Abstract
(1) Background: Breast cancer is the most prevalent cancer found in women in Mali. The aim of the current study was to determine the association between metabolites circulating in the blood, 25(OH)D and 1,25(OH)2D, and vitamin D levels with the risk of breast cancer in Malian women. (2) Methods: We conducted a prospective case–control study from August 2021 to March 2022. Control subjects were matched to cases according to age (within 5 years). The patients’ clinical stage was determined by the oncologist according to the tumour–nodes–metastasis (TNM) classification system. (3) Results: We observed no differences in the mean 25(OH)D (p = 0.221) and 1,25(OH)2D (p = 0.285) between cases and controls. However, our findings indicate a more pronounced inverse association in the first level of plasma 25(OH)D, while the risk function decreases at higher levels. This observation takes strength with 1,25(OH)2D by a significant association between the first quartile and breast cancer as a risk factor (p = 0.03; OR = 71.84; CI: 1.36–3785.34). (4) Conclusions: These outcomes showed a possible association between 25(OH)D and 1,25(OH)2D in decreasing the risk of breast cancer.
Keywords: breast cancer; 25-hydroxyvitamin D; 1,25-dihydroxyvitamin D; hypovitaminosis D; metastasis
1. Introduction
Breast cancer (BC) is the most prevalent cause of death by cancer among women [1], particularly in Africa [2]. In Mali, breast cancer represented 24.8% of cancers in women in 2020 according to the GLOBOCAN analysis report, making it the most common form of cancer in women in the country [3].
For a long time, vitamin D (vitD) has been understood to have certain effects on phosphocalcic metabolism and bone mineralisation. Our fundamental and clinical knowledge of the multitissue influence of this steroid has, however, evolved in recent years. The natural form of vitamin D, vitamin D3 (cholecalciferol), is obtained from dietary sources and is also generated in the human skin under the influence of sunlight (ultraviolet B radiation) from its precursor, 7-dehydrocholesterol [4,5,6]. Two hydroxylation steps generate the biologically active form of vitamin D3, the 1α,25-dihydroxyvitamin D (1,25(OH)2D). The first hydroxylation starts with carbon 25 by CYP2R1/CYP27A1 (cytochrome P450 enzymes) in the liver and the next in the kidney by CYP27B1, which hydroxylates 25-hydroxyvitamin D (25(OH)D) at carbon 1. 1,25(OH)2D3 exercises its biological functions by binding to the vitamin D receptor (VDR), a transcription factor that regulates gene expression in vitamin D target tissues [7,8]. It is known to have multiple antiproliferative, proapoptotic, prodifferentiating, and anti-inflammatory effects on malignant cells, as in BC [7].
However, epidemiological evidence for the relationship between plasma 25(OH)D and BC incidence is limited and conflicting [9,10]. Indeed, several longitudinal studies on serum 25(OH)D and multiple cancer risks have concluded that 25(OH)D concentrations are inversely associated with the incidence of colorectal cancer [10,11] but not with prostate cancer or BC incidence [10,12,13], while many studies suggest that vitamin D may reduce the risk of BC [14,15,16] and is also subtype-specific [17].
Racial differences in both BC and vitamin D metabolism have been described [17,18]. Dark skin pigmentation may play an important role in the deficit of circulating vitamin D levels [19]. And geography likely plays a part too. Mali is a sub-Saharan country that receives large amounts of sunlight and has prolonged periods of high temperatures (the maximum temperature varies between 34 °C and 37 °C). Its climate is both dry, with the Sahara desert in the north, and tropical, with the Sahel region running through the centre. Some studies have been published on 25(OH)D [15,20] in Africa. To our knowledge, no published studies explore 25(OH)D and 1,25(OH)2D levels and their relationship to breast cancer in sub-Saharan countries, such as Mali.
The aim of the current study was to determine the association between the blood-circulating metabolite 25(OH)D and the active metabolite 1,25(OH)2D of vitamin D levels with the risk of breast cancer amongst Malian women.
2. Materials and Methods
2.1. Study Population
This is a prospective case–control study in a population of Malian women. This study was carried out over a period of 8 months, from August 2021 to March 2022. The questionnaire and methodology for this study was approved by the Ethics Committee of the UNIVERSITY OF SCIENCE, TECHNIQUES AND TECHNOLOGIES OF BAMAKO (Ethics approval number: 2021/236/USTTB).
The recruitment of breast cancer cases was carried out after histological confirmation in the medical oncology departments of two university hospitals: the Luxembourg “Mère-Enfant” and that of Point G. Analysis took place at the Rodolphe Merieux Laboratory (RML) at the Charles Merieux Center for Infectiology (CMCI) in Mali.
This current study included 110 women with breast cancer and 110 women without breast cancer for 25(OH)D assays. Randomly, we measured 1,25(OH)2D in 35 women from the BC group and 35 women in the control group, with no selection criteria. This study was restricted to females aged 18 and above.
We included all patients living in Mali for more than a year before inclusion with newly diagnosed breast cancer in the 2 departments, regardless of the grade, who had not initiated chemotherapy treatment. The patients’ clinical stage was determined by the oncologist according to the tumour–nodes–metastasis (TNM) classification system [21]. This categorisation divides breast cancer into 4 stages, from I to IV, based on the following criteria: localised invasive breast cancer (stages I and II); inoperable locally advanced invasive breast cancer (stage III); and metastatic disease (stage IV).
