Abstract
Simple Summary
Adoptive immunotherapy has emerged as an effective alternative of mounting impact to the current standard of care in cancer, viral infections, and recently, autoimmunity. Key players in maintaining immune homeostasis are the regulatory T cells (Tregs), a major immunosuppressive cell subset and, therefore, an attractive candidate for the cellular therapy of autoimmune disorders or allo-responses in the transplantation setting. Notwithstanding the safety and tolerability of Tregs in early trials, their efficacy remains rather ill-defined, being limited by poor persistence and a lack of specificity, thus hindering widespread clinical application. However, the better biological understanding of in vivo Treg performance and the recent advances in genetic engineering have led to the next-generation Treg immunotherapy era, enabling the introduction of new features in Tregs and generating more potent and targeted Treg cellular therapies. In this review, we discuss the current achievements and existing challenges towards clinically translating Tregs into a living drug therapy for a variety of inflammatory conditions.
Abstract
Regulatory T cells (Tregs) are fundamental to maintaining immune homeostasis by inhibiting immune responses to self-antigens and preventing the excessive activation of the immune system. Their functions extend beyond immune surveillance and subpopulations of tissue-resident Treg cells can also facilitate tissue repair and homeostasis. The unique ability to regulate aberrant immune responses has generated the concept of harnessing Tregs as a new cellular immunotherapy approach for reshaping undesired immune reactions in autoimmune diseases and allo-responses in transplantation to ultimately re-establish tolerance. However, a number of issues limit the broad clinical applicability of Treg adoptive immunotherapy, including the lack of antigen specificity, heterogeneity within the Treg population, poor persistence, functional Treg impairment in disease states, and in vivo plasticity that results in the loss of suppressive function. Although the early-phase clinical trials of Treg cell therapy have shown the feasibility and tolerability of the approach in several conditions, its efficacy has remained questionable. Leveraging the smart tools and platforms that have been successfully developed for primary T cell engineering in cancer, the field has now shifted towards “next-generation” adoptive Treg immunotherapy, where genetically modified Treg products with improved characteristics are being generated, as regards antigen specificity, function, persistence, and immunogenicity. Here, we review the state of the art on Treg adoptive immunotherapy and progress beyond it, while critically evaluating the hurdles and opportunities towards the materialization of Tregs as a living drug therapy for various inflammation states and the broad clinical translation of Treg therapeutics.
Keywords: regulatory T cells, adoptive immunotherapy, autoimmune diseases, transplantation, CAR tregs
1. Introduction
Regulatory T cells (Tregs), consist of a small, albeit critical for maintaining immune equilibrium, fraction of T cells, that prevent or dampen immune responses to self-antigens, thus preserving self-tolerance while suppressing excessive immune activation to non-self-antigens ([1,2,3]). According to the recommendations on Treg cell nomenclature [4], Tregs can be classified based on their origin of differentiation as (i) emerging de novo in the thymus, thus bearing a T cell receptor (TCR) with specificity towards self-antigens [thymus-derived Tregs [5], tTregs, previously called natural Tregs (nTregs)]; (ii) differentiating in the periphery, therefore having a non-self-antigen-specific TCR [peripherally derived Tregs, pTregs, previously known as induced or adaptive Tregs (iTregs or aTregs)]; and (iii) being generated ex vivo (in vitro-induced Tregs, iTregs) and clearly distinguished from the in vivo-generated Tregs. Despite their heterogeneity, there is a lack of specific markers distinguishing human tTregs from pTregs. The classically defined Tregs are CD4+ cells, constitutively expressing high levels of CD25 (interleukin-2 receptor alpha chain, IL-2Rα) and the transcription factor forkhead box P3 (Foxp3). These markers are expressed by the majority of tTregs and also a subpopulation of pTregs [6]. Two additional Foxp3- pTreg subsets, T-helper 3 (Th3) and type-1 Treg (Tr1), the suppressive functions of which rely on transcription growth factor beta (TGF-β) and interleukin-10 (IL-10) secretion, respectively, have also been well-described [7,8]. To date, there is also convincing, albeit substantially less than for CD4+ Tregs, evidence for the existence of CD8+ Tregs with properties similar to their CD4+ counterparts [9]. Table 1 outlines the main extracellular markers and transcription factors expressed by various Treg subtypes, along with their respective mechanism of immunosuppressive action.
Tregs reshape immune responses with precision, executing their regulatory function in a sophisticated and tailored manner as opposed to the conventional, general immunosuppressive approaches. This precise immune regulation, particularly in contexts like autoimmunity and transplantation is of highest importance. Strategies boosting polyclonal Treg numbers and function in vivo by the administration of Treg-promoting proteins or pharmacological agents such as interleukin-2 (IL-2) [10,11,12], anti-IL-2 complexes [13], intravenous immunoglobulin alone or in combination with rapamycin [14,15,16,17], antibody-mediated agonistic stimulation of tumor necrosis factor superfamily receptor 25 (TNFRSF25), and cytokine-targeted antibodies, which modify the pro-inflammatory environment rescuing Treg function [18,19,20,21,22], have enhanced the in vivo tolerance in preclinical studies and early clinical trials.
Adoptive immunotherapy with Tregs, which includes the isolation and ex vivo expansion of autologous Tregs, has emerged as an attractive therapeutic option to restore the immune balance in autoimmunity and transplantation. Multiple studies using the adoptive transfer of ex vivo-expanded polyclonal Tregs have demonstrated significant potential for inducing tolerance and preventing graft rejection following solid organ transplantation [23], as well as treating autoimmune-mediated diseases, including type 1 diabetes (T1D) [24], rheumatoid arthritis [25], multiple sclerosis [26], and systemic lupus erythematosus (SLE), or graft-versus-host disease (GvHD) in the allogeneic hematopoietic cell transplantation setting, and recently, coronavirus disease 2019 (COVID-19) [27,28,29]. However, increasing evidence suggests that Tregs are functionally impaired in patients with autoimmune diseases and transplant recipients, due to the instability of Foxp3 expression, impaired suppressive function, decreased migratory capacity, and increased apoptosis [30,31,32]. The early results of the first clinical trials, although promising, have questioned the efficacy of Tregs, as only modest clinical responses were achieved. In this review, we focus on the obstacles limiting the clinical utility of Treg adoptive immunotherapy in the context of autoimmunity and transplantation and discuss strategies to overcome these impediments and improve the outcomes with Treg cell therapy (Table 2).
Table 1.
Extracellular markers, transcription factors, and mechanisms of actions of various Treg subtypes.
| Treg Subset | Origin | Markers | Transcription Factors | Suppressive Mechanism | References |
|---|---|---|---|---|---|
| tTregs | Generating in the thymus | CD4+, CD25hi, CD27lo, CTLA-4+, LAG-3+, TIGIT+, TIM-3+, PD-1+ | FOXP3pos | Cell-contact-dependent immunosuppression via receptors like CTLA-4 and PD-1 | [33] |
| pTregs | Differentiating from peripheral naive CD4+ T cells | CD4+, CD25hi, CD27lo, CTLA-4+, LAG-3+, TIGIT+, Tim-3+, PD-1+ | FOXP3pos | Inhibitory function via soluble factors such as TGF-β1 and IL-10 | [33] |
| Tr1 Tregs | Differentiating from peripheral naive CD4+ T cells | CD4+, CD25, CD49b+, LAG-3+ [3] | Tbet, Blimp-1, FOXP3neg [1] | Inhibitory function via IL-10 production | [34,35] |
| Th3 Tregs | Differentiating from peripheral naive CD4+ T cells | CD4+, CD25+, CD69+, LAP+ | TGF-β, FOXP3neg | Inhibitory function via TGF-β production | [36] |
| CD8+ Tregs | Differentiating from peripheral naive CD8+ T cells | CD8+, CD25+, CD122+, CD49d+ | FOXP3pos, Eomes, Helios, TGF-β | TGF-dependent control of Helios and homeostatic cytokine IL-15 [4] | [37,38] |
tTregs: thymus-derived Tregs, pTregs: peripherally derived Tregs, Tr1 Tregs: Type 1 Tregs, Th3 Tregs: T helper T cells, CTLA-4: Cytotoxic T-lymphocyte-associated protein 4, LAG-3: Lymphocyte-activation gene 3, TIGIT: T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains, TIM3: T-cell immunoglobulin and mucin domain-containing protein 3, PD-1: Programmed cell death protein 1, FOXP3: Forkhead box P3, TGF-β: Transforming Growth Factor β 1, IL-10: Interleukin-10, Tbet: T-box expressed in T cells, Blimp-1: B lymphocyte-induced maturation protein 1, LAP: Latency-associated peptide positive, Eomes: Eomesodermin, IL-15: Interleukin-15.
Table 2.
Clinical trials of adoptive immunotherapy with Tregs.
| Cell Product | Source | Disease | Treg Manufacturing | Study Phase | Patients | Safety | Efficacy | Trial ID | References |
|---|---|---|---|---|---|---|---|---|---|
| Tregs for autoimmune diseases | |||||||||
| Polyclonal Tregs | Autologous | T1D | Isolation/enrichment and ex vivo expansion | I | 12 | No AEs | 8/12 clinical remission | ISRCTN06128462 | Marek-Trzonkowska et al., 2014 [39] |
| Polyclonal Tregs | Autologous | T1D | Isolation/enrichment and ex vivo expansion | I | 14 | Well-tolerated. No cell therapy-related high-grade AEs | Not powered to detect improvement in metabolic function | NCT01210664 | Bluestone et al., 2015 [24] |
| Polyclonal Tregs | Autologous | T1D | Ex vivo expansion | II, randomized placebo-controlled double blind | 110 | Well-tolerated | No improvement in the preservation of C-peptide levels vs. placebo | NCT02691247 | Caladrius Biosciences, 2019 [40] |
| Polyclonal Tregs | UCB | T1D | Isolation/enrichment and ex vivo expansion | I/II, randomized, parallel assignment, open label | Recruiting | NCT02932826 | |||
| Combinational: polyclonal Tregs + low-dose IL-2 | Autologous | T1D | Isolation/enrichment and ex vivo expansion | I | 7 | Off-target effect of low-dose IL-2 (dramatic reduction in C-peptide production and potential shift of the immune balance toward activation rather than tolerance)—terminated | No preservation or improvement of C-peptide production | NCT02772679 | Dong et al., 2021 [41] |
| Combinational: polyclonal Tregs + anti-CD20 | Autologous | T1D | Isolation/enrichment and ex vivo expansion | I/II, randomized, three-arm, open-label, single-blinded | 36 paediatric (Tregs only n = 13, Tregs + rituximab n = 12, control n = 11) | AEs in 80% of pts (combined group and Tregs only group). AEs, such as infections, needed special surveillance | Tregs+anti-CD20 were superior than Tregs in controlling recent-onset T1DM regarding C-peptide levels and remission | TregVAC2.0; EudraCT: 2014-004319-35 | Zieliński et al., 2022 [42] |
| Combinational: polyclonal Tregs + Liraglutide | UCB | T1D | Isolation/enrichment and ex vivo expansion | I/II, randomized, parallel assignment, open label | Recruiting | NCT03011021 | |||
| Polyclonal Tregs | Autologous | MS | Tregs for iv: isolation/enrichment and ex vivo expansion Tregs for IT: isolation/enrichment |
1b/2a (randomized to iv or IT Treg administration) | 14 (iv n = 11, IT n = 3) | No severe AEs | 5/11 relapses (iv-treated), 0/3 relapses (IT-treated). The statistical results may be underpowered due to the low number of patients | EudraCT 2014–004320-22 | Chwojnick et al., 2021 [43] |
| Polyclonal Tregs | Autologous | Autoimmune hepatitis | Isolation/enrichment and ex vivo expansion | I/II | Unknown status | NCT02704338 | |||
| Polyclonal Tregs | Autologous | Active cutaneous lupus | Isolation/enrichment and ex vivo expansion | I | 1 | Terminated due to participant recruitment feasibility | Stable clinical status | NCT02428309 | Dall’Era et al., 2019 [28] |
| Polyclonal Tregs | Autologous | Active Pemphigus | Isolation/enrichment and ex vivo expansion | I | 5 | Terminated due to recruitment issues and the impact of the coronavirus infectious disease 19 (COVID-19) pandemic | NCT03239470 | ||
| Ag-specific, ovalbumin-specific type 1 Tregs (ova-Tregs) | Autologous | Refractory Crohn’s disease | Isolation/enrichment and ex vivo expansion | I/IIa | 29 enrolled, 20 treated | Well-tolerated, good safety profile for this small patient cohort—significant AEs primarily related to the gastrointestinal system and the underlying CD | 8/20 (40%) total clinical improvement and 6/8 (75%) clinical response in the low-dose group (reducing dose-dependent efficacy) | NCT02327221 | Desreumaux et al., 2012 [44] |
| Polyclonal Tregs | Autologous | Crohn’s disease | Isolation/enrichment and ex vivo expansion | I | Recruiting | NCT03185000 | |||
| Tregs for solid organ transplantation | |||||||||
| Donor-alloantigen-specific Tregs | Autologous | Liver transplantation | Ex vivo expansion | I/IIa | 10 | Good safety profile | 10/10 normal graft function and histology. 7/10 successful cessation of immunosuppressive drugs. 3/10 required conventional low-dose immunotherapy | n/a | Todo et al., 2016 [45] |
| Donor-alloantigen-specific Tregs | Autologous | Liver transplantation | Ex vivo expansion | I | 15 | Terminated as it could not be completed within the grant timeline | NCT02188719 (darTregs) in Liver Transplantation (deLTa) | ||
| Donor-alloantigen-specific Tregs | Autologous | Liver transplantation | Isolation/enrichment and ex vivo expansion | I | Unknown status | NCT01624077 | |||
| Donor-alloantigen-specific Tregs | Autologous | Liver transplantation | Isolation/enrichment and ex vivo expansion | I/II | 15 | Not sufficiently powered to assess safety or efficacy (only n = 5 finally received Tregs) | NCT02474199 (ARTEMIS) | Tang Q et al., 2022 [46] | |
| Polyclonal Tregs | Autologous | Liver transplantation | Ex vivo expansion | I/II | 9 (3 received 106 Tregs/kg, 6 received 4.5 × 106 Tregs/kg) | Good safety profile | 6/6 of the high-dose-treated demonstrated reduced donor-specific T cell responses | NCT02166177 (ThRIL) | Sánchez-Fueyo et al., 2020 [47] |
| Donor-alloantigen-specific Tregs | Autologous | Liver transplantation | Isolation/enrichment and ex vivo expansion | I/II | Active, not recruiting | NCT03577431(ITN073ST) | |||
| HLA-A∗02-CAR Tregs | Autologous | Liver transplantation | Ex vivo expansion and genetic engineering | I/II | Recruiting | NCT05234190 (LIBERATE) | |||
| HLA-A∗02-CAR Tregs | Autologous | Kidney transplantation | Ex vivo expansion and genetic engineering | I/II | Recruiting | NCT04817774 (Steadfast) | Schreeb et al., 2022 [48] | ||
| Polyclonal Tregs | Autologous | Kidney transplantation | Isolation/enrichment and ex vivo expansion | I | 3 | Well-tolerated | 2/3 improvement in follow-up biopsies | NCT02088931 (TASKp pilot trial) | Chandran et al., 2017 [49] |
| Polyclonal Tregs | Autologous | Kidney transplantation | Ex vivo expansion | I | 9 | Good safety profile | All pts survived for at least 2 years | NCT02145325 (TRACT trial) | Mathew et al., 2018 [50] |
| Polyclonal Tregs | Autologous | Kidney transplantation | Isolation/enrichment and ex vivo expansion | n/a | Recruiting | NCT03284242 | |||
| Combinational: polyclonal Tregs+ donor bone marrow cells + Tocilizumab | Autologous | Kidney transplantation | Isolation/enrichment and ex vivo expansion | I/IIa | Active, not recruiting | NCT03867617 (Trex001) | Oberbauer et al., 2021 [51] | ||
| Polyclonal Tregs | Autologous | Kidney transplantation | Isolation/enrichment and ex vivo expansion | I/II | Unknown status | NCT01446484 (RSMU-001) | |||
| Polyclonal vs. donor-specific Tregs | Autologous | Kidney transplantation | Ex vivo expansion | I/II randomized open-label | n/a | Completed. No results posted yet | NCT02711826 (TASK, CTOT-21) | ||
| Polyclonal and donor-antigen reactive Tregs, tolerogenic dendritic cell and regulatory macrophage cells | Autologous | Kidney transplantation | Isolation/enrichment and/or ex vivo expansion | 7 phase I/II trials | 66 cell-treated group vs. 38 reference-group | Good safety profile | Lower infection rates; rates of biopsy-confirmed acute rejection (BCAR) comparable between the standard immunosuppressive group and the cell-based therapy group. Successfully weaned off immunosuppression within the first year post-transplantation to monotherapy in nearly all cell-treated patients |
NCT02371434, NCT02129881 (polyclonal Treg), NCT02244801, NCT02091232 (donor-antigen reactive Treg), NCT02252055 (tolerogenic dendritic cell), NCT02085629 (regulatory mac rophage cell), NCT01656135 (reference group) (ONE study) |
Sawitzki et al., 2020 [52] |
| Combinational: total lymphoid irradiation (TLI), total body irradiation (TBI), anti-thymocyte globulin (ATG), donor HSCs and polyclonal Tregs | Autologous | Kidney transplantation | Ex vivo expansion | I | Recruiting | NCT03943238 | |||
| Polyclonal Tregs | Autologous | Kidney transplantation | Ex vivo expansion | IIb, randomized | Recruiting | ISRCTN11038572 (Two study) | Brook et al., 2022 [53] | ||
| Polyclonal Tregs | Autologous | Heart transplantation | Isolation/enrichment and ex vivo expansion | I/II, randomized | Recruiting | NCT04924491 (THYTECH) | Bernaldo-de-Quirós et al., 2022 [54] | ||
| Polyclonal Tregs | Autologous | Islet transplantation | Isolation/enrichment and ex vivo expansion | I | Active, not recruiting | NCT03444064 | |||
| Tregs for COVID-19 | |||||||||
| Polyclonal Tregs | Allogeneic, UCB | COVID-19 | Isolation/enrichment and ex vivo expansion | I, randomized, double-blinded, placebo-controlled clinical trial | 45 (15 pts placebo, 15 pts 100 × 106 Tregs, 15 pts 300 × 106, 3 doses Tregs) | Good safety profile | No definitive conclusions with respect to efficacy due to to the low number of patients | NCT04468971 | Gladstone et al., 2023 [55] |
| Tregs for GvHD | |||||||||
| Polyclonal HLA-G + induced T-regulatory cells (iG-Tregs) | Allogeneic, HLA-identical sibling donor-derived | GvHD prophylaxis | Ex vivo expansion | I/II | Recruiting | EUDRACT-2021-006367-26 | Lysandrou et al., 2023 [56] | ||
| Polyclonal Tregs | Allogeneic, HLA-matched sibling donor-derived |
GvHD treatment | Isolation/enrichment and ex vivo expansion | I | 2 | Temporal control of grade IV acute GvHD refractory to all other immunosuppressants used/significant alleviation of chronic GvHD accompanied by reduced pharmacologic immunosuppression | NKEBN/458-310/2008 | Trzonkowski et al., 2009 [57] | |
| Polyclonal Tregs | Allogeneic, partially HLA-matched third UCB | GvHD prophylaxis | Isolation/enrichment | I | 23 | No infusional toxicities | No adverse effect in terms of infection, relapse, or early mortality/decreased incidence of grade II–IV acute GVHD vs. identically treated historical controls | NCT00602693 | Brunstein et al., 2011 [58] |
| Polyclonal Tregs | Allogeneic, HSC donor-derived | Severe refractory GvHD treatment | Isolation/enrichment | I/II | Completed. No results posted yet | NCT02749084 | |||
| Polyclonal Tregs | Allogeneic, UCB donor-derived | GvHD prophylaxis | Not specified | II | 3 | 2/3 AEs/Treg-cell infusion toxicity | 2/3 grade II-IV acute GvHD; 1/3 bacterial infection; 2/3 viral infection. Terminated due to the consideration of new technology for the product |
NCT02991898 | |
| Polyclonal Tregs | Allogeneic, HLA-matched sibling donor-derived |
Steroid dependent/refractory chronic GvHD treatment | Not specified | I | Completed. No results posted yet | NCT01911039 | |||
| Combinational: polyclonal Tregs + low-dose IL-2 | Allogeneic, HSC donor-derived | Steroid refractory chronic GvHD treatment | Isolation/enrichment | I | 25 | Good safety profile | 5/25 (20%) PR; 10/25 (40%) stable disease | NCT01937468 | Whangbo et al., 2022 [59] |
| Polyclonal Tregs | Allogeneic, HSC donor-derived | Steroid refractory chronic GvHD treatment | Isolation/enrichment | I/II | Unknown status | NCT02385019 | |||
| Combinational: polyclonal Tregs + IL-2 + rapamycin | Allogeneic, HSC donor-derived | Chronic GvHD treatment | Isolation/enrichment | II | Teriminated due to slow recruitment | NCT01903473 | |||
| Combinational: polyclonal Tregs + Tcon | Allogeneic, HSC donor-derived | GvHD prophylaxis + GvL augmentation in pts with high-risk hematological malignancies undergoing allogeneic myeloablative (MA) HCT with a T cell-depleted graft | Isolation/enrichment | I/II | Interim results: 12 (initial group: 5 pts with frozen Tregs, modified groupI:7 pts with fresh Tregs and single-agent GVHD prophylaxis) | No infusion reaction | Initial group: 2/5 grade II GvHD; modified group: 0/7 GvHD |
NCT01660607 | Meyer et al., 2019 [60] |
| Polyclonal, fucosylated Tregs | Allogeneic UCB-derived | GvHD prophylaxis | Isolation/enrichment and ex vivo expansion | I | 5 | No infusion reaction | 5/5 ≥grade II acute GVHD. No longterm complications for 4/5 alive pts | NCT02423915 | Kellner et al., 2018 [61] |
| Alloantigen-specific Tr1 cells | Allogeneic,HSC donor-derived | GvHD prophylaxis | Ex vivo expansion | I | 3 (preliminary results) | No AEs post infusion | 3/3 alive, disease-free and acute GvHD-free at 1 year post-HCT | NCT03198234 | Chen et al., 2021 [62] |
| Polyclonal Tregs | Allogeneic,HSC donor-derived | GvHD prophylaxis | Isolation/enrichment and ex vivo expansion | I | 14 | No severe infusional toxicities | Pts receiving sirolimus/MMF: 2/2 grade III acute GvHD pts receiving CSA/MMF: 5/12 acute GvHD grade II-III, 6/12 chronic GvHD |
NCT01634217 | MacMillan et al., 2021 [63] |
| Polyclonal Tregs | Allogeneic, HSC donor-derived | Steroid refractory chronic GvHD treatment | Isolation/enrichment | II | Recruiting | NCT05095649 | |||
| CD6-CAR Tregs | Allogeneic,HSC donor-derived | Chronic GvHD treatment | Ex vivo expansion and genetic engineering | I | Not yet recruiting | NCT05993611 | |||
Treg: regulatory T cells. T1D: type 1 diabetes. AEs: adverse events. MS: multiple sclerosis. Ag-specific: antigen-specific. UCB: umbilical cord blood. Tr1 cells: type 1 regulatory T cells. GvHD: graft versus host disease. Pt: patient; PR: partial response. IL: interleukin. IT: intrathecal. MMF: mycophenolate mofetil. CSA: cyclosporine. HSC: hematopoietic stem cells.
