Abstract
The genes encoding enzymes of the Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway in Rhodobacter capsulatus are organized in at least two operons, each preceded by a separate cbbR gene, encoding potential LysR-type transcriptional activators. As a prelude to studies of cbb gene regulation in R. capsulatus, the nucleotide sequence of a 4,537-bp region, which included cbbRII, was determined. This region contained the following open reading frames: a partial pgm gene (encoding phosphoglucomutase) and a complete qor gene (encoding NADPH:quinone oxidoreductase), followed by cbbRII, cbbF (encoding fructose 1,6-bisphosphatase), cbbP (encoding phosphoribulokinase), and part of cbbT (encoding transketolase). Physiological control of the CBB pathway and regulation of the R. capsulatus cbb genes were studied by using a combination of mutant strains and promoter fusion constructs. Characterization of mutant strains revealed that either form I or form II ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO), encoded by the cbbLS and cbbM genes, respectively, could support photoheterotrophic and autotrophic growth. A strain with disruptions in both cbbL and cbbM could not grow autotrophically and grew photoheterotrophically only when dimethyl sulfoxide was added to the culture medium. Disruption of cbbP resulted in a strain that did not synthesize form II RubisCO and had a phenotype similar to that observed in the RubisCO-minus strain, suggesting that there is only one cbbP gene in R. capsulatus and that this gene is cotranscribed with cbbM. Analysis of RubisCO activity and synthesis in strains with disruptions in either cbbRI or cbbRII, and β-galactosidase determinations from wild-type and mutant strains containing cbbIp- and cbbIIp-lacZ fusion constructs, indicated that the cbbI and cbbII operons of R. capsulatus are within separate CbbR regulons.
Purple nonsulfur photosynthetic bacteria display exceptional metabolic versatility (20, 31) and assimilate CO2 via the highly regulated Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway (12, 17, 48). During photo- and chemoautotrophic growth, CO2 is the sole source of cellular carbon, and maximal levels of the key CBB pathway enzymes, ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) and phosphoribulokinase (PRK), are observed (48). Photoheterotrophic growth results in much lower yet substantial levels of RubisCO and PRK; however, under these conditions the CBB pathway functions primarily to help maintain the redox balance of the cell by allowing CO2 to serve as an electron sink. Alternate electron acceptors such as dimethyl sulfoxide (DMSO) can function in place of CO2 (7, 43, 56).
The organization and regulation of structural genes encoding enzymes of the CBB pathway have been extensively studied in Rhodobacter sphaeroides, and there are at least three major operons which comprise the cbb regulon of this organism. Two major operons, the cbbI, or form I, operon and the cbbII, or form II, operon, are comprised of structural genes of the CBB pathway, some of which are duplicated (13–15). The third operon consists of the cbbXYZ genes, encoding two proteins of unknown function and phosphoglycolate phosphatase, respectively, and is downstream of the cbbI operon (18). Transcription of both the cbbI and cbbII operons is positively regulated by the product of the cbbR gene, which is upstream and divergently transcribed from the R. sphaeroides cbbI operon (16). By contrast, the form I RubisCO genes (cbbLS) of R. capsulatus are not associated with any CBB pathway structural genes (38, 39), and an open reading frame (ORF) with sequence similarity to cbbQ, which is also downstream of cbbLS of Pseudomonas hydrogenothermophila and Chromatium vinosum (62), is found downstream of R. capsulatus cbbLS (38). The cbbQ gene product has no known function in R. capsulatus (20a). In addition, there are two cbbR genes in R. capsulatus; cbbRI is upstream and divergently transcribed from the cbbLS genes (38), while cbbRII is upstream and divergently transcribed from the cbbFPTGAM genes (39).
The recent description of variant cbb gene organization in R. capsulatus and R. sphaeroides, particularly the presence of two cbbR genes in R. capsulatus, suggests potential differences in cbb gene regulation. For example, unlike R. sphaeroides, R. capsulatus does not synthesize form I RubisCO when the organism is grown photoheterotrophically on malate (39, 46). Furthermore, the R. capsulatus form I enzyme is immunologically distinct from the form I enzyme of R. sphaeroides (15, 39) and appears to have been acquired by horizontal gene transfer (38). Thus, to initiate and provide a framework for cbb gene regulation studies in R. capsulatus, specific cbb gene disruption strains and cbb promoter fusions were constructed and characterized.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
Plasmids and R. capsulatus strains used or constructed are listed in Table 1. Escherichia coli JM107 (60), JM109 λpir, and S17-1 λpir (40) were grown aerobically on LB medium (2) at 37°C. Aerobic cultures of R. capsulatus were grown in PYE medium (57) at 30°C. Photosynthetic cultures were grown in Ormerod’s medium (37) supplemented with thiamine (1 μg/ml), nicotinic acid (1 μg/ml), and biotin (0.1 μg/ml). Photo- and chemoautotrophic growth conditions were previously described (38, 39). Antibiotic concentrations used for R. capsulatus strains were as follows: rifampin, 100 μg/ml; kanamycin, 5 μg/ml; spectinomycin, 10 μg/ml; and tetracycline, 2 μg/ml for plasmid maintenance or 0.1 μg/ml for screening during gene disruption experiments. For E. coli, antibiotic concentrations were 30 μg/ml for kanamycin, 50 μg/ml for spectinomycin, 12.5 μg/ml for tetracycline, and 200 μg/ml for trimethoprim. DMSO was used at 30 mM.
TABLE 1.
Plasmids and bacterial strains used this study
| Plasmid or strain | Relevant characteristics; phenotypea | Reference |
|---|---|---|
| Plasmids | ||
| pK18, pK19 | Kmr, pUC derivatives | 42 |
| pTZ18R | Apr, pUC derivative | 33 |
| pUC1318 | Apr, pUC derivative with modified multiple cloning site | 21 |
| pUC1813 | Apr, pUC derivative with modified multiple cloning site | 21 |
| pRL648 | Kmr Apr, pUC derivative with the Tn5 Kmr gene cassette | 6 |
| pRK415 | Tcr, broad-host-range cloning vector, lacZα | 22 |
| pRPS-1 | Tcr, broad-host-range expression vector containing R. rubrum cbbM promoter and cbbR gene in pRK404 | 7 |
| pRPSKm | Kmr derivative of pRPS-1 | 8a |
| pJP5603 | Kmr, mobilizable suicide vector | 40 |
| pTC5603 | Tcr derivative of pJP5603 | This work |
| pHP45Ω | Apr Spr, containing Ω cassette encoding Spr | 41 |
| pVK101 | Tcr Kmr, broad-host-range vector | 23 |
| pUC1318K | Apr Kmr, pUC1318 with a 1.5-kb HindIII-SalI fragment from Tn5 encoding Kmr | 35 |
| pUC1813K | Apr Kmr, pUC1813 with a 1.5-kb SalI fragment from pUC1318K encoding the Tn5 Kmr | This work |
| pXBA601 | Tcr, broad-host-range lacZ translational fusion vector | 1 |
| pRCFII | R. capsulatus cosmid library clone containing the cbbII genes | 39 |
| pK18FIIEH | pK18 containing the R. capsulatus cbbRII, cbbF, cbbP, cbbT, cbbG, and cbbA genes on an 8.4-kb EcoRI-HindIII fragment from pRCFII | This work |
| pRKFIIEH | pRK415 containing the EcoRI-HindIII fragment from pK18FIIEH | This work |
| pRKFIP | pRK415 with a 9-kb PstI fragment containing the R. capsulatus cbbRI, cbbLS, and cbbQ genes | 39 |
| pUC1813::FIB | pUC1813 containing the R. capsulatus cbbLS genes on a 4.7-kb BamHI fragment from pRKFIP | This work |
| pUC1813::FIΩ | pUC1318::FIB Δ EcoRI::Spr | This work |
| pJP::FIΩ | pJP5603 containing the BamHI insert of pUC1813::FIΩ | This work |
| pK18FIIS2-I | pK18 containing the R. capsulatus cbbM gene on a 2-kb SalI fragment | 39 |
| pUC1318FII | pUC1318 containing the R. capsulatus cbbM gene cloned on a 2-kb SalI fragment | This work |
| pUC1318::FIIKm | pUC1318FII with the Δ HindIII::Kmr cartridge inserted | This work |
| pTC::FIIKm | pTC5603 with the SalI fragment of pUC1318::FIIKm | This work |
| pK18FIIB2.3 | pK18 with a 2.3-kb BamHI fragment containing the R. capsulatus cbbRII, cbbF, and cbbP genes | 39 |
| pK18FIIBSm | pK18 containing part of R. capsulatus cbbP on a 543-bp BamHI-SmaI fragment | This work |
| pK18CBBPΩ | pK18FIIBSm with the Spr cartridge from pHP45Ω cloned into the SalI site of cbbP | This work |
| pJP::CBBPΩ | pJP5603 containing the cbbP::Ω interposon of pK18CBBPΩ cloned as a HindIII-EcoRI fragment | This work |
| pK18FIIS4.4 | pK18 with a 4.4-kb SalI fragment containing the 5′ end of the R. capsulatus cbbII cluster | 39 |
| pTZ::FII3.7 | pTZ18R containing the R. capsulatus cbbRII and cbbF genes on a 3.7-kb SalI-SmaI fragment from pK18FIIEH | This work |
| pTZ::CbbRKm | pTZ::FII3.7 with a Kmr gene cartridge cloned into the BamHI site of cbbRII | This work |
| pTCTZ::CbbRKm | pTZ::CbbRKm cloned into vector pTC5603 by linearizing with XbaI | This work |
| pJN940A | The R. sphaeroides cbbPI gene cloned as an AvaI fragment into pK18 | 36a |
| pUC1813::RsPI | pUC1813 containing the R. sphaeroides cbbPI gene cloned as a 1-kb AvaI fragment from plasmid pJN940A | This work |
| pRPS::RsPIA | pRPSKm containing the R. sphaeroides cbbPI gene cloned from pUC1813::RsPI as an XbaI fragment | This work |
| pRPS::RsPIB | pRsPIA containing the XbaI insert in the opposite orientation | This work |
| pUC1813S4.4 | pUC1813 containing the R. capsulatus cbbRII, cbbF, and cbbP genes on a 4.4-kb SalI fragment | This work |
| pEULA4 | 4-kb EcoRI fragment containing R. capsulatus cbbL, cbbRI, anfA, and uncharacterized sequence between cbbRI and anfA in pK19 | 38 |
| pVK::CbbRI | pVK101 containing the the 4-kb EcoRI fragment from pEULA4 | This work |
| pVK::CbbRII | 4.4-kb SalI fragment containing the R. capsulatus cbbRII, cbbF, and cbbP genes cloned into the XhoI site of pVK101 | This work |
| pEULA4Ω | pEULA4 with a SmaI Spr cassette cloned into the unique SspI site within cbbRI | This work |
| pJPLA4Ω | pJP5603 containing the cbbRI::SprEcoRI fragment from pEULA4Ω | This work |
| pK18FISN | pK18 containing the 3.2-kb SalI-NcoI fragment from pEULA4 such that the cbbL start codon is fused to lacZα | This work |
| pXLB | pXBA601 containing the 3.2-kb BamHI fragment from pK18FISN, cbbIp fusion to lacZ | This work |
| pXLBP | pXBA601 containing the 367-bp PstI-BamHI fragment from pK18FISN, cbbIp fusion to lacZ | This work |
| pK18FIISN | pK18 containing the 2.44-kb SalI-NcoI fragment from pK18FIIS4.4 such that the cbbF start codon is fused to lacZα | This work |
| pXFB | pXBA601 containing the 722-bp BamHI fragment from pK18FIISN, cbbIIp fusion to lacZ | This work |
| R. capsulatus strains | ||
| SB1003 | Rifr derivative of strain B10; PH+ PA+ CA+ | 61 |
| SBI− | cbbL::Spr derivative of SB1003; PH+ PA+ CA+ | This work |
| SBII− | cbbM::Kmr derivative of SB1003; PH+ PA+ CA+ | This work |
| SBI-II | cbbL::Spr, cbbM::Kmr derivative of SB1003; PH− PA− CA− | This work |
| SBP− | cbbP::Spr derivative of SB1003; PH− PA− CA− | This work |
| SBRI− | cbbRI::Spr derivative of SB1003; PH+ PA+ CA+ | This work |
| SBRII− | cbbRII::Kmr derivative of SB1003; PH+ PA− CA− | This work |
Ability of strains to grow under various conditions: PH, photoheterotrophically with malate; PA, photoautotrophically; CA, aerobic chemoautotrophically.
