Abstract
The effects of stress (restraint plus tail shock) on hippocampus-dependent trace eyeblink conditioning and hippocampal excitability were examined in C57BL/6 male mice. The results indicate that the stressor significantly increased the concentration of circulating corticosterone, the amount and rate of learning relative to nonstressed conditioned mice, and the excitability of CA1 hippocampal pyramidal neurons. Behaviorally, there was no effect of the stressor on control mice that received unpaired presentations of the tone and periorbital shock, i.e., neither stressed nor nonstressed control mice showed an increase in conditioned responding that was above baseline levels. Biophysically, the stressor significantly decreased the amplitude of the post-burst afterhyperpolarization (AHP) and decreased spike frequency accommodation relative to cells from nonstressed control mice. The effect was significant for mice that were stressed either 1 h or 24 h earlier. The results suggest that the stressor increases the excitability of hippocampal pyramidal neurons and that the mechanism underlying this increase may contribute to the more rapid acquisition of hippocampally dependent eyeblink conditioning.
The hippocampus is critically important for the formation of memories, as observed in human patients with temporal lobe damage (Scoville and Milner 1957; Daum et al. 1993; Zola and Squire 2001) and as demonstrated by lesion studies in several species, including rabbits (Solomon et al. 1986; Moyer Jr. et al. 1990), rats (Weiss et al. 1999a) and mice (Takehara et al. 2002; Tseng et al. 2004). Electrophysiological studies using both extracellular single neuron recordings (Berger et al. 1983; Weiss et al. 1996; McEchron and Disterhoft 1997) and intracellular recordings of the biophysical properties of hippocampal pyramidal neurons (Disterhoft et al. 1986; Moyer Jr. et al. 1996) have also revealed learning and memory-specific changes in the firing patterns and biophysical properties of these neurons. These neurons are particularly responsive to behaviorally salient stimuli, especially those that help inform the animal of where it is in space (Wilson and McNaughton 1993) and those that predict the temporal occurrence of another stimulus (Berger et al. 1976).
This innate responsiveness is also sensitive to adverse environmental conditions or stressors, which can either facilitate or inhibit the formation of new memories (Kim and Yoon 1998; de Kloet et al. 1999). The relationship between facilitation and inhibition for a particular stressor is likely due to the stimulus intensity in terms of magnitude, frequency, or duration (Shors and Servatius 1997), as well its timing relative to another event (Shors 2001). This relationship is often referred to as having an inverted U function; i.e., some stress facilitates behavior, but too much stress is detrimental. A better understanding of the relation which governs the effects of stress and behavior will be beneficial for understanding the neurobiological basis for stress-related disorders (Brewin 2001; McEwen 2001; Bremner 2003; Liberzon et al. 2003; Nutt and Malizia 2004) as well as the mechanisms underlying stress-facilitated learning (Shors et al. 1992).
Stress-facilitated learning of the eyeblink conditioning (EBC) task has been well characterized in the rat by Shors and colleagues (2000). This task requires the subject to associate an auditory conditioning stimulus with a brief periorbital shock or puff of air to the eye (Weiss et al. 1999b). This is an excellent system to examine interactions of stress and learning, but the mouse would be a better subject for the purpose of examining the genetic basis of these interactions. Several laboratories have already demonstrated EBC in the mouse (Chen et al. 1999; Takehara et al. 2002; Weiss et al. 2002), including hippocampally dependent trace EBC (Takehara et al. 2002; Tseng et al. 2004). The aims of the present set of experiments were to determine if stressors that facilitate EBC in the rat also facilitate EBC in the mouse, and to determine if the facilitation is due in part to an increased excitability of CA1 hippocampal pyramidal neurons. The results from these experiments can then form the basis for a genetic analysis of stress-related interactions with learning and memory.
Results
First the effect of the stressor on circulating levels of corticosterone was examined as a neuroendocrine assay for the effectiveness of the stressor. Second, the effect of stress on acquisition of hippocampally dependent EBC (Tseng et al. 2004) was determined. Third, the effect of the stress on the biophysical properties of CA1 pyramidal neurons was examined.