Control subjects were matched to cases according to age (within 5 years). They were all apparently healthy, cancer-free women accompanying the patients, coming from gynaecology services or coming to the LRM for their assessment. We included only the women who had evidence of a recent consultation or a follow-up with a gynaecologist attesting to their health or women with normal mammography data. Patients with a history of cancers other than breast cancer were excluded, as were patients with diseases that would prevent them from exposing themselves to the sun. Pregnant, lactating women and women using vitamin D supplements were excluded at the time of enrolment from both cases and controls. Patients whose blood samples underwent haemolysis were also excluded. All patients with and without cancer provided written informed consent, following which they were given a questionnaire to fill in during an interview. All participants were informed about the aim of the study by a general information document.
The questionnaire contained variables regarding information on sociodemographic data, cancer history, parity, chronic diseases (such as diabetes mellitus, high blood pressure, etc.), TNM classification of cases, hormone receptor status (if available), amount and frequency of food consumption, sun exposure status, and physical activity.
2.2. Samples Collection
In view of the COVID-19 pandemic, barrier measures were scrupulously respected. A blood sample of 10 mL was collected into 2 sodium heparinate tubes from patients who had fasted for 8 h. The samples collected were placed in a refrigerated bag with a gel pack (allowing storage between 2 °C and 8 °C) and sent immediately to the CMIC. They were centrifuged at 2314 rcf (Relative Centrifugal Force) for 10 min and then aliquoted with at least 200 μL of plasma into cryovials and stored at −80 °C for 7 months until the end of sampling. The samples were anonymised using codes that combined their origin and their order of sampling.
2.3. Analysis of Vitamin D Metabolites
The concentration of vitamin D metabolites in the blood was determined by an automatic chemiluminescence method produced on an Immuno Diagnostic System (IDS-iSYS) device (Auxois, France). For the 25(OH)D assay, we used the reagents IDS 25VIT Ds, IDS 25VIT Ds Control Set for control kit and IDS 25VIT Ds Cal Set for calibrators. For the 1,25(OH)2D assay, we used the following kits: IDS 1,25VitD Xp, in which calibrators were included, and IDS 1,25VitD Xp Control Set for control reagent.
The 25(OH)D needs a pretreatment step to denature the Vitamin D Binding Protein (VDBP). After neutralisation in a buffer solution, an anti-25(OH)D antibody labelled with biotin was added. After 2 incubation steps, 25(OH)D labelled with acridinium and the magnetic particles bound to streptavidin were added successively. After the last incubation step, the complex was recovered using a magnet and washing was performed to remove any unbound analyte. Activation reagents were added, and the resulting light emitted by acridinium labelling was inversely proportional to the concentration of 25(OH)D present in the starting sample. The interpretation of vitamin D adopted is presented below: deficient level < 20 ng/mL; insufficient level between 20 ng/mL and 30 ng/mL; and normal levels between 30 and 50 ng/mL.
The 1,25(OH)2D needs an immunoextraction step. First, in a cuvette, the sample was delipidated and then incubated using magnetic particles coated with specific anti-1,25(OH)2D antibodies. After incubation, the magnetic particles were washed and the 1,25(OH)2D of the sample was eluted. This eluate was transferred into a second cuvette in which the assay was based on the same chemiluminescent method as 25(OH)D.
The interpretation of 1,25(OH)2D adopted is the following normal range: 15.2 to 90.1 pg/mL.
2.4. Statistical Analysis
Data were recorded in Excel 2022 and analysed using the IBM Statistical Package of Social Science (SPSS.21) software (IBM corp, New York, NY, USA).
Quantitative variables were expressed as mean ± standard deviation, and qualitative variables were given as number (n) and percentage (%). Student’s t test was used to compare means, Chi2 test to compare qualitative variables, and Pearson’s r was used for correlation tests. We then used multivariable conditional logistic regression to verify the effects between the qualitative dependent variable and the other factors. The results are considered statistically significant for a value of p < 0.05.
3. Results
We included a total of 220 patients, consisting of 110 cases of breast cancer that were matched to 110 controls according to age (within 5 years). We excluded 12 patients (6 cases and 6 controls) due to technical issues, so our study population included 208 patients with 104 cases and 104 matched healthy women.
3.1. Sociodemographic and Clinico-Pathological Characteristics of the Study Population
The characteristics of the study population are shown in Table 1. The mean age of the study population was 48.09 ± 12.42 years for the cases, ranging from 21 to 85 years, and 47.79 ± 12.42 years for controls, with 20 to 84 years for the range.
Table 1.
Sociodemographic and clinico-pathological characteristics.