DNA manipulations.
Routine DNA manipulations, including plasmid preparation, restriction endonuclease digestion, agarose gel electrophoresis, fragment ligation, and bacterial transformation, were performed by standard methods (2). R. capsulatus chromosomal DNA was prepared as previously described (19). For gene disruption experiments, plasmid pJP5603 derivatives were conjugated into R. capsulatus SB1003 by using E. coli S17-1 λpir (40). For complementation of mutant strains, plasmids were conjugated into R. capsulatus by triparental matings on filter pads as previously described (57), using the helper plasmid pRK2013 (10).
Southern blotting and hybridization.
Southern transfer experiments were performed by using GeneScreen Plus (NEN, DuPont, Boston, Mass.) or Hybond N+ (Amersham, Arlington Heights, Ill.) membranes. Hybridizations were conducted according to the protocols provided by NEN, DuPont, using formamide under stringent conditions. Probes were labeled with [α-32P]dCTP (NEN, DuPont) by the random prime labeling method (9), using a kit purchased from United States Biochemical Corporation (Cleveland, Ohio).
DNA sequencing and analysis.
Nucleotide sequences were determined with an ABI Prism 310 Genetic Analyzer. A thermal cycler and dye terminator cycle sequencing kit were used as described by the manufacturer (Perkin-Elmer, Foster City, Calif.). The M13/pUC forward 23-base primer, M13 reverse (−48) primer, and sequence-specific synthetic primers were used to complete the double-stranded sequence. Sequence analysis was carried out with the University of Wisconsin Genetics Computing Group software, the EGCG extension programs (The Sanger Centre, Hinxton, England), and the MacVector sequence analysis software (International Biotechnology, Inc., New Haven, Conn.).
Preparation of cell extracts and enzyme assays.
Culture samples (20 to 30 ml) were taken in late log phase (A660 = 0.9 to 1.2) and washed twice in cold buffer (100 mM Tris-HCl, 1 mM EDTA [pH 8.0]) before freezing at −70°C. Thawed pellets were resuspended in 1 ml of TEM buffer (50 mM Tris-HCl, 1 mM EDTA, 5 mM β-mercaptoethanol [pH 7.5]) and disrupted by sonication in an ice bath. Cell debris was removed by centrifugation for 10 min in a microcentrifuge at 4°C.
RubisCO activity was measured as ribulose 1,5-bisphosphate-dependent 14CO2 fixation into acid-stable 3-phosphoglycerate (14). PRK was assayed as previously described (47) except that ribulose 5-phosphate was not added directly but generated from ribose 5-phosphate by the addition of 5 U of phosphoriboisomerase (Sigma Chemical, St. Louis, Mo.).
β-Galactosidase was measured by continuous assays in Z buffer (50 mM sodium phosphate [pH 7.0], 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol) (36) containing 0.8 mg of o-nitrophenol β-d-galactopyranoside (ONPG) per ml. The production of o-nitrophenol from ONPG was measured by monitoring the increase in A405. β-Galactosidase activities were calculated by using an extinction coefficient for o-nitrophenol of 3.1 × 103 cm2/mmol (55).
Protein concentrations were determined by a modified Lowry procedure (32) using bovine serum albumin as a standard.
Western immunoblot analysis.
Antibodies raised against R. sphaeroides form II RubisCO and form I PRK (PRK I) were used to detect R. capsulatus form II RubisCO and PRK, respectively. Although R. capsulatus form I RubisCO reacts poorly with antibody raised against R. sphaeroides form I RubisCO, anti-Synechococcus strain PCC 6301 RubisCO antibody cross-reacts well (38, 39) and was used to detect R. capsulatus form I RubisCO. Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (28). After SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.), using a Bio-Rad Transblot semidry cell (Bio-Rad, Hercules, Calif.) and established protocols (50). The blots were developed by using the Vistra ECF fluorescent detection system (Amersham Corporation, Buckinghamshire, England) and visualized with a Molecular Dynamics Storm 840 imaging system (Molecular Dynamics, Sunnyvale, Calif.).
Construction of mutant strains.
R. capsulatus strains with disruptions in cbbL, cbbM, cbbP, cbbRI, and cbbRII were constructed by mobilizing the appropriate pJP5603 derivative into strain SB1003 from E. coli S17-1 λpir. Homologous recombination of the plasmid-borne disrupted gene into the wild-type copy in the chromosome was forced because pJP5603 does not replicate in R. capsulatus. Recombinant strains were selected by aerobic growth on PYE plates supplemented with the antibiotic corresponding to the disrupting cassette. Rifampin was used to select against the E. coli donor. Resistant clones were screened for sensitivity to the plasmid-encoded antibiotic resistance marker to identify strains that may had undergone a second recombination event. Double recombination was confirmed by Southern blotting and hybridization analysis of chromosomal DNA from the mutant and wild-type strains (data not shown). Specific plasmid and strain constructions are described below.
Strain SBI− (cbbL).
A 4.7-kb BamHI fragment, containing the R. capsulatus cbbLS genes, was cloned from pRKFIP into pUC1813. The resulting plasmid, pUC1813::FIB, lacked any EcoRI sites in the multiple cloning region so that the 639-bp EcoRI fragment within cbbL could be removed and replaced by the spectinomycin resistance (Spr) gene from pHP45Ω. The 6.5-kb BamHI fragment containing the disrupted gene was moved from pUC1813::FIΩ to pJP5603, resulting in plasmid pJP::FIΩ. Plasmid pJP::FIΩ was mobilized into R. capsulatus SB1003 from E. coli S17-1 λpir. Six of the Spr exconjugants screened were sensitive to kanamycin (Kms). Southern blot analysis of chromosomal DNA prepared from the six Spr Kms isolates revealed that five of them resulted from double recombination. One of these strains was used for subsequent experiments.
Strain SBII− (cbbM).
The 2-kb SalI fragment encoding the R. capsulatus cbbM gene was cloned from plasmid pK18FIIS2-I into plasmid pUC1318. The resulting construct, pUC1318FII, lacked HindIII sites within its multiple cloning region. To generate a Kmr cassette with flanking HindIII sites, a 1.4-kb SalI fragment encoding the Tn5 Kmr gene was cloned from pUC1318K into plasmid pUC1813, generating pUC1813K. The 650-bp HindIII fragment within the cbbM gene in vector pUC1318FII was removed and replaced by the HindIII fragment containing the Tn5 Kmr gene from plasmid pUC1813K. The resulting cbbM deletion fragment was cloned as an XbaI fragment into plasmid pTC5603, yielding pTC::FIIKm. E. coli S17-1 λpir was used to mobilize pTC::FIIKm into R. capsulatus SB1003. Three hundred Kmr clones were screened for tetracycline sensitivity (Tcs). All of the exconjugants were sensitive to 2 μg of tetracyline per ml, but only five clones were sensitive to 0.1 μg/ml. Due to the very low resistance to tetracycline, the 300 clones were examined for loss of pTC5603 by colony hybridization. The five Tcs clones did not hybridize to the pTC5603 probe, but the 295 Tcs clones did hybridize to the probe. Three of the five Tcs clones were screened by Southern blot hybridization analysis of chromosomal DNA and found to be the result of double recombination. One of these recombinants was used for subsequent experiments.