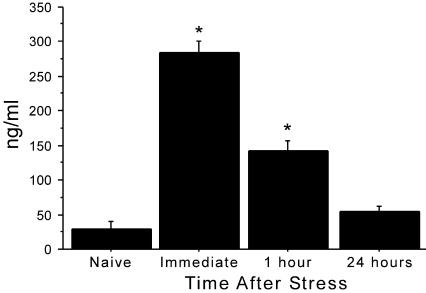
The data from the corticosterone assay are presented in Figure 1. An ANOVA indicated that there was a significant time-dependent change in the level of circulating corticosterone following the stressor (F(3,20) = 79.9, P < 0.0001). Post hoc least significant difference (LSD) tests indicated that the level immediately after the stressor was significantly elevated relative to controls (P < 0.0001). The level from blood taken 1 h after the stress was still significantly elevated relative to controls (P < 0.0001), but the values had dropped significantly relative to the data from blood taken immediately after the stress (P < 0.0001). Data from the 24-h time point were not significantly different from those of the naive controls.
Figure 1.
Stress significantly increased circulating levels of corticosterone when measured from blood samples taken either immediately after the stress or 1 h after the stress. Samples taken 24 h after the stress were not significantly different from that of naive control mice. There were six mice per group, and each mouse was only sampled one time. *P < 0.05.
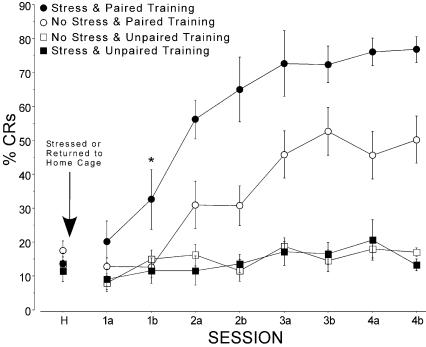
The effect of the stressor on learning was assayed with hippocampally dependent trace EBC (Tseng et al. 2004). The results indicate that there was a general facilitation of EBC in mice that were exposed to the stressor (Fig. 2). The nonstressed mice exhibited an asymptotic level of ∼48% conditioned responses (CRs) during the last four sessions; the stressed mice had an asymptotic level of ∼75% CRs. An ANOVA confirmed that the stressor significantly increased the level of conditioned responding (F(1,27) = 0.3, P = 0.008) in comparison to nonstressed mice. There was also a significant interaction (F(1,27) = 9.3, P = 0.005) of stress and training (conditioned or pseudoconditioned), which was due to the conditioned groups exhibiting CRs while the pseudoconditioned control groups did not exhibit a significant increase in the percent of trials with CRs whether they were stressed or not.
Figure 2.
Stress significantly facilitated trace eyeblink conditioning in mice but had no effect on control mice that received explicitly unpaired presentations of the CS and US. Mice received one session of habituation and then two sessions (a and b) of conditioning per day for 4 d. Data are mean ± SE for each session. The asterisk shows the first session for which post hoc tests revealed a significant increase in the stressed group relative to unstressed controls.
Post hoc t-tests of the percentage of trials with CRs for each session indicated that the first session in which a significant difference was found between stressed and nonstressed conditioned mice was session 1b, i.e., the second session of the first day of conditioning (t = 2.22, df = 17, P = 0.041). This session is indicated by an asterisk in Figure 2. Finally, there was no statistical difference among the groups during the habituation session (which occurred prior to giving the stress).
The increased learning rate among stressed mice suggested to us that the stressor may have increased the excitability of CA1 pyramidal neurons and thus increased hippocampus-dependent learning. We recorded biophysical data from eight nonstressed control mice (26 cells), seven mice stressed 1 h before slice preparation (22 cells), and seven mice stressed 24 h prior to slice preparation (22 cells). All recordings were done blind to the behavioral manipulation. The holding potential was -68 mV for all cells.
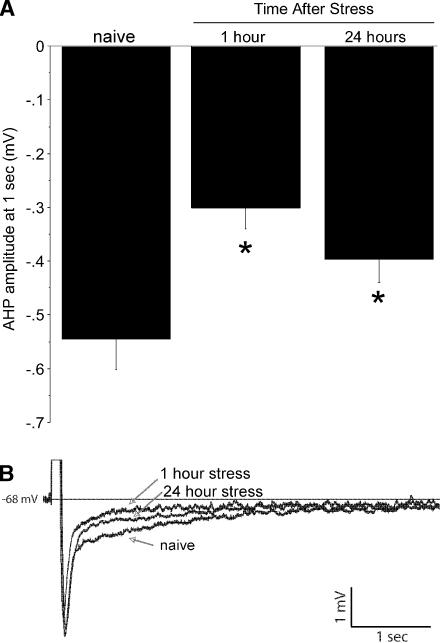
An ANOVA of the amplitude of the AHP at 1 sec indicated that stress differentially affected CA1 neurons (F(2,67) = 6.8, P = 0.002). A graph of the results showing the group means is shown in Figure 3A. Post hoc Fisher's LSD tests indicated that the stressed mice had a significantly smaller mean AHP at 1 h (P = 0.0005) and 24 h (P = 0.031) after the stress compared with nonstressed control mice (-0.30 and -0.40 versus -0.55 mV, respectively). An example of the change in the AHP due to the stressor is shown in Figure 3B.
