Abstract
In Rhizobium meliloti (Sinorhizobium meliloti) cultures, the endo-1,3-1,4-β-glycanases ExoK and ExsH depolymerize nascent high-molecular-weight (HMW) succinoglycan to yield low-molecular-weight (LMW) succinoglycan. We report here that the succinyl and acetyl modifications of succinoglycan influence the susceptibility of succinoglycan to cleavage by these glycanases. It was previously shown that exoH mutants, which are blocked in the succinylation of succinoglycan, exhibit a defect in the production of LMW succinoglycan. We have determined that exoZ mutants, which are blocked in the acetylation of succinoglycan, exhibit an increase in production of LMW succinoglycan. For both wild-type and exoZ mutant strains, production of LMW succinoglycan is dependent on the exoK+ and exsH+ genes, implying that the ExoK and ExsH glycanases cleave HMW succinoglycan to yield LMW succinoglycan. By supplementing cultures of glycanase-deficient strains with exogenously added ExoK or ExsH, we have demonstrated directly that the absence of the acetyl group increases the susceptibility of succinoglycan to cleavage by ExoK and ExsH, that the absence of the succinyl group decreases the susceptibility of succinoglycan to cleavage, and that the succinyl effect outweighs the acetyl effect for succinoglycan lacking both modifications. Strikingly, nonsuccinylated succinoglycan actually can be cleaved by ExoK and ExsH to yield LMW succinoglycan, but only when the glycanases are added to cultures at greater than physiologically relevant concentrations. Thus, we conclude that the molecular weight distribution of succinoglycan in R. meliloti cultures is determined by both the levels of ExoK and ExsH glycanase expression and the susceptibility of succinoglycan to cleavage.
Bacterial polysaccharides are crucial for the establishment of the nitrogen fixing symbiosis between the soil bacterium Rhizobium meliloti (Sinorhizobium meliloti) and its host plant, alfalfa (14, 29). R. meliloti has the capacity to produce two exopolysaccharides (EPSs), succinoglycan and EPS II, as well as a capsular polysaccharide, KPS (22, 37, 40). Each polysaccharide can be produced in symbiotically active or inactive forms, and the bacterium must produce at least one of these polysaccharides in a symbiotically active form in order to invade root nodules successfully (15, 18, 28, 29, 41). For example, wild-type strain Rm1021, which fails to produce EPS II and which produces KPS in a symbiotically inactive form, depends on the production of succinoglycan in a symbiotically active form for successful root nodule invasion. The symbiotically active forms of these polysaccharides may function as signals to facilitate the nodule invasion process (2, 18).
Succinoglycan is a polymer of an octasaccharide repeating unit consisting of one galactose and seven glucose units; the polymer has approximately one acetyl, one succinyl, and one pyruvyl modification per repeating unit (1, 24, 37). Wild-type R. meliloti produces succinoglycan in low-molecular-weight (LMW) forms, consisting of short oligomers of the octasaccharide repeating unit, and in high-molecular-weight (HMW) forms, consisting of hundreds to thousands of repeating units (2, 27). The molecular weight distribution of succinoglycan (as well as that of EPS II) seems to be relevant to symbiotic activity (2, 18); specific LMW forms of succinoglycan, highly charged tetramers of the octasaccharide repeating unit, have been proposed to be active in mediating nodule invasion (2).
The steps of octasaccharide synthesis and modification are well defined genetically (3–5, 16, 17, 29, 32, 34). A series of genes (termed exo) involved in succinoglycan biosynthesis have been cloned and sequenced (3–5, 16, 17, 32, 34). Most of these genes have been assigned functions in the synthesis and modification of the octasaccharide repeating unit, based on analyses of radiolabeled lipid-linked intermediates of succinoglycan biosynthesis that accumulate in various exo mutants and based on nucleotide sequence data (16, 17, 39).
How R. meliloti accomplishes the production of two distinct size classes of succinoglycan (LMW and HMW) from pools of lipid-linked octasaccharide repeating units is less clear. Becker et al. (7) have proposed that ExoP regulates the extent of succinoglycan polymerization (7, 8), and González et al. (19) have reported that ExoT and ExoQ are required for the direct synthesis of LMW and HMW succinoglycan, respectively. Thus, R. meliloti apparently can produce LMW and HMW succinoglycan by conducting limited polymerization as well as extensive polymerization of octasaccharide repeating units.
In addition, we have obtained genetic and biochemical evidence that R. meliloti can produce LMW succinoglycan by a second mechanism, depolymerization of HMW succinoglycan (44, 45). The R. meliloti exoK and exsH genes encode endo-1,3-1,4-β-glycanases (3, 17, 44) that can depolymerize succinoglycan to yield monomers or multimers of the octasaccharide repeating unit (45). ExoK and ExsH have been implicated in the production of LMW succinoglycan in cultures, based on our observations that exoK exsH double mutants exhibit a dramatic defect in the production of LMW succinoglycan (44) and that the exogenous addition of ExoK or ExsH to cultures of the exoK exsH strain restores the production of LMW succinoglycan (45). Curiously though, we observed that neither ExoK nor ExsH can efficiently cleave HMW succinoglycan in vitro (45). We resolved this apparent contradiction by determining that ExoK and ExsH specifically depolymerize nascent succinoglycan, but not succinoglycan that has accumulated in culture supernatants, to yield LMW succinoglycan (45). Apparently succinoglycan undergoes a transition from a glycanase-susceptible form to a glycanase-refractory form in cultures. This transition may correspond to a change in the physical structure of succinoglycan molecules, such as a change from random coils to helices or from individual molecules to aggregates (11), for example.
We were interested in determining whether the succinyl and acetyl modifications of succinoglycan might affect the molecular weight distribution of succinoglycan by influencing the susceptibility of succinoglycan to cleavage by ExoK and ExsH. The fact that exoH mutants, which synthesize nonsuccinylated succinoglycan (28), and exoK exsH mutants, which fail to express the ExoK and ExsH glycanases (44), produce almost exclusively HMW succinoglycan had suggested that the absence of the succinyl modification from succinoglycan inhibits glycanase-mediated cleavage of the polysaccharide. The molecular weight distribution of the succinoglycan produced by exoZ mutants, which synthesize nonacetylated succinoglycan (38), had not been examined. However, it had been shown previously that deacetylation of certain other bacterial polysaccharides, including alginate produced by Pseudomonas aeruginosa and Azotobacter vinelandii, gellan produced by Sphingomonas strains, and surface polysaccharides produced by Rhizobium leguminosarum bv. trifolii, increases the susceptibility of these polysaccharides to cleavage by specific polysaccharide lyases (23, 26, 43).
