Abstract
Holins are a diverse group of small integral membrane proteins elaborated by bacteriophages to lyse bacterial hosts and effect release of progeny phages in a precisely timed manner. Recently, the holin S gene of phage λ was overexpressed and the holin protein was purified to homogeneity by means of an oligohistidine tag procedure and immobilized metal affinity chromatography (D. L. Smith, D. K. Struck, J. M. Scholtz, and R. Young, J. Bacteriol. 180:2531–2540, 1998). Numerous locations within the S gene were tested as sites for an oligohistidine-tag-encoding insertion which preserves holin function. The lysis phenotypes of these alleles, expressed from moderate-copy-number transactivation plasmids, were characterized. A striking class of mutants, previously referred to as early-dominant, have been found to have severe lysis defects but are fully functional in the presence of wild-type protein. Results presented here reveal that the early-dominance phenotype is independent of S107 inhibitor function. The results provide insight into the mechanism of hole formation and indicate that, while oligomerization is required in the pathway to hole formation, a nucleation event may also be required.
The bacteriophage λ has four lysis genes: S, R, Rz, and Rz1 (see Fig. 1A), of which S and R are absolutely required for disruption of the host envelope under normal laboratory growth conditions (12, 16, 19, 32, 33, 43, 47). R encodes λ endolysin, a soluble transglycosylase which degrades peptidoglycan by breaking the glycosidic linkages (3). S encodes the λ holin, a protein required to enable R to pass across the cytoplasmic membrane, because the latter protein lacks a secretory signal sequence (32, 33). The S protein forms a lethal membrane lesion, or hole, which mediates the escape of the endolysin. Although nothing is known directly about the structure of the hole, it is apparently nonspecific because it can function with another endolysin of completely unrelated structure and function (e.g., the gene 19 lysozyme of P22) (34) and must have a large effective diameter, because the endolysins accumulate fully folded and active within the cytoplasm before holin-mediated release occurs. S protein forms sodium dodecyl sulfate-resistant oligomers, and after cross-linking, S-containing oligomers with up to six mers have been detected, with spacing consistent with homooligomerization (7, 48). The S gene is also lethal in Saccharomyces cerevisiae, which indicates that no specific host protein interactions are required (13). Purified S protein can cause the release of dye from liposomes (41). Taken together, these observations support the notion that the hole is an oligomeric form of S. Holins are unique in biology since they are apparently the only type of cytolytic protein which forms holes from within the cell, rather than from without. Moreover, S, and presumably all holins, exists only in a membrane-bound form (1), as opposed to the cytolytic toxins, such as colicins (35), α-hemolysin (2), and the mammalian complement system (23), which exist as soluble, monomeric proteins before assembly on the target cell membrane.
FIG. 1.
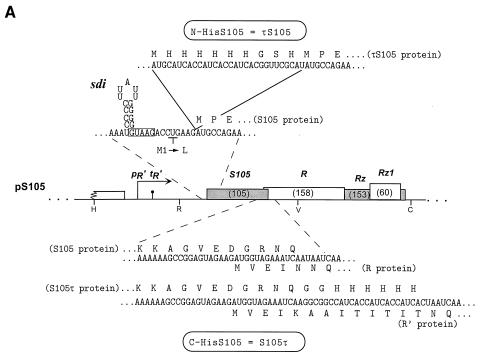
(A) The λ lysis gene region from plasmid pS105 is shown, with the predicted size in amino acid residues shown for each of the four lysis genes. Expanded views are shown for sequences of the mRNA sdi structure and N-terminal positions of the S105 gene. The boxed sequence in the mRNA indicates the Shine-Dalgarno sequence for the S105 gene (5). sdi is the RNA stem-loop structure known to participate in the regulation of the normal S gene dual start and cause dominant-negative lysis defects (31). The pR′ promoter and tR′ terminator for the λ late gene transcriptional unit are shown upstream of the EcoRI site. Restriction sites are indicated (H, HindIII; R, EcoRI; V, EcoRV; and C, ClaI). pS105 contains a HindIII-ClaI fragment corresponding to the wild-type lysis cassette of λ (45), inserted into the cognate sites in pOR19 (41). In pS105 the M1L mutation prevents synthesis of S107 inhibitor protein. Below the lysis gene map is an expanded view of the region of overlap between the S and R genes. The mRNA sequence and protein translations for S105, τS105, S105τ, R, and R′ are depicted. (B) The S105 sequence is depicted with charged residues denoted above the sequence, as well as with arrows indicating the positions at which oligohistidine tag insertions (τ) have been isolated. Putative α-helices and turn or loop regions are modeled below the sequence. Aberrant insertions and subsequent frameshifts after codons 88 and 94 are depicted above the S105 sequence. Residue numbers are as in the full-length S protein, i.e., the initial residue of S105 is Met3. (C) Topological models A and B for the λ holin. S may have either two or three α-helical transmembrane domains depending on whether the putative domain 3 (dashed box) exists as a membrane-spanning domain (6, 46).
This distinction raises the question of how hole formation by the membrane-bound holin is regulated. S accumulates in the membrane from about 8 min after infection until, under standard conditions, hole formation is spontaneously triggered at about 50 min. In the absence of endolysin, this triggering is observed as a sudden cessation in the accumulation of culture mass and is associated with a rapid loss of cell viability, in terms of CFU (15). The time delay is critical to allow phage morphogenesis in quantity, and the timing is thus under strict, genetically programmed control. S mutants which cause hole formation as early as 20 min after infection, or at almost any time after 50 min, have been isolated (22, 30, 31). The energized membrane plays a role in this timing mechanism. Adding an energy poison, like cyanide, at any time after 20 to 30 min postinduction but before the normal time of triggering, instantly triggers premature hole formation (14, 32, 33). Part of the “lysis clock” is explained by the fact that the S gene has a dual-start motif, with the first three codons encoding sequence beginning with MKM. Both Met codons are used for translational initiations in vivo, and thus two proteins, designated S107 and S105 for their lengths in amino acid residues, are made (5, 8). S107 differs from S105 only in having two extra N-terminal residues (M and K). It has been conclusively demonstrated that the two proteins have opposing functions in vivo: the shorter product, S105, is the functional holin, whereas the longer product, S107, acts as an inhibitor of S105 function, with the functional difference being the additional positively charged lysine residue (4, 9). The presence of two opposing products complicates genetic analysis, because, for example, a loss of hole-forming function may be due to a defect in the activity of S105 or an increase in the inhibitor ability of S107.
In the absence of an in vitro assay, purification of the small, membrane-embedded, low-abundance S holin in its native form was technically difficult, if not impractical. We resorted to oligohistidine tag methodology because it has been successfully applied to a number of proteins which have resisted purification and confers real advantages to a membrane protein purification scheme because it facilitates detergent exchange steps. Here we report the results of our efforts to find a site within the reading frame of the S gene in which to insert an oligohistidine-encoding sequence. The properties of several insertion alleles are discussed in terms of current models for S structure, topology, and function.
MATERIALS AND METHODS
Strains, phages, plasmids, and oligonucleotides.
All Escherichia coli strains, bacteriophages, and plasmids used in this study are listed in Table 1. Oligonucleotides are listed in Table 2 and were purchased from the Gene Technologies Laboratory in the Department of Biology at Texas A&M University. Luria-Bertani (LB) medium and LB-Amp medium (LB medium supplemented with 100 μg of ampicillin per ml) were prepared according to the method of Miller (24). Chemicals were obtained from Sigma (St. Louis, Mo.).
TABLE 1.
