Abstract
Language development starts during the fetal period when the brain is sensitive to endocrine disruptions from environmental contaminants. This systematic review aims to systematically summarize the existing literature on early-life exposure to PFAS and children’s language and communication development, which is an indicator of neurocognitive development. A structured literature search was conducted using three databases, PubMed, Scopus, and CINAHL, last updated in April 2023. The population was defined as children and young adults. PFAS exposure was assessed pre- or postnatally. The outcome was defined as a language and communication ability assessed with validated instruments, parental self-reports, or clinical language disorder diagnoses. In total, 15 studies were identified for subsequent analyses. Thirteen were performed in background-exposed populations and two in highly exposed populations. There were some indications of potential adverse effects; however, these were not consistent across child sex, age of assessment, or PFAS exposure levels. No systematic effect of early-life PFAS exposure on language and communication development was found. These inconclusive findings may partly be explained by the use of general test instruments with limited validity as to children’s language and communication development. Further studies over a wider exposure range using specific language test instruments are needed.
Keywords: child language development, developmental language disorder, environmental contaminants, perfluoroalkyl substances, prenatal exposure, postnatal exposure
1. Introduction
Children’s language, cognitive, and motor development follows universal milestones and develops in parallel [1]. Language development starts during the fetal period [2,3,4] and continues through preschool age [5] and well into adolescence [6].
Developmental language disorder (DLD) is one of the most common developmental disorders and affects 7–8% of all children [7,8]. The aetiology of DLD is multifactorial, involving both genetic and environmental risk factors [9,10]. Children with DLD have significant difficulties in one or more areas of spoken or written language, comprehension, and communication, including social interactions. If delayed or disordered language and communication persist at 5 years of age, the condition is considered permanent [9,11], and it is likely to be a risk factor for adverse social and educational performance [12]. Language and communication development is an important part of children’s general development [7,13,14,15]. Late language development or DLD is subsequently associated with other neurodevelopmental disorders and co-occurs with attention-deficit/hyperactivity disorder (ADHD), autism, and intellectual developmental disabilities [14,16,17].
Many hormones affect the development of the fetal brain [18], and prenatal language development is sensitive to the influence of sex hormones [19,20,21]. Early-life exposure to endocrine-disrupting chemicals may, thus, harm the developing brain [22,23,24,25,26]. Adverse effects can occur if exogenous substances pass across the placenta and reach fetal circulation or if they interfere with normal placenta functioning [24]. Exposure to endocrine-disrupting environmental contaminants such as polychlorinated biphenyls, lead, methylmercury, polybrominated diphenyl ethers, and organophosphates have been associated with the impaired development of both receptive (i.e., understanding of words and sentences) and expressive language (i.e., decreased mean length of utterances) [27].
A group of endocrine-disrupting contaminants that are widely spread in the environment and for which human exposure is ubiquitous are perfluoroalkyl substances (PFAS) [28,29]. PFASs are synthetically produced chemicals that are extremely stable and accumulate in humans [30,31,32,33,34]. PFAS transfers effectively from mother to child during pregnancy and lactation [32,33,34,35,36] and may interfere with thyroid functions that are essential for brain development [36].
Animal studies have indicated developmental toxicity as a sensitive target after prenatal PFAS exposure [37]. While a few epidemiological studies have reported adverse associations between PFAS serum levels and general neurodevelopment [36,38,39], the overall evidence is inconsistent [37]. These previous studies have focused on the potential impact of PFAS on children’s general development, but there has been a lack of studies on the impact of children’s language and communication, which is an important indicator of neurocognitive development. Therefore, we performed a systematic review of the existing literature on pre- and postnatal exposure to PFAS with a focus on children’s language and communication development.
2. Materials and Methods
We performed a systematic review according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) model [40] and the Swedish Agency for Health Technology Assessment and Assessment of Social Services (2017) [41].
We preregistered the study protocol at the Open Science frameworks on 29 October 2020, https://osf.io/y45ez/.
2.1. Eligibility Criteria and Research Definition
We applied the Population–Exposure–Comparison–Outcome model (PECO) [42] to define our research question. The population was defined as children and young adults aged 0–21 years. Exposure was assessed as measured PFAS concentrations or as residential history in a highly exposed population. To this, we added outcomes for language and communication ability based on the use of valid test instruments, parental self-reports, or clinical diagnoses. We included original peer-reviewed scientific studies published in the English language.
2.2. Information Sources and Search Methods
A stepwise literature search was performed with the help of two trained librarians at Gothenburg University Library to determine search concepts in the following three areas: (1) language and communication, including stuttering, voice, and swallowing, (2) neurodevelopmental ability problems where an assessment of language and communication abilities may be included and (3) PFAS and terms related to PFAS substances. The first search in PubMed, Scopus, and CINAHL was performed by the librarians on 16 June 2020. The specification for all databases and search concepts is available in a Supplementary File (see Supplementary File S1). An updated search to capture recently published papers was performed in April 2023, and five new records were added. During a pre-search, we found no study with language or communication as the primary study outcome using specific language test instruments. Therefore, the broader search concept was used to include proxy information about language and communication domains through instruments assessing children’s general development. Moreover, reviews and systematic reviews were checked for references that might be of interest.
2.3. Study Selection
The relevance assessment of record titles and abstracts that included PFAS as the exposure was first conducted by two speech and language pathologists independently, with a pre-specified intention to “include rather than exclude”. For inclusion, we required an assessment of children’s language and communication and an assessment of PFAS. In the next step, an epidemiologist and a researcher in environmental medicine scrutinized the titles and abstracts that were included to assess whether the exposure assessments and study designs seemed to be relevant.
2.4. Data Extraction
We extracted the following metadata from the articles: the author, year, title, continent, country or state, possible connection to other screened articles, aim, population, study design, the name of study or study cohort, ages, counts, gender distribution, and the developmental domains assessed (e.g., cognitive, motor, language, and communication). In addition, information on which test instrument was used for the assessment of language and communication and if this assessment was comprehensive or only a part of broader cognitive testing was extracted. We noted reported PFAS substances, sampling periods (e.g., gestational week and child age), and matrix (whole blood, serum, plasma, or breast milk) or modeled exposure. We focused on the legacy PFAS perfluorohexane sulfonic acid (PFHxS), perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorononanoic acid (PFNA).
An additional search in the Supplementary Material was also performed to obtain the information needed for decisions on inclusion and quality assessment. The validation of data followed the extraction of data, and during this process, decisions for inclusion and exclusion were made. Specific information on outcomes related to language ability was extracted from the main and supplementary text, tables, and figures. The summary measures of the results, i.e., medians, means, risk ratio, odds ratio, hazard ratio, and standard deviation, were extracted from the main and Supplementary Material.
2.5. Risk of Bias Assessment
The assessment was completed using the Critical Appraisal Skills Programme and checklist for cohorts [43]. When necessary, additional information was added from supplements. All questions in the checklist were answered, but we primarily focused on a quality assessment concerning the following questions:
-
-
“Were the participants recruited acceptably?”
-
-
“Was the exposure accurately measured to minimize bias?”
-
-
“Was the outcome accurately measured to minimize bias?”
-
-
“Have the authors identified all the important confounding factors?”
-
-
“Have they taken into account the confounding factors in the design and/or analysis?”