Strain SBI-II (cbbL cbbM).
To construct a strain lacking genes for both forms of RubisCO, the cbbM deletion plasmid pTC::FIIKm was mobilized into R. capsulatus cbbL strain SBI−. Two hundred Kmr colonies were screened, and two were Tcs. Both of these Tcs clones had lost the pTC5603 vector as determined by colony hybridization using pTC5603 as a probe. Southern blot analysis using cbbM and cbbL probes revealed that these strains were the result of double recombination, leaving behind a deletion within the chromosomal copy of cbbM, with the cbbL gene deletion still present.
Strain SBP− (cbbP).
A 543-bp SmaI-BamHI fragment encoding part of the R. capsulatus cbbP gene was cloned from pK18FIIB2.3 into pK18, resulting in plasmid pK18::BSm. The cbbP gene was disrupted by cloning the Spr gene from pHP45Ω as a SalI fragment into the unique XhoI site within the cbbP gene fragment in plasmid pK18::BSm, yielding plasmid pK18CBBPΩ. The resulting disrupted gene fragment was cloned as a BamHI-SmaI fragment into pJP5603, yielding plasmid pJP::CBBPΩ. E. coli S17-1 λpir was used to mobilize plasmid pJP::CBBPΩ into R. capsulatus SB1003. Two hundred fifty Spr exconjugants were screened, and seven were Kms. One of these strains was screened by Southern blot hybridization analysis of the chromosomal DNA and found to be the result of a double recombination. This strain, SBP−, was characterized further.
Strain SBRI− (cbbRI).
The Spr cassette from pHP45Ω was cloned as a SmaI fragment into the unique SspI site within the cbbRI gene in plasmid pEULA4 to yield pEULA4Ω. The cbbRI disruption was cloned from pEULA4Ω into the EcoRI site in pJP5603. The resulting plasmid, pJPLA4Ω, was mobilized into R. capsulatus SB1003 via E. coli S17-1 λpir. Of the 1,500 Spr colonies screened, 35 were Kms. Chromosomal DNA was prepared from eight Kms clones, and Southern blot and hybridization analysis confirmed that each of the clones was the result of double recombination. One strain, SBRI−, was characterized further.
Strain SBRII− (cbbRII).
The 3.7-kb SalI-SmaI fragment containing the R. capsulatus cbbRII and cbbF genes was cloned from pK18FIIS4.4 into plasmid pTZ18R, generating pTZ::FII3.7. Removal of the SalI-SmaI fragment from the multiple cloning region of pTZ18R during the construction of pTZ::FII3.7 deleted the BamHI site. This allowed disruption of the cbbRII gene in pTZ::FII3.7 by insertion of a BamHI fragment containing the Tn5 Kmr gene from plasmid pRL648 into the unique BamHI site within cbbRII. The resulting construct, pTZ::CBBRKm, was linearized with XbaI and ligated to XbaI-digested pTC5603, resulting in plasmid pTZTC::CBBRKm. This plasmid was mobilized into R. capsulatus SB1003 from E. coli S17-1 λpir. Three hundred Kmr colonies were screened, and 299 were sensitive to Tc. Hybridization of colony blots from the 300 Kmr clones using a probe derived from the Tcr region of pTC5603 (EcoRI to PvuII fragment of pBR322) revealed that only the single Tcr clone contained the Tcr gene. Chromosomal DNA was prepared from three of the Tcs and the Tcr clone. The Tcr clone was the result of a single recombination of pTZTC::CBBRKm into the SB1003 chromosome, and each of the three Tcs clones was the result of double recombination. One of the Kmr Tcs double-recombinant clones, strain SBRII−, was used in subsequent experiments.
Construction of cbb promoter fusions.
The translational fusion vector pXBA601 (1) was used for construction of cbbL (cbbIp) and cbbF (cbbIIp) promoter fusions to lacZ. pXBA601 requires that the fusion end of the promoter fragment be ligated to the unique BamHI site within this vector. For construction of the cbbIp fusion, the ends of a 3.2-kb SalI-NcoI fragment from pEULA4 were filled with the Klenow fragment of DNA polymerase I. The blunt-ended fragment was cloned into the SmaI site of pK18, yielding plasmid pK18FISN. This resulted in an in-frame fusion of the cbbL ATG initiation codon that is within the NcoI recognition site to lacZ. After screening for the proper orientation, the fusion was confirmed by sequencing. A PstI-BamHI fragment and a BamHI fragment were cloned from pK18FISN into pXBA601, resulting in constructs with 367 bp and 3.2 kb upstream of the cbbL initiation codon fused to lacZ, pXLBP and pXLB, respectively. Inserts were detected by colony blot hybridization, and the orientation of the insert in pXLB was determined by restriction enzyme digestion. The cbbIIp fusion was constructed by first filling the ends of the 2.44 kb SalI-NcoI fragment from plasmid pKFIIS4.4 with the Klenow fragment of DNA polymerase I and ligating it to SmaI-cut pK18, yielding pK18FIISN. After screening for the orientation of the insert, the fusion was confirmed by nucleotide sequencing. This resulted in an in-frame lacZ fusion to the cbbF ATG initiation codon that is within the NcoI recognition site. A 722-bp BamHI fragment was subcloned from pK18FIISN into pXBA601. The presence of an insert was determined by colony blot hybridization, and the orientation of the insert was determined by nucleotide sequencing. The resulting construct, pXFB, contained 722 bp upstream of cbbF fused to lacZ at the cbbF start codon.
Nucleotide sequence accession number.
The nucleotide sequences reported in this paper have been submitted to the GenBank database under accession no. U87282.
RESULTS
Nucleotide sequence analysis and amino acid sequence comparisons.
The DNA upstream of the presumptive R. capsulatus cbbII operon contains at least two regions of interest with respect to cbb gene regulation: the cbbRII gene, encoding a putative cbb transcriptional activator, and the cbbRII-cbbF intergenic region, containing the presumptive cbbII operon promoter. As a prelude to further studies of R. capsulatus cbb gene regulation, the nucleotide sequence of a 4,537-bp region, from the SalI site 1.4 kb downstream of cbbRII to the 5′ end of cbbTII,, was determined (Fig. 1). In addition to cbbRII, cbbF, cbbP, and part of the cbbT gene known to be present in this region (39), database searches revealed one full-length ORF and one partial ORF downstream of cbbRII. One end of the sequenced region contained a partial ORF (Fig. 1) encoding 83 amino acids that were 56.6% identical (74.7% similar) to the C-terminal portion of Agrobacterium tumefaciens phosphoglucomutase and 49.4% identical (69.9% similar) to the human PGM *1+ isoform of phosphoglucomutase (Fig. 2A). A phosphoglucomutase gene had not been previously identified in nonsulfur purple photosynthetic bacteria. An ORF encoding a 322-amino-acid gene product was directly downstream from cbbRII (Fig. 1). The deduced amino acid sequence of this ORF showed 47.7 and 43.3% identity to the NAD(P)H quinone oxidoreductase (QOR) from Pseudomonas aeruginosa and E. coli, respectively. QOR from E. coli has been crystallized (49), and every residue known to be involved in substrate binding or catalysis is conserved in the R. capsulatus enzyme (Fig. 2B). The AXXGXXG sequence (Fig. 2B) is an unusual nucleotide binding fingerprint motif found only among the QORs (49).
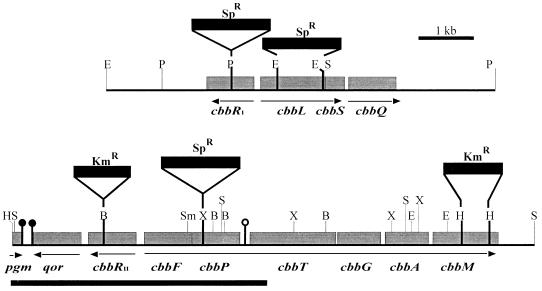
FIG. 1.
Map of R. capsulatus cbb genes. Arrows indicate direction and size of potential transcripts. The sites of gene disruptions in the mutant strains are indicated. The dark bar denotes the region that was sequenced for this study. , potential transcriptional terminator hairpin structure; , hairpin preceded by an RNase E recognition sequence. Gene designations are as follows: cbbR, LysR-type transcriptional regulator; cbbL, form I RubisCO large subunit; cbbS, form I RubisCO small subunit; cbbQ, gene of unknown function; pgm, phosphoglucomutase; qor, NAD(P)H quinone oxidoreductase; cbbF, fructose 1,6-sedoheptulose 1,7-bisphosphatase; cbbP, phosphoribulokinase; cbbT, transketolase; cbbG, glyceraldehyde 3-phosphate dehydrogenase; cbbA, fructose 1,6-bisphosphate aldolase; cbbM, form II RubisCO. Restriction sites: B, BamHI, E, EcoRI, H, HindIII; S, SalI; Sm, SmaI, X, XhoI.