2. Specificity of Tolerance
Although there are a number of ongoing clinical trials for autoimmune disorders using polyclonal Tregs (NCT0469123, NCT02772679), the use of polyclonal Tregs, exhibiting a plethora of different TCR specificities, has been hampered by fundamental limitations including the lack of antigen specificity, the heterogeneity of the cell population, and an exhausted Treg phenotype during ex vivo expansion. The suppressive activity of polyclonal Tregs is shaped after ex vivo Ag activation via their TCR, prior to adoptive transfer; however, once stimulated, activated Tregs exert non-specific suppression in an Ag-independent manner post in vivo administration. Such generalized immunosuppression may enhance the risk of opportunistic infections or tumor growth in transplanted or tumor-bearing hosts, respectively [64,65,66].
In addition, as polyclonal Tregs do not consist of a homogeneous population, an infusion of large numbers of cells is required for clinical benefit, yet at the expense of nonspecific immune suppression. At last, the observed loss of the Treg phenotype and attenuation of their immunosuppressive function upon repetitive polyclonal TCR and CD28 co-receptor-mediated stimulation during ex vivo expansion [67] further limits polyclonal Treg potency. To overcome these hurdles, many groups are engaged in pursuing alternatives to polyclonal Tregs.
Generating Antigen-Specific Tregs to Overcome Polyclonal Treg Limitations
In contrast to polyclonal Tregs, an enriched population of antigen-specific Tregs, which would mainly migrate towards the sites of cognate antigen presentation, may provide the advantage of on-target specificity, without global immunosuppression. In addition, due to the enhanced trafficking to, and the targeted immunosuppression in diseased tissues, lower numbers of antigen-specific Tregs are required for clinically relevant outcomes over their unselected, polyclonal counterparts [68]. Thus, immunotherapy with antigen-specific Tregs may be both safer and more potent than Tregs of a polyclonal TCR repertoire in inducing immune tolerance in a disease-specific manner.
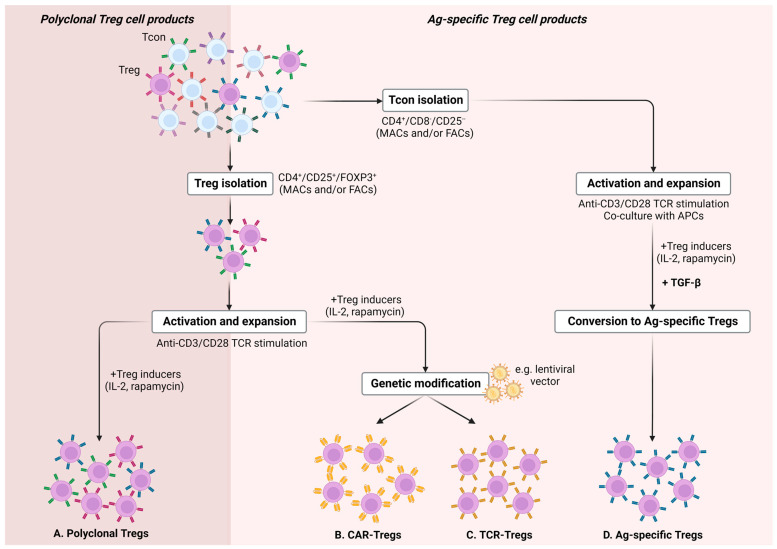
Indeed, numerous studies attest to the clinical superiority of antigen- or alloantigen-specific Tregs, showcasing their increased suppressive efficacy, improved migration patterns to the target tissue, and limited off-target effects as compared to polyclonal Tregs [68,69,70,71,72,73]. Nevertheless, the ex vivo, large-scale expansion of disease-relevant, antigen-specific Tregs is hampered mainly by their low frequency in peripheral blood (merely 1–3% of the circulating CD4+ T cells [74]). Thus, expanding sufficient doses for clinical use requires the implementation of prolonged, labor-intensive, and costly protocols, largely yielding Treg products of suboptimal quality with compromised Treg suppressive ability. Hence, current efforts are focusing on the ex vivo manufacturing of antigen-specific Tregs, either by converting antigen-specific conventional T cells into FOXP3+ cells with suppressive function or redirecting the specificity of polyclonal Treg cells by genetic engineering to express a synthetic antigen receptor that recognizes a disease-relevant antigen (Figure 1) [75].
Figure 1.
Strategies to ex vivo generate Treg cells for adoptive immunotherapy. (A) A polyclonal Treg cell population can be generated after isolation of Tregs, CD3/CD28 activation, and ex vivo expansion in the presence of high-dose IL-2 or other Τreg inducers. (B,D) Antigen-specific Treg cell products can be produced either by genetically modifying isolated polyclonal Treg cells to express a chimeric antigen receptor (CAR-Tregs) (B) or an artificial T cell receptor (TCR-Tregs) targeting a disease-relevant antigen of interest (C) or by converting antigen-specific Tcons to antigen-specific Tregs, e.g., via culture in the presence of TGF-ß (D). Tcon: T conventional cells, Treg: T regulatory cells, Ag: antigen, CAR: chimeric antigen receptor, TCR: T cell receptor, APCs: antigen-presenting cells, IL-2: interleukin-2, MACs: Magnetic Cell Separation, FACs: fluorescence-activated cell sorting, TGF-ß: transforming growth factor beta. Created with BioRender.com (accessed on 7 December 2023).
The first approach was initially reported by Stephens et al., who converted naive CD4+ Foxp3− T cells specific for a naturally expressed autoantigen (H+/K+ ATPase) into self-antigen-specific Foxp3+ Tregs, by stimulation in the presence of TGF-β [76]. Those naïve organ-specific Tregs proved effective at preventing autoimmunity in a murine model of autoimmune gastritis. More recently, Akamatsu et al. showed that epigenetic modification induced by the chemical inhibition of the cyclin-dependent kinase 8 (CDK8) and CDK19, enabled the conversion of antigen-specific effector/memory T cells into Foxp3+ cells [75]. The in vivo inhibition of CDK8/19 generated functionally stable FoxP3+Tregs, capable of suppressing immune responses in mouse models of multiple sclerosis, allergy, and diabetes.
The second approach of converting primary T cells into antigen-specific Tregs towards targeted immune suppression involves a plethora of genetic engineering technologies, including retro/lenti-viral transduction or non-viral transfection methods, such as DNA-based transposons, CRISPR/Cas9 technology, or the direct transfer of in vitro transcribed messenger RNA (mRNA), allowing the introduction and ultimately the expression of either artificial TCRs (TCR-Tregs) or chimeric antigen receptors (CAR-Tregs) into Tregs [77]) (Figure 1).
TCRs isolated from islet-specific human T cells and delivered into polyclonal Tregs provided the proof of concept for the development of islet-specific Treg therapies for the effective treatment of Type 1 diabetes (T1D) [78]. Similarly, Tregs expressing a myelin basic protein-specific (MBP) TCR ameliorated the severity of disease in mouse models of multiple sclerosis [79,80]. Given that in many autoimmune diseases, the causative antigen is often not defined, Wright et al. leveraged the ability of Tregs to also promote bystander suppression once activated—in other words, the ability of activated Tregs to recognize unrelated antigens in the local microenvironment and create a regulatory milieu suppressing conventional T cells (Tcons), independently of antigen specificity [66,81]—and explored whether Tregs, transduced with a TCR specific to a disease-unrelated antigen, could direct their suppressive function to selective sites in vivo and ameliorate the autoimmune disease. Indeed, adoptive therapy with ovalbumin-specific TCR-Tregs in an established arthritis model resulted in the amelioration of arthritis via bystander suppressive pathways, in the absence of cognate recognition of disease-initiating antigen [82], suggesting a clear clinical benefit by tissue-specific TCR Tregs in the treatment of autoimmune diseases even when the disease-causing autoantigens remain unknown. Beyond autoimmunity, the combination of TCR-Tregs specific for allogeneic major histocompatibility complex (MHC) class II molecules with short-term adjunctive immunosuppression, favored transplantation tolerance in mice, implying clinical potential for the administration of Tregs bearing a TCR specific for donor antigens. Likewise, Tregs derived from TCR transgenic mice targeting the minor histocompatibility antigen (miHAg) HY, which is expressed solely in male mice, were highly effective in controlling GvHD in an antigen-dependent manner while sparing the GVL effect in haploidentical and miHAg-mismatched murine bone marrow transplantation models [83,84].
Tregs have been also engineered to express CARs towards suppressing Ag-specific immune responses in various diseases and several proof-of-concept studies demonstrated the utility of CAR Tregs in the setting of autoimmunity and transplantation. CARs recognize a specific antigen in an MHC-independent mode via an extracellular fusion protein of the variable regions of the heavy and light chain of a specific immunoglobulin which is linked via a transmembrane domain to the intracellular signaling domain CD3z, allowing for T cell activation upon antigen encounter.
Elinav et al. first reported that the adoptive transfer of CAR-Tregs targeting the colitis-associated antigen 2,4,6-trinitrophenol (TNP) for the treatment of induced colitis [85] ameliorated experimental colitis over wild type Tregs, thus paving the way for the treatment of inflammatory diseases using CAR-Tregs. Following this study, many groups reported data suggesting the preliminary success of CAR-Tregs in experimental models of autoimmune and chronic inflammatory diseases, including inflammatory bowel disease [86,87,88], multiple sclerosis [89], T1D [90], asthma [91], and hemophilia [92]. Apart from autoantigens, Tregs can be also engineered to suppress alloimmune responses and promote transplantation tolerance via the CAR targeting of donor MHC molecules. CAR-Tregs targeting human leukocyte antigen (HLA)-A2, the most common, frequently mismatched, antigen in transplantation, have been shown to efficiently prevent lethal GvHD [93,94], and induce graft-specific tolerance after pancreatic islet, skin, or heart graft in mouse models [95,96,97,98,99]. These promising findings led to the authorization of the first-in-human trials assessing the safety and tolerability of autologous CAR-Tregs in HLA-A*02-negative recipients receiving renal and liver transplants, from an HLA-A*02-positive donor (NCT04817774 and NCT05234190, respectively [48]). Lastly, CD19 has also been targeted by CAR-Tregs, as autoantibodies secreted from B cells are thought to induce various autoimmune diseases [100]. In a xenograft mouse model, CD19-CAR-Tregs showed the efficient suppression of IgG antibody by B cells and the differentiation of B cells, without inducing GvHD, providing a novel strategy to treat autoantibody-mediated autoimmune diseases [101]. In this context, conventional CD19-CAR T cells, yet not CD19-CAR Treg cells, have been shown to successfully treat refractory SLE in humans [102].
Technological advances in engineering Tcons for cancer therapy have also inspired their integration into Treg immunotherapy. Biswas’s group redirected the specificity of Tregs towards the coagulation factor (F)VIII, either by delivering a high-affinity CAR (second generation CAR) or a TCR fusion construct (TRuC) synthesized by fusing the FVIII single-chain variable fragment (scFv) to the TCRε subunit, enabling T cell activation independently of a peptide–MHC complex, and compared those two Treg cell products side by side [103]. Surprisingly, CAR-Treg engagement induced a robust effector phenotype resulting in the loss of their suppressive function. In contrast, TruC Tregs delivered controlled antigen-specific signaling via the engagement of the entire TCR complex and successfully suppressed the FVIII-specific antibody response, implicating that cellular therapies employing engineered receptor Tregs may require the fine-tuning of activation thresholds to optimize their suppressive performance. CARs engineered with a modular approach are called UniCARs and have also been employed in CAR-Treg therapy. In Uni CARs, the antigen recognition domain is split from the signaling domain of a conventional CAR. This CAR system contains a signaling module that binds to a specific epitope on a switching/targeting module, which is a bispecific fusion molecule harboring one binding domain directed against a tumor-associated Ag and an epitope specifically recognized by the UniCAR. Hence, UniCAR T cells are switchable and remain dormant until they encounter the targeting module and are cross-linked to target cells. The target antigen can be readily adjusted if needed, by targeting module exchange, without the requirement of re-engineering the CAR T cells [104]. These CARs can be therefore applied universally. The rationale behind UniCAR Tregs has been tested to a limited extent thus far. UniCAR Tregs have been generated from patients with autoimmune or inflammatory diseases or healthy volunteers and when infused in mouse models, they were localized at specific sites and mitigated inflammatory or allograft responses in a spatiotemporal manner [95,105]. Although limited, these findings provide evidence for the feasibility of UniCAR adaptation in Tregs. To our knowledge, UniCAR Tregs have not yet been tested in the clinical setting. However, immunotherapy with UniCAR Tregs with an on/off switchable potential may offer a safer approach, enabling a flexible, albeit precise, modular targeting for Treg adoptive immunotherapy of inflammation-related diseases including GvHD, autoimmunity, or transplant rejection. In another context, De Paula Pohl et al. developed a CAR-analogous, chimeric B-cell antibody receptor, called BAR, containing the immunodominant A2 domain of FVIII to generate BAR-Tregs targeting FVIII-specific B cells which are responsible for persistent anti-FVIII neutralizing antibodies (inhibitors) in hemophilia A patients [106]. This in vitro study demonstrated that only A2-FVIII domain-expressing BAR Tregs, but not A2-BAR Tcons, could efficiently target and suppress FVIII-specific memory B cells. Other approaches driving the antigen specificity of Tcons, such as third- and fourth-generation CARs, and TCR-like CARs (CAR T cells with a TCR-like antibody) could also be applied to Treg-based therapies [107,108,109,110].