Bacterial strains, bacteriophages, and plasmids
| Strain, phage, or plasmid | Relevant feature(s) | Reference or source |
|---|---|---|
| Bacterial strains | ||
| MC4100 | E. coli K-12 F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | 39 |
| XL1-Blue | E. coli K-12 recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15::Tn10 (Tetr)] | Stratagene |
| Phages | ||
| λ Cmr Δ(SR) | stf::cat::tfa cI857 Δ(SR); replacement of stf and tfa genes (λ nt 19996–22220) with cat gene (37); Δ(SR); loss of λ nt 45136–45815 (31) | Laboratory stock |
| λ 111 | cI857 Sam7 | 16 |
| λ RG1 | b519 b515 b2::Tn903 cI857 nin5 S+ | 22 |
| λ S105 | stf::cat::tfa cI857 S with M1L (makes only S105) | This study |
| Plasmids | ||
| pOR19 | pBR322 with EcoRI site deleted by blunt ending and ligation | Laboratory stock |
| pKB110 | Derivative of pOR19; λ lysis gene region cloned as HindIII-ClaI fragment (λ nt 44141–46440) | 7 |
| pSG38P | S with G38P; SmaI site created in codons 38 and 39 | 41 |
| pSG39P | S with G39P; ApaI site created in codons 38 and 39 | This study |
| pS105 | S with M1L; derivative of pSG38P; bears S105 only | 41 |
| pτS105 | Derivative of pSG38P; insert at the N terminus of S105 (MH6GSH) | 41 |
| pS105R33L | S with R33L; derivative of pS105 | This study |
| pS105A52G | S with A52G; derivative of pS105 | This study |
| pS105A52V | S with A52V; derivative of pS105 | This study |
| pS105τ | Derivative of pSG38P; insert at the C terminus of S105 (G2H6) | 41 |
| pS105τ9x | Altered insertion after L9 codon in S105 (G2H6PA) | This study |
| pS105τ9z | Altered insertion after L9 codon in S105 (G2H6RG) | This study |
| pS105τ21 | Insertion after I21 codon in S105 (G2H6G2) | This study |
| pS105τ38* | Insertion after G38 codon in S105 (glycine-free tag; H6) | This study |
| pS105τ38 | Insertion after G38 codon in S105 (G2H6G2) | This study |
| pS105τ38x | Altered insertion after G38 codon in S105 (G2H6G3H6G2) | This study |
| pS105τ49 | Insertion after T49 codon in S105 (G2H6G2) | This study |
| pS105τ49x | Insertion after T49 codon in S105 (G2H6G2 with C51Y mutation) | This study |
| pS105τ63 | Insertion after D63 codon in S105 (G2H6G2) | This study |
| pS105τ63x | Insertion after D63 codon in S105 (G2H6G2 with F64L mutation) | This study |
| pS105τ75 | Insertion after T75 codon in S105 (G2H6G2) | This study |
| pS105τ75x | Altered insertion after T75 codon in S105 (G2H3G2) | This study |
| pS105τ83 | Insertion after G83 codon in S105 (G2H6G2) | This study |
| pS105τ88 | Insertion after G88 codon in S105 (G2H6G2) | This study |
| pS105τ88x | Altered insertion after G88 codon in S105 (G2H6D) | This study |
| pS105τ88z | Altered insertion after G88 codon in S105 with frameshift (G2H4PRLAYQTLRC) | This study |
| pS105τ94 | Insertion after F94 codon in S105 (G2H6G2) | 41 |
| pS105τ94x | Altered insertion after F94 codon with frameshift in S105 (G2H3QKSITITAALLKKPE) | 41 |
| pS105τ94z | Insertion after F94 codon with frameshift in S105 (G2H6G2LLKKPE) | 41 |
| pS105t94R33L | S105 with R33L; derivative of pS105τ94 | This study |
| pS105τ94A52G | S105 with A52G; derivative of pS105τ94 | This study |
| pS105τ94A52V | S105 with A52V; derivative of pS105τ94 | 41 |
TABLE 2.
Oligonucleotides
| Oligo- nucleotide | Sequencea | Relevant feature(s) |
|---|---|---|
| ForLamRI | GCCACTGTCTGTCCT | Forward primer to amplify λ lysis genes; anneals directly upstream of native EcoRI site (λ nt 44956–44972) |
| RevLamCla | TTCGTAAATGGATTGCTCGGTTTTA | Reverse primer to amplify λ lysis genes; anneals downstream of the native ClaI site beyond RZ; λ nt 46536–46512 |
| ForSG39P | GATATAATGGgcccGCGTTTACAAAAACAG | Same as ForSG38P (λ nt 45289–45318) but creates an ApaI site and the G39P mutation |
| RevSG39P | GTAAACGCgggcCCATTATATCTGCCGCG | Reverse primer used in conjunction with ForSG39P (λ nt 45316–45282) |
| SeqpR′ | tgtaaaacgacggccagtGCATGATTGCCACGGATGGC | Forward primer that anneals upstream of pR′ (λ nt 44512–44531); contains Seqman primer landing site |
| SRev36 | gcggatccagggcgagcgcGTCCGTTATCAGTTCCCTC | Reverse primer that anneals 36 bp downstream of S gene stop codon (λ nt 45565–45557); contains ERevBam landing site |
| Seqman | TGTAAAACGACGGCCAGT | Forward universal primer used for automated sequencing of PCR products |
| ERevBam | GCGGATCCAGGGCGAGCGC | Reverse universal primer used for automated sequencing of PCR products |
| ForL9τ | agttactcttctatcaccatcacggcggcTTGGCCGCCATTCTCGCG | Forward primer for Seamless Cloning insertion of oligohistidine codons after codon 9 (λ nt 45213–45230) with the restriction enzyme Eam1104I |
| RevL9τ | agttactcttctgatgatggtggccgccCAGGTCATGTTTTTCTGGC | Reverse primer to be used in conjunction with ForL9τ (λ nt 45212–45194) |
| ForS107M3L | CTTATTGGGGGTAAGACATGAAGcTGCCAGAAAAACATGACC | Forward primer for QuikChange mutagenesis; creates M3L to prevent S105 synthesis (anneals to λ nt 45169–45210) |
| RevS107M3L | GGTCATGTTTTTCTGGCAgCTTCATGTCTTACCCCCAATAAG | Reverse primer to be used in conjunction with ForS107M3L (anneals to λ nt 45210–45169) |
Letters in lowercase represent either mismatches or insertions, and new restriction sites are indicated by boldface type.
DNA manipulations.
Restriction enzymes were purchased from Promega (Madison, Wis.), and restriction of DNA was performed according to the manufacturer’s instructions. A Qiaprep Spin Miniprep kit (Qiagen, Chatsworth, Calif.) and a Jet Star kit (Genomed, Raleigh, N.C.) were used according to the manufacturers’ recommendations to obtain plasmid mini- and maxi-preparations, respectively. Gel-purified DNA fragments for cloning were excised from agarose gels and purified with Qiaex gel extraction spin columns (Qiagen) per the manufacturer’s instructions. A Rapid DNA Ligation kit from Boehringer Mannheim (Indianapolis, Ind.) was used per the manufacturer’s suggestion. Transformation-competent E. coli cells were prepared according to the method of Chung et al. (10).
DNA amplification.
DNA fragments for subcloning and automated fluorescence sequencing were amplified by PCR and sequenced as previously described (41).
Mutagenesis of S by PCR overlap extension mutagenesis.
Plasmid pSG39P was created by the PCR overlap extension method of Ho et al. (21). A unique ApaI site was introduced into the putative periplasmic-loop-encoding region of the S gene (Fig. 1B) in the pKB110 plasmid by modifying the G38 codon (GGT to GGG; λ nucleotides [nt] 45297 to 45299) and mutating the G39 codon to a proline (GGC to CCC; λ nt 45300 to 45302). Basically, complementary primers were used in separate reactions to generate two DNA fragments with overlapping ends. The overlap allowed these two fragments to be melted, annealed, and extended. The product of complete extension was then used as a template for standard PCR amplification and subsequent cloning. The overlapping fragments used to clone pSG39P were made with the primer pairs ForLamRI and RevSG39P and ForSG39P and RevLamCla. The fully extended PCR product was digested with EcoRI and ClaI and inserted into pKB110 by replacing the wild-type S allele and lysis cassette. The correct clone was confirmed by digestion with ApaI and sequencing.
Mutagenesis of S by modified Seamless Cloning.
Most manipulations of S were done with the reading frame for S105, beginning with codon 3 and terminating with codon 107. For simplicity, this reading frame will be designated S105 throughout this work. The modified version of the Seamless Cloning (Stratagene, La Jolla, Calif.) protocol has been described previously (41) and was used with the oligonucleotides listed in Table 2 to insert sequences encoding oligohistidine tags into the S105 allele after codons 9, 21, 38, 49, 75, 83, 88, and 94 under cognate λ late gene expression signals. These plasmids and their derivatives were used as transactivation plasmids by introducing them into MC4100 [λ Δ(SR)]. Induction of the lysis-defective prophage resulted in transactivation of the pR′ promoter and thus transcription of the lysis genes on the plasmid.
QuikChange mutagenesis.