| Characteristic | Cases (n = 104) | Controls (n = 104) | p-Value | |
|---|---|---|---|---|
| Age (y *), mean ± SD ** | 48.09 ± 12.42 | 47.79 ± 12.42 | 0.863 | |
| Age at menarche (y), mean ± SD | 14.32 ± 1.52 | 14.23 ± 1.35 | 0.715 | |
| First pregnancy age (y), mean ± SD | 20.16 ± 4.12 | 22.56 ± 4.96 | 0.001 | |
| BMI *** (kg/m2), mean ± SD | 26.39 ± 6.15 | 28.35 ± 6.21 | 0.024 | |
| Parity, mean ± SD | 5.53 ± 2.93 | 3.01 ± 2.13 | <10−3 | |
| Months of breastfeeding, mean ± SD | 20.41 ± 8.76 | 15.92 ± 8.05 | <10−3 | |
| Sleep time (hours) | 7.17 ± 1.19 | 6.90 ± 1.61 | 0.172 | |
| Sun exposure time (hours) | 2.48 ± 0.62 | 1.80 ± 0.80 | <10−3 | |
| Residence | Urban, n (%) | 67 (64.40) | 99 (95.20) | <10−3 |
| Rural, n (%) | 37 (35.60) | 5 (4.80) | ||
| Menopausal status | Menopausal women, n (%) | 52 (50) | 52 (50) | 1 |
| Premenopausal women, n (%) | 52 (50) | 52 (50) | ||
| Use of oral contraceptives | Yes, n (%) | 29 (27.90) | 51 (49) | 0.002 |
| No, n (%) | 75 (72.10) | 53 (51) | ||
| Menstrual cycle | Regular, n (%) | 90 (86.50) | 84 (80.80) | 0.261 |
| Irregular, n (%) | 14 (13.5) | 20 (19.20) | ||
| Smoking status | Current, n (%) | 37 (35.60) | 22 (21.20) | 0.176 |
| Never, n (%) | 67 (64.40) | 82 (78.80) | ||
| Professional status | Cleaning lady, n (%) | 64 (61.53) | 48 (46.15) | <10−3 |
| Entrepreneur, n (%) | 25 (24.03) | 28 (26.92) | ||
| State official, n (%) | 9 (8.65) | 11 (10.57) | ||
| Student, n (%) | 0 (0) | 2 (1.92) | ||
| Retiree, n (%) | 4 (3.84) | 11 (10.57) | ||
| Health official, n (%) | 2 (1.92) | 4 (3.84) | ||
| Socioeconomic status **** | High, n (%) | 10 (9.60) | 24 (23.10) | <10−3 |
| Moderate, n (%) | 21 (20.20) | 60 (57.70) | ||
| Low, n (%) | 73 (70.20) | 20 (19.20) | ||
| Education | Uneducated, n (%) | 77 (74) | 14 (13.50) | <10−3 |
| High school, n (%) | 9 (8.70) | 18 (17.30) | ||
| College graduate, n (%) | 6 (5.80) | 10 (9.60) | ||
| University, n (%) | 12 (11.50) | 62 (59.6) | ||
| Family history of cancer | Yes, n (%) | 13 (12.50) | 23 (22.10) | 0.049 |
| No, n (%) | 91 (87.50) | 81 (77.90) | ||
| Past abortion | Yes, n (%) | 47 (45.20) | 52 (50) | 0.277 |
| No, n (%) | 57 (54.80) | 52 (50) | ||
| Dress habits ***** | Loincloth and scarf set, n (%) | 88 (84.51) | 87 (83.65) | 0.569 |
| Hijab set, n (%) | 16 (15.39) | 17 (16.35) | ||
| Diet | Yes, n (%) | 79 (75.90) | 33 (31.70) | <10−3 |
| No, n (%) | 25 (24.10) | 71 (68.30) | ||
| Dairy products | At least once a day, n (%) | 55 (52.88) | 61 (58.65) | 0.248 |
| Sometimes, n (%) | 12 (11.50) | 14 (13.50) | ||
| Rarely, n (%) | 37 (35.6) | 25 (24) | ||
| Never, n (%) | 0 | 4 (3.80) | ||
| Meat consumption | At least once a day, n (%) | 87 (83.65) | 70 (67.30) | 0.059 |
| Sometimes, n (%) | 11 (10.60) | 22 (21.20) | ||
| Rarely, n (%) | 16 (15.80) | 11 (10.60) | ||
| Never, n (%) | 0 | 1 (1.00) | ||
| Fish consumption | At least once a day, n (%) | 84 (80.76) | 81 (77.88) | 0.892 |
| Sometimes, n (%) | 17 (16.30) | 18 (17.30) | ||
| Rarely, n (%) | 3 (2.90) | 5 (4.80) | ||
| Fruit consumption | At least once a day, n (%) | 86 (82.69) | 81 (77.88) | 0.044 |
| Sometimes, n (%) | 12 (11.50) | 16 (15.40) | ||
| Rarely, n (%) | 6 (5.80) | 7 (6.70) | ||
| Physical activity | Yes, n (%) | 100 (96.15) | 104 (100) | 0.043 |
| No, n (%) | 4 (3.80) | 0 (0.00) | ||
| Tumour grade | T1, n (%) | 2 (2.10) | ||
| T2, n (%) | 11 (11.7) | |||
| T3, n (%) | 17 (18.10) | |||
| T4, n (%) | 64 (68.10) | |||
| Nodal status | N0, n (%) | 12 (12.80) | ||
| N1, n (%) | 63 (67.00) | |||
| N2, n (%) | 16 (17.00) | |||
| N3, n (%) | 3 (2.60) | |||
| Metastasis | M0, n (%) | 69 (73.40) | ||
| M1, n (%) | 25 (26.60) | |||
| Clinical stage | I, n (%) | 2 (2.10) | ||
| II, n (%) | 15 (16.00) | |||
| III, n (%) | 52 (55.30) | |||
| IV, n (%) | 25 (26.60) | |||
* y: years; ** SD: standard deviation; *** BMI: body mass index; **** socioeconomic status: the criteria for socioeconomic status stratification was an estimation based on the answers to questions about income per month; ***** dress habits: most of our subjects were dressed as Malian women with a combination of loincloth and scarf sets. Loincloths are a complete cloth wrapped around the waist and with a top to match.
The majority of both case and control populations were from urban areas, while 35.6% of the cases and only 4.8% of the controls were from rural locations. There was no difference in the mean age at menarche between the two groups (p = 0.715), while we noticed some significant differences in the age at which subjects had their first pregnancy (p = 0.001), BMI (p = 0.024), parity (p < 10−3) and in the months of breastfeeding (p < 10−3). With half of each group consisting of menopausal women, our population exposure time was significantly higher in the BC group than in the control group (p < 10−3). The majority of the women in the control group had a moderate socioeconomic status (57.70%), while women in the BC group were largely of low socioeconomic status (70.20%) (p < 10−3).