3. Treg Functional Stability versus Plasticity
The T cell phenotype is inseparably linked to its activity; thus, any phenotypic alteration of T cells will significantly skew their function. Studies over the past few decades have established that within an inflammatory niche, some Tregs present lineage instability, losing the expression of FoxP3, the master regulator of Treg cell differentiation and function [6,111], and thus, the ability to sustain repressor functions or/and an unexpected plasticity enabling rapid cell fate conversion from a suppressive to an active, effector T cell immune phenotype and function (as reviewed in [112,113,114,115,116,117,118]). An inflammatory local milieu within an overall lymphopenic environment has been incriminated for the inhibition of Treg function and their cell fate conversion to effector T cells (ex-Foxp3 cells), which then secrete inflammatory cytokines, increasing the risk of disease aggravation [119]. Although the dynamic regulation of Foxp3 expression is crucial in enabling the immune system to most flexibly control pathogens under various physiological conditions, a reverted, effector phenotype of ex-FoxP3 cells and the transition from a regulatory to an inflammatory program may trigger a pathogenic autoreactive T cell immunity or cytotoxic activity with serious sequelae in the context of in vivo or adoptively transferred Tregs, respectively [117]. Hence, Treg instability and plasticity are features of great importance in the pathogenesis of immunological diseases, while they represent significant barriers to the broader clinical adaptation of Tregs.
Stabilizing Treg Phenotype to Overcome Plasticity
Since the efficacy of Treg cell therapy is closely related to their phenotypic stability in vivo, successful Treg immunotherapy requires an inflexible phenotypic profile and sustained immunosuppressive functions against a destabilizing inflammatory microenvironment. Regulatory T cells induced ex vivo (iTregs) demonstrate functional instability over nTregs as a result of the lack of iTreg-specific epigenetic changes and in particular, DNA hypomethylation at the enhancer regions of FOXP3 and other signature genes that drive phenotypic stability [120]. iTregs display only incomplete DNA demethylation despite high Foxp3 expression. Towards maintaining Treg functional benefits in vivo, fine-tuning transcriptional and epigenetic signals and pathways is a sine qua non requirement to ensure the stability of Tregs and minimize the risk of skewing into pathogenic T cells under a highly inflammatory environment.
The forced expression of Foxp3 in TCR-Tregs has been shown to counteract the consequences of endogenous FoxP3 downmodulation, thus preventing the accumulation of effector T cells in vivo, and also to convert contaminating conventional CD4 cells into Treg-like cells displaying long-term persistence [121].
The mere expression of FoxP3 is not sufficient for iTreg generation; the Treg-specific epigenome needs to be also induced in iTreg cells, in particular, Treg-specific DNA hypomethylation. The expression of Foxp3 is regulated by combinatorial epigenetic modifications and mainly relies on the methylation status of Foxp3 gene loci, and in particular, the promoter and Treg-specific demethylated region (TSDR) in the Foxp3 gene, which become demethylated in functional Tregs [120,122,123]. Indeed, the stabilization of Foxp3 expression has been successfully achieved by the epigenetic modifications of TSDR or within the promoter and enhancer regions of the Foxp3 locus, using DNA methyltransferase (DNMT) or histone deacetylase (HDACs) inhibitors or dCas9 fused to transcriptional activators (VPR), repressors (KRAB), or histone acetyltransferases (HATs, p300) [124,125,126,127,128,129]. Targeting intronic cis-regulatory elements in the Foxp3 loci (CNS1 and CNS2) by metabolic reprogramming using small molecules such as vitamin C, a cofactor for ten-eleven-translocation (TET) enzyme mediating DNA demethylation, also conferred epigenetic modulation in a TET-2-dependent manner, leading to stable Foxp3 expression and improved suppressive Treg activity [130,131,132].
Since a variety of extrinsic and intrinsic cell factors control the epigenetic, transcriptional, translational, and post-translational regulation of Foxp3 expression (reviewed in [133,134]), their direct regulation could be another key strategy to “lock in” Foxp3 expression, and subsequently Treg stability. To further add to the list of potential targets enhancing the Foxp3 stability, super-enhancers, a cluster of highly active, cell type-specific enhancers orchestrating the expression of Foxp3 and other Treg cell lineage-defining genes, as well as chromatin organizers, such as Satb1, which plays an essential role in establishing and activating Treg-specific super-enhancers have also been identified [135,136,137].
Another approach to ensure the stabilization of Foxp3 is to protect it from its negative regulators or proteins leading to polyubiquitination and subsequent proteasomal degradation. To this end, the inhibition of Foxp3 negative regulators including the Deleted in Breast Cancer 1 (DBC1) protein or Janus kinase2 (JAK2), licensed Tregs to retain high Foxp3 expression and maintain their suppressive function in experimental models of autoimmunity and GvHD, respectively [138,139]. Likewise, in proof-of-principle studies, the short hairpin RNA (shRNA)-mediated inhibition of Stub1, a ubiquitin ligase responsible for the polyubiquitination of Foxp3, or the ectopic expression of the deubiquitinase USP7, resulted in stable or increased Foxp3 expression and enhanced Treg suppressive function even within a hostile inflammatory microenvironment [140,141].
Helios, a transcription factor expressed in a large subset of Foxp3+ Tregs, mainly in tTregs, which exhibit a more stable suppressive phenotype than pTregs and iTregs by virtue of a more stabilized epigenetic signature [142], has attracted researchers’ attention as another potential target towards enhancing Treg stability. Indeed, the ectopic expression of Helios along with Foxp3 in Tregs resulted in superior suppressive function as compared to only Foxp3- and only Helios-expressing Tregs in a murine GvHD model [143].
4. Inhibitory Treg Signaling by the Tumor Microenvironment
In addition to tumor and stromal cells, immune cells are an essential component of the tumor microenvironment (TME) where the tumor-immune cell interplay plays a key role in tumorigenesis. In contrast to effector T cells, NK cells, DCs, and M1 macrophages, Tregs comprise tumor-promoting immune cells in which the suppressive activity is mediated by key molecules including IL-10, TGF-β, CTLA4, and IL-35. In contrast, inflammatory signals by cytokines, like TNF-a or IL-6, can decrease Treg activity probably as a homeostatic mechanism against Treg interference with immune responses to pathogens [144,145,146,147]. In addition to these signals, Treg function can be reduced via inhibitory signals directed to their TCR, which can provide negative feedback to Treg-mediated suppression. In particular, CD4+CD25+ Treg cells have a significant defect in the phosphorylation of AKT upon TCR-mediated activation, resulting in the decreased activity of downstream effectors. This defect is tightly associated with Treg suppressive function as the TCR-independent conditional activation of exogenous AKT reversed their suppressive capacities [148]. In recent years, immune checkpoint molecules including CTLA-4, PD-1, LAG-3, TIM-3, and TIGIT have been recognized as critical mediators in the biology of TME that promote cancer progression by exerting inhibitory antitumor mechanisms, whereas immune checkpoint inhibitors (ICIs) induce impressive effector T cell antitumor immune responses [149,150,151,152,153,154,155]. However, as Tregs are known to express several immune checkpoint inhibitor targets, their numbers and function may be altered by ICI immunotherapy, thus shaking the balance between effector T cell activation and suppressive effector PD-1+ Treg cell proliferation at the tumor site. Should the effect on suppressive effector Tregs be dominant, the inhibition of anticancer immunity with uncontrollable tumor growth may occur, resulting in the development of hyperprogressive disease (HPD) [156,157]. This paradoxical acceleration of the disease in a subset of patients treated with ICIs should be promptly acknowledged and urgently managed to counteract a potentially deleterious flare-up. The presence of actively proliferating PD-1+ effector Treg cells at tumor sites has been suggested as a reliable marker for HPD and their depletion in tumor tissues as a means of treating and preventing HPD in PD-1 blockade cancer immunotherapy [156]. In addition, a PD-1 and CTLA-4 combination blockade has been shown to increase effector Teff infiltration, resulting in highly advantageous Teff-to-regulatory T-cell ratios within the tumor [158].
Functional Treg Enhancement against Inflammatory Cytokine Signaling
Expanding knowledge on the negative signal that inflammatory cytokines exert on Tregs has triggered investigation towards establishing a more robust Treg function. One such strategy is rendering Tregs resistant to factors of the inflammatory milieu driving the negative feedback of their function, such as Protein kinase C theta (PKC-θ), an inhibitor of Tregs’ suppressive function being selectively recruited to the central supramolecular activation complex (cSMAC) region of the immunological synapse (IS) between an antigen-stimulated T cell and an antigen-presenting cell. Tregs modify the type of IS that is established between the naïve T cell and the peptide-loaded dendritic cell by inhibiting the recruitment of PKC-θ to the IS [159,160]. The blockade or silencing of PKC-θ shielded Tregs from the negative effects of cytokines associated with an inflammatory milieu and ultimately enhanced their ability to prevent autoimmune colitis, as well as restored the function of defective Tregs derived from rheumatoid arthritis patients [159]. Ex vivo-generated iTregs, in which PKC-θ was neutralized by an antibody using a synthetic, cell-penetrating peptide mimic, presented enhanced immunosuppression and stability and were highly effective in preventing GvHD in a mouse model while maintaining anti-tumor surveillance [161].
An alternative to making Tregs resistant to pro-inflammatory cytokines is to neutralize cytokines in vivo. In fact, in vivo treatment with cytokine- or cytokine receptor-targeted monoclonal antibodies, such as anti-tumor necrosis factor α (anti-TNF-α) or anti-IL-6 receptor, has been shown to neutralize inflammatory cytokines while rescued Treg function in patients with rheumatoid arthritis and kidney transplant recipients, respectively [19,162,163]. It may therefore be possible to enhance Treg cell function and improve outcomes by combining adoptive Treg transfer with monoclonal Abs targeting cytokines or cytokine receptors.
In addition, Tregs could also be engineered to self-secrete neutralizing agents like monoclonal antibodies or to express receptors counteracting extrinsic, repressive signals. Using this concept, albeit in a different context, genetically engineered, anti-tumor T cell products with integrated artificial receptors engaging transforming growth factor β (TGF-β), an inhibitor of effector T and NK cells and tumor antigen-specific cellular immunity, were capable of overcoming the TGF-β-induced tumor immune evasion, being shielded from the inhibitory effects of TGF-β or functionally empowered via the conversion of TGF-β suppressive signal to an activating signal [164,165,166,167]. A similar rationale may be adapted to generate Treg products able to “tame” their corresponding inhibitory molecules even within a hostile inflammatory environment.
Besides providing the means to balance the extrinsic microenvironmental factors, genetic engineering also offers the opportunity to manipulate Tregs to secrete anti-inflammatory cytokines and thus, acquire an intrinsic advantage. The co-expression of IL-10 as additional payload in HLA-A2 CAR-Tregs further enhanced their capacity to suppress alloresponses in vitro [168], although IL-10-overexpressing FVIII-CAR Tregs unexpectedly developed a robust effector phenotype and failed to control inhibitory immune responses in a murine model of hemophilia A [103]. TGF-beta or IL-34, both suppressive Treg-specific and tolerogenic cytokines [169], could serve as additional candidates for co-expression in CAR-Tregs in order to augment their suppressive functions. Nevertheless, in engineered receptor Tregs, the tight regulation of their signal output and the determination of activation thresholds are critical to avoid unwanted toxicity.
5. Tissue Homeostatic Repair
In addition to being potent immune suppressors, Tregs have recently been recognized as also expressing tissue repair/regeneration signatures [170]. Unique populations of Treg cells with a broad phenotypic and functional diversity, have been discovered in a variety of non-lymphoid tissues, including the skeletal and cardiac muscle, skin, gut, lung, liver, and the CNS [171,172,173,174,175,176]. Tissue Tregs take over in the early phase of the inflammatory response, to foster the transition to a tissue milieu that favors regeneration via promoting tissue barrier repair, the proliferation and/or differentiation of non-lymphoid cell precursors, and the tissue remodeling to dampen fibrosis or astrogliosis [172,177]. These pro-regenerative effects of tissue Tregs may result from either cell–cell contacts or paracrine effects with general or tissue-specific soluble factors.
Tissue-resident Tregs, which have been aptly characterized as “regulatory chameleons” [178], share a common FOXP3+CD4+ precursor located in lymphoid organs that undergoes definitive specialization once in the target tissue, following complex transcriptional programs. A conserved transcriptional and epigenetic signature, common in mice and humans, that defines tissue-resident Tregs was identified as BATF+CCR8+ Treg cells in peripheral blood [172]. Notably, CCR8+Tregs from healthy tissues presented multiple similarities with CCR8+ Tregs isolated from tumor sites, thus strongly implicating the contribution of these cells to the human tissue repair program in both health and disease [172]. Another highly suppressive population of Treg cells, CD161+Treg cells having an all-trans retinoic acid (ATRA)-regulated gene signature, has been identified as also mediating wound healing. These CD161+Tregs were enriched in the intestinal lamina propria, particularly in Crohn’s disease, where CD161 expression on Treg cells was induced by ATRA. CD161 was co-stimulatory, and co-ligation with the TCR-induced cytokine secretion accelerated the gut epithelial barrier healing [176].
Promoting Tissue Homeostatic Regeneration by Treg Cells
Modifications promoting homeostatic tissue repair could enhance Treg function to restore tissue damage caused by chronic inflammation, in addition to suppressing local inflammation. Examples of such modifications include engineered Tregs to overexpress Amphiregulin (AREG), a ligand for the epidermal growth receptor, and a wound-repair factor or Cellular Communication Network Factor 3 (CCN3), a growth regulatory protein, implicated in the regeneration of various tissues, including muscle [172], demyelinated neurons, and skin [179,180,181]. Engineered AREG-producing Tregs presented an enhanced ability to polarize monocytes toward an M2-like tolerogenic phenotype, which usually drives the natural wound-healing process, suggesting that engineered Tregs may further promote tissue repair [182]. The tissue-repair capacity of human AREG+ Tregs seems to operate independently from their classical suppressive function, as TCR-induced proliferation/differentiation coincided with a progressive loss of AREG [182].
6. Site-Specific Treg Cell Migration
The question of whether Tregs act primarily in the draining lymph node or the target tissue has drawn conflicting conclusions in different studies; however, they collectively point to the requirement of trafficking and migration to both inflamed tissues and draining lymphoid organs for effective Treg cell function in vivo [183]. Nevertheless, the trafficking properties of Treg cells proved to be highly dynamic and only the sequential migration from blood to the inflamed tissue and then to the draining lymph nodes using a panel of trafficking molecules and chemokine receptors (CCR2/CCR4/CCR5/CCR7 and P- and E-selectins) orienting their migration, ensured efficient Treg differentiation and the full execution of their immunosuppressive function [184], in an islet allograft transplantation. By entering in a coordinated fashion, both the diseased and the priming site, Tregs may limit effector T cell migration at both sites or control their priming via releasing IL-10 and TGF-b. The two sequential stages of migration seem functionally tightly linked, as the suppressive capabilities of Treg cells became limited when one migration phase was prevented [184].
Enhancing Treg Cell Recruitment In Vivo
To be effective, adoptively transferred Tregs must home to and mediate their function at the target tissues [185]. To this end, the manipulation of Treg cell differentiation and dynamic trafficking may be therapeutically beneficial for Treg immunotherapy.
To ensure precise trafficking to specific sites/tissues in vivo, homing-receptor-tailored Tregs orchestrating the tissue-targeted migration of adoptively transferred Tregs have been developed. Tailoring thymic Tregs to express specific homing receptors for targeted migration by ex vivo expansion in Th1-polarizing conditions induced by the addition of interferon-γ and IL-12 or retinoic acid, generated epigenetically stable Tregs under prolonged exposure to inflammatory conditions, that were directed towards Th1-inflammation sites or the gut, respectively [186].
Alternatively, tissue-directed Treg recruitment was achieved by the controlled release of the C-C-Motif Chemokine 22 (CCL22) through microparticle formulations enabling the preferential recruitment of CCR4-expressing Tregs to a local site in vivo [187]. This microparticle-based system prolonged hindlimb allograft survival and promoted donor-specific tolerance [188].
7. Survival and Persistence
The limited to-date efficacy of adoptive immunotherapy with Tregs is, at least in part, attributed to their poor in vivo persistence. Both apoptosis and the loss of proliferation advantage could be incriminated for the observed poor persistence. Indeed, due to low Bcl-2 expression or induced oxidative stress, freshly isolated CD4+CD25+ Tregs were prone to apoptosis as compared to their CD25- counterparts or activated Tregs were driven to apoptosis upon encountering a specific antigen, respectively [189,190]. Moreover, in contrast to the well-described phenomenon of exhaustion with Tcon cells resulting from chronic stimulation and leading to poor in vivo T cell performance, Treg susceptibility to exhaustion remained an outstanding question, although the repetitive cycles of stimulation and prolonged culture required for Treg expansion were expected to affect their phenotypes, functionality, and fitness [191]. Indeed, the repetitive TCR-driven stimulation and prolonged ex vivo expansion were shown to be associated with epigenetic remodeling at loci important for Treg function and identity, including the promoter hypomethylation of genes known to downregulate T cell activation with the concomitant promoter hypermethylation of genes positively regulating TCR signaling and strong promoter hypomethylation in genes implicated in Tcon cell exhaustion [192], thus posing a risk for functional Treg exhaustion similar to what was previously reported for effector T cells [193].
To address the issue of potential Treg susceptibility to exhaustion, as it occurs with Tcons, and overcome the limitations posed by Treg capacity to normally express exhaustion-related inhibitory receptors which are often associated with enhanced Treg suppressive potential [194,195,196], Lamarche et al. used a model of tonic-signaling CAR to ask whether exhaustion has the potential to limit the in vivo efficacy of Tregs. This recent study revealed for the first time that Tregs can develop a functional deficit consistent with the concept of exhaustion, acquiring phenotypic, functional, and epigenetic changes accompanied by the complete loss of their suppressive function in vivo [197]. Therefore, Treg susceptibility to chronic stimulation-driven dysfunction must be considered and mitigated as we move forward with sophisticated adoptive Treg cellular therapies.
Improving Treg Survival and Persistence
Given that IL-2 is indispensable, yet not Treg secretable, for Treg development in the thymus and survival in the periphery, one strategy to exploit the high sensitivity of Tregs to this cytokine and expand Treg numbers in vivo while avoiding the activation of Tcons, is by using low doses of IL-2 after adoptive Treg transfer [198,199]. The low, in contrast to high, IL-2 doses are not associated with toxicity, while they can safely expand endogenous Tregs in various disease contexts [200,201,202,203,204,205,206]. The high sensitivity of Tregs to very low IL-2 doses, based on a reduced IL-2 signaling threshold compared to effector cells, is attributed to the constitutive expression of high-affinity IL-2 receptor α chain (CD25) in Tregs, in stark contrast to intermediate affinity CD25 expressed in antigen-experienced effector cells [207]. Despite the expansion of circulating Tregs and the promising clinical results in early trials of hepatitis C virus-induced vasculitis [202], GvHD [200,208], T1D [12], SLE [209,210], and alopecia areata [211], low IL-2 monotherapy in double-blind, placebo-controlled trials was not sufficient to provide clinically relevant improvements [212,213], nor promote liver allograft tolerance [214].