The specific mutations M3L, R33L, A52G, and A52V were introduced into plasmids pS105 and pS105τ94 by site-directed mutagenesis with a QuikChange kit from Stratagene. The manufacturer’s instructions were specifically followed. Only the forward primer set for S107 with the mutation M3L (ForS107M3L) is listed in Table 2. The primer pairs for the remaining four alleles were named in the same manner and contained 17 to 23 nt of homology to either side of the altered nucleotide. Constructs were confirmed by diagnostic PCR and sequencing as previously described (41).
Induction of transactivation plasmids harboring the S gene.
For each induction, MC4100 [λ (ΔSR)] harboring the transactivation plasmid derived from pKB110 was induced under carefully controlled conditions, as previously described (41). Briefly, aerobic cultures grown at 30°C in LB-Amp medium were shifted to 42°C at an A550 of 0.2, aerated at 42°C for 15 min, and then aerated at 37°C.
Membrane protein sample preparation and analysis.
Detergent-solubilized preparations of inner membrane proteins were obtained and analyzed as described previously (9, 40).
RESULTS
System for analysis of function of tagged S derivatives.
In order to assess the functionality of oligohistidine-tagged versions of S, a plasmid vector system which could provide expression of S under its cognate transcriptional and translational signals was required. Moreover, in order to avoid the potential ambiguity of whether the phenotype associated with a particular oligohistidine tag insertion was due to its effect on the active holin product, S105, or the inhibitor product, S107, or both, it was necessary to have a parental allele for mutagenesis in which the start codon for the S107 reading frame was inactivated. Accordingly, plasmid pS105, which bears the pR′ late gene promoter and the entire λ lysis gene cassette, was constructed (Fig. 1A) (41). (Here and throughout, the altered S gene carrying the M1L change is designated S105). Thermal induction of a λ lysogen harboring the thermolabile repressor allele, cI857, results in induction and subsequent synthesis of the late gene transcriptional antiterminator, Q, which transactivates the plasmid-borne lysis genes as well as the late genes of the prophage (17, 44). If the prophage carries a null S allele, then the lysis phenotype can be ascribed to the plasmid-borne S gene, whereas if the prophage carries a functional S allele, the dominant or recessive character of plasmid-borne S can be assessed. The efficacy of this system is demonstrated in Fig. 2, in which the lysis phenotypes of two S alleles encoding either an N- or a C-terminal oligohistidine tag are assessed. (Here and below, the presence of the oligohistidine tag, defined as shown in Table 1, is indicated by τ. Thus, τS105 has a tag sequence at the N terminus.) This τS105 allele, when it is expressed with the transcriptional and translational signals of the T7 φ10 gene, affects culture growth and cellular morphology indistinguishably from S105 but, as reported previously, exhibits a defective phenotype when it is expressed from the transactivation vector (Fig. 2A) (41). Moreover, the τS105 allele like S107 has inhibitor function, as was shown by its dominant effect over S+ (which encodes both the S107 and S105 proteins) and S105 in the isogenic transactivation experiments (Fig. 2C and D). Interestingly, immunoblot analysis revealed that this allele generates two protein products, one corresponding to the full-length oligohistidine-tagged protein and the other apparently corresponding to S105 (Fig. 3).
FIG. 2.
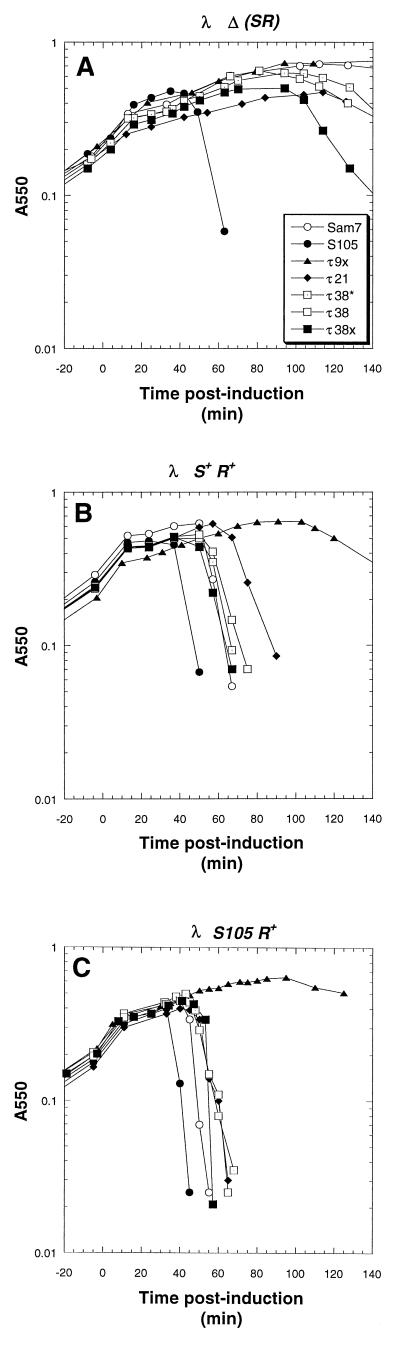
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids bearing the S105 gene with inserts encoding an oligohistidine tag at either the N or C terminus. (A) Inductions of MC4100 [λ Δ(SR)] cells harboring pKB1 (Sam7) (○), pS105 (•), pτS105 (□), and pS105τ (⧫) were performed as described in Materials and Methods. Similar plasmid transactivations were performed with the following different lysogens: MC4100 [λ 111] (Sam7) (B), MC4100 [λ S+] (C), and MC4100 [λ S105] (D).
FIG. 3.
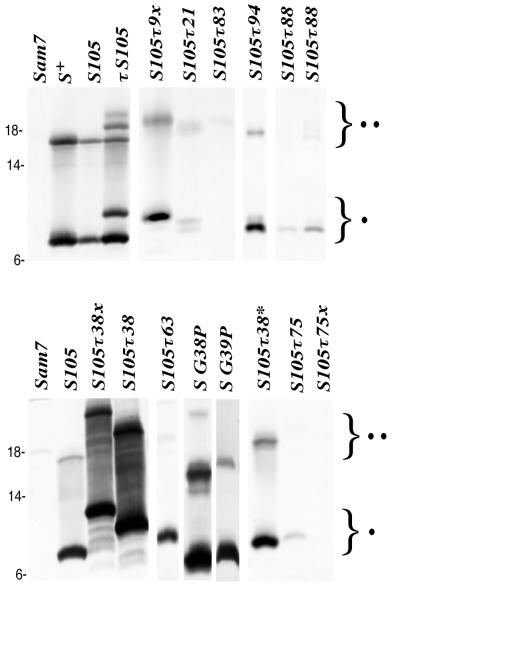
Various S alleles were tested for membrane localization and accumulation of stable protein by Western immunoblot analysis. Approximately equal amounts of membrane extract were applied to the lanes. The positions of monomeric (•) and dimeric (••) species of S are indicated with braces. Apparently higher levels of the holin in the samples from lysis-defective alleles are due to continued synthesis in the induced cells. Molecular mass (in kilodaltons) is indicated on the left. The S allele in each lane is designated.
A construct in which an oligohistidine tag is placed at the C terminus of S105, pS105τ, has a different kind of lysis defect. Lysis is apparently triggered at about the normal time, but the rate of lysis is severely affected, suggesting that the release of endolysin to the periplasm through the holes is retarded (Fig. 2A). Part of this defect derives from the fact that appending the multiple histidine codons to the terminus of the S105 gene also disrupts the R gene, which overlaps the S sequence by 14 nt (λ nt 45493 to 45506) (30) (Fig. 1A). However, even when functional R endolysin is supplied in trans, the rate of lysis is substantially altered (Fig. 2B), indicating that the C-terminal tag interferes with the hole-forming function of S105 protein.
The sensitivity of the transactivation assay can also be exploited to detect more subtle characteristics of the tagged proteins. Lysis timing is sensitive to gene dosage, as can be seen from the fact that a plasmid-borne S105 allele accelerates the onset of lysis when it is in trans to a prophage S105 or S+ allele (Fig. 2C and D). Despite its intrinsic lysis defect shown in trans to S null alleles, the S105τ allele is phenotypically indistinguishable from the parental S105 allele, when it is in trans to S+ or S105 on the prophage (Fig. 2C and D). That is, S105τ accelerates the onset of lysis by the same time interval as does S105. This effect has been reported before, with different missense alleles of S being paired with S+ in tandem prophages. Strictly, this is a dominance effect, and the term early dominance has been used to distinguish such alleles from those with the more negative dominance (lysis delay) (31) (see below).
Systematic mutagenesis of S105 with oligohistidine-tag-encoding oligonucleotide insertions.