Moreover, there was a significant difference between the control group and the cases group with regard to a family history of cancer (p = 0.04).
The majority of women in our cases group had metastasis (73.40%), including a high number of advanced breast cancer, of which 81.90% were either stage III or stage IV.
3.2. Vitamin D Metabolite Levels in Breast Cancer Patients
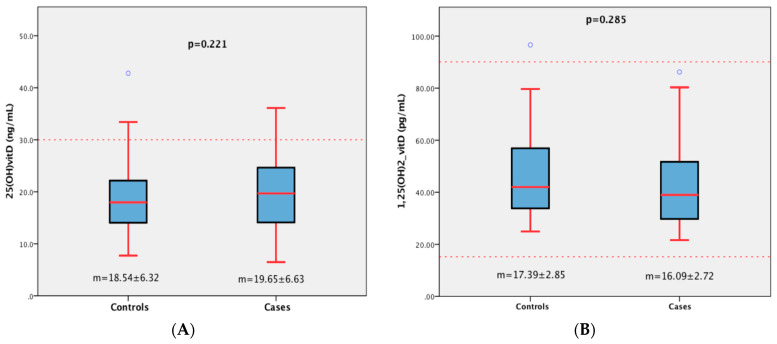
Across all 208 women, the mean values of 25(OH)D and 1,25(OH)2D were similar between the case and control groups (p = 0.221 and p = 0.285, respectively), as shown in Figure 1. Despite the insufficiency of 25(OH)D in the two groups, 1,25(OH)2D remained within the normal range (Figure 1).
Figure 1.
Plasma levels of 25(OH)D (A) and 1,25(OH)2D (B) in women with and without breast cancer. m: mean.
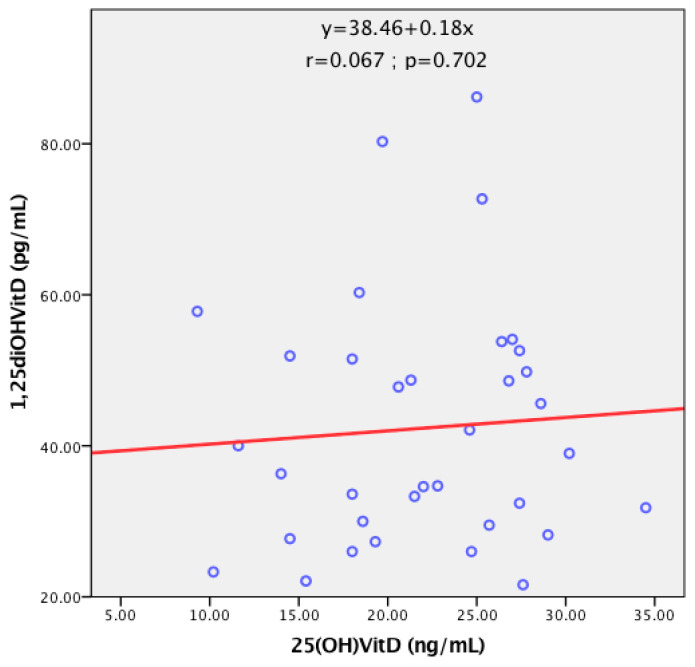
There was no correlation between levels of 25(OH)D and 1,25(OH)2D among subjects in the BC group, as shown in the scatterplot of Figure 2. This absence of correlation between precursor and active substance suggests the possible presence of unknown mechanisms that could cause their fluctuation in BC women, while the presence of a correlation indicates an interdependence of the levels of the two markers.
Figure 2.
Scatterplot of the relationship between circulating levels of 25(OH)D and 1,25(OH)2D.
Using the 30 ng/mL cut-off of 25(OH)D, the majority of the subjects in both groups were in hypovitaminosis D with 93.9% and 95.2% for cases and controls, respectively. The opposite is found with 1,25(OH)2D concentrations for cases and control groups, both of which were largely in the normal range (Table 2).
Table 2.
Vitamin D levels and stage of advanced breast cancer.
| Study Cohort | p | |||
|---|---|---|---|---|
| Cases | Controls | |||
| Plasma 25(OH)D | Deficient, n (%) | 54 (51.9) | 65 (62.5) | 0.303 |
| Insufficient, n (%) | 44 (42.3) | 34 (32.7) | ||
| Normal, n (%) | 6 (5.8) | 5 (4.8) | ||
| Plasma 1,25(OH)2D | Normal, n (%) | 35 (100) | 34 (97.3) | 0.327 |
| Low, n (%) | 0 (0) | 1 (2.7) | ||
| High, n (%) | 0 (0) | 0 (0) | ||
Among patients with breast cancer, we noticed no difference in 25(OH)D and 1,25(OH)2D plasma concentration between subjects with early (clinical stages I and II) and advanced (clinical stages III and IV) disease. Table 3 shows a nonsignificant decrease in these two metabolites from subjects without metastasis to ones with metastasis.
Table 3.
Plasma levels of 25(OH)D (ng/mL) and 1,25(OH)2D (pg/mL) in case subjects classified by clinical stage and metastasis.
| 25(OH)D | 1,25(OH)2D | ||
|---|---|---|---|
| Clinical Stages I and II | (n = 17) 17.10 ± 6.50 1 | (n = 6) 34.95 ± 12.53 2 | |
| Clinical Stages III and IV | (n = 77) 20.08 ± 6.57 1 | (n = 29) 43.84 ± 16.50 2 | |
| Metastasis | No | (n = 69) 19.71 ± 6.77 3 | (n = 25) 42.99 ± 17.79 4 |
| Yes | (n = 25) 18.89 ± 6.12 3 | (n = 10) 39.62 ± 11.60 4 | |
student t test; 1 0.101; 2 0.170; 3 0.578; 4 0.528.