Therefore, several groups leveraged the ability to selectively increase immunosuppressive Tregs via the high-affinity IL-2Rαβγc using the combination of the adoptive transfer of Tregs with IL-2 administration in vivo (Figure 2). In a nonhuman primate model, adding low-dose IL-2 to rapamycin in a setting of clinically relevant immunosuppression doubled the number of circulating Tregs and logarithmically prolonged the persistence of adoptively transferred ex vivo-expanded Tregs, which resulted in transcriptomic similarity to endogenous resting Tregs with increasing time after transfer [215]. Nevertheless, in patients with T1D or skin allografted mice, the low-dose IL-2 treatment post the adoptive transfer of polyclonal Tregs although it increased the frequency of circulating Tregs, led to only limited therapeutic benefit [41,216], probably due to the inferior in vivo performance of polyclonal Tregs over antigen-specific Tregs, as discussed earlier. In fact, when IL-2 was combined with donor-specific Tregs, but not with polyclonal Tregs, it preferentially enhanced the proliferation of the allospecific Tregs and a synergistic effect in prolonging skin allograft survival was observed [216]. Nevertheless, IL-2 receptor complexes are also expressed on immune cells other than CD4+ T cells, making them responsive to IL-2. Therefore, low IL-2 dosing may come at the expense of the activation of CD8+ and NK cells. In a clinical trial assessing the efficacy of low IL-2 to suppress allospecific immune responses and allow the complete discontinuation of maintenance immunosuppression in liver transplant recipients, rejection episodes were reported for four of five participants who initiated immunosuppression withdrawal [214]. Interestingly, exogenous IL-2, even at low doses, has been shown to induce conflicting effects on Tregs in the allo-HCT setting depending on the immune environment of the host; in a mild inflammatory state, low IL-2 regulated Treg homeostasis and suppressed GvHD, whereas in an intense inflammatory environment, the same IL-2 doses enhanced activated T cells rather than Tregs and exacerbated GvHD in a mouse model [217].
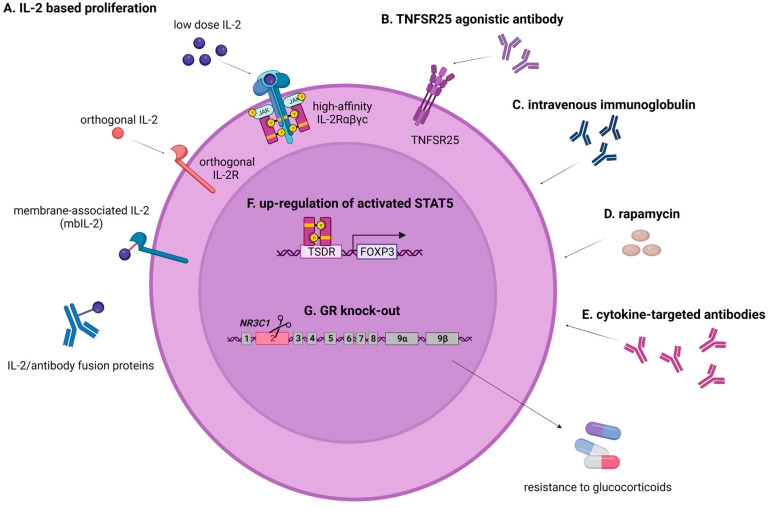
Figure 2.
Strategies to enhance the survival and persistence of adoptively transferred Tregs. The figure depicts different approaches to enhance the in vivo survival and persistence of Tregs after adoptive Treg transfer; these include administration of low-dose IL-2 or its mutants for selective in vivo stimulation of Tregs and not other immune cells (A), administration of other molecules that can also in vivo boost adoptively transferred Tregs such as TNFSR25 agonistic antibody (B), immunoglobulin (C), rapamycin (D), and cytokine-targeted antibodies (E), upregulation of STAT5 for sustained, IL-2-independent Foxp3 expression, and (F) knock-out of the glucocorticoid receptor (GR) to render Tregs resistant to glucocorticoids (G). Created with BioRender.com (accessed on 7 December 2023).
To overcome the pleiotropy of IL-2 leading to the simultaneous stimulation and suppression of immune responses as well as systemic toxicity and to specifically target transferred Tregs without activating other immune cells, Garcia’s group engineered orthogonal IL-2/IL-2 receptor (IL-2R) pairs that interact with one another, but do not interact with the natural IL-2 or IL-2R counterparts, thereby enabling the selective stimulation of target cells in vivo [218]. Following the adoptive transfer of Tregs incorporating an orthogonal IL-2R into a murine mixed hematopoietic chimerism model, orthogonal IL-2 injection selectively promoted ortho IL-2Rβ+ Treg cell proliferation without increasing other T cell subsets and facilitated donor hematopoietic cell engraftment followed by heart transplantation tolerance [219]. Likewise, in a murine major histocompatibility complex-disparate GVHD model, this approach led to enhanced GVHD survival, the in vivo selective expansion of Tregs, and importantly, the maintenance of graft-versus-tumor (GVT) responses, whereas the adoptive transfer of ortho-hIL-2Rβ+ CAR T cells into immunodeficient mice bearing CD19+Nalm6 leukemia xenografts in combination with ortho-hIL-2 administration led to 1000-fold ortho-hIL-2Rβ+CAR T expansion and rescued the antileukemic effect of an otherwise suboptimal CAR T cell dose [220,221].
Another IL-2 mutein therapeutic approach currently being evaluated in clinical trials (NCT3451422) [222], makes use of an IL-2 Fc fusion protein (Efavaleukin alfa), in which an introduced mutation decreases binding to IL-2Rβ and increases dependence to IL-2α (CD25). This preferential binding to the high-affinity IL-2R leads to enhanced cell surface retention and selective Treg signaling over recombinant IL-2. Similarly, human cytokine/antibody fusion proteins introduced into Tregs conferred protection in mouse models of colitis and checkpoint inhibitor-induced diabetes mellitus [223], while CAR-Tregs bearing membrane-associated IL-2 (mbIL-2) showed superior activity compared to control CAR-Tregs in a preclinical humanized mouse model [224]. Though promising, the efficacy of those IL-2 Treg mutants remains to be determined in clinical trials.
Other molecules known to also expand Tregs such as TNFRSF25 agonistic antibody, intravenous immunoglobulin, rapamycin, and cytokine-targeted antibodies [14,15,16,20,21,22] could be used to in vivo boost adoptively transferred Tregs in the recipient (Figure 2). Alternatively, such molecules could be administered to the donor to increase Tregs’ numbers and their potential, prior to ex vivo expansion, as it has been promisingly shown in animal models [225,226,227,228]; however, such an approach raises ethical concerns and clinical translation seems rather unrealistic.
Moreover, targeting the downstream IL-2 signaling, for instance, using signal transducer and activator of transcription 5 (STAT5)-transduced Tregs, may result in the disruption of Treg dependency on IL-2 and sustained Foxp3 expression, thus ensuring Tregs’ long-term persistence [229]. Indeed, modulating the Th2 cytokine production in vivo through STAT5 overexpression in transgenic CD4+ cells resulted in more efficient Treg expansion in vivo and reduced GvHD lethality compared to wild type Tregs in an in vivo relevant model [230]. These data implicate that the upregulation of constitutively active forms of STAT5 in Tregs by either pharmacological methods or genetic engineering could prove useful in preventing or controlling GvHD or autoimmunity.
In the context of transplantation, immunosuppression, although a sine qua non for the prevention or treatment of GvHD and graft rejection, severely compromises the endogenous or any potential adoptively transferred T cell immunity. Hence, by making Tregs resistant to specific immunosuppressive agents, they may acquire a survival advantage and remain functional even under the unfavorable conditions of intense immunosuppression (Figure 2). In the setting of adoptive T cell therapy with virus-specific T cells, our group has developed steroid-resistant, pathogen-specific T cells by the CRISPR/CAS9 genetic disruption of the glucocorticoid receptor, and other groups have also generated specific T cells with engineered resistance to various immunosuppressive agents [231] (reviewed in [232]). We foresee that this approach could be adopted for the generation of immunosuppression-resistant Treg cell products, which after adoptive transfer into transplanted patients, could remain functional and effective, thus broadening the applicability of immunotherapy with Tregs.
8. Treg Safety Considerations
Treg cell immunotherapy products consist of “living drugs” with potential long-term dynamics and as such, safety is of utmost importance in moving this therapy to the bedside. The non-targeted specificity of polyclonal Tregs, phenotypic instability, and potential plasticity may lead to unwanted and even deleterious effects. Genetically-modified Tregs bear additional risks associated with genetic engineering, including genotoxicity, off-tumor/on-target toxicity, and hyperactivation syndromes.
Optimizing Treg Safety
As discussed, polyclonal, non-specific Treg cell products might entail safety risks due to their potential to lead to systemic, off-target immunosuppression and therefore, suboptimal immune responses against opportunistic infections and possibly cancer development. The use of antigen-specific Tregs, described in detail above, could decrease this risk, however, even with enriched antigen-specific Treg products, an unintentional contamination of Treg cell products with effector Tcons or a potential phenotype switching in vivo, can lead to unwanted immune responses with severe consequences. The prevention of contamination with Tcons during Treg manufacturing, ideally by magnetic-activated or fluorescence-activated cell sorting purification as a last production step, could remove the undesired Tcons, at the expense, however, of some loss of an already limited Treg population. The isolation of antigen-specific T cells expressing Treg markers, along with an in-depth assessment of their regulatory activity and ability to produce immunosuppressive factors upon antigen-specific challenge, will enable the release of safer cell products. To better control the Treg in vivo plasticity risk and further improve the safety of adoptive immunotherapy with antigen-specific Tregs, the strategies mentioned above enhancing the stability of Tregs, such as enforced Foxp3 expression [121], can be applied.
Genetically-modified Tregs having enhanced activation and expansion capabilities, may be advantageous over conventional Treg therapy against autoimmunity and allo-responses due to their specificity and potency. Notwithstanding the reported long-term safety of retro- or lentiviral-based CAR T cells in thousands of patients receiving CAR-transduced Tcons [233,234], genetic modification per se creates legitimate concerns regarding the risk of genotoxicity and insertional mutagenesis that could also apply to genetically-modified Tregs [235,236,237,238,239,240]. Inducible suicide genes or chromatin insulators have been proposed as potential means to generate safer viral vectors and consequently safer gene and cell therapy [241,242,243]. Should the employment of (viral or non-viral) delivery methods in nuclease-based genome editing with CRISPR/Cas9 or TALENs be concerned, it is vital to minimize potential undesirable, off-target mutations by performing extended in silico, in vitro, and in vivo off-target analysis which may help limit the introduction of unintended genetic changes that could affect the safety and efficacy of engineered cells and by validating precision targeting [244,245,246,247].
Although the potential of gene-modified Tcon cells to elicit off-tumor/on-target toxicity, CRS, or ICANS is well-recognized and could also apply to CAR-Treg immunotherapy, the risk of these toxicities being observed with gene-engineered CAR Tregs (should they not be contaminated with large numbers of Tcon cells or not be profoundly unstable in vivo) is, at least theoretically, substantially lower; this is due to the Treg capacities to counteract the T-effector cell function in vivo, disfavor macrophage activation, and potentially prevent the cytokine storm [248,249,250].
Towards mitigating CAR Tcon or TCR Tcon cells’ toxicity, various safety features have been incorporated in CAR-Tcons, including suicide genes (RQR8, huEGFRt, HSV-tk, iCasp9) that make the cells highly vulnerable to lysis by monoclonal antibodies or small molecules administered on demand, enabling the deletion of cells in case of severe adverse events [241,242,251].
The use of antigen-specific TCR-Tregs is associated with the risk of the potential mismatched pairing of the transgenic with the endogenous TCRs that may lead to impaired transgenic TCR expression, or in the worst-case scenario, undesirable, and even dangerous off-target effects (as reviewed in [252]). Strategies including the deletion or silencing of endogenous TCR by gene editing or RNA interference, respectively, or the cysteine modification of the transgenic TCR, making the unproductive mispairing with the endogenous TCR unlikely [253,254,255,256,257], have been proposed to prevent TCR mispairing in cancer therapy with TCR-engineered Tcons and could also be considered in TCR-Treg immunotherapy.
The potential immunogenicity of CARs induced by the presence of non-human sequences (scFvs) in the CAR construct, other components of the CAR-T, or the presence of residual viral or other non-human origin proteins during CAR T cell manufacturing represents another safety challenge to consider regarding CAR-Tregs [258]. Humanized alloantigen-specific CAR (A2-CAR)-Tregs to annihilate the risk of undesirable immune responses have been developed and shown to be effective in suppressing HLA-A2+ cell-mediated xenogeneic GvHD and diminish the rejection of human HLA-A2+ skin allografts [259].
9. Conclusions
The feasibility and safety of adoptive immunotherapy with Tregs have been demonstrated in pivotal clinical studies that have suggested Treg cell therapy as a promising therapeutic option for patients suffering from autoimmune diseases or the immunological complications of hematopoietic cell or solid organ transplantation (Table 2). Independent of their suppressive activity, a new role for Tregs has been recently recognized with regard to promoting tissue repair and wound healing, thus opening the potential for also treating non-autoimmune disorders with Tregs.
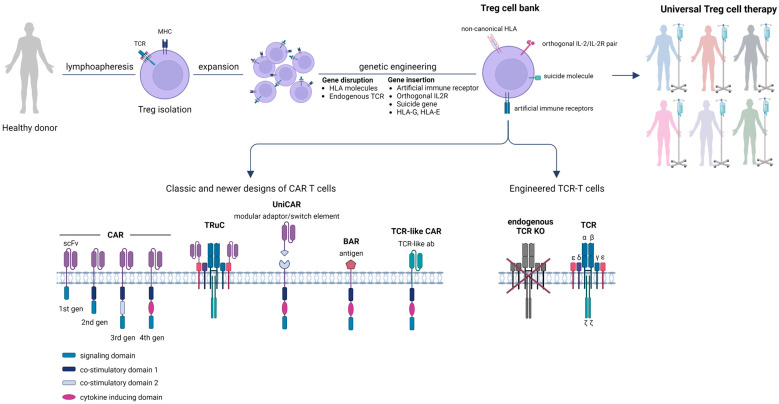
Nevertheless, the efficacy of Treg adoptive immunotherapy to reshape the immune balance toward a specific and long-lasting tolerance still faces a plethora of challenges and the establishment of immune tolerance remains rather elusive. In the recent two decades, the dramatic progress in our understanding of basic Treg cell biology and its association with the development of autoimmunity or allo-responses in transplantation, along with the advent of new genetic engineering tools, has led to the concept and development of “designer” Tregs towards enhancing the potency, long-lasting effect, and safety of this tolerogenic therapy [260]. Unequivocally, genetically-modified Tregs have attracted increasing scientific, as well as commercial attention. An important step further, towards the broader applicability of adoptive Treg cell therapy especially in the autoimmunity context, where autologous and possibly suboptimally performing ex vivo and in vivo cells are being used, will be the development of Treg biobanks with universal, off-the-shelf products (Figure 3). Such universal Tregs will enable the use of a single batch of a Treg cell product to treat multiple patients while minimizing the manufacturing time and cost of those living drugs. In this case, it would be ideal, in order to escape immune recognition by the host, to use either HLA-deficient, gene-edited Tregs or Tregs having the non-classical HLA-E or HLA-G either ectopically expressed or epigenetically reprogrammed, to also bypass the NK cell-mediating killing [56,261].
Figure 3.
Universal or “one-size-fits-all” Treg cell therapeutics. Universal Treg cell therapy will be based on the development of biobanks of healthy donor-derived, off-the-shelf Treg cell products generated by multiplex genetic engineering to overcome the HLA barriers by disrupting the HLA molecules or knocking in non-classical HLAs (HLA-E/-G) and to express an artificial disease-relevant immune receptor—instead of their native TCR—for antigen specificity (classic and newer designer CARs or transgenic TCRs), an orthogonal IL2/IL-2R pair for enhanced in vivo persistence and/or a suicide gene as a safety switch. Treg: T regulatory cells, TCR: T cell receptor, MHC: major histocompatibility complex, HLA: human leukocyte antigen, IL-2: interleukin-2, IL-2R: interleukin-2 receptor, HLA-G: human leukocyte antigen G, HLA-E: human leukocyte antigen E, CAR: chimeric antigen receptor, scFv: single-chain variable fragment, gen: generation, TRuC: TCR fusion construct, UniCAR: universal CAR-T cells, BAR: chimeric B-cell antibody receptor, ab: antibody, KO: knockout. Created with BioRender.com (accessed on 7 December 2023).
The numerous gene engineering tools and the smart strategies for redirecting cell specificity that are currently available or under development, along with new semi- or fully automated systems for manufacturing have generated an exciting, yet challenging, new era in Treg adoptive immunotherapy [262] towards finding the proper balance between immune tolerance and immune surveillance, thus bringing us closer to definitive treatments for difficult-to-cure diseases.
