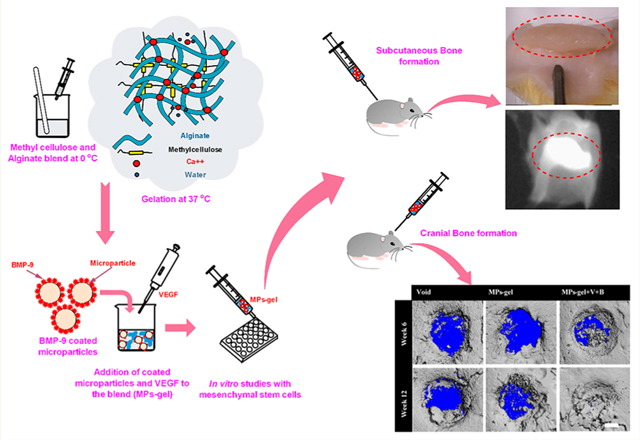
Abstract
Bone morphogenetic protein-9 (BMP-9) has been shown to be the most osteogenic BMP. Most of these experiments, however, involve an adenovirus-transfection strategy. Here, we used the scaffold-based strategy to study the bone forming ability of recombinant BMP-9 combined with vascular endothelial growth factor (VEGF). A robust, injectable, multicomponent-releasing scaffold in the form of a composite gel was developed by combining chitosan microparticles (MPs) with thermosensitive gel (MPs-gel). The MPs acted as the carriers for BMP-9 and the gel was loaded with VEGF. The developed gel consisted of hydrophobic chains of methyl cellulose (MC) and the cross-linked structures of alginate (Alg) and calcium. Gelation was achieved at physiological temperature and thus facilitated the injection and localization of MPs enabling an increased efficacy of incorporated growth factors at the target site. A release profile of incorporated growth factors over a two-week period showed higher release of VEGF at each time point compared to that of BMP-9. Human mesenchymal stem cells (hMSCs) encapsulated within the MPs-gel maintained their viability. BMP-9 enhanced the proliferation of hMSCs along the surface of MPs. Furthermore, BMP-9 potently induced the osteogenic differentiation of encapsulated hMSCs elucidated by the increased alkaline phosphatase (ALP) activity and the higher expression of ALP, collagen 1, and osteocalcin genes. In addition, in vivo experiments demonstrated that MPs-gel with the combination of BMP-9-VEGF could significantly enhance both subcutaneous and cranial bone formation (p < 0.05). Taken together, the results here strongly suggest that BMP-9-VEGF incorporated MPs-gel holds promise as an injectable bone tissue engineering platform.
Keywords: bone morphogenic protein-9, injectable, methylcellulose, microparticles
Graphical Abstract
INTRODUCTION
The inherent limitations of currently available bone repair techniques using autograft and allograft have prompted a demand for new scaffolds that can effectively combine three key components of bone tissue engineering: osteogenic progenitor cells, osteoinductive factors, and osteoconductive materials.1,2 In particular, there is a need for injectable scaffolds that can undergo sol−gel transition at physiological conditions.3 These in situ gelling scaffolds offer good mobility at room temperature making them easy to inject in liquid form. This leads to the additional benefits of scaffold including adaptation to the defect margins, good bioadhesion, and a suitable niche for growth factors and cells.4,5 With these taken into consideration, a myriad of studies have been performed on injectable hydrogels using various polymers.3−5
Methylcellulose (MC) and alginate (Alg) are naturally derived biocompatible polymers readily soluble in water. MC has been widely used as a binder or viscosity-enhancing polymer in the food, pharmaceutical, and ceramic industries. When MC solution is heated to lower critical temperature, MC precipitates out of the solution and forms a gel. The use of MC only as a hydrogel for tissue regeneration applications is mostly limited by its gelation temperature which is higher than the physiological temperature.6 Furthermore, gel strength in cell culture and in vivo conditions is problematic as a slight variation in temperature can affect its stability. One of the ways to modify or enhance the physical properties of MC is by blending it with another polymer.6,7 Alg has been studied in tissue engineering and drug delivery applications for a long time. It forms a cross-linked complex with polyvalent cations. The complexes with calcium are widely used in the form of microgels, microcapsules, scaffolds, and hydrogels in biomedical applications.8 MC blended with Alg had been explored in early days by a few researchers to develop a pH-sensitive controlled release system.9,10 MC is also being used to improve the extrusion based 3-D printing of alginate owing to the high viscosity of MC.11
While injectable hydrogels have shown immense potential in therapeutic delivery over the past few decades, the concept of a complex multicomponent system capable of releasing multiple therapeutics in a temporally controlled manner is still being explored.12−14 One of the ways to develop such a system is to preload the protein into polymeric microparticles (MPs) and integrate them into the hydrogel containing another therapeutics.13 In tissue regeneration studies, the MPs with the injectable size ranging from 10 to 1000 μm are being used as solid scaffolds mostly through sintering or chemical agglomeration.15,16 These processes, however, take away the injectable nature of these MPs. The MPs-incorporated composite hydrogel can be a better scaffold option since injectability can be preserved. Chitosan (CS), a naturally derived biocompatible polymer, has been used to prepare the MPs through a facile fabrication technique in benign conditions.17,18 These MPs have been used in a wide array of drug delivery and tissue regeneration applications. Tissue specific regeneration has been achieved with these MPs through various biological and chemical surface treatments.19,20
BMPs are potent osteoinductive factors. BMP-2/4/6/7/9 have been known as osteogenic BMPs, as they have the potential to induce the osteoblast differentiation and bone formation.21−23 Several comprehensive studies have shown BMP-9 to be the most osteogenic and shown that the SMAD phosphorylation induced by BMP-9, a major pathway in osteogenic differentiation, was not prevented by Noggin which is a well-known antagonist of the BMP pathway.24,25 Most of the studies showing the osteogenic application of BMP-9 in bone regeneration, however, involves the BMP-9 expressing adenoviral transfection of osteoprogenitor or stem cells.26−28 While the results with gene therapy are efficient, the viral nature and lack of control make this technique problematic in bone regeneration.22,29 Recombinant proteins, on the other hand, provide safer, reliable, and established alternatives to study protein induced bone formation. So far, we just found one study where the ability of recombinant BMP-9 (BMP-9 hereafter), delivered through a scaffold material, to induce ectopic bone formation at the quadriceps has been investigated. The study highlighted the importance of the delivery system and the need for a muscle extracellular matrix environment for BMP-9 to induce ectopic bone formation.22,30
In the current study, we report the first results of bone formation induced by BMP-9 at low doses when codelivered with vascular endothelial growth factor (VEGF) using CS MPs incorporated thermoresponsive gel. The dose of BMP-9 used in the present study is much lower than the previous study reported for BMP-9 to induce the in vivo bone formation.30 VEGF is considered one of the key regulators of angiogenesis during bone formation and has been shown to disrupt the normal fracture healing when inhibited.13 The multicomponent releasing MPs-gel system mimics a temporal release profile of BMP-9 and VEGF during the normal bone healing process where VEGF expression is higher compared to that of BMPs.13,31 The in situ gelling combination of MC and Alg was able to localize the BMP-9 coated MPs at the subcutaneous injection site of rats and thus is expected to improve the therapeutic efficacy of BMP-9 at target site.
EXPERIMENTAL PROCEDURES
Materials.
Methylcellulose with a viscosity of 15 cP (2% w/v in water at 20 °C), sodium alginate, chitosan (75−85% deacetylation), sodium tripolyphosphate (85%), β-glycerol phosphate (≥98.0%), L-ascorbic acid (cell culture tested), and dexamethasone were all purchased from Sigma-Aldrich (St. Louis, MO). Calcium chloride was purchased from Fisher Scientific (Waltham, MA). Recombinant BMP-9 and VEGF along with their corresponding ELISA kits were purchased from R&D Systems (Minneapolis, MN). Human bone marrow mesenchymal stem cells at passage 4, xenofree media (StemPro), Dulbecco’s minimum essential media (DMEM, Gibco), alpha minimum essential media (α-MEM), fetal bovine serum (FBS, Gibco), penicillin−streptomycin (Gibco), gentamicin (Gibco), and live and dead cell assay kit (Invitrogen) were all purchased from ThermoFisher Scientific (Waltham, MA). Alkaline phosphatase (ALP) assay kit was purchased from BioVision Incorporated (San Francisco, CA).
Synthesis of MPs.
Chitosan (CS) MPs were fabricated using a simple coacervation technique with sodium tripolyphosphate (TPP) as a cross-linker as explained in our previous studies.32,33 Briefly, 2% (w/v) CS solution was added dropwise to 2% (w/v) TPP solution using a syringe and a 30G needle under stirring condition for 1 h 30 min at 300 rpm. Thus, obtained CS beads were then separated from the TPP solution, washed with deionized (DI) water, and dried inside the fume hood to get the MPs. The dried MPs were further rinsed with DI water under stirring condition for 1 h and dried again inside the fume hood. This process allowed us to get the slightly reduced size MPs that made them suitable for injection application using 13 or 16 G needles.
Synthesis of Gel.
