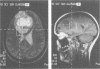
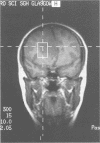
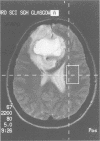
Abstract
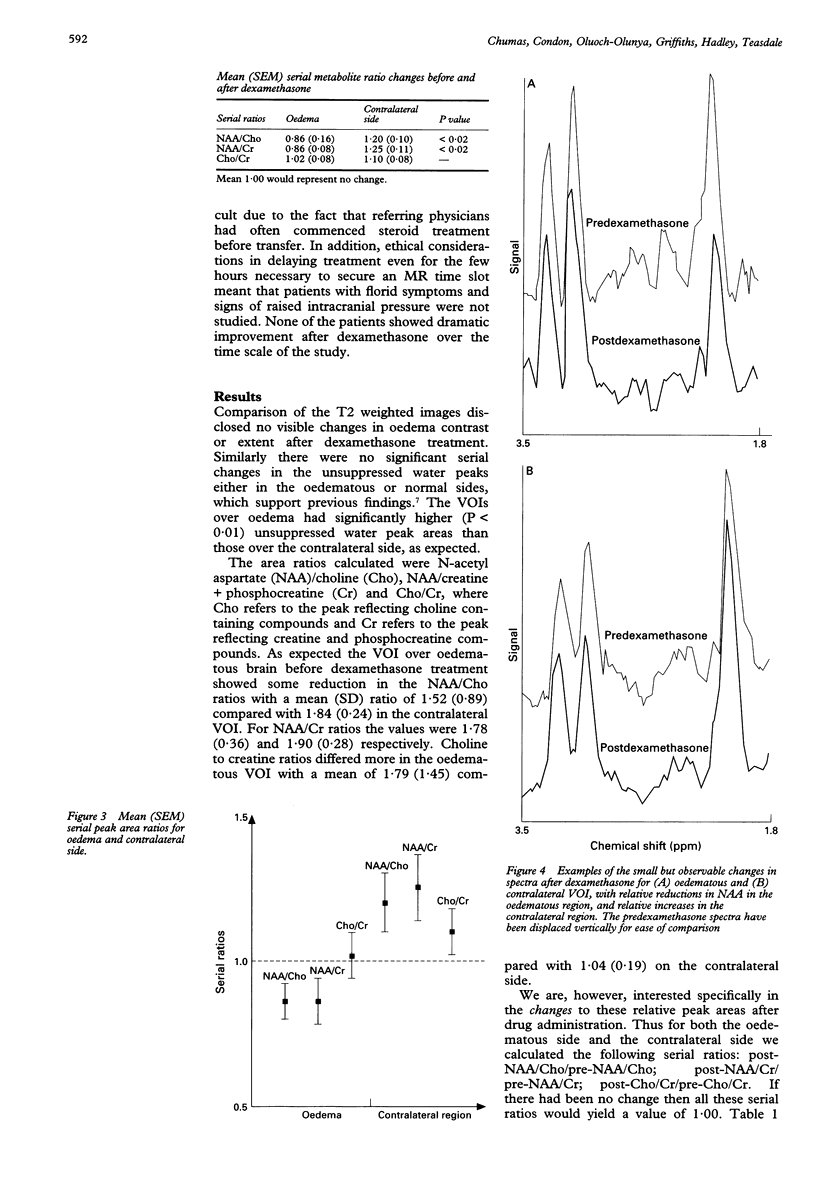
AIMS: To study the mechanism of action of steroids in patients with peritumorous oedema. METHODS: To investigate early cerebral metabolic changes proton magnetic resonance spectroscopy (1H-MRS) was used before and 11 to 14 hours after treatment with dexamethasone (12 mg oral loading and 4 mg four times daily maintenance). Nine patients (two men, seven women, mean age 54) with pronounced oedema associated with various intracranial tumours (two astrocytomas, three meningiomas, two glioblastoma, and two metastases) were examined using MRI and MRS. SE1500/135 volume selected MRS (mean volume 21 ml) were performed on an oedematous region and a contralateral region. All spectra were acquired with and without water suppression. Metabolite peak area ratios were determined. RESULTS: Regions of oedema had significantly (P < 0.01) higher unsuppressed water than the contralateral regions, as expected. There was no change at this early time point after dexamethasone. The ratio of the area of choline containing compounds to that creatine and phosphocreatine compounds was determined after which the serial ratios of these before and after were calculated (a serial ratio of 1.0 would indicate no change in the choline to creatine ratios after steroid administration). The mean serial ratios for the area of oedema were 1.02 (SEM 0.08) and 1.10 (0.08) for the contralateral volume of interest, indicating no significant changes. However, significant changes (P < 0.02) were found in the N-acetyl-aspartate (NAA)/choline serial ratios (0.86 (0.06) in the area of oedema, 1.20 (0.10) in contralateral brain) and the NAA/creatine serial ratios (0.86 (0.08) for the oedema, 1.25 (0.11) in contralateral brain). CONCLUSIONS: Such rapid changes may be explained either by relatively large alterations in the relaxation characteristics of NAA or, more controversially, by actual changes in the amounts of NAA. It is proposed that steroids act primarily by causing early metabolic changes that are later expressed in improvements in intracranial volume relations.