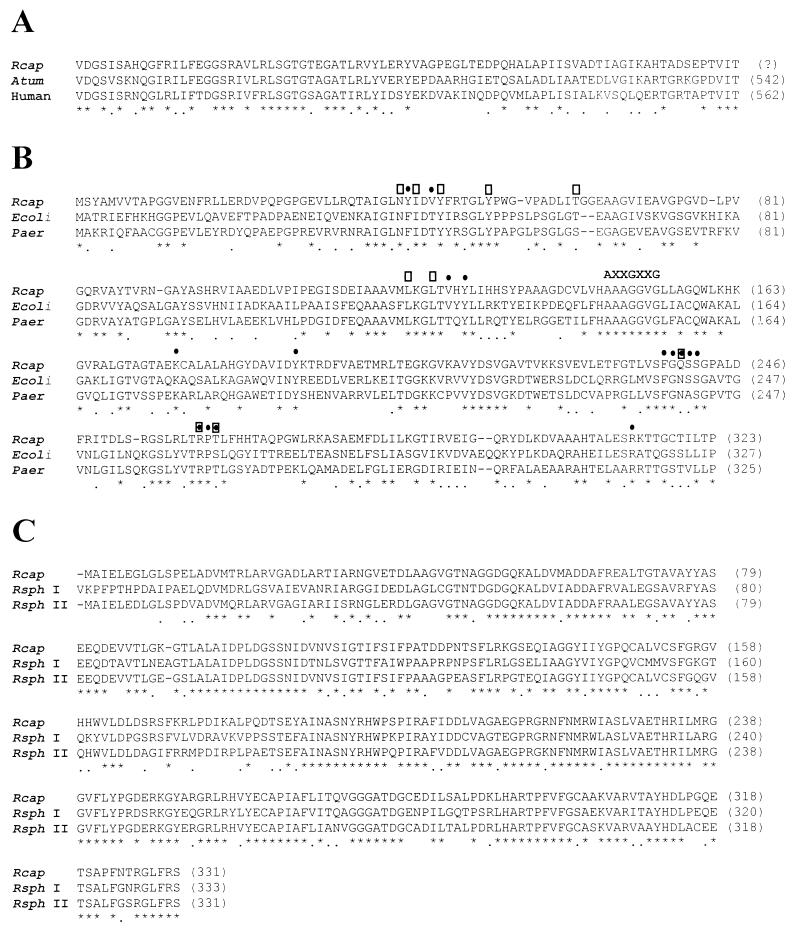
FIG. 2.
Comparison and alignment of deduced amino acid sequences to sequences of known proteins. Invariant amino acids are indicated by asterisks and conservative changes are indicated by dots below the amino acids. (A) Alignment of the deduced amino acid sequence of the R. capsulatus (Rcap) partial ORF with that of A. tumefaciens (Atum) (51) and human phosphoglucomutase (58) amino acid sequences. In each case, the threonine residue is the C-terminal residue. (B) Amino acid sequence alignment of R. capsulatus QOR with QOR from E. coli (29) and P. aeruginosa (Paer) (GenBank accession no. X85015). AXXGXXG is an unusual nucleotide binding motif found in this protein (49). Additional residues in contact with the bound NADPH (•) and those that are within the catalytic site (□) are indicated. (C) FBPase amino acid alignment. The R. capsulatus deduced amino acid sequence is aligned with the amino acid sequences of R. sphaeroides FBPase I (14) and FBPase II (13) (Rsph I and Rsph II). (D) PRK amino acid sequence alignment. The R. capsulatus PRK amino acid sequence is aligned with the amino acid sequences of R. sphaeroides PRK I (14) and PRK II (13) (Rsph I and Rsph II). The putative ATP binding domain is indicated by the shaded box, and the pyridine nucleotide binding site is indicated by the bar. Residues implicated in the R. sphaeroides form I enzyme in sugar phosphate binding (#) and catalysis (∧) are noted. (E) Alignment of the R. capsulatus (Rc) cbbT partial deduced amino acid sequence with R. capsulatus TktA (4) and R. sphaeroides (Rs) CbbT (13) sequences.
The CbbR proteins comprise a family of LysR-type transcriptional activators that are involved in the regulation of cbb genes, from which they are usually divergently transcribed (17). The R. capsulatus cbbRII gene is immediately upstream and divergently transcribed from cbbF (Fig. 1). A second cbbR gene, cbbRI, was found upstream and divergently transcribed from the R. capsulatus cbbLS genes (38). Amino acid sequence comparisons and phylogenetic analyses of the R. capsulatus CbbR proteins with other CbbRs, presented elsewhere (38), showed that R. capsulatus CbbR II is most homologous to R. sphaeroides CbbR (55.2% identity) and less homologous to the R. capsulatus CbbR I (42.5% identity).
The R. capsulatus cbbF gene product is most similar to the fructose 1,6-bisphosphatases (FBPases) from R. sphaeroides, showing 84.0% identity with R. sphaeroides FBPase II and 66.8% identity to R. sphaeroides FBPase I (Fig. 2C). It has been suggested, by analogy to the R. sphaeroides cbbII operon, that the R. capsulatus cbbF promoter may control the entire cbbFPTGAM (cbbII) gene cluster (39). In this respect, it is interesting that three potential CbbR binding sites (17) are present upstream of cbbF (sequence not shown).
The R. capsulatus cbbP gene, encoding a putative PRK, was immediately downstream of cbbF (Fig. 1). A potential ribosome binding site was 8 nucleotides upstream of the cbbP start codon and within the cbbFII coding region; thus, cbbF and cbbP may be translationally coupled and are almost certainly cotranscribed. This arrangement is similar to that of R. sphaeroides cbbFI-cbbPI (14) and cbbFII-cbbPII (13). The predicted molecular weight, 33,244, is very similar to the subunit molecular weight determined for purified R. capsulatus PRK (47). R. capsulatus PRK is highly similar to R. sphaeroides PRK I (86.2% identity) and PRK II (87.0% identity) (Fig. 2D). The domains involved in ATP (24, 25) and pyridine nucleotide (3, 13) binding are indicated in Fig. 2D. Residues implicated by site-directed mutagenesis of the R. sphaeroides PRK I in sugar phosphate binding (44) and catalysis (3) are also noted.
The 46 N-terminal amino acids of the cbbT gene are encoded by 138 nucleotides at the 3′ end of the sequenced region (Fig. 1). Over this portion of the protein, the R. capsulatus cbbT gene product is more similar to R. sphaeroides CbbT (91.3% identity) than to R. capsulatus TktA (69.6% identical), a second transketolase found in R. capsulatus (4) (Fig. 2E). Presumably, the latter deduced amino acid sequence is encoded by a heterotrophic transketolase.
An inverted repeat preceded by a sequence which matches a consensus RNase E cleavage site [(G/A)AUU(A/U)] (5) was found to be present within the 83-nucleotide cbbP-cbbT intergenic region. Since the combination of a hairpin preceded by an RNase cleavage site has recently been shown to be sufficient for cleavage of puf mRNA by an RNase E-like enzyme in R. capsulatus (11), this potential RNase E cleavage site could function in cbbII mRNA processing.
Construction and phenotypes of R. capsulatus cbb mutant strains.
Strains SBI− (cbbL), SBII− (cbbM), SBI-II (cbbL cbbM), SBP− (cbbP), SBRI− (cbbRI), and SBRII− (cbbRII) were constructed as described in Materials and Methods. The ability of the wild-type and mutant strains to grow under photoheterotrophic, photoautotrophic, and chemoautotrophic conditions was determined on solid media, and the results are presented in Table 1.
Characterization and complementation of RubisCO-minus strains.
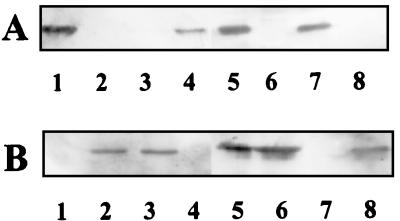
Analysis of R. sphaeroides cbbL and cbbM mutant strains revealed that disruption of the gene(s) encoding one RubisCO led to enhanced levels of RubisCO gene transcription, greater amounts of RubisCO protein, and enhanced enzyme activity of the remaining RubisCO. Indeed, the observed level of activity met or exceeded that present in the wild-type strain (14). To determine if a similar compensatory regulatory effect occurred in R. capsulatus, RubisCO activities and protein levels were assessed in cbbL and cbbM strains. The disruption of the cbbL and cbbM genes in R. capsulatus SBI− and SBII−, respectively, was confirmed by hybridization analyses of Southern blots (data not shown), and Western immunoblotting confirmed the lack of RubisCO protein corresponding to each mutated gene (Fig. 3). Unlike the wild-type strain, in which form II RubisCO is present in both photoheterotrophically and photoautotrophically grown cells (Fig. 3B, lanes 2 and 5), form II RubisCO was not present in extracts of either photoheterotrophically or photoautotrophically grown SBII− (Fig. 3B, lanes 4 and 7). Since wild-type strain SB1003 did not synthesize detectable levels of form I RubisCO under photoheterotrophic conditions (Fig. 3A, lane 2), the lack of form I RubisCO in strain SBI− was confirmed by Western blot analysis of extracts from photoautotrophically grown cells (Fig. 3A, lane 6). Despite the fact that form I RubisCO is not detectable in photoheterotrophically grown wild-type cells, either form I (strain SBII−) or form II (SBI−) RubisCO supported photoheterotrophic, photoautotrophic, and chemoautotrophic growth (Table 1); however, the doubling times for the mutant strains were slightly longer than for the wild-type under photoheterotrophic and photoautotrophic conditions (Table 2).
FIG. 3.
Western immunoblot analysis of R. capsulatus wild-type and RubisCO-minus strains. Purified Synechococcus sp. strain PCC 6301 RubisCO and purified R. sphaeroides form II RubisCO were loaded into lanes 1 and 8, respectively. Crude extracts (approximately 10 μg of protein) were loaded as follows: lane 2, photoheterotrophically grown SB1003; lane 3, photoheterotrophically grown SBI−; lane 4, photoheterotrophically grown SBII−; lane 5, photoautotrophically grown SB1003; lane 6, photoautotrophically grown SBI−; lane 7, photoautotrophically grown SBII−. Blots were incubated with antibody raised against Synechococcus strain PCC 6301 RubisCO (A) and R. sphaeroides form II RubisCO (B). The figure was generated as follows: the region of interest of each blot was converted to TIFF files by using the software provide with the Molecular Dynamics Storm 840 imaging system (Molecular Dynamics), the TIFF files were imported into CorelDraw 7.0 (Corel Corporation, Ottawa, Ontario, Canada), where frames and numbers were added, and the images were printed on a Kodak ColorEase PS printer.