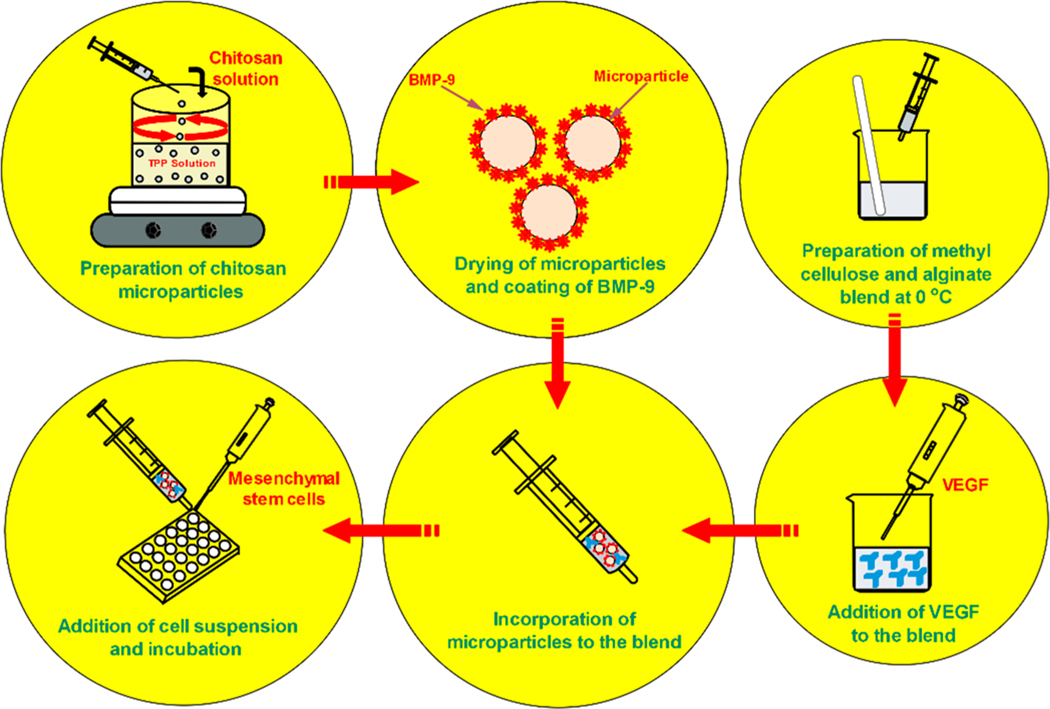
All of the polymer solutions used in the synthesis of the gel were prepared under the sterile condition. 10% (w/v) methyl cellulose (MC) solution was prepared by dispersing the MC on a beaker containing hot DI water under stirring condition (1000 rpm, 85 °C) for 15 min. The temperature was then reduced to 55 °C and 0.3% (w/v) calcium chloride (CaCl2) was added to the dispersion and the stirring was continued for 15 min. The dispersion was then cooled down by transferring the beaker to an ice bath and letting the MC solubilize. As the temperature was lowered, a clear MC solution was obtained. The solution was allowed to equilibrate by storing it overnight at 4 °C and was stored at same conditions until further use. 5% (w/v) alginate (Alg) solution was prepared by slowly adding the Alg powder to DI water at room temperature under stirring condition (500 rpm, 2 h). The clear solution obtained was then stored at 4 °C until further use. To prepare the gel, a polymer blend of MC and Alg was prepared by mixing them at the volume ratio of 1:1. The concentration of CaCl2 (0.3% w/v) in MC was optimized so that the liquid-like behavior of the blend at lower temperature would not be affected by the cross-linking between Alg and CaCl2. When subjected to 37 °C, the blend transformed to a gel within 15 min of incubation. 20 mg of MPs was introduced to the polymer blend before gelation and dispersed uniformly to develop the MPs-gel scaffolds (Scheme 1).
Scheme 1.
Schematic Representation of Experimental Design for the Preparation and in Vitro Studies of MPs-Gel System
Measurement of Viscoelastic Property.
A parallel plate rheometer (RDA III, Rheometric Scientific) was used to measure the rheological properties of the gel only and the MPs-gel scaffolds. Dynamic viscoelastic parameters such as dynamic shear storage modulus (G′) and loss modulus (G′′) were measured as a function of angular frequency at 37 °C. The gel was formed on a glass plate and transferred within the space between two parallel metal plates. The top plate was lowered to a gap distance of 0.6 mm and a frequency sweep test from 0.1 to 100 rad/s at 20% strain amplitude was performed.
Incorporation and Release of Growth Factors.
BMP-9 and VEGF were incorporated into the MPs-gel scaffolds to promote the bone regeneration. The release of these growth factors was tailored by incorporating BMP-9 to the MPs and VEGF to the gel enabling a temporal release profile. The BMP-9 was incorporated noncovalently to the MPs through a passive absorption process. 20 mg of MPs was immersed in 500 ng of BMP-9 solution and incubated at 4 °C for complete absorption. Equal amounts of MPs were immersed in sterile PBS and incubated at 4 °C to prepare the noncoated MPs. These MPs were incorporated into the polymer blend before gelation. 1 μg of VEGF was directly added to 0.5 mL of the polymer blend and mixed properly before gelation. The final concentration of BMP-9 and VEGF on the MPs-gel was thus 1 and 2 μg/mL, respectively. Three groups of the MPs-gel were prepared and denoted as MPs-gel (without growth any growth factors), MPs-gel+V (with VEGF only), and MPs-gel+V+B (with VEGF and BMP-9 coated MPs).
The release of BMP-9 was studied from coated MPs and both BMP-9 and VEGF from MPs-gel+V+B. ELISA based detection was used for the quantitation of released BMP-9 and VEGF. For release studies, coated MPs only or MPs-gel+V+B were placed in a glass vial and 2 mL of 1× sterile PBS was added to the vials, which were then subjected to 37 °C on an orbital shaker set to 50 rpm. At predetermined time points (1 h, 3 h, 5 h, day 1, day 3, day 5, day 7, and day 14), the PBS from the vial was collected and replaced with fresh PBS. The collected PBS was stored at −20 °C before performing the ELISA. The remaining amount of BMP-9 was determined by dissolving the MPs on acetic acid and the remaining amount of VEGF on MPs-gel+V+B was determined by dissolving the gel in cold water at the end of the release study. The release profile is presented as a percent cumulative release based on the total amount present on MPs and MPs-gel+V+B scaffolds.
Biological Activity of BMP-9.
The characterization of BMP-9 was done in terms of its effects on cytotoxicity, proliferation, and differentiation of hMSCs on a 24 well plate (2-D culture) and within the MPs-gel system (3-D culture). Prior to studies with MPs-gel, the bioactivity of BMP-9 coated on the MPs was determined by studying the attachment and proliferation of hMSCs and rat MSCs (rMSCs) on the surface of MPs. rMSCs were harvested from the femur bone marrow of rats. (Please refer to Supporting Information (SI) for detailed protocol.)
2-D Culture System.
Human bone marrow derived mesenchymal stem cells (hMSCs) obtained at passage 4 were expanded 3 to 4 times on serum-free media (StemPro XenoFree supplemented with GlutaMAX). To study the effects of BMP-9 on cell viability and proliferation, hMSCs were harvested from the culture dishes and seeded to the 24 well plate at the density of 20,000 cells/well with DMEM complete media containing MSC qualified FBS and penicillin−streptomycin. BMP-9 was added to the cells at the concentration of 0, 10, and 100 ng/mL. During each media change, new BMP-9 was added to the fresh media to maintain the required concentration of BMP-9. At days 3 and 7, live and dead cell assay was performed and the qualitative analysis of viability and proliferation of hMSCs was performed using fluorescence imaging.
The osteogenic differentiation of hMSCs on 2-D culture was studied using ALP quantification and alizarin red staining. The concentration of BMP-9 was maintained at 100 ng/mL for the cells seeded at the density of 30,000 cells/well. For ALP quantification, the cells were grown in four different groups as normal medium (NM) with and without BMP-9, as well as osteogenic medium (OM) (normal medium supplemented with 10 nM dexamethasone, 50 μM ascorbic acid, and 10 mM β-glycerol phosphate) with and without BMP-9. At days 3 and 7, ALP assay was performed to quantify the ALP activity. The cells in the well were washed with PBS followed by the addition of 250 μL of lysis buffer and kept under shaking condition for 2 min. The lysed cell suspension was collected, centrifuged, and the supernatant was mixed with p-nitrophenyl phosphate (pNPP) substrate and the kit instructions were followed. The ALP activity was normalized with the total protein content of the sample which was determined using a Coomassie Plus protein assay kit (ThermoFisher Scientific, MA). To further study the mineralization induced by the osteogenic differentiation, Alizarin red staining was performed at day 14. The cells were fixed with 2.5% buffered glutaraldehyde and washed with PBS. The washed cells were left to stain with the Alizarin red solution for 15 min and washed again with DI water few times. The staining of calcium mineral deposits was observed under color bright field microscopy.
3-D MPs-Gel System.
Prior to the cell studies with the MPs-gel system, the bioactivity of BMP-9 coated on the surface of MPs was determined. MPs coated with BMP-9 was seeded with the hMSCs and rMSCs at the density of 20,000 cells per 20 mg MPs in a 24 well plate. Species-specific BMP-9 was used for hMSCs and rMSCs. To increase the contact between the cells and MPs, 200 μL of cell suspension was first added to MPs and incubated for 3 h followed by the addition of the remaining 800 μL of media. At days 3 and 7, the MPs from cell seeded wells were transferred to a new well and washed with PBS. The qualitative analysis of the attachment and viability of MSCs growing on MPs was performed using the live/dead assay kit. After confirming the bioactivity of BMP-9 coated on the MPs, a series of in vitro studies were performed to determine the in vitro osteogenic properties of the MPs-gel system.
Seeding and Viability of hMSCs within MPs-Gel.
50 μL of the cell suspension containing 40,000 hMSCs was added to the MPs-polymer blend (0.5 mL) on a glass-chambered slide and incubated at 37 °C, 5% CO2 for 30 min. The polymer blend with MPs underwent gelation forming MPs-gel with hMSCs encapsulated within it. 1 mL of complete culture medium was added to the chambers and returned to the incubator. 500 μL of medium was removed every 3 days and replaced with the same amount of fresh medium. At days 3 and 10, live and dead cell assay was performed. The culture medium was removed from the chamber slides and the MPs-gel was washed with PBS. Live and dead cell stain diluted in DPBS was added directly to the chamber and incubated for 30 min. Confocal microscopy was performed to qualitatively observe the viability of hMSCs encapsulated within the MPs-gel. To maintain the stability of gel during imaging, the temperature of the microscope chamber was maintained at 37 °C.
In Vitro Osteogenic Differentiation.