Here we report that the acetyl and succinyl modifications of succinoglycan do indeed influence the susceptibility of succinoglycan to cleavage by the ExoK and ExsH glycanases. By integrating the analyses of mutants blocked in the modification of succinoglycan and/or the expression of glycanases with the reconstitution of glycanase activity in cultures, we have determined that nonacetylated succinoglycan has a high degree of susceptibility to cleavage by ExoK and ExsH, that normally modified succinoglycan has an intermediate degree of susceptibility to cleavage, and that nonsuccinylated succinoglycan has a low degree of susceptibility to cleavage. We have also determined that, like normally modified succinoglycan, nonacetylated and nonsuccinylated forms of succinoglycan undergo transitions from glycanase-susceptible to glycanase-refractory forms as they accumulate in cultures. Our results indicate that the molecular weight distribution of each of the variously modified forms of succinoglycan can be manipulated with a high degree of control simply by culturing the various R. meliloti strains in the presence of various amounts of exogenously added glycanase. Furthermore, the cleavage of succinoglycan by glycanases may have important implications for biological phenomena, such as the establishment of nitrogen fixing symbiosis and biofilm formation by R. meliloti.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and Calcofluor halo assay.
Strains used in this study are listed in Table 1. The following growth media were used: Luria-Bertani medium (33), MGS medium (potassium phosphate [100 mM, pH 7.3], mannitol [55 mM], monosodium glutamate [5 mM], sodium chloride [8 mM]), and GMS medium (containing mannitol at a final concentration of 27.5 mM) (46). MGS and GMS media were also supplemented with a mixture of magnesium sulfate (1 mM), calcium chloride (0.25 mM), biotin (100 μg/liter), and thiamine (100 μg/liter for GMS medium) after autoclaving the media.
TABLE 1.
Bacterial strains
| Strain | Genotype | Source or reference |
|---|---|---|
| Rm1021 | RmSU47 streptomycin resistant; wild type | F. Ausubel |
| Rm7210 | exoY210::Tn5 | J. Leigh |
| Rm7225 | exoH225::Tn5-233 | 28 |
| Rm8341 | exoZ341::Tn5 | 32 |
| Rm8445 | exoK445::Tn5 | 32 |
| Rm8800 | exoY210::Tn5-Tp | 44 |
| Rm8801 | exoK445::Tn5-233 | 44 |
| Rm8822 | exsH13::Tn5 | 44 |
| Rm8823 | exsH13::Tn5-132 | 44 |
| Rm8826 | exoK445::Tn5-233 exsH13::Tn5 | 44 |
| Rm8829 | exoH225::Tn5-233 exoK445::Tn5 | 44 |
| Rm8830 | exoH225::Tn5-233 exsH13::Tn5 | 44 |
| Rm8831 | exoH225::Tn5-233 exoK445::Tn5 exsH13::Tn5-132 | 44 |
| Rm8834 | exoY210::Tn5-Tp exoK445::Tn5-233 exsH13::Tn5 | 44 |
| Rm8835 | exoZ341::Tn5-Tp | This work |
| Rm8836 | exoZ341::Tn5-Tp exoK445::Tn5-233 | This work |
| Rm8837 | exoZ341::Tn5-Tp exsH13::Tn5 | This work |
| Rm8838 | exoZ341::Tn5-Tp exoK445::Tn5-233 exsH13::Tn5 | This work |
| Rm8839 | exoH225::Tn5-233 exoZ341::Tn5 | L. Reuber |
| Rm8840 | exoH225::Tn5-233 exoZ341::Tn5-Tp exoK445::Tn5 | This work |
| Rm8841 | exoH225::Tn5-233 exoZ341::Tn5 exsH13::Tn5-132 | This work |
| Rm8842 | exoH225::Tn5-233 exoZ341::Tn5-Tp exoK445::Tn5 exsH13::Tn5-132 | This work |
| Rm8843 | exoY210::Tn5-Tp exoH225::Tn5-233 | This work |
| Rm8847 | exoY210::Tn5-Tp exoZ341::Tn5 | This work |
| Rm8851 | exoY210::Tn5-Tp exoH225::Tn5-233 exoZ341::Tn5 | This work |
Cultivation of R. meliloti strains and analyses of extracellular proteins.
To measure and compare production of extracellular carbohydrate or expression of glycanases by various R. meliloti strains, we incubated Luria-Bertani cultures to saturation, determined the optical densities at 600 nm of the cultures, washed the cells in sterile 0.85% saline, inoculated 50 ml of GMS or MGS cultures in 250-ml flasks with equal titers of cells, and incubated the cultures at 30°C with aeration until a given time point or until cultures reached a given level of extracellular carbohydrate.
For experiments involving analyses of extracellular proteins, cells were removed from aliquots of culture supernatants by centrifugation (20,800 × g, 5 min). The proteins in 5-μl aliquots of cell-free supernatants were separated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide separating gels) and analyzed by use of a Tropix Western Light protein detection kit (Bedford, Massachusetts). Polyclonal antibodies that recognize ExoK and ExsH have been described previously (45).
Analyses of extracellular carbohydrate in culture supernatants.
Strains were cultivated in GMS medium as described above. Cells were removed from culture supernatants by centrifugation (27,000 × g, 20 min); samples that appeared viscous were diluted in 5 volumes of distilled water and were subjected to further centrifugation (20,000 × g, 20 min). We used previously described approaches for Biogel A-5 chromatographic analyses (27). For A-5 column chromatography, samples consisting of up to 2 mg of carbohydrate from lyophilized culture supernatants dissolved in a final volume of 20 ml of column buffer (sodium phosphate [50 mM, pH 7.0], sodium chloride [100 mM]) were applied to the column. Succinoglycan consistently elutes in distinct HMW (excluded-volume) and LMW (included-volume) fractions.
Carbohydrate concentrations and relative reducing end concentrations were determined by the anthrone-sulfuric acid method (31) and the Lever method (30), respectively. It is important to note that the Lever assay overestimates the actual concentration of reducing ends for a given polysaccharide sample, presumably due to alkaline hydrolysis of polysaccharide chains during the course of the assay (21). We use the Lever assay here not to measure the precise degree of polymerization of polysaccharides but to provide a relative measure of the degree of polymerization between samples.