Author Contributions
Conceptualization and design: P.C. and A.P.; initial manuscript draft writing: P.C., C.P. and A.P.; figure design: C.P. and E.Y.; writing—review and editing: P.C., C.P., N.P., I.S., E.Y. and A.P.; editing and further drafts: N.P. and E.Y.; supervision of the work, review and revision of the text: E.Y. and A.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interest relevant to the subject matter of this article.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Edinger M., Hoffmann P., Ermann J., Drago K., Garrison Fathman C., Strober S., Negrin R.S. CD4+CD25+ Regulatory T Cells Preserve Graft-versus-Tumor Activity While Inhibiting Graft-versus-Host Disease after Bone Marrow Transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J.L., Trenado A., Vasey D., Klatzmann D., Salomon B.L. CD4+CD25+ Immunoregulatory T Cells: New Therapeutics for Graft-versus-Host Disease. J. Exp. Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Abbas A.K., Benoist C., Bluestone J.A., Campbell D.J., Ghosh S., Hori S., Jiang S., Kuchroo V.K., Mathis D., Roncarolo M.G., et al. Regulatory T Cells: Recommendations to Simplify the Nomenclature. Nat. Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 5.Wyss L., Stadinski B.D., King C.G., Schallenberg S., Mccarthy N.I., Lee J.Y., Kretschmer K., Terracciano L.M., Anderson G., Surh C.D., et al. Affinity for Self Antigen Selects Treg Cells with Distinct Functional Properties. Nat. Immunol. 2016;17:1093–1101. doi: 10.1038/ni.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori S., Nomura T., Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. J. Immunol. 2017;198:981–985. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 7.Zeng H., Zhang R., Jin B., Chen L. Type 1 Regulatory T Cells: A New Mechanism of Peripheral Immune Tolerance. Cell. Mol. Immunol. 2015;12:566–571. doi: 10.1038/cmi.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner H.L., da Cunha A.P., Quintana F., Wu H. Oral Tolerance. Immunol. Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra S. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021;12:708874. doi: 10.3389/fimmu.2021.708874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinberg-Bleyer Y., Baeyens A., You S., Elhage R., Fourcade G., Gregoire S., Cagnard N., Carpentier W., Tang Q., Bluestone J., et al. IL-2 Reverses Established Type 1 Diabetes in NOD Mice by a Local Effect on Pancreatic Regulatory T Cells. J. Exp. Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosmaczewska A. Low-Dose Interleukin-2 Therapy: A Driver of an Imbalance between Immune Tolerance and Autoimmunity. Int. J. Mol. Sci. 2014;15:18574–18592. doi: 10.3390/ijms151018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartemann A., Bensimon G., Payan C.A., Jacqueminet S., Bourron O., Nicolas N., Fonfrede M., Rosenzwajg M., Bernard C., Klatzmann D. Low-Dose Interleukin 2 in Patients with Type 1 Diabetes: A Phase 1/2 Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 13.Webster K.E., Walters S., Kohler R.E., Mrkvan T., Boyman O., Surh C.D., Grey S.T., Sprent J. In Vivo Expansion of t Reg Cells with Il-2-Mab Complexes: Induction of Resistance to Eae and Long-Term Acceptance of Islet Allografts without Immunosuppression. J. Exp. Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H.J., Baker J., Leveson-Gower D.B., Smith A.T., Sega E.I., Negrin R.S. Rapamycin and IL-2 Reduce Lethal Acute Graft-versus-Host Disease Associated with Increased Expansion of Donor Type CD4+CD25 +Foxp3+ Regulatory T Cells. Blood. 2011;118:2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka K.I., Koreth J., Kim H.T., Bascug G., McDonough S., Kawano Y., Murase K., Cutler C., Ho V.T., Alyea E.P., et al. Low-Dose Interleukin-2 Therapy Restores Regulatory T Cell Homeostasis in Patients with Chronic Graft-versus-Host Disease. Sci. Transl. Med. 2013;5:179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M.-G. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 17.Trinath J., Hegde P., Sharma M., Maddur M.S., Rabin M., Vallat J.M., Magy L., Balaji K.N., Kaveri S.V., Bayry J. Intravenous Immunoglobulin Expands Regulatory T Cells via Induction of Cyclooxygenase-2-Dependent Prostaglandin E2 in Human Dendritic Cells. Blood. 2013;122:1419–1427. doi: 10.1182/blood-2012-11-468264. [DOI] [PubMed] [Google Scholar]
- 18.Bayry J., Sibéril S., Triebel F., Tough D.F., Kaveri S.V. Rescuing CD4+CD25+ Regulatory T-Cell Functions in Rheumatoid Arthritis by Cytokine-Targeted Monoclonal Antibody Therapy. Drug Discov. Today. 2007;12:548–552. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Nadkarni S., Mauri C., Ehrenstein M.R. Anti-TNF-α Therapy Induces a Distinct Regulatory T Cell Population in Patients with Rheumatoid Arthritis via TGF-β. J. Exp. Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marfil-Garza B.A., Pawlick R.L., Szeto J., Kroger C., Tahiliani V., Hefler J., Dadheech N., Seavey M.M., Wolf J., Jasuja R.R., et al. Tumor Necrosis Factor Receptor Superfamily Member 25 (TNFRSF25) Agonists in Islet Transplantation: Endogenous in Vivo Regulatory T Cell Expansion Promotes Prolonged Allograft Survival. Am. J. Transplant. 2022;22:1101–1114. doi: 10.1111/ajt.16940. [DOI] [PubMed] [Google Scholar]
- 21.Wolf D., Schreiber T.H., Tryphonopoulos P., Li S., Tzakis A.G., Ruiz P., Podack E.R. Tregs Expanded in Vivo by TNFRSF25 Agonists Promote Cardiac Allograft Survival. Transplantation. 2012;94:569–574. doi: 10.1097/TP.0b013e318264d3ef. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber T.H., Wolf D., Tsai M.S., Chirinos J., Deyev V.V., Gonzalez L., Malek T.R., Levy R.B., Podack E.R. Therapeutic Treg Expansion in Mice by TNFRSF25 Prevents Allergic Lung Inflammation. J. Clin. Investig. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orozco G., Gupta M., Gedaly R., Marti F. Untangling the Knots of Regulatory T Cell Therapy in Solid Organ Transplantation. Front. Immunol. 2022;13:883855. doi: 10.3389/fimmu.2022.883855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bluestone J.A., Buckner J.H., Fitch M., Gitelman S.E., Gupta S., Hellerstein M.K., Herold K.C., Lares A., Lee M.R., Li K., et al. Type 1 Diabetes Immunotherapy Using Polyclonal Regulatory T Cells. Sci. Transl. Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezaei Kahmini F., Shahgaldi S., Azimi M., Mansourabadi A.H. Emerging Therapeutic Potential of Regulatory T (Treg) Cells for Rheumatoid Arthritis: New Insights and Challenges. Int. Immunopharmacol. 2022;108:108858. doi: 10.1016/j.intimp.2022.108858. [DOI] [PubMed] [Google Scholar]
- 26.Kohm A.P., Carpentier P.A., Anger H.A., Miller S.D. Cutting Edge: CD4+CD25+ Regulatory T Cells Suppress Antigen-Specific Autoreactive Immune Responses and Central Nervous System Inflammation During Active Experimental Autoimmune Encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 27.Gladstone D.E., Kim B.S., Mooney K., Karaba A.H., D’Alessio F.R. Regulatory T Cells for Treating Patients with COVID-19 and Acute Respiratory Distress Syndrome: Two Case Reports. Ann. Intern. Med. 2020;173:852–853. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dall’Era M., Pauli M.L., Remedios K., Taravati K., Sandova P.M., Putnam A.L., Lares A., Haemel A., Tang Q., Hellerstein M., et al. Adoptive Treg Cell Therapy in a Patient With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71:431–440. doi: 10.1002/art.40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hippen K.L., Hefazi M., Larson J.H., Blazar B.R. Emerging Translational Strategies and Challenges for Enhancing Regulatory T Cell Therapy for Graft-versus-Host Disease. Front. Immunol. 2022;13:926550. doi: 10.3389/fimmu.2022.926550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Wang G., Xia H. Molecular Mechanism for Impaired Suppressive Function of Tregs in Autoimmune Diseases: A Summary of Cell-Intrinsic and Cell-Extrinsic Factors. J. Cell. Mol. Med. 2020;24:11056–11063. doi: 10.1111/jcmm.15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehouse G., Gray E., Mastoridis S., Merritt E., Kodela E., Yang J.H.M., Danger R., Mairal M., Christakoudi S., Lozano J.J., et al. IL-2 Therapy Restores Regulatory T-Cell Dysfunction Induced by Calcineurin Inhibitors. Proc. Natl. Acad. Sci. USA. 2017;114:7083–7088. doi: 10.1073/pnas.1620835114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su X., Wang Q., Guo W., Pei X., Niu Q., Liu M., Liu Y., Chen S., Feng S., He Y., et al. Loss of Lkb1 Impairs Treg Function and Stability to Aggravate Graft-versus-Host Disease after Bone Marrow Transplantation. Cell. Mol. Immunol. 2020;17:483–495. doi: 10.1038/s41423-019-0312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Povoleri G.A.M., Scottá C., Nova-Lamperti E.A., John S., Lombardi G., Afzali B. Thymic versus Induced Regulatory T Cells-Who Regulates the Regulators? Front. Immunol. 2013;4:169. doi: 10.3389/fimmu.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P., Lee J.S., Gartlan K.H., Schuster I.S., Comerford I., Varelias A., Ullah M.A., Vuckovic S., Koyama M., Kuns R.D., et al. Eomesodermin Promotes the Development of Type 1 Regulatory T (TR1) Cells. Sci. Immunol. 2017;2:eaah7152. doi: 10.1126/sciimmunol.aah7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Y., Wang N., Chen L., Fang L. Tr1 Cells as a Key Regulator for Maintaining Immune Homeostasis in Transplantation. Front. Immunol. 2021;12:671579. doi: 10.3389/fimmu.2021.671579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chien C.H., Chiang B.L. Regulatory T Cells Induced by B Cells: A Novel Subpopulation of Regulatory T Cells. J Biomed Sci. 2017;24:86. doi: 10.1186/s12929-017-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieyra-Lobato M.R., Vela-Ojeda J., Montiel-Cervantes L., López-Santiago R., Moreno-Lafont M.C. Description of CD8+ Regulatory T Lymphocytes and Their Specific Intervention in Graft-versus-Host and Infectious Diseases, Autoimmunity, and Cancer. J. Immunol. Res. 2018;2018:3758713. doi: 10.1155/2018/3758713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra S., Liao W., Liu Y., Yang M., Ma C., Wu H., Zhao M., Zhang X., Qiu Y., Lu Q., et al. TGF-β and Eomes Control the Homeostasis of CD8+regulatory T Cells. J. Exp. Med. 2021;218:e20200030. doi: 10.1084/jem.20200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marek-Trzonkowska N., Myśliwiec M., Dobyszuk A., Grabowska M., Derkowska I., Juścińska J., Owczuk R., Szadkowska A., Witkowski P., Młynarski W., et al. Therapy of Type 1 Diabetes with CD4+CD25highCD127-Regulatory T Cells Prolongs Survival of Pancreatic Islets—Results of One Year Follow-Up. Clin. Immunol. 2014;153:23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Caladrius Biosciences, Inc. Caladrius Biosciences Reports Top-Line Data for the Phase 2a Sanford Project: T-Rex Trial of CLBS03 for Recent Onset Type 1 Diabetes. Caladrius Biosciences, Inc.; Basking Ridge, NJ, USA: 2019. [Google Scholar]
- 41.Dong S., Hiam-Galvez K.J., Mowery C.T., Herold K.C., Gitelman S.E., Esensten J.H., Liu W., Lares A.P., Leinbach A.S., Lee M., et al. The Effect of Low-Dose IL-2 and Treg Adoptive Cell Therapy in Patients with Type 1 Diabetes. JCI Insight. 2021;6:e147474. doi: 10.1172/jci.insight.147474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zieliński M., Żalińska M., Iwaszkiewicz-Grześ D., Gliwiński M., Hennig M., Jaźwińska-Curyłło A., Kamińska H., Sakowska J., Wołoszyn-Durkiewicz A., Owczuk R., et al. Combined Therapy with CD4+CD25highCD127− T Regulatory Cells and Anti-CD20 Antibody in Recent-Onset Type 1 Diabetes Is Superior to Monotherapy: Randomized Phase I/II Trial. Diabetes Obes. Metab. 2022;24:1534–1543. doi: 10.1111/dom.14723. [DOI] [PubMed] [Google Scholar]
- 43.Chwojnicki K., Iwaszkiewicz-Grześ D., Jankowska A., Zieliński M., Łowiec P., Gliwiński M., Grzywińska M., Kowalczyk K., Konarzewska A., Glasner P., et al. Administration of CD4+CD25highCD127−FoxP3+ Regulatory T Cells for Relapsing-Remitting Multiple Sclerosis: A Phase 1 Study. BioDrugs. 2021;35:47–60. doi: 10.1007/s40259-020-00462-7. [DOI] [PubMed] [Google Scholar]
- 44.Desreumaux P., Foussat A., Allez M., Beaugerie L., Hébuterne X., Bouhnik Y., Nachury M., Brun V., Bastian H., Belmonte N., et al. Safety and Efficacy of Antigen-Specific Regulatory T-Cell Therapy for Patients with Refractory Crohn’s Disease. Gastroenterology. 2012;143:1207–1217.e2. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 45.Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Aoyagi T., Suzuki T., Shimamura T., et al. A Pilot Study of Operational Tolerance with a Regulatory T-cell-based Cell Therapy in Living Donor Liver Transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 46.Tang Q., Leung J., Peng Y., Sanchez-Fueyo A., Lozano J.-J., Lam A., Lee K., Greenland J.R., Hellerstein M., Fitch M., et al. Selective Decrease of Donor-Reactive T Regs after Liver Transplantation Limits Treg Therapy for Promoting Allograft Tolerance in Humans. Sci. Transl. Med. 2022;14:eabo2628. doi: 10.1126/scitranslmed.abo2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez-Fueyo A., Whitehouse G., Grageda N., Cramp M.E., Lim T.Y., Romano M., Thirkell S., Lowe K., Fry L., Heward J., et al. Applicability, Safety, and Biological Activity of Regulatory T Cell Therapy in Liver Transplantation. Am. J. Transplant. 2020;20:1125–1136. doi: 10.1111/ajt.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreeb K., Culme-Seymour E., Ridha E., Dumont C., Atkinson G., Hsu B., Reinke P. Study Design: Human Leukocyte Antigen Class I Molecule A∗02-Chimeric Antigen Receptor Regulatory T Cells in Renal Transplantation. Kidney Int. Rep. 2022;7:1258–1267. doi: 10.1016/j.ekir.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandran S., Tang Q., Sarwal M., Laszik Z.G., Putnam A.L., Lee K., Leung J., Nguyen V., Sigdel T., Tavares E.C., et al. Polyclonal Regulatory T Cell Therapy for Control of Inflammation in Kidney Transplants. Am. J. Transplant. 2017;17:2945–2954. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew J.M., H-Voss J., LeFever A., Konieczna I., Stratton C., He J., Huang X., Gallon L., Skaro A., Ansari M.J., et al. A Phase i Clinical Trial with Ex Vivo Expanded Recipient Regulatory T Cells in Living Donor Kidney Transplants. Sci. Rep. 2018;8:7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberbauer R., Edinger M., Berlakovich G., Kalhs P., Worel N., Heinze G., Wolzt M., Lion T., Wekerle T. A Prospective Controlled Trial to Evaluate Safety and Efficacy of in Vitro Expanded Recipient Regulatory T Cell Therapy and Tocilizumab Together With Donor Bone Marrow Infusion in HLA-Mismatched Living Donor Kidney Transplant Recipients (Trex001) Front. Med. 2021;7:634260. doi: 10.3389/fmed.2020.634260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawitzki B., Harden P.N., Reinke P., Moreau A., Hutchinson J.A., Game D.S., Tang Q., Guinan E.C., Battaglia M., Burlingham W.J., et al. Regulatory Cell Therapy in Kidney Transplantation (The ONE Study): A Harmonised Design and Analysis of Seven Non-Randomised, Single-Arm, Phase 1/2A Trials. Lancet. 2020;395:1627–1639. doi: 10.1016/S0140-6736(20)30167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brook M.O., Hester J., Petchey W., Rombach I., Dutton S., Bottomley M.J., Black J., Abdul-Wahab S., Bushell A., Lombardi G., et al. Transplantation Without Overimmunosuppression (TWO) Study Protocol: A Phase 2b Randomised Controlled Single-Centre Trial of Regulatory T Cell Therapy to Facilitate Immunosuppression Reduction in Living Donor Kidney Transplant Recipients. BMJ Open. 2022;12:893576. doi: 10.1136/bmjopen-2022-061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernaldo-de-Quirós E., Cózar B., López-Esteban R., Clemente M., Gil-Jaurena J.M., Pardo C., Pita A., Pérez-Caballero R., Camino M., Gil N., et al. A Novel GMP Protocol to Produce High-Quality Treg Cells From the Pediatric Thymic Tissue to Be Employed as Cellular Therapy. Front. Immunol. 2022;13:893576. doi: 10.3389/fimmu.2022.893576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gladstone D.E., D’Alessio F., Howard C., Lyu M.-A., Mock J.R., Gibbs K.W., Abrams D., Huang M., Zeng K., Herlihy J.P., et al. Randomized, Double-Blinded, Placebo-Controlled Trial of Allogeneic Cord Blood T-Regulatory Cells for Treatment of COVID-19 ARDS. Blood Adv. 2023;7:3075–3079. doi: 10.1182/bloodadvances.2022009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lysandrou M., Stamou P., Kefala D., Pierides C., Kyriakou M., Savvopoulos N., Christofi P., Papadopoulou A., Yannaki E., Costeas P., et al. Hypomethylation-Induced Regulatory Programs in T Cells Unveiled by Transcriptomic Analyses. Front. Immunol. 2023;14:1235661. doi: 10.3389/fimmu.2023.1235661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trzonkowski P., Bieniaszewska M., Juścińska J., Dobyszuk A., Krzystyniak A., Marek N., Myśliwska J., Hellmann A. First-in-Man Clinical Results of the Treatment of Patients with Graft versus Host Disease with Human Ex Vivo Expanded CD4+CD25+CD127- T Regulatory Cells. Clin. Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Brunstein C.G., Miller J.S., Cao Q., McKenna D.H., Hippen K.L., Curtsinger J., DeFor T., Levine B.L., June C.H., Rubinstein P., et al. Infusion of Ex Vivo Expanded T Regulatory Cells in Adults Transplanted with Umbilical Cord Blood: Safety Profile and Detection Kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whangbo J.S., Nikiforow S., Kim H.T., Wahl J., Reynolds C.G., Rai S.C., Kim S., Burden A., Alho A.C., Lacerda J.F., et al. A Phase 1 Study of Donor Regulatory T-Cell Infusion plus Low-Dose Interleukin-2 for Steroid-Refractory Chronic Graft-vs-Host Disease. Blood Adv. 2022;6:5786–5796. doi: 10.1182/bloodadvances.2021006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer E.H., Laport G., Xie B.J., MacDonald K., Heydari K., Sahaf B., Tang S.W., Baker J., Armstrong R., Tate K., et al. Transplantation of Donor Grafts with Defined Ratio of Conventional and Regulatory T Cells in HLA-Matched Recipients. JCI Insight. 