Since the S proteins tagged at either chain terminus were unsuitable candidates for the purification and functional assay, an internal location for an oligohistidine tag was sought. A number of alleles harboring oligohistidine-tag-encoding inserts were prepared, by a technique which does not require modification of the target sequence other than by the addition of the insertion (41) (see Materials and Methods). In each mutagenesis experiment, the objective was to insert oligonucleotides encoding the standard sequence G2H6G2 after a designated codon, without modification of the DNA flanking the insertion site (Fig. 1B). We isolated insertions encoding hexahistidine sequences from a total of 11 positions within the S105 reading frame, most of which encoded the standard G2H6G2 oligohistidine tag but some of which encoded missense, partially deleted, or duplicated variants of that sequence. Most of these insertions resulted in lysis-defective phenotypes, although some of these had informative properties. Modifications at two positions in the C-terminal domain yielded S105 proteins with essentially normal timing and lysis profiles. This collection of insertion alleles, listed in order of the insertion site beginning from the start codon, is described below.
Insertions after codon 9.
The position after codon 9 is predicted to be adjacent to the first putative transmembrane domain (Fig. 1B). No standard insertion encoding G2H6G2 after residue 9 was obtained, but two aberrant, lysis-defective alleles, S105τ9x (tag sequence, G2H5RG) and S105τ9z (tag sequence, G2H6PA), were isolated (Fig. 4A). The S105τ9z allele did not accumulate stable protein and was recessive in trans to both S+ and S105 alleles (data not shown). The S105τ9x allele accumulated stable protein and was strongly dominant (Fig. 3 and 4B).
FIG. 4.
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids bearing the S105 gene bearing inserts after codon 9, 21, or 38. (A) MC4100 [λ Δ(SR)] cells harboring pKB1 (○), pS105 (•), pS105τ9x (▴), pS105τ21 (⧫), pS105τ38* (⊡), pS105τ38 (□), and pS105τ38x (■) were induced as described in the legend to Fig. 2. (B and C) The Hosts were MC4100 [λ S+] and MC4100 [λ S105], respectively.
Insertion after codon 21.
The position after codon 21 is within the putative amino-terminal transmembrane domain of S (Fig. 1B). The standard insertion allele is lysis defective and generates a partially unstable membrane protein (Fig. 3 and 4A). Surprisingly, this allele is weakly dominant in trans to S+ (Fig. 4B) but is largely recessive in trans to S105+ (Fig. 4C).
Insertions after codon 38.
Among the first sites to be tested as suitable for an oligopeptide insertion was the connector loop between the first two putative transmembrane domains (Fig. 1B), which we considered the most likely site to tolerate the oligopeptide insertion. We postulated that the adjacent glycine codons at positions 38 and 39 within this putative loop provided flexibility, since alteration of these residues leads to decreased function (30, 31) (Fig. 1B). Before the technique of inserting DNA without respect to restriction sites became available, the unique restriction sites SmaI and ApaI were introduced independently by altering the nucleotide sequences of glycine codons 38 and 39 to proline in, in these cases, the S+ reading frame (see Materials and Methods). With both, however, despite the relatively conservative nature of these substitutions, the mutant alleles had lysis-defective phenotypes (Fig. 5A). The G38P allele was nonlytic and recessive, and it accumulated larger than normal amounts of S protein in the membrane (Fig. 3 and 5B). The G39P allele was also lysis defective and accumulated stable protein but exhibited a strongly dominant character (Fig. 3 and 5).
FIG. 5.
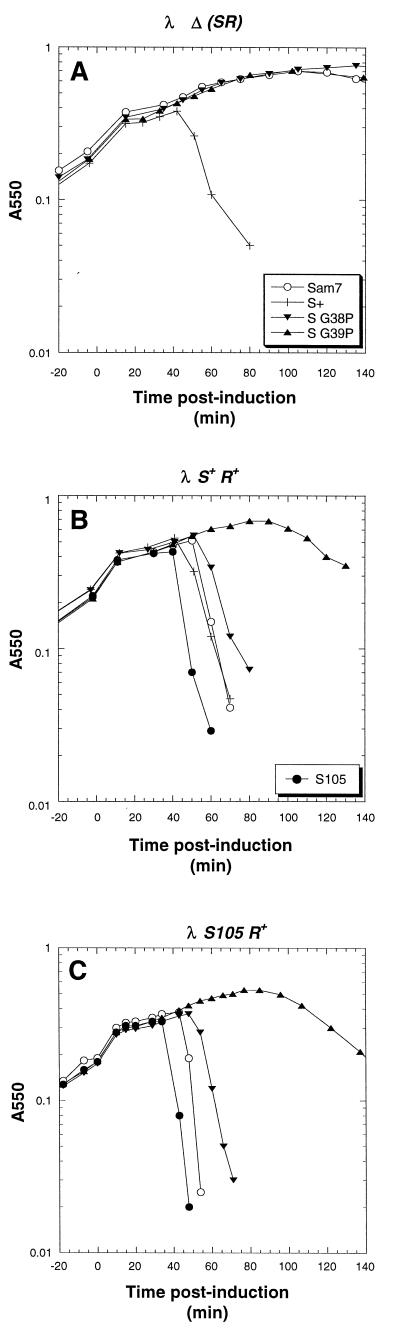
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids bearing the S105 gene with the mutations in the region encoding the first connector loop. (A) MC4100 [λ Δ(SR)] cells harboring pKB1 (○), pKB110 (+), pSG38P (▾), and pSG39P (▴) were induced as described in the legend to Fig. 2. (B and C) The hosts were MC4100 [λ S+] and MC4100 [λ S105], respectively. MC4100 [λ S+] cells harboring pS105 (•) were also induced.
After the restriction site-independent mutagenesis technique was developed, three insertions were isolated in the S105 reading frame between G38 and G39 encoding (i) G2H6G2 (designated S105τ38); (ii) H6 (designated S105τ38*); and (iii) G2H6G3H6G2 (designated S105τ38x), an aberrant insert. All of these exhibited severe lysis defects, although the last showed a reasonable but delayed-onset lytic profile, starting at about 90 min after induction (Fig. 4A). All three alleles were recessive and accumulated stable S protein in the membrane, as judged by Western immunoblotting (Fig. 3 and 4B and C).
Insertions after codon 49.
The position after codon 49 is within the putative second transmembrane domain of S (Fig. 1B). The standard insertion and two other alleles were isolated, one with an additional missense change and the other with a frameshift generating a nonsense polypeptide. All three alleles were recessive and absolute lysis defective, and none accumulated stable S protein in the membrane (data not shown).
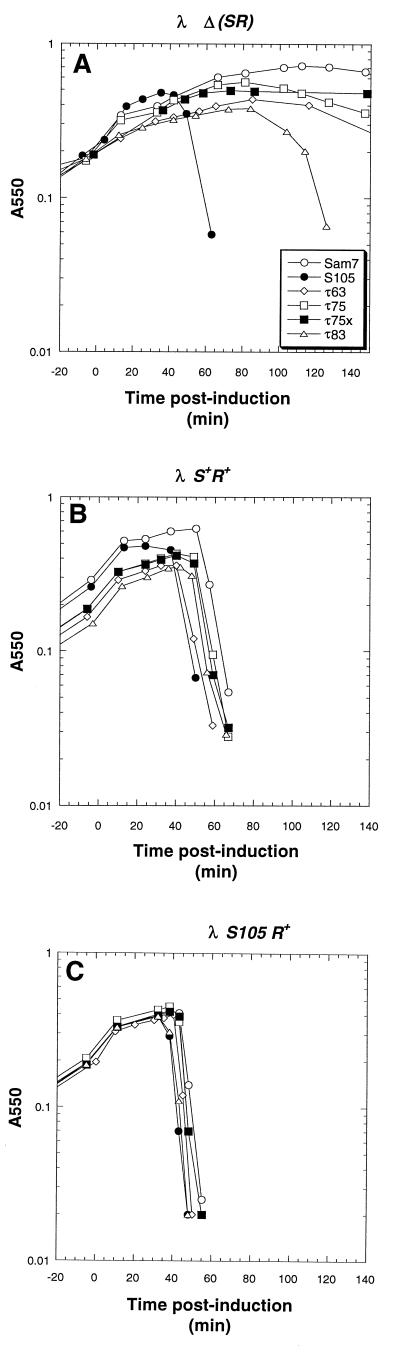
Insertions after codon 63.