Depolymerization of succinoglycan by ExoK and ExsH added exogenously to culture supernatants.
Expression and purification of ExoK and ExsH, by use of the pET5a vector (Promega, Madison, Wis.) in Escherichia coli, has been described previously (45). For experiments involving the supplementation of cultures with exogenously added ExoK or ExsH, 2-ml aliquots of R. meliloti cultures were transferred from 50-ml cultures (in 250-ml flasks) to test tubes at the time of the addition of enzyme (or the addition of water as a control) and these cultures were further incubated with aeration. For experiments in which glycanases were added to growth medium at the same time that the growth medium itself was inoculated with bacteria, 2-ml aliquots of culture were incubated in test tubes with aeration for the entire cultivation period. For experiments in which glycanases were added to cultures at physiologically relevant levels (200 ng/ml for ExsH) over the time course of culture incubation, glycanases were added to cultures at the following time points to the cumulative final concentrations that are listed: 18 h, to 40 ng/ml; 42 h, to 160 ng/ml; and 66 h, to 200 ng/ml. For experiments in which much higher levels of glycanases (10-μg/ml final concentration) were added over the time course of culture incubation, glycanases were added to cultures in eight equal aliquots at approximately 12-h intervals, beginning at the time of inoculation of the cultures with bacteria. Otherwise, glycanases were added to cultures at the time points and to the final concentrations indicated in the Results section. As a control we had previously determined that the addition of purified ExoK and ExsH to R. meliloti cultures does not cause the bacteria to produce any extracellular carbohydrate in addition to the succinoglycan that is normally produced (45).
RESULTS
The exoH mutation decreases production of LMW succinoglycan, and the exoZ mutation increases production of LMW succinoglycan.
To establish whether the succinyl and acetyl modifications of succinoglycan may influence the susceptibility of succinoglycan to cleavage by glycanases, we determined the ratio of LMW succinoglycan to HMW succinoglycan produced by strains defective in the expression of glycanases and/or defective in the modification of succinoglycan. Specifically, we tested R. meliloti strains representing each combination of the wild-type and mutant alleles for the glycanase genes exoK and exsH, the succinyl transferase gene exoH, and the acetyltransferase gene exoZ. For these experiments, we cultivated strains for 5 days in GMS minimal medium, in which succinoglycan constitutes approximately 97% of the extracellular carbohydrate produced by R. meliloti (27, 44), and then separated the succinoglycan into HMW and LMW fractions by Biogel A-5 gel filtration chromatography.
Leigh and Lee (27) had previously determined that the wild-type strain, when grown under these conditions, produces approximately half of its succinoglycan in HMW forms and half in LMW forms (Table 2). We subsequently demonstrated that this production of wild-type LMW succinoglycan is almost entirely dependent on the expression of the glycanases ExoK and ExsH (44). Thus, exoK mutants exhibit a slight defect in the production of LMW succinoglycan, exsH mutants exhibit a more dramatic defect, and exoK exsH mutants exhibit the most severe defect, producing approximately 3% of their total extracellular carbohydrate in LMW forms (Table 2) (44). The phenotypes of these mutants reflect a decrease in the conversion of HMW succinoglycan to LMW succinoglycan by glycanases (44).
TABLE 2.
Percentage of total extracellular carbohydrate in culture supernatants that is in LMW formsa
| Glycanase genotype | % Total extracellular carbohydrate in LMW form for indicated succinylation/acetylation genotype
|
|||
|---|---|---|---|---|
| Wild type | exoZ | exoH | exoH exoZ | |
| Wild type | 48 | 86b | 5 | 7 |
| exoK | 34 | 80 | n.d.c | n.d. |
| exsH | 9 | 33d | n.d. | n.d. |
| exoK exsH | 3 | 9 | 3 | 4 |
Under the incubation conditions used here, approximately 97% of total extracellular carbohydrate is succinoglycan. Data represent averages of two cultures. The standard deviation (SD) is ≤3.5% except where indicated.
SD = 6%.
n.d., not determined.
SD = 12.5%.
As reported by Leigh and Lee (27), exoH mutants, which are defective in the succinylation of succinoglycan, exhibit a severe defect in the production of LMW succinoglycan (Table 2). The defect of exoH mutants is of approximately the same magnitude as that of exoK exsH mutants, and is only minimally affected by the additional mutation of both the exoK and exsH genes (Table 2), suggesting that under these cultivation conditions nonsuccinylated succinoglycan is a poor substrate for cleavage by ExoK and ExsH.
Strikingly, we determined that exoZ mutants, which are defective in the acetylation of succinoglycan, exhibit a phenotype opposite to those of exoH and exoK exsH mutants in terms of the molecular weight distribution of succinoglycan (Table 2). The exoZ mutant exhibits an increase in the proportion of its succinoglycan that is present in LMW forms. To determine which mutations would be epistatic in multiply mutant strains, we tested exoZ exoK, exoZ exsH, and exoZ exoK exsH mutants and determined that production of LMW succinoglycan by exoZ mutants is almost entirely dependent on the exoK+ and exsH+ genes. This result implies that in cultures of exoZ mutants, as in the wild-type strain, ExoK and ExsH cleave HMW succinoglycan to yield LMW succinoglycan (Table 2) and suggests that nonacetylated succinoglycan is a better substrate for cleavage than is normally modified succinoglycan. Analyses of exoH exoZ mutants indicate that the production of LMW succinoglycan by exoZ mutants is also dependent on the exoH+ gene, suggesting that the negative effect on the production of LMW succinoglycan associated with the absence of the succinyl modification almost entirely outweighs the positive effect associated with the absence of the acetyl modification.
Changes in levels of succinoglycan production or glycanase expression are not sufficient to account for the effects of the exoH and exoZ mutations on the molecular weight distribution of succinoglycan.