2019;4:e127244. doi: 10.1172/jci.insight.127244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kellner J.N., Delemarre E.M., Yvon E., Nierkens S., Boelens J.J., Mcniece I., Olson A., Nieto Y., Ciurea S., Popat U., et al. Third Party, Umbilical Cord Blood Derived Regulatory T-Cells for Prevention of Graft versus Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation: Feasibility, Safety and Immune Reconstitution. Oncotarget. 2018;9:35611–35622. doi: 10.18632/oncotarget.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen P.P., Cepika A.-M., Agarwal-Hashmi R., Saini G., Uyeda M.J., Louis D.M., Cieniewicz B., Narula M., Amaya Hernandez L.C., Harre N., et al. Alloantigen-Specific Type 1 Regulatory T Cells Suppress through CTLA-4 and PD-1 Pathways and Persist Long-Term in Patients. Sci. Transl. Med. 2021;13:eabf5264c. doi: 10.1126/scitranslmed.abf5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacMillan M.L., Hippen K.L., McKenna D.H., Kadidlo D., Sumstad D., Defor T.E., Brunstein C.G., Holtan S.G., Miller J.S., Warlick E.D., et al. First-in-Human Phase 1 Trial of Induced Regulatory T Cells for Graft-versus-Host Disease Prophylaxis in HLA-Matched Siblings. Blood Adv. 2021;5:1425–1436. doi: 10.1182/bloodadvances.2020003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunstein C.G., Blazar B.R., Miller J.S., Cao Q., Hippen K.L., McKenna D.H., Curtsinger J., McGlave P.B., Wagner J.E. Adoptive Transfer of Umbilical Cord Blood-Derived Regulatory T Cells and Early Viral Reactivation. Biol. Blood Marrow Transplant. 2013;19:1271–1273. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noyes D., Bag A., Oseni S., Semidey-Hurtado J., Cen L., Sarnaik A.A., Sondak V.K., Adeegbe D. Tumor-Associated Tregs Obstruct Antitumor Immunity by Promoting T Cell Dysfunction and Restricting Clonal Diversity in Tumor-Infiltrating CD8+ T Cells. J. Immunother. Cancer. 2022;10:e004605. doi: 10.1136/jitc-2022-004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornton A.M., Shevach E.M. Suppressor Effector Function of CD4+CD25+ Immunoregulatory T Cells Is Antigen Nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmann P., Boeld T.J., Eder R., Huehn J., Floess S., Wieczorek G., Olek S., Dietmaier W., Andreesen R., Edinger M. Loss of FOXP3 Expression in Natural Human CD4+ CD25+ Regulatory T Cells upon Repetitive in Vitro Stimulation. Eur. J. Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 68.Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. In Vitro-Expanded Antigen-Specific Regulatory T Cells Suppress Autoimmune Diabetes. J. Exp. Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tarbell K.V., Yamazaki S., Olson K., Toy P., Steinman R.M. CD25+ CD4+ T Cells, Expanded with Dendritic Cells Presenting a Single Autoantigenic Peptide, Suppress Autoimmune Diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagoo P., Ali N., Garg G., Nestle F.O., Lechler R.I., Lombardi G. Human Regulatory T Cells with Alloantigen Specificity Are More Potent Inhibitors of Alloimmune Skin Graft Damage than Polyclonal Regulatory T Cells. Sci. Transl. Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Golshayan D., Jiang S., Tsang J., Garin M.I., Mottet C., Lechler R.I. In Vitro-Expanded Donor Alloantigen-Specific CD4+CD25 + Regulatory T Cells Promote Experimental Transplantation Tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 72.Kim Y.C., Zhang A.H., Su Y., Rieder S.A., Rossi R.J., Ettinger R.A., Pratt K.P., Shevach E.M., Scott D.W. Engineered Antigen-Specific Human Regulatory T Cells: Immunosuppression of FVIII-Specific T- and B-Cell Responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahic M., Henjum K., Yaqub S., Bjørnbeth B.A., Torgersen K.M., Taskén K., Aandahl E.M. Generation of Highly Suppressive Adaptive CD8+CD25+FOXP3+ Regulatory T Cells by Continuous Antigen Stimulation. Eur. J. Immunol. 2008;38:640–646. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 74.Safinia N., Scotta C., Vaikunthanathan T., Lechler R.I., Lombardi G. Regulatory T Cells: Serious Contenders in the Promise for Immunological Tolerance in Transplantation. Front. Immunol. 2015;6:438. doi: 10.3389/fimmu.2015.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akamatsu M., Mikami N., Ohkura N., Kawakami R., Kitagawa Y., Sugimoto A., Hirota K., Nakamura N., Ujihara S., Kurosaki T., et al. Conversion of Antigen-Specific Effector/Memory T Cells into Foxp3-Expressing Tregcells by Inhibition of CDK8/19. Sci. Immunol. 2019;4:eaaw2707. doi: 10.1126/sciimmunol.aaw2707. [DOI] [PubMed] [Google Scholar]
- 76.DiPaolo R.J., Brinster C., Davidson T.S., Andersson J., Glass D., Shevach E.M. Autoantigen-Specific TGFβ-Induced Foxp3+ Regulatory T Cells Prevent Autoimmunity by Inhibiting Dendritic Cells from Activating Autoreactive T Cells. J. Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 77.Bittner S., Hehlgans T., Feuerer M. Engineered Treg Cells as Putative Therapeutics against Inflammatory Diseases and Beyond. Trends Immunol. 2023;44:468–483. doi: 10.1016/j.it.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Hull C.M., Nickolay L.E., Estorninho M., Richardson M.W., Riley J.L., Peakman M., Maher J., Tree T.I.M. Generation of Human Islet-Specific Regulatory T Cells by TCR Gene Transfer. J. Autoimmun. 2017;79:63–73. doi: 10.1016/j.jaut.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Kim Y.C., Zhang A.H., Yoon J., Culp W.E., Lees J.R., Wucherpfennig K.W., Scott D.W. Engineered MBP-Specific Human Tregs Ameliorate MOG-Induced EAE through IL-2-Triggered Inhibition of Effector T Cells. J. Autoimmun. 2018;92:77–86. doi: 10.1016/j.jaut.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stephens L.A., Malpass K.H., Anderton S.M. Curing CNS Autoimmune Disease with Myelin-Reactive Foxp3+ Treg. Eur. J. Immunol. 2009;39:1108–1117. doi: 10.1002/eji.200839073. [DOI] [PubMed] [Google Scholar]
- 81.Karim M., Feng G., Wood K.J., Bushell A.R. CD25+CD4+ Regulatory T Cells Generated by Exposure to a Model Protein Antigen Prevent Allograft Rejection: Antigen-Specific Reactivation in Vivo Is Critical for Bystander Regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 82.Wright G.P., Notley C.A., Xue S.A., Bendle G.M., Holler A., Schumacher T.N., Ehrenstein M.R., Stauss H.J. Adoptive Therapy with Redirected Primary Regulatory T Cells Results in Antigen-Specific Suppression of Arthritis. Proc. Natl. Acad. Sci. USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Heinrichs J., Haarberg K., Semple K., Veerapathran A., Liu C., Anasetti C., Yu X.-Z. HY-Specific Induced Regulatory T Cells Display High Specificity and Efficacy in the Prevention of Acute Graft-versus-Host Disease. J. Immunol. 2015;195:717–725. doi: 10.4049/jimmunol.1401250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsang J.Y.S., Tanriver Y., Jiang S., Xue S.A., Ratnasothy K., Chen D., Stauss H.J., Bucy R.P., Lombardi G., Lechler R. Conferring Indirect Allospecificity on CD4+CD25+ Tregs by TCR Gene Transfer Favors Transplantation Tolerance in Mice. J. Clin. Investig. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elinav E., Waks T., Eshhar Z. Redirection of Regulatory T Cells With Predetermined Specificity for the Treatment of Experimental Colitis in Mice. Gastroenterology. 2008;134:2014–2024. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 86.Elinav E., Adam N., Waks T., Eshhar Z. Amelioration of Colitis by Genetically Engineered Murine Regulatory T Cells Redirected by Antigen-Specific Chimeric Receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 87.Blat D., Zigmond E., Alteber Z., Waks T., Eshhar Z. Suppression of Murine Colitis and Its Associated Cancer by Carcinoembryonic Antigen-Specific Regulatory T Cells. Mol. Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu D., Wong M.Q., Vent-Schmidt J., Boardman D.A., Steiner T.S., Levings M.K. A Method for Expansion and Retroviral Transduction of Mouse Regulatory T Cells. J Immunol Methods. 2021;488:112931. doi: 10.1016/j.jim.2020.112931. [DOI] [PubMed] [Google Scholar]
- 89.Fransson M., Piras E., Burman J., Nilsson B., Essand M., Lu B.F., Harris R.A., Magnusson P.U., Brittebo E., Loskog A.S.I. CAR/FoxP3-Engineered T Regulatory Cells Target the CNS and Suppress EAE upon Intranasal Delivery. J. Neuroinflamm. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tenspolde M., Zimmermann K., Weber L.C., Hapke M., Lieber M., Dywicki J., Frenzel A., Hust M., Galla M., Buitrago-Molina L.E., et al. Regulatory T Cells Engineered with a Novel Insulin-Specific Chimeric Antigen Receptor as a Candidate Immunotherapy for Type 1 Diabetes. J. Autoimmun. 2019;103:102289. doi: 10.1016/j.jaut.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 91.Skuljec J., Chmielewski M., Happle C., Habener A., Busse M., Abken H., Hansen G. Chimeric Antigen Receptor-Redirected Regulatory T Cells Suppress Experimental Allergic Airway Inflammation, a Model of Asthma. Front. Immunol. 2017;8:1125. doi: 10.3389/fimmu.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon J., Schmidt A., Zhang A.H., Königs C., Kim Y.C., Scott D.W. FVIII-Specific Human Chimeric Antigen Receptor T-Regulatory Cells Suppress T- and B-Cell Responses to FVIII. Blood. 2017;129:238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dawson N.A.J., Rosado-Sánchez I., Novakovsky G.E., Fung V.C.W., Huang Q., McIver E., Sun G., Gillies J., Speck M., Orban P.C., et al. Functional Effects of Chimeric Antigen Receptor Co-Receptor Signaling Domains in Human Regulatory T Cells. Sci. Transl. Med. 2020;12:eaaz3866. doi: 10.1126/scitranslmed.aaz3866. [DOI] [PubMed] [Google Scholar]
- 94.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., Broady R., Levings M.K. Alloantigen-Specific Regulatory T Cells Generated with a Chimeric Antigen Receptor. J. Clin. Investig. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pierini A., Iliopoulou B.P., Peiris H., Pérez-Cruz M., Baker J., Hsu K., Gu X., Zheng P.P., Erkers T., Tang S.W., et al. T Cells Expressing Chimeric Antigen Receptor Promote Immune Tolerance. JCI Insight. 2017;2:e92865. doi: 10.1172/jci.insight.92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller Y.D., Ferreira L.M.R., Ronin E., Ho P., Nguyen V., Faleo G., Zhou Y., Lee K., Leung K.K., Skartsis N., et al. Precision Engineering of an Anti-HLA-A2 Chimeric Antigen Receptor in Regulatory T Cells for Transplant Immune Tolerance. Front. Immunol. 2021;12:686439. doi: 10.3389/fimmu.2021.686439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boardman D.A., Philippeos C., Fruhwirth G.O., Ibrahim M.A.A., Hannen R.F., Cooper D., Marelli-Berg F.M., Watt F.M., Lechler R.I., Maher J., et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am. J. Transplant. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 98.Noyan F., Zimmermann K., Hardtke-Wolenski M., Knoefel A., Schulde E., Geffers R., Hust M., Huehn J., Galla M., Morgan M., et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am. J. Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 99.Wagner J.C., Ronin E., Ho P., Peng Y., Tang Q. Anti-HLA-A2-CAR Tregs Prolong Vascularized Mouse Heterotopic Heart Allograft Survival. Am. J. Transplant. 2022;22:2237–2245. doi: 10.1111/ajt.17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rawlings D.J., Metzler G., Wray-Dutra M., Jackson S.W. Altered B Cell Signalling in Autoimmunity. Nat. Rev. Immunol. 2017;17:421–436. doi: 10.1038/nri.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Imura Y., Ando M., Kondo T., Ito M., Yoshimura A. CD19-Targeted CAR Regulatory T Cells Suppress B Cell Pathology without GvHD. JCI Insight. 2020;5:e136185. doi: 10.1172/jci.insight.136185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mackensen A., Müller F., Mougiakakos D., Böltz S., Wilhelm A., Aigner M., Völkl S., Simon D., Kleyer A., Munoz L., et al. Author Correction: Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022;28:2124–2132. doi: 10.1038/s41591-022-02017-5. [DOI] [PubMed] [Google Scholar]
- 103.Rana J., Perry D.J., Kumar S.R.P., Muñoz-Melero M., Saboungi R., Brusko T.M., Biswas M. CAR- and TRuC-Redirected Regulatory T Cells Differ in Capacity to Control Adaptive Immunity to FVIII. Mol. Ther. 2021;29:2660–2676. doi: 10.1016/j.ymthe.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu D., Zhao J., Song Y. Engineering Switchable and Programmable Universal CARs for CAR T Therapy. J. Hematol. Oncol. 2019;12:69. doi: 10.1186/s13045-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koristka S., Kegler A., Bergmann R., Arndt C., Feldmann A., Albert S., Cartellieri M., Ehninger A., Ehninger G., Middeke J.M., et al. Engrafting Human Regulatory T Cells with a Flexible Modular Chimeric Antigen Receptor Technology. J Autoimmun. 2018;90:116–131. doi: 10.1016/j.jaut.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Pohl A.D.P., Venkatesha S.H., Zhang A.H., Scott D.W. Suppression of FVIII-Specific Memory B Cells by Chimeric BAR Receptor-Engineered Natural Regulatory T Cells. Front. Immunol. 2020;11:693. doi: 10.3389/fimmu.2020.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akatsuka Y. TCR-Like CAR-T Cells Targeting MHC-Bound Minor Histocompatibility Antigens. Front. Immunol. 2020;11:257. doi: 10.3389/fimmu.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdeladhim M., Zhang A.H., Kropp L.E., Lindrose A.R., Venkatesha S.H., Mitre E., Scott D.W. Engineered Ovalbumin-Expressing Regulatory T Cells Protect against Anaphylaxis in Ovalbumin-Sensitized Mice. Clin. Immunol. 2019;207:49–54. doi: 10.1016/j.clim.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G., et al. Therapy of Autoimmune Disease. Science. 2017;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramírez-Chacón A., Betriu-Méndez S., Bartoló-Ibars A., González A., Martí M., Juan M. Ligand-Based CAR-T Cell: Different Strategies to Drive T Cells in Future New Treatments. Front. Immunol. 2022;13:932559. doi: 10.3389/fimmu.2022.932559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. J. Immunol. 2017;198:986–992. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 112.Sakaguchi S., Vignali D.A.A., Rudensky A.Y., Niec R.E., Waldmann H. The Plasticity and Stability of Regulatory T Cells. Nat. Rev. Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 113.Qiu R., Zhou L., Ma Y., Zhou L., Liang T., Shi L., Long J., Yuan D. Regulatory T Cell Plasticity and Stability and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2020;58:52–70. doi: 10.1007/s12016-018-8721-0. [DOI] [PubMed] [Google Scholar]
- 114.Dupage M., Bluestone J.A. Harnessing the Plasticity of CD4+ T Cells to Treat Immune-Mediated Disease. Nat. Rev. Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 115.Zhou X., Bailey-Bucktrout S.L., Jeker L.T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J.A. Instability of the Transcription Factor Foxp3 Leads to the Generation of Pathogenic Memory T Cells in Vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bailey-Bucktrout S.L., Martinez-Llordella M., Zhou X., Anthony B., Rosenthal W., Luche H., Fehling H.J., Bluestone J.A. Self-Antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-Hora M., Kodama T., Tanaka S., Bluestone J.A., Takayanagi H. Pathogenic Conversion of Foxp3 + T Cells into TH17 Cells in Autoimmune Arthritis. Nat. Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 118.Hua J., Inomata T., Chen Y., Foulsham W., Stevenson W., Shiang T., Bluestone J.A., Dana R. Pathological Conversion of Regulatory T Cells Is Associated with Loss of Allotolerance. Sci. Rep. 2018;8:7059. doi: 10.1038/s41598-018-25384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Komatsu N., Mariotti-Ferrandiz M.E., Wang Y., Malissen B., Waldmann H., Hori S. Heterogeneity of Natural Foxp3+ T Cells: A Committed Regulatory T-Cell Lineage and an Uncommitted Minor Population Retaining Plasticity. Proc. Natl. Acad. Sci. USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Floess S., Freyer J., Siewert C., Baron U., Olek S., Polansky J., Schlawe K., Chang H.D., Bopp T., Schmitt E., et al. Epigenetic Control of the Foxp3 Locus in Regulatory T Cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGovern J., Holler A., Thomas S., Stauss H.J. Forced Fox-P3 Expression Can Improve the Safety and Antigen-Specific Function of Engineered Regulatory T Cells. J. Autoimmun. 2022;132:102888. doi: 10.1016/j.jaut.2022.102888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janson P.C.J., Winerdal M.E., Marits P., Thörn M., Ohlsson R., Winqvist O. FOXP3 Promoter Demethylation Reveals the Committed Treg Population in Humans. PLoS ONE. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baron U., Floess S., Wieczorek G., Baumann K., Grützkau A., Dong J., Thiel A., Boeld T.J., Hoffmann P., Edinger M., et al. DNA Demethylation in the Human FOXP3 Locus Discriminates Regulatory T Cells from Activated FOXP3+ Conventional T Cells. Eur. J. Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 124.Okada M., Kanamori M., Someya K., Nakatsukasa H., Yoshimura A. Stabilization of Foxp3 Expression by CRISPR-DCas9-Based Epigenome Editing in Mouse Primary T Cells. Epigenetics Chromatin. 2017;10:24. doi: 10.1186/s13072-017-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forstnerič V., Oven I., Ogorevc J., Lainšček D., Praznik A., Lebar T., Jerala R., Horvat S. CRISPRa-Mediated FOXP3 Gene Upregulation in Mammalian Cells. Cell Biosci. 2019;9:93. doi: 10.1186/s13578-019-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Polansky J.K., Floess S., Freyer J., Hamann A., Huehn J. Epigenetic regulation of Foxp3 expression in regulatory T cells. Epigenetics Autoimmune Dis. 2009;182:21–38. doi: 10.1002/9780470743553.ch2. [DOI] [Google Scholar]