TABLE 2.
Growth rates, RubisCO activities, and PRK activities of R. capsulatus wild-type and cbb mutant and complemented strains
| Strain | Growth conditiona | Doubling timeb (h) | Activity (nmol min−1
mg−1)
|
|
|---|---|---|---|---|
| RubisCO | PRK | |||
| SB1003 (wild type) | MAL | 5.0 | 30.7 ± 16.5 | 112.9 ± 12.4 |
| PA | 12.5 | 459.6 ± 51.0 | 451.1 ± 9.8 | |
| MAL/DMSO | 12.5 | 32.4 ± 8.4 | 95.3 ± 14.2 | |
| SBI− (cbbL) | MAL | 6.5 | 51.3 ± 3.1 | 76.5 ± 15.7 |
| PA | 15.5 | 362.8 ± 5.6 | 489.9 ± 36.2 | |
| SBII− (cbbM) | MAL | 7.0 | 31.7 ± 1.3 | 193.0 ± 25.3 |
| PA | 15.5 | 313.6 ± 18.4 | 506.3 ± 28.9 | |
| SBI-II (cbbL− cbbM) | MAL/DMSO | 14.5 | 0.0 | 25.7 ± 5.8 |
| SBI-II(pRPSFI-I) | MAL | 9.5 | 34.5 ± 0.4 | NDc |
| PA | 49.5 | 269.4 ± 13.9 | ND | |
| SBI-II(pRPSFII-I) | MAL | 10.5 | 29.8 ± 4.9 | ND |
| PA | 40.0 | 492.8 ± 39.2 | ND | |
| SBP− (cbbP) | MAL/DMSO | 8.0 | 3.0 ± 1.1 | 0.0 |
| SBP−(pRPS::RsPIA) | MAL | 16.5 | 12.8 ± 1.8 | 922.8 ± 202.4 |
| SBRI− (cbbRI) | MAL | 3.3 | 19.2 ± 0.6 | ND |
| PA | 13.3 | 237.8 ± 33.7 | ND | |
| SBRI−(pVK::CbbRI) | MAL | 2.9 | 16.2 ± 0.7 | ND |
| PA | 12.6 | 256.4 ± 21.5 | ND | |
| SBRII− (cbbRII) | MAL | 10.5 | 10.1 ± 1.9 | 11.3 ± 1.3 |
| SBRII−(pVK::CbbRII) | MAL | 7.5 | 11.8 ± 0.1 | 17.8 ± 3.4 |
| PA | 47.0 | 49.1 ± 3.5 | 64.5 ± 6.9 | |
MAL, photoheterotrophic growth on malate; PA, photoautotrophic growth on 1.5% CO2–98.5% H2; MAL/DMSO, photoheterotrophic growth on malate in the presence of 30 mM DMSO.
Average of at least two independent determinations, with no more than a 15% discrepancy for any one growth rate.
ND, not determined.
Strain SBII−, which was unable to make form II RubisCO, was capable of photoheterotrophic growth. Apparently the lack of form II RubisCO synthesis resulted in the compensatory synthesis of form I RubisCO under photoheterotrophic conditions (Fig. 3A, lane 4). The level of RubisCO activity in photoheterotrophically grown strain SBII−, which must be attributed to only form I RubisCO, was approximately the same as that observed for photoheterotrophically grown wild-type strain (Table 2). Likewise, the activity of form II RubisCO in photoheterotrophically grown SBI− was similar to but somewhat higher than that observed for the wild-type strain. Under photoautotrophic growth conditions, the levels of activity for the two RubisCO mutants approached that obtained in the wild-type strain (Table 2). This sort of compensation in RubisCO activity is analogous to that observed in R. sphaeroides form I or form II RubisCO-minus strains (14). The level of PRK activity in strains SBI− and SBII− did not vary significantly from that in the wild-type strain under either photoheterotrophic or photoautotrophic conditions (Table 2).
We constructed a strain lacking both forms of RubisCO (SBI-II) to determine if the CBB pathway was absolutely required for CO2 fixation during photosynthetic and chemoautotrophic growth of R. capsulatus and whether this strain had the potential to serve as a host for recombinant RubisCO synthesis. R. capsulatus cbbL cbbM strain SBI-II was unable to grow autotrophically or photoheterotrophically in the absence of an alternate electron acceptor (Table 1) but could grow photoheterotrophically on malate with a doubling time of 14.5 h when DMSO was supplied as an alternate electron acceptor (Table 2). Despite the fact that DMSO did not have a significant effect on RubisCO or PRK activity in the wild-type strain (Table 2), strain SBI-II lacked detectable RubisCO activity when grown photoheterotrophically on malate with DMSO (Table 2), and neither form I nor form II RubisCO was detectable in extracts from these cultures (data not shown). Strain SBI-II exhibited PRK activity, but at a reduced level compared to strains SB1003, SBI−, and SBII− (Table 2). R. capsulatus SBI-II could be complemented to photoheterotrophic and photoautotrophic growth with the R. capsulatus cbbLS or cbbM gene in plasmid pRPSFI-I or pRPSFII-I, respectively. Although RubisCO activity levels of the complemented strains were comparable to those in the wild-type strain, the complemented strains grew much more slowly than strain SB1003, particularly under photoautotrophic conditions (Table 2), similar to the situation with R. sphaeroides (7).
Characterization and complementation of the PRK-minus strain.
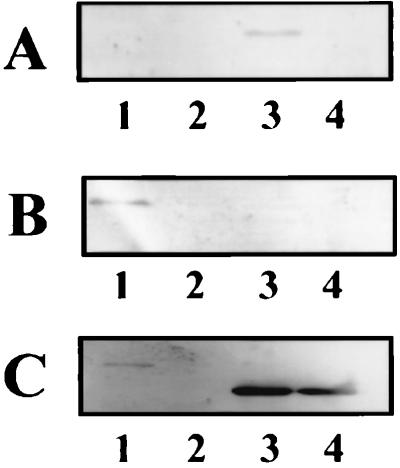
Unlike R. sphaeroides, R. capsulatus appears to have only a single copy of cbbP (39). Further evidence that there is only one copy of cbbP in R. capsulatus was provided by low-stringency Southern blot analysis of R. capsulatus genomic DNA, using a probe derived from the R. capsulatus cbbP gene. In each case, the size of the hybridizing fragment corresponded to the size predicted for cbbP-containing fragment for the cbbFPTGAM region (data not shown). Hybridization and wash conditions were used such that a second copy of cbbP would be predicted to be less than 60% identical to the cbbP probe. PRK is the only enzyme, other than RubisCO, that is unique to the CBB pathway; therefore, disruption of the R. capsulatus cbbP gene should abolish the CBB pathway. In addition, if the R. capsulatus cbbFPTGAM genes form an operon, disruption of cbbP would be expected to have a polar effect on the expression of downstream genes, including cbbM. The cbbP deletion strain, SBP−, was unable to grow photoautotrophically or chemoautotrophically and grew photoheterotrophically only when DMSO was supplied as an exogenous electron acceptor (Table 1). Strain SBP− lacked detectable PRK activity when grown photoheterotrophically with DMSO (Table 2) despite the fact that the presence of DMSO did not significantly reduce the level of PRK activity in the wild-type strain (Table 2). Additionally, Western immunoblot analysis showed low but detectable amounts of PRK in extracts from strain SB1003 grown photoheterotrophically in the presence of DMSO, while strain SBP− lacked detectable levels of PRK protein (Fig. 4C, lanes 1 and 2). A concomitant loss of detectable levels of form II RubisCO protein was also observed in SBP− (Fig. 4B, lanes 1 and 2). The level of RubisCO activity in strain SBP− grown photoheterotrophically in the presence of DMSO was much lower than that in wild-type strain SB1003 (Table 2), and unlike in strain SBII−, the compensatory synthesis of form I RubisCO was not observed (Fig. 4A, lane 2). Complementation of R. capsulatus SBP− with R. sphaeroides cbbPI in expression vector pRPS-1 (pRPS::RsPI) resulted in photoheterotrophic growth without a requirement for DMSO. Complementation was dependent on the proper orientation of the inserted DNA fragment. Despite very high PRK activity and PRK protein synthesis in the complemented strain (Table 2; Fig. 4C, lane 3), plasmid pRPS::RsPI did not complement strain SBP− to photoautotrophic growth. A fourfold increase in RubisCO activity was also noted when strain SBP− was complemented with plasmid pRPS::RsPI, and only form I RubisCO protein was detected (Table 2; Fig. 4A and B, lane 3). The loss of form II RubisCO protein in strain SBP− and the synthesis of only form I RubisCO in the complemented strain provide additional evidence that the cbbII genes are cotranscribed.
FIG. 4.
Western blot analysis the R. capsulatus wild-type and PRK-minus strains. Crude extracts were prepared and loaded (approximately 10 μg of protein) as follow: lane 1, SB1003 grown photoheterotrophically with malate in the presence of DMSO; lane 2, SBP− grown photoheterotrophically with malate in the presence of DMSO; lane 3, strain SBP− with plasmid pRPS::RsPI grown photoheterotrophically with malate; lane 4, purified R. sphaeroides PRK I. Blots were incubated with antibody raised against Synechococcus strain PCC 6301 RubisCO (A), R. sphaeroides form II RubisCO (B), and R. sphaeroides PRK I (C). The figure was generated as described in the legend to Fig. 3.
Characterization and complementation of CbbR I- and CbbR II-minus strains.