Perhaps the simplest hypothesis to explain our data is that the acetyl and succinyl modifications of succinoglycan influence the susceptibility of succinoglycan to cleavage by ExoK and ExsH. However, a plausible alternative hypothesis is that the exoH and/or exoZ mutations cause changes in levels of glycanase expression or succinoglycan production, resulting in shifts in the ratio of glycanase to succinoglycan that cause shifts in the molecular weight distribution of succinoglycan. For example, Becker et al. (4) have reported that exoH::Tn5 mutations exhibit a highly polar effect on the transcription of the downstream exoK gene, raising the possibility that exoH mutants are defective in the production of LMW succinoglycan due to decreased glycanase expression. Furthermore, Buendia et al. (10) have reported that when colonies are cultivated on growth medium containing the succinoglycan-binding dye Calcofluor and are visualized under UV light, exoZ mutant colonies exhibit a delay in the onset of fluorescence relative to colonies of the wild-type strain. This suggests the possibility that exoZ mutants exhibit a decreased rate of production of succinoglycan.
To test whether exoH and exoZ mutations affect glycanase expression, we cultivated exoY, exoY exoH, exoY exoZ, and exoY exoH exoZ strains in minimal media, removed cells from cultures by centrifugation, and measured levels of extracellular ExoK and ExsH by use of polyclonal antibodies that recognize ExoK and ExsH (45) (Fig. 1). We tested exoY strains, rather than the corresponding exoY+ strains, in order to improve the efficiency of removal of cells from cultures by centrifugation; exoY mutants are blocked in the production of succinoglycan (39) and therefore yield cultures with relatively low viscosities.
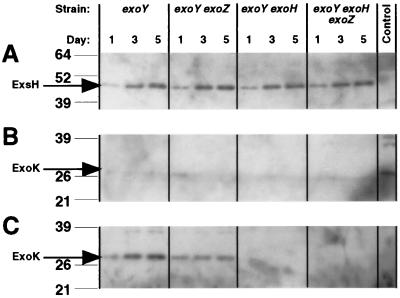
FIG. 1.
Western blots measuring the effect of the exoZ and exoH mutations on the extracellular accumulation of ExsH (by use of anti-ExsH polyclonal antibodies) (A) and ExoK (by use of anti-ExoK polyclonal antibodies) (B and C). The exoY, exoY exoZ, exoY exoH, and exoY exoH exoZ strains were cultivated in GMS medium (A, B) or in MGS medium (C) for a total of 5 days. Each lane contains a 5-μl aliquot of cell-free culture supernatant from day 1, day 3, or day 5 cultures. For blots A and C, the control lane corresponds to the negative control exoY exoK exsH strain. For blot B, the control lane corresponds to the exoY strain cultivated in MGS medium, which serves as a positive control for detection of extracellular ExoK. Each control sample corresponds to cell-free supernatants of a day 5 culture. Arrows indicate expected positions of ExsH and ExoK. Lines indicate positions of molecular weight markers (in kilodaltons).
Our results indicate that the effects of exoH and exoZ mutations on the molecular weight distribution of succinoglycan are not due to effects of these mutations on glycanase expression. We have previously determined that in cultures of the exoY strain cultivated in GMS medium, ExsH accumulates extracellularly to approximately 200 ng/ml, whereas ExoK fails to accumulate extracellularly to a detectable extent (<6 ng/ml) (45). Our results here indicate that for strains cultivated in GMS medium, neither the exoH nor the exoZ mutation has any apparent effect on levels of extracellular ExsH (Fig. 1A) or extracellular ExoK (Fig. 1B).
Interestingly, the exoH mutation does have a detectable, negative effect on levels of extracellular ExoK in MGS medium. MGS medium is similar to GMS medium, except that MGS medium contains higher concentrations of phosphate and mannitol than does GMS medium and lacks certain salts that are added in trace amounts to GMS medium. We had previously determined that in cultures of the exoY mutant cultivated in MGS medium ExsH and ExoK both accumulate to approximately 200 ng/ml (45). We determined here that in cultures of exoH mutant strains cultivated in MGS medium ExoK fails to accumulate extracellularly to a detectable extent (<6 ng/ml) (Fig. 1C). This result is consistent with the previously mentioned polar effect of the exoH::Tn5 mutation on transcription of the downstream exoK gene (4).
Given that ExoK accumulates in GMS cultures to levels below the limit of detection for the detection method that we utilized here, our results do not rule out the possibility that the exoH mutation also affects levels of extracellular ExoK in GMS cultures. Yet, given that ExsH makes a far greater contribution than does ExoK in terms of the production of LMW succinoglycan by R. meliloti strains grown in GMS medium (44) and given that the exoH mutation has no effect on levels of extracellular ExsH (Fig. 1A), our results clearly indicate that the severe defect in the production of LMW succinoglycan associated with the exoH strain cultivated in GMS medium is not simply due to a negative effect of the exoH mutation on the expression of glycanases. In addition, our results indicate that the exoZ mutation does not cause an increase in the production of extracellular ExoK or ExsH (Fig. 1), which rules out the possibility that the increased production of LMW succinoglycan associated with the exoZ mutant strain is due to an increase in the expression of glycanases.
To test the possibility that the exoH and exoZ mutations cause changes in levels of total succinoglycan production, we cultivated wild-type strains and exoH and exoZ mutants in parallel and compared levels of succinoglycan production over an incubation period of 5 days. The exoH mutant exhibited no change in levels of succinoglycan production in comparison to the wild-type strain (data not shown). The exoZ mutant exhibited an approximately 25% decrease in succinoglycan production relative to the wild-type strain, consistent with the delay in the appearance of Calcofluor fluorescence associated with colonies of this strain. However, we have determined directly that this difference is too subtle to account for differences in the molecular weight distribution of succinoglycan in wild-type versus exoZ cultures (see below).
The absence of the acetyl modification of succinoglycan increases the susceptibility of succinoglycan to cleavage by ExoK and ExsH.
To directly test whether the acetyl and succinyl modifications of succinoglycan influence cleavage of the polysaccharide by glycanases, we proceeded to reconstitute normal levels of extracellular glycanase in cultures of exoK exsH glycanase-deficient strains, to see whether this would restore the production of LMW succinoglycan to levels typical of those of the corresponding exoK+ exsH+ glycanase-producing strains. Given that ExsH makes a greater contribution to the production of LMW succinoglycan than does ExoK (for glycanase-producing strains cultivated in GMS medium), we focused first on ExsH. To cultures of the glycanase-deficient exoK exsH, exoZ exoK exsH, exoH exoK exsH, and exoH exoZ exoK exsH strains we added physiologically relevant amounts of ExsH (45) gradually to a cumulative final concentration of 200 ng/ml, over a time course of 4 days. We then separated the succinoglycan present in cultures into HMW and LMW fractions by Biogel A-5 column chromatography. As expected, we observed a close match between levels of LMW succinoglycan in cultures of the various glycanase-deficient strains to which ExsH had been added exogenously (Table 3), in comparison to those in cultures of the corresponding glycanase-producing strains to which no exogenous glycanase had been added (Table 2). Clearly, nonsuccinylated succinoglycan is cleaved to little or no extent, normally modified succinoglycan is cleaved to a moderate extent, and nonacetylated succinoglycan is cleaved to a large extent.
