Abstract
Objectives
The aims of this study were to validate a commercially available chemiluminescent assay for measurement of feline plasma adrenocorticotropic hormone concentration (ACTH), to determine the normal reference interval (RI) of plasma ACTH in healthy cats, to assess plasma ACTH in cats with naturally occurring hypercortisolism (HC), primary hypoadrenocorticism (PH) and other diseases (OD), and to evaluate the effect of aprotinin on plasma ACTH degradation.
Methods
Forty healthy cats, 10 with HC, 11 with PH and 30 with OD, were included. The chemiluminescent enzyme immunometric assay was evaluated by measurement of intra-assay precision, interassay precision and linearity. The RI for plasma ACTH in healthy cats was established using robust methods. Plasma ACTH of samples collected with and without aprotinin, stored at 4°C and assayed over a 6-day period, was measured.
Results
The intra-assay coefficients of variance (CVs) ranged from 2.7% to 4.3% and interassay CVs from 3.3% to 10.7%. Dilution studies showed excellent accuracy (R2 >0.99). The RI for plasma ACTH in healthy cats was 32–370 pg/ml. Plasma ACTH was not significantly different between healthy cats and the OD group. Cats with pituitary-dependent hypercortisolism (PDH) and PH had significantly higher plasma ACTH than the other groups. Plasma ACTH did not show significant differences when samples collected with and without aprotinin were compared.
Conclusions and relevance
The Immulite chemiluminescent assay is a valid technique for measuring plasma ACTH in cats and the RI of plasma ACTH is quite wide. Owing to the low overlap between healthy or OD cats and cats with HC or PH, the measurement of plasma ACTH appears to be useful and should be included in the diagnostic work-up when HC or PH are suspected. Furthermore, the measurement of plasma ACTH may be an accurate test for differentiating PDH from adrenal-dependent hypercortisolism.
Keywords: Feline plasma ACTH, adrenocorticotropic hormone, chemiluminescent assay, reference interval, Cushing’s syndrome, Addison’s disease, aprotinin
Introduction
Naturally occurring hypercortisolism (HC) and primary hypoadrenocorticism (PH) are rare conditions in cats,1–9 and their diagnosis can be challenging. However, adrenocortical axis disorders in cats are increasingly recognised and often included in the list of differential diagnoses. Once a diagnosis of HC is established, it is crucial to differentiate between pituitary-dependent HC (PDH) and adrenal-dependent HC (ADH) so that appropriate treatment can be instituted. Evaluation of plasma adrenocorticotropic hormone concentration (ACTH) can be useful when one of these conditions is suspected and can assist in determining the cause of feline HC. Although studies10–22 have been undertaken to evaluate the hypothalamic–pituitary–adrenal (HPA) axis in healthy cats, cats with pituitary or adrenal disorders, and cats with other diseases (OD), only a few have mentioned the measurement of plasma ACTH.10,15,16,18,21,22 This may be due, at least partly, to the lack of a current validated chemiluminescent assay for measurement of feline plasma ACTH and of a reference interval (RI) for plasma ACTH in healthy cats.Immunoradiometric assays (IRMAs) showed good performance in cats;18,22 however, the advantages of chemiluminescent methods over IRMA include lack of exposure to radioisotopes, easy integration into laboratory processes and rapid turnaround times. Currently, the chemiluminescent assay has been validated for the measurement of plasma ACTH in dogs, 23 but there are no studies that have evaluated its use in cats. A validated commercially available chemiluminescent assay may be used as a reference for future clinical studies investigating feline adrenocortical axis diseases. Moreover, the identification of an RI for feline plasma ACTH would help veterinary practitioners in the diagnosis of differential HPA disorders. Hence, the objectives of this study were to validate a chemiluminescent enzyme immunometric assay to measure feline plasma ACTH, determine the normal RI of plasma ACTH in healthy cats and assess plasma ACTH in cats with HC and PH, and to determine if OD may influence plasma ACTH.
Plasma ACTH is considered to be highly unstable in blood because of proteolytic degradation.24–27 For this reason, care must be taken during the pre-analytical phase and it is recommended that a constant low temperature be maintained from the point of blood sampling until analysis. 28 In clinical practice, however, this procedure may present logistical problems because samples for plasma ACTH measurement are frequently shipped to an external laboratory. Therefore, an additional aim of the present study was to determine if aprotinin, a protease inhibitor previously investigated for use in human and canine samples,23,29–31 may help prevent ACTH degradation in feline plasma samples.