ALP activity of the cells within the MPs-gel was determined to study the osteogenic differentiation of encapsulated hMSCs. 105 hMSCs were cultured in the presence of OM and half the amount of OM was replenished every 3 days. To liberate the encapsulated cells, MPs-gel was flash-frozen by immersing in liquid nitrogen followed by its pulverization. The disrupted powder was transferred to the lysis buffer and a similar process as explained was followed to quantify the ALP activity at days 7 and 14. To further observe the calcium deposition within the MPs-gel, at day 10, MPs-gel were processed into histological slides embedded in paraffin and von Kossa staining was performed. Briefly, the slides were first deparaffinized by immersing in a series of xylene, 100% and 30% ethanol, and washed thoroughly with DI water. Silver nitrate solution was added to the washed slides placed on aluminum foil and illuminated with 60 W bulb for 20 min. The slides were washed again and observed under bright field microscopy.
Gene Expression Study.
An osteoblast specific gene expression study was performed at days 7 and 14 with the cells cultured in the presence of OM. To isolate the RNA from the encapsulated cells, MPs-gel samples were flash frozen in liquid nitrogen and pulverized. The pulverized powder was added to the Trizol reagent (VWR International, PA) for the RNA isolation followed by its purification using the RNeasy kit (Qiagen Group, USA). The purified RNA was quantified using a Nanodrop-1000 spectrophotometer (ThermoFisher Scientific, MA). The RNA amount for the reverse transcription into complementary DNA (cDNA) was done based on the lowest concentration of RNA among the samples, and the cDNA synthesis kit (Verso kit, ThermoFisher Scientific, USA) was used for cDNA synthesis following the manufacturer’s instruction. The early osteogenic markers such as ALP and collagen 1 (Col1) as well as a late marker such as osteocalcin (OCN) was analyzed. The primers used for these genes are shown in Table S1. A quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed using a SYBR green qPCR kit (Smart Biosciences, USA) and StepOne Plus thermal cycler (Applied Biosystems, CA). The obtained Ct values for the target genes were normalized using GAPDH as the housekeeping gene and the fold change for MPs-gel+V and MPs-gel +V+B was expressed relative to the MPs-gel only group.
Animal Studies.
All the animal tests were conducted with approval from the Institutional Animal Care and Use Committee (IACUC) at the University of Toledo. Eight-week-old male rats (Charles River laboratories, MA) were anaesthetized using isoflurane inhalation (2−3% isoflurane vaporized in O2). The anaesthetized rats were maintained at 1.5% isoflurane for both injection and surgical procedure.
Ectopic Bone Formation.
Rats were divided into three groups (n = 5) based on the scaffold composition. MPs-gel, MPs-gel+V+B, and MPs-gel+V+B with osteoinduced rMSCs (cultured with OM for 5 days) were loaded to a 1 mL syringe and kept on ice until injection. Two injections of the same group of scaffold per rat were done to the subcutaneous pocket using a 13 G needle. Immediately after injection, the rats were transferred to the recovery chamber and housed individually after recovery.
After 2 and 4 weeks post-injection, the rats were sacrificed and the MPs-gel with surrounding tissues were harvested. For histological analysis, the harvested tissues were fixed in 10% (v/v) neutral buffered formalin, dehydrated in sequentially increasing ethanol solutions to 100% (v/v) ethanol, immersed in xylene, and embedded in paraffin. The tissue samples were cross-sectioned to 5 μm thickness and stained with hematoxylin−eosin (H&E) and Masson’s Trichrome to observe the bone tissue formation. The tissue samples after 4 weeks were harvested with the outer skin and subjected to dual-energy X-ray absorptiometry (DEXA) before performing the histological analysis. Since the shape of the new tissue was different for the different samples, the total bone mineral content (BMC) on the new tissue was determined and reported instead of bone mineral density (BMD).
Cranial Bone Formation.
Animal Surgery.
The surgical site was shaved and disinfected with a series of isopropanol, betadine scrub, and betadine solution. A 0.8 mm incision was made along the midline of the skull and the periosteum was set aside to expose the bone. Two full circular defects of 3.5 mm diameter were created, using a microdrill and burr, on the calvarium of each rat. The defect site was regularly flushed with sterile saline to remove the bone debris and minimize the heat production during drilling. Each defect was injected with 0.2 mL of MPs-gel. The study groups were divided into void group, MPs-gel group, and MPs-gel+V+B group with each group consisting of 5 animals (total 10 defects per group). The animals were euthanized at 12 weeks post-operation and the bone formation along the defects was analyzed using microcomputed tomography (microCT).
MicroCT and Histological Evaluation.
The bone samples harvested from the rats were positioned in a 34-mm-diameter specimen holder and scanned using a microCT system (μCT100 Scanco Medical, Bassersdorf, Switzerland). Scan settings were as follows: voxel size 18 μm, 70 kVp, 114 μA, 0.5 mm AL filter, and integration time 500 ms. Analysis was performed using the manufacturer’s evaluation software, and a fixed global threshold of 21% (210 on a grayscale of 0−1000) was used to segment bone from nonbone. A cylindrical sample the width of the skull with a diameter of 3.5 mm and centered over the defect was analyzed.
The harvested bone samples were decalcified for a week before they were embedded in the liquid paraffin for sectioning. 5-μm-thick sections were fixed on the microscope slides for staining with H&E.
Statistical Analysis.
The statistical analysis was performed using SPSS software. Two-tailed paired sample Student’s t tests were performed to evaluate gene expression. Analysis of variance (ANOVA) followed by Tukey’s post hoc analysis was performed to test the significance between the groups. A value of p < 0.05 was considered significant.
RESULTS AND DISCUSSION
Synthesis of MPs and MPs-Gel.
CS MPs prepared using the coacervation technique by cross-linking with TPP have been used in many drug delivery and tissue regeneration applications.19,20,32,33 Spherical MPs with the size ranging from 500 to 1000 μm can be fabricated using this technique where drops of CS solution are dripped to the TPP solution under stirring condition.32,33 The size of the MPs fabricated using an air-drying technique in this study ranged from 600 to 700 μm. These MPs were difficult to inject using 13 or 16 G needles due to their size. We further rinsed these air-died MPs in water under stirring condition and air-dried them again. This process enabled us to slightly reduce the size of the MPs to 500 to 600 μm, thus making them easier to inject using 13 or 16 G needles.
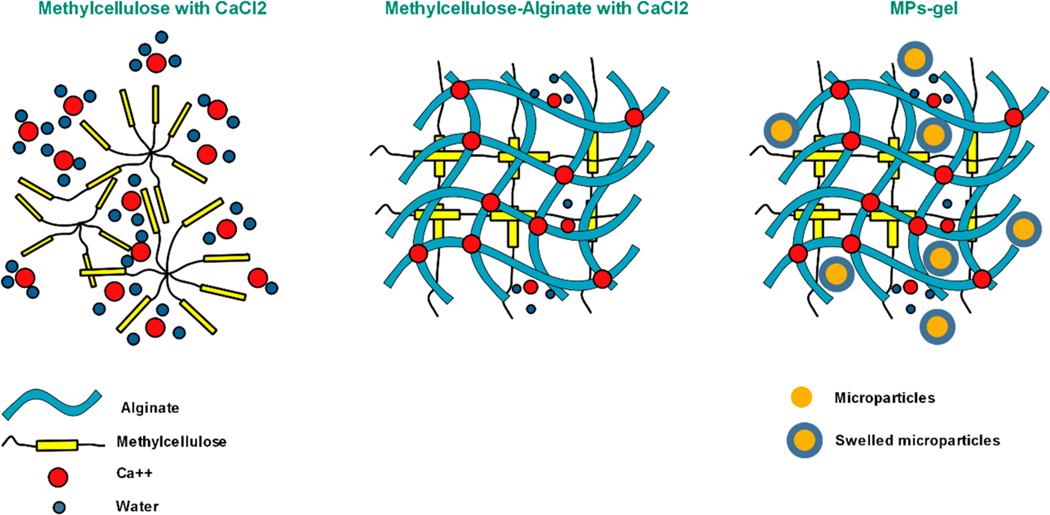
The MC solution itself undergoes gelation at higher temperature depending on the molecular weight. MC used in this study at the concentration of 10% (w/v) has been reported to have a gelation temperature of 40.1 °C through calorimetry experiments.34 Increasing the concentration beyond 10% might reduce the gelation temperature and bring it more toward the physiological temperature, but those solutions are hard to handle due to high viscosity. Another method to lower the gelation temperature is through the addition of salts, but it has been shown to reduce the strength of the gel when subjected to aqueous or cell culture environment.6,7 In this study, we wanted to develop a gel that can undergo gelation at 37 °C with better gel strength. We used the strategy of blending two polymers and blended MC with calcium chloride and Alg. This polymer blend formed a strong cross-linked gel at 37 °C (Scheme 2). It slightly lost the liquid-like flow after blending due to the cross-linking between Alg and calcium chloride and was more of semiliquid at lower temperature, but when subjected to 37 °C, the gelation occurred in less than 10 min of incubation. This gel was developed as an injectable medium for MPs so that a good retention of MPs could be achieved at the injection or defect site. Furthermore, we hypothesized that the release of BMP-9 coated on the MPs would be controlled from the MPs-gel system due to the additional diffusion barrier presented by the cross-linked gel. The gelation of polymer the blend was not affected by the addition of MPs; instead, faster gelation was observed with MPs-gel compared to gel only (Figure 1a). This is due to the absorption of water by MPs from the blend solution, causing the viscosity to increase faster. This was qualitatively confirmed as the MPs were observed to be swelling within the gel. The FTIR analysis (Figure 1b) showed the characteristic peak of MC at 3426 cm−1 (O−H stretching) and 2903 cm−1 (C−H stretching in CH2 and CH3). The characteristic peaks of Alg were observed at 1599 and 1406 cm−1 corresponding to the asymmetric and symmetric stretching of COO−, respectively. The O−H stretching peak for Alg was observed at 3248 cm−1. The peak at 1634 cm−1 in MPs spectrum corresponds to the shifted amide I band in CS which has been explained elsewhere.35 The polymer blend of MC and Alg showed the O−H stretching peak at 3298 cm−1 which was shifted to a lower value compared to MC and a higher value compared to Alg. This peak was slightly narrow compared to that observed in Alg. These changes might be due to the interaction of Ca with Alg and a result of polymer blending. There was no change in the peak position of asymmetrical stretching of COO− observed at 1599 cm−1 which is normally observed in calcium alginate complexes. The spectrum of MPs-gel showed all the peaks observed in the MC-Alg blend except that the O−H stretching peak was shifted to 3420 cm−1 with slight peak broadening. This suggests that introduction of MPs to the blend increased the hydrogen bonding as evidenced through the swelling of MPs when introduced to the blend.