TABLE 3.
LMW extracellular carbohydrate (expressed as percentage of total extracellular carbohydrate) generated by the addition of glycanase (final concentration of 200 ng/ml) to culturesa
| Strain genotype | % Extracellular carbohydrate in LMW form when indicated enzyme was added:
|
|||
|---|---|---|---|---|
| Throughout the course of culture incubation
|
Entirely upon inoculation of cultures
|
|||
| ExsH | ExoK | ExsH | ExoK | |
| exoK exsH | 54 | 9 | 87 | 41 |
| exoK exsH exoZ | 90 | 35 | 100 | 83 |
| exoK exsH exoH | 2 | 7 | 3 | 5 |
| exoK exsH exoH exoZ | 13 | 7 | 7 | 13 |
Under the incubation conditions used here, approximately 97% of total extracellular carbohydrate is succinoglycan.
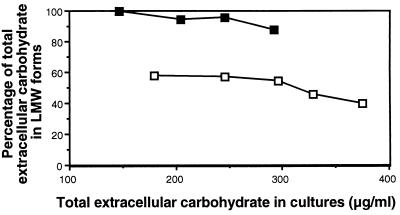
We proceeded to test directly whether subtle differences in total succinoglycan production might account for the large differences in the production of LMW succinoglycan by exoK exsH versus exoZ exoK exsH cultures. We inoculated a series of cultures with either of the two strains, varying the titers of inocula over a wide range; added identical, physiologically relevant levels of ExsH to each culture; and then compared the ratio of HMW to LMW succinoglycan present in cultures of the two strains in which various total amounts of succinoglycan had been produced over the course of culture incubation. The difference in the production of LMW succinoglycan in exoK exsH versus exoZ exoK exsH cultures is apparent across a wide range of levels of total succinoglycan production (Fig. 2). Apparently, the absence of the acetyl group from the succinoglycan produced by exoZ mutants greatly increases the susceptibility of this succinoglycan to cleavage by ExsH.
FIG. 2.
Graph of LMW carbohydrate (expressed as percentage of total extracellular carbohydrate in culture) generated by the addition of ExsH to cultures of glycanase-deficient strains versus the total amount of extracellular carbohydrate that had accumulated in cultures of these strains after 4 days of incubation. Note that approximately 97% of the total extracellular carbohydrate is succinoglycan. exoK exsH strain, open squares; exoZ exoK exsH strain, solid squares. ExsH was added to cultures gradually over the course of 4 days to the final, physiologically relevant concentration of 200 ng/ml.
We extended these analyses by adding ExsH to a final concentration of 200 ng/ml entirely at the time of inoculation of cultures, to test whether the timing of the addition of ExsH would affect the yield of LMW succinoglycan. Although there was no effect on exoH exoK exsH and exoH exoZ exoK exsH cultures, exoK exsH and exoZ exoK exsH cultures both exhibited a dramatic increase in the conversion of HMW succinoglycan to LMW succinoglycan (Table 3). Thus, it is not the case that some fixed proportions of wild-type and exoZ mutant succinoglycans are produced in glycanase-refractory forms. Instead, the timing of glycanase addition as well as glycanase concentration determines how much HMW succinoglycan is converted to LMW succinoglycan.
We extended these analyses further by testing ExoK (at a final concentration of 200 ng/ml), added either throughout the course of culture incubations or at the time of inoculation of cultures. Interestingly, although ExoK is less effective than ExsH in producing LMW succinoglycan under these conditions, the addition of ExoK at the onset of culture inoculations did result in a detectable increase in the production of LMW succinoglycan (Table 3). Apparently, under these conditions ExoK has a substrate preference similar to that of ExsH but is less active than ExsH.
The absence of the succinyl modification from succinoglycan dramatically decreases but does not absolutely block cleavage of succinoglycan by ExoK and ExsH.
At this point we wanted to determine whether the nonsuccinylated succinoglycan produced by exoH mutants is absolutely refractory to cleavage by the glycanases ExoK and ExsH or whether it can be cleaved but at a much lower efficiency than normally modified succinoglycan. To distinguish between these two possibilities, we tested the effect of supplementing cultures of glycanase-deficient strains with glycanases at levels that are greatly in excess of those found physiologically. We added either ExoK or ExsH to cultures to a final concentration of 10 μg/ml, which is approximately 50-fold higher than the physiological level of ExsH in GMS cultures of the wild-type strain. When the enzymes are added gradually throughout the period of culture incubation, ExsH and, to a lesser extent, ExoK partially convert the nonsuccinylated HMW succinoglycan normally produced by exoH exoK exsH and exoH exoZ exoK exsH strains to LMW succinoglycan (Table 4). Thus, nonsuccinylated succinoglycan has a low degree of susceptibility to cleavage by ExoK and ExsH, but it can be cleaved extensively when these enzymes are added to cultures at sufficiently high concentrations. In addition, virtually all of the normally modified and nonacetylated succinoglycan produced by exoK exsH and exoZ exoK exsH strains is converted to LMW succinoglycan under these conditions (Table 4), consistent with our results involving lower concentrations of glycanase.
TABLE 4.
LMW extracellular carbohydrate (expressed as percentage of total extracellular carbohydrate) generated by the addition of glycanase (final concentration of 10 μg/ml) to culturesa
| Strain genotype | % Extracellular carbohydrate in LMW form when indicated enzyme was added:
|
|||||
|---|---|---|---|---|---|---|
| Throughout culture incubation
|
Halfway through culture incubation
|
After removal of cells from culture
|
||||
| ExsH | ExoK | ExsH | ExoK | ExsH | ExoK | |
| exoK exsH | 100 | 93 | 43 | 54 | 0 | 0 |
| exoK exsH exoZ | 100 | 100 | 46 | 51 | 4 | 5 |
| exoK exsH exoH | 59 | 21 | 9 | 6 | 0 | 0 |
| exoK exsH exoH exoZ | 61 | 39 | 9 | 6 | 0 | 0 |
Under the incubation conditions used here, approximately 97% of total extracellular carbohydrate is succinoglycan.