3.3. Vitamin D Metabolites and Breast Cancer Risk
In a univariate statistical analysis of our population, we found a number of factors that were associated with breast cancer, as shown in Table 1. We also performed a multiple binary logistic regression model for breast cancer for 25(OH)D and 1,25(OH)2D, both stratified by quartiles, as shown in Table 4.
Table 4.
Multiple binary logistic regression model showing the association between quartile concentrations of 25(OH)D and 1,25(OH)2D in women in the case and control groups.
| p-Value | OR 2 | 95% CI 3 | ||
|---|---|---|---|---|
| Lower | Upper | |||
| 25(OH)D (n = 208) * | 0.13 | 1.08 | 0.98 | 1.18 |
| Q 11 (<14.10 ng/mL) | 0.25 | 0.40 | 0.08 | 1.91 |
| Q2 (14.10–18.84 ng/mL) | 0.16 | 0.32 | 0.06 | 1.60 |
| Q3 (18.85–23.51 ng/mL) | 0.23 | 0.38 | 0.08 | 1.80 |
| Q4 (≥23.52 ng/mL) | 1.00 | |||
| 1,25(OH)2D (n = 70) ** | 0.18 | 0.97 | 0.92 | 1.02 |
| Q1 (<31.92 pg/mL) | 0.03 | 71.84 | 1.36 | 3785.34 |
| Q2 (31.92–39.79 pg/mL) | 0.98 | 0.96 | 0.07 | 14.28 |
| Q3 (39.80–54.01 pg/mL) | 0.98 | 0.96 | 0.05 | 19.50 |
| Q4 (≥54.02 pg/mL) | 1.00 | |||
1 Q: quartile; 2 OR: odds ratio; 3 95% CI: 95% confidence interval. * Logistic regression model, adjusted for menopausal status, age, age of menarche, body mass index, residence, smoking status, use of oral contraceptives, work, socioeconomic status, sleep time, education, family history of cancer, parity, first pregnancy age, months of breastfeeding, diet, dress habits, sun exposure time, and physical activity. ** Logistic regression model with restricted adjustment for menopausal status, age, age of menarche, body mass index, smoking status, use of oral contraceptives, socioeconomic status, sleep time, education, family history of cancer, parity, and months of breastfeeding.
There was no association between metabolites of vitamin D, 25(OH)D, and 1,25(OH)2D and breast cancer. However, it is noteworthy that our findings indicate a more pronounced inverse association in the first level of plasma 25(OH)D, while the risk function decreases at higher levels. This observation takes strengths with 1,25(OH)2D by a significant association between the first quartile and breast cancer as a risk factor (p = 0.03; OR= 71.84; CI: 1.36–3785.34). The risk function flattens and remains the same with increased concentrations.
4. Discussion
As the widely accepted marker for its stability and reliability, we chose 25(OH)D as the main metabolite measured in the current study. The second one, 1,25(OH)2D, as the biologically active form of vitamin D, was measured to allow us to explain some of the variations in 25(OH)D levels. In fact, the lack of correlation between these two metabolites could make us hypothesize the presence of another unknown mechanism, which could constitute new lines of research.
Several studies have addressed the biological effects of vitamin D metabolites, including 1,25-dihydroxyvitamine D (1,25(OH)2D) and its precursor 25(OH)D, on breast cancer [7,22,23], seeking to determine an association with breast cancer disease in diverse populations.
We observed no difference in the mean 25(OH)D (p = 0.221) between cases and controls, thus suggesting a lack of association between vitamin D and breast cancer risk. The same results are found in studies by Lyra et al. [24], Janowsky et al. [18], and Eliassen et al. [25], which reported no significant changes in 25(OH)D concentrations. These findings were in contrast to many studies, such as those by Husain et al. [15], Patel et al. [16], Abboud et al. [26], and Khedr et al. [27], which detected a significantly lower concentration of 25(OH)D in subjects with breast cancer. These differences could be explained by the differences between the populations included in these studies, especially with respect to skin pigmentation, and differences between the analytical methods used. Indeed, our results show a hypovitaminosis D of 94.2% in women with breast cancer and 95.2% in women without breast cancer. The main mechanism of vitamin D deficiency results from the racial characteristic of being black, i.e., a cutaneous pigmentation. The melanin produced in the deep layers of the epidermis works as a filter that absorbs ultraviolet B radiation (UVB) in competition with 7-dehydrocholesterol [19]. Intense sunlight in sub-Saharan Africa may therefore compensate for low sun absorption through the skin [17]. In the current study, the higher concentration of 25(OH)D in the BC group could be explained by the significantly higher (p < 103) sun exposure time for women with breast cancer (2.48 ± 0.62 h) compared with those without breast cancer (1.80 ± 0.80 h). Moreover, Chauveau et al. [19] have proven that UV doses must be multiplied by six in people of sub-Saharan African origin in order to obtain an identical concentration of vitamin D to that found in Caucasians. It is therefore worth noting that, in the current study, the mean exposure time in the cases and the control group was not sufficient, which probably led to 25(OH)D deficiency in comparison groups.