3. Results
3.1. Study Selection
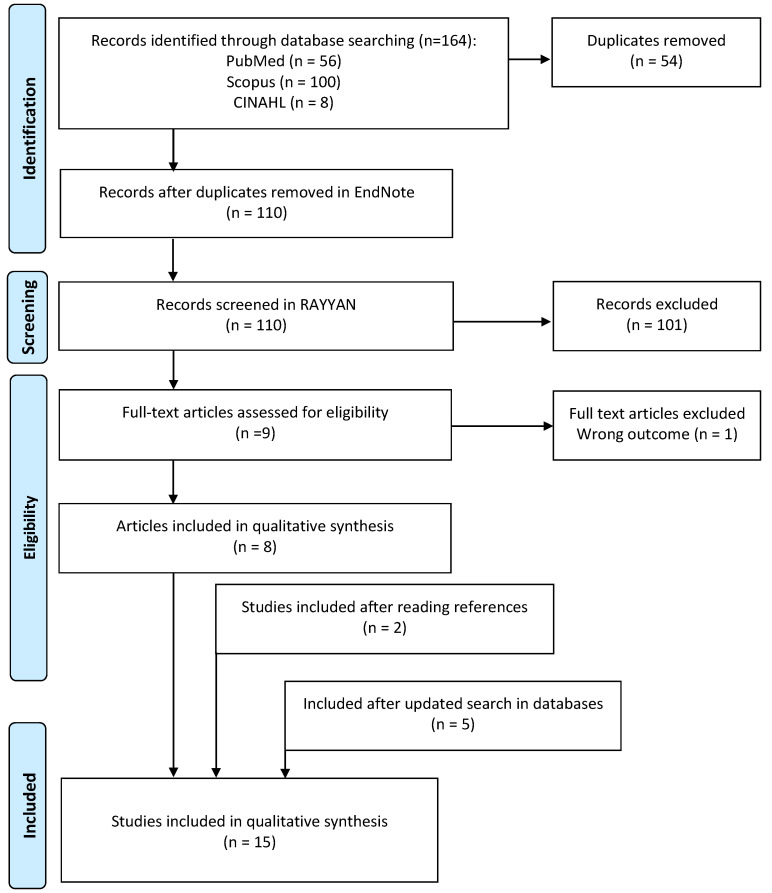
The final study sample consisted of 15 studies (Table 1) published between 2013 and 2023. The initial literature search identified 164 records, and after the removal of 54 duplicates via EndNote 20, [44], the remaining 110 titles and abstracts were imported to the Rayyan review app [45]. After screening titles and abstracts, there were nine articles imported to EndNote in full text. We performed full-text screening for relevance. During this process, one article was excluded because the outcome was irrelevant to the research question. After searching the reference lists of the included articles, we identified and included two additional studies. An updated literature search in April 2023 added five articles that had been published after the initial search. This process is shown in the PRISMA flow diagram (Figure 1).
Figure 1.
PRISMA flow chart diagram of study selection.
3.2. Study Characteristics
Twelve studies were population-based birth cohorts (Table 1), all with maternal pregnancy or cord blood samples (Table 2). In addition, two studies collected childhood serum samples.
Three studies were initiated in response to a point source of exposure. One was a birth cohort that was part of a program collecting data on contaminants and health outcomes after the collapse of the World Trade Center [46]. Two studies were part of large research programs assessing health effects after exposure to contaminated drinking water. The first was performed within the C8 Study Panel framework with exposure dominated by PFOA [47]. The second was part of the Ronneby PFAS Research Program, with exposure dominated by PFOS and PFHxS [48,49]. Both studies included prenatal exposure assessments.
Thirteen studies focused on children’s general cognitive development and included verbal subscales (Table 3), whereas two studies had a specific focus on language and communication development (i.e., Jeddy et al., 2017 and Stübner et al., 2023) [49,50].
The publications were from three continents, including six from Asia (China, Japan, Taiwan), five from Europe (Denmark, England, Norway, Spain, Sweden), and four from the U.S. The number of participants ranged from 120 to 11,895. Two publications reported data from the same cohort at different ages of follow-up [51,52].
One study included only girls [50]. In the remaining studies, there was an even distribution between the sexes.
Table 1.
General characteristics of studies included in the systematic review.
| Author Year | Geographical Area | Cohort | Enrollment Year |
Age 1 at Outcome Assessment | Number of Participants 2 | Sex Distribution boys, % |
|---|---|---|---|---|---|---|
| Carrizosa et al., 2021 [53] |
Europe, Spain |
INfancia y Medio Ambiente (Environment and Childhood) (INMA) |
2003–2008 | 14 months 4–5 years |
1131 1192 |
51 |
| Chen et al., 2013 [54] |
Asia, Taiwan |
Taiwan Birth Panel Study (TBPS) | 2004–2005 | 2 years | 239 | 55 |
| Goudarzi et al., 2016 [55] |
Asia, Japan |
The Hokkaido Study on Environment and Children’s health | 2002–2005 | 6 months 18 months |
173 133 |
48 50 |
| Harris et al., 2018 [56] |
USA, Boston |
Project Viva | 1999–2002 | 3 years 7 years |
986 865 |
52 52 |
| Jeddy et al., 2017 [50] |
Europe, England |
The Avon Longitudinal Study of Parents and Children (ALSPAC) | 1991–1992 | 15 months 38 months |
432 | 0 |
| Liew et al., 2018 [57] |
Europe, Denmark |
Lifestyle During Pregnancy Study nested within the Danish National Birth Cohort | 2003–2008 | 5 years | 1592 | 52 |
| Luo et al., 2022 [51] |
Asia, China |
Shanghai Birth Cohort (SBC) | 2013–2016 | 2 years | 2257 | 51 |
| Oh et al., 2022 [58] |
Asia, Japan |
Hamamutsu Birth Cohort (HBC Study) | 2007–2012 | Eight times between 4 to 40 months |
~550 | 52 |
| Skogheim et al., 2020 [59] |
Europe, Norway |
ADHD Study nested within the Norwegian Mother, Father, and Child Cohort Study | 1999–2008 | 3.5 years | 944 | 51 |
| Spratlen et al., 2020 [46] |
USA, New York |
A cohort with prenatal exposure to the World Trade Center disaster | 2001–2002 | 1 year 2 years 3 years 4 years 6 years |
156 157 127 124 110 |
50 |
| Stein et al., 2013 [47] |
USA, West Virginia, and Ohio |
A child subcohort from the C8 Health Project | 2005–2010 | 6–12 years (mean age 10 years) |
320 | 47 |
| Stübner et al., 2023 [49] |
Europe, Sweden |
Register-based cohort study in Ronneby, Sweden | 1998–2013 | 3.2–4.6 years | 11,895 | 54 |
| Vuong et al., 2019 [60] |
USA, Ohio |
Health Outcomes and Measures of the Environment (HOME) | 2003–2006 | 8 years | 221 | 45 |
| Wang et al., 2015 [38] |
Asia, Taiwan |
The Taiwan Maternal and Infant Cohort Study | 2000–2001 | 5 years 8 years |
120 120 |
52 3 50 |
| Wang et al., 2023 [52] |
Asia, China |
Shanghai Birth Cohort | 2013–2016 | 4 years | 2031 | 52 |
1 For studies with repeated outcome assessments, the number of participants at each assessment is given. 2 No numbers for sex at each age are reported. 3 Only information about means for the first speech and language pathologist visit was reported.
Table 2.
Exposure characteristics, including the PFAS sampling period, matrix, and exposure levels for PFOA, PFOS PFHxS, and PFNA.