Scheme 2. Gelation of MC, MC-Alg, MPs-Gel at 37 °Ca.
a The MPs within the gel are in swelled form that causes faster gelation of the MC-Alg blend.
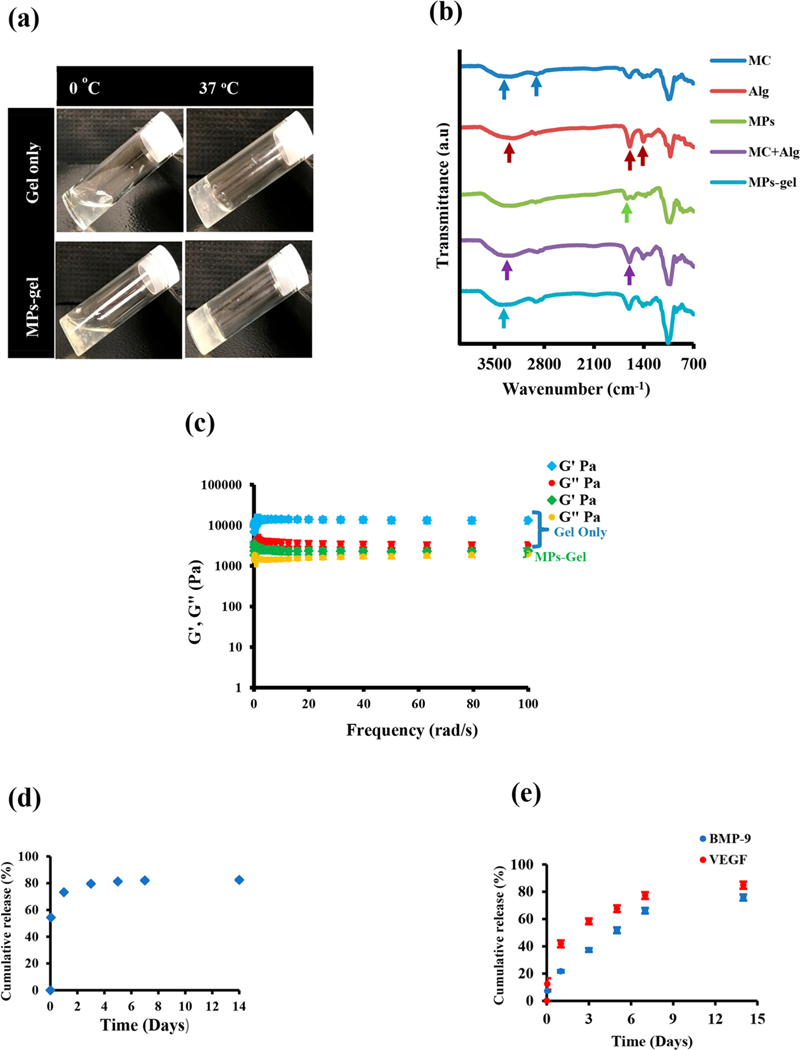
Figure 1.
Physical appearance (a), FTIR analysis spectra (b), and rheological property of developed gel and MPs-gel (c). Cumulative release profile of BMP-9 from MPs only (d) and BMP-9 and VEGF from MPs-gel scaffolds (e).
Viscoelastic Property of Gel.
The frequency sweep test for gel only and MPs-gel was performed at 37 °C using parallel plate rheometer. The result in Figure 1c shows that G′ (storage modulus) remains almost constant at higher frequencies for both gel only and MPs-gel. This indicated the stiff nature of the gel and is predicted to remain stable over a period of time. This is one of the important properties required for the injectable gel. The G′′ (loss modulus) also followed a similar pattern for both samples. The actual magnitude of G′ and G′′ for the MPs-gel system was lower than that for gel-only samples. The gelation of MC at a higher temperature occurs due to the formation of intra- and intermolecular chain hydrophobic interactions. The strength of the MC-based gel thus is due to the formation of a hydrophobically cross-linked network.6 In the current study, the ionic interaction between the negatively charged group in Alg and CaCl2 also contributes to the overall strength of the gel. The addition of MPs, however, lowers this ionic interaction as CS itself can undergo ionic complexation with negatively charged groups. This might have resulted in the lower strength of MPs-gel scaffolds compared to gel only.
Release of BMP-9 and VEGF.
The delivery system plays an important role in determining the efficacy of BMPs-based treatments. The necessary dose of this protein to induce bone formation may be significantly decreased, if the protein can be retained at the intended site for an extended period of time in a bioactive form.36,37 The release of BMP-9 from coated MPs was demonstrated first. Considering the physical absorption of BMP-9 on the surface, we expected the release to be burst and the result showed that more than 70% of BMP-9 was released within 24 h (Figure 1d). It has been reported that encapsulation of BMPs into the MPs can slow down their release, but the problems with the lower encapsulation and the requirement of harsh chemical treatments significantly affects the bioactivity of BMPs.37 Furthermore, the use of MPs only to study their bone forming ability in in vivo models such as ectopic bone formation is virtually impossible, as they cannot be localized at a specific site. In this study, we aimed to use these burst-releasing coated MPs as BMP-9 carriers incorporated into an injectable gel. The gel acted as a reservoir to control the release of BMP-9 while also protecting it from in vivo degradation. These coated MPs were incorporated into the polymer blend containing VEGF. VEGF is considered one of the key regulators of angiogenesis during the bone formation process.13 Angiogenesis involves the formation of new blood vessels from pre-existing blood vessels. During the normal bone healing process, the expression of VEGF is high during earlier time points followed by the expression of BMPs later.13,38 The MPs-gel system was developed to mimic similar temporal release profiles with faster and more VEGF release in the earlier days. After 24 h, about 40% of VEGF was released from MPs-gel compared to 20% BMP-9 release. The release of BMP-9 from MPs-gel was thus slower and less than VEGF release, as well as being more controlled than the burst release from MPs only (Figure 1e). The slower release of BMP-9 from the MPs-gel system increases its bioavailability within the gel, which is important especially if the developed hydrogel is designed to encapsulate the stem cells.
In Vitro Bioactivity of BMP-9 in 2-D Culture.
While BMP-2 and BMP-7 have shown promise in a series of clinical trials in both the spine and orthopedic trauma, it is unclear if these are the most effective BMPs for the promotion of fracture healing.23 Endogenous BMP-9, however, has been studied to be the most osteogenic among all BMPs. Studies done with hMSCs transfected with BMP-9-expressing adenovirus have been shown to significantly improve the ALP expression by hMSCs even at the low concentration of expressed BMP-9 (600 pg/mL).39 Very few studies done with recombinant BMP-9 have shown that the addition of BMP-9 to a 2-D culture of preosteoblasts and adipose derived stem cells results in a significant improvement of osteogenic activity of these cells.23,24 The potent osteogenic activity of BMP-9 has been attributed to the fact that BMP-9 is resistant to the BMP antagonist Noggin which generally inhibits the BMP-induced SMAD signaling pathways.25 The studies showing the osteogenic potential of recombinant BMP-9 are performed mostly with preosteoblasts, and the studies involving BMP-9 and hMSCs have been done mostly with adenovirus transfection. In order to study the therapeutic level and osteogenic activities of recombinant BMP-9 with hMSCs, we studied two different concentrations of BMP-9 and characterized the effects in terms of toxicity, proliferation, and differentiation of hMSCs in 2-D culture. As shown in Figure 2a, the addition of BMP-9 to the 2-D culture of hMSCs at two different concentrations improved their proliferation without causing any toxic effects. Qualitatively, we observed higher proliferation with 100 ng/mL concentration at both days 3 and 7. An ALP expression study was performed to study the osteogenic differentiation of hMSCs in 2-D culture at a BMP-9 concentration of 100 ng/mL and in the presence of normal medium (NM) and osteogenic medium (OM). The results in Figure 2b show that at both days 3 and 7, osteogenic differentiation of hMSCs in the presence of BMP-9 was taking place with both NM and OM indicated by the higher expression of ALP. The ALP expression was comparatively lower in the absence of BMP-9. At day 7, the scenario slightly changed with significantly high differentiation of hMSCs in the presence of BMP-9 and OM. The cells growing with BMP-9 in NM and only OM had similar ALP activity which was significantly higher than that with NM only. The result here shows the potential of BMP-9 to induce the early osteogenic differentiation of hMSCs even without the osteogenic supplements. The osteogenic differentiation, however, is best achieved with BMP-9 in the presence of OM as indicated by the significantly higher expression of ALP with this group. The mineralization induced by the osteogenic differentiation was evaluated using Alizarin red staining at day 14 for the hMSCs with BMP-9 (100 ng/mL) and without BMP-9 in OM. As shown in Figure 2c, the mineralized deposits were present in both groups as indicated by the presence of red spots across the well. We qualitatively observed that these deposits were denser and more uniform along the whole surface area of the well containing BMP-9 when compared to that without BMP-9 where deposits were observed in a few areas. This result is consistent with the ALP expression data where osteogenic differentiation was higher for hMSCs grown in OM with BMP-9. The overall results here show the significant potential of BMP-9 to induce the osteogenic differentiation of hMSCs in 2-D culture.
Figure 2.