Analysing 1,25(OH)2D allows us to better understand 25(OH)D levels. A nonsignificant (p = 0.285) decrease in the second metabolite, 1,25(OH)2D, has been observed in women with breast cancer compared with women without breast cancer (Figure 1). Only a few studies have looked at the assay of both metabolites, 25(OH)D and 1,25(OH)2D, in parallel [18,24,28,29]. Compared with our outcomes, Lyra et al. [24] found a lower significant level of 1,25(OH)2D (p = 0.011) in the BC group. In keeping with our data, Janowsky et al. [18] found no significant fluctuation of 1,25(OH)2D between black women in their case and control groups, while there was a significantly lower concentration in white women with breast cancer compared with the controls. In the same study, there were no differences in mean value for either group with respect to 25(OH)D. Our results are in agreement with those of Hiatt et al. [30] and Bertone-Johnson et al. [28], with no difference in 1,25(OH)2D concentrations between cases and matched control subjects. The differences between all these studies and the current one confirm just how controversial the influence of plasma vitamin D on breast cancer is, showing that it could be explained using the same reasons cited above for 25(OH)D. In light of our results, there may be racial differences in the relationship between vitamin D and breast cancer. Indeed, our data agree with other findings regarding outcomes in black women, such as Janowsky et al.’s [18], while we observe the opposite with outcomes in white women [28,30]. The hypothesis of racial disparities is now common. Yao and Ambrosone [17] have affirmed that the high prevalence of vitamin D deficiency in women of African ancestry could be attributed to some degree to their ancestral genetic background, shaped over millennia in Africa. Differences in the timing of measurement could explain some of the variability in the study’s findings.
We performed a multivariate logistic regression model to better understand the involvement of vitamin D metabolites as factors linked to the risk of breast cancer. By stratifying by quartile levels, we found it noteworthy that there was a decrease in risk from the first quartile. These results show a possible association of 25(OH)D and 1,25(OH)2D in the decrease in breast cancer risk. Indeed, a number of studies have demonstrated 1,25(OH)2D’s antiproliferative, proapoptotic, prodifferentiating, and anti-inflammatory effects on malignant cells in breast cancer [7,31,32].
Our data indicate that 1,25(OH)2D remained in the normal range and has no level differences (Figure 1), despite mean values close to the lower limit of our technique showing a drop in concentration across the two groups. This decrease in levels of 1,25(OH)2D in the cases and controls suggests that its local production seems impaired and may be correlated with low levels of 25(OH)D detected in the same groups. This lends weight to the possibility that mechanisms in addition to hypovitaminosis D could be responsible for the association between breast cancer risk and the lower levels of 25(OH)D. Bertone Jonhson’s study [33] asserts that in case–control studies, the presence of a tumour may affect circulating vitamin D levels, either by altering 25(OH)D metabolism or by altering a patient’s dietary intake of vitamin D or sunlight exposure. Recent studies have also suggested that 25(OH)D is hydroxylated to form 1,25(OH)2D at extrarenal sites, including breast tissue [28]. The 1,25(OH)2D produced through this mechanism seems to act only as an autocrine or paracrine hormone, and it does not enter general circulation and may not be measurable by standard plasma assay [28,34]. In contrast, 25(OH)D levels are more sensitive to changes in diet and sunlight exposure and may not reflect the level of 1,25(OH)2D ultimately available to the target tissue [28,35].
The levels of vitamin D metabolites according to the stages of BC are necessary for a good understanding of the effects of metabolites. The majority of our breast cancer subjects were in advanced stages of the disease, with 83% for 1,25(OH)2D and 74.03% for 25(OH)D. We noticed no differences in plasma concentrations between early and advanced breast cancer levels of 25(OH)D and 1,25(OH)2D. There were also some similar concentrations of these metabolites between women with metastasis and without metastasis. These results could be explained by the small size of early breast cancer and women with metastasis. According to many studies, hypovitaminosis D in breast cancer could also be explained by the presence of VDR, because its expression in BC tissue decreases during tumour progression, making it less sensitive to vitamin D3 [36,37,38,39].
However, this study has some limitations, such as a lack of an evaluation of the enzymes 1α-hydroxylase, 24-hydroxylase, and the VDR in breast cancer tissue samples. This evaluation could provide more information about the mechanisms involved in the downregulation of 1,25(OH)2D. Another possible limitation is the low sample size of 1.25(OH)2D.
5. Conclusions
In the last decade, breast cancer has become the most frequently diagnosed cancer in Mali, and it continues to be a serious public health problem. Our data lend weight to the multifactorial aspects of this disease’s occurrence. In summary, our results prove that the population always consults late, which leads to a high rate of advanced breast cancer. The current study showed a possible association of 25(OH)D and 1,25(OH)2D in a decreased risk of breast cancer. Despite all the findings regarding the role of vitamin D in breast cancer disease, a number of questions remain to be answered, including the function of the vitamin D mechanism of dysregulation in breast cancer. Our study is the first to analyse 25(OH)D and 1,25(OH)2D in the blood of breast cancer patients in Mali. Given the level of disagreement in this field, further studies are needed to clarify the real impact of vitamin D metabolites in breast cancer disease.
