Abstract
Manipulations that reduce or enhance the activity of basolateral amygdala (BLA) neurons in the minutes to hours after training have been shown to respectively impair or facilitate retention on the inhibitory avoidance task. Although this suggests that BLA activity is altered after emotional arousal, such changes have not been directly demonstrated. To test this, we devised a feline analog of the inhibitory avoidance task and recorded BLA unit activity before and after a single inescapable footshock. Single-unit recordings revealed that the firing rate of many BLA neurons gradually increased after the footshock, peaking 30–50 min post-shock and then subsiding to baseline levels 2 h later. During this period of increased activity, the discharges of simultaneously recorded BLA cells were more synchronized than before the shock. Although it was known that pairing innocuous (conditioned stimulus, CS) and noxious stimuli modifies the responsiveness of BLA neurons to the CS, our results constitute the first demonstration that emotional arousal produces lasting increases in the spontaneous firing rates of BLA neurons. We propose that these changes in BLA activity may promote Hebbian interactions between coincident but spatially distributed activity patterns in BLA targets, facilitating the consolidation of emotional memories.
Much evidence indicates that emotional arousal generally improves memory consolidation (Christianson 1992) and that the basolateral amygdala (BLA) is responsible for this effect (McGaugh 2004). It was first shown that systemic administration of adrenal stress hormones after learning could facilitate retention (Gold et al. 1975) in appetitively or aversively motivated tasks (for review, see Cahill and McGaugh 1998). The effects of stress hormones and their pharmacological analogs were time-dependent, their impact on retention decreasing as the interval between the training and hormone treatment increased. These results suggested that the extent of memory consolidation depended on the motivational or arousing consequences of an experience, as expressed by the release of stress hormones (Gold and McGaugh 1975).
Subsequently, it was reported that the memory modulation produced by manipulations of stress hormone levels could be blocked by lesioning or inactivating the BLA or stria terminalis, but not the central amygdaloid nucleus (Liang and McGaugh 1983; Liang et al. 1990; Introini-Collison et al. 1991; Roozendaal and McGaugh 1996; Roozendaal et al. 1996). Consistent with these findings, post-training injections of drugs that presumably enhanced or reduced BLA activity facilitated or impaired retention, respectively. In particular, reduced retention was seen with local intra-amygdala injections of GABA agonists (Castellano et al. 1989), β-adrenergic antagonists (Gallagher et al. 1977), and glutamate receptor antagonists (Izquierdo and Medina 1993). Enhanced retention was seen with intra-amygdaloid injections of bicuculline (Dickinson et al. 1993), and agonists of β-adrenergic (Ferry and McGaugh 1999; Hatfield and McGaugh 1999) and muscarinic (Salinas et al. 1997) receptors. Similarly, post-training intra-amygdaloid injections of glucocorticoids were also reported to enhance memory consolidation (for review, see Roozendaal 1999). Importantly, as was found in the stress hormone studies, the effects of these post-learning treatments decreased as the interval between the training and the treatment increased, generally vanishing after 2–3 h (however see Sacchetti et al. 1999).
Taken together, these results suggest that stress hormones released in emotionally arousing conditions increase the activity of BLA neurons. In turn, the enhanced activity of BLA cells would facilitate memory consolidation in other brain areas via their widespread cortical and subcortical projections. However, other interpretations are possible. For instance, it is conceivable that the activity of BLA cells is unchanged during emotional arousal but that widespread increases in the concentration of key neuromodulators modify the impact of BLA inputs on target neurons.
Thus, the present study was undertaken to determine whether emotional arousal modifies the firing rates of BLA neurons in a sustained manner, as predicted by the pharmaco-behavioral evidence reviewed above. To this end, we developed an analog of the inhibitory avoidance task that could be conducted in head-restrained animals, thus maximizing recording stability.
Results
Behavioral observations
All tested cats (n = 3) behaved similarly in this paradigm (Fig. 1; see Materials and Methods). During the initial learning (Phase 1), they quickly learned to refrain from lever-pressing during the lights-off periods. All cats reached criterion by day 7. Figure 2A shows the behavior of a representative animal on the first and last days of training. In this and following panels, the top trace indicates lever-presses and the second trace shows when the light was turned on and off. As illustrated in Figure 2B, performance leveled off on day 7.
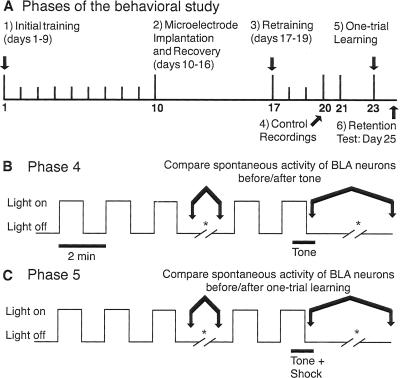
Figure 1.