| Author Year |
PFAS Included in Statistical Analysis | Sampling Period | Matrix | PFOA (ng/mL) | PFOS (ng/mL) | PFHxS (ng/mL) | PFNA (ng/mL) |
|---|---|---|---|---|---|---|---|
| Carrizosa et al., 2021 [53] | PFOA, PFOS, PFHxS, PFNA | Trimester 1 | Maternal plasma | Median (IQR) 2.4 (1.6, 3.3) |
Median (IQR) 6.1 (4.4, 7.8) |
Median (IQR) 0.6 (0.4, 0.8) |
Median (IQR) 0.7 (0.5, 0.9) |
| Chen et al., 2013 [54] |
PFOA, PFOS | Delivery | Plasma from cord blood | Mean (SD) 2.5 (2.6) |
Mean (SD) 7.0 (5.8) |
- | - |
| Goudarzi et al., 2016 [55] |
PFOA, PFOS | After 2nd trimester | Maternal serum | Median (IQR) 1.2 (0.8, 1.7) |
Median (IQR) 5.7 (4.4, 7.4) |
- | - |
| Harris et al., 2018 [56] |
PFOA, PFOS, PFHxS, PFNA, PFDeA, MeFOSAA, EtFOSAA, |
Trimester 1, 2 Postnatal |
Maternal plasma Child plasma midchildhood at Median age 7.7 years (6.6–10.9 year) |
Maternal Median (IQR) 5.6 (4.1, 7.7) Childhood Median (IQR) 4.4 (3.1, 6.0) |
Maternal Median (IQR) 24.9 (18.4, 34.4) Childhood Median (IQR) 6.2 (4.2, 9.7) |
Maternal Median (IQR) 2.4 (1.6, 3.7) Childhood Median (IQR) 1.9 (1.2, 3.4) |
Maternal Median (IQR) 0.6 (0.5, 0.9) Childhood Median (IQR) 1.5 (1.1, 2.3) |
| Jeddy et al., 2017 [50] |
PFOA, PFOS, PFNA, PFHxS, | Median week 15 | Maternal serum | Median (IQR) 3.7 (2.8, 4.8) |
Median (IQR) 19.8 (15.0, 24.95) |
Median (IQR) 1.6 (1.2, 2.2) |
Median (IQR) 0.5 (0.4, 0.7) |
| Liew et al., 2018 [57] |
PFOA, PFOS, PFHxS, PFNA, PFHpS, PFDA, PFOSA |
Trimester 1 | Maternal plasma | Median (IQR) 4.3 (3.2, 5.5) |
Median (IQR) 28.1 (21.6, 35.8) |
Median (IQR) 1.1 (0.8, 1.4) |
Median (IQR) 0.46 (0.36, 0.57) |
| Luo et al., 2022 [51] |
PFOA, PFOS, PFHxS, PFNA PFHpA, PFDeA, PFUnDA, PFDoA, PFBS |
Trimester 1 | Maternal plasma | Median (IQR) 11.90 (9.30, 15.20) |
Median (IQR) 9.56 (6.65, 13.87) |
Median (IQR) 0.54 (0.42, 0.68) |
Median (IQR) 1.74 (1.25, 2.39) |
| Oh et al., 2022 [58] |
PFOA, PFOS | Postnatal | Serum from cord blood | Median (IQR) 1.2 (0.8, 1.8) |
Median (IQR) 1.2 (0.9, 1.7) |
- | - |
| Skogheim et al., 2020 [59] |
PFOA, PFOS, PFHxS, PFNA, PFHpS, PFDA, PFUnDA |
Trimester 2 | Maternal plasma | Median (IQR) 2.5 (1.8, 3.2) |
Median (IQR) 11.5 (8.8, 14.8) |
Median (IQR) 0.7 (0.5, 0.9) |
Median (IQR) 0.4 (0.3, 0.5) |
| Spratlen et al., 2020 [46] |
PFOA, PFOS, PFHxS, PFNA | Delivery | Serum from cord blood and maternal blood day after delivery | Geometric Mean (Range) Cord: 2.31 (0.18, 8.14) Maternal 2.42 (0.88, 5.06) |
Geometric Mean (Range) Cord: 6.03 (1.05, 33.7) Maternal: 11.9 (2.90, 30.9) |
Geometric Mean (Range) Cord: 0.67 (0.08, 15.8) Maternal: 0.94 (0.35, 3.20) |
Geometric Mean (Range) Cord: 0.43 (<LOQ, 10.3) Maternal: 0.43 (<LOQ, 10.3) |
| Stein et al., 2013 [47] |
PFOA | Estimated prenatal Postnatal |
Modeled prenatal exposure Serum collected postnatally at mean age 5.7 years |
Estimated in utero mean (SD) 115.9 (164.6) Childhood mean (SD) 91.1 (139.8) |
Measured childhood mean (SD) 21.1 (13.3) | Measured childhood mean (SD) 9.8 (14.1) |
Measured childhood mean (SD) 1.9 (1.1) |
| Stübner et al., 2023 [49] | PFOA, PFOS, PFHxS | Estimated early life | Maternal residential history, i.e., with or without highly PFAS-contaminated drinking water, during the five-year period before childbirth was used as a proxy for early-life exposure. | High exposure Median 9 ng/mL Intermediate Median 3 ng/mL Background Median 2 ng/mL |
High exposure Median 169 ng/mL Intermediate Median 48 ng/mL Background Median 4 ng/mL |
High exposure Median129 ng/mL Intermediate Median 40 ng/mL Background Median 0.8 ng/mL |
- |
| Vuong et al., 2019 [60] |
PFOA, PFOS, PFHxS, PFNA | Trimester 2 or 3 Postnatal |
Maternal serum, gestation week 16 ± 3, or 26, or within 24 h of parturition. If more than one measure were taken, an average of were used. Child serum, 3 and 8 years of age |
Prenatal 2nd Tertile <3.9 4.0, 6.3 ≥ 6.4 3 years 2nd Tertile <4.1 4.1, 6.7 ≥ 6.8 8 years 2nd Tertile <2.0 2.0, 2.8 ≥ 2.9 |
Prenatal 2nd Tertile <10.0 10.0, 15.6 ≥ 15.7 3 years 2nd Tertile <5.0 5.0, 7.9 ≥ 8.0 8 years 2nd Tertile <3.0 3.0, 4.5 ≥ 4.6 |
Prenatal 2nd Tertile <0.9 0.9, 1.8 ≥ 1.9 3 years 2nd Tertile <1.2 1.2, 2.4 ≥ 2.5 8 years 2nd Tertile <1.0 1.0, 1.5 ≥ 1.6 |
Prenatal 2ndTertile <0.7 0.7, 0.9 ≥ 1.0 3 years 2nd Tertile <1.0 1.0, 1.5 ≥ 1.6 8 years 2nd Tertile <0.6 0.6, 0.8 ≥ 0.9 |
| Wang et al., 2015 [38] |
PFOA, PFOS, PFHxS, PFNA, PFDeA, PFUnDA, PFDoDA |
Trimester 3 | Maternal serum | 5 years Median (IQR) 2.5 (1.5, 3.4) Geometric mean (95% CI) 2.0 (1.8, 2.3) 8 years Median (IQR) 2.5 (1.5, 3.3) Geometric mean (95% CI) 2.0 (1.7, 2.3) |
5 years Median (IQR) 13.3 (9.8, 17.5) Geometric mean (95% CI) 11.9 (10.4–13.6) 8 years Median (IQR) 12.3 (9.5, 16.3) Geometric mean (95% CI) 11.5 (10.2, 13.1) |
5 years Median (IQR) 0.7 (0.1, 1.1) Geometric mean (95% CI) 0.4 (0.3, 0.6) 8 years Median (IQR) 0.7 (0.1, 1.1) Geometric mean (95% CI) 0.5 (0.4, 0.6) |
5 years Median (IQR) 1.6 (0.8, 2.4) Geometric mean (95% CI) 1.4 (1.2, 1.7) 8 years Median (IQR) 1.4 (0.8, 2.3) Geometric mean (95% CI) 1.3 (1.1, 1.6) |
| Wang et al., 2023 [52] | PFOA, PFOS, PFHxS, PFNA, PFHpA PFDeA, PFUnDA, PFDoA, PFBS |
Trimester 1-2 | Maternal plasma | Median (IQR) 13.1 (9.4, 15.5) |
Median (IQR) 11.3 (6.7, 13.7) |
Median (IQR) 0.6 (0.4, 0.7) |
Median (IQR) 2.1 (1.3, 2.5) |
Perfluoroalkyl substances (PFAS), Perfuorooctanoic acid (PFOA), Perfluorooctane sulfonic acid (PFOS), Perfluorohexane sulfonic acid (PFHxS), Perfluorononanoic acid (PFNA), Perfluoroundecanoic acid (PFUnDA),Perfluorododecanoic acid FDoDA/PFDoA), Perfluorodecanoic acid (PFDA/PFDeA), Perfuoroheptane sulfonic acid (PFHpS), Methyl perfluorooctane sulfonamido acetate (MeFOSAA), Ethyl perfluorooctane sulfonamido acetate (EtFOSAA), Perfluorooctane sulfonamide (FOSA/PFOSA), Perfluoroheptanoic acid (PFHpA), Perfluorobutane sulfonate (PFBS), Perfluorododecanoic acid (PFDoDA/PFDoA) interquartil range (IQR), confidence interval (CI), standard deviation (SD).