- 127.Polansky J.K., Kretschmer K., Freyer J., Floess S., Garbe A., Baron U., Olek S., Hamann A., von Boehmer H., Huehn J. DNA Methylation Controls Foxp3 Gene Expression. Eur. J. Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 128.Tao R., de Zoeten E.F., Ozkaynak E., Wang L., Li B., Greene M.I., Wells A.D., Hancock W.W. Histone Deacetylase Inhibitors and Transplantation. Curr. Opin. Immunol. 2007;19:589–595. doi: 10.1016/j.coi.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tao R., De Zoeten E.F., Özkaynak E., Chen C., Wang L., Porrett P.M., Li B., Turka L.A., Olson E.N., Greene M.I., et al. Deacetylase Inhibition Promotes the Generation and Function of Regulatory T Cells. Nat. Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 130.Sasidharan Nair V., Song M.H., Oh K.I. Vitamin C Facilitates Demethylation of the Foxp3 Enhancer in a Tet-Dependent Manner. J. Immunol. 2016;196:2119–2131. doi: 10.4049/jimmunol.1502352. [DOI] [PubMed] [Google Scholar]
- 131.Yue X., Trifari S., Äijö T., Tsagaratou A., Pastor W.A., Zepeda-Martínez J.A., Lio C.W.J., Li X., Huang Y., Vijayanand P., et al. Control of Foxp3 Stability through Modulation of TET Activity. J. Exp. Med. 2016;213:377–397. doi: 10.1084/jem.20151438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi H., Chi H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Front. Immunol. 2019;10:2716. doi: 10.3389/fimmu.2019.02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Colamatteo A., Carbone F., Bruzzaniti S., Galgani M., Fusco C., Maniscalco G.T., Di Rella F., de Candia P., De Rosa V. Molecular Mechanisms Controlling Foxp3 Expression in Health and Autoimmunity: From Epigenetic to Post-Translational Regulation. Front. Immunol. 2020;10:3136. doi: 10.3389/fimmu.2019.03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joudi A.M., Reyes Flores C.P., Singer B.D. Epigenetic Control of Regulatory T Cell Stability and Function: Implications for Translation. Front. Immunol. 2022;13:861607. doi: 10.3389/fimmu.2022.861607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kitagawa Y., Ohkura N., Kidani Y., Vandenbon A., Hirota K., Kawakami R., Yasuda K., Motooka D., Nakamura S., Kondo M., et al. Guidance of Regulatory T Cell Development by Satb1-Dependent Super-Enhancer Establishment. Nat. Immunol. 2017;18:173–183. doi: 10.1038/ni.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feng D., Chen Y., Dai R., Bian S., Xue W., Zhu Y., Li Z., Yang Y., Zhang Y., Zhang J., et al. Chromatin Organizer SATB1 Controls the Cell Identity of CD4+ CD8+ Double-Positive Thymocytes by Regulating the Activity of Super-Enhancers. Nat. Commun. 2022;13:5554. doi: 10.1038/s41467-022-33333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohkura N., Sakaguchi S. Transcriptional and Epigenetic Basis of Treg Cell Development and Function: Its Genetic Anomalies or Variations in Autoimmune Diseases. Cell Res. 2020;30:465–474. doi: 10.1038/s41422-020-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao Y., Tang J., Chen W., Li Q., Nie J., Lin F., Wu Q., Chen Z., Gao Z., Fan H., et al. Inflammation Negatively Regulates FOXP3 and Regulatory T-Cell Function via DBC1. Proc. Natl. Acad. Sci. USA. 2015;112:E3246–E3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iamsawat S., Daenthanasanmak A., Voss J.H., Nguyen H., Bastian D., Liu C., Yu X.-Z. Stabilization of Foxp3 by Targeting JAK2 Enhances Efficacy of CD8 Induced Regulatory T Cells in the Prevention of Graft-versus-Host Disease. J. Immunol. 2018;201:2812–2823. doi: 10.4049/jimmunol.1800793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.vanLoosdregt J., Fleskens V., Fu J., Brenkman A.B., Bekker C.P.J., Pals C.E.G.M., Meerding J., Berkers C.R., Barbi J., Gröne A., et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity. 2013;39:259–271. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Z., Barbi J., Bu S., Yang H.Y., Li Z., Gao Y., Jinasena D., Fu J., Lin F., Chen C., et al. The Ubiquitin Ligase Stub1 Negatively Modulates Regulatory T Cell Suppressive Activity by Promoting Degradation of the Transcription Factor Foxp3. Immunity. 2013;39:272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng Y., Josefowicz S., Chaudhry A., Peng X.P., Forbush K., Rudensky A.Y. Role of Conserved Non-Coding DNA Elements in the Foxp3 Gene in Regulatory T-Cell Fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seng A., Krausz K.L., Pei D., Koestler D.C., Fischer R.T., Yankee T.M., Markiewicz M.A. Coexpression of FOXP3 and a Helios Isoform Enhances the Effectiveness of Human Engineered Regulatory T Cells. Blood Adv. 2020;4:1325–1339. doi: 10.1182/bloodadvances.2019000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wan S., Xia C., Morel L. IL-6 Produced by Dendritic Cells from Lupus-Prone Mice Inhibits CD4+CD25+ T Cell Regulatory Functions. J. Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 145.Valencia X., Stephens G., Goldbach-Mansky R., Wilson M., Shevach E.M., Lipsky P.E. TNF Downmodulates the Function of Human CD4+CD25hi T-Regulatory Cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. A Unique Subset of CD4+CD25highFoxp3+ T Cells Secreting Interleukin-10 and Transforming Growth Factor-Β1 Mediates Suppression in the Tumor Microenvironment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 147.Pasare C., Medzhitov R. Toll Pathway-Dependent Blockade of CD4+CD25+ T Cell-Mediated Suppression by Dendritic Cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 148.Crellin N.K., Garcia R.V., Levings M.K. Altered Activation of AKT Is Required for the Suppressive Function of Human CD4 CD25 T Regulatory Cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 149.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 150.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Larkin J., Minor D., Angelo S.D., Neyns B., Smylie M., Miller W.H., Gutzmer R., Linette G., Chmielowski B., Lao C.D., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. 2017;36:383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.-L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang B., Chikuma S., Hori S., Fagarasan S., Honjo T. Nonoverlapping Roles of PD-1 and FoxP3 in Maintaining Immune Tolerance in a Novel Autoimmune Pancreatitis Mouse Model. Proc. Natl. Acad. Sci. USA. 2016;113:8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang C.-T., Workman C.J., Flies D., Pan X., Marson A.L., Zhou G., Hipkiss E.L., Ravi S., Kowalski J., Levitsky H.I., et al. Role of LAG-3 in Regulatory T Cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic Self-Tolerance Maintained by Cd25+Cd4+Regulatory T Cells Constitutively Expressing Cytotoxic T Lymphocyte–Associated Antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kamada T., Togashi Y., Tay C., Ha D., Sasaki A., Nakamura Y., Sato E., Fukuoka S., Tada Y., Tanaka A., et al. PD-1+ Regulatory T Cells Amplified by PD-1 Blockade Promote Hyperprogression of Cancer. Proc. Natl. Acad. Sci. USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim C.G., Kim K.H., Pyo K.H., Xin C.F., Hong M.H., Ahn B.C., Kim Y., Choi S.J., Yoon H.I., Lee J.G., et al. Hyperprogressive Disease during PD-1/PD-L1 Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2019;30:1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 158.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K.L., Brown M., Blazar B.R., Abramson S.B., Lafaille J.J., Dustin M.L. Protein Kinase C-θ Mediates Negative Feedback on Regulatory T Cell Function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Altman A., Villalba M. Protein Kinase C-θ (PKCθ): It’s All about Location, Location, Location. Immunol. Rev. 2003;192:53–63. doi: 10.1034/j.1600-065X.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 161.Ozay E.I., Shanthalingam S., Sherman H.L., Torres J.A., Osborne B.A., Tew G.N., Minter L.M. Cell-Penetrating Anti-Protein Kinase C Theta Antibodies Act Intracellularly to Generate Stable, Highly Suppressive Regulatory T Cells. Mol. Ther. 2020;28:1987–2006. doi: 10.1016/j.ymthe.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chandran S., Leung J., Hu C., Laszik Z.G., Tang Q., Vincenti F.G. Interleukin-6 Blockade with Tocilizumab Increases Tregs and Reduces T Effector Cytokines in Renal Graft Inflammation: A Randomized Controlled Trial. Am. J. Transplant. 2021;21:2543–2554. doi: 10.1111/ajt.16459. [DOI] [PubMed] [Google Scholar]
- 163.Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A., Mauri C. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFα Therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burga R.A., Yvon E., Chorvinsky E., Fernandes R., Cruz C.R., Bollard C.M. Engineering the TGFb Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin. Cancer Res. 2019;25:4400–4412. doi: 10.1158/1078-0432.CCR-18-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Z., Guo L., Song Y., Zhang Y., Lin D., Hu B., Mei Y., Sandikin D., Liu H. Augmented Anti-Tumor Activity of NK-92 Cells Expressing Chimeric Receptors of TGF-ΒR II and NKG2D. Cancer Immunol. Immunother. 2017;66:537–548. doi: 10.1007/s00262-017-1959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Foster A.E., Dotti G., Lu A., Khalil M., Brenner M.K., Heslop H.E., Rooney C.M., Bollard C.M. Antitumor Activity of EBV-Specific T Lymphocytes Transduced with a Dominant Negative TGF-β Receptor. J. Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bollard C.M., Tripic T., Cruz C.R., Dotti G., Gottschalk S., Torrano V., Dakhova O., Carrum G., Ramos C.A., Liu H., et al. Tumor-Specific t-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients with Relapsed Hodgkin Lymphoma. J. Clin. Oncol. 2018;36:1128–1139. doi: 10.1200/JCO.2017.74.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mohseni Y.R., Saleem A., Tung S.L., Dudreuilh C., Lang C., Peng Q., Volpe A., Adigbli G., Cross A., Hester J., et al. Chimeric Antigen Receptor-Modified Human Regulatory T Cells That Constitutively Express IL-10 Maintain Their Phenotype and Are Potently Suppressive. Eur. J. Immunol. 2021;51:2522–2530. doi: 10.1002/eji.202048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bézie S., Picarda E., Ossart J., Tesson L., Usal C., Renaudin K., Anegon I., Guillonneau C. IL-34 Is a Treg-Specific Cytokine and Mediates Transplant Tolerance. J. Clin. Investig. 2015;125:3952–3964. doi: 10.1172/JCI81227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zaiss D.M., Minutti C.M., Knipper J.A. Immune- and Non-Immune-Mediated Roles of Regulatory T-Cells during Wound Healing. Immunology. 2019;157:190–197. doi: 10.1111/imm.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dial C.F., Tune M.K., Doerschuk C.M., Mock J.R. Foxp31 Regulatory t Cell Expression of Keratinocyte Growth Factor Enhances Lung Epithelial Proliferation. Am. J. Respir. Cell Mol. Biol. 2017;57:162–173. doi: 10.1165/rcmb.2017-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y., Sefik E., Tan T.G., Wagers A.J., Benoist C., et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mock J.R., Garibaldi B.T., Aggarwal N.R., Jenkins J., Limjunyawong N., Singer B.D., Chau E., Rabold R., Files D.C., Sidhaye V., et al. Foxp3 + Regulatory T Cells Promote Lung Epithelial Proliferation. Mucosal. Immunol. 2014;7:1440–1451. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Weirather J., Hofmann U.D.W., Beyersdorf N., Ramos G.C., Vogel B., Frey A., Ertl G., Kerkau T., Frantz S. Foxp3+ CD4+ T Cells Improve Healing after Myocardial Infarction by Modulating Monocyte/Macrophage Differentiation. Circ. Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 175.Li J., Yang K.Y., Tam R.C.Y., Chan V.W., Lan H.Y., Hori S., Zhou B., Lui K.O. Regulatory T-Cells Regulate Neonatal Heart Regeneration by Potentiating Cardiomyocyte Proliferation in a Paracrine Manner. Theranostics. 2019;9:4324–4341. doi: 10.7150/thno.32734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Povoleri G.A.M., Nova-Lamperti E., Scottà C., Fanelli G., Chen Y.C., Becker P.D., Boardman D., Costantini B., Romano M., Pavlidis P., et al. Human Retinoic Acid–Regulated CD161 + Regulatory T Cells Support Wound Repair in Intestinal Mucosa. Nat. Immunol. 2018;19:1403–1414. doi: 10.1038/s41590-018-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Arpaia N., Green J.A., Moltedo B., Arvey A., Hemmers S., Yuan S., Treuting P.M., Rudensky A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Muñoz-Rojas A.R., Mathis D. Tissue Regulatory T Cells: Regulatory Chameleons. Nat. Rev. Immunol. 2021;21:597–611. doi: 10.1038/s41577-021-00519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dombrowski Y., O’Hagan T., DIttmer M., Penalva R., Mayoral S.R., Bankhead P., Fleville S., Eleftheriadis G., Zhao C., Naughton M., et al. Regulatory T Cells Promote Myelin Regeneration in the Central Nervous System. Nat. Neurosci. 2017;20:674–680. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shime H., Odanaka M., Tsuiji M., Matoba T., Imai M., Yasumizu Y., Uraki R., Minohara K., Watanabe M., Bonito A.J., et al. Proenkephalin+ Regulatory T Cells Expanded by Ultraviolet B Exposure Maintain Skin Homeostasis with a Healing Function. Proc. Natl. Acad. Sci. USA. 2020;117:20696–20705. doi: 10.1073/pnas.2000372117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Delacher M., Simon M., Sanderink L., Hotz-Wagenblatt A., Wuttke M., Schambeck K., Schmidleithner L., Bittner S., Pant A., Ritter U., et al. Single-Cell Chromatin Accessibility Landscape Identifies Tissue Repair Program in Human Regulatory T Cells. Immunity. 2021;54:702–720.e17. doi: 10.1016/j.immuni.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lam A.J., MacDonald K.N., Pesenacker A.M., Juvet S.C., Morishita K.A., Bressler B., Pan J.G., Sidhu S.S., Rioux J.D., Levings M.K. Innate Control of Tissue-Reparative Human Regulatory T Cells. J. Immunol. 2019;202:2195–2209. doi: 10.4049/jimmunol.1801330. [DOI] [PubMed] [Google Scholar]
- 183.Belkaid Y., Tarbell K.V. Arming Treg Cells at the Inflammatory Site. Immunity. 2009;30:322–323. doi: 10.1016/j.immuni.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Zhang N., Schröppel B., Lal G., Jakubzick C., Mao X., Chen D., Yin N., Jessberger R., Ochando J.C., Ding Y., et al. Regulatory T Cells Sequentially Migrate from Inflamed Tissues to Draining Lymph Nodes to Suppress the Alloimmune Response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lim H.W., Broxmeyer H.E., Kim C.H. Regulation of Trafficking Receptor Expression in Human Forkhead Box P3+ Regulatory T Cells1. J. Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 186.Hoeppli R.E., MacDonald K.N., Leclair P., Fung V.C.W., Mojibian M., Gillies J., Rahavi S.M.R., Campbell A.I.M., Gandhi S.K., Pesenacker A.M., et al. Tailoring the Homing Capacity of Human Tregs for Directed Migration to Sites of Th1-Inflammation or Intestinal Regions. Am. J. Transplant. 2019;19:62–76. doi: 10.1111/ajt.14936. [DOI] [PubMed] [Google Scholar]
- 187.Jhunjhunwala S., Raimondi G., Glowacki A.J., Hall S.J., Maskarinec D., Thorne S.H., Thomson A.W., Little S.R. Bioinspired Controlled Release of CCL22 Recruits Regulatory T Cells in Vivo. Adv. Mater. 2012;24:4735–4738. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fisher J.D., Zhang W., Balmert S.C., Aral A.M., Acharya A.P., Kulahci Y., Li J., Turnquist H.R., Thomson A.W., Solari M.G., et al. In Situ Recruitment of Regulatory T Cells Promotes Donor-Specific Tolerance in Vascularized Composite Allotransplantation. Sci. Adv. 2020;6:eaax8429. doi: 10.1126/sciadv.aax8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang Y.Y., Feng B.S., Zhang H., Yang G., Jin Q.R., Luo X.Q., Ma N., Huang Q.M., Yang L.T., Zhang G.H., et al. Modulating Oxidative Stress Counteracts Specific Antigen-Induced Regulatory T-Cell Apoptosis in Mice. Eur. J. Immunol. 2021;51:1748–1761. doi: 10.1002/eji.202049112. [DOI] [PubMed] [Google Scholar]
- 190.Taams L.S., Smith J., Rustin M.H., Salmon M., Poulter L.W., Akbar A.N. Human Anergic/Suppressive CD4+CD25+ T Cells: A Highly Differentiated and Apoptosis-Prone Population. Eur. J. Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::AID-IMMU1122>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 191.MacDonald K.N., Piret J.M., Levings M.K. Methods to Manufacture Regulatory T Cells for Cell Therapy. Clin. Exp. Immunol. 2019;197:52–63. doi: 10.1111/cei.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ou K., Hamo D., Schulze A., Roemhild A., Kaiser D., Gasparoni G., Salhab A., Zarrinrad G., Amini L., Schlickeiser S., et al. Strong Expansion of Human Regulatory T Cells for Adoptive Cell Therapy Results in Epigenetic Changes Which May Impact Their Survival and Function. Front. Cell Dev. Biol. 2021;9:751590. doi: 10.3389/fcell.2021.751590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wherry E.J., Kurachi M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gautron A.-S., Dominguez-Villar M., de Marcken M., Hafler D.A. Enhanced Suppressor Function of TIM-3+FoxP3+ Regulatory T Cells. Eur. J. Immunol. 2014;44:2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gianchecchi E., Fierabracci A. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol. 2018;9:2374. doi: 10.3389/fimmu.2018.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lamarche C., Ward-Hartstonge K., Mi T., Lin D.T.S., Huang Q., Brown A., Edwards K., Novakovsky G.E., Qi C.N., Kobor M.S., et al. Tonic-Signaling Chimeric Antigen Receptors Drive Human Regulatory T Cell Exhaustion. Proc. Natl. Acad. Sci. USA. 2023;120:e2219086120. doi: 10.1073/pnas.2219086120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thornton A.M., Donovan E.E., Piccirillo C.A., Shevach E.M. Cutting Edge: IL-2 Is Critically Required for the In Vitro Activation of CD4+CD25+ T Cell Suppressor Function. J. Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 199.Bayer A.L., Yu A., Adeegbe D., Malek T.R. Essential Role for Interleukin-2 for CD4+CD25+ T Regulatory Cell Development during the Neonatal Period. J. Exp. Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koreth J., Matsuoka K., Kim H.T., McDonough S.M., Bindra B., Alyea E.P., Armand P., Cutler C., Ho V.T., Treister N.S., et al. Interleukin-2 and Regulatory T Cells in Graft-versus-Host Disease. N. Engl. J. Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.He J., Zhang X., Wei Y., Sun X., Chen Y., Deng J., Jin Y., Gan Y., Hu X., Jia R., et al. Low-Dose Interleukin-2 Treatment Selectively Modulates CD4+ T Cell Subsets in Patients with Systemic Lupus Erythematosus. Nat. Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 202.Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Klatzmann P.C.D. Regulatory T-Cell Responses to Low-Dose Interleukin-2 in HCV-Induced Vasculitis. N. Engl. J. Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 203.Ito S., Bollard C.M., Carlsten M., Melenhorst J.J., Biancotto A., Wang E., Chen J., Kotliarov Y., Cheung F., Xie Z., et al. Ultra-Low Dose Interleukin-2 Promotes Immune-Modulating Function of Regulatory t Cells and Natural Killer Cells in Healthy Volunteers. Mol. Ther. 2014;22:1388–1395. doi: 10.1038/mt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rosenberg S.A., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., Seipp C.A., Einhorn J.H., White D.E. Treatment of 283 Consecutive Patients With Metastatic Melanoma or Renal Cell Cancer Using High-Dose Bolus Interleukin 2. JAMA. 1994;271:907–913. doi: 10.1001/jama.1994.03510360033032. [DOI] [PubMed] [Google Scholar]