Behavioral paradigm. (A) Time-line of the behavioral study. (B,C) Timing of data acquisition during the electrophysiological experiments of Phases 4 (B) and 5 (C).
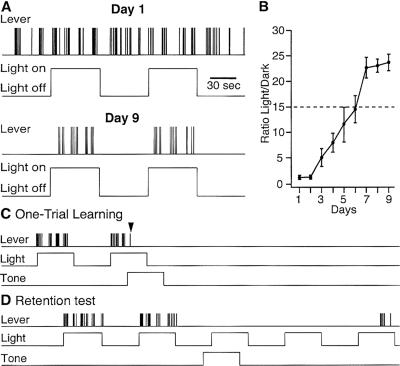
Figure 2.
Behavior of a representative cat in Phases 1 (A), 5 (C), and 6 (D). In A, C, and D, the top trace indicates lever presses, and the second trace shows when the lights were turned on and off. The third trace in C,D indicates when the tone was turned on. (B) Progression of lever-pressing rate during Phase 1. Ratio of the number of lever presses in the “lights-on” to “lights-off” periods (y-axis) as a function of time (x-axis). Average of three cats. Dashed lines indicate criterion. When the cats did not press the lever during the “lights-off” period, the ratio was equal to the number of lever presses during the “lights-on” period. Panel C begins after the extended “lights-off” epoch during which the spontaneous activity of BLA neurons was recorded. During the last “lights-on” period, a tone was presented and the animal received a footshock after the first lever-press following tone onset. Upon receiving the shock, the cat stopped pressing the lever. Neuronal recordings resumed after the lights and tone were turned off. (D) Two days later, the cat behaved normally before the tone was turned on, but refrained from pressing the lever for several minutes after presentation of the tone.
After electrode implantation and retraining to criterion (Fig. 2B, dashed line), the animals continued to lever-press at the same rate when an unpaired auditory conditioned stimulus (CS) was presented for the first time during a lights-on period (21.6 ± 2.3 vs. 20.4 ± 3.7 times/min before vs. during the auditory CS). On the one-trial learning day (Phase 5), the first lever-press after tone onset (Fig. 2C, third trace) triggered a footshock. As expected, all cats immediately stopped lever-pressing when the shock was delivered (Fig. 2C, arrowhead). Two days later, during the retention test (Phase 6, Fig. 2D), they exhibited normal lever-pressing at first, but refrained from pressing the lever for >4 min after tone onset (average duration of lever-press cessation: 5.4 ± 0.8 min), demonstrating that they had learned the tone-shock association.
Activity of BLA neurons during the control phase and one-trial learning task
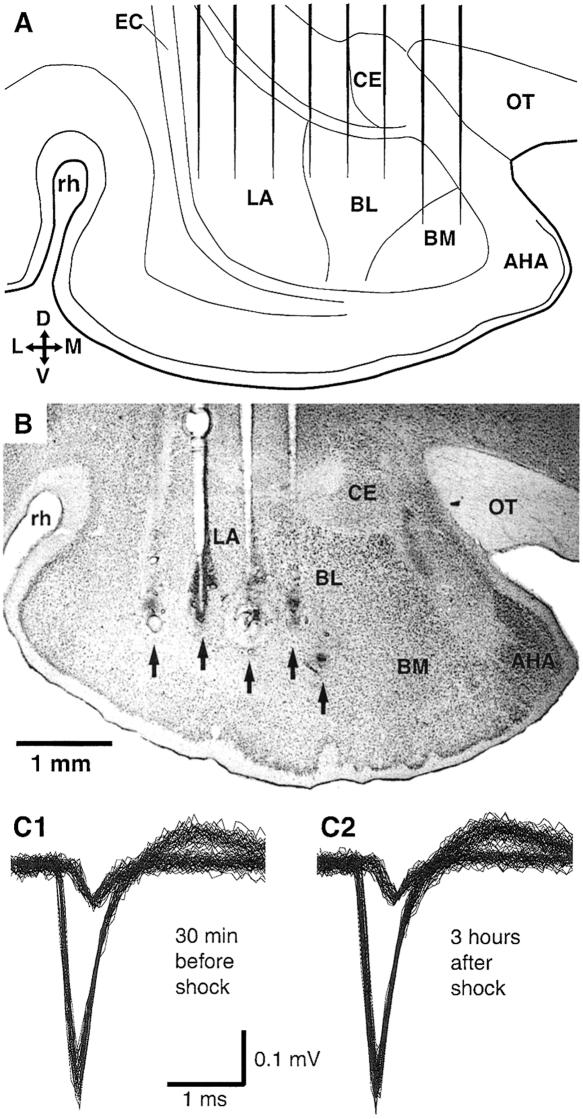
The spontaneous activity of BLA neurons was recorded extracellularly by means of two arrays of eight microelectrodes (Fig. 3A) implanted bilaterally. In order to be included in the analysis, recorded cells had to meet the following criteria. First, they had to be located within the confines of the BLA, as determined in histological controls (Fig. 3B). Second, the spikes they generated had to be clearly distinct from the noise and remain stable for the entire duration of the recording session (Fig. 3C). Third, their baseline firing rate in waking (W) had to be ≤5 Hz, as previous work suggests that BLA neurons with high firing rates are inter-neurons (see Discussion). A total of 42 neurons met these criteria in the control recording session (Phase 4), and 54 during the one-trial learning (Phase 5). Note that Phase 4 and 5 neurons were recorded within 300 μm of each other, along the same electrode tracks.