The position after codon 63 is immediately after D63, which is located near the end of the putative second transmembrane domain of S. The standard insertion along with a variant encoding the missense change F64L was isolated. The S105τ63 alleles were absolute lysis defectives (Fig. 6A) and accumulated stable S protein in the membrane (Fig. 4). Since the two alleles were indistinguishable, only the standard insertion is presented in Fig. 4 and 6. Like the partially defective S105τ allele, S105τ63 also exhibited an early-dominance phenotype in trans to both the S+ and S105 alleles (Fig. 6B and C).
FIG. 6.
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids bearing the S105 gene with insertions after codons 63, 75, and 83. (A) MC4100 [λ Δ(SR)] cells harboring the plasmids pKB1 (○), pS105 (•), pS105τ63 (◊), pS105τ75 (□), pS105τ75x (■), and pS105τ83 (▵) were induced as described in the legend to Fig. 2. (B and C) The hosts were MC4100 [λ S+] and MC4100 [λ S105], respectively.
Insertions after codon 75.
The position after codon 75 is in the middle of the putative third transmembrane domain of S, according to model A (Fig. 1B and C). The standard insertion, S105τ75, exhibited a largely defective lysis profile, although gradual loss of turbidity began at about 80 min after induction of the prophage (Fig. 6A). Interestingly, an aberrant insertion, S105τ75x, encoding G2H3G2 was triggered at a much earlier time, about 50 to 60 min, as was determined by the cessation of accumulation of culture mass (Fig. 6A). However, no lysis was observed after triggering, suggesting that the hole which is formed, although sufficient to collapse the membrane potential and halt cellular metabolism, does not have the capacity to release endolysin. Both alleles with insertions after codon 75 were recessive (Fig. 6B and C), possibly because neither allele accumulated protein to normal levels, and in fact, the S105τ75x protein was not detectable on immunoblots (Fig. 3).
Insertion after codon 83.
The position after codon 83 is near the end of the third putative transmembrane domain, as defined in model A for S topology (Fig. 1C). The allele encoding the standard insertion, S105τ83, exhibited a lysis proficiency phenotype which was triggered very late, at about 80 min, although ultimately it supported complete lysis (Fig. 6A). However, in trans to a functional S+ or S105 allele on the induced prophage, S105τ83 accelerated lysis as well as the S105 allele (Fig. 6B and C). Despite its ability to support a delayed lysis event, the S105τ83 monomer was not detected in immunoblots of isolated membrane samples (Fig. 3). However, the dimer band, normally only a fraction of the total reactive material (40, 41), was detected.
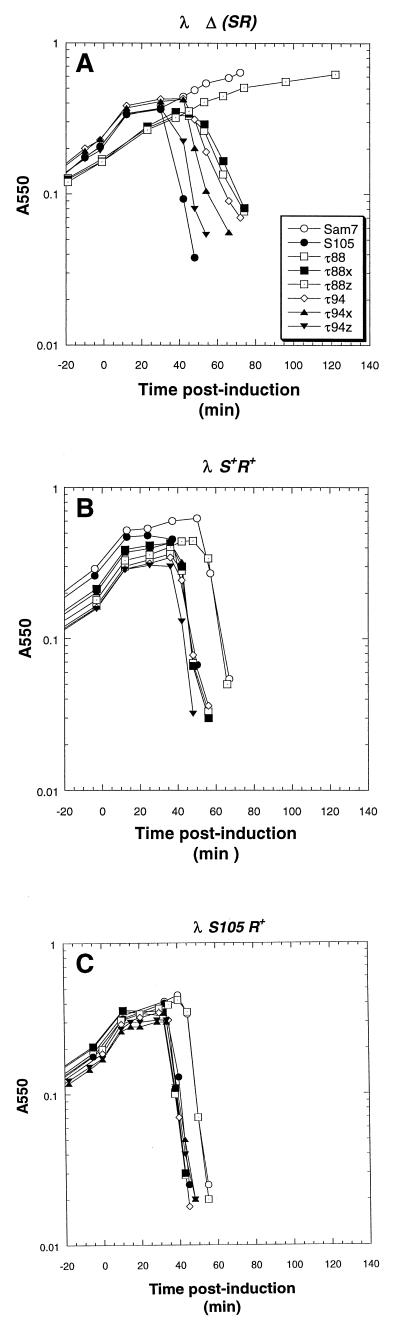
Insertions after codon 88.
Three insertions behind codon 88, including the standard insertion, an insertion encoding the sequence G2H6D (S105τ88x), and an insertion encoding G2H4 truncated with a frameshift (S105τ88z), were isolated. While the last was a recessive null allele, the first and second insertion alleles supported lysis profiles that were essentially the same as that generated by S105 (Fig. 7), although the level of protein detected in membrane extracts in each case was reduced, suggesting proteolytic instability (Fig. 3). In trans to S105, S105τ88 actually accelerated lysis triggering slightly, but reproducibly, more than the parental allele (Fig. 7B and C).
FIG. 7.
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids bearing the S105 gene with inserts after codons 88 and 94. (A) Plasmids pKB1 (○), pS105 (•), pS105τ88 (□), pS105τ88x (■), pS105τ88z (⊡), pS105τ94 (◊), pS105τ94x (▴), and pS105τ94z (▾) were induced as described in the legend to Fig. 2. (B and C) The hosts were MC4100 [λ S+] and MC4100 [λ S105], respectively.
Insertions after codon 94.
The allele with the standard insertion after residue 94 has been described and was used as a source for oligohistidine-tagged S105 protein to work out a purification system and in vitro assay for hole formation (41). Essentially, it is very similar to the S105τ88 allele described above, in terms of lysis triggering, except that it supports the accumulation of approximately the same level of S protein in the membrane as the parent (Fig. 3 and 7A). In addition, two insertions with accompanying frameshifts were isolated. In one, S105τ94x, the insertion and frameshift encode the sequence G2H3QKSITITAALLKKPE after F94 (Fig. 1B). In the second, S105τ94z, the insertion encodes the sequence G2H6G2LLKKPE after F94 (Fig. 1B). Despite the loss of the C-terminal hydrophilic domain, both of these frameshift alleles were functional and, in fact, the induced S105τ94z frameshift allele triggered lysis essentially as well as S105 (Fig. 7A). Interestingly, the S105τ94z allele actually supported reproducibly faster onset of lysis than did S105 in trans to S+ but not in trans to S105 (Fig. 7B and C).
Mutant alleles of S in the S105τ94 context.
A collection of missense S alleles with recessive, dominant, and early-dominant characters have been isolated and characterized previously (22, 31). Several of these changes were transferred to the contexts of the S105τ94 reading frame and, in parallel, the S105 parental reading frame by site-directed mutagenesis. In all but one instance, the phenotype of the original mutation was reproduced in both the S105 and S105τ94 contexts (Fig. 8). In both the S105 and S105τ94 contexts the plasmid-borne A52V mutation conferred a defective, but slightly dominant, phenotype (Fig. 8B and C), which is in contrast to the originally reported recessive phenotype reported for the A52V allele (31).
FIG. 8.
Plasmid transactivation profiles of MC4100 harboring λ lysogens and transactivation plasmids carrying missense mutations in the S105 or S105τ94 gene. (A) Plasmids pKB1 (○), pS105 (•), pS105R33L (■), pS105τ94R33L (□), pS105A52G (⧫), pS105τ94A52G (◊), pS105A52V (▾), and pS105τ94A52V (▿) were induced as described in the legend to Fig. 2. (B and C) The hosts were MC4100 [λ S+] and MC4100 [λ S105], respectively.
In addition, the SA52G allele does not support plaque formation in the phage context, because it triggers lysis too early, before the first virion has been assembled (22). The timing defect may have been due to a loss of function in the S107 inhibitor protein equally as well as a gain of function in the S105 effector. However, the S105A52G and S105τ94A52G alleles were triggered early, just like SA52G (Fig. 8A). With S105τ94A52G, lysis began after a 20-min delay, suggesting that the oligohistidine-tagged peptide was impeding the formation of productive holes at low concentrations of S protein (Fig. 8A).
DISCUSSION
We have described a collection of insertion mutations created during an effort to insert an oligohistidine tag into the λ holin protein, S. Most insertions fundamentally changed the character of the protein and abolished its lethal holin activity. Some insertions conferred new properties on S, and only two allowed S to maintain normal holin function. The phenotypes of these insertion alleles are useful for analysis of S function and topology.
Locations of oligohistidine tags in holins.