Figure 3.
Stress reduces the amplitude of the post-burst afterhyperpolarization of CA1 hippocampal pyramidal neurons, which increases their excitability. (A) The mean and SE for each group. (B) An individual example of the AHP for each group.
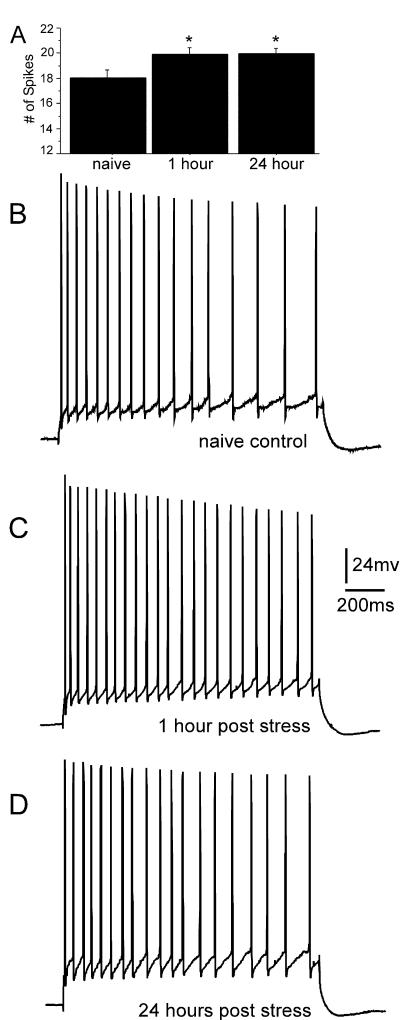
The results for the measure of accommodation paralleled the results for changes to the AHP (F(2,66) = 4.2, P = 0.019); i.e., the mean number of action potentials evoked by the 800-msec current pulse was significantly increased at 1 h (P = 0.017) and 24 h (P = 0.014) after the stress, relative to nonstressed control mice (19.99 ± 0.51 and 19.95 ± 0.46 versus 18.05 ± 0.60). A graph of the group results is shown in Figure 4A, and examples showing the change in accommodation are presented in Figure 4, B through D.
Figure 4.
Stress reduces the spike frequency accommodation of CA1 hippocampal pyramidal neurons. (A) The mean and SE for each group. The effect on accommodation at 1 h after stress was more robust than at 24 h after stress, but the data from both time points were significantly different from that of neurons from nonstressed control mice. (B–D) An individual example of spike frequency accommodation for each group.
Discussion
The results of these experiments clearly indicate that an acute stress of restraint plus tail shock persistently facilitates the subsequent acquisition of hippocampally dependent trace EBC in young adult C57BL/6 male mice; i.e., the stressed mice consistently exhibited a greater level of conditioned responding than did the nonstressed mice, even though both groups had a similar level of baseline responding as measured during the habituation session. Furthermore, the facilitation appears to be specific for associative learning since neither the group of stressed mice receiving explicitly unpaired stimuli nor the group of nonstressed mice receiving explicitly unpaired stimuli exhibited any increase in conditioned responding.
The facilitation that did occur in the stressed and conditioned mice was substantial and extends the results of previous experiments that examined the effects of stress on delay EBC using male Fischer-344 rats (Shors et al. 1992) and trace EBC using Sprague-Dawley rats (Beylin and Shors 1998). Although we did not test the effects of the stressor on delay EBC, it too is likely to be facilitated since the hippocampus is activated in a learning-specific manner during delay conditioning in addition to trace conditioning (Berger et al. 1976, 1983). Furthermore, the inclusion of the mouse into these data sets is important to begin a genetic analysis of stress-facilitated learning mechanisms.