Figure 3.
Inclusion criteria in physiological experiments. (A) Microelectrode array positioned stereotaxically in the BLA. (B) Histological determination of recording sites. Arrows point to electrolytic lesions made after the retention test to mark the position of the recording sites. (C) Two BLA neurons recorded simultaneously by the same microelectrode 30 min before (C1) and 3 h after the footshock in Phase 5 (C2).
Because the firing rates of BLA neurons are known to vary depending on the state of vigilance (Jacobs and McGinty 1971; White and Jacobs 1975; Paré and Gaudreau 1996), data obtained in W and slow-wave sleep (SWS) were processed separately. Data obtained in paradoxical sleep was excluded because the animals spent too little time in this state. Stable epochs of quiet W or SWS (>30 sec) were identified using spontaneous EEG fluctuations in the power of the 1–4 Hz band, as detailed in Materials and Methods. The firing rate of BLA neurons was then calculated in 10-sec bins and the data plotted using different colors for W (Fig. 4, black) and SWS (Fig. 4, red).
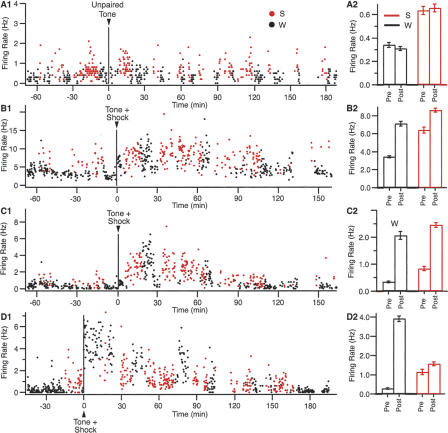
Figure 4.
Firing rate of BLA neurons during Phases 4 (A) and 5 (B–D). Panels A–D show the activity of four different neurons. Left: Graphs plotting firing rate (y-axis) as a function of time (x-axis, 10-sec bins). Black and red symbols indicate data obtained in W and SWS, respectively. The vertical line marks a 3.5-min interruption when the tone (A) or tone plus footshock (B–D) were presented. Right: Histograms showing averaged firing rates in W (black) and SWS (red), pre- (left) and post-tone (right).
Note that in the two following sections, we compare the firing rates of BLA neurons before versus after the tone (Phase 4) or after the tone plus shock (Phase 5).
Control recording day (Phase 4)
Other than state-related fluctuations in activity, the firing rate of BLA cells varied little during the control recording sessions. Presentation of the tone alone did not produce lasting modifications in firing rate. An example of this is shown in Figure 4A1 for a BLA neuron that was generally more active in SWS (red dots) than W (black dots), as was typically the case in our sample. The impression of stability gained by visual inspection of the rate meter (Fig. 4A1) was confirmed when separate averages of all the data points were computed in W (Fig. 4A2, black bars) and SWS (Fig. 4A2, red bars), before versus 70 min after the tone (left and right bars of Fig. 4A2, respectively). Only between-states differences in firing rates reached significance (t-test, P < 0.05); within-states differences did not (P > 0.05, Fig. 4A2).
When the same analyses were repeated on all control BLA cells, 98% of them yielded the same conclusion: no within-states differences before versus after the tone. Overall, firing rates in W averaged 0.39 ± 0.15 Hz before the tone, compared to 0.41 ± 0.11 Hz in the 70 min that followed the tone (t-test, P > 0.05). In SWS, they averaged 1.61 ± 0.29 Hz before the tone, compared to 1.69 ± 0.32 Hz after the tone (t-test, P > 0.05). The higher firing rates seen in SWS compared to W (t-test, P < 0.05) is consistent with previous reports (Jacobs and McGinty 1971; White and Jacobs 1975; Paré and Gaudreau 1996).
One-trial learning (Phase 5)
The stable firing rates of BLA cells during the control recording sessions (Phase 4; Fig. 4A) contrasted with the results obtained when the footshock was introduced (Phase 5; Fig. 4B–D). Indeed, a cursory survey of rate meters suggested that firing rates in W increased for hours after the tone-footshock pairing, in all tested animals. This impression was confirmed by using t-tests (P value of 5%) to compare the firing rates of each cell in W before versus in the 70 min after the shock, when firing rates seemed highest. Note that although such repeated comparisons increase the likelihood of false positives, by chance alone no more than 5% type II errors are expected. Yet, significant increases in firing rates were detected in as many as 48% of BLA cells (or 26/54) recorded in Phase 5; nearly 10 times more than expected by chance. For convenience, these neurons are hereafter called responsive or “R-cells,” whereas the term nonresponsive or “NR-cells” is used when referring to the rest of the sample.
As shown in Figure 4B–D, accelerations in firing rate took two forms. Most R-cells (77% or 20 of 26, Fig. 4B,C) reached their peak firing rate after a long delay (on average 39 ± 4 min after the footshock; range 29–67 min). These cells were encountered in the medial part of the lateral nucleus and throughout the basal nuclei. In a minority of R-cells (23% or 6 of 26, Fig. 4D), firing rates peaked within seconds after the footshock. These cells were only encountered in the basolateral nucleus. In both subsets of R-cells, firing rates returned to control levels 100–160 min post-shock (137 ± 5 min). The origin of these different behaviors is unclear. It is possible that they reflect distinct mechanisms of activation (e.g., neuronal vs. hormonal).
Surprisingly, post-shock accelerations in firing rate were seen in both W and SWS. To address this point, separate averages of all the data points were computed for each R-cell in W (Fig. 4B2–D2, black bars) and SWS (Fig. 4B2–D2, red bars), before versus 70 min post-shock (left and right bars of Fig. 4B2–D2, respectively). This analysis revealed that on average, the firing rates of R-cells increased by 449 ± 77% in W (Fig. 4B–D, right column, black bars). In SWS, post-shock firing rates were significantly higher than pre-shock values in 69% of R-cells, close to 14 times more than expected by chance. However, the post-shock increases in firing rates were significantly smaller in SWS (an increase of 68 ± 33%) than in W (t-test, P < 0.05).
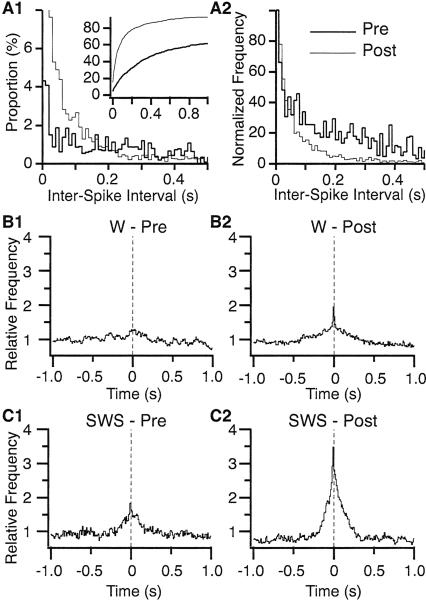
To determine whether these long-lasting increases in discharge rates were accompanied by modifications in firing pattern, we computed interspike interval (ISI) histograms for all R-cells before versus after the shock. For the post-shock data, we limited our analysis to 25–45 min post-shock, when firing rates peaked. As expected, this analysis revealed that in both W and S, the proportion of short ISIs increased after (Fig. 5A, thin line) compared to before (Fig. 5A thick line) the shock (see details in figure legend). However, the frequency distribution of ISIs remained Poisson-like after the shock (Fig. 5A2), suggesting that the arousal it produced did not cause lasting modifications in firing pattern.
Figure 5.
Inter-spike interval (ISI) histograms and cross-correlation of BLA activity. (A) ISI histograms in the waking state before (thick line) vs. after (thin line) the shock. A1, frequency distribution (10-msec bins). Note that the first bin of the post-ISI histogram was truncated for clarity. The true peak was 23%. Inset: Cumulative frequency distribution. A2, same as A1 after normalization so that the histogram peaks are equal. (B,C) Population cross-correlograms of BLA unit activity in W (B) and SWS (C). Cross-correlograms of unit discharges were computed for all available pairs (n = 71) of simultaneously recorded R-cells, and a normalized average was obtained. Left and right columns of panels show activity recorded before and after the shock, respectively.
To test whether the emotional arousal caused by the shock produced changes in the probability of synchronized firing among BLA cells, cross-correlograms were computed for all pairs of R-cells recorded simultaneously in Phase 5 (n = 71). Individual cross-correlograms were computed separately for the data acquired before and after the shock. The resulting correlograms were then normalized to an average count of 1 and pooled in separate population histograms, depending on the behavioral state (W, Fig. 5B; SWS, Fig. 5C). In both W and SWS, the post-shock correlograms (right) have a much higher central peak (post-to pre-shock ratio of 153 ± 0.2% in W and 174 ± 0.15% in SWS; P < 0.05). Note that since the correlograms were normalized to an average bin count of 1, this effect cannot be due to the random influence of increased firing rates. Consistent with this, no correlation was found between the change in firing rates and normalized cross-correlations before versus after the shock (W, r = 0.09; SWS, r = -0.16; P > 0.05).