Oligohistidine tags have been increasingly used as a primary sequence modification to facilitate protein purification. In several instances, oligohistidine tags have been inserted in the sequence of membrane proteins, which are especially difficult to purify because of the necessity of the presence of a detergent for solubilization (25, 26, 36). Holins constitute a large class of integral membrane proteins with novel functions and may be good choices for biochemical and biophysical analysis because of the simplicity of their primary sequences and apparent functional autonomy. Because of the small size, membrane localization, and lethal nature of holins, oligohistidine tag methodology appears to be an ideal methodology. Other systems involving fusion of large purifiable domains have not been useful with holins, because the detergent present to maintain holin solubility interferes with removal of the fusion domain (7). The simplest choices for oligohistidine tag positions have been at the N and C termini, in part because of the availability of plasmids with high-efficiency gene expression systems (11, 28). Neither polypeptide terminus of S was found to tolerate the insertion of an oligohistidine tag sequence without unacceptable phenotypic alteration, which led us to conduct a systematic mutagenesis of the primary sequence of the S105 reading frame by inserting oligohistidine-tag-encoding sequences. Ultimately, insertions after positions 88 and 94 were found to be tolerated without significant alteration of the lysis properties. The protein products of these S105τ94 alleles accumulated to normal levels in the membrane fraction, and the proteins have been successfully purified to homogeneity (41). Moreover, the phenotypes of several important missense alleles originally isolated in the S+ context were found to be conserved in the context of the S105τ94 gene, suggesting that the C-terminal domain of S does not have essential interactions with the interior of the molecule. With this information in hand, inspection of the sequences of the charged C-terminal domains of other holins suggests that there are homologous insertion points for creating oligohistidine-tag-encoding alleles with minimal risk of disrupting the function of the protein (45, 46).
It is not clear why location of the oligohistidine tag at the extreme C terminus caused dysfunction of the S gene. We cannot rule out proteolytic instability of the C-terminally tagged product, because the antibody for S was raised against a synthetic peptide corresponding to the C-terminal sequence and does not recognize the S105τ protein. However, S105τ was detected, without evidence of degradation, with two reagents designed to specifically recognize oligohistidine tags and it accumulates to Coomassie blue-detectable levels when it is overexpressed (data not shown).
Early-dominance phenotype not due to titration of inhibitor.
S105τ exhibits the early-dominance phenotype, first noted for other lysis-defective alleles in the original analysis of S (30). The presence of a functional S allele in trans to a prophage S+ allele results in an accelerated onset of lysis (Fig. 2C), and thus, in such a context, the defective S105τ allele was expected to exert either no effect on lysis timing, indicating recessive character, or a delay in lysis timing, indicating negative-dominant character. Remarkably, despite its lysis defect, S105τ accelerates lysis as well as S105 (Fig. 2C and D). This type of dominance, previously designated early dominance, in some lysis-defective alleles has been ascribed to a titration of the inhibitor species of the S+ allele used in trans (30). According to this view, the defective holin acts as a sink for the S107 inhibitor species from the wild-type allele, without interacting with the S105 effector. Thus, the early-dominant character results from an indirect effect on normal holin regulation. However, for this allele and some others (see below), early-dominant behavior is clearly found even when the functional allele in trans is S105 and thus no inhibitor species is present at all. (See below.)
Insertions before the first transmembrane domain.
Western immunoblot analysis of τS105 indicated that two protein species were present. The smaller of the two may be a fortuitous proteolytic event generating a cleavage product identical to S105. More likely, it represents secondary translational starts from the Met codon for S105, some 30 nt downstream. τS105 is defective in hole formation but has been demonstrated to affect normal hole formation in a dominant-negative manner (Fig. 2C and D). This finding suggests that the τS105 protein assumes a normal topology which allows it to interact with functional S105 protein.
Although no standard insertion was isolated after codon 9, the properties of the abnormal insertions are instructive. That the S105τ9x allele was strongly dominant clearly suggests that the positively charged arginine residue in S105τ9x confers inhibitor capacity to this tagged S105 derivative (41). Given that inhibitor capacity is dependent on positive charge (4, 42), this result indicates that the positioning of the charged residue within the N-terminal domain abutting the first putative transmembrane domain is not critical for the inhibition. Insertion of the sequence G2H5RG after L9 results in a lysis-defective allele, S105τ9x, which, in trans to an induced prophage bearing an S105 allele, retards lysis even more dramatically (125 min) than an allele producing both S107 and S105 (105 min) under isogenic conditions. This result demonstrates that the precise location of the required positively charged residue within the N terminus is not critical in terms of the requirements for being an inhibitor protein, consistent with the finding that adding or subtracting positively charged residues between positions 2 and 8 has the same lysis retardation effect as increasing the population of S107 molecules within the pool of S holin proteins (9, 42). The potent inhibitor function of the S105τ9x allele contrasts clearly with the recessive nature of the allele with a different aberrant insertion after residue 9, S105τ9z, which encodes the tag sequence G2H6PA. The fact that this allele does not accumulate stable protein and is also nonfunctional demonstrates that the N-terminal domain has at least some structural requirement other than serving as a site for positively charged residues. Apparently this structural requirement cannot be met in the S105τ9z allele, possibly because of the proline residue in the aberrant inserted sequence.
Insertions within putative transmembrane domains.
There is no structural information to guide mutagenesis of holins. Although the membrane topology of λ S is still uncertain, the sequence of this protein is so short that only two reasonable models for its membrane topology can be imagined. In Fig. 1, model A, there are three transmembrane domains, whereas in model B, there are only two transmembrane domains. Circular dichroism studies reveal that S105τ94 is about 40% α-helix, which indicates that fewer than 45 residues are in α-helical conformation in detergent, which is more consistent with the model B bitopic topology (41). In general, the oligohistidine tags after positions 21 and 49, corresponding to insertions within the first and second transmembrane domains, respectively, generated nonfunctional proteins. τ21 is dominant in trans to S+, suggesting that disruption of the first putative transmembrane domain does not abolish intermolecular interactions with other S molecules (Fig. 4B). However, the absence of significant dominant character may indicate that the weak dominance exhibited in Fig. 4B is an indirect effect, possibly resulting from preferential proteolysis (see below).
An insertion after codon 75 within the ambiguous domain left the protein with partial function, in the sense that it appears to trigger, albeit somewhat late, but then never allows release of the endolysin, suggesting that a defective hole is being formed. These data appear to be less compatible with model A, where disruption of the third transmembrane domain would almost certainly lead to topological disorganization. Thus, the facts that 10 residues can be inserted at this point and partial function can be retained suggest that position 75 is unlikely to be in the middle of a transmembrane domain. Failure to accumulate S105τ75x protein, as assessed by Western immunoblot analysis, indicates a drastically decreased level of S105τ75x protein. Nevertheless, sufficient S105τ75x protein is available to collapse the membrane potential without concomitant release of endolysin (Fig. 6A). Proteolysis may explain the inability to visualize the protein in Western immunoblots. Alternatively, the decreased level of S105τ75x protein may reduce the total number of holes formed per cell and thus limit endolysin release. Yet another possibility is that holes are large enough to be lethal but too small to allow endolysin unrestricted passage to the periplasm. In any case, the segregation of lethality and hole formation are novel results.
The dominant character of the S105τ21 allele in the presence of an S+ allele, but not an S105 allele, is surprising, considering that the insertion should disrupt the first putative transmembrane domain and thus potentially alter the topological organization of the protein. However, we favor the interpretation that in this case, the weak early dominance is due to an indirect effect, namely proteolysis. Several missense S proteins which exhibit no defect in membrane localization but do not oligomerize properly are found to be unstable at physiological levels of expression (18). These same proteins when overexpressed accumulate normally in the membrane fraction, indicating that the protease activities involved can be titrated. To account for how S105τ21 can inhibit the lysis function of S+, but not S105, the simplest model is that somehow the production of S105τ21 increases the relative activity of S107, the holin inhibitor produced in S+, but not in S105. This possibility implies that normally some S107 is proteolytically degraded but that the production of S105τ21 at least partially titrates out the protease activity. The S105τ21 protein may thus, by partially titrating endogenous membrane proteolytic activity, increase the likelihood of successful S107-dependent inhibition of hole formation. The only pulse-chase labeling experiments done with S were designed to test the hypothesis that S107 is proteolytically processed to S105 (8). Although the label in the two species appeared to be constant during an extended chase period, the conditions were distinctly nonphysiological, being based on a T7 RNA polymerase expression system, and a role for proteolysis in the lysis timing mechanism under normal conditions was not ruled out. It should be noted that an integral membrane protease has been shown to be involved in other regulatory events in the λ life cycle (38). It will be of interest to see if the S105-S107 regulatory system and the unexpected phenotypes of S105τ21 and other insertion genes are affected by inactivation of this and other known proteolytic systems in E. coli.