Materials and methods
Validation of the chemiluminescent assay
Plasma ACTH was measured using a chemiluminescent enzyme immunometric assay (Immulite 2000 ACTH; Diagnostic Product Corporation), which measures the intact ACTH molecule (amino acids 1–39). The method uses a combination of a monoclonal murine anti-ACTH antibody and an alkaline phosphatase-labelled polyclonal rabbit anti-ACTH antibody directed against the C-terminal region and the N-terminal region, respectively. The manufacturer’s instructions indicated an analytical sensitivity for the assay of 5 pg/ml and a calibration range of up to 1250 pg/ml. There is some cross-reactivity (16.6–17.3%) with ACTH fragment 18–39 (corticotropin-like intermediate lobe peptide). There is no reported interference with alpha-melanocyte-stimulating hormone (α-MSH). 32
Intra-assay variability was determined by calculating the coefficients of variance (CVs) for eight replicates of three plasma samples having different concentrations of ACTH; analyses were repeated eight times within the same run. Interassay variability was determined by calculating the CVs for eight replicates of three plasma samples, having different concentrations of ACTH, analysed eight times on eight different days. Linearity was evaluated by measurement of two samples, one containing moderate concentrations of ACTH and one containing high concentrations of ACTH, before and after dilution (1:2, 1:4 and 1:8, respectively), using the diluent provided by the manufacturer. To assess linearity, the measured ACTH concentrations were plotted against expected values in a linear regression analysis. Accuracy after spiking was determined by adding 100 µl samples containing moderate and high concentrations of ACTH to samples containing a low concentration of ACTH. The measured ACTH concentrations were then compared with predicted ACTH concentrations.
Animals
Healthy cats
In order to establish the RI of feline plasma ACTH, 40 healthy client-owned cats, including 20 males (10 castrated) and 20 females (17 spayed) with a median age of 4.0 years (range 1.0–15.6 years), were included. Breeds comprised 32 domestic shorthairs (DSHs), four Maine Coons, two Kurilian Bobtails, one British Shorthair and one Siberian. The cats were considered healthy based on history and results of physical examination, complete blood count (CBC), biochemistry profile and urinalysis. None of the cats had received any medications for at least 3 months before enrolment in the study except for routine vaccination and ectoparasite or endoparasite prophylaxis. All cats were prospectively included according to the study protocol, which was approved by the Scientific Ethics Committee of the University of Bologna (no. 747/2016).
Cats with naturally occurring hypercortisolism and primary hypoadrenocorticism
Ten cats with HC and 11 with PH were retrospectively identified from the medical records of three referral institutions – (1) Veterinary Teaching Hospital, University of Bologna, Bologna, Italy; (2) Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland; and (3) Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Utrecht, Utrecht, The Netherlands – which use the same method for measuring plasma ACTH (Immulite 2000 ACTH).Cats with HC included six castrated males and four spayed females with a median age of 12.4 years (range 6.10–15.0 years). Breeds comprised eight DSHs and two Persians. Cats were considered to have been appropriately diagnosed with HC if clinical signs and laboratory findings were consistent with the disease and at least one of the following endocrine tests yielded a positive result: (1) low-dose dexamethasone suppression test (LDDST); (2) ACTH stimulation test (ACTHST); or (3) urinary cortisol:creatinine ratio. Differentiation of PDH from ADH was based on the results of the LDDST and the appearance of the adrenal and pituitary glands determined by ultrasonography, CT or both methods. On the basis of the above, PDH was diagnosed in eight cats and ADH in two cats.
Eleven cats with PH, including nine castrated males and two spayed females with a median age of 8.0 years (range 2.0–11.0 years), were retrospectively enrolled. Breeds comprised six British Shorthairs, three DSHs, one Bengal and one Chartreux. Hypoadrenocorticism was diagnosed by means of an ACTHST; the disease was considered primary owing to electrolyte abnormalities (hyponatremia and/or hyperkalaemia) and high plasma ACTH.
The LDDST was performed by injecting dexamethasone (Dexadreson; Intervet) at 0.1 mg/kg IV and collecting blood at baseline and after 4 and 8 h. The ACTHST was performed by collecting blood samples at 0 and 60 mins after the intravenous administration of 0.125 mg tetracosactide esacetate – synthetic ACTH (Synacthen; Novartis).