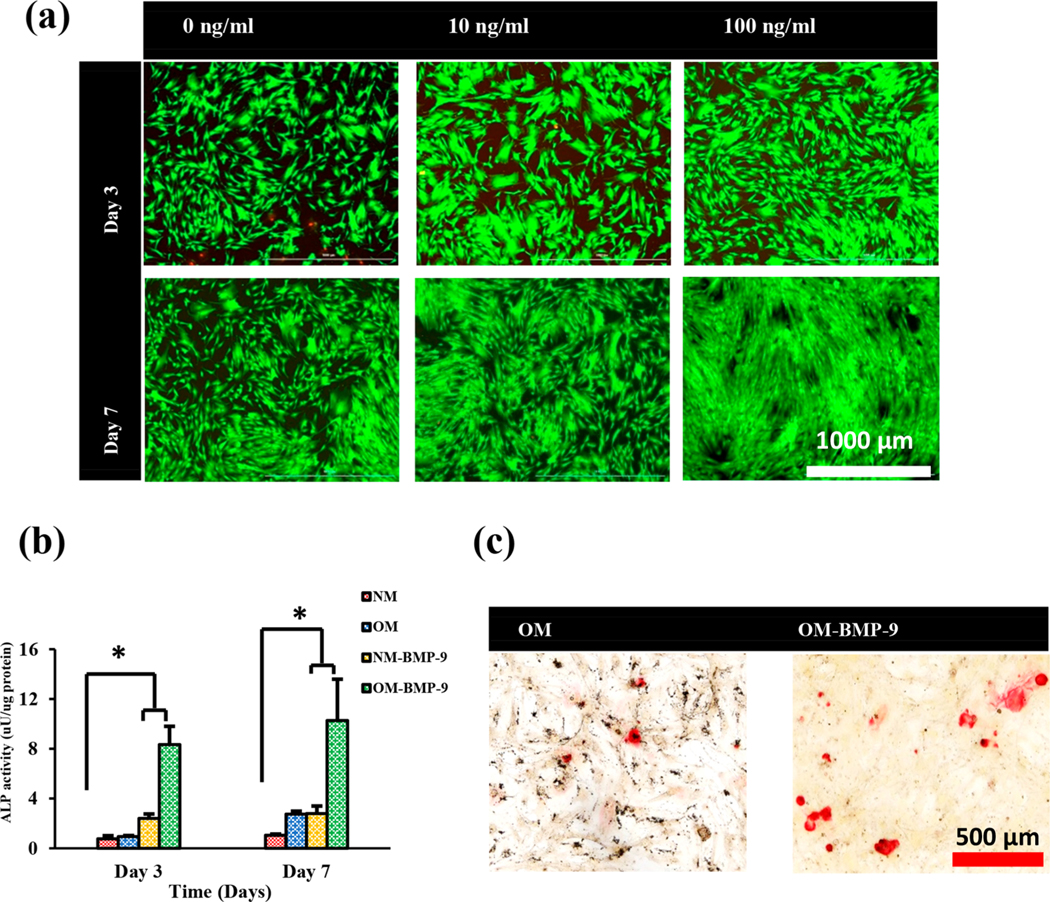
hMSCs growth and proliferation at different concentrations of BMP-9 (a). Osteogenic differentiation of hMSCs in 2-D culture with and without 100 ng/mL BMP-9 and in the presence of normal media and osteogenic media determined using ALP expression (b) and Alizarin red staining in the presence of osteogenic media (c).
In Vitro Bioactivity of BMP-9 Coated MPs.
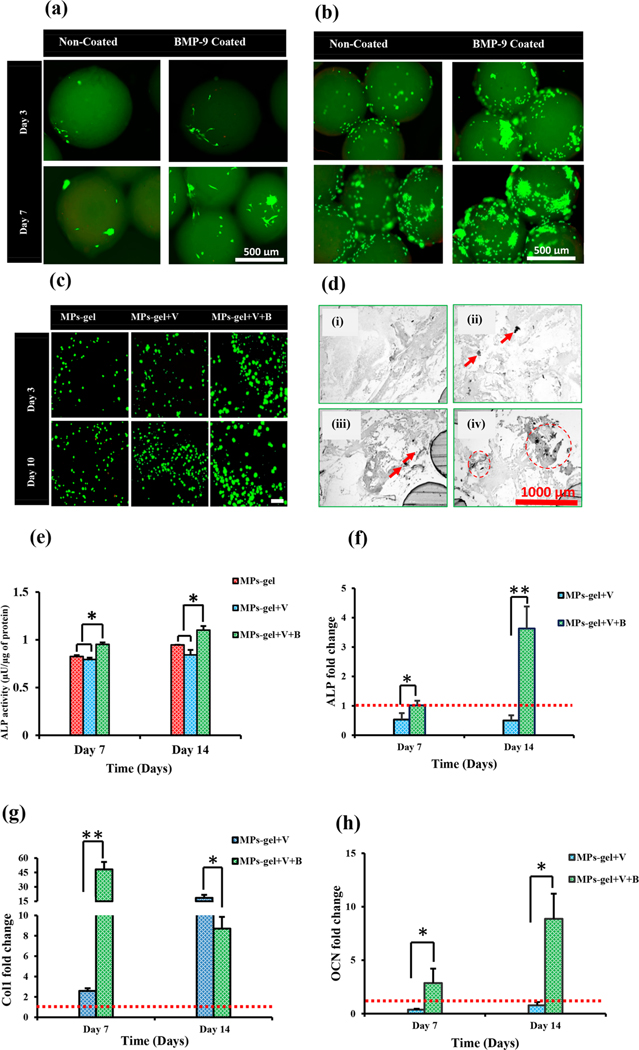
The osteogenic response of BMP-9 combined with VEGF delivered through the MPs-gel was studied. As a first step to the study, the bioactivity of BMP-9 coated on the MPs was determined to analyze the ability of CS MPs to be used as BMP-9 carriers. The CS MPs are prepared in very mild conditions without the use of harsh chemical treatment. The direct physical absorption of BMPs, though results in the bulk release quickly, has been stated to improve their bioactivity compared to direct grafting to the materials.40 Bioactivity of coated BMP-9 was studied by observing the attachment and proliferation of hMSCs and rMSCs on the MPs. The results (Figure 3a and b) showed that the attachment and proliferation of MSCs at both days 3 and 7 was higher in BMP-9 coated MPs than noncoated MPs. The cells on coated MPs grew in big colonies along the surface. The rMSCs growth (Figure 5b) was denser than that of hMSCs (Figure 5a), which could be due to the larger size of hMSCs and the curvature of the MPs limiting their attachment to the MPs. The results, however, showed that the BMP-9 coated on the surface of MPs was bioactive and enhanced the attachment and proliferation of MSCs.
Figure 3.
In vitro response of hMSCs and rMSCs in MPs and MPs-gel scaffolds. Attachment and proliferation of hMSCs (a) and rMSCs (b) on BMP-9 coated and noncoated MPs. Viability of hMSCs encapsulated within MPs-gel (c) and their mineral deposition shown by von Kossa staining (i, without cells; ii, MPs-gel; iii, MPs-gel+V; iv, MPs-gel+V+B) (d). Osteogenic differentiation of hMSCs within MPs-gel determined using ALP quantification (e), and osteogenic gene expressions (f,g,h). The dotted red line shows the reference level of MPs-gel as control.
Figure 5.
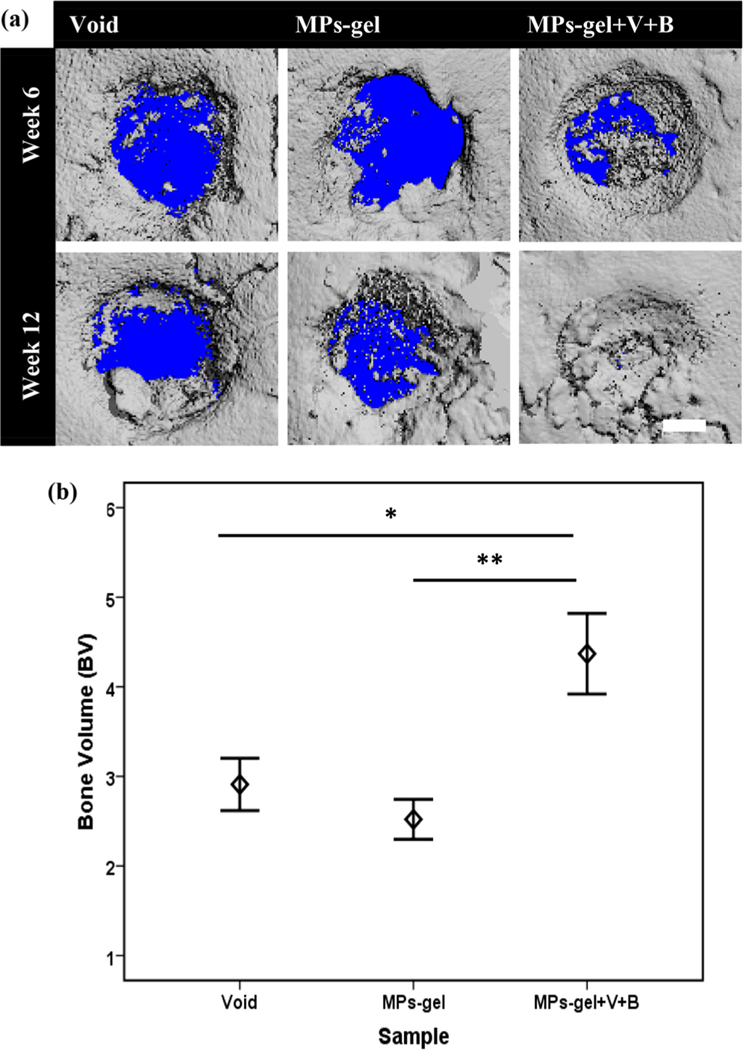
MicroCT evaluation of bone formation on cranial defects of rats. 3-D reconstructed images of defect site after weeks 6 and 12 (a). Quantification of bone volume along the defect site with the diameter of 3.5 mm at week 12 (b).
In Vitro Response of MPs-Gel Scaffolds.