3.3. Exposure
The exposure characteristics of the studies are provided in Table 2. Fourteen studies relied on measured PFAS levels, albeit in different matrices, i.e., whole blood, serum, or plasma. The time of sampling varied between the first pregnancy trimester up to the age of 11 years. Eight studies relied on maternal exposure measurements and two on measurements in the cord blood, whereas four studies used a combination of maternal and cord/child measurements. All studies investigated exposure to PFOA, including fourteen for PFOS, eleven for PFHxS, and ten for PFNA. Three of the studies at background PFAS exposures had higher serum levels than the others [50,56,57]. Two studies investigated highly exposed populations living in areas with contaminated drinking water. One was dominated by PFOA exposure, assessed by measured child PFAS levels, and modeled maternal exposure levels during pregnancy [47]. The second study used maternal residential history and the municipal distribution of contaminated drinking water over time to construct a proxy variable of prenatal exposure and assessed its validity against measured serum levels in a smaller dataset [49]. Here, exposure to PFOS and PFHxS dominated to a lesser extent than PFOA.
3.4. Outcome Assessment
The child’s age at the outcome assessment ranged from 4 months to 12 years. Seven studies performed repeated outcome assessments over periods spanning from 4 months up to 8 years (Table 1).
Thirteen studies used objective instruments assessing children’s general cognitive abilities with subscales for language and communication (Table 3). One study used a valid parental questionnaire targeting children’s vocabulary, which was developed specifically to capture language and communication development [50], while another study included preschool teacher reporting [59]. Harris et al. [56] used a specific language test instrument assessing vocabulary comprehension as a language sub-domain in their 3-year assessment. Finally, one study defined the clinical outcome of a developmental language disorder according to diagnostic codes (International Classification of Disorders, tenth revision, (ICD-10) [61], set by speech and language pathologists, using data from an administrative healthcare register with the population coverage of children that had been referred to the regional speech and pathology clinic by child nurses performing routine language screening at child health care centers [49].
Table 3.
Characteristics of outcome assessment tests or questionnaires including the domains tested and subtests including language and communication.
| Acronym | Outcome Test | Developmental Domains Tested | Scoring 1 | Subtests Including Language and Communication Domains | Validated Age Range | Used by |
|---|---|---|---|---|---|---|
| BSID-I | Bayley Scales of Infant and Toddler Development (Bayley, 1969, ref 1993) [62] |
Developmental functioning, mental scale and motor scale | Standard scores (M = 100, SD = 15) |
Receptive and expressive language presented in the mental scale | 3–28 months | Carrizosa et al., 2021 [53] |
| BSID-II | Bayley Scales of Infant and Toddler Development -2nd Edition, (Bayley, 1993) [6] | Developmental functioning, mental developmental index [MDI]) and motor development psychomotor developmental index (PDI) | Standard scores (M = 100, SD = 15) |
Receptive and expressive language presented in the mental developmental index (MDI) | 1–42 months | Goudarzi et al., 2016 [55] Spratlen et al., 2020 [46] |
| BSID-III | Bayley Scales of Infant and Toddler Development –Third Edition, Chinese version, (Hua et al., 2019, Yue et al., 2019) [63,64] | Developmental functioning in five domains, cognitive, language, motor, social–emotional, and adaptive behavior scales. | Standard scores (M = 100, SD = 15) |
Receptive and expressive communication | 1–42 months | Luo et al., 2022 [51] |
| CDIIT | Comprehensive Developmental Inventory for Infants and Toddlers (Liao et al., 2008) [65] | Developmental areas cognition, language, motor, social, and self-care skills | Standard scores (M = 100, SD = 15) |
62 items (language) | 3–71 months | Chen et al., 2013 [54] |
| KBIT-2 | Kaufman brief intelligence test–second edition (KBIT-2) (Kaufman, 2004) [66] | Cognitive ability and processing skills | Standard scores (M = 100, SD = 15), age equivalents, and percentile ranks |
Verbal standard score consists of verbal knowledge, answers given by pointing to pictures. For the riddles subtest, answers given by pointing to a picture or saying a word | 4–90 years | Harris et al., 2018 [56] |
| MSEL | Mullen scale of early learning (Mullen, 1995) [67] |
Visual reception, fine motor, receptive language, and expressive language | T-scores (M = 50, SD = 10), percentile ranks, and age equivalents for each of the five domains and the single composite (M = 100, SD = 15) |
Expressive language, and receptive language | 0–68 months | Oh et al., 2022 [58] |
| MSCA | McCarthy Scales of Children’s Abilities, (Kaufman and Kaufman, 1977) [68] |
Cognitive ability | Standard scores (M = 100, SD = 15) |
Verbal scale including subtests of pictorial memory, word knowledge, verbal memory, verbal fluency, and opposite analogies |
2–8 years | Carrizosa et al., 2021 [53] |
| NEPSY-II | Developmental Neuropsychological Assessment 2 edition, NEPSY-II, (Korkman et al., 2007) [69] | Neurocognitive processes, 32 subtests for use in a neuropsychological assessment with preschoolers, children, and adolescents. | Scale scores (1–19, M = 10, SD = 3) |
Body part naming and identification comprehension of instructions, Oro motor sequences, phonological processing, the repetition of nonsense words, speeded naming and word generation | 3–16 years | Stein et al., 2013 [47] |
| PPVT-III | Peabody Picture Vocabulary Test, (Dunn, 1997) [70] | Vocabulary | Raw scores to percentile ranks, age equivalents, or standard scores (M = 100, SD = 15) |
Receptive vocabulary | 2–90 years | Harris et al., 2018 [65] |
| SB-5 | Stanford-Binet Intelligence Scale, Fifth Edition, (Roid, 2003) [71] | Cognitive strengths and weaknesses | Standard scores (M = 100, SD = 15) scaled scores (M = 10, SD = 3), percentile scores, confidence intervals, age equivalents |
Verbal fluid reasoning, verbal knowledge, verbal quantitative reasoning, verbal visual-spatial processing, verbal working memory | 2–85 year | Skogheim et al., 2020 [59] |
| WASI | The Wechsler Abbreviated Scale of Intelligence, (Wechsler, 1999) [72] | General intellectual ability | Standard scores (M = 100, SD = 15) |
Vocabulary, similarities | 6–90 year | Stein et al., 2013 [47] |
| WPPSI-R | Wechsler Preschool and Primary Scale of Intelligence–Revised, (Wechsler, 1990) [73] | Intellectual ability | Standard scores (M = 100, SD = 15) |
Verbal scale subtests: information, comprehension, arithmetic, vocabulary, similarities, and sentences | 3–7 years | Liew et al., 2018 [57] Spratlen et al., 2020 [46] Wang et al., 2015 [38] (5 year assessment) |
| WPPSI-IV | Wechsler Preschool and Primary Scale of Intelligence (Wechsler, 2012) [74] | Intellectual ability | Standard scores (M = 100, SD = 15) |
Verbal scale subtests: information, similarities | 2–7 years | Wang et al., 2023 [52] |
| WISC III | Wechsler Intelligence Scale for Children 3rd. edition, (Wechsler, 1991) [75] | General cognitive ability | Standard scores (M = 100, SD = 15) |
The verbal scale mandatory subtests: information, similarities, arithmetic, vocabulary, and comprehension. The supplementary subtest: digit span | 6–16 year | Wang et al., 2015 [38] (8 year assessment) |
| WISC-IV | Wechsler Intelligence Scale for Children 4th edition, (Wechsler, 2003) [76] | General cognitive ability | Standard scores (M = 100, SD = 15) |
The verbal scale core subtest: similarities, vocabulary, comprehension, supplementary: information, word reasoning | 6–16 year | Vuong et al., 2019 [60] Stein 2013 [47] |
| Outcome questionnaire | ||||||
| CDI | Child Development Inventory, (Ireton and Glascoe, 1995) [77] | Teacher questionnaires. Measure the child’s present development in eight areas. Include general Development Scale and items to identify parent’s concerns about child’s health and growth, vision and hearing, development, and behavior | Percentile scores, age equivalents | Expressive language | 15 months– 6 years |
Skogheim et al., 2020 [59] |
| MCDI/MB-CDI | MacArthur-Bates Communicative Development Inventories, Second Edition, (Fenson et al., 2007) [78] |
Parent questionnaire. Evaluate communication in young children | Percentile scores | Communicative skills, comprehension, early vocabulary, and early grammar | 8–30 months | Jeddy et al., 2017 [50] |
1. Higher scores indicate better performance in all tests and questionnaires. Bayley Scales of Infant and Toddler Development—2nd Edition (BSID-II), Comprehensive Developmental inventory for infants and toddlers, diagnostic test (CDIIT), Kaufman Assessment Battery for Children, Second Edition (KBIT-2), McCarthy Scales of Children’s Abilities (MSCA), Developmental NEuroPSychological Assessment, 2nd edition (NEPSY-II), Peabody Picture Vocabulary Test (PPVT-III), Stanford–Binet Intelligence Scale, Fifth Edition (SB-5), The Wechsler Abbreviated Scale of Intelligence (WASI), Wechsler Preschool and Primary Scale of Intelligence–Revised (WPPSI-R), Wechsler Intelligence Scale for Children, 3rd. edition (WISC III), Wechsler Intelligence Scale for Children, 4th edition (WISC-IV), Child Development Inventory (CDI), MacArthur-Bates Communicative Development Inventories, Second Edition (MCDI or MB-CDI), * Maternal translation occurred if primary language was not English or Chinese.