The persistent facilitation that we observed occurred even though there was only a temporary elevation in circulating levels of corticosterone. This initial increase in corticosterone is likely to be necessary and sufficient for the initial effect of the stress, but not for the persistent effect (Shors 2001; Beylin and Shors 2003). A more long-lasting effect of the stress may require protein synthesis (Karst and Joels 1991) and may be mediated by increases in the excitability of hippocampal neurons since we found that CA1 pyramidal neurons from mice exposed to the stressor had a significantly smaller slow AHP and significantly decreased accommodation to pulses of injected current than did control mice, which were not stressed. Such biophysical changes have previously been associated with faster learning of EBC in rabbits and rats; i.e., drugs that reduce the age-related increase in the amplitude of the AHP facilitate acquisition of EBC (Moyer Jr. and Disterhoft 1994; Weiss et al. 2000; Power et al. 2001), and acquisition of EBC in otherwise naive animals is associated with reductions of the AHP in CA1 pyramidal neurons (Disterhoft et al. 1986; Coulter et al. 1989; de Jonge et al. 1990; Moyer Jr. et al. 1996, 2000). The excitability of CA1 pyramidal neurons is also increased by the application of CRF via a protein kinase A–mediated reduction of sIAHP in rat (Haug and Storm 2000), and as assayed with tests of long-term potentiation from slices of naive mice (Blank et al. 2002, 2003a). These changes in the AHP also appear to affect a more general mechanism that affects learning of other tasks such as finding the location of a hidden platform in a water maze (Oh et al. 2003) or associating a neutral conditioning stimulus with a fear evoking unconditioned stimulus (Blank et al. 2002).
Our finding that the stressor we used increased the excitability of CA1 pyramidal neurons may at first seem at odds with reports that stress impairs long-term potentiation (Foy et al. 1987; Diamond and Rose 1994) and facilitates long-term depression (Xu et al. 1997), but the difference may be due to a shift in the baseline excitability of hippocampal neurons; i.e., if stress increases, the excitability of neurons prior to LTP, then the currents mediating LTP may be maximized already and incapable of exhibiting a further increase.
Other possibilities for a more persistent effect of the stress include increased density of spines on the apical (Shors et al. 2001a; Beylin and Shors 2003) and basal dendrites of CA1 pyramidal neurons (Leuner et al. 2003), increased levels of acetylcholine in the hippocampus and prefrontal cortex (Mark et al. 1996), and suppression of glutamatergic mediated neuronal activity in the basolateral nucleus of the amygdala (Shors 1999).
Our results from the biophysical experiments indicate that we can use the AHP as an assay for excitability changes that are associated with stress or the processes that are related to stress, regardless of the mechanism underlying changes in the AHP. Since these changes are more persistent and downstream of the behavioral manipulation, we should also be able to use genetically modified mice to examine the mechanisms underlying the biophysical and behavioral changes we observed. Changes in the small conductance potassium channels (SK) (Vergara et al. 1998) may be an example of a mechanism that mediates changes in the excitability of hippocampal neurons (Sah 1996; Oh et al. 2000; Stackman et al. 2002; Blank et al. 2003b). These calcium activated potassium channels are of particular interest due to age-related memory impairments and age-related changes in neuronal calcium regulation (Blank et al. 2003b). These changes could be due to any of the three genes that encode SK subunits (SK1, SK2, and SK3) (Köhler et al. 1996). These subunits form channels that are voltage-insensitive and activated by submicromolar intracellular Ca2+ concentration. The SK1 and SK2 subunits are highly expressed in the neocortex and hippocampus according to in situ hybridization (Stocker and Pedarzani 2000). The SK1 channel is insensitive to the bee venom peptide apamin, whereas the SK2 channel is highly sensitive to it (Köhler et al. 1996, Vergara et al. 1998, Bond et al. 1999). Furthermore, the SK2 channel could contribute in an increase in excitability if elevated corticosterone levels repress the transcription factor nuclear factor-κB as shown in PC12 cells transfected with a murine SK2 promoter-luciferase reporter gene construct (Kye et al. 2004).
Another possibility for examining the genetic mechanisms underlying the effects of stress is to examine mice with and without the receptors for corticotrophin-releasing factor. This initiator of the endocrine stress response has profound effects on hippocampal excitability, learning, and behaviors affected by stress (Blank et al. 2002, 2003a).
Last, we should consider the impact of neurogenesis on hippocampally dependent learning paradigms since trace EBC has been found to enhance the survival of newly generated neurons in the adult rat hippocampus (Shors et al. 2000) and inhibition of neurogenesis impairs acquisition of trace but not delay EBC in rats (Shors et al. 2001b).