Mutations and insertions within the first connector loop.
It is surprising that the alteration of the GG residues at positions 38 and 39, between the first two putative transmembrane domains, to either PG or GP completely inactivates the holin without conferring proteolytic instability. Even more surprising are the facts that the GG→GP change is strongly dominant and that the GG→PG change is largely recessive. These unexpected defective phenotypes suggest that the flexibility expected in the adjacent glycine residues in the connector domain must play an important role in hole formation, perhaps to allow reorientation of the two transmembrane domains flanking the loop. These phenotypes parallel those observed for missense mutations in the same residues, obtained by a biological selection for loss of the lethal activity of S (30, 31). Although both the mutations G38S and G39D confer a lysis-defective phenotype, the former allele is leaky, allowing a grossly delayed lysis, whereas the latter is an absolute nonlytic allele in a phage context (31). Also, G38S is negatively codominant, whereas G39D is strongly early dominant (31). Thus, unlike other polytopic transmembrane proteins where connector loops have been shown to be largely irrelevant in terms of structure or function, the first connector loop in S is surprisingly sensitive to missense substitution. Moreover, the dramatically opposite dominance phenotypes of missense changes in these adjacent glycine residues suggest that this dipeptide sequence plays a key role in the conformational change involved in converting S from its prehole, chronic state, which persists throughout the late period of gene expression, to its lethal hole or acute state. At position 39, the fact that replacing a glycine, which has the maximum intrinsic flexibility, with a proline, which may require covalent catalysis to change its cis or trans orientation with regard to the N-terminally adjacent residue, creates a dominant-negative nonlytic allele suggests that the conformational change in triggering of lysis involves movement of the two flanking transmembrane domains with respect to one another. Presumably, the G39P change locks the holin in an untriggerable state, which may or may not correspond to the normal prehole conformation but which is fully capable of interacting with other S monomers. Interestingly, an adventitiously isolated insertion of a random hexapeptide of neutral amino acids (VMVMMV) between positions 38 and 39 was found partially to suppress the lysis defect, suggesting that sufficient flexibility to accomplish the triggering change can be restored by simply increasing the chain length of the loop (data not shown). From this perspective, it is surprising that all of the oligohistidine insertions between positions 38 and 39 were lysis defective, recessive, and stable. Thus, the connector sequence (residues 33 to 39) between the two putative transmembrane domains is intolerant of oligopeptide insertion and, if altered, as with these insertions or with the missense mutations isolated previously in 4 of the 7 residues (30), leads to a nonfunctional S holin. However, if this loop is located in the periplasm, where acidic conditions predominate, substantial cationic charge may be associated with the modified loop domains, which may account for an inability to oligomerize productively in homomeric complexes (with other oligohistidine-tagged holins) or with the parental S105 protein, which already has two cationic residues in the loop region. In any case, the availability of the putatively locked holin mutant which accumulates stable protein should be a useful tool now that structural investigation of the holin is becoming practical.
Early-dominance insertions after codons 63 and 83.
Insertions behind codons 63 and 83 should interrupt a cytoplasmic domain, according to the bitopic model for S topology, or flank the third transmembrane domain, according to the tritopic model (Fig. 1B). Both alleles exhibited severe lysis defects, but surprisingly, in the presence of the S105 allele, these defective insertions exhibited early dominance and were indistinguishable from S105. Thus, the lysis-defective insertion alleles at codons 63, 83, and 107 (S105τ) of the S105 allele all exhibit early-dominant character. One interpretation of early dominance, in the context of the S system, is that the S105 product of the mutant allele is capable of participating in hole formation only after some sort of nucleation or templating event is achieved by the functional S105 protein. The strong early-dominance phenotype exhibited by, for example, the S63τ allele (Fig. 7) is even more provocative, considering that the plasmid-borne allele is expressed at a much higher relative rate than the prophage allele (18). Thus, only a small amount of wild-type S105 is needed to nucleate or template the hole formation event onto the otherwise incompetent S105τ63 proteins. Thus, S105τ63, S105τ83, and S105τ might be proficient at perpetuating a conformational change in oligomeric secondary, tertiary, or quaternary structure but not in initiating the change. The presence of dimeric S105τ83 protein in the absence of the monomer suggests proteolytic degradation of the oligohistidine-tagged holin after induction during membrane isolation and protein extraction. Thus, it may be that the early-dominant holin is unstably folded or self-associated and that admixture of proportions of the wild-type proteins raises the overall stability above a threshold level. If the nucleation hypothesis is correct, the proportions of parental holin molecules required to restore lysis in cells expressing the early-dominance alleles might be much less than stoichiometric. Experiments to distinguish between these possibilities are under way in our laboratory. We conclude that at least for this allele, the S105τ protein is capable of participating in lethal hole formation in the presence of the wild-type S105 protein but that it cannot do so alone.
Insertions near the C terminus.
The fact that the region near codon 88 can tolerate a large insertion suggests that it does not have a critical structure and serves primarily as a linker or connector domain, consistent with the fact that no missense mutations were isolated between codons 83 and 102 during selections for mutations conferring loss of lethality on S (27, 29, 30). More dramatically, the functional activity of the S105τ94z frameshift allele strongly suggests that the C-terminal domain of S has no essential structure but that it instead serves primarily as a reservoir of charged residues acting as an important topology determinant and also in the regulation of S by interaction with the energized membrane. It may be that the C terminus has evolved as a fine-tuning domain which, in the absence of a critical role in hole formation, can be mutated to accelerate or decelerate lysis timing, to optimize the length of the vegetative cycle for whatever host context is currently predominant.
Mutant alleles of S in the S105τ94 context.
The parallels between the phenotypes of S+ and S105 or S105τ94 alleles extends to dominance-recessivity tests, with one exception, the mutation A52V. This mutation was recessive in the phage context in trans to a second prophage with the S+ allele (31). However, when it is mounted on a multicopy plasmid, it exhibits some dominance, retarding the onset of lysis when it is in trans to a prophage carrying S105 (Fig. 8). This result may reflect quantitative differences, because the mutant allele is present on a multicopy plasmid at the time of the onset of late gene transcription, which is different from the situation with an induced lysogen, where late gene expression begins about 8 min after induction, before significant amplification of the prophage template (9, 20). Testing of other recessive alleles in this plasmid context should resolve this question. In any case, the results from transactivation of previously characterized mutations in an S105 only context are significant because the associated defects could not be attributed to decreased effector function of S105 or increased function of S107, the holin inhibitor (31). Clearly, these data unequivocally prove the defect in each case lies in the S105 effector protein.
ACKNOWLEDGMENTS
The Ni-nitrilotriacetic acid biotin-streptavidin horseradish peroxidase conjugate was a kind gift from Michael Brigham-Burke (Smith-Kline Beecham). We are indebted to the members of the Young laboratory past and present for their help and encouragement and to Sharyll Pressley for her competent clerical assistance. We especially thank Paul K. Piper, Matilda Powers, and George Han for assistance with plasmid constructions, sequencing, and inductions.
This work was funded by grant GM27099 from the National Institute of General Medical Sciences, National Institutes of Health, to R.Y. and by funds from the College of Agriculture at Texas A&M University.