3.5. Association between PFAS Exposure and Language and Communication Outcomes
The findings of the included studies are summarized in Table 4.
Among the 13 studies with background PFAS exposure levels, the majority found no statistically significant associations. Two studies reported better language ability at higher PFAS levels [53,57]. Adverse associations were reported by Luo, Chen, Yu, Huo, Wang, Nian, Tian, Xu, Zhang, and Zhang [51], and two studies reported both positive and negative effect estimates [50,58].
The cohort established after the World Trade Center disaster [46] exhibited PFAS levels comparable to the background exposure cohorts, albeit with a more complex situation of chemical and psychophysiological exposure for pregnant mothers. Here, no effect of PFAS exposure was seen for the verbal scales while simultaneously indicating better general neurodevelopment–mental outcomes with increasing PFAS levels.
Two studies investigated children with substantially higher PFAS exposure. A small study (n = 320) reported no associations between PFOA exposure and language-related test outcomes [47]. A large, registry-based study (n = 11,895) found an increased risk for DLD after high exposure dominated by PFOS and PFHxS, but only in girls [49].
Five of seven studies with repeated outcome measurements found different results between examinations [38,46,50,53,58]. Eight out of thirteen studies that reported results for several PFAS compounds showed consistent results between the compounds.
Effect modification by sex was found in four of the eleven studies where it was investigated. Three studies had indications of an adverse risk for girls [49,55,58], while Spratlen et al. (2020) [46] found a favorable effect in girls at the age of 2 years.
In summary, the majority of the studies did not demonstrate an association between PFAS and language and communication development, while seven reported favorable or adverse associations.
Table 4.
Summary of study results.
| Author Year |
Associations between PFAS Exposure and Language Development 1 | Overall Summary of Results 2 | Consistency between Outcome Assessment at Different Ages | Consistency between Repeated Exposure Measurements | Consistency between PFAS Compounds | Effect Modification by Sex | |
|---|---|---|---|---|---|---|---|
| Favorable 3 | Adverse 4 | ||||||
| Carrizosa et al., 2021 [53] | No associations at age 14 months for PFOS, PFHxS, PFOA, or PFNA. At age 4–5 years, a favorable association between PFOS and verbal subscale was also indicated for PFNA. |
Yes | No | No | n.a. 5 | No | No |
| Chen et al., 2013 [54] |
No association at age 2 years for PFOA or PFOS. | No | No | n.a. | n.a. | Yes | n.a. |
| Goudarzi et al., 2016 [55] |
No associations at age 6 and 18 years for PFOA or PFOS. | No | No | Yes | n.a. | Yes | Girls in highest PFOA quartile had a tendency to produce lower scores at 6 months, but not at 18 months |
| Harris et al., 2018 [56] | No associations at age 3 and 8 years between verbal IQ scores and PFOS, PFHxS, PFOA, or PFNA | No | No | Yes | Yes | Yes | No |
| Jeddy et al., 2017 [50] | At 15 months, there is a favorable association between verbal comprehension and vocabulary. At 38 months, both a favorable and adverse association for PFOS, PFHxS, PFOA, or PFNA is found. | Yes | Yes | No | n.a. | Yes | n.a. |
| Liew et al., 2018 [57] |
No associations for PFOS, PFHxS or PFOA at age 5 years. Indication of favorable association between verbal IQ score and PFNA at age 5 years. | Yes | No | n.a. | n.a. | No | No |
| Luo et al., 2022 [51] |
Adverse association between PFHxS and PFNA, but not PFOS and PFOA for language scores at age 2 years. | No | Yes | n.a. | n.a. | No | n.a. |
| Oh et al., 2022 [58] |
No associations were observed except an adverse association between PFOA and receptive language at 10 months and a favorable association between PFOS and expressive language at 24 and 32 months. Longitudinal changes in scores from 4 to 40 months of age indicated a favorable association between receptive and expressive language and PFOA and PFOS. |
Yes | Yes | No | n.a. | No | Effect modification with a worse outcome for girls at 10, 18 and 40 months, but not at other timepoints |
| Skogheim et al., 2020 [59] | No associations for PFOS, PFHxS, PFOA or PFNA at age 3.5 years. | No | No | n.a. | n.a. | Yes | No |
| Spratlen et al., 2020 [46] | No association between PFOS, PFHxS, PFOA or PFNA and MDI at age 1 year, but a tendency for favorable scores at age 2 and 3 years. No associations were observed for the verbal IQ score at age 4 years |
No | No | No | n.a. | Yes | Stronger positive association between PFOS and MDI for girls at age 2 years. |
| Stein et al., 2013 [47] |
No associations for PFOA at age 6–12 years. | No | No | n.a. | Yes | n.a. | No |
| Stübner et al., 2023 [49] | Adverse association between PFAS exposure and a clinical diagnosis of delayed language disorder in pre-school girls, but not in boys. | No | Yes | n.a. | n.a. | n.a. | Adverse effect in girls but not boys |
| Vuong et al., 2019 [60] | No associations for PFOS, PFHxS and PFOA and verbal comprehension at age 8 years. For PFNA, a trend for a favorable outcome was indicated. |
No | No | n.a. | No | No | No |
| Wang et al., 2015 [38] | No associations for PFOS, PFHxS, PFOA or PFNA at age 5 years. At 8 years, no associations except an adverse association for PFNA |
No | Yes | No | n.a. | No | n.a. |
| Wang et al., 2023 [52] | No associations for PFOS, PFHxS, PFOA or PFNA at age 4 years. | No | No | n.a. | n.a. | Yes | No |
1 The tests used are reported in Table 3. 2 Confidence interval for beta estimates either below or above 0 and a confidence interval for relative risk estimates not including 1.0 or p < 0.05. 3 Better outcomes at higher PFAS levels. 4 Worse outcomes at higher PFAS levels. 5 Not applicable.
3.6. Quality Assessment
3.6.1. Selection Bias
We identified no evident risk of selection bias from conditioning participation on both exposure and outcome in any of the studies. The study by Chen et al. [54], excluded children with physician-diagnosed neurodevelopmental disorders at age 2 years. The study by Skogheim et al. [59], had an enriched sample (20%) of children with ADHD symptoms at age 3 years according to parental interviews, and excluded a few children with high questionnaire scores on autistic symptoms. However, as only a very small proportion of children with neurodevelopmental disorders such as autism or ADHD receive a clinical diagnosis at such an early age [79], we assume the impact to be of minor importance for the generalizability of their results.
3.6.2. Information Bias
All PFAS analyses were performed in established laboratories.
The studies that used a modeled exposure assessment provided cross-validation between modeled and measured data [47,49]. Moreover, in these studies, the exposure levels spanned over a very wide range, which reduced the risk of exposure misclassification. By contrast, analytical uncertainty has a larger relative impact at low exposure levels.
There was a lack of information about blinding regarding exposure in three studies with objective outcome assessments [46,54,59] and in the study using parental questionnaires [50]. The remaining eleven studies with objective outcome assessments had assessors blinded to participants’ PFAS exposure levels.
Most studies relied on instruments assessing general cognitive abilities with verbal subscales. Although these tests can provide essential information about language difficulties, it is important to remember that such tests are not primarily designed to assess language ability.