Full text
PDF
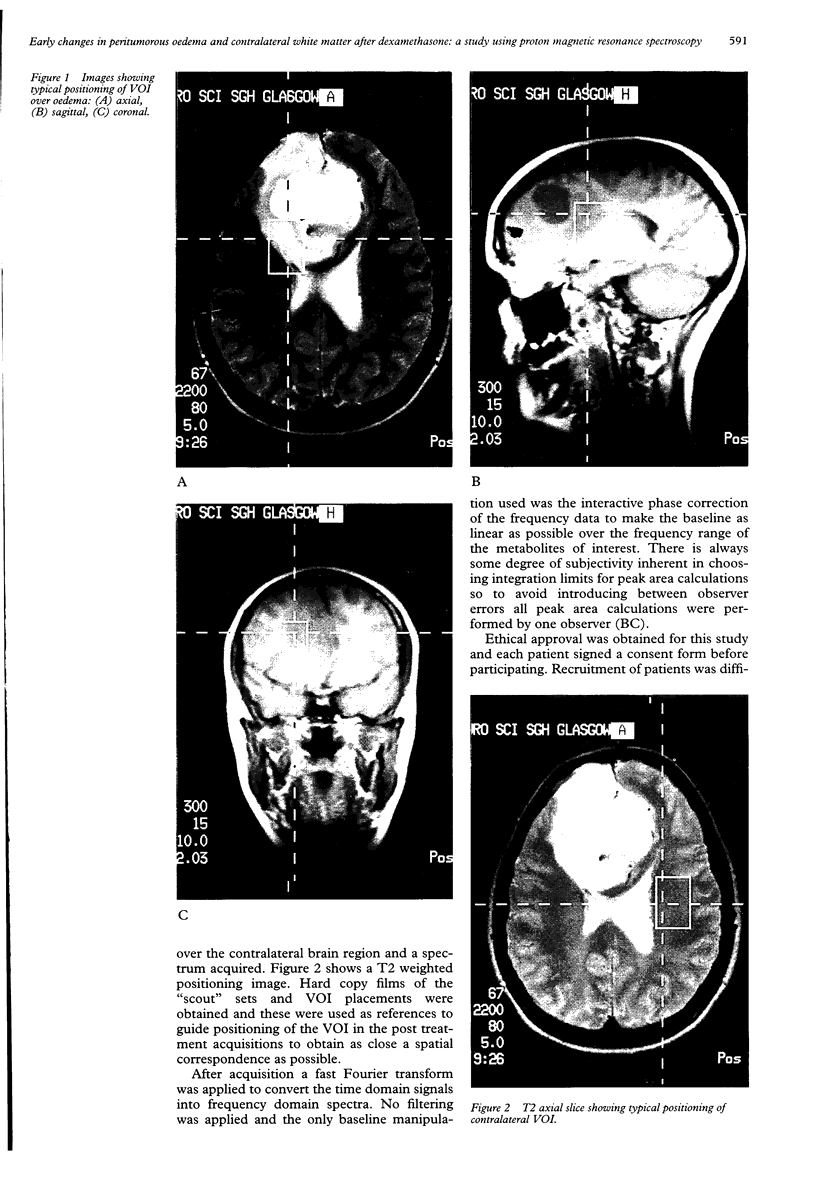



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti E., Hartmann A., Schütz H. J., Schreckenberger F. The effect of large doses of dexamethasone on the cerebrospinal fluid pressure in patients with supratentorial tumors. J Neurol. 1978 Feb 14;217(3):173–181. doi: 10.1007/BF00312958. [DOI] [PubMed] [Google Scholar]
- Andersen C., Astrup J., Gyldensted C. Quantitation of peritumoural oedema and the effect of steroids using NMR-relaxation time imaging and blood-brain barrier analysis. Acta Neurochir Suppl (Wien) 1994;60:413–415. doi: 10.1007/978-3-7091-9334-1_112. [DOI] [PubMed] [Google Scholar]
- Andersen C., Haselgrove J. C., Doenstrup S., Astrup J., Gyldensted C. Resorption of peritumoural oedema in cerebral gliomas during dexamethasone treatment evaluated by NMR relaxation time imaging. Acta Neurochir (Wien) 1993;122(3-4):218–224. doi: 10.1007/BF01405532. [DOI] [PubMed] [Google Scholar]
- Bell B. A., Smith M. A., Kean D. M., McGhee C. N., MacDonald H. L., Miller J. D., Barnett G. H., Tocher J. L., Douglas R. H., Best J. J. Brain water measured by magnetic resonance imaging. Correlation with direct estimation and changes after mannitol and dexamethasone. Lancet. 1987 Jan 10;1(8524):66–69. doi: 10.1016/s0140-6736(87)91908-8. [DOI] [PubMed] [Google Scholar]
- Birken D. L., Oldendorf W. H. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989 Spring;13(1):23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Chumas P. D., Del Bigio M. R., Drake J. M., Tuor U. I. A comparison of the protective effect of dexamethasone to other potential prophylactic agents in a neonatal rat model of cerebral hypoxia-ischemia. J Neurosurg. 1993 Sep;79(3):414–420. doi: 10.3171/jns.1993.79.3.0414. [DOI] [PubMed] [Google Scholar]
- D'Adamo A. F., Jr, Gidez L. I., Yatsu F. M. Acetyl transport mechanisms. Involvement of N-acetyl aspartic acid in de novo fatty acid biosynthesis in the developing rat brain. Exp Brain Res. 1968;5(4):267–273. doi: 10.1007/BF00235902. [DOI] [PubMed] [Google Scholar]
- Duyn J. H., Gillen J., Sobering G., van Zijl P. C., Moonen C. T. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993 Jul;188(1):277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- Frahm J., Bruhn H., Gyngell M. L., Merboldt K. D., Hänicke W., Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magn Reson Med. 1989 Jul;11(1):47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]