The osteogenic response of BMP-9 combined with VEGF delivered through MPs-gel was studied. All of the polymers used in the development of MPs-gel scaffolds are naturally derived with established biocompatibility properties. While CS and Alg have been shown to have excellent cytocompatibility, MC might have differing responses to the cells depending on molecular weight (MW). In general, polysaccharides with high MW are studied to be less cytocompatible compared to those with low MW. The MC used in the current study has been shown to have an excellent response to the cells. The viability of hMSCs encapsulated within the MPs-gel system is shown in Figure 3c. The MPs-gel system maintained an excellent cell viability up to 10 days of encapsulation shown by green fluorescence with a sparse number of dead cells indicated by the red/yellowish fluorescence. The cells mostly stayed with the spherical morphology on all groups of MPs-gel with very little spreading on both days. Furthermore, there were no qualitative differences in cell proliferation among the different groups of MPs-gel. This shows the very small role of BMP-9 in stimulating the MSCs elongation and proliferation in a 3-D gel. Indeed, studies have shown that the proliferation of MSCs during bone formation is mostly dominated by TGF-β1 and other pathways. The combined effects of TGF-β1 and BMP-9 was able to induce MSCs proliferation in the early stages of cell cycle.41,42 Furthermore, the fact that BMP-9 can stimulate the osteogenic differentiation of MSCs during the early stages might implicate its little role in their proliferation. The results here show the ability of MPs-gel to encapsulate the MSCs and maintain their long-term viability. We observed that both BMP-9 and VEGF have nonsignificant roles in improving the hMSCs proliferation within the developed gel.
The differentiation of encapsulated hMSCs to the osteogenic lineage within the MPs-gel system was studied to evaluate the osteoinductive potential of BMP-9 on the 3-D culture system where we observed their different adhesion and limited stretching pattern compared to the 2-D culture system. Very few studies have reported the ability of recombinant BMP-9 to promote in vitro osteogenic differentiation in the semirigid 3-D gel matrix, whereas there are many studies showing the ability of BMP-9 to enhance the ALP expression by MSCs and preosteoblasts through adenoviral transfection in 2-D and rigid 3-D culture environments.39,43,44 Results here showed that the ALP expression (Figure 3e) by the cells cultured within MPs-gel+V+B was significantly higher (p < 0.05) than that of other groups at both time points. The expression of ALP by cells within the MPs-gel with and without VEGF was almost the same indicating that VEGF itself had no role in directly influencing the osteogenic differentiation of hMSCs. Mineral deposition is one of the important events that takes place during the osteogenic differentiation. The calcium deposits within the MPs-gel were observed using the von Kossa assay at day 14 after processing the MPs-gel−cell construct into the histological slides. As shown in the microscopy images on Figure 3d, there were no black deposits on the MPs-gel samples without any hMSCs. The samples encapsulating hMSCs showed some mineral deposition indicated by the black dots on the images showing the onset of mineralization within the gel by the differentiated cells. Comparatively, the black deposits were more visible in the MPs-gel+V+B and were present over the larger surface area than that on the MPs-gel and MPs-gel+V.
Osteogenic differentiation of MSCs is accompanied by the upregulation of osteogenic gene expression. In the current study, we focused on three specific genes pertinent to bone development and mineralization. ALP and Col1 are relatively early markers of osteogenic differentiation. The expression of these genes shows the early osteoblast lineage commitment of MSCs. Col1 is a major organic component of bone extracellular matrix. OCN, on the other hand, is a relatively late marker whose expression is accompanied by the matrix mineralization. OCN is mainly secreted by the mature osteoblasts and is one of the most abundant non-collagenous protein of the extracellular matrix.5 The fold change in expression of genes by MPs-gel+V and MPs-gel+V+B is expressed relative to the expression of genes by the MPs-gel group. As shown in Figure 3f, MPs-gel+V+B upregulated the expression of ALP on both days 7 and 14 while that by MPs-gel+V is slightly downregulated compared to the MPs-gel group on both days. The expression of Col1 was significantly upregulated on MPs-gel+V+B group on day 7 (Figure 3g). Interestingly, the expression of Col1 on day 14 was decreased; however, the expression of OCN was upregulated (Figure 3h). Thus, the group containing BMP-9 showed significant differences in the expression of all three genes demonstrating the ability of BMP-9 to induce the osteogenic differentiation of MSCs within the 3-D gel. The results here show that BMP-9 can significantly improve the osteogenic differentiation of MSCs not only in 2-D culture but also in a 3-D gel. Both early and late osteogenic differentiation markers were significantly upregulated in BMP-9 containing groups. Many studies have been and are still being done to identify the mechanism of BMP-9’s potent osteogenic ability. Almost all of these studies are able to establish that all osteogenic BMPs except BMP-9 induced osteogenic differentiation are inhibited by Noggin, a common antagonist for other BMPs. Results have also indicated that BMP-9 induced bone formation is also resistant to BMP-3, which is another potent antagonist to BMPs.24,25
Ectopic Bone Formation.
The subcutaneous bone formation in rodents was studied, as it allows one to test if the developed system could support bone formation, eliminating the effect of bone-stimulating cytokines, bone forming cells, and potentially bone-stimulating mechanotransduction.26,45 Many studies have shown the ability of BMP-9 to induce ectopic bone formation. However, almost all of those studies utilized osteoprogenitor cells or stem cells of different origins transduced with BMP-9 expressing viral vectors.26,27,46 Recombinant proteins, on the other hand, provide a safer, reliable, and established alternative to study protein induced processes. So far, we just found one study where the ability of recombinant BMP-9, delivered through a scaffold material, to induce ectopic bone formation at the quadriceps has been investigated. The study highlighted the importance of the delivery system and the need for a muscle extracellular matrix environment for BMP-9 to induce ectopic bone formation.22,30 In the current study, we report the first results of subcutaneous bone formation induced by BMP-9 incorporated thermoresponsive gel. In order to specifically study the role of BMP-9 and VEGF within the MPs-gel, the samples injected included MPs-gel which was more of a control group for MPs-gel+V+B and MPs-gel+V+B+osteo-induced rMSCs. The group with rMSCs was included to study if those cells would accelerate the ectopic bone formation process. We observed that all groups of injected MPs-gel were well retained within the subcutaneous injection site and bonded well to the subcutaneous tissues. We also observed that MPs remained with the gel at the injection site, indicating the gel was able to retain the MPs at the target site (Figure 4a). After 4 weeks, MPs-gel+V+B with cells were noticeably more rigid and larger in size when compared to the other two groups and appeared more resistant to deformation during isolation and handling. Figure 4b,c shows the representative H&E and Masson’s trichrome stained sections, respectively, for the tissue samples harvested from MPs-gel. At 2 weeks (Figure S1a,b), the gel structure mostly remained intact with the huge infiltration of fibroblasts. At 4 weeks, the gel seemed to be degrading away, and dispersed collagen fibers were present shown by the pink stains in panel b and blue stains in panel c. There was, however, no evidence to support that these collagenous materials were remodeling into bone as indicated by the absence of osteoblast-like cells and blood vessels.
Figure 4.
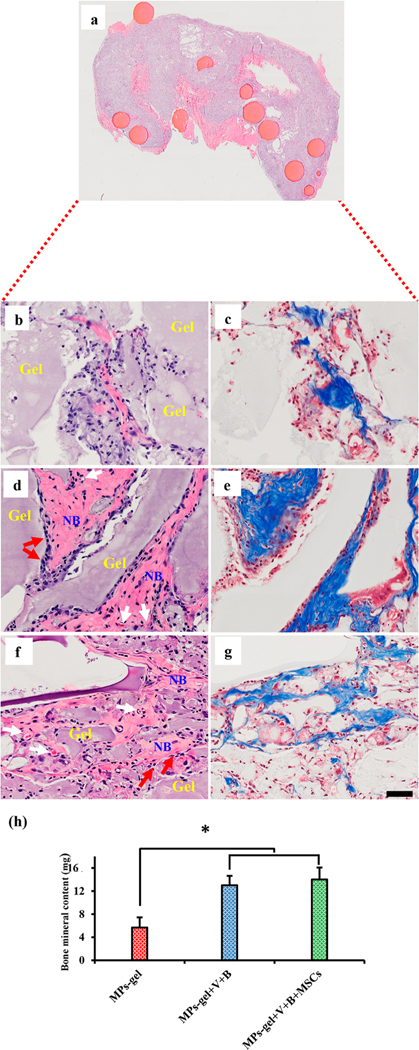
Histological sections stained with H&E and Masson’s trichrome stains to show the subcutaneous bone formation in rats after 4 weeks. The top section shows the localization of MPs within the harvested tissue after 4 weeks of injection (a). The left and right panels show the H&E and Masson’s trichrome sections, respectively, for MPs-gel (b,c), MPs-gel+V+B (d,e), and MPs-gel+V+B with hMSCs (f,g). Total bone mineral content was quantified using DEXA for the tissue harvested after 4 weeks (h). (NB: new bone.)
The representative stained sections of tissue sample harvested from MPs-gel+V+B are shown in Figure 4d and e. This group showed the presence of fibrous collagenous tissue at 2 weeks indicating the onset of ectopic bone formation (Figure S1c,d). The role of VEGF was evident through the presence of abundant blood vessels. At 4 weeks, the woven bone-like structure was evident throughout the harvested tissue. They were found to be lined with osteoblasts (red arrows) and the blood vessels continued to form along these structures. This indicates that BMP-9 was able to induce ossification within the multiple site of the MPs-gel and VEGF induced neo-vascularization.
Another experimental group included MPs-gel+V+B with 1 × 106 rMSCs derived from the femoral bone marrow of a 3-week-old rat. This group was included to study if the ectopic bone formation due to MSCs in MPs-gel+V+B would be different than that without MSCs. Studies have shown that the ectopically injected or implanted scaffolds with MSCs show better bone formation than with only BMPs.47,48 Qualitatively, we observed that the tissue sections harvested from this group were larger in size compared to those from other groups. As shown in Figure S1e,f, fibrous collagenous materials were present with abundant blood vessels at 2 weeks. At 4 weeks (Figure 4f,g), woven bone structures were observed lined with osteoblasts. The onset and remodeling of ectopic bone formation at 2 and 4 weeks on this group was similar to that without MSCs except for the presence of bone structure in more regions of harvested tissue in this group.