Each of the variously modified forms of succinoglycan becomes refractory to cleavage as it accumulates in cultures.
We previously determined that normally modified succinoglycan becomes refractory to cleavage as it accumulates in cultures. We proceeded to test whether the other variously modified forms of succinoglycan exhibit the same property. We determined that the addition of glycanase to cultures at a point about halfway through the period of culture incubations resulted in a decrease in the yield of LMW succinoglycan (Table 4), relative to that in cases for which glycanase was added throughout the period of culture incubation. This is consistent with our previously reported observation that succinoglycan becomes refractory to cleavage by glycanases as it accumulates in cultures (45). We then determined that the addition of the entire glycanase sample at the end of the period of culture incubation (after removal of cells by centrifugation), followed by a 24-h period of incubation, resulted in little or no production of LMW succinoglycan for any of the strains (Table 4). These results demonstrate that all of the variously modified forms of succinoglycan share the common property that, as they accumulate in culture supernatants, they become refractory to cleavage by the ExoK and ExsH glycanases.
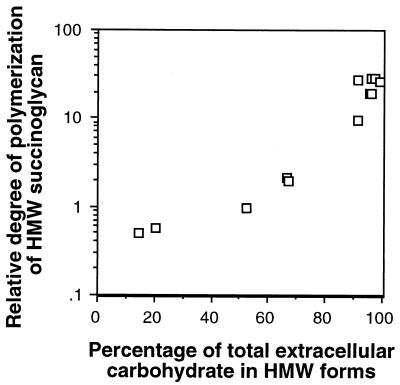
Increasing conversion of HMW succinoglycan to LMW succinoglycan by glycanases correlates with a decreasing degree of polymerization of the remaining HMW succinoglycan in cultures.
The observation that ExoK and ExsH cleave HMW succinoglycan in cultures to yield LMW succinoglycan suggests that increasing production of LMW succinoglycan across a series of strains would likely correlate with a decreasing degree of polymerization of the remaining HMW succinoglycan in cultures of these strains, particularly if depolymerization is neither an extremely rapid nor strictly processive process. To test this idea, we measured the relative degree of polymerization (concentration of carbohydrate per concentration of reducing ends) of HMW succinoglycan recovered from cultures of the wild-type strain and from cultures of various strains defective in expression of glycanases and/or acetylation and succinylation of succinoglycan (Table 5) and we observed the expected correlation (Fig. 3). Our results indicate that glycanase activity influences the molecular weight distribution of succinoglycan, not just in terms of generating LMW succinoglycan, but also in terms of controlling the average degree of polymerization of the remaining pool of HMW succinoglycan. Importantly, the average degree of polymerization of HMW succinoglycan produced by exoH mutants is similar to that produced by exoK exsH mutants, again implying that the nonsuccinylated succinoglycan produced by the exoH strain is a poor substrate for cleavage by physiologically relevant levels of ExoK and ExsH.
TABLE 5.
Degree of polymerization of HMW succinoglycan samples, expressed relative to degree of polymerization of wild-type HMW succinoglycana
| Glycanase genotype | Relative degree of polymerization for indicated succinylation/acetylation genotype
|
|||
|---|---|---|---|---|
| Wild type | exoZ | exoH | exoH exoZ | |
| Wild type | 1.0b | 0.52 | 20 | 20 |
| exoK | 2.2b | 0.57 | n.d.c | n.d. |
| exsH | 9.6b | 2.0b | n.d. | n.d. |
| exoK exsH | 29 | 28 | 27 | 29 |
Data represent averages for succinoglycan from two cultures. The standard deviation (SD) is <20% of the average degree of polymerization for a given sample, except as indicated.
SD ≤ 30% of the average degree of polymerization for a given sample.
n.d. not determined.
FIG. 3.
Degree of polymerization of HMW succinoglycan samples, expressed relative to degree of polymerization of wild-type HMW succinoglycan (data from Table 5), plotted versus the percentage of total extracellular carbohydrate in cultures that is in HMW forms (data from Table 2). Note that approximately 97% of total extracellular carbohydrate in cultures is succinoglycan.
DISCUSSION
Our results indicate that the acetyl and succinyl modifications of succinoglycan dramatically affect the molecular weight distribution of succinoglycan in R. meliloti cultures, apparently by influencing the susceptibility of the polysaccharide to cleavage by the ExoK and ExsH glycanases. In particular, the nonacetylated succinoglycan produced by exoZ mutants has a high degree of susceptibility to cleavage, the normally modified succinoglycan produced by the wild-type strain has an intermediate degree of susceptibility to cleavage, and the nonsuccinylated succinoglycan produced by exoH mutants has a low degree of susceptibility to cleavage. These characteristics pertain specifically to succinoglycan as it is actively being produced by R. meliloti strains; each of the variously modified forms of succinoglycan undergoes a transition from a glycanase-susceptible to a glycanase-refractory form as it accumulates in cultures. Thus, the actual molecular weight distribution of succinoglycan in a given R. meliloti culture is determined by both the susceptibility of the polysaccharide to cleavage and the timing and levels of endogenous expression or exogenous addition of glycanase to the cultures.
Our results are consistent with a simple model, whereby R. meliloti strains cultivated in GMS medium synthesize almost all of their succinoglycan in long-chain, HMW forms. Then, in proportion to the extent that a given strain expresses the ExoK and ExsH glycanases and to the extent that the succinoglycan produced by this given strain is susceptible to cleavage, the HMW succinoglycan is cleaved by these glycanases to yield LMW succinoglycan and residual, shorter-chain forms of HMW succinoglycan.
How do the acetyl and succinyl modifications of succinoglycan affect the susceptibility of succinoglycan to cleavage by glycanases? One possibility is that the modifications influence the conformation of succinoglycan prior to its transition from glycanase-susceptible to glycanase-refractory forms. For example, Gravanis et al. (20) have proposed that the precise conformation of individual, nonsuccinylated succinoglycan chains may differ from that of normally modified succinoglycan chains. Thus, the different susceptibilities to cleavage associated with the variously modified forms of succinoglycan may reflect a different fit for each substrate in the active sites of ExoK and ExsH.