- GALICICH J. H., FRENCH L. A., MELBY J. C. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961 Feb;81:46–53. [PubMed] [Google Scholar]
- Henriksen O., Wieslander S., Gjerris F., Jensen K. M. In vivo 1H-spectroscopy of human intracranial tumors at 1.5 tesla. Preliminary experience at a clinical installation. Acta Radiol. 1991 Mar;32(2):95–99. doi: 10.1177/028418519103200201. [DOI] [PubMed] [Google Scholar]
- INGRAHAM F. D., MATSON D. D., McLAURIN R. L. Cortisone and ACTH as an adjunct to the surgery of craniopharyngiomas. N Engl J Med. 1952 Apr 10;246(15):568–571. doi: 10.1056/NEJM195204102461502. [DOI] [PubMed] [Google Scholar]
- Jarden J. O., Dhawan V., Poltorak A., Posner J. B., Rottenberg D. A. Positron emission tomographic measurement of blood-to-brain and blood-to-tumor transport of 82Rb: the effect of dexamethasone and whole-brain radiation therapy. Ann Neurol. 1985 Dec;18(6):636–646. doi: 10.1002/ana.410180603. [DOI] [PubMed] [Google Scholar]
- Johnston I., Gilday D. L., Hendrick E. B. Experimental effects of steroids and steroid withdrawal on cerebrospinal fluid absorption. J Neurosurg. 1975 Jun;42(6):690–695. doi: 10.3171/jns.1975.42.6.0690. [DOI] [PubMed] [Google Scholar]
- Kamada K., Houkin K., Hida K., Matsuzawa H., Iwasaki Y., Abe H., Nakada T. Localized proton spectroscopy of focal brain pathology in humans: significant effects of edema on spin-spin relaxation time. Magn Reson Med. 1994 May;31(5):537–540. doi: 10.1002/mrm.1910310510. [DOI] [PubMed] [Google Scholar]
- Kamada K., Houkin K., Iwasaki Y., Abe H., Kashiwaba T. In vivo proton magnetic resonance spectroscopy for metabolic changes of human brain edema. Neurol Med Chir (Tokyo) 1994 Oct;34(10):676–681. doi: 10.2176/nmc.34.676. [DOI] [PubMed] [Google Scholar]
- Koide T., Wieloch T. W., Siesjö B. K. Chronic dexamethasone pretreatment aggravates ischemic neuronal necrosis. J Cereb Blood Flow Metab. 1986 Aug;6(4):395–404. doi: 10.1038/jcbfm.1986.72. [DOI] [PubMed] [Google Scholar]
- Kostron H., Fischer J. Regional, cellular, and subcellular distribution of [3H]dexamethasone in rat brain edema. Surg Neurol. 1983 Jul;20(1):48–54. doi: 10.1016/0090-3019(83)90105-2. [DOI] [PubMed] [Google Scholar]