REFERENCES
- 1.Altman E, Altman R K, Garrett J M, Grimaila R J, Young R. S gene product: identification and membrane localization of a lysis control protein. J Bacteriol. 1983;155:1130–1137. doi: 10.1128/jb.155.3.1130-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bienkowska-Szewczyk K, Lipinska B, Taylor A. The R gene product of bacteriophage λ is the murein transglycosylase. Mol Gen Genet. 1981;184:111–114. doi: 10.1007/BF00271205. [DOI] [PubMed] [Google Scholar]
- 4.Bläsi U, Chang C-Y, Zagotta M T, Nam K, Young R. The lethal λ S gene encodes its own inhibitor. EMBO J. 1990;9:981–989. doi: 10.1002/j.1460-2075.1990.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bläsi U, Nam K, Hartz D, Gold L, Young R. Dual translational initiation sites control function of the λ S gene. EMBO J. 1989;8:3501–3510. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bläsi U, Young R. Two beginnings for a single purpose: the dual-start holins in the regulation of phage lysis. Mol Microbiol. 1996;21:675–682. doi: 10.1046/j.1365-2958.1996.331395.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang C-Y. Synthesis, function and regulation of the λ holin. Ph.D. dissertation. College Station, Tex: Texas A&M University; 1994. [Google Scholar]
- 8.Chang C-Y, Nam K, Bläsi U, Young R. Synthesis of two bacteriophage λ S proteins in an in vivo system. Gene. 1993;133:9–16. doi: 10.1016/0378-1119(93)90218-r. [DOI] [PubMed] [Google Scholar]
- 9.Chang C-Y, Nam K, Young R. S gene expression and the timing of lysis by bacteriophage λ. J Bacteriol. 1995;177:3283–3294. doi: 10.1128/jb.177.11.3283-3294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clontech. TALON metal affinity resin user manual. Palo Alto, Calif: Clontech Laboratories, Inc.; 1996. pp. 1–31. [Google Scholar]
- 12.Del Campillo-Campbell A, Campbell A. Endolysin from mutants of bacteriophage λ. Biochem Z. 1965;342:485–491. [PubMed] [Google Scholar]
- 13.Garrett J, Bruno C, Young R. Lysis protein S of phage λ functions in Saccharomyces cerevisiae. J Bacteriol. 1990;172:7275–7277. doi: 10.1128/jb.172.12.7275-7277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett J, Fusselman R, Hise J, Chiou L, Smith-Grillo D, Schulz R, Young R. Cell lysis by induction of cloned λ lysis genes. Mol Gen Genet. 1981;182:326–331. doi: 10.1007/BF00269678. [DOI] [PubMed] [Google Scholar]
- 15.Garrett J, Young R. Lethal action of bacteriophage λ S gene. J Virol. 1982;44:886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg A R, Howe M. New mutations in the S cistron of bacteriophage λ affecting host cell lysis. Virology. 1969;38:200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- 17.Grayhack E J, Yang X, Lau L F, Roberts J W. Phage λ gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell. 1985;42:259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- 18.Grüendling, A., and R. Young. Unpublished data.
- 19.Harris A W, Mount D W A, Fuerst C R, Siminovitch L. Mutations in bacteriophage λ affecting host cell lysis. Virology. 1967;32:553–569. doi: 10.1016/0042-6822(67)90032-3. [DOI] [PubMed] [Google Scholar]
- 20.Herskowitz I, Signer E R. A site essential for expression of all late genes in bacteriophage λ. J Mol Biol. 1970;47:545–556. doi: 10.1016/0022-2836(70)90321-9. [DOI] [PubMed] [Google Scholar]
- 21.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson-Boaz R, Chang C-Y, Young R. A dominant mutation in the bacteriophage λ S gene causes premature lysis and an absolute defective plating phenotype. Mol Microbiol. 1994;13:495–504. doi: 10.1111/j.1365-2958.1994.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 23.Malinski J A, Nelsestuen G L. Membrane permeability to macromolecules mediated by the membrane attack complex. Biochemistry. 1989;28:61–70. doi: 10.1021/bi00427a010. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.Mitchell D M, Gennis R B. Rapid purification of wildtype and mutant cytochrome c oxidase from Rhodobacter sphaeroides by Ni2+-NTA affinity chromatography. FEBS Lett. 1995;368:148–150. doi: 10.1016/0014-5793(95)00626-k. [DOI] [PubMed] [Google Scholar]
- 26.Moeck G S, Tawa P, Xiang H, Ismail A A, Turnbull J L, Coulton J W. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K-12. Mol Microbiol. 1996;22:459–471. doi: 10.1046/j.1365-2958.1996.00112.x. [DOI] [PubMed] [Google Scholar]
- 27.Neal G S. Mutational analysis of the λ S gene. M.S. thesis. College Station, Tex: Texas A&M University; 1984. [Google Scholar]
- 28.Qiagen. The QIAexpressionist. Chatsworth, Calif: Qiagen; 1997. [Google Scholar]
- 29.Raab R. The structure, function and regulation of the S gene of bacteriophage λ. Ph.D. dissertation. College Station, Tex: Texas A&M University; 1988. [Google Scholar]
- 30.Raab R, Neal G, Garrett J, Grimaila R, Fusselman R, Young R. Mutational analysis of bacteriophage λ lysis gene S. J Bacteriol. 1986;167:1035–1042. doi: 10.1128/jb.167.3.1035-1042.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab R, Neal G, Sohaskey C, Smith J, Young R. Dominance in λ S mutations and evidence for translational control. J Mol Biol. 1988;199:95–105. doi: 10.1016/0022-2836(88)90381-6. [DOI] [PubMed] [Google Scholar]
- 32.Reader R W, Siminovitch L. Lysis defective mutants of bacteriophage λ: genetics and physiology of S cistron mutants. Virology. 1971;43:607–622. doi: 10.1016/0042-6822(71)90286-8. [DOI] [PubMed] [Google Scholar]
- 33.Reader R W, Siminovitch L. Lysis defective mutants of bacteriophage λ: on the role of the S function in lysis. Virology. 1971;43:623–637. doi: 10.1016/0042-6822(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 34.Rennell D, Poteete A R. Phage P22 lysis genes: nucleotide sequences and functional relationships with T4 and λ genes. Virology. 1985;143:280–289. doi: 10.1016/0042-6822(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 35.Riley M A. Molecular mechanisms of colicin evolution. Mol Biol Evol. 1993;10:1380–1395. doi: 10.1093/oxfordjournals.molbev.a040081. [DOI] [PubMed] [Google Scholar]
- 36.Scheel A A, Pelham H R B. Purification and characterization of the human KDEL receptor. Biochemistry. 1996;35:10203–10209. doi: 10.1021/bi960807x. [DOI] [PubMed] [Google Scholar]
- 37.Schweizer H P. The pUC18CM plasmids: a chloramphenicol resistance gene cassette for site-directed insertion and deletion mutagenesis in Escherichia coli. BioTechniques. 1990;8:612–616. [PubMed] [Google Scholar]
- 38.Shotland Y, Koby S, Teff D, Mansur N, Oren D A, Tatematsu K, Tomoyasu T, Kessel M, Burkau B, Ogura T, Oppenheim A B. Proteolysis of the phage λ CII regulatory protein by FtsH (HflB) of Escherichia coli. Mol Microbiol. 1997;24:1303–1310. doi: 10.1046/j.1365-2958.1997.4231796.x. [DOI] [PubMed] [Google Scholar]
- 39.Silhavy T J, Berman M L, Enquist L W. Bacterial strains. In: Silhavy T J, Berman M L, Enquist L W, editors. Experiments with gene fusions. 1st ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. pp. xi–xiii. [Google Scholar]
- 40.Smith D L, Chang C-Y, Young R. The λ holin accumulates beyond the lethal triggering concentration under hyper-expression conditions. Gene Expr. 1998;7:39–52. [PMC free article] [PubMed] [Google Scholar]
- 41.Smith D L, Struck D K, Scholtz J M, Young R. Purification and biochemical characterization of the λ holin. J Bacteriol. 1998;180:2531–2540. doi: 10.1128/jb.180.9.2531-2540.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steiner M, Bläsi U. Charged amino-terminal amino acids affect the lethal capacity of λ lysis proteins S107 and S105. Mol Microbiol. 1993;8:525–533. doi: 10.1111/j.1365-2958.1993.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 43.Taylor A, Kedzierska S, Wawrzynów A. Bacteriophage λ lysis gene product modified and inserted into Escherichia coli outer membrane: Rz1 lipoprotein. Microb Drug Resist. 1996;2:147–153. doi: 10.1089/mdr.1996.2.147. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Hart C M, Grayhack E J, Roberts J W. Transcription antitermination by phage λ gene Q protein requires a DNA segment spanning the RNA start site. Genes Dev. 1987;1:217–226. doi: 10.1101/gad.1.3.217. [DOI] [PubMed] [Google Scholar]
- 45.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young R, Bläsi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 47.Young R, Way S, Yin J, Syvanen M. Transposition mutagenesis of bacteriophage λ: a new gene affecting cell lysis. J Mol Biol. 1979;132:307–322. doi: 10.1016/0022-2836(79)90262-6. [DOI] [PubMed] [Google Scholar]
- 48.Zagotta M T, Wilson D B. Oligomerization of the bacteriophage λ S protein in the inner membrane of Escherichia coli. J Bacteriol. 1990;172:912–921. doi: 10.1128/jb.172.2.912-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]