A second possibility is that the acetyl and succinyl modifications of succinoglycan affect the rate of transition of succinoglycan from glycanase-susceptible to glycanase-refractory forms. Although we have determined that all of the variously modified forms of succinoglycan undergo a transition to glycanase-refractory forms in culture, the nature of the transition itself is not known. Previous analyses of the physical properties of succinoglycan imply that purified succinoglycan samples can undergo disorder-order conformational transitions in solution, consisting of random-coil-to-helix transitions and aggregation (11). Either or both might account for the transition of succinoglycan from glycanase-susceptible to glycanase-refractory forms. Interestingly, analyses of nonsuccinylated succinoglycan, as recovered from exoH mutant cultures or as generated by chemical desuccinylation of wild-type succinoglycan, indicate that the absence of the succinyl group results in an increase in the order-disorder transition temperature of the polysaccharide (13, 42). Thus, the absence of the succinyl modification seems to increase the stability of ordered forms of succinoglycan. Also, increasing salt concentrations in succinoglycan solutions promote the disorder-order transition (11), and increasing salt concentrations in R. meliloti cultures promote a shift toward accumulation of more HMW succinoglycan and less LMW succinoglycan (8, 9). Whether the latter effect is due to decreased depolymerization of succinoglycan or whether it is a function of regulation of the extent of polymerization of succinoglycan remains to be determined.
The dramatic differences in generation of LMW succinoglycan associated with adding glycanase to cultures early versus late in the course of cultivation suggest that shifts in the timing and levels of glycanase expression, relative to the timing and levels of succinoglycan production, could have dramatic effects on the molecular weight distribution of the succinoglycan produced by R. meliloti strains. Several mutants that exhibit increased succinoglycan production (exoR95, exoS96, exoX363, and exsB mutants), decreased succinoglycan production (mucR and exoX319 mutants), or decreased expression of the exoK gene (a mucR mutant) have been described previously (6, 12, 25, 36). Components of growth media can also affect levels of succinoglycan production (12) and the extracellular accumulation of ExoK (45). The identification and characterization of additional genetic and environmental factors that cause changes in glycanase expression or succinoglycan production should enable further refinement of the evolving model for how R. meliloti controls the molecular weight distribution of succinoglycan.
Our findings may have implications beyond understanding succinoglycan production by cultures of R. meliloti. Glycanases may prove useful as tools in characterizing the differences in the physical structures of normally modified versus nonsuccinylated succinoglycan, which in turn may provide new insights into why exoH mutants, but not exoK exsH mutants, exhibit a defect in invasion of alfalfa root nodules during the establishment of symbiosis (28, 44). Fluctuations in the rates of transition of succinoglycan to glycanase-refractory forms and in the levels of active, extracellular glycanases may cause spatial and temporal heterogeneity of succinoglycan, in terms of the molecular weight distribution of the polysaccharide as it is being produced by R. meliloti in cultures or in natural habitats. In general such heterogeneity might have important consequences for the development of bacterial-polysaccharide biofilms (35). Finally, our results may serve to bridge research on polysaccharide physical properties and polysaccharide molecular weight control, such that results from both fields can provide context and relevance for each other. Increased understanding of polysaccharide physical properties should help to further elucidate how glycanases control polysaccharide molecular weight, and the ability to engineer polysaccharides within particular molecular weight ranges should enable the testing of assumptions about how physical properties of polysaccharides are influenced by polysaccharide molecular weight.
ACKNOWLEDGMENTS
We thank Latoya Maynard, who carried out research as part of the Undergraduate Research Opportunities Program at the Massachusetts Institute of Technology, for construction of the exoK exoH strain.
This work was supported by Public Health Service grant GM31030 from the National Institutes of Health.
REFERENCES
- 1.Aman P, McNeil M, Franzen L-E, Darvill A G, Albersheim P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr Res. 1981;95:263–282. [Google Scholar]
- 2.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker A, Kleickmann A, Arnold W, Pühler A. Analysis of the Rhizobium meliloti exoH, exoK, exoL fragment: ExoK shows homology to excreted endo-β 1,3-1,4 glucanases and ExoH resembles membrane proteins. Mol Gen Genet. 1993;238:145–154. doi: 10.1007/BF00279541. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Kleickmann A, Keller M, Arnold W, Pühler A. Identification and analysis of the Rhizobium meliloti exoAMONP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol Gen Genet. 1993;241:367–379. doi: 10.1007/BF00284690. [DOI] [PubMed] [Google Scholar]
- 5.Becker A, Kleickmann A, Küster H, Keller M, Arnold W, Pühler A. Analysis of the Rhizobium meliloti genes exoU, exoV, exoW, exoT, and exoI involved in exopolysaccharide biosynthesis and nodule invasion: exoU and exoW probably encode glucosyltransferases. Mol Plant-Microbe Interact. 1993;6:735–744. doi: 10.1094/mpmi-6-735. [DOI] [PubMed] [Google Scholar]
- 6.Becker A, Küster H, Niehaus K, Pühler A. Extension of the Rhizobium meliloti succinoglycan biosynthesis gene cluster: identification of the exsA gene encoding an ABC transporter protein, and the exsB gene which probably codes for a regulator of succinoglycan biosynthesis. Mol Gen Genet. 1995;249:487–497. doi: 10.1007/BF00290574. [DOI] [PubMed] [Google Scholar]
- 7.Becker A, Niehaus K, Pühler A. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol Microbiol. 1995;16:191–203. doi: 10.1111/j.1365-2958.1995.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 8.Becker A, Pühler A. Specific amino acid substitutions in the proline-rich motif of the Rhizobium meliloti ExoP protein result in enhanced production of low-molecular-weight succinoglycan at the expense of high-molecular-weight succinoglycan. J Bacteriol. 1998;180:395–399. doi: 10.1128/jb.180.2.395-399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedveld M W, Zevenhuizen L P T M, Zehnder A J B. Osmotically induced oligo- and polysaccharide synthesis by Rhizobium meliloti SU-47. J Gen Microbiol. 1990;136:2511–2519. [Google Scholar]