Cats with OD
Thirty cats with OD, including 20 males (18 castrated) and 10 females (seven spayed) with a median age of 10.2 years (range 1.0–19.0 years), were prospectively enrolled in the study. Breeds included 26 DSHs, and one each of Exotic Shorthair, Chartreux, Don Sphynx and Norwegian Forest Cat. The cats had been diagnosed with diabetes mellitus (n = 10), hyperthyroidism (n = 5), gastrointestinal disease (n = 6), chronic kidney disease (n = 2), acute kidney injury (n = 2), hyperaldosteronism (n = 2), central diabetes insipidus (n = 1), hypovolemic shock (n = 1) and septic shock (n = 1). All cats underwent a complete work-up, including physical examination, CBC, biochemistry profile and urinalysis. The decision on whether to carry out additional diagnostics (ie, diagnostic imaging, tests for concurrent diseases) was the responsibility of the clinician managing the case.
Sample collection and processing
Blood samples for the determination of plasma ACTH were collected from the jugular or cephalic vein into EDTA-coated plastic tubes placed on ice. The samples were immediately centrifuged at 4°C, 500 g for 8 mins, and plasma was immediately transferred to plastic tubes, stored at 4°C and analysed within 8 h or, alternatively, stored at −80°C and thawed at room temperature immediately before analysis. All analyses, except for retrospectively collected data (ie, cats with HC and PH), were performed in duplicate and the results averaged.
To determine the effect of aprotinin on ACTH degradation, three sets of blood samples with different ACTH concentrations collected with and without aprotinin (150 kallikrein inhibitor units aprotinin/ml whole blood) were assayed over a 6-day period. The plasma from each sample was divided into six aliquots, stored at 4°C and measured on days 0, 1, 2, 3, 4 and 5 after blood collection.
Statistical analysis
Descriptive statistics were generated to characterise the study population. Continuous variables were presented as mean ± SD or median and range (minimum and maximum value), depending on whether the data were normally or not normally distributed, respectively. Categorical variables were described with frequencies, proportions or percentages. Plasma ACTH values above the upper limit of quantification of the assay were reported as >1250 pg/ml and values below the analytical sensitivity of the assay were reported as <5 pg/ml. The RI of plasma ACTH was calculated using robust methods. 33 The Kruskal–Wallis test with Dunn’s post-test was used for comparison of plasma ACTH among groups (healthy, PDH, PH, OD). The two cats diagnosed with ADH were excluded from the analysis. Differences between samples at a given time point with or without aprotinin were compared using a repeated measures ANOVA. Values of P <0.05 were considered significant.
Results
Assay validation and RI
CVs for intra-assay precision for plasma sample 1, plasma sample 2 and plasma sample 3, having different concentrations of ACTH (sample 1: 98.9 ± 4.0 pg/ml; sample 2: 334.2 ± 9.1 pg/ml; sample 3: 539.2 ± 23.2 pg/ml), were 4.1%, 2.7% and 4.3%, respectively. CVs for interassay precision of plasma sample 1, plasma sample 2 and plasma sample 3 (sample 1: 58.8 ± 6.3 pg/ml; sample 2: 302.1 ± 21.5 pg/ml; sample 3: 900.2 ± 30.1 pg/ml) were 10.7%, 7.1% and 3.3%, respectively. Linear regression analysis of ACTH assay provided R 2 values of 0.994 (P = 0.002) for plasma samples containing moderate concentrations of ACTH and 0.997 (P = 0.001) for plasma samples containing high concentrations of ACTH. Recovery of ACTH after spiking ranged from 88.1% to 91.4% of the predicted concentration.
The RI for plasma ACTH was 32–370 pg/ml (median 99 pg/ml; range 24–400 pg/ml) (Figure 1). The 90% confidence interval (CI) of the lower limit was 24–43 pg/ml and the 90% CI of the upper limit was 277–478 pg/ml.
Figure 1.