3.6.3. Confounding
In general, studies were adjusted for the confounders that we a priori considered to be relevant (i.e., maternal age, socioeconomic status, parity, and smoking during pregnancy), and presented a theoretical ground as to why they were included. All studies presented both unadjusted and adjusted results in the main text or the Supplementary Materials.
4. Discussion
This systematic review identified fifteen studies that investigated the association between early-life exposure to PFAS (PFOA, PFOS, PFHxS, and PFNA) and language and communication development. Overall, there were no consistent findings for associations between early-life exposure to PFAS and language and communication development, neither adverse nor favorable. Sex dimorphic effects were reported in some studies, albeit in different directions.
All but two studies were performed in birth cohorts, with substantial variation concerning the timepoint for outcome assessments. The majority of studies used well-known, validated developmental or cognitive test instruments, capturing crude measures of language and speech domains as subtests within general test batteries. In marked contrast, one study relied on standardized expert diagnoses of DLD [49], thus detecting clinically important outcomes.
One explanation for the inconsistent findings might be related to the lack of specific language test instruments for outcome assessments. Although developmental tests or cognitive tests aim to assess the intellectual capacity of children and adolescents and can provide valuable information about possible language difficulties, these tests were not primarily designed to assess language difficulties. For example, children with expressive language disorder who struggle with grammar and speech sound pronunciation problems may still have full marks on these cognitive tests. It is also possible for children with reading and writing difficulties to pass the test because the tasks are administered and answered verbally. Thus, there is a risk that estimates may have become attenuated from outcome misclassification when general instruments are used.
Specific language test instruments are more sensitive to picking up nuances in language and communication, and they are, thus, expected to increase the validity of the outcome assessment. In addition, the use of caregiver questionnaires on language development can be a cost- and time-efficient method to capture important aspects of functional everyday communication [80,81]. This approach was used in two of the included studies [50,59].
An alternative strategy for outcome assessment is to use clinical developmental language disorder diagnoses to define the outcome when healthcare data are available. In Stübner et al. (2023) [49], the outcomes were defined as (a) routine language screening with validated instrument child healthcare nurses followed by referral to a speech and language pathology clinic and (b) a subsequent diagnosis set by a speech and language pathologist. An advantage of this approach is that it captures clinically relevant outcomes, but it requires routine healthcare data for the study population to be available.
Only two studies were based on cohorts with high exposure levels of PFAS, and they reported diverging findings.
The remaining 13 studies investigated background-exposed cohorts with small exposure contrasts. Thus, there is insufficient evidence to evaluate exposure–response relationships, particularly at intermediate exposure levels.
Self-selection in longitudinal cohorts is an inherent risk, and parents with better socioeconomic characteristics are more likely to enroll as well as have higher compliance over time [82]. In populations with background exposure, a higher PFAS exposure has been associated with higher parental socioeconomic status (SES) [83]. Given that language ability is positively associated with parental SES [84], the association between language ability and PFAS exposure risk is biased toward the null in background-exposed birth cohorts. In contrast, the study by Stübner et al. (2023) [49] used routinely collected administrative healthcare data to define the outcome status for the entire population. With such a study design, selection bias can be avoided.
5. Conclusions
We found no consistent association between early-life exposure to PFAS and language and communication development. Most previous research was performed in populations with background levels of exposure; the timepoint of exposure and outcome assessment varied substantially, and most studies used general cognitive instruments for outcome assessment. However, the observation of an increased risk for the clinical diagnosis of developmental language disorders in highly exposed girls is of concern. Thus, research at intermediate exposure levels is needed to clarify the exposure–response relationship between early-life PFAS exposure and speech and language development. In future studies, instruments developed to assess language and communication abilities, preferably in collaboration with speech- and language pathologists with clinical expertise, should be used.
Acknowledgments
The authors are grateful to Librarians Eva Hessman and Linda Hammarbäck at Gothenburg University for their help with building search concepts and guiding the search process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20247170/s1, Supplementary material File S1: Search terms.
Author Contributions
C.S.: Conceptualization, Data curation, Investigation, Writing—Original draft preparation, Visualization, Project administration. C.N.: Methodology, Supervision, Validation, Writing—Reviewing and Editing. K.J.: Methodology, Supervision, Validation, Writing—Reviewing and Editing. C.G.: Writing—Reviewing and Editing. C.M.: Conceptualization, Project administration, Supervision, Validation, Writing—Reviewing and Editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This article is based on publicly available data and the sources are provided in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work is supported by The Swedish Research Council for Health, Working Life and Welfare (FORTE) (grant number 2018-00289) to CM. In-kind support from the University of Gothenburg, Sweden and Lund University, Sweden.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lightfoot C.M., Cole S.R. Development of Children. 8th ed. Worth Publishers Inc.; New York, NY, USA: 2018. [Google Scholar]
- 2.Gervain J. Plasticity in early language acquisition: The effects of prenatal and early childhood experience. Curr. Opin. Neurobiol. 2015;35:13–20. doi: 10.1016/j.conb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Karmiloff K., Karmiloff-Smith A., Karmiloff K. Pathways to Language: From Fetus to Adolescent. Harvard University Press; Cambridge, MA, USA: 2009. [Google Scholar]
- 4.Moon C., Lagercrantz H., Kuhl P.K. Language experienced in utero affects vowel perception after birth: A two-country study. Acta Paediatr. 2013;102:156–160. doi: 10.1111/apa.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rescorla L., Mirak J. Normal language acquisition. Semin. Pediatr. Neurol. 1997;4:70–76. doi: 10.1016/S1071-9091(97)80022-8. [DOI] [PubMed] [Google Scholar]
- 6.Nippold M.A. Language development during the adolescent years: Aspects of pragmatics, syntax, and semantics. Top. Lang. Disord. 2000;20:15–28. doi: 10.1097/00011363-200020020-00004. [DOI] [Google Scholar]
- 7.Norbury C.F., Gooch D., Wray C., Baird G., Charman T., Simonoff E., Vamvakas G., Pickles A. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. J. Child Psychol. Psychiatry. 2016;57:1247–1257. doi: 10.1111/jcpp.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomblin J.B., Records N.L., Buckwalter P., Zhang X., Smith E., O’brien M. Prevalence of specific language impairment in kindergarten children. J. Speech Lang. Hear. Res. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop D.V.M., Snowling M.J., Thompson P.A., Greenhalgh T. Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J. Child Psychol. Psychiatry. 2017;58:1068–1080. doi: 10.1111/jcpp.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomblin J.B., Smith E., Zhang X. Epidemiology of specific language impairment: Prenatal and perinatal risk factors. J. Commun. Disord. 1997;30:325–344. doi: 10.1016/S0021-9924(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 11.Norbury C.F., Vamvakas G., Gooch D., Baird G., Charman T., Simonoff E., Pickles A. Language growth in children with heterogeneous language disorders: A population study. J. Child Psychol. Psychiatry. 2017;58:1092–1105. doi: 10.1111/jcpp.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stothard S.E., Snowling M.J., Bishop D.V.M., Chipchase B.B., Kaplan C.A. Language-impaired preschoolers: A follow-up into adolescence. J. Speech Lang. Hear. Res. 1998;41:407–418. doi: 10.1044/jslhr.4102.407. [DOI] [PubMed] [Google Scholar]
- 13.Buschmann A., Jooss B., Rupp A., Dockter S., Blaschtikowitz H., Heggen I., Pietz J. Children with developmental language delay at 24 months of age: Results of a diagnostic work-up. Dev. Med. Child Neurol. 2008;50:223–229. doi: 10.1111/j.1469-8749.2008.02034.x. [DOI] [PubMed] [Google Scholar]
- 14.Sim F., O’Dowd J., Thompson L., Law J., Macmillan S., Affleck M., Gillberg C., Wilson P. Language and social/emotional problems identified at a universal developmental assessment at 30 months. BMC Pediatr. 2013;13:206. doi: 10.1186/1471-2431-13-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snowling M.J., Bishop D.V.M., Stothard S.E., Chipchase B., Kaplan C. Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. J. Child Psychol. Psychiatry. 2006;47:759–765. doi: 10.1111/j.1469-7610.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- 16.Conti-Ramsden G., Durkin K. Language development and assessment in the preschool period. Neuropsychol. Rev. 2012;22:384–401. doi: 10.1007/s11065-012-9208-z. [DOI] [PubMed] [Google Scholar]
- 17.Miniscalco C., Nygren G., Hagberg B., Kadesjö B., Gillberg C. Neuropsychiatric and neurodevelopmental outcome of children at age 6 and 7 years who screened positive for language problems at 30 months. Dev. Med. Child Neurol. 2006;48:361–366. doi: 10.1017/S0012162206000788. [DOI] [PubMed] [Google Scholar]
- 18.Hiller-Sturmhöfel S., Bartke A. The endocrine system: An overview. Alcohol. Health Res. World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- 19.Quast A., Hesse V., Hain J., Wermke P., Wermke K. Baby babbling at five months linked to sex hormone levels in early infancy. Infant Behav. Dev. 2016;44:1–10. doi: 10.1016/j.infbeh.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Schaadt G., Hesse V., Friederici A.D. Sex hormones in early infancy seem to predict aspects of later language development. Brain Lang. 2015;141:70–76. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Wermke K., Quast A., Hesse V. From melody to words: The role of sex hormones in early language development. Horm. Behav. 2018;104:206–215. doi: 10.1016/j.yhbeh.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Futran Fuhrman V., Tal A., Arnon S. Why endocrine disrupting chemicals (EDCs) challenge traditional risk assessment and how to respond. J. Hazard. Mater. 2015;286:589–611. doi: 10.1016/j.jhazmat.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogg K., Price E.M., Hanna C.W., Robinson W.P. Prenatal and Perinatal Environmental Influences on the Human Fetal and Placental Epigenome. Clin. Pharmacol. Ther. 2012;92:716. doi: 10.1038/clpt.2012.141. [DOI] [PubMed] [Google Scholar]
- 25.Nesan D., Kurrasch D.M. Gestational Exposure to Common Endocrine Disrupting Chemicals and Their Impact on Neurodevelopment and Behavior. Annu. Rev. Physiol. 2020;82:177–202. doi: 10.1146/annurev-physiol-021119-034555. [DOI] [PubMed] [Google Scholar]
- 26.Schettler T. Toxic threats to neurologic development of children. Environ. Health Perspect. 2001;109((Suppl. S6)):813–816. doi: 10.1289/ehp.01109s6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzwilewski K.L., Schantz S.L. Prenatal chemical exposures and child language development. J. Commun. Disord. 2015;57:41–65. doi: 10.1016/j.jcomdis.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trudel D., Horowitz L., Wormuth M., Scheringer M., Cousins I.T., Hungerbühler K. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 29.Winkens K., Vestergren R., Berger U., Cousins I.T. Early life exposure to per- and polyfluoroalkyl substances (PFASs): A critical review. Emerg. Contam. 2017;3:55–68. doi: 10.1016/j.emcon.2017.05.001. [DOI] [Google Scholar]
- 30.Borg D., Lund B.O., Lindquist N.G., Håkansson H. Cumulative health risk assessment of 17 perfluoroalkylated and polyfluoroalkylated substances (PFASs) in the Swedish population. Environ. Int. 2013;59:112–123. doi: 10.1016/j.envint.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Calafat A.M., Kuklenyik Z., Caudill S.P., Reidy J.A., Needham L.L. Perfluorochemicals in Pooled Serum Samples from United States Residents in 2001 and 2002. Environ. Sci. Technol. 2006;40:2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- 32.Johansson N., Fredriksson A., Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Piekarski D., Diaz K., McNerney M. Perfluoroalkyl chemicals in neurological health and disease: Human concerns and animal models. Neurotoxicology. 2020;77:155–168. doi: 10.1016/j.neuro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Spulber S., Kilian P., Ibrahim W.N.W., Onishchenko N., Ulhaq M., Norrgren L., Negri S., Tuccio M.D., Ceccatelli S. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. (Report) PLoS ONE. 2014;9:e94227. doi: 10.1371/journal.pone.0094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blomberg A.J., Norén E., Haug L.S., Lindh C., Sabaredzovic A., Pineda D., Jakobsson K., Nielsen C. Estimated Transfer of Perfluoroalkyl Substances (PFAS) from Maternal Serum to Breast Milk in Women Highly Exposed from Contaminated Drinking Water: A Study in the Ronneby Mother—Child Cohort. Environ. Health Perspect. 2023;131:017005. doi: 10.1289/EHP11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rappazzo K.M., Coffman E., Hines E.P. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature. Int. J. Environ. Res. Public Health. 2017;14:691. doi: 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services; 2022. [(accessed on 3 March 2023)]. The Agency for Toxic Substances and Disease Registry (ATSDR), Per- and Polyfluoroalkyl Substances (PFAS) and Your Health. Available online: https://www.atsdr.cdc.gov/ [Google Scholar]
- 38.Wang Y., Rogan W.J., Chen H.Y., Chen P.C., Su P.H., Chen H.Y., Wang S.L. Prenatal exposure to perfluroalkyl substances and children’s IQ: The Taiwan maternal and infant cohort study. Int. J. Hyg. Environ. Health. 2015;218:639–644. doi: 10.1016/j.ijheh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B., Wang Z., Zhang J., Dai Y., Feng C., Lin Y., Zhang L., Guo J., Qi X., Chang X. Prenatal perfluoroalkyl substances exposure and neurodevelopment in toddlers: Findings from SMBCS. Chemosphere. 2023;313:137587. doi: 10.1016/j.chemosphere.2022.137587. [DOI] [PubMed] [Google Scholar]
- 40.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Statens Beredning för Medicinsk och Social Utvärdering (SBU) Utvärdering av Metoder i hälso- och Sjukvården och Insatser i Socialtjänsten: En Metodbok. [(accessed on 1 May 2020)]. Stockholm. Available online: https://www.sbu.se/metodbok.
- 42.Morgan R.L., Whaley P., Thayer K.A., Schünemann H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. (Pt 1)Environ. Int. 2018;121:1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CASP Critical Appraisal Skills Programme CASP Cohort Study Checklist. [(accessed on 1 June 2020)]. Available online: https://casp-uk.b-cdn.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf.
- 44.The EndNote Team . EndNote 20. Clarivate; Philadelphia, PA, USA: 2013. [Google Scholar]
- 45.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spratlen M.J., Perera F.P., Lederman S.A., Rauh V.A., Robinson M., Kannan K., Trasande L., Herbstman J. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ. Pollut. 2020;263:114444. doi: 10.1016/j.envpol.2020.114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein C.R., Savitz D.A., Bellinger D.C. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y., Li Y., Scott K., Lindh C.H., Jakobsson K., Fletcher T., Ohlsson B., Andersson E.M. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ. Res. 2020;181:108923. doi: 10.1016/j.envres.2019.108923. [DOI] [PubMed] [Google Scholar]
- 49.Stübner C., Ebel M., Jakobsson K., Gillberg C., Nielsen C., Miniscalco C. Developmental language disorders in preschool children after high exposure to perfluoroalkyl substances from contaminated drinking water in Ronneby, Sweden. Environ. Epidemiol. 2023;7:e233. doi: 10.1097/EE9.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeddy Z., Hartman T.J., Taylor E.V., Poteete C., Kordas K. Prenatal concentrations of Perfluoroalkyl substances and early communication development in British girls. Early Hum. Dev. 2017;109:15–20. doi: 10.1016/j.earlhumdev.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo F., Chen Q., Yu G., Huo X., Wang H., Nian M., Tian Y., Xu J., Zhang J., Zhang J. Exposure to perfluoroalkyl substances and neurodevelopment in 2-year-old children: A prospective cohort study. Environ. Int. 2022;166:107384. doi: 10.1016/j.envint.2022.107384. [DOI] [PubMed] [Google Scholar]
- 52.Wang H., Luo F., Zhang Y., Yang X., Zhang S., Zhang J., Tian Y., Zheng L. Prenatal exposure to perfluoroalkyl substances and child intelligence quotient: Evidence from the Shanghai birth cohort. Environ. Int. 2023;174:107912. doi: 10.1016/j.envint.2023.107912. [DOI] [PubMed] [Google Scholar]
- 53.Carrizosa C., Murcia M., Ballesteros V., Costa O., Manzano-Salgado C.B., Ibarluzea J., Iñiguez C., Casas M., Andiarena A., Llop S., et al. Prenatal perfluoroalkyl substance exposure and neuropsychological development throughout childhood: The INMA Project. J. Hazard. Mater. 2021;416:125185. doi: 10.1016/j.jhazmat.2021.125185. [DOI] [PubMed] [Google Scholar]
- 54.Chen M.H., Ha E.H., Liao H.F., Jeng S.F., Su Y.N., Wen T.W., Lien G.W., Chen C.Y., Hsieh W.S., Chen P.C. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology. 2013;24:800–808. doi: 10.1097/EDE.0b013e3182a6dd46. [DOI] [PubMed] [Google Scholar]
- 55.Goudarzi H., Nakajima S., Ikeno T., Sasaki S., Kobayashi S., Miyashita C., Ito S., Araki A., Nakazawa H., Kishi R. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Sci. Total Environ. 2016;541:1002–1010. doi: 10.1016/j.scitotenv.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Harris M.H., Oken E., Rifas-Shiman S.L., Calafat A.M., Ye X., Bellinger D.C., Webster T.F., White R.F., Sagiv S.K. Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFASs) and child cognition. Environ. Int. 2018;115:358–369. doi: 10.1016/j.envint.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liew Z., Ritz B., Bach C.C., Asarnow R.F., Bech B.H., Nohr E.A., Bossi R., Henriksen T.B., Bonefeld-Jorgensen E.C., Olsen J. Prenatal Exposure to Perfluoroalkyl Substances and IQ Scores at Age 5 a Study in the Danish National Birth Cohort. (Research) (Medical condition overview) Environ. Health Perspect. 2018;126:067004. doi: 10.1289/EHP2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh J., Shin H.-M., Nishimura T., Rahman M.S., Takahashi N., Tsuchiya K.J. Perfluorooctanoate and perfluorooctane sulfonate in umbilical cord blood and child cognitive development: Hamamatsu Birth Cohort for Mothers and Children (HBC Study) Environ. Int. 2022;163:107215. doi: 10.1016/j.envint.2022.107215. [DOI] [PubMed] [Google Scholar]
- 59.Skogheim T.S., Villanger G.D., Weyde K.V.F., Engel S.M., Surén P., Øie M.G., Skogan A.H., Biele G., Zeiner P., Øvergaard K.R., et al. Prenatal exposure to perfluoroalkyl substances and associations with symptoms of attention-deficit/hyperactivity disorder and cognitive functions in preschool children. Int. J. Hyg. Environ. Health. 2020;223:80–92. doi: 10.1016/j.ijheh.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vuong A.M., Yolton K., Xie C., Dietrich K.N., Braun J.M., Webster G.M., Calafat A.M., Lanphear B.P., Chen A. Prenatal and childhood exposure to poly- and perfluoroalkyl substances (PFAS) and cognitive development in children at age 8 years. Envrion. Res. 2019;172:242–248. doi: 10.1016/j.envres.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO. Socialstyrelsen W. International Classification of Disorders. 10th ed. Almquist & Wiksell; Uppsala, Sweden: 1996. Klassifikation av sjukdomar och hälsoproblem 1997. [Google Scholar]
- 62.Bayley N. Bayley Scales of Infant Development. Harcourt Brace; San Diego, CA, USA: 1993. [Google Scholar]
- 63.Hua J., Li Y., Ye K., Ma Y., Lin S., Gu G., Du W. The reliability and validity of Bayley-III cognitive scale in China’s male and female children. Early Hum. Dev. 2019;129:71–78. doi: 10.1016/j.earlhumdev.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Yue A., Jiang Q., Wang B., Abbey C., Medina A., Shi Y., Rozelle S. Concurrent validity of the ages and stages questionnaire and the Bayley scales of infant development III in China. PLoS ONE. 2019;14:e0221675. doi: 10.1371/journal.pone.0221675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao H.-F., Yao G., Wang T.-M. Concurrent Validity in Taiwan of the Comprehensive Developmental Inventory for Infants and Toddlers Who Were Full-Term Infants. Percept. Mot. Ski. 2008;107:29–44. doi: 10.2466/PMS.107.5.29-44. [DOI] [PubMed] [Google Scholar]
- 66.Kaufman A.S. Kaufman Brief Intelligence Test. 2nd ed. American Guidance Service; Circle Pines, MN, USA: 2004. KBIT-2. [Google Scholar]
- 67.Mullen E.M. Mullen Scales of Early Learning. AGS; Circle Pines, MN, USA: 1995. [Google Scholar]
- 68.Kaufman A.S., Kaufman N.L. Clinical Evaluation of Young Children with the McCarthy Scales. Grune & Stratton; New York, NY, USA: 1977. [Google Scholar]
- 69.Korkman M., Kirk U., Kemp S. NEPSY-II: A developmental neuropsychological assessment San Antonio. Tex. Psychol. Corp. 2007;16:80–101. [Google Scholar]
- 70.Dunn L.M. PPVT-III: Peabody Picture Vocabulary Test. 3rd ed. American Guidance Service; Circle Pines, MN, USA: 1997. [Google Scholar]
- 71.Gale H.R. Stanford Binet Intelligence Scales. 5th ed. Riverside Pub.; Itasca, IL, USA: 2003. [Google Scholar]
- 72.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; Harcourt Brace & Company; New York, NY, USA: 1999. [Google Scholar]
- 73.Wechsler D. Manual for Wechsler Preschool and Primary Scale of Intelligence-Revised (British Amendments) Psychological Corporation; Kent, UK: 1990. [Google Scholar]
- 74.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th ed. The Psychological Corporation; San Antonio, TX, USA: 2012. [Google Scholar]
- 75.Wechsler D. WISC-III: Wechsler Intelligence Scale for Children: Manual. Psychological Corporation; San Antonio, TX, USA: 1991. [Google Scholar]
- 76.Wechsler D. WISC-IV: Administration and Scoring Manual. Psychological Corporation; San Antonio, TX, USA: 2003. [Google Scholar]
- 77.Ireton H., Glascoe F.P. Assessin Children’s Development Using Parents’ Reports: The Child Development Inventory. Clin. Pediatr. 1995;34:248–255. doi: 10.1177/000992289503400504. [DOI] [PubMed] [Google Scholar]
- 78.Fenson L., Marchman V.A., Thal D.J., Dale P.S., Reznick J.S., Bates E. MacArthur-Bates Communicative Development Inventories. 2nd ed. Brookes Publishing; Towson, MD, USA: 2007. [Google Scholar]
- 79.Fernell E., Gillberg C. Autism under the umbrella of ESSENCE. Front. Psychiatry. 2023;14:1002228. doi: 10.3389/fpsyt.2023.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thal D.J., O’Hanlon L., Clemmons M., Fralin L. Validity of a parent report measure of vocabulary and syntax for preschool children with language impairment. J. Speech Lang. Hear. Res. 1999;42:482–496. doi: 10.1044/jslhr.4202.482. [DOI] [PubMed] [Google Scholar]
- 81.Paul R. Language Disorders from Infancy Through Adolescence: Listening, Speaking, Reading, Writing, and Communicating. 5th ed. Elsevier; St. Louis, MI, USA: 2018. pp. 441–444, 903–909. [Google Scholar]
- 82.Bliddal M., Liew Z., Pottegård A., Kirkegaard H., Olsen J., Nohr E.A. Examining Nonparticipation in the Maternal Follow-up Within the Danish National Birth Cohort. Am. J. Epidemiol. 2018;187:1511–1519. doi: 10.1093/aje/kwy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montazeri P., Thomsen C., Casas M., de Bont J., Haug L.S., Maitre L., Papadopoulou E., Sakhi A.K., Slama R., Saulnier P.J., et al. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int. J. Hyg. Environ. Health. 2019;222:864–872. doi: 10.1016/j.ijheh.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madigan S., Prime H., Graham S.A., Rodrigues M., Anderson N., Khoury J., Jenkins J.M. Parenting behavior and child language: A meta-analysis. Pediatrics. 2019;144 doi: 10.1542/peds.2018-3556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article is based on publicly available data and the sources are provided in the article.