- 205.Rosenberg S.A., Lotze M.T., Yang J.C., Aebersold P.M., Linehan W.M., Seipp C.A., White D.E. Experience with the Use of High-Dose Interleukin-2 in the Treatment of 652 Cancer Patients. Ann. Surg. 1989;210:474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lim T.Y., Martinez-Llordella M., Kodela E., Gray E., Heneghan M.A., Sanchez-Fueyo A. Low-Dose Interleukin-2 for Refractory Autoimmune Hepatitis. Hepatology. 2018;68:1649–1652. doi: 10.1002/hep.30059. [DOI] [PubMed] [Google Scholar]
- 207.Ward N.C., Yu A., Moro A., Ban Y., Chen X., Hsiung S., Keegan J., Arbanas J.M., Loubeau M., Thankappan A., et al. IL-2/CD25: A Long-Acting Fusion Protein That Promotes Immune Tolerance by Selectively Targeting the IL-2 Receptor on Regulatory T Cells. J. Immunol. 2018;201:2579–2592. doi: 10.4049/jimmunol.1800907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kennedy-Nasser A.A., Ku S., Castillo-Caro P., Hazrat Y., Wu M.F., Liu H., Melenhorst J., Barrett A.J., Ito S., Foster A., et al. Ultra Low-Dose IL-2 for GVHD Prophylaxis after Allogeneic Hematopoietic Stem Cell Transplantation Mediates Expansion of Regulatory t Cells without Diminishing Antiviral and Antileukemic Activity. Clin. Cancer Res. 2014;20:2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Von Spee-Mayer C., Siegert E., Abdirama D., Rose A., Klaus A., Alexander T., Enghard P., Sawitzki B., Hiepe F., Radbruch A., et al. Low-Dose Interleukin-2 Selectively Corrects Regulatory T Cell Defects in Patients with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2016;75:1407–1415. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 210.Humrich J.Y., Von Spee-Mayer C., Siegert E., Alexander T., Hiepe F., Radbruch A., Burmester G.R., Riemekasten G. Rapid Induction of Clinical Remission by Low-Dose Interleukin-2 in a Patient with Refractory SLE. Ann. Rheum. Dis. 2015;74:791–792. doi: 10.1136/annrheumdis-2014-206506. [DOI] [PubMed] [Google Scholar]
- 211.Speeckaert R., Lambert J., Van Geel N. Clinical Significance of Serum Soluble CD Molecules to Assess Disease Activity in Vitiligo. JAMA Dermatol. 2016;152:1194–1200. doi: 10.1001/jamadermatol.2016.2366. [DOI] [PubMed] [Google Scholar]
- 212.Rosenzwajg M., Salet R., Lorenzon R., Tchitchek N., Roux A., Bernard C., Carel J.C., Storey C., Polak M., Beltrand J., et al. Low-Dose IL-2 in Children with Recently Diagnosed Type 1 Diabetes: A Phase I/II Randomised, Double-Blind, Placebo-Controlled, Dose-Finding Study. Diabetologia. 2020;63:1808–1821. doi: 10.1007/s00125-020-05200-w. [DOI] [PubMed] [Google Scholar]
- 213.Le Duff F., Bouaziz J.D., Fontas E., Ticchioni M., Viguier M., Dereure O., Reygagne P., Montaudié H., Lacour J.P., Monestier S., et al. Low-Dose IL-2 for Treating Moderate to Severe Alopecia Areata: A 52-Week Multicenter Prospective Placebo-Controlled Study Assessing Its Impact on T Regulatory Cell and NK Cell Populations. J. Investig. Dermatol. 2021;141:933–936.e6. doi: 10.1016/j.jid.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 214.Lim T.Y., Perpiñán E., Londoño M.C., Miquel R., Ruiz P., Kurt A.S., Kodela E., Cross A.R., Berlin C., Hester J., et al. Low Dose Interleukin-2 Selectively Expands Circulating Regulatory T Cells but Fails to Promote Liver Allograft Tolerance in Humans. J. Hepatol. 2023;78:153–164. doi: 10.1016/j.jhep.2022.08.035. [DOI] [PubMed] [Google Scholar]
- 215.Furlan S.N., Singh K., Lopez C., Tkachev V., Hunt D.J., Hibbard J., Betz K.M., Blazar B.R., Trapnell C., Kean L.S. IL-2 Enhances Ex Vivo-Expanded Regulatory T-Cell Persistence after Adoptive Transfer. Blood Adv. 2020;4:1594–1605. doi: 10.1182/bloodadvances.2019001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ratnasothy K., Jacob J., Tung S., Boardman D., Lechler R.I., Sanchez-Fueyo A., Martinez-Llordella M., Lombardi G. IL-2 Therapy Preferentially Expands Adoptively Transferred Donor-Specific Tregs Improving Skin Allograft Survival. Am. J. Transplant. 2019;19:2092–2100. doi: 10.1111/ajt.15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meguri Y., Asano T., Yoshioka T., Iwamoto M., Ikegawa S., Sugiura H., Kishi Y., Nakamura M., Sando Y., Kondo T., et al. Responses of Regulatory and Effector T-Cells to Low-Dose Interleukin-2 Differ Depending on the Immune Environment after Allogeneic Stem Cell Transplantation. Front. Immunol. 2022;13:891925. doi: 10.3389/fimmu.2022.891925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sockolosky J.T., Trotta E., Parisi G., Picton L., Su L.L., Le A.C., Chhabra A., Silveria S.L., George B.M., King I.C., et al. Selective Targeting of Engineered T Cells Using Orthogonal IL-2 Cytokine-Receptor Complexes. Science. 2018;359:1037–1042. doi: 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hirai T., Ramos T.L., Lin P.Y., Simonetta F., Su L.L., Picton L.K., Baker J., Lin J.X., Li P., Seo K., et al. Selective Expansion of Regulatory T Cells Using an Orthogonal IL-2/IL-2 Receptor System Facilitates Transplantation Tolerance. J. Clin. Investig. 2021;131:e139991. doi: 10.1172/JCI139991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Q., Hresko M.E., Picton L.K., Su L., Hollander M.J., Nunez-Cruz S., Zhang Z., Assenmacher C.A., Sockolosky J.T., Garcia K.C., et al. A Human Orthogonal IL-2 and IL-2Rβ System Enhances CAR T Cell Expansion and Antitumor Activity in a Murine Model of Leukemia. Sci. Transl. Med. 2021;13:abg6986. doi: 10.1126/scitranslmed.abg6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ramos T.L., Bolivar-Wagers S., Jin S., Thangavelu G., Simonetta F., Lin P.-Y., Hirai T., Saha A., Koehn B., Su L.L., et al. Prevention of Acute GVHD Using an Orthogonal IL-2/IL-2Rβ System to Selectively Expand Regulatory T Cells in Vivo. Blood. 2023;141:1337–1352. doi: 10.1182/blood.2022018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Harris F., Berdugo Y.A., Tree T. IL-2-Based Approaches to Treg Enhancement. Clin. Exp. Immunol. 2023;211:149–163. doi: 10.1093/cei/uxac105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.VanDyke D., Iglesias M., Tomala J., Young A., Smith J., Perry J.A., Gebara E., Cross A.R., Cheung L.S., Dykema A.G., et al. Engineered Human Cytokine/Antibody Fusion Proteins Expand Regulatory T Cells and Confer Autoimmune Disease Protection. Cell Rep. 2022;41:111478. doi: 10.1016/j.celrep.2022.111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kremer J., Henschel P., Simon D., Riet T., Falk C., Hardtke-Wolenski M., Wedemeyer H., Noyan F., Jaeckel E. Membrane-Bound IL-2 Improves the Expansion, Survival, and Phenotype of CAR Tregs and Confers Resistance to Calcineurin Inhibitors. Front. Immunol. 2022;13:1005582. doi: 10.3389/fimmu.2022.1005582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wolf D., Barreras H., Bader C.S., Copsel S., Lightbourn C.O., Pfeiffer B.J., Altman N.H., Podack E.R., Komanduri K.V., Levy R.B. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017;23:757–766. doi: 10.1016/j.bbmt.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mavers M., Simonetta F., Nishikii H., Ribado J.V., Maas-Bauer K., Alvarez M., Hirai T., Turkoz M., Baker J., Negrin R.S. Activation of the DR3-TL1A Axis in Donor Mice Leads to Regulatory T Cell Expansion and Activation with Reduction in Graft-versus-Host Disease. Front. Immunol. 2019;10:1624. doi: 10.3389/fimmu.2019.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Copsel S., Wolf D., Kale B., Barreras H., Lightbourn C.O., Bader C.S., Alperstein W., Altman N.H., Komanduri K.V., Levy R.B. Very Low Numbers of CD4+ FoxP3+ Tregs Expanded in Donors via TL1A-Ig and Low-Dose IL-2 Exhibit a Distinct Activation/Functional Profile and Suppress GVHD in a Preclinical Model. Biol. Blood Marrow Transplant. 2018;24:1788–1794. doi: 10.1016/j.bbmt.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim B.S., Nishikii H., Baker J. Treatment with Agonistic DR3 Antibody Results in Expansion of Donor Tregs and Reduced Graft-versus-Host Disease. Transplantation. 2015;99:2005–2006. doi: 10.1182/blood-2015-04-637587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Passerini L., Allan S.E., Battaglia M., Di Nunzio S., Alstad A.N., Levings M.K., Roncarolo M.G., Bacchetta R. STAT5-Signaling Cytokines Regulate the Expression of FOXP3 in CD4+CD25+ Regulatory T Cells and CD4+ CD25- Effector T Cells. Int. Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 230.Vogtenhuber C., Bucher C., Highfill S.L., Koch L.K., Goren E., Panoskaltsis-Mortari A., Taylor P.A., Farrar M.A., Blazar B.R. Constitutively Active Stat5b in CD4+ T Cells Inhibits Graft-versus-Host Disease Lethality Associated with Increased Regulatory T-Cell Potency and Decreased T Effector Cell Responses. Blood. 2010;116:466–474. doi: 10.1182/blood-2009-11-252825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Koukoulias K., Papayanni P.G., Georgakopoulou A., Alvanou M., Laidou S., Kouimtzidis A., Pantazi C., Gkoliou G., Vyzantiadis T.A., Spyridonidis A., et al. “Cerberus” T Cells: A Glucocorticoid-Resistant, Multi-Pathogen Specific T Cell Product to Fight Infections in Severely Immunocompromised Patients. Front. Immunol. 2021;11:608701. doi: 10.3389/fimmu.2020.608701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Papadopoulou A., Alvanou M., Karavalakis G., Tzannou I., Yannaki E. Pathogen-Specific T Cells: Targeting Old Enemies and New Invaders in Transplantation and Beyond. Hemasphere. 2023;7:E809. doi: 10.1097/HS9.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Marcucci K.T., Jadlowsky J.K., Hwang W.T., Suhoski-Davis M., Gonzalez V.E., Kulikovskaya I., Gupta M., Lacey S.F., Plesa G., Chew A., et al. Retroviral and Lentiviral Safety Analysis of Gene-Modified T Cell Products and Infused HIV and Oncology Patients. Mol. Ther. 2018;26:269–279. doi: 10.1016/j.ymthe.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G., et al. Decade-Long Safety and Function of Retroviral-Modified Chimeric Antigen Receptor T Cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.-L., Fraser C.C., Cavazzana-Calvo M., et al. A Serious Adverse Event after Successful Gene Therapy for X-Linked Severe Combined Immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 237.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal Systemic Inflammatory Response Syndrome in a Ornithine Transcarbamylase Deficient Patient Following Adenoviral Gene Transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 238.Chandler R.J., Sands M.S., Venditti C.P. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum. Gene Ther. 2017;28:314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hanlon K.S., Kleinstiver B.P., Garcia S.P., Zaborowski M.P., Volak A., Spirig S.E., Muller A., Sousa A.A., Tsai S.Q., Bengtsson N.E., et al. High Levels of AAV Vector Integration into CRISPR-Induced DNA Breaks. Nat. Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nguyen G.N., Everett J.K., Kafle S., Roche A.M., Raymond H.E., Leiby J., Wood C., Assenmacher C.A., Merricks E.P., Long C.T., et al. A Long-Term Study of AAV Gene Therapy in Dogs with Hemophilia A Identifies Clonal Expansions of Transduced Liver Cells. Nat. Biotechnol. 2021;39:47–55. doi: 10.1038/s41587-020-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Philip B., Kokalaki E., Mekkaoui L., Thomas S., Straathof K., Flutter B., Marin V., Marafioti T., Chakraverty R., Linch D., et al. A Highly Compact Epitope-Based Marker/Suicide Gene for Easier and Safer T-Cell Therapy. Blood. 2014;124:1277–1287. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 242.Wang X., Chang W.-C., Wong C.W., Colcher D., Sherman M., Ostberg J.R., Forman S.J., Riddell S.R., Jensen M.C. A Transgene-Encoded Cell Surface Polypeptide for Selection, in Vivo Tracking, and Ablation of Engineered Cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Papayanni P.-G., Psatha N., Christofi P., Li X.-G., Melo P., Volpin M., Montini E., Liu M., Kaltsounis G., Yiangou M., et al. Investigating the Barrier Activity of Novel, Human Enhancer-Blocking Chromatin Insulators for Hematopoietic Stem Cell Gene Therapy. Hum. Gene Ther. 2021;32:1186–1199. doi: 10.1089/hum.2021.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yee J.K. Off-Target Effects of Engineered Nucleases. FEBS J. 2016;283:3239–3248. doi: 10.1111/febs.13760. [DOI] [PubMed] [Google Scholar]
- 245.Naeem M., Majeed S., Hoque M.Z., Ahmad I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells. 2020;9:1608. doi: 10.3390/cells9071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., Kim J.S. Analysis of Off-Target Effects of CRISPR/Cas-Derived RNA-Guided Endonucleases and Nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu C., Zhang L., Liu H., Cheng K. Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic Applications. J. Control. Release. 2017;266:17–26. doi: 10.1016/j.jconrel.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gogishvili T., Langenhorst D., Lühder F., Elias F., Elflein K., Dennehy K.M., Gold R., Hünig T. Rapid Regulatory T-Cell Response Prevents Cytokine Storm in CD28 Superagonist Treated Mice. PLoS ONE. 2009;4:e4643. doi: 10.1371/journal.pone.0004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Goswami T.K., Singh M., Dhawan M., Mitra S., Bin Emran T., Rabaan A.A., Mutair A.A., Alawi Z.A., Alhumaid S., Dhama K. Regulatory T Cells (Tregs) and Their Therapeutic Potential against Autoimmune Disorders–Advances and Challenges. Hum. Vaccin Immunother. 2022;18:2035117. doi: 10.1080/21645515.2022.2035117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Galani I.E., Wendel M., Stojanovic A., Jesiak M., Müller M.M., Schellack C., Suri-Payer E., Cerwenka A. Regulatory T Cells Control Macrophage Accumulation and Activation in Lymphoma. Int. J. Cancer. 2010;127:1131–1140. doi: 10.1002/ijc.25132. [DOI] [PubMed] [Google Scholar]
- 251.Serafini M., Manganini M., Borleri G., Bonamino M., Imberti L., Biondi A., Golay J., Rambaldi A., Introna M. Characterization of CD20-Transduced T Lymphocytes as an Alternative Suicide Gene Therapy Approach for the Treatment of Graft-Versus-Host Disease. Hum. Gene Ther. 2004;15:63–76. doi: 10.1089/10430340460732463. [DOI] [PubMed] [Google Scholar]
- 252.Liu Y., Yan X., Zhang F., Zhang X., Tang F., Han Z., Li Y. TCR-T Immunotherapy: The Challenges and Solutions. Front. Oncol. 2022;11:794183. doi: 10.3389/fonc.2021.794183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Provasi E., Genovese P., Lombardo A., Magnani Z., Liu P.Q., Reik A., Chu V., Paschon D.E., Zhang L., Kuball J., et al. Editing T Cell Specificity towards Leukemia by Zinc Finger Nucleases and Lentiviral Gene Transfer. Nat. Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Legut M., Dolton G., Mian A.A., Ottmann O.G., Sewell A.K. CRISPR-Mediated TCR Replacement Generates Superior Anticancer Transgenic t Cells. Blood. 2018;131:311–322. doi: 10.1182/blood-2017-05-787598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mastaglio S., Genovese P., Magnani Z., Ruggiero E., Landoni E., Camisa B., Schiroli G., Provasi E., Lombardo A., Reik A., et al. NY-ESO-1 TCR Single Edited Stem and Central Memory T Cells to Treat Multiple Myeloma without Graft-versus-Host Disease. Blood. 2017;130:606–618. doi: 10.1182/blood-2016-08-732636. [DOI] [PubMed] [Google Scholar]
- 256.Ochi T., Fujiwara H., Okamoto S., An J., Nagai K., Shirakata T., Mineno J., Kuzushima K., Shiku H., Yasukawa M. Novel Adoptive T-Cell Immunotherapy Using a WT1-Specific TCR Vector Encoding Silencers for Endogenous TCRs Shows Marked Antileukemia Reactivity and Safety. Blood. 2011;118:1495–1503. doi: 10.1182/blood-2011-02-337089. [DOI] [PubMed] [Google Scholar]
- 257.Cohen C.J., Li Y.F., El-Gamil M., Robbins P.F., Rosenberg S.A., Morgan R.A. Enhanced Antitumor Activity of T Cells Engineered to Express T-Cell Receptors with a Second Disulfide Bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gorovits B., Koren E. Immunogenicity of Chimeric Antigen Receptor T-Cell Therapeutics. BioDrugs. 2019;33:275–284. doi: 10.1007/s40259-019-00354-5. [DOI] [PubMed] [Google Scholar]
- 259.Dawson N.A.J., Lamarche C., Hoeppli R.E., Bergqvist P., Fung V.C.W., McIver E., Huang Q., Gillies J., Speck M., Orban P.C., et al. Systematic Testing and Specificity Mapping of Alloantigen-Specific Chimeric Antigen Receptors in Regulatory T Cells. JCI Insight. 2019;4:e123672. doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tuomela K., Salim K., Levings M.K. Eras of Designer Tregs: Harnessing Synthetic Biology for Immune Suppression. Immunol. Rev. 2023;320:250–267. doi: 10.1111/imr.13254. [DOI] [PubMed] [Google Scholar]
- 261.Gornalusse G.G., Hirata R.K., Funk S.E., Riolobos L., Lopes V.S., Manske G., Prunkard D., Colunga A.G., Hanafi L.A., Clegg D.O., et al. HLA-E-Expressing Pluripotent Stem Cells Escape Allogeneic Responses and Lysis by NK Cells. Nat. Biotechnol. 2017;35:765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ganeeva I., Zmievskaya E., Valiullina A., Kudriaeva A., Miftakhova R., Rybalov A., Bulatov E. Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies. Bioengineering. 2022;9:808. doi: 10.3390/bioengineering9120808. [DOI] [PMC free article] [PubMed] [Google Scholar]