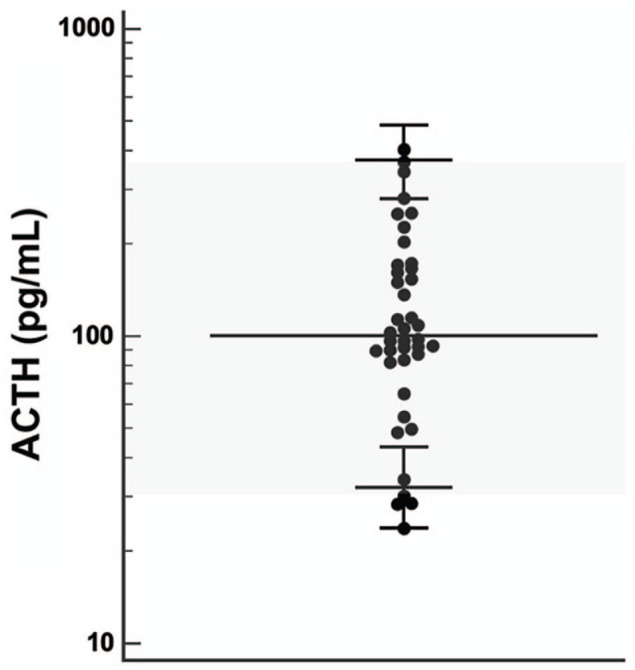
Reference interval for plasma adrenocorticotropic hormone (ACTH) in 40 healthy cats. Horizontal bars represent median value and 90% confidence interval (calculated using robust methods)
Plasma ACTH
Plasma ACTH results of healthy cats and cats with PDH, PH and OD are reported in Table 1. Plasma ACTH did not show significant differences between healthy and OD groups (P >0.99), and in cats with PDH compared with PH cats (P >0.99). Cats with PDH and PH had significantly higher plasma ACTH values compared with cats in the other two groups (P <0.001). Plasma ACTH in only one of the eight PDH cats (⩾331, minimum) overlapped with concentrations found in healthy (⩽400, maximum) and OD cats (⩽355, maximum). Nevertheless, there were overlapping results between two cats with PDH (>1250, maximum) and PH cats (>1250, minimum) (Figure 2). The two cats diagnosed with ADH both showed plasma ACTH values <5 pg/ml (ie, below the detection limit of the assay).
Table 1.
Range and median values of plasma adrenocorticotropic hormone (ACTH) in healthy cats, and in cats with pituitary-dependent hypercortisolism (PDH), primary hypoadrenocorticism (PH) and other diseases (OD)
| Variable (unit) | Healthy | PDH | PH | OD |
|---|---|---|---|---|
| ACTH (pg/ml) | 24–400 (99)* | 331–>1250 (690)† | >1250† | 22–355 (86)* |
Different symbols indicate statistically significant differences between the groups (P <0.05)
Figure 2.
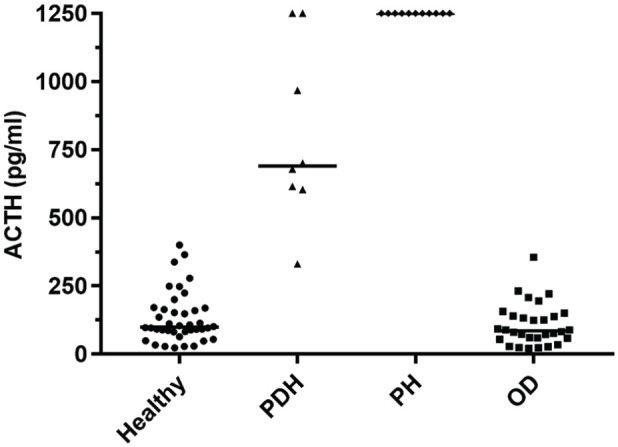
Plasma adrenocorticotropic hormone (ACTH) in healthy cats (n = 40), and cats with pituitary-dependent hypercortisolism (PDH, n = 8), primary hypoadrenocorticism (PH, n = 11) and other diseases (OD, n = 30). Horizontal bars represent median values
The plasma ACTH values in the presence and absence of aprotinin were not significantly different in the three sets of samples analysed (cat 1, P = 0.12; cat 2, P = 0.14; cat 3, P = 0.07). As expected, for each set of samples there was a decreasing trend of measured plasma ACTH with time. After only 1 day of storage at 4°C, the decline in measured plasma ACTH ranged from 0.7% to 29% (median 16%). The change over time in plasma ACTH of samples collected with and without the addition of aprotinin is shown in Table 2.
Table 2.