- Leenders K. L., Beaney R. P., Brooks D. J., Lammertsma A. A., Heather J. D., McKenzie C. G. Dexamethasone treatment of brain tumor patients: effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurology. 1985 Nov;35(11):1610–1616. doi: 10.1212/wnl.35.11.1610. [DOI] [PubMed] [Google Scholar]
- MCINTOSH J. C., COOPER J. R. FUNCTION OF N-ACETYL ASPARTIC ACID IN THE BRAIN: EFFECTS OF CERTAIN DRUGS. Nature. 1964 Aug 8;203:658–658. doi: 10.1038/203658a0. [DOI] [PubMed] [Google Scholar]
- Martins A. N., Ramirez A., Solomon L. S., Wiese G. M. The effect of dexamethasone on the rate of formation of cerebrospinal fluid in the monkey. J Neurosurg. 1974 Nov;41(5):550–554. doi: 10.3171/jns.1974.41.5.0550. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991 Apr;12(4):141–147. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Leech P. Effects of mannitol and steroid therapy on intracranial volume-pressure relationships in patients. J Neurosurg. 1975 Mar;42(3):274–281. doi: 10.3171/jns.1975.42.3.0274. [DOI] [PubMed] [Google Scholar]
- Sato O., Hara M., Asai T., Tsugane R., Kageyama N. The effect of dexamethasone phosphate on the production rate of cerebrospinal fluid in the spinal subarachnoid space of dogs. J Neurosurg. 1973 Oct;39(4):480–484. doi: 10.3171/jns.1973.39.4.0480. [DOI] [PubMed] [Google Scholar]
- Shapiro W. R., Hiesiger E. M., Cooney G. A., Basler G. A., Lipschutz L. E., Posner J. B. Temporal effects of dexamethasone on blood-to-brain and blood-to-tumor transport of 14C-alpha-aminoisobutyric acid in rat C6 glioma. J Neurooncol. 1990 Jun;8(3):197–204. doi: 10.1007/BF00177352. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H. Studies on the distribution of N-acetyl-L-aspartic acid in brain. J Biol Chem. 1957 Jan;224(1):41–45. [PubMed] [Google Scholar]
- Thurston J. H., Hauhart R. E., Dirgo J. A., Jones E. M. Mechanisms of increased brain glucose and glycogen after hydrocortisone: possible clinical significance. Ann Neurol. 1980 Jun;7(6):515–523. doi: 10.1002/ana.410070604. [DOI] [PubMed] [Google Scholar]
- Thurston J. H., Pierce R. W. Increase of glucose and high energy phosphate reserve in the brain after hydrocortisone. J Neurochem. 1969 Jan;16(1):107–111. doi: 10.1111/j.1471-4159.1969.tb10348.x. [DOI] [PubMed] [Google Scholar]
- Vielkind U., Walencewicz A., Levine J. M., Bohn M. C. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res. 1990 Nov;27(3):360–373. doi: 10.1002/jnr.490270315. [DOI] [PubMed] [Google Scholar]