Discussion
Although it is well known that pairing a neutral CS to a noxious unconditioned stimulus (US) produces long-term increases in the CS-responsiveness of BLA neurons (Quirk et al. 1995; Collins and Paré 2000; Maren 2000; Repa et al. 2001), the effect of mild emotional arousal on the spontaneous activity of BLA cells had received little attention before. The seminal study of Quirk et al. (1995) reported that a few LA neurons exhibited short-lived (<1 h) alterations in firing rate following fear conditioning. However, because the authors' focus was on the sensory responsiveness of LA neurons, few time points were analyzed and the confounding effect of behavioral states was not considered.
Our interest in this question stemmed from a series of pharmaco-behavioral studies where it was shown that enhancing or inhibiting BLA activity in the 2–3 h post-training could respectively facilitate or reduce performance on an inhibitory avoidance task, tested days later. Consistent with this pharmaco-behavioral evidence, our present results suggest that emotional arousal does produce long-lasting increases in the spontaneous activity of BLA neurons. These results are also consistent with functional imaging studies in humans indicating a strong correlation between amygdala activation at encoding and long-term recall of emotional material (Cahill et al. 1996, 2004; Dolcos et al. 2004).
Correspondence between electrophysiologically and behaviorally determined windows of increased BLA activity
In the pharmaco-behavioral studies mentioned in the introduction, it was observed that the effects of post-training BLA treatments (i.e., local drug infusions) decreased as the interval between the training and the treatment increased, generally vanishing after 2–3 h. Namely, drugs that facilitated or reduced BLA activity enhanced or diminished retention (McGaugh 2004). Interestingly, this interval corresponds to the time required for amygdala noradrenaline levels to return to baseline after emotional arousal (McIntyre et al. 2002).
While these observations suggest that the emotional arousal produced during training increases BLA activity for a few hours, other interpretations are possible. For instance, the activity of BLA cells might remain stable during emotional arousal, but its impact would be modified because of widespread increases in the concentration of key neuromodulators in target structures. While the present study cannot exclude the contribution of neuromodulators, it indicates that emotional arousal does enhance the activity of BLA neurons. Moreover, our electrophysiological estimate of the duration of increased BLA activity agrees remarkably well with that derived from the pharmaco-behavioral data.
Identity of R-neurons
Previous work revealed that the BLA contains two main cell types (for review, see McDonald 1992). Most neurons are glutamatergic projection cells, and a minority of neurons are local-circuit cells immunopositive for γ-aminobutyric acid (GABA; McDonald 1985). Thus, by chance alone, our sample of BLA neurons should be mainly comprised of projection cells. To further restrict our attention to projection cells, we only considered those that fired at 5 Hz or less in W, an activity pattern that was found to be characteristic of projection cells in previous electrophysiological studies (Paré and Gaudreau 1996; Lang and Paré 1998).
Nevertheless, the behavior of BLA cells was heterogeneous after the footshock, with nearly half the cells increasing their firing rate and the rest keeping the same level of activity. At present, the origin of this heterogeneity is unclear. In the pre-shock period, the spontaneous activity of NR- and R-cells was indistinguishable. Moreover, the position of R- and NR-cells overlapped extensively in the BLA. Given the evidence suggesting that noradrenaline and glucocorticoids constitute a critical link between emotional arousal and the facilitation of memory consolidation by the amygdala (Quirarte et al. 1997), it is possible that this heterogeneity reflects cell-to-cell variations in the expression of receptors for these neuromodulators.
Implication for mechanisms of BLA-mediated facilitation of memory consolidation by emotion
The presence of R-cells in much of the BLA has important consequences for how the BLA facilitates memory in emotionally arousing conditions. Indeed, the various BLA nuclei have different projection sites. As a group, they project to the rhinal cortices, hippocampus, insula, prefrontal cortex, striatum, etc. (for review, see Pitkänen 2000). This implies that the emotional arousal produced by the footshock caused long-lasting activity enhancements in neurons that project to functionally heterogeneous brain structures. Thus, these considerations suggest that the memory-modulating role of the BLA would not depend on the specific activation of particular groups of BLA neurons, but on the activity patterns taking place in BLA projection sites when the emotional arousal occurred.
The fact that post-shock BLA discharges are more synchronized than baseline activity also has important implications. Indeed, the conduction times of BLA axons are adjusted to compensate for variations in distance between the BLA and its targets (Pelletier and Paré 2002). This property might allow BLA neurons to generate short time windows of depolarization that facilitate Hebbian associations between coincident but spatially distributed activity patterns in target structures. However, this view implies that the activity patterns that took place during the emotionally arousing event are replayed in both waking and slow-wave sleep. In support of this idea, there is now much evidence of replay in slow-wave sleep (Pennartz et al. 2002, 2004) and waking (Abeles et al. 1995; Seidemann et al. 1996; Nadasdy et al. 1999) at multiple levels of the central nervous system.
Materials and Methods
Animals
The experiments were conducted in three adult cats (2.5–3.5 kg). This species was chosen for the following reasons. First, the large size of the cat brain facilitates the placement of multiple microelectrodes within the amygdala. Second, cats tolerate head restraint very well, and immobilization of the head is optimal for obtaining prolonged extracellular neuronal recordings. Experiments were carried out at Université Laval (Québec, Canada) in agreement with the guidelines of the Canadian Council for Animal Care.
Experimental procedures
The behavioral part of the study was divided into six phases (Fig. 1A). Note that we did not aim to implicate the amygdala in the acquisition of this task, only to test whether the emotional arousal produced as part of this task is accompanied by lasting changes in the spontaneous activity of BLA cells.
1. Initial training
Over a period of 9 d, cats that had been trained to press a lever repetitively for food learned that lever-pressing would only be rewarded when a light was turned on (“lights-on”), not when it was off (“lights-off”). Cats received one training session per day during which fifteen 1-min “lights-on or off” epochs were presented. Performance on this task was monitored by computing the ratio of the number of lever presses when the light CS was on to when it was off.
2. Microelectrode implantation
Then, as described in detail below, the animals were anesthetized and implanted with two arrays of eight high-impedance tungsten electrodes stereotaxically aimed at the BLA.
3. Retraining
After recovery from surgery, the animals were retrained to criterion (15 times more lever presses during “lights-on” than “lights-off” epochs, dashed line in Fig. 2B). Then, extended “lights-off”periods were introduced in the middle (1 h) and at the end (4 h) of each training session over a period of 3 d. These epochs were introduced to enable prolonged periods of neuronal recordings in Phases 4 and 5.
4. Control recordings
On the first recording day (Fig. 1B), the animals were presented three alternating “lights-on and off” epochs, each lasting 1 min, followed by an extended “lights-off” epoch during which the spontaneous activity of BLA neurons was recorded in both waking and slow-wave sleep. Then, the “lights-on and off” alternation resumed with the exception that a second stimulus (a 1 kHz tone, 50 db, 1 min) was presented during the second “lights-on” epoch (Fig. 1B). After the lights and tone were turned off, the spontaneous activity of BLA neurons was recorded for the next 3–4 h.
5. One-trial learning
On the second recording day (Fig. 1C), the cats received the same stimuli as in Phase 4 with the exception that the first lever press following tone onset triggered a single brief footshock (0.5 sec, 0.5 mA). The lights and tone were then turned off and recordings performed as in Phase 4.
6. Retention test
Two days after the introduction of the footshock, cats were given the opportunity to press the lever for food again. After two “lights-on” and “lights off” epochs, the tone was presented 15 sec before the onset of a “lights-on” epoch and left on for 1 min. Then, the alternation between “lights-on and off” epochs continued normally. The number of lever presses and latency of the first lever press following tone onset were measured. On this day, no footshocks were administered.
Electrode implantation
The electrode implantation was performed in sterile conditions. Anesthesia was induced with ketamine (15 mg/kg, i.m.) and atropine sulfate (0.05 mg/kg, i.m.) was administered to prevent secretions. Then, sodium pentobarbital was injected gradually (pentobarbital, ≈15 mg/kg, i.v.). To record eye movements (EOG), two silver-ball electrodes were fixed into the supraorbital cavity with dental cement. Two Teflon-insulated wires were inserted in the neck muscles to monitor electromyographic activity (EMG), and stainless steel screws were anchored to the bone overlying the pericruciate area to monitor the EEG.
The bone overlying the amygdala was removed and the dura mater opened bilaterally. Then, two arrays of eight tungsten electrodes (2–6 MΩ at 1 kHz; outer diameter of 80 μm; FHC) were lowered until they reached the dorsal aspect of the amygdala in each hemisphere (for details, see Collins and Paré 1999). Their length was adjusted so that recordings could be performed simultaneously in the different nuclei of the BLA.
Finally, four screws were cemented to the skull. These screws were later used to fix the cat's head in a stereotaxic position without pain or pressure. Bicillin (i.m. daily for 3 d) and buprenorphine (0.03 mg/kg, i.m. every 12 h for 24 h) were administered post-operatively. Recording sessions began 10–12 d after the surgery.
The recording methods used in Phases 4 and 5 were identical. At the beginning of both sessions, the electrode array was slowly lowered 80–300 μm, until the activity of a satisfactory group of neurons could be observed. To ensure mechanical stability, we waited at least 30 min before beginning the recordings.
Identification of recording sites
At the end of the experiments, the animals were deeply anesthetized with sodium pentobarbital, and recording sites were marked with electrolytic lesions (0.5 mA for 5 sec). Following this, the animals were perfused with 500 mL of a cold saline solution (0.9%) followed by 1 L of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffered saline (pH 7.4). The brains were later sectioned on a vibrating microtome (at 80 μm), and stained with thionin to verify the position of the recording electrodes. The microelectrode tracks were reconstructed by combining micrometer readings with the histological controls. Despite the high number of electrodes, it was easy to determine the position of recorded neurons as the relative electrode position was known. Data were only included in the analyses after histological determination of the recording sites.
Analysis
Analyses were performed off-line with the software IGOR (Wave-metrics) and home-made software running on Macintosh microcomputers. Spikes were detected with a window discriminator after digital filtering (0.3–20 kHz) of the data. To identify behavioral states, the power in the 1–4-Hz band of the EEG signal was computed in 1-sec windows sliding in steps of 100 msec. In all cats, plotting the 1–4-Hz power over time revealed that it oscillated between two quasi-stable states of high and low 1–4-Hz power. To be classified as waking, epochs had to be characterized by a 1–4-Hz power no more than 10% higher than that seen when the animals were eagerly anticipating the lights-on period to begin. Slow-wave sleep was operationally defined as having an integrated 1–4-Hz power ≥ 3 times that seen during these epochs of expectancy. We only considered uninterrupted periods of waking and slow-wave sleep epochs at least 30 sec in duration. The rare periods of paradoxical sleep could be identified by the muscular atonia characteristic of that state. They were excluded from the analysis. We used a fixed level of significance (P < 0.05) for all statistical tests. Values are expressed as means ± standard error.
Acknowledgments
This work was supported by FCAR and by grant R01MH-06685601 from NIMH.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.88605.
References
- Abeles, M., Bergman, H., Gat, I., Meilijson, I., Seidemann, E., Tishby, N., and Vaadia, E. 1995. Cortical activity flips among quasi-stationary states. Proc. Natl. Acad. Sci. 92: 8616-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L. and McGaugh, J.L. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21: 294-299. [DOI] [PubMed] [Google Scholar]
- Cahill, L., Haier, R.J., Fallon, J., Alkire, M.T., Tang, C., Keator, D., Wu, J., and McGaugh, J.L. 1996. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc. Natl. Acad. Sci. 93: 8016-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L., Uncapher, M., Kilpatrick, L., Alkire, M.T., and Turner, J. 2004. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An FMRI investigation. Learn. Mem. 11: 261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano, C., Brioni, J.D., Nagahara, A.H., and McGaugh, J.L. 1989. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav. Neural Biol. 52: 170-179. [DOI] [PubMed] [Google Scholar]
- Christianson, S.A. 1992. Handbook of emotion and memory: Current research and theory. Erlbaum, Hillsdale, NJ.
- Collins, D.R. and Paré D. 1999. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J. Neurosci. 19: 836-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2000. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(-). Learn. Mem. 7: 97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A.H., Mesches, M.H., Coleman, K., and McGaugh, J.L. 1993. Bicuculline administered into the amygdala blocks benzodiazepine-induced amnesia. Behav. Neural Biol. 60: 1-4. [DOI] [PubMed] [Google Scholar]
- Dolcos, F., LaBar, K.S., and Cabeza, R. 2004. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42: 855-863. [DOI] [PubMed] [Google Scholar]
- Ferry, B. and McGaugh, J.L. 1999. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol. Learn. Mem. 72: 8-12. [DOI] [PubMed] [Google Scholar]
- Gallagher, M., Kapp, B.S., Musty, R.E., and Driscoll, P.A. 1977. Memory formation: Evidence for a specific neurochemical system in the amygdala. Science 198: 423-425. [DOI] [PubMed] [Google Scholar]
- Gold, P.E. and McGaugh, J.L. 1975. A single-trace, two process view of memory storage processes. In Short-term memory (eds. D. Deutsch and J.A. Deutsch), pp. 355-378. Academic Press, New York.
- Gold, P.E., van Buskirk, R.B., and McGaugh, J.L. 1975. Effects of hormones on time-dependent memory storage processes. Prog. Brain Res. 42: 210-211. [DOI] [PubMed] [Google Scholar]
- Hatfield, T. and McGaugh, J.L. 1999. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol. Learn. Mem. 71: 232-239. [DOI] [PubMed] [Google Scholar]
- Introini-Collison, I.B., Miyazaki, B., and McGaugh, J.L. 1991. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology (Berl) 104: 541-544. [DOI] [PubMed] [Google Scholar]
- Izquierdo, I. and Medina, J.H. 1993. Role of the amygdala, hippocampus and entorhinal cortex in memory consolidation and expression. Braz. J. Med. Biol. Res. 26: 573-589. [PubMed] [Google Scholar]
- Jacobs, B.L. and McGinty, D.J. 1971. Amygdala unit activity during sleep and waking. Exp. Neurol. 33: 1-15. [DOI] [PubMed] [Google Scholar]
- Lang, E.J. and Paré, D. 1998. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience 83: 877-889. [DOI] [PubMed] [Google Scholar]
- Liang, K.C. and McGaugh, J.L. 1983. Lesions of the stria terminalis attenuate the enhancing effect of post-training epinephrine on retention of an inhibitory avoidance response. Behav. Brain Res. 9: 49-58. [DOI] [PubMed] [Google Scholar]
- Liang, K.C., McGaugh, J.L., and Yao, H.Y. 1990. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 508: 225-233. [DOI] [PubMed] [Google Scholar]
- Maren, S. 2000. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 12: 4047-4054. [DOI] [PubMed] [Google Scholar]
- McDonald, A.J. 1985. Morphology of peptide-containing neurons in the rat basolateral amygdaloid nucleus. Neurosci. Lett. 53: 203-207. [DOI] [PubMed] [Google Scholar]
- ———. 1992. Cell types and intrinsic connections of the amygdala. In The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction (ed. J.P. Aggleton), pp. 67-96. Wiley-Liss, New York.
- McGaugh, J.L. 2004. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27: 1-28. [DOI] [PubMed] [Google Scholar]
- McIntyre, C.K., Hatfield, T., and McGaugh, J.L. 2002. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci. 16: 1223-1226. [DOI] [PubMed] [Google Scholar]
- Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J., and Buzsaki, G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19: 9497-9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré, D. and Gaudreau, H. 1996. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J. Neurosci. 16: 3334-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, J.G. and Paré, D. 2002. Uniform range of conduction times from the lateral amygdala to distributed perirhinal sites. J. Neurophysiol. 87: 1213-1221. [DOI] [PubMed] [Google Scholar]
- Pennartz, C.M., Uylings, H.B., Barnes, C.A., and McNaughton, B.L. 2002. Memory reactivation and consolidation during sleep: From cellular mechanisms to human performance. Prog. Brain. Res. 138: 143-166. [DOI] [PubMed] [Google Scholar]
- Pennartz, C.M., Lee, E., Verheul, J., Lipa, P., Barnes, C.A., and McNaughton B.L. 2004. The ventral striatum in off-line processing: Ensemble reactivation during sleep and modulation by hippocampal ripples. J. Neurosci. 24: 6446-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen, A. 2000. Connectivity of the rat amygdaloid complex. In The Amygdala: A functional analysis (ed. J.P. Aggleton), pp. 31-115. Oxford University Press, Oxford, UK.
- Quirarte, G.L., Roozendaal, B., and McGaugh J.L. 1997. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. 94: 14048-14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk, G.J., Repa, C., and LeDoux, J.E. 1995. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron 15: 1029-1039. [DOI] [PubMed] [Google Scholar]
- Repa, J.C., Muller, J., Apergis, J., Desrochers, T.M., Zhou, Y., and LeDoux, J.E. 2001. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat. Neurosci. 4: 724-731. [DOI] [PubMed] [Google Scholar]
- Roozendaal, B. 1999. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25: 213-238. [DOI] [PubMed] [Google Scholar]
- Roozendaal, B. and McGaugh, J.L. 1996. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Res. 709: 243-250. [DOI] [PubMed] [Google Scholar]
- Roozendaal, B., Portillo-Marquez, G., and McGaugh, J.L. 1996. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav. Neurosci. 110: 1074-1083. [DOI] [PubMed] [Google Scholar]
- Sacchetti, B., Lorenzini, C.A., Baldi, E., Tassoni, G., and Bucherelli, C. 1999. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J. Neurosci. 19: 9570-9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas, J.A., Introini-Collison, I.B., Dalmaz, C., and McGaugh, J.L. 1997. Posttraining intraamygdala infusions of oxotremorine and propranolol modulate storage of memory for reductions in reward magnitude. Neurobiol. Learn. Mem. 68: 51-59. [DOI] [PubMed] [Google Scholar]
- Seidemann, E., Meilijson, I., Abeles, M., Bergman, H., and Vaadia, E. 1996. Simultaneously recorded single units in the frontal cortex go through sequences of discrete and stable states in monkeys performing a delayed localization task. J. Neurosci. 16: 752-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, T.J. and Jacobs, B.L. 1975. Single unit activity in the lateral amygdala of the cat during sleep and waking. Electroencephalogr. Clin. Neurophysiol. 38: 331-333. [DOI] [PubMed] [Google Scholar]