The subcutaneous bone formation was further investigated by measuring BMC using DEXA. Results show the significantly higher mineral content in the MPs-gel+V+ B and MPs-gel+V +B+MSCs groups compared to the MPs-gel group (Figure 4h). This further shows the ability of BMP-9 and VEGF to induce ectopic bone formation within a month of injection. There was no significant difference in mineral content between the growth factor loaded groups with and without cells. The similar results with and without MSCs indicate that the host cells can rapidly infiltrate into the MPs-gel where they are eventually differentiated to the bone forming osteoblasts. BMP-9 delivered with VEGF through MPs-gel, thus, can induce the subcutaneous bone formation in the absence of bone forming cells.
Cranial Defects.
The encouraging results from the ectopic bone formation model directed us to move forward with the orthotopic bone formation model. The MPs-gel+V+B group with MSCs was omitted from this study as the subcutaneous bone formation results showed no particular difference in bone formation with this group compared to that without MSCs. The cranial defects were created on rats and the gel samples were injected, and the bone regeneration was studied using microCT for 12 weeks. MicroCT analysis and 3D rendering were performed for some samples at 6 weeks to observe the defect site and bone ingrowth. As observed in Figure 5a, after 6 weeks, the bone fragments were growing toward the defect in all groups. The bone ingrowth, however, looked more prominent in the MPs-gel+V+B group with significant bone growing in the defect region. The results at 12 weeks further verified this observation with superior bone formation observed in this group with new bone covering the whole defect region. The quantitative analysis of new bone formation measured as bone volume (BV) further showed a significantly higher value of BV in the MPs-gel+V+B group compared to the MPs-gel (p < 0.001) and void (p < 0.05) groups. These results (Figure 5b) show that the combination of VEGF with BMP-9 when delivered using MPs-gel induced higher bone regeneration in the defects at much lower doses of BMP-9 than the ones that were reported earlier.
The bone regeneration was further studied using H&E staining at 12 weeks. Histological analysis revealed the nature of bone formation along the defect region. As shown in Figure 6a, most of the new bone formation on the void defect was along the defect margin with few bone fragments on the central defect region, but the majority of it was filled with fibrous connective tissue. The MPs-gel group also showed similarly lower bone formation with most of the defect region filled with fibrous connective tissues (Figure 6c). The new bone formation on both of these groups looked immature, lacking osteoid and blood vessels, as shown in the magnified images in Figure 6b and d. In the case of MPs-gel+V+B, complete bone formation was observed with new bone bridging the gap between the defects (Figure 6e). The newly formed bone showed the abundant presence of osteoid and blood vessels (Figure 6f).
Figure 6.
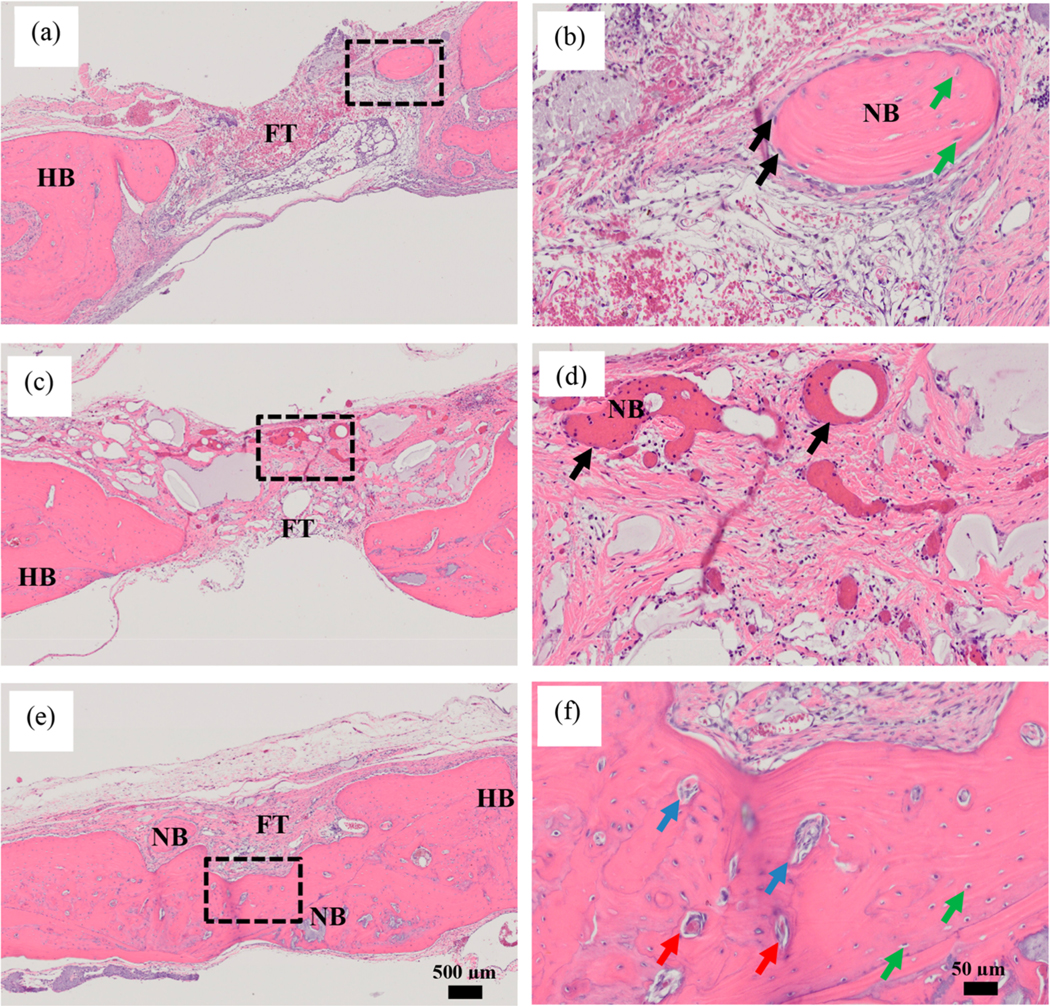
Histological sections of defect region harvested at 12 weeks and stained with H&E. Void defect (a, b). MPs-gel (c, d). MPs-gel+V+B (e, f). The dotted black rectangle shows the area magnified at 20× shown in the panel on the right. FT: Fibrous connective tissue. HB: Host bone. NB: New bone. Black arrows: osteoblasts. Green arrows: Osteocytes. Blue arrows: Osteoid. Red arrows: Blood vessels.
CONCLUSIONS
In this work, we developed multicomponent releasing MPs-gel scaffolds to facilitate BMP-9 induced bone formation at low dose. The thermosensitive gel was developed as an injectable medium for BMP-9 coated MPs so that their injectability property could be preserved while also increasing the efficacy of BMP-9 at the injection site through the localization of MPs. BMP-9 coated on the MPs and VEGF introduced to the thermosensitive gel were both bioactive, and their temporal release profile enabled improved osteogenic responses. MPs-gel maintained the long-term viability of encapsulated hMSCs. Furthermore, the MPs-gel with BMP-9 and VEGF were found to enhance both subcutaneous and cranial bone formation indicated by significantly higher bone formation induced by the MPs-gel+V+B group. Taken together, our results show that BMP-9 combined with VEGF when delivered through injectable MPs-gel composites would enhance the bone formation at low dose of BMP-9. Future studies will be focused toward the comparative analysis of BMP-9 with other BMPs at similar doses.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health (R01DE023356). We would like to acknowledge Mr. Amit Chougule for his help with DEXA.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.9b00082.