Change in plasma adrenocorticotropic hormone (ACTH) over time in three sets of samples collected with and without the addition of aprotinin (150 KIU/ml), stored at 4°C and assayed over a 6-day period
| Time interval (day) |
ACTH in cat 1, which had PDH
(pg/ml) |
ACTH in cat 2, which was healthy
(pg/ml) |
ACTH in cat 3, which had PDH
(pg/ml) |
|||
|---|---|---|---|---|---|---|
| Aprotinin | No aprotinin | Aprotinin | No aprotinin | Aprotinin | No aprotinin | |
| T0 | 720 | 765 | 63 | 58 | 955 | 941 |
| T1 | 659 (−8.5%) | 638 (−16%) | 53 (−16%) | 57 (−1%) | 677 (−29%) | 668 (−29%) |
| T2 | 564 (−21%) | 533 (−30%) | 54 (−15%) | 44 (−23%) | 592 (−38%) | 495 (−47%) |
| T3 | 519 (−28%) | 419 (−45%) | 43 (−32%) | 32 (−45%) | 514 (−46%) | 417 (−56%) |
| T4 | 473 (−34%) | 361 (−53%) | 41 (−35%) | 30 (−48%) | 317 (−67%) | 376 (−60%) |
| T5 | 393 (−45%) | 299 (−61%) | 32 (−49%) | 25 (−57%) | 271 (−72%) | 241 (−74%) |
Data in parentheses represent the percentage of change in hormone concentration compared with T0
KIU = kallikrein inhibitor units; PDH = pituitary-dependent hypercortisolism
Discussion
The chemiluminescent enzyme immunometric assay used in the present study is precise and accurate for the measurement of ACTH concentration in feline plasma samples. This assay showed acceptable intra- (<5%) and interassay (<15%) CVs. Results of dilution studies demonstrated excellent accuracy of the method (R 2 >0.99). The performance of the assay used in the current study compared favourably with the data reported in the literature for the IRMA. 22 It is also worth mentioning that the chemiluminescent assay does not require exposure to radioisotopes and appears to be easy to integrate into laboratory functions. Based on these considerations, it should be recommended to laboratories performing endocrine tests.
In our study, the RI for plasma ACTH in cats was wide (32–370 pg/ml) and different from that observed in dogs (6–58 pg/ml). 34 The reference values reported here appear similar to those reported by Javadi et al, 18 who used an immunoradiometric assay in 130 healthy house cats (ACTH 14–359 pg/ml). The wide distribution of plasma ACTH may be due, at least partly, to the episodic secretion of this hormone from the pituitary gland. 35 Samples may have been collected during a pulse of hormone release. Another important aspect concerns the sensitivity of cats to stress. It has been demonstrated that even relatively mild stresses, such as handling or intradermal skin testing, may cause a sharp increase in plasma concentrations of cortisol, ACTH and α-MSH. 36 For this reason, the stress of transport, handling and blood sampling may have caused high plasma ACTH in some cats. However, for the analysis of the RI, we decided not to remove the outliers, in order to have a result that reflects the real clinical setting.
ACTH is a polypeptide tropic hormone synthesised and secreted by the anterior pituitary gland. ACTH is derived from a high-molecular-weight precursor molecule called pro-opiomelanocortin (POMC). POMC is first processed to pro-ACTH which is, in turn, cleaved into ACTH by the prohormone convertase 1. 37 A recent study in human medicine, involving 87 clinical laboratories, reported a POMC interference in the measurement of plasma ACTH by immunometric methods. 38 The mean cross-reactivity reported by Monaghan et al 38 for the chemiluminescent assay used in the present study was 2.2% (range 1.1–3.5%). This cross-reactivity was reflected in a large percentage increase in ACTH measured of up to 261% (range 52–261%), depending on the concentration of POMC and the method used. Several studies reported that plasma ACTH precursors (POMC and pro-ACTH) are increased in large and aggressive pituitary tumours in both humans and dogs.34,39–41 Moreover, a recent investigation demonstrated high plasma ACTH precursors in feline patients with PDH. 21 In the same study, the concentration of plasma ACTH precursors in healthy cats was up to 450 pg/ml. Unfortunately, in our study, the concentration of plasma ACTH precursors was not evaluated. However, considering the above, it is possible that POMC-derived peptides might have interfered with the ACTH measurement by the assay.
In the present study, we were able to demonstrate that the measurement of plasma ACTH can help in differentiating cats with HC and PH from those with mimicking diseases and healthy cats. Plasma ACTH did not show significant differences between healthy cats and the OD group, suggesting that the influence of disorders other than HC or PH on the plasma concentration of ACTH might be considered marginal. Cats with PDH and PH showed plasma ACTH significantly higher than the other two groups and only one PDH cat had a plasma ACTH within the RI.In dogs with suspected HC, the most common problem with the ACTH assay is poor sensitivity, 42 as often the concentrations of ACTH do not differ between healthy dogs and those with PDH.43,44 Interestingly in cats, unlike the canine species, the ACTH assay exhibited a greater sensitivity. In fact, in the present study, 87% of cats with PDH (n = 7/8) showed a plasma ACTH above the normal RI. It is also noteworthy that, in the current study, all cats with PH (n = 11) showed a plasma ACTH greater than the upper limit of quantification of the method (>1250 pg/ml). However, such clear differentiation between healthy cats and those with adrenocortical-axis disorders may, at least partly, reflect the low number of cases included. It is not possible to exclude the possibility that more cats with PDH or PH would have shown a plasma ACTH concentration within the normal RI if a greater number of cases had been included.Data from the present study support the proposal that the measurement of plasma ACTH in cats may be regarded as a useful test and should always be part of the diagnostic work-up when HC or PH are suspected. Furthermore, our results suggest that measurement of plasma ACTH may be an accurate test, as in dogs, 44 for differentiating PDH from ADH. The advantages of measuring plasma ACTH include the requirement of a single sample and the test can definitively diagnose ADH. Otherwise, pulsatile ACTH secretion, the sensitivity of cats to stress and inappropriate sample handling allowing ACTH degradation increases the likelihood of a false-positive result in healthy cats. For this reason, it is strongly suggested that a positive result is confirmed through the use of further diagnostic tests (ie, LDDST, ACTHST or diagnostic imaging). However, further prospective investigations involving a large cohort of cats are necessary to determine the exact performance of the ACTH assay and its clinical utility.
Previous investigations in humans30,31 and dogs23,29 have suggested that the use of aprotinin, a protease inhibitor, may help prevent ACTH proteolytic degradation after sample collection. In our study, plasma ACTH values in the presence and absence of aprotinin were not significantly different in the three sets of samples analysed. These results might be explained by the fact that the proteolytic properties of plasma may vary among different species and, conceivably, the concentration of aprotinin used in the present study was not an adequate volume to prevent the enzymatic degradation of ACTH in the feline patient. Based on our findings, if plasma samples are handled appropriately (ie, collected and maintained under cold conditions until analysis), the presence of aprotinin might not be necessary for avoiding ACTH degradation. However, the results described here need to be confirmed in a prospective study including a greater number of samples for analysis.
The main limitation of the current study is the small number of cases included. This is partly due to the fact that diseases affecting the adrenocortical axis are rarer in cats compared with dogs. A further limitation is that the performance of the chemiluminescent assay was not directly compared with a previously validated IRMA method.
Conclusions
The results of the present study demonstrate that the Immulite chemiluminescent assay is a valid technique for measuring ACTH concentration in feline plasma samples. The RI of feline plasma ACTH is wide and different from that of the dog. Owing to the low overlap between healthy cats or cats with OD and those with PH or HC, the measurement of plasma ACTH appears to be useful and should be included in the diagnostic work-up when PH or HC are suspected. In addition, plasma ACTH measurement appears to be an accurate test for determining the aetiology of feline HC.
Acknowledgments
The authors thank Professor Francesco Dondi for the help with the validation tests.
Footnotes
Accepted: 20 April 2020
Author note: This paper was presented in abstract form at the 29th ECVIM-CA Congress in Milan, Italy, 19–21 September 2019.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals (owned or unowned) and procedures that differed from established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Federico Fracassi  https://orcid.org/0000-0003-3121-2199
https://orcid.org/0000-0003-3121-2199
References
- 1. Nelson RW, Feldman EC, Smith MC. Hyperadrenocorticism in cats: seven cases (1978–1987). J Am Vet Med Assoc 1988; 193: 245–250. [PubMed] [Google Scholar]
- 2. Peterson ME, Greco DS, Orth DN. Primary hypoadrenocorticism in ten cats. J Vet Intern Med 1989; 3: 55–58. [DOI] [PubMed] [Google Scholar]
- 3. Bell R, Mellor DJ, Ramsey I, et al. Decreased sodium:potassium ratios in cats: 49 cases. Vet Clin Pathol 2005; 34: 110–114. [DOI] [PubMed] [Google Scholar]
- 4. Valentin SY, Cortright CC, Nelson RW, et al. Clinical findings, diagnostic test results, and treatment outcome in cats with spontaneous hyperadrenocorticism: 30 cases. J Vet Intern Med 2014; 28: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldman EC. Hyperadrenocorticism in cats. In: Feldman EC, Nelson RW, Reusch CE, et al. (eds). Canine and feline endocrinology. 4th ed. St Louis, MO: Elsevier, 2015, pp 452–484. [Google Scholar]
- 6. Scott-Moncrieff JC. Hypoadrenocorticism. In: Feldman EC, Nelson RW, Reusch CE, et al. (eds). Canine and feline endocrinology. 4th ed. St Louis, MO: Elsevier, 2015, pp 485–520. [Google Scholar]
- 7. Boland LA, Barrs VR. Peculiarities of feline hyperadrenocorticism: update on diagnosis and treatment. J Feline Med Surg 2017; 19: 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galac S, Rosenberg D, Pey P. Cushing’s syndrome (hypercortisolism). In: Feldman EC, Fracassi F, Peterson ME. (eds). Feline endocrinology. Milan: Edra, 2019, pp 363–380. [Google Scholar]
- 9. Ramsey I. Feline hypoadrenocorticism. In: Feldman EC, Fracassi F, Peterson ME. (eds). Feline endocrinology. Milan: Edra, 2019, pp 410–421. [Google Scholar]
- 10. Smith MC, Feldman EC. Plasma endogenous ACTH concentrations and plasma cortisol responses to synthetic ACTH and dexamethasone sodium phosphate in healthy cats. Am J Vet Res 1987; 48: 1719–1724. [PubMed] [Google Scholar]
- 11. Zerbe CA, Refsal KR, Peterson ME, et al. Effect of nonadrenal illness on adrenal function in the cat. Am J Vet Res 1987; 48: 451–454. [PubMed] [Google Scholar]
- 12. Peterson ME, Graves TK. Effects of low dosages of intravenous dexamethasone on serum cortisol concentrations in the normal cat. Res Vet Sci 1988; 44: 38–40. [PubMed] [Google Scholar]
- 13. Sparkes AH, Adams DT, Douthwaite JA, et al. Assessment of adrenal function in cats: response to intravenous synthetic ACTH. J Small Anim Pract 1990; 31: 1–4. [Google Scholar]
- 14. Peterson ME, Kemppainen RJ. Comparison of intravenous and intramuscular routes of administering cosyntropin for corticotropin stimulation testing in cats. Am J Vet Res 1992; 53: 1392–1395. [PubMed] [Google Scholar]
- 15. Peterson ME, Kemppainen RJ, Orth DN. Plasma concentration of immunoreactive proopiomelanocortin peptides and cortisol in clinically normal cats. Am J Vet Res 1994; 55: 295–300. [PubMed] [Google Scholar]
- 16. Willemse T, Mol JA. Comparison of in vivo and in vitro corticotrophin-releasing hormone-stimulated release of proopiomelanocortin-derived peptides in cats. Am J Vet Res 1994; 55: 1677–1681. [PubMed] [Google Scholar]
- 17. Schoeman JO, Evans HJ, Childs D, et al. Cortisol response to two different doses in intravenous synthetic ACTH (Tetracosactrin) in overweight cats. J Small Anim Pract 2000; 41: 552–557. [DOI] [PubMed] [Google Scholar]
- 18. Javadi S, Slingerland LI, van de Beck MG, et al. Plasma renin activity, and plasma concentrations of aldosterone, cortisol, adrenocorticotropic hormone, and α-melanocyte-stimulating hormone in normal cats. J Vet Intern Med 2004; 18: 625–631. [DOI] [PubMed] [Google Scholar]
- 19. Chatdarong K, Pnglowhapan S, Karisson A, et al. The effect of ACTH stimulation on cortisol and progesterone concentrations in intact and ovariohysterectomized domestic cats. Theriogenology 2006; 66: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 20. DeClue AE, Martin LG, Behrend EN, et al. Cortisol and aldosterone response to various doses of cosyntropin in healthy cats. J Am Vet Med Assoc 2011; 238: 176–182. [DOI] [PubMed] [Google Scholar]
- 21. Benchekroun G, de Fornel-Thibaud P, Dubord M, et al. Plasma ACTH precursors in cats with pituitary-dependent hyperadrenocorticism. J Vet Intern Med 2012; 26: 575–581. [DOI] [PubMed] [Google Scholar]
- 22. Eiler KC, Bruyette DS, Behrend EN, et al. Comparison of intravenous versus intramuscular administration of corticotropin-releasing hormone in healthy cats. J Vet Intern Med 2013; 27: 516–521. [DOI] [PubMed] [Google Scholar]
- 23. Scott-Moncrieff JC, Koshko MA, Brown JA, et al. Validation of a chemiluminescent enzyme immunometric assay for plasma adrenocorticotropic hormone in the dog. Vet Clin Pathol 2003; 32: 180–187. [DOI] [PubMed] [Google Scholar]
- 24. Ghosh BN, Smith EL, Sayers G. Adrenocorticotrophic hormone: stability studies. Proc Soc Exp Biol Med 1952; 79: 23–27. [DOI] [PubMed] [Google Scholar]
- 25. Meakin J, Tingey W, Nelson D. The catabolism of adrenocorticotrophic hormone: the stability of adrenocorticotrophic hormone in blood, plasma, serum and saline. Endocrinology 1960; 66: 59–72. [DOI] [PubMed] [Google Scholar]
- 26. Diver MJ, Hughes JG, Hutton JL, et al. The long-term stability in whole blood of 14 commonly requested hormone analytes. Ann Clin Biochem 1994; 31: 561–565. [DOI] [PubMed] [Google Scholar]
- 27. Evans MJ, Livesey JH, Ellis MJ, et al. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem 2001; 34: 107–112. [DOI] [PubMed] [Google Scholar]
- 28. Wu ZQ, Xu HG. Preanalytical stability of adrenocorticotropic hormone depends on both time to centrifugation and temperature. J Clin Lab Anal 2017; 31: e1–e3. DOI: 10.1002/jcla.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemppainen RJ, Clark TP, Peterson ME. Preservative effect of aprotinin on canine plasma immunoreactive adrenocorticotropin concentrations. Domest Anim Endocrinol 1994; 11: 355–362. [DOI] [PubMed] [Google Scholar]
- 30. Casati M, Cappellani A, Perlangeli V, et al. Adrenocorticotropic hormone stability in preanalytical phase depends on temperature and proteolytic enzyme inhibitor. Clin Chem Lab Med 2013; 5: e45–e47. DOI: 10.1515/cclm-2012-0431. [DOI] [PubMed] [Google Scholar]
- 31. Tekkeşin N. The effect of temperature and aprotinin on the preanalytical stability of adrenocorticotrophic hormone. Turk J Biochem 2015; 40: 169–174. [Google Scholar]
- 32. Diagnostic Products Corporation. Immulite 2000 ACTH Technical Bulletin (PIL2KAC-18). Los Angeles, CA: Diagnostic Products Corporation, 2018. [Google Scholar]
- 33. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012; 41: 441–453. [DOI] [PubMed] [Google Scholar]
- 34. Granger N, de Fornel P, Devauchelle P, et al. Plasma pro-opiomelanocortin, pro-adrenocorticotropin hormone, and pituitary adenoma size in dogs with Cushing’s disease. J Vet Intern Med 2005; 19: 23–28. [DOI] [PubMed] [Google Scholar]
- 35. Kemppainen RJ, Peterson ME. Domestic cats show episodic variation in plasma concentrations of adrenocorticotropin, α-melanocyte-stimulating hormone (α-MSH), cortisol and thyroxine with circadian variation in plasma α-MSH concentrations. Eur J Endocrinol 1996; 134: 602–609. [DOI] [PubMed] [Google Scholar]
- 36. Willemse T, Vroom MW, Mol JA, et al. Changes in plasma cortisol, corticotropin, and alpha-melanocyte-stimulating hormone concentrations in cats before and after physical restraint and intradermal testing. Am J Vet Res 1993; 54: 69–72. [PubMed] [Google Scholar]
- 37. White A, Gibson S. ACTH precursors: biological significance and clinical relevance. Clin Endocrinol 1998; 48: 251–255. [DOI] [PubMed] [Google Scholar]
- 38. Monaghan PJ, Kyriacou A, Sturgeon C, et al. Proopiomelanocortin interference in the measurement of adrenocorticotrophic hormone: a United Kingdom National External Quality Assessment Service study. Clin Endocrinol 2016; 85: 569–574. [DOI] [PubMed] [Google Scholar]
- 39. Gibson S, Ray DW, Crosby SR, et al. Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab 1996; 81: 497–502. [DOI] [PubMed] [Google Scholar]
- 40. Raffin-Sanson ML, Massias JF, Dumont C, et al. High plasma proopiomelanocortin in aggressive adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab 1996; 81: 4272–4277. [DOI] [PubMed] [Google Scholar]
- 41. Bosje JT, Rijnberk A, Mol JA, et al. Plasma concentrations of ACTH precursors correlate with pituitary size and resistance to dexamethasone in dogs with pituitary-dependent hyperadrenocorticism. Domest Anim Endocrinol 2002; 22: 201–210. [DOI] [PubMed] [Google Scholar]
- 42. Behrend EN, Kooistra HS, Nelson R, et al. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J Vet Intern Med 2013; 27: 1292–1304. [DOI] [PubMed] [Google Scholar]
- 43. Hanson JM, Kooistra HS, Mol JA, et al. Plasma profiles of adrenocorticotropic hormone, cortisol, α-melanocyte-stimulating hormone, and growth hormone in dogs with pituitary-dependent hyperadrenocorticism before and after hypophysectomy. J Endocrinol 2006; 190: 601–609. [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez Pineiro MI, Benchekroun G, de Fornel-Thibaud P, et al. Accuracy of an adrenocorticotropic hormone (ACTH) immunoluminometric assay for differentiating ACTH-dependent from ACTH-independent hyperadrenocorticism in dogs. J Vet Intern Med 2009; 23: 850–855. [DOI] [PubMed] [Google Scholar]