Author Contributions
Conceptualisation, A.D.T.B. and Z.O.; methodology, A.D.T.B. and Z.O.; validation, P.R., Z.O. and M.L.; formal analysis, F.M.S.; investigation, A.E.H.A.; resources, B.K.; data curation, M.T. and L.G.T.; writing—original draft preparation, A.D.T.B.; writing—review and editing, A.D.T.B. and N.O.K.B.; visualisation, B.S.K.; supervision, P.R. and Z.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics committee of the University of Science, Techniques and Technologies, Bamako (protocol number: 2021/236/USTTB; 30 September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to patient privacy regulations.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sancho-Garnier H., Colonna M. Épidémiologie des cancers du sein. La Presse Médicale. 2019;48:1076–1084. doi: 10.1016/j.lpm.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Zaki H.M., Garba-Bouda O., Garba S.M., Nouhou H. Profil Épidémiologique et Anatomopathologique Du Cancer Du Sein Au Niger. J. Afr. Du Cancer/Afr. J. Cancer. 2013;5:185–191. doi: 10.1007/s12558-013-0274-9. [DOI] [Google Scholar]
- 3.Mali Source Globocan 2020 . International Agency for Research on Cancer. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
- 4.Jeon S.-M., Shin E.-A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018;50:1–14. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christakos S., Li S., De La Cruz J., Bikle D.D. New Developments in Our Understanding of Vitamin Metabolism, Action and Treatment. Metabolism. 2019;98:112–120. doi: 10.1016/j.metabol.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhevel J., Verlinden L., Doms S., Wildiers H., Verstuyf A. The Role of Vitamin D in Breast Cancer Risk and Progression. Endocr.-Relat. Cancer. 2022;29:R33–R55. doi: 10.1530/ERC-21-0182. [DOI] [PubMed] [Google Scholar]
- 8.Voutsadakis I.A. Vitamin D Receptor (VDR) and Metabolizing Enzymes CYP27B1 and CYP24A1 in Breast Cancer. Mol. Biol. Rep. 2020;47:9821–9830. doi: 10.1007/s11033-020-05780-1. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand K.A., Rosner B., Eliassen A.H., Hankinson S.E., Rexrode K.M., Willett W., Tamimi R.M. Premenopausal Plasma 25-Hydroxyvitamin D, Mammographic Density, and Risk of Breast Cancer. Breast Cancer Res. Treat. 2015;149:479–487. doi: 10.1007/s10549-014-3247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain S., Beydoun M.A., Beydoun H.A., Chen X., Zonderman A.B., Wood R.J. Vitamin D and Breast Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. ESPEN. 2019;30:170–184. doi: 10.1016/j.clnesp.2018.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H., Lin H., Hu Y., Li X., He W., Jin X., Gao J., Zhao N., Liu Z., Gao X. Serum 25-Hydroxyvitamin D Levels Are Associated with Carotid Atherosclerosis in Normotensive and Euglycemic Chinese Postmenopausal Women: The Shanghai Changfeng Study. BMC Cardiovasc. Disord. 2014;14:197. doi: 10.1186/1471-2261-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen P., Hu P., Xie D., Qin Y., Wang F., Wang H. Meta-Analysis of Vitamin D, Calcium and the Prevention of Breast Cancer. Breast Cancer Res. Treat. 2010;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 13.Gandini S., Boniol M., Haukka J., Byrnes G., Cox B., Sneyd M.J., Mullie P., Autier P. Meta-Analysis of Observational Studies of Serum 25-Hydroxyvitamin D Levels and Colorectal, Breast and Prostate Cancer and Colorectal Adenoma. Int. J. Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 14.Hemida M.A., AbdElmoneim N.A., Hewala T.I., Rashad M.M., Abdaallah S. Vitamin D Receptor in Breast Cancer Tissues and Its Relation to Estrogen Receptor Alpha (ER-α) Gene Expression and Serum 25-Hydroxyvitamin D Levels in Egyptian Breast Cancer Patients: A Case-Control Study. Clin. Breast Cancer. 2019;19:e407–e414. doi: 10.1016/j.clbc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Husain N., Suliman A., Abdelrahman I., Bedri S., Musa R., Osman H., Mustafa A., Gafer N., Farah E., Satir A., et al. Serum Vitamin D Level, Sun-Exposed Area, Dietary Factors, and Physical Activity as Predictors of Invasive Breast Cancer Risk among Sudanese Women: A Case–Control Study. J. Fam. Med. Prim. Care. 2019;8:1706. doi: 10.4103/jfmpc.jfmpc_197_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S.R., Patel K.D., Patel K.R., Gokani R.A., Patel J.B., Patel P.S., Shah F.D. Clinical Significance of Serum 25 Hydroxyvitamin D in Breast Cancer: An Indian Scenario. J. Steroid Biochem. Mol. Biol. 2020;202:105726. doi: 10.1016/j.jsbmb.2020.105726. [DOI] [PubMed] [Google Scholar]
- 17.Yao S., Ambrosone C.B. Associations between Vitamin D Deficiency and Risk of Aggressive Breast Cancer in African-American Women. J. Steroid Biochem. Mol. Biol. 2013;136:337–341. doi: 10.1016/j.jsbmb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Janowsky E.C., Lester G.E., Weinberg C.R., Millikan R.C., Schildkraut J.M., Garrett P.A., Hulka B.S. Association between Low Levels of 1,25-Dihydroxyvitamin D and Breast Cancer Risk. Public Health Nutr. 1999;2:283–291. doi: 10.1017/S1368980099000385. [DOI] [PubMed] [Google Scholar]