Primers used for RT-PCR experiment (Table S1); Detailed protocol on isolating and culturing the MSCs from the bone marrow of rats; Histological sections of tissues harvested at 2 weeks of injection of MPs-gel scaffolds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Bohner M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13 (1), 24–30. [Google Scholar]
- (2).Ho-Shui-Ling A; Bolander J; Rustom LE; Johnson AW; Luyten FP; Picart C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kretlow JD; Young S; Klouda L; Wong M; Mikos AG Injectable Biomaterials for Regenerating Complex Craniofacial Tissues. Adv. Mater 2009, 21 (32−33), 3368–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Qu Y; Wang B; Chu B; Liu C; Rong X; Chen H; Peng J; Qian Z. Injectable and Thermosensitive Hydrogel and PDLLA Electrospun Nanofiber Membrane Composites for Guided Spinal Fusion. ACS Appl. Mater. Interfaces 2018, 10 (5), 4462–4470. [DOI] [PubMed] [Google Scholar]
- (5).Sivashanmugam A; Charoenlarp P; Deepthi S; Rajendran A; Nair SV; Iseki S; Jayakumar R. Injectable Shear-Thinning CaSO4/ FGF-18-Incorporated Chitin−PLGA Hydrogel Enhances Bone Regeneration in Mice Cranial Bone Defect Model. ACS Appl. Mater. Interfaces 2017, 9 (49), 42639–42652. [DOI] [PubMed] [Google Scholar]
- (6).Tang Y; Wang X; Li Y; Lei M; Du Y; Kennedy JF; Knill CJ Production and characterisation of novel injectable chitosan/ methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym 2010, 82 (3), 833–841. [Google Scholar]
- (7).Rangabhatla ASL; Tantishaiyakul V; Oungbho K; Boonrat O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm 2016, 499 (1−2), 110–118. [DOI] [PubMed] [Google Scholar]
- (8).Lee KY; Mooney DJ Alginate: Properties and biomedical applications. Prog. Polym. Sci 2012, 37 (1), 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Liang HF; Hong MH; Ho RM; Chung CK; Lin YH; Chen CH; Sung HW Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules 2004, 5 (5), 1917–25. [DOI] [PubMed] [Google Scholar]
- (10).Ramesh Babu V; Sairam M; Hosamani KM; Aminabhavi TM Preparation of sodium alginate−methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym 2007, 69 (2), 241–250. [Google Scholar]
- (11).Li H; Tan YJ; Leong KF; Li L. 3D Bioprinting of Highly Thixotropic Alginate/Methylcellulose Hydrogel with Strong Interface Bonding. ACS Appl. Mater. Interfaces 2017, 9 (23), 20086–20097. [DOI] [PubMed] [Google Scholar]
- (12).Barati D; Shariati SRP; Moeinzadeh S; Melero-Martin JM; Khademhosseini A; Jabbari E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Controlled Release 2016, 223, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kempen DH; Lu L; Heijink A; Hefferan TE; Creemers LB; Maran A; Yaszemski MJ; Dhert WJ Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30 (14), 2816–25. [DOI] [PubMed] [Google Scholar]
- (14).Buwalda SJ; Vermonden T; Hennink WE Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18 (2), 316–330. [DOI] [PubMed] [Google Scholar]
- (15).Singh M; Sandhu B; Scurto A; Berkland C; Detamore MS Microsphere-based scaffolds for cartilage tissue engineering: Using subcritical CO2 as a sintering agent. Acta Biomater. 2010, 6 (1), 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Jiang T; Abdel-Fattah WI; Laurencin CT In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 2006, 27 (28), 4894–4903. [DOI] [PubMed] [Google Scholar]
- (17).Wang X; Shi J; Li Z; Zhang S; Wu H; Jiang Z; Yang C; Tian C. Facile One-Pot Preparation of Chitosan/Calcium Pyrophosphate Hybrid Microflowers. ACS Appl. Mater. Interfaces 2014, 6 (16), 14522–14532. [DOI] [PubMed] [Google Scholar]
- (18).Ko JA; Park HJ; Hwang SJ; Park JB; Lee JS Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm 2002, 249 (1), 165–174. [DOI] [PubMed] [Google Scholar]
- (19).Custódio CA; Cerqueira MT; Marques AP; Reis RL; Mano JF Cell selective chitosan microparticles as injectable cell carriers for tissue regeneration. Biomaterials 2015, 43, 23–31. [DOI] [PubMed] [Google Scholar]
- (20).Leonor IB; Baran ET; Kawashita M; Reis RL; Kokubo T; Nakamura T. Growth of a bonelike apatite on chitosan microparticles after a calcium silicate treatment. Acta Biomater. 2008, 4 (5), 1349–1359. [DOI] [PubMed] [Google Scholar]
- (21).Cheng H; Jiang W; Phillips FM; Haydon RC; Peng Y; Zhou L; Luu HH; An N; Breyer B; Vanichakarn P; Szatkowski JP; Park JY; He TC Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). Journal of bone and joint surgery. American volume 2003, 85 (8), 1544–52. [DOI] [PubMed] [Google Scholar]
- (22).Leblanc E; Drouin G; Grenier G; Faucheux N; Hamdy R. From skeletal to non skeletal: The intriguing roles of BMP-9: A literature review. Adv. Biosci. Biotechnol 2013, 04 (10), 16. [Google Scholar]
- (23).Rivera J; Strohbach C; Wenke J; Rathbone C. Beyond osteogenesis: an in vitro comparison of the potentials of six bone morphogenetic proteins. Front. Pharmacol 2013, 4 (125), 1 DOI: 10.3389/fphar.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Fuchigami S; Nakamura T; Furue K; Sena K; Shinohara Y; Noguchi K. Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur. J. Oral Sci 2016, 124 (2), 151–7. [DOI] [PubMed] [Google Scholar]
- (25).Wang Y; Hong S; Li M; Zhang J; Bi Y; He Y; Liu X; Nan G; Su Y; Zhu G; Li R; Zhang W; Wang J; Zhang H; Kong Y; Shui W; Wu N; He Y; Chen X; Luu HH; Haydon RC; Shi LL; He TC; Qin J. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J. Orthop. Res 2013, 31 (11), 1796–803. [DOI] [PubMed] [Google Scholar]
- (26).Nie L; Yang X; Duan L; Huang E; Pengfei Z; Luo W; Zhang Y; Zeng X; Qiu Y; Cai T; Li C. The healing of alveolar bone defects with novel bio-implants composed of Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci. Rep 2017, 7 (1), 6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kang Q; Sun MH; Cheng H; Peng Y; Montag AG; Deyrup AT; Jiang W; Luu HH; Luo J; Szatkowski JP; Vanichakarn P; Park JY; Li Y; Haydon RC; He TC Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312. [DOI] [PubMed] [Google Scholar]
- (28).Zou Y; Qazvini NT; Zane K; Sadati M; Wei Q; Liao J; Fan J; Song D; Liu J; Ma C; Qu X; Chen L; Yu X; Zhang Z; Zhao C; Zeng Z; Zhang R; Yan S; Wu T; Wu X; Shu Y; Li Y; Zhang W; Reid RR; Lee MJ; Wolf JM; Tirrell M; He T-C; de Pablo JJ; Deng Z-L Gelatin-Derived Graphene−Silicate Hybrid Materials Are Biocompatible and Synergistically Promote BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2017, 9 (19), 15922–15932. [DOI] [PubMed] [Google Scholar]
- (29).Phillips JE; Gersbach CA; García AJ Virus-based gene therapy strategies for bone regeneration. Biomaterials 2007, 28 (2), 211–229. [DOI] [PubMed] [Google Scholar]
- (30).Bergeron E; Leblanc E; Drevelle O; Giguere R; Beauvais S; Grenier G; Faucheux N. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng., Part A 2012, 18 (3−4), 342–52. [DOI] [PubMed] [Google Scholar]
- (31).Grosso A; Burger MG; Lunger A; Schaefer DJ; Banfi A; Di Maggio N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol 2017, 5 (68), 1 DOI: 10.3389/fbioe.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Mantripragada VP; Jayasuriya AC Injectable chitosan microparticles incorporating bone morphogenetic protein-7 for bone tissue regeneration. J. Biomed. Mater. Res., Part A 2014, 102 (12), 4276–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gaihre B; Uswatta S; Jayasuriya AC Nano-scale characterization of nano-hydroxyapatite incorporated chitosan particles for bone repair. Colloids Surf., B 2018, 165, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Thirumala S; Gimble JM; Devireddy RV Methylcellulose Based Thermally Reversible Hydrogel System for Tissue Engineering Applications. Cells 2013, 2 (3), 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Gaihre B; Lecka-Czernik B; Jayasuriya AC Injectable nanosilica−chitosan microparticles for bone regeneration applications. J. Biomater. Appl 2018, 32 (6), 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lee K; Silva EA; Mooney DJ Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc., Interface 2011, 8 (55), 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Vo TN; Kasper FK; Mikos AG Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Delivery Rev 2012, 64 (12), 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Uchida S; Sakai A; Kudo H; Otomo H; Watanuki M; Tanaka M; Nagashima M; Nakamura T. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone 2003, 32 (5), 491–501. [DOI] [PubMed] [Google Scholar]
- (39).Ren X; Weisgerber DW; Bischoff D; Lewis MS; Reid RR; He TC; Yamaguchi DT; Miller TA; Harley BA; Lee JC Nanoparticulate Mineralized Collagen Scaffolds and BMP-9 Induce a Long-Term Bone Cartilage Construct in Human Mesenchymal Stem Cells. Adv. Healthcare Mater 2016, 5 (14), 1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bouyer M; Guillot R; Lavaud J; Plettinx C; Olivier C; Curry V; Boutonnat J; Coll J-L; Peyrin F; Josserand V; Bettega G; Picart C. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Li XL; Liu YB; Ma EG; Shen WX; Li H; Zhang YN Synergistic effect of BMP9 and TGF-beta in the proliferation and differentiation of osteoblasts. GMR, Genet. Mol. Res 2015, 14 (3), 7605–15. [DOI] [PubMed] [Google Scholar]
- (42).Lamplot JD; Qin J; Nan G; Wang J; Liu X; Yin L; Tomal J; Li R; Shui W; Zhang H; Kim SH; Zhang W; Zhang J; Kong Y; Denduluri S; Rogers MR; Pratt A; Haydon RC; Luu HH; Angeles J; Shi LL; He TC BMP9 signaling in stem cell differentiation and osteogenesis. Am. J. Stem Cells 2013, 2 (1), 1–21. [PMC free article] [PubMed] [Google Scholar]
- (43).Shui W; Zhang W; Yin L; Nan G; Liao Z; Zhang H; Wang N; Wu N; Chen X; Wen S; He Y; Deng F; Zhang J; Luu HH; Shi LL; Hu Z; Haydon RC; Mok JM; He TC Characterization of scaffold carriers for BMP9-transduced osteoblastic progenitor cells in bone regeneration. J. Biomed. Mater. Res., Part A 2014, 102 (10), 3429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Dumanian ZP; Tollemar V; Ye J; Lu M; Zhu Y; Liao J; Ameer GA; He T-C; Reid RR Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS One 2017, 12 (3), No. e0172327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Scott MA; Levi B; Askarinam A; Nguyen A; Rackohn T; Ting K; Soo C; James AW Brief review of models of ectopic bone formation. Stem Cells Dev. 2012, 21 (5), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ye J; Wang J; Zhu Y; Wei Q; Wang X; Yang J; Tang S; Liu H; Fan J; Zhang F; Farina EM; Mohammed MK; Zou Y; Song D; Liao J; Huang J; Guo D; Lu M; Liu F; Liu J; Li L; Ma C; Hu X; Haydon RC; Lee MJ; Reid RR; Ameer GA; Yang L; He TC A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells. Biomedical materials (Bristol, England) 2016, 11 (2), No. 025021. [DOI] [PubMed] [Google Scholar]
- (47).Kim HK; Shim WS; Kim SE; Lee KH; Kang E; Kim JH; Kim K; Kwon IC; Lee DS Injectable in situ-forming pH/ thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng., Part A 2009, 15 (4), 923–33. [DOI] [PubMed] [Google Scholar]
- (48).Liao HT; Chen CT; Chen JP Osteogenic differentiation and ectopic bone formation of canine bone marrow-derived mesenchymal stem cells in injectable thermo-responsive polymer hydrogel. Tissue Eng., Part C 2011, 17 (11), 1139–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.