Each of these possibilities can eventually be tested by generating mice with and without different receptors, or receptor subunits, and then testing those mice for stress facilitated EBC and learning-related reductions in the AHP. Those mice could also be tested to analyze the genetic basis of stress-related learning impairments. Although this report has focused on EBC and stress-related facilitation of learning and memory, stress-related facilitation of contextual fear conditioning has been reported (Cordero et al. 2003), and stress-related impairments of spatial memory have been documented (see de Quervain et al. 1998; Conrad et al. 2004), as has the lack of a stress-related effect on spatial memory (Warren et al. 1991). The basis for the difference among the outcomes is likely to be due to differences in the timing and context of different tasks relative to stressors of different magnitudes, and to the balance of receptor activation by CRF and glucocorticoids and mineralcorticoids (Joels and de Kloet 1991; Joels and Vreugdenhil 1998). An analysis of these effects is important to understand learning and memory processes more fully and may ultimately lead to the development of therapeutic agents for disorders related to stress and anxiety.
Materials and Methods
Subjects
Three-month-old C57BL/6J male mice were obtained from the National Institute of Aging. Animals were housed two to four per cage and maintained on a 14-h light/10-h dark cycle (lights on at 6:00 a.m.) with ad libitum access to food and water. The mice typically weighed between 26 and 32 g.
Surgery
All mice used for behavioral conditioning were anesthetized with avertin (670 mg/kg tribromoethanol in 1.3% tertiary amyl alcohol) and given supplements as necessary. Animals were then positioned in a stereotaxic frame, and the skull was exposed between bregma and lambda. A connector with four Teflon-coated stainless steel wires (75 μm) and one bare stainless steel wire was positioned on the skull. Two of the coated wires were subcutaneously passed through the upper eyelid of the right eye to record EMG activity of the orbicularis oculi muscle; the two other coated wires (shock electrodes) were subcutaneously passed through the periorbital region caudal to the eye. The bare wire was secured to two stainless steel skull screws (00–90 × 1/16 in.) to serve as an electrical ground. The connector was cemented to the skull with dental acrylic (Grip Cement; Caulk Dentsply). The remaining four wires were pulled taut, stripped of insulation at the distal ends, shortened, and crimped over the eyelid so that ∼2 mm of exposed wire was through or under the eyelid, and 2 mm was on top of the eyelid. All animals were then placed on a warm isothermal pad following surgery. The mice exhibited no obvious abnormal behaviors following the surgery. The Northwestern University Animal Care and Use Committee approved all procedures for these experiments in accordance with National Institute of Health guidelines.
Stressor
The stressor was similar to that used by Shors et al. (1992). The mice were restrained in a tube (the base of a 250-mL graduated cylinder cut off at about the 70-mL mark, which had ventilation holes added) and their tail was placed through the hole of a rubber stopper. The tail was then placed through a cuff electrode containing two wire contacts, and the cuff was prevented from slipping by taping it in place. Each mouse received 90 shocks (1 mA AC, 1 sec, 1/min) via a Colbourn Instruments HS13-16 animal shocker (our pilot data indicated that 60 shocks were not as effective as 90 shocks) (c.f. Shors and Servatius 1997).
Blood collection and analysis
The effect of the stressor was evaluated by assaying the plasma for levels of circulating corticosterone. We collected ∼500 μL of blood from the retro-orbital sinus within a maximum of 40 sec and stored the blood on ice until the plasma was separated out by centrifugation (3000g, 15 min, 4°C, ∼10 mM EDTA). Plasma samples were stored at -80°C until they were assayed by radioimmunoassay. We collected blood from a group of naive control mice and from three other groups that had been stressed either immediately before sampling, 1 h before sampling, or 24 h prior to sampling (N = 6 per group). The data were analyzed by ANOVA (StatView) with Fisher's LSD tests for post hoc analyses.