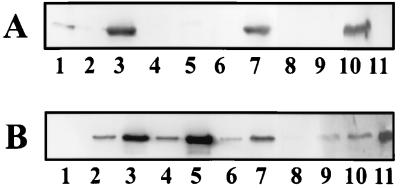
R. capsulatus strains in which cbbRI and cbbRII were insertionally inactivated were constructed to establish a physiological role for the respective cbbR gene products. A strain in which the cbbRI gene was disrupted, strain SBRI−, exhibited no phenotype (Table 1), and under photoheterotrophic conditions, RubisCO activity in this strain was not significantly lower than the level in the wild-type strain (Table 2). Interestingly, about one-half of the wild-type RubisCO activity was detected in strain SBRI− grown under photoautotrophic conditions (Table 2). Since form I RubisCO is not synthesized in photoheterotrophically grown SB1003, these results (wild-type RubisCO activity under photoheterotrophic conditions and reduced RubisCO activity under photoautotrophic conditions) would be consistent with the absence of form I RubisCO synthesis in strain SBRI−. Western immunoblot analysis confirmed that RubisCO activity in strain SBRI− was due solely to form II RubisCO synthesis (Fig. 5). Form II RubisCO synthesis in strain SBRI− was qualitatively similar to that in the wild-type strain under both photoheterotrophic and photoautotrophic conditions (Fig. 5B, lanes 2 to 5). As in the wild-type strain, no form I RubisCO was present in extracts prepared from photoheterotrophically grown SBRI− (Fig. 5A, lanes 2 and 4), but unlike wild-type strain SB1003, strain SBRI− did not synthesize form I RubisCO even under photoautotrophic conditions (Fig. 5A, lanes 3 and 5). Although a slight reaction with the anti-form I RubisCO is visible in Fig. 5A, lane 5, this was not due to the presence form I RubisCO in extracts of photoautotrophically grown SBRI− because the cross-reacting protein is of higher apparent molecular weight than the form I RubisCO and it was not detected in subsequent Western immunoblots (data not shown). Introduction of the R. capsulatus cbbRI gene into strain SBRI− on plasmid pVK::CbbRI restored the ability to synthesize high levels of form I RubisCO under photoautotrophic conditions (Fig. 5A, lane 7) without an apparent effect on form I RubisCO synthesis under photoheterotrophic conditions (Fig. 5A, lane 6).
FIG. 5.
Western immunoblot analysis of R. capsulatus wild-type and CbbR-minus strains. Purified Synechococcus sp. strain PCC 6301 RubisCO and purified R. sphaeroides form II RubisCO were loaded into lanes 1 and 11, respectively. Crude extracts were loaded (approximately 10 μg of protein) as follows: lane 2, photoheterotrophically grown SB1003; lane 3, photoautotrophically grown SB1003; lane 4, photoheterotrophically grown SBRI−; lane 5, photoautotrophically grown SBRI−; lane 6, photoheterotrophically grown SBRI− with pVK::CbbRI; lane 7, photoautotrophically grown SBRI− with pVK::CbbRI; lane 8, photoheterotrophically grown SBRII−; lane 9, photoheterotrophically grown SBRII− with plasmid pVK::CbbRII; lane 10, photoautotrophically grown SBRII− with plasmid pVK::CbbRII. Blots were incubated with antibody raised against Synechococcus strain PCC 6301 RubisCO (A) and R. sphaeroides form II RubisCO (B). The figure was generated as described in the legend to Fig. 3.
A strain in which cbbRII was insertionally inactivated, SBRII−, was unable to grow photo- or chemoautotrophically (Table 1) but grew photoheterotrophically on malate at a reduced rate (Table 2). RubisCO and PRK activities in the cbbRII− strain were reduced to 33 and 10%, respectively, of the activity observed in photoheterotrophically grown wild-type strain SB1003 (Table 2). Form II RubisCO protein was barely detectable in strain SBRII− compared to wild-type strain SB1003 (Fig. 5B, lanes 2 and 8). Unlike the response in strain SBII− (Fig. 3A), there was no apparent synthesis of form I RubisCO in response to the drastically reduced levels of form II RubisCO in strain SBRII− (Fig. 5A, lane 8). Strain SBRII− was complemented to photoautotrophic growth by the cbbRII gene on plasmid pVK::CbbRII. Despite the ability to complement strain SBRII− to autotrophic growth, the introduction of plasmid pVK::CbbRII did not restore the PRK or RubisCO activities to wild-type levels under either photoheterotrophic or photoautotrophic conditions (Table 2), and the photoautotrophic growth rate of the complemented strain was severely reduced relative to the wild-type rate (Table 2). Lack of complementation to wild-type enzyme activities might be due to the presence of the cbbII promoter, but not the cbbII genes, on complementing plasmid pVK::CbbRII. Binding of CbbRII to the cbbII promoter on the plasmid could titrate the activator away from the chromosomal cbbII promoter without leading to productive transcription. The complementation of strain SBRII− by plasmid pVK::CbbRII restored form II RubisCO synthesis under photoheterotrophic conditions (Fig. 5B, lane 9), and both form I and form II RubisCO were synthesized in photoautotrophically grown strain SBRII−(pVK::CbbRII) (Fig. 5A and B, lanes 10).
Analysis of R. capsulatus cbb promoter fusion constructs.
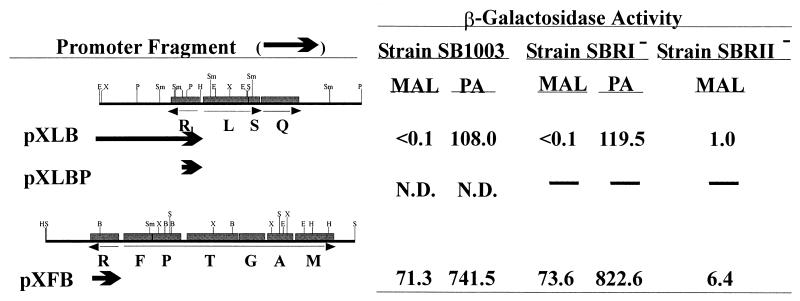
Promoter fusions were constructed to further examine the regulation of the cbb operons under photoheterotrophic and photoautotrophic conditions. β-Galactosidase activity was measured in extracts from SB1003 containing plasmid-borne fusions of lacZ to cbbIp and cbbIIp (Table 1; Fig. 6). Under photoheterotrophic conditions, β-galactosidase activity was nearly undetectable in strain SB1003 containing the cbbIp-lacZ fusion pXLB (Fig. 6), consistent with the finding that form I RubisCO was not detected in R. capsulatus grown photoheterotrophically on malate (Table 2; Fig. 3). In agreement with previous studies (39, 46) and data presented here (Table 2; Fig. 3), which show that form II RubisCO is synthesized under photoheterotrophic conditions, β-galactosidase activities in photoheterotrophically grown SB1003 containing a cbbIIp-lacZ fusion (pXFB) indicated that transcription occurred from cbbIIp under these conditions (Fig. 6). Increased β-galactosidase activity was observed in photoautotrophically grown SB1003 harboring either pXLB or pXFB, suggesting that transcription from cbbIp and cbbIIp is induced under photoautotrophic conditions.
FIG. 6.
β-Galactosidase activity from R. capsulatus cbbp-lacZ fusions. The cbb promoter fragments that were fused to the lacZ gene in vector pXBA601 are represented by arrows. In plasmids pXLB and pXLBP, cbbIp was fused to lacZ at the ATG start codon of cbbL. cbbIIp was fused to the ATG start codon of cbbF in plasmid pXFB. β-Galactosidase activity is expressed in nanomoles/minute/milligram. The growth conditions were photoheterotrophically with malate as a carbon source (MAL) and photoautotrophically (PA). Strain SBRII− did not grow photoautotrophically, and so the β-galactosidase activity could not be determined. N.D., not detectable; —, not determined. The values are averages derived from multiple assays of two independent cultures for each strain.
The role of CbbR II as a transcriptional activator of cbbII was further established by measuring β-galactosidase activities in photoheterotrophically grown strain SBRII−(pXFB). The level of β-galactosidase activity expressed from the cbbIIp fusion construct in the cbbRII mutant strain was about 9% of that observed in the wild-type strain (Fig. 6). Any role for CbbR II in transcriptional activation at cbbIp could not be addressed directly because the cbbIp-lacZ fusion did not result in significant β-galactosidase activity in either strain SB1003 or strain SBRII−, and the latter strain did not grow under photoautotrophic conditions. In addition, although a difference in β-galactosidase activity was observed in strains SB1003 and SBRII− containing pXLB, the activities were too low to determine if the differences were significant.
Direct evidence for transcriptional activation at cbbIp by CbbR I could not be obtained by introducing the cbbIp-lacZ fusions into strain SBRI−. A cbbIp-lacZ fusion containing a truncated cbbRI (pXLBP) was constructed (Table 1; Fig. 6) but did not yield detectable β-galactosidase in the wild-type strain even under photoautotrophic conditions (Fig. 6). β-Galactosidase activity in SBRI−(pXLB) was similar to that measured in SB1003 containing plasmid pXLB (Fig. 6), but this was probably due to the complementation of strain SBRI− by the copy of the cbbRI gene on the promoter fragment in this construct. The levels of β-galactosidase activity in SBRI−(pXFB) under photoheterotrophic and photoautotrophic growth conditions were very similar to or slightly higher than those measured in the wild-type strain containing pXFB (Fig. 6), suggesting that CbbR I does not act as a positive regulator at cbbIIp.
DISCUSSION
Previous studies established that cbb gene organization in R. capsulatus is different from the situation for the cbb regulon of the closely related organism R. sphaeroides (38, 39). The present study elaborated additional features of cbb gene organization and control in R. capsulatus as a prelude to further detailed investigations of cbb regulation in this organism. The finding that the cbbP ribosomal binding site was within the cbbF coding region and the polar effect of the cbbP disruption on form II RubisCO synthesis provided strong evidence that the R. capsulatus cbbFPTGAM genes make up an operon (cbbII operon). Moreover, the presence of a potential RNase E cleavage site within the R. capsulatus cbbP-cbbT intergenic region hints that posttranscriptional processing of the R. capsulatus cbbII message may occur, reminiscent of the suggested posttranscriptional processing of cbb operon transcripts of R. sphaeroides (14). Despite these similarities, a very significant difference between the R. capsulatus and R. sphaeroides cbbII operons is the presence of a cbbR gene, cbbRII, divergently transcribed from the R. capsulatus cbbII operon. In addition, a second cbbR gene, cbbRI, is upstream and divergently transcribed from the cbbLS (cbbI) operon. It has become well established that CbbR is involved in the regulation of cbb gene expression in a number of autotrophic bacteria, including C. vinosum (54), Ralstonia eutropha (formerly Alcaligenes eutrophus) (59), Xanthobacter flavus (34), R. sphaeroides (16), Rhodospirillum rubrum (8), and Thiobacillus ferrooxidans (26). These studies are buttressed by the finding that CbbR binds to the promoter region of the cbb operon (26, 27, 52, 53), with a physiological role for CbbR now well established (16, 52, 59). In R. eutropha (59), R. sphaeroides (16), and X. flavus (52), the product of a single cbbR gene regulates transcription from at least two different promoters. Consequently, transcription from these operons is coordinately activated within a single CbbR regulon. Only Thiobacillus denitrificans (30) and R. capsulatus have two potentially functional cbbR genes. The presence of two CbbR proteins in R. capsulatus raises questions concerning the involvement of each form of CbbR in the expression of the two or more cbb promoters found in this organism. The likelihood that CbbR I controls only the cbbI operon was previously suggested since the cbbRI, cbbLS, and cbbQ genes were all apparently acquired by horizontal gene transfer (38). Thus, to probe the role of the two CbbRs in R. capsulatus cbb gene regulation, strains with disruptions in cbbRI and cbbRII were constructed and characterized. A strain (SBRII−) in which the cbbRII gene was disrupted was unable to grow autotrophically and grew at a reduced rate photoheterotrophically, showing reduced levels of both PRK and RubisCO activity and form II RubisCO protein. In addition, β-galactosidase activity derived from cbbIIp-lacZ fusion pXFB in strain SBRII− was only about 9% of the activity of the wild-type strain under photoheterotrophic conditions. These results clearly implicate CbbR II in activation of transcription at cbbIIp. The presence of PRK activity in strain SBRII− indicates that some transcription from cbbIIp occurred in the absence of CbbR II, albeit at an apparently reduced rate. Whether this was due to cross-talk activation by CbbR I remains to be established; however, it should be noted that transcription from cbbIIp is not entirely dependent on CbbR in R. sphaeroides (16). The lack of form I RubisCO in photoautotrophically grown strain SBRI− provides evidence that CbbR I is involved in the regulation of form I RubisCO synthesis, probably by activating transcription at cbbIp. Furthermore, the absence of form I RubisCO in photoautotrophically grown strain SBRI− indicates that CbbR II is unable to activate transcription from cbbIp. In addition, the ability of strain SBRI− to grow under photoheterotrophic and autotrophic conditions, and the apparent normal level of form II RubisCO synthesis in this strain, demonstrate that CbbR I is not required for expression of the cbbII operon. The data strongly indicate that the CbbR I and CbbR II proteins are necessary for normal regulation of the cbbI and cbbII operons, respectively, and that cross-talk activation of the cbb operons by the opposite CbbR protein does not occur. These studies thus provide the first indication that the cbb operons may belong to independent CbbR regulons.
LysR-type transcriptional activators generally bind to the promoter they activate, even under noninducing conditions, and the binding of a low-molecular-weight coinducer molecule to the LysR-type protein is required, in most cases, to activate transcription (45). It will be interesting to determine if independent regulation of the R. capsulatus cbb operons by the cognate CbbR proteins correlates with activation by unique coinducer molecules. Activation of transcription at cbbIIp by CbbR II under photoheterotrophic conditions, and lack of transcriptional activation at cbbIp by CbbR I under the same conditions, indicate that either a repressor binds to cbbIp under photoheterotrophic conditions, different inducer molecules bind to the different CbbR proteins, or the CbbR proteins bind the same inducer with different affinities. In the latter case, it is possible that the intracellular concentration of the inducing metabolite increases under photoautotrophic conditions, resulting in activation of transcription at cbbIp by CbbR I. Certainly, the presence of two different CbbR proteins raises additional questions about DNA binding specificity. Since CbbR probably binds to the cbb promoter region in the absence of an inducer molecule, binding must be specific to prevent repressive effects on the opposite promoter (i.e., binding of CbbR II to the cbbI promoter may not activate transcription but could prevent the binding of CbbR I). Current studies are directed at examining the specificity of CbbR I and CbbR II in vitro.
The product of the qor gene discovered downstream of cbbRII may also serve to regulate cbb gene expression. This gene encodes a soluble NAD(P)H QOR that catalyzes the reversible transfer of electrons from reduced pyridine nucleotides, with a preference for NADPH, to membrane-bound quinones. On the basis of the reaction catalyzed by this enzyme, QOR could function to sense or maintain the redox state of the membrane quinone pool. Interestingly, NADPH has been implicated as the coinducer of CbbR transcriptional activation in X. flavus (53). Thus, as a redox sensor, QOR could be involved in the regulation of cbb gene expression or perhaps in regulating the CBB pathway enzymes.
Although the evidence discussed above demonstrates that the cbbI and cbbII operons are differentially regulated by the two CbbR proteins, additional evidence suggests that regulation of these operons is also coordinated. When either cbbM or cbbL was disrupted, the absence of the missing RubisCO was compensated for, such that levels of RubisCO did not differ significantly from that in the wild-type strain. This compensation is analogous to what was observed in R. sphaeroides (14). However, the compensation effect was most dramatically demonstrated in R. capsulatus by the ability of strain SBII− to grow photoheterotrophically, concomitant with the synthesis of form I RubisCO. Since the wild-type strain did not synthesize detectable levels of form I RubisCO under photoheterotrophic conditions, the absence of form II RubisCO synthesis in strain SBII− somehow signaled the cell to compensate, by making form I RubisCO. However, compensation of form I RubisCO synthesis (Table 2) was not manifested by the cbbP mutant (strain SBP−), in which form II RubisCO is not synthesized due to a polar effect of this mutation on cbbM (Fig. 4). These results thus suggest that the balance of various intermediates of the CBB pathway might regulate gene expression, which is an area that is currently being explored.
In summary, R. capsulatus cbb gene regulation is quite complex and differs markedly from that in R. sphaeroides. Two different CbbR transcriptional activators that allow autonomous regulation of the cbbI and cbbII operons, perhaps by binding different inducer molecules, are present in R. capsulatus. Obviously, to allow efficient regulation, the CbbR proteins must bind specifically to their respective cbb promoters. The presence of a potential RNase E recognition site within the cbbII message suggests that it is posttranscriptionally processed. Further study of R. capsulatus cbb gene regulation will not only provide a better understanding of the control of CO2 fixation but also address more general questions of gene regulation, such as the specificity of DNA-protein interactions and the significance of mRNA processing in prokaryotes.
ACKNOWLEDGMENTS
We thank J. T. Beatty for providing plasmid pXBA601 and Wenona Stankiewicz for technical assistance.
P.V. acknowledges the support provided by a Royal Thai graduate student scholarship. This work was supported by Public Health Service grant GM45404 from the National Institutes of Health.
REFERENCES
- 1.Adams C W, Forrest M E, Cohen S N, Beatty J T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus pufoperon. J Bacteriol. 1989;171:473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- 3.Charlier H A, Jr, Runquist J A, Miziorko H M. Evidence supporting catalytic roles for aspartate residues in phosphoribulokinase. Biochemistry. 1994;33:9343–9350. doi: 10.1021/bi00197a039. [DOI] [PubMed] [Google Scholar]
- 4.de Sury d’Aspremont R, Toussaint B, Vignais P M. Isolation of Rhodobacter capsulatus transketolase: cloning and sequencing of its structural tklAgene. Gene. 1996;169:81–84. doi: 10.1016/0378-1119(95)00796-2. [DOI] [PubMed] [Google Scholar]
- 5.Ehretsmann C P, Carpousis A J, Krisch H M. Specificity of Escherichia coli endoribonuclease RNase E: in vivo and in vitroanalysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992;6:149–159. doi: 10.1101/gad.6.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 7.Falcone D L, Tabita F R. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J Bacteriol. 1991;173:2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falcone D L, Tabita F R. Complementation analysis and regulation of CO2 fixation gene expression in a ribulose 1,5-bisphosphate carboxylase/oxygenase deletion strain of Rhodospirillum rubrum. J Bacteriol. 1993;175:5066–5077. doi: 10.1128/jb.175.16.5066-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Falcone, D. L., and F. R. Tabita. Unpublished data.