- 10.Buendia A M, Enenkel B, Köplin R, Niehaus K, Arnold W, Pühler A. The Rhizobium meliloti exoZ/exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose-4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar. viciae strain TOM. Mol Microbiol. 1991;5:1519–1530. doi: 10.1111/j.1365-2958.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 11.Burova T V, Golubeva I A, Grinberg N V, Mashkevich A Y, Grinberg V Y, Usov A I, Navarini L, Cesáro A. Calorimetric study of the order-disorder conformational transition in succinoglycan. Biopolymers. 1996;39:517–529. [Google Scholar]
- 12.Doherty D, Leigh J A, Glazebrook J, Walker G C. Rhizobium meliloti mutants that overproduce the R. meliloti acidic Calcofluor-binding exopolysaccharide. J Bacteriol. 1988;170:4249–4256. doi: 10.1128/jb.170.9.4249-4256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidanza M, Dentini M, Crescenzi V, Del Vecchio P. Influence of charged groups on the conformational stability of succinoglycan in dilute aqueous solution. Int J Biol Macromol. 1989;11:372–376. doi: 10.1016/0141-8130(89)90010-x. [DOI] [PubMed] [Google Scholar]
- 14.Finan T M, Hirsch A M, Leigh J A, Johansen E, Kuldau G A, Deegan S, Walker G C, Signer E R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985;40:869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- 15.Glazebrook J, Walker G C. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 16.Glucksmann M A, Reuber T L, Walker G C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J Bacteriol. 1993;175:7033–7044. doi: 10.1128/jb.175.21.7033-7044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González, J. E., C. E. Semino, L. E. Castellano-Torres, and G. C. Walker. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 20.Gravanis G, Milas M, Rinaudo M, Tinland B. Comparative behavior of the bacterial polysaccharides xanthan and succinoglycan. Carbohydr Res. 1987;160:259–265. [Google Scholar]
- 21.Greenwood C T, Milne E A. Starch degrading and synthesizing enzymes: a discussion of their properties and action pattern. Adv Carbohydr Chem. 1968;23:282–366. doi: 10.1016/s0096-5332(08)60171-x. [DOI] [PubMed] [Google Scholar]
- 22.Her G-R, Glazebrook J, Walker G C, Reinhold V N. Structural studies of a novel exopolysaccharide produced by a mutant of Rhizobium meliloti strain Rm1021. Carbohydr Res. 1990;198:305–312. doi: 10.1016/0008-6215(90)84300-j. [DOI] [PubMed] [Google Scholar]
- 23.Hollingsworth R I, Abe M, Sherwood J E, Dazzo F B. Bacteriophage-induced acidic heteropolysaccharide lyases that convert the acidic heteropolysaccharides of Rhizobium trifolii into oligosaccharide units. J Bacteriol. 1984;160:510–516. doi: 10.1128/jb.160.2.510-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansson P-E, Kenne L, Lindberg B, Ljunggren H, Ruden U, Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of R. meliloti by sequential degradation. J Am Chem Soc. 1977;99:3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- 25.Keller M, Roxlau A, Weng W M, Schmidt M, Quandt J, Karsten N, Jording D, Arnold W, Pühler A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succinoglycan and galactoglucan. Mol Plant-Microbe Interact. 1995;8:267–277. doi: 10.1094/mpmi-8-0267. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy L, McDowell K, Sutherland I W. Alginases from Azotobacter species. J Gen Microbiol. 1992;138:2465–2471. [Google Scholar]
- 27.Leigh J A, Lee C C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988;170:3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh J A, Reed J W, Hanks J F, Hirsch A M, Walker G C. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 29.Leigh J A, Signer E R, Walker G C. Exopolysaccharide-deficient mutants of R. meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972;47:273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- 31.Loewus F A. Improvement in the anthrone method for determination of carbohydrates. Anal Chem. 1952;24:219. [Google Scholar]
- 32.Long S, Reed J W, Himawan J, Walker G C. Genetic analysis of a cluster of genes required for synthesis of the Calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988;170:4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 34.Müller P, Hynes M, Kapp D, Niehaus K, Pühler A. Two classes of Rhizobium meliloti infection mutants differ in exopolysaccharide production and in coinoculation properties with nodulation mutants. Mol Gen Genet. 1988;211:17–26. [Google Scholar]
- 35.Palmer R J, Jr, White D C. Developmental biology of biofilms: implications for treatment and control. Trends Microbiol. 1997;5:435–440. doi: 10.1016/S0966-842X(97)01142-6. [DOI] [PubMed] [Google Scholar]
- 36.Reed J W, Capage M, Walker G C. Rhizobium meliloti exoG and exoJ mutations affect the ExoX-ExoY system for modulation of exopolysaccharide production. J Bacteriol. 1991;173:3776–3788. doi: 10.1128/jb.173.12.3776-3788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhold B B, Chan S Y, Reuber T L, Marra A, Walker G C, Reinhold V N. Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J Bacteriol. 1994;176:1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuber T L, Walker G C. The acetyl substituent of succinoglycan is not necessary for alfalfa nodule invasion by Rhizobium meliloti Rm1021. J Bacteriol. 1993;175:3653–3655. doi: 10.1128/jb.175.11.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 40.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuhs B L, Williams M N V, Kim J S, Carlson R W, Côté F. Suppression of the Fix− phenotype of Rhizobium meliloti exoB mutants by lpsZ is correlated to a modified expression of the K polysaccharide. J Bacteriol. 1995;177:4289–4296. doi: 10.1128/jb.177.15.4289-4296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridout M J, Brownsey G J, York G M, Walker G C, Morris V J. Effect of o-acyl substituents on the functional behavior of Rhizobium meliloti succinoglycan. Int J Biol Macromol. 1997;20:1–7. doi: 10.1016/s0141-8130(96)01140-3. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland I W, Kennedy L. Polysaccharide lyases from gellan-producing Sphingomonas spp. Microbiology. 1996;142:867–872. doi: 10.1099/00221287-142-4-867. [DOI] [PubMed] [Google Scholar]
- 44.York G M, Walker G C. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol Microbiol. 1997;25:117–134. doi: 10.1046/j.1365-2958.1997.4481804.x. [DOI] [PubMed] [Google Scholar]
- 45.York G M, Walker G C. The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. Proc Natl Acad Sci USA. 1998;95:4912–4917. doi: 10.1073/pnas.95.9.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zevenhuizen L P T M, van Neerven A R W. (1-2)-β-d-Glucan and acidic oligosaccharides produced by Rhizobium meliloti. Carbohydr Res. 1983;118:127–134. [Google Scholar]