Behavioral apparatus, training, and analysis
The mice were placed within Plexiglas cylinders (5-in. diameter) on a wire rack floor in a single light and sound-attenuated Industrial Acoustics chamber. We trained a maximum of four mice at a time with each mouse in its own cylinder. A tether was attached to the connector on the mouse that allowed the mice to be freely moving. A speaker delivered the tone CS (250 msec, 2 kHz, 80 dB, 5-msec rise/fall), which was of lower volume than our typical 85 dB in order to prevent a ceiling effect (cf. Tseng et al. 2004). A DC biphasic shocker (Bak Electronics) delivered the square wave shock US (100 msec train, 60 Hz, 0.5-msec pulses per phase). The trace interval between the CS and US was 250 msec. Mice were given 30 trials per session and two sessions per day. Trials were separated by a random intertrial interval (ITI) that varied from 30–60 sec. The control group used to detect pseudoconditioning was given random presentations of explicitly unpaired tones and shocks with a 15–30 sec ITI and 60 trials per session to maintain the same stimulus density as the experimental group. Sessions were separated by 3–4 h. The EMG activity was amplified 5000 times, filtered between 100–5000 Hz, sampled at 5000 Hz, and stored on a computer. EMG activity during each trial was collected, rectified, and integrated by using specially designed LabView routines (Knuttinen et al. 2001). All mice received one habituation session the day prior to the start of training. They were connected to the tether and the computer collected EMG activity as if a training session was being given, but no stimuli were presented. The mice were either returned to their home cage immediately after the habituation session, or stressed (as described above) and then returned to their home cage. There were 10 mice that were conditioned with no stress, nine mice that were stressed and conditioned, seven mice that were not stressed and used to test for pseudoconditioning, and five mice that were tested to determine if stress induced pseudoconditioning.
All behavioral data were analyzed by using custom LabView routines, and statistical analyses were done with StatView software. The factors were conditioning (conditioned, pseudoconditioned) and stress (stressed, nonstressed). There was also a repeated measure of training (eight sessions). A CR was defined as integrated EMG activity that was four standard deviations greater than the mean of a 250-msec baseline immediately prior to CS onset. A CR also needed to have an onset latency of at least 35 msec, occur before US onset, and have a duration of at least 15 msec. CRs with an onset latency <35 msec were considered α responses. These analyses were done on tone alone trials for the pseudoconditioned control animals.
Biophysical recording methods
Slice preparation
Animals were killed by decapitation under general halothane anesthesia. The brains were quickly removed and immediately cooled in ice-cold (4°C) artificial cerebrospinal solution (aCSF). aCSF for slice preparation consisted of (in mM): 124 NaCl, 3 KCl, 1.25 KH2PO4, 1.0 CaCl2, 2.4 MgCl2, 26 NaHCO3, and 10 glucose; the concentrations of MgCl2 were reduced to 2.0 mM and CaCl2 was increased to 2.4 mM for recording purposes. Both solutions were saturated with 95% O2/5% CO2 for slice oxygenation and to maintain pH (7.4).
The middle part of the hippocampus was dissected from both hemispheres and mounted on the stage of a vibratome (Vibratome Series 1000, TPI) by using cyanoacrylate glue and supporting agar. Transverse slices (300 μm) were cut in ice-cold aCSF. Slices were then transferred into an incubation chamber with aCSF. Slices were stored for at least 1 h prior to any recording and then transferred to the submerged small glass-bottom recording chamber mounted onto the stage of an upright microscope (DM LFS, Leica Microsystem; Axioskop, Carl Zeiss, Inc.) with a long-distance water-immersion objective (×40). The recording chamber was constantly perfused with aCSF (2 mL/min). All recordings were performed at room temperature (∼23°C).
Whole-cell patch-clamp recording
Whole-cell patch-clamp recordings were obtained by the “cell-target” method by using near-infrared differential interference contrast (IR DIC) microscopy according to standard protocols (Edwards et al. 1989; Stuart et al. 1993). Individual cells were visualized on a black-and-white video-enhanced TV system (video camera model NC-70, Dage-MTI, Inc.; B/W TV monitor Model PVM-137, Sony Co.). Patch-pipettes with resistances of 3-6 MΩ were pulled from borosilicate glass (1.5 mm outer diameter, 0.86 mm inner diameter with inner filament, AM System) using a Brown-Flaming horizontal puller (Model P-87, Sutter Instrument Co.). The tips were heat polished using a microforge (Model MF-930, Narishige International). Pipettes for whole-cell recording were filled with the following solution (in mM): 130 K methylsulphate, 10 KCl, 10 HEPES, 1 MgCl2, 2 K2-ATP (pH 7.3–7.4 adjusted with NaOH), osmolarity 290±10 mOsm. No correction was made for the bath-pipette solution junction potential (∼5–8 mV) during experiments. A tight seal (1–10 GΩ) was formed on the cell membrane before rupturing into whole-cell mode. The access resistance was fully compensated. After membrane rupture, the basic parameters were measured (resting membrane potential, cell input resistance, whole-cell capacitance). All recordings were made after 5–10 min of stabilization.
A current-clamp protocol was employed for measuring post-burst AHP and accommodation. All recorded CA1 pyramidal neurons were held at -68 mