen in higher grade tumours, consistent with the findings in the present study.
Very few studies have applied the canine STS grading system to feline STSs. Two such studies3,11 focused solely on FISS, and neither found grading to be of prognostic merit in these tumours. Porcellato et al reported that the STS grade, depth of infiltration, surgical margin and Ki67 score of these tumours bore no relation to the likelihood of local recurrence, while the size of the tumour and the mitotic count were more useful (a cut-off of 20 mitotic figures per 10 high power fields was used). 11 The grading system used in the present study is a modified version of the one applied in these two FISS studies. Having evaluated potential factors individually, those that were found to be significantly associated with survival time were then incorporated into two different grading systems (the second system was not as strongly associated with outcome and was therefore not included here).
The canine STS grading system is based on a human scheme. 13 Some sarcomas that are not typically included within the canine STS group were included in the original human study, 13 which found that histological grade was predictive of survival and also disease-free intervals. Interestingly, the histological grading of adult human STSs is predictive of metastatic disease (rather than local recurrence).13,18 Metastatic disease is considered uncommon in canine STSs; it is reported to be rare in canine grade I STSs, of uncertain frequency in grade II STSs and to be most frequent in grade III tumours. Published studies do not generally verify metastatic disease histologically, and are further hampered by the relatively rare occurrence of metastatic disease and the small numbers of grade III STSs diagnosed in dogs; thus it is difficult to be certain of the true metastatic rate of these tumours. 1 Metastatic disease has been reported affecting lymph nodes and the lungs.19–21 In the current study, four of the 22 cats that died of TRD were suspected to have metastatic disease (out of the 51 cases with clinical follow-up available); however, this was not confirmed histologically. Those with suspected metastatic disease had grade II (n = 1) and grade III (n = 3) tumours; these numbers are too small to draw any conclusions but would appear to be consistent with the previous understanding that these tumours are unlikely to metastasise. This may also reflect that metastatic disease is slow to occur and/or metastatic lesions are slow to grow to a size likely to be clinically detectable or to pose a significant threat to life, particularly in older patients that often have comorbidities.
There is no clear consensus as to whether the histological grade of canine STSs is associated with overall survival; 1 in one study, the grade was prognostic for survival 19 but in several others it was not.12,21–23 McSporran demonstrated that histological grade was a predictor of local recurrence after excisional biopsy, but not survival. 12 Canine STSs were most often grade I or II, with relatively few grade III tumours, 1 whereas in the current study feline STSs were evenly spread across the three grades. This may reflect the differences between the two grading systems used, differences between the two species and/or may be impacted by the presence of FISS within the feline population, which themselves appear to be typically of higher grade than other feline STSs.
In the present study, there were relatively few cases with both clinical follow-up and margins available for assessment, meaning that it is difficult to determine the effect of margin completeness on the likelihood of local recurrence and survival time. Margins could only be measured for those cases with excisional biopsies (or amputations). Furthermore, the mass was not available for gross assessment, meaning that measurement of margins had to be performed on the tumour represented in the histological sections. Other limitations of the study include the small number of cases with clinical follow-up available, for what is undoubtedly a common but heterogeneous group of tumours with a range of potential biological behaviours. The FISS phenomenon further complicates the picture for our feline patients; if FISS was easier to differentiate from other forms of feline STSs, it might be more prudent to consider them as a separate entity. However, at present their identification can sometimes be problematic. The diversity of the study population with respect to time between the mass being noted and presentation to the primary veterinarian, the stage of disease and the treatment status are also limitations of this study, as is the loss of cases to follow-up.
Conclusions
Despite the mentioned limitations, this is the first study that has applied a histological grading system to feline cutaneous and subcutaneous STSs, which are a common form of neoplasia in cats, and which have a range of potential biological behaviours. For those cats whose tumours were treated surgically, local recurrence appears to be the main cause of a poor outcome, with metastatic disease apparently rare. The proposed grading system incorporates presence and extent of intratumoural necrosis, mitotic count and degree of inflammation, all of which can be assessed on routine HE-stained sections. In this small scale study, the histological grade of the tumour appears to be associated with survival time, although larger scale studies are required to confirm this finding.
Acknowledgments
The authors would like to thank all of the staff at Finn Pathologists for their assistance with accessing the database and producing the tissue sections. This research was performed as part of a final year research project (VR) supported by the Royal Veterinary College.
Footnotes
Accepted: 18 June 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or nonexperimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Melanie J Dobromylskyj  https://orcid.org/0000-0002-0781-5726
https://orcid.org/0000-0002-0781-5726
References
- 1. Dennis MM, McSporran KD, Bacon NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol 2011; 48: 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Gross TL, Ihrke PJ, Walder EJ, et al. Skin diseases of the dog and cat. 2nd ed. Oxford: Blackwell, 2005. [Google Scholar]
- 3. Couto SS, Griffey SM, Duarte PC, et al. Feline vaccine-associated fibrosarcoma: morphological distinctions. Vet Pathol 2002; 39: 33–41. [DOI] [PubMed] [Google Scholar]
- 4. Davidson EB, Gregory CR, Kass PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg 1997; 26: 265–269. [DOI] [PubMed] [Google Scholar]
- 5. Mauldin GN. Soft tissue sarcomas. Vet Clin North Am Small Anim Pract 1997; 27: 139–148. [DOI] [PubMed] [Google Scholar]
- 6. Ho NT, Smith KC, Dobromylskyj MJ. Retrospective study of more than 9000 feline cutaneous tumours in the UK: 2006–2013. J Feline Med Surg 2018; 20: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller MA, Nelson SL, Turk JR, et al. Cutaneous neoplasia in 340 cats. Vet Pathol 1991; 28: 389–395. [DOI] [PubMed] [Google Scholar]
- 8. Graf R, Grüntzig K, Hässig M, et al. Swiss Feline Cancer Registry: a retrospective study of the occurrence of tumours in cats in Switzerland from 1965 to 2008. J Comp Pathol 2015; 153: 266–277. [DOI] [PubMed] [Google Scholar]
- 9. Bostock DE. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br Vet J 1986; 142: 1–19. [DOI] [PubMed] [Google Scholar]
- 10. Jörger K. Skin tumors in cats. Occurrence and frequency in the research material (biopsies from 1984–1987) of the Institute for Veterinary Pathology, Zurich. Schweiz Arch Tierheilkd 1988; 130: 559–569. [PubMed] [Google Scholar]
- 11. Porcellato I, Menchetti L, Brachelente C, et al. Feline injection-site sarcoma: matrix remodelling and prognosis. Vet Pathol 2017; 54: 204–211. [DOI] [PubMed] [Google Scholar]
- 12. McSporran KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol 2009; 46: 928–933. [DOI] [PubMed] [Google Scholar]
- 13. Trojani M, Contesso G, Rousse J, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984; 33: 37–42. [DOI] [PubMed] [Google Scholar]
- 14. Melville K, Smith KC, Dobromylskyj MJ. Feline cutaneous mast cell tumours: a UK-based study comparing signalment and histological features with long-term outcomes. J Feline Med Surg 2015; 17: 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015; 17: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graf R, Grüntzig K, Boo G, et al. Swiss Feline Cancer Registry 1965–2008: the influence of sex, breed and age on tumour types and tumour locations. J Comp Pathol 2016; 154: 195–210. [DOI] [PubMed] [Google Scholar]
- 17. Doddy FD, Glickman LT, Glickman NW, et al. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol 1996; 114: 165–174. [DOI] [PubMed] [Google Scholar]
- 18. Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med 2006; 130: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 19. Kuntz CA, Dernell WS, Powers BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996). J Am Vet Med Assoc 1997; 211: 1147–1151. [PubMed] [Google Scholar]
- 20. Bacon NJ, Dernell WS, Ehrhart N, et al. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999–2004). J Am Vet Med Assoc 2007; 230: 548–554. [DOI] [PubMed] [Google Scholar]
- 21. Simon D, Ruslander DM, Rassnick KM, et al. Orthovoltage radiation and weekly low dose of doxorubicin for the treatment of incompletely excised soft-tissue sarcomas in 39 dogs. Vet Rec 2007; 160: 321–326. [DOI] [PubMed] [Google Scholar]
- 22. Ettinger SN, Scase TJ, Oberthaler KT, et al. Association of argyrophilic nucleolar organizing regions, Ki-67, and proliferating cell nuclear antigen scores with histologic grade and survival in dogs with soft tissue sarcomas: 60 cases (1996–2002). J Am Vet Med Assoc 2006; 228: 1053–1062. [DOI] [PubMed] [Google Scholar]
- 23. McKnight JA, Mauldin GN, McEntee MC, et al. Radiation treatment for incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc 2000; 217: 205–210. [DOI] [PubMed] [Google Scholar]