- 19.Chauveau P., Aparicio M. Ethnicité et vitamine D. Néphrologie Thérapeutique. 2013;9:398–402. doi: 10.1016/j.nephro.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Moukal A., El Farouqi A., Aghrouch M., Chadli S., Zekhnini A., Izaabel E.H. Serum 25-Hydroxyvitamin D and the Risk of Breast Cancer in Women from Southern Morocco: A Case-Control Study. MNM. 2022;15:361–368. doi: 10.3233/MNM-211564. [DOI] [Google Scholar]
- 21.Zhu H., Doğan B.E. American Joint Committee on Cancer’s Staging System for Breast Cancer, Eighth Edition: Summary for Clinicians. Eur. J. Breast Health. 2021;17:234–238. doi: 10.4274/ejbh.galenos.2021.2021-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de La Puente-Yagüe M., Cuadrado-Cenzual M.A., Ciudad-Cabañas M.J., Hernández-Cabria M., Collado-Yurrita L. Vitamin D: And Its Role in Breast Cancer. Kaohsiung J. Med. Sci. 2018;34:423–427. doi: 10.1016/j.kjms.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Narvaez C.J., Matthews D., LaPorta E., Simmons K.M., Beaudin S., Welsh J. The Impact of Vitamin D in Breast Cancer: Genomics, Pathways, Metabolism. Front. Physiol. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lyra E.C., da Silva I.A., Katayama M.L.H., Brentani M.M., Nonogaki S., Góes J.C.S., Folgueira M.A.A.K. 25(OH)D3 and 1,25(OH)2D3 Serum Concentration and Breast Tissue Expression of 1alpha-Hydroxylase, 24-Hydroxylase and Vitamin D Receptor in Women with and without Breast Cancer. J. Steroid Biochem. Mol. Biol. 2006;100:184–192. doi: 10.1016/j.jsbmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Eliassen A.H., Spiegelman D., Hollis B.W., Horst R.L., Willett W.C., Hankinson S.E. Plasma 25-Hydroxyvitamin D and Risk of Breast Cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011;13:R50. doi: 10.1186/bcr2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abboud S.F., Saleh B.O., Ahmed S.J., Al Mussawi K.K. Association between vitamin d receptor gene taqi (731236) polymorphisms and breast cancer risk in iraqi women. Biochem. Cell. Arch. 2022:3713–3718. doi: 10.51470/bca.2022.22.2.3713. [DOI] [Google Scholar]
- 27.Khedr M.I., Sharaf S.A.F., Aal A.N.A., Dessouky I.S., Soliman M. Serum 25-Hydroxyvitamin D Level and Breast Cancer Risk in Egyptian Females. Asian J. Oncol. 2022;8:76–80. doi: 10.1055/s-0042-1748494. [DOI] [Google Scholar]
- 28.Bertone-Johnson E.R., Chen W.Y., Holick M.F., Hollis B.W., Colditz G.A., Willett W.C., Hankinson S.E. Plasma 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D and Risk of Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 29.Freedman D.M., Chang S.-C., Falk R.T., Purdue M.P., Huang W.-Y., McCarty C.A., Hollis B.W., Graubard B.I., Berg C.D., Ziegler R.G. Serum Levels of Vitamin D Metabolites and Breast Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomark. Prev. 2008;17:889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiatt R.A., Krieger N., Lobaugh B., Drezner M.K., Vogelman J.H., Orentreich N. Prediagnostic Serum Vitamin D and Breast Cancer. JNCI J. Natl. Cancer Inst. 1998;90:461–463. doi: 10.1093/jnci/90.6.461. [DOI] [PubMed] [Google Scholar]
- 31.LaPorta E., Welsh J. Modeling Vitamin D Actions in Triple Negative/Basal-like Breast Cancer. J. Steroid Biochem. Mol. Biol. 2014;144:65–73. doi: 10.1016/j.jsbmb.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y., Trivedi T., Lin R.C., Fong-Yee C., Nolte R., Manibo J., Chen Y., Hossain M., Horas K., Dunstan C., et al. Loss of the Vitamin D Receptor in Human Breast and Prostate Cancers Strongly Induces Cell Apoptosis through Downregulation of Wnt/β-Catenin Signaling. Bone Res. 2017;5:17023. doi: 10.1038/boneres.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertone-Johnson E.R. Vitamin D and Breast Cancer. Ann. Epidemiol. 2009;19:462–467. doi: 10.1016/j.annepidem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Welsh J., Wietzke J.A., Zinser G.M., Byrne B., Smith K., Narvaez C.J. Vitamin D-3 Receptor as a Target for Breast Cancer Prevention. J. Nutr. 2003;133:2425S–2433S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 35.Stoll F., Akladios C.Y., Mathelin C. Vitamine D et cancer du sein: Y a-t-il un lien? Gynécologie Obs. Fertil. 2013;41:242–250. doi: 10.1016/j.gyobfe.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lopes N., Sousa B., Martins D., Gomes M., Vieira D., Veronese L.A., Milanezi F., Paredes J., Costa J.L., Schmitt F. Alterations in Vitamin D Signalling and Metabolic Pathways in Breast Cancer Progression: A Study of VDR, CYP27B1 and CYP24A1 Expression in Benign and Malignant Breast Lesions Vitamin D Pathways Unbalanced in Breast Lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh J. Function of the Vitamin D Endocrine System in Mammary Gland and Breast Cancer. Mol. Cell. Endocrinol. 2017;453:88–95. doi: 10.1016/j.mce.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray A., Madden S.F., Synnott N.C., Klinger R., O’Connor D., O’Donovan N., Gallagher W., Crown J., Duffy M.J. Vitamin D Receptor as a Target for Breast Cancer Therapy. Endocr. Relat. Cancer. 2017;24:181–195. doi: 10.1530/ERC-16-0463. [DOI] [PubMed] [Google Scholar]
- 39.Francis I., AlAbdali N., Kapila K., John B., Al-Temaimi R.A. Vitamin D Pathway Related Polymorphisms and Vitamin D Receptor Expression in Breast Cancer. Int. J. Vitam. Nutr. Res. 2019;91:124–132. doi: 10.1024/0300-9831/a000615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to patient privacy regulations.