- 9.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 10.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritsch J, Rothfuchs R, Rauhut R, Klug G. Identification of an mRNA element promoting rate-limiting cleavage of the polycistronic puf mRNA in Rhodobacter capsulatusby an enzyme similar to RNase E. Mol Microbiol. 1995;15:1017–1029. doi: 10.1111/j.1365-2958.1995.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 12.Gibson J L. Genetic analysis of CO2 fixation genes. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publisher; 1995. pp. 1107–1124. [Google Scholar]
- 13.Gibson J L, Chen J-H, Tower P A, Tabita F R. The form II fructose 1,6-bisphosphatase and phosphoribulokinase genes form part of a large operon in Rhodobacter sphaeroides: primary structure and insertional mutagenesis analysis. Biochemistry. 1990;29:8085–8093. doi: 10.1021/bi00487a014. [DOI] [PubMed] [Google Scholar]
- 14.Gibson J L, Falcone D L, Tabita F R. Nucleotide sequence, transcriptional analysis and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991;266:14646–14653. [PubMed] [Google Scholar]
- 15.Gibson J L, Tabita F R. Isolation and preliminary characterization of two forms of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas capsulata. J Bacteriol. 1977;132:818–823. doi: 10.1128/jb.132.3.818-823.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson J L, Tabita F R. Nucleotide sequence and functional analysis of CbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J Bacteriol. 1993;175:5778–5784. doi: 10.1128/jb.175.18.5778-5784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson J L, Tabita F R. The molecular regulation of the reductive pentose phosphate pathway in proteobacteria and cyanobacteria. Arch Microbiol. 1996;166:141–150. doi: 10.1007/s002030050369. [DOI] [PubMed] [Google Scholar]
- 18.Gibson J L, Tabita F R. Analysis of the cbbXYZ operon of Rhodobacter sphaeroides. J Bacteriol. 1997;179:663–669. doi: 10.1128/jb.179.3.663-669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimberg J, Maguire S, Belluscio L. A simple method for the preparation of plasmid and chromosomal E. coliDNA. Nucleic Acids Res. 1989;17:8893. doi: 10.1093/nar/17.21.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen T A, van Gemerden H. Sulfide utilization by purple nonsulfur bacteria. Arch Microbiol. 1972;86:49–56. doi: 10.1007/BF00412399. [DOI] [PubMed] [Google Scholar]
- 20a.Horken, K. M., and F. R. Tabita. Unpublished data.
- 21.Kay R, McPherson J. Hybrid pUC vectors for addition of new restriction enzyme sites to the ends of DNA fragments. Nucleic Acids Res. 1987;15:2778. doi: 10.1093/nar/15.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 23.Knauf V C, Nester E. Wide host range cloning vectors: a cosmid bank of an AgrobacteriumTi plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 24.Kreiger T J, Mende-Mueller L, Miziorko H M. Phosphoribulokinase: isolation and sequence determination of the cysteine-containing active-site peptide modified by 5′-p-fluorosulfonylbenzoyladenosine. Biochim Biophys Acta. 1987;915:112–119. doi: 10.1016/0167-4838(87)90130-0. [DOI] [PubMed] [Google Scholar]
- 25.Kreiger T J, Miziorko H M. Affinity labeling and purification of spinach leaf ribulose-5-phosphate kinase. Biochemistry. 1986;25:247–252. doi: 10.1021/bi00360a003. [DOI] [PubMed] [Google Scholar]
- 26.Kusano T, Sugawara K. Specific binding of Thiobacillus ferrooxidans RbcR to the intergenic sequence between the rbc operon and the rbcRgene. J Bacteriol. 1993;175:1019–1025. doi: 10.1128/jb.175.4.1019-1025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusian B, Bowien B. Operator binding of the CbbR protein, which activates the duplicate cbb CO2 assimilation operons of Alcaligenes eutrophus. J Bacteriol. 1995;177:6568–6574. doi: 10.1128/jb.177.22.6568-6574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lilley P E, Stamford N P J, Vasudevan S G, Dixon N E. The 92-min region of the Escherichia coli chromosome: location and cloning of the ubiA and alrgenes. Gene. 1993;129:9–16. doi: 10.1016/0378-1119(93)90690-5. [DOI] [PubMed] [Google Scholar]
- 30.Lorbach S C, Shively J M. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Identification, isolation, and sequencing of the ribulose bisphosphate carboxylase/oxygenase regulatory genes (cbbRI and cbbRII) in Thiobacillus denitrificans, abstr. H207; p. 528. [Google Scholar]
- 31.Madigan M T, Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2as the energy source. J Bacteriol. 1979;137:524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 33.Mead D A, Szczesna-Skorupa E, Kemper B. Single-stranded DNA ’blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986;1:67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Meijer W G, Arnberg A C, Enequist H G, Terpstra P, Lidstrom M E, Dijkhuizen L. Identification and organization of the carbon dioxide fixation genes in Xanthobacter flavusH4-14. Mol Gen Genet. 1991;225:320–330. doi: 10.1007/BF00269865. [DOI] [PubMed] [Google Scholar]
- 35.Meijer W G, Tabita F R. Isolation and characterization of the nifUSVW-rpoN gene cluster from Rhodobacter sphaeroides. J Bacteriol. 1992;174:3855–3866. doi: 10.1128/jb.174.12.3855-3866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 36a.Novak, J. S., and F. R. Tabita. Unpublished data.
- 37.Ormerod J G, Ormerod K S, Gest H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by the photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- 38.Paoli G C, Soyer F, Shively J M, Tabita F R. Rhodobacter capsulatus genes encoding form I ribulose-1,5-bisphosphate carboxylase/oxygenase (cbbLS) and neighbouring genes were acquired by a horizontal gene transfer. Microbiology. 1998;144:219–227. doi: 10.1099/00221287-144-1-219. [DOI] [PubMed] [Google Scholar]
- 39.Paoli G C, Strom-Morgan N, Shively J M, Tabita F R. Expression of the cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch Microbiol. 1995;164:396–405. [PubMed] [Google Scholar]
- 40.Penfold R J, Pemberton J M. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene. 1992;118:145–146. doi: 10.1016/0378-1119(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 41.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 42.Pridmore R D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 43.Richardson D J, King G F, Kelly D J, McEwan A G, Ferguson S J, Jackson J B. The role of auxiliary oxidants in maintaining redox balance during growth of Rhodobacter capsulatuson propionate and butyrate. Arch Microbiol. 1988;150:131–137. [Google Scholar]
- 44.Sandbaken M G, Runquist J A, Barbieri J T, Miziorko H M. Identification of the phosphoribulokinase sugar phosphate binding domain. Biochemistry. 1992;31:3715–3719. doi: 10.1021/bi00129a022. [DOI] [PubMed] [Google Scholar]
- 45.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 46.Shively J M, Davidson E, Marrs B L. Depression of the synthesis of the intermediate and large forms of ribulose-1,5-bisphosphate carboxylase/oxygenase in Rhodopseudomonas capsulatus. Arch Microbiol. 1984;138:233–236. doi: 10.1007/BF00402127. [DOI] [PubMed] [Google Scholar]
- 47.Tabita F R. Pyridine nucleotide control and subunit structure of phosphoribulokinase from photosynthetic bacteria. J Bacteriol. 1980;143:1275–1280. doi: 10.1128/jb.143.3.1275-1280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabita F R. The biochemistry and molecular regulation of carbon metabolism and CO2 fixation in purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 885–914. [Google Scholar]
- 49.Thorn J M, Barton J D, Dixon N E, Ollis D L, Edward K J. Crystal structure of the Escherichia coliquinone oxidoreductase complexed with NADPH. J Mol Biol. 1995;249:785–799. doi: 10.1006/jmbi.1995.0337. [DOI] [PubMed] [Google Scholar]
- 50.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uttaro A D, Ugalde R S. A chromosomal cluster of genes encoding ADP-glucose synthetase, glycogen synthase, and phosphoglucomutase in Agrobacterium tumefaciens. Gene. 1994;150:117–122. doi: 10.1016/0378-1119(94)90869-9. [DOI] [PubMed] [Google Scholar]
- 52.van den Bergh E R E, Dijkhuizen L, Meijer W G. CbbR, a LysR-type transcriptional activator, is required for expression of the autotrophic CO2 fixation enzymes of Xanthobacter flavus. J Bacteriol. 1993;175:6097–6104. doi: 10.1128/jb.175.19.6097-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Keulen G, Girbal L, van den Bergh E R E, Dijkhuizen L, Meijer W G. The LysR-type transcriptional regulator CbbR controlling autotrophic CO2 fixation by Xanthobacter flavusis an NADPH sensor. J Bacteriol. 1998;180:1141–1147. doi: 10.1128/jb.180.6.1411-1417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viale A M, Kobayashi H, Akazawa T, Henikoff S. rbcR, a gene coding for a member of the LysR family of transcriptional regulators, is located upstream of the expressed set of ribulose 1,5-bisphosphate carboxylase/oxygenase genes in the photosynthetic bacterium Chromatium vinosum. J Bacteriol. 1991;173:5224–5229. doi: 10.1128/jb.173.16.5224-5229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallenfels K. β-Galactosidase (crystalline) Methods Enzymol. 1962;5:212–219. [Google Scholar]
- 56.Wang X, Falcone D L, Tabita F R. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroidesand evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain redox balance of the cell. J Bacteriol. 1993;175:3372–3379. doi: 10.1128/jb.175.11.3372-3379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weaver K E, Tabita F R. Isolation and partial characterization of Rhodopseudomonas sphaeroidesmutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983;156:507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitehouse D B, Putt W, Lovegrove J U, Morrison K, Hollyoake M, Fox M F, Hopkinson D A, Edwards Y H. Phosphoglucomutase 1: complete human and rabbit mRNA sequences and direct mapping of this highly polymorphic marker on human chromosome 1. Proc Natl Acad Sci USA. 1992;89:411–415. doi: 10.1073/pnas.89.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Windhovel U, Bowien B. Identification of cfxR, an activator gene of autotrophic CO2 fixation in Alcaligenes eutrophus. Mol Microbiol. 1991;5:2695–2705. doi: 10.1111/j.1365-2958.1991.tb01978.x. [DOI] [PubMed] [Google Scholar]
- 60.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains; nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 61.Yen H C, Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulatus. J Bacteriol. 1976;126:619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoyama K, Hayashi N R, Arai S Y C H, Igarashi Y, Kodama T. Genes encoding RubisCO in Pseudomonas hydrogenothermophila are followed by a novel cbbQ gene similar to nirQ of the denitrification gene cluster from Pseudomonasspecies. Gene. 1995;153:75–79. doi: 10.1016/0378-1119(94)00808-6. [DOI] [PubMed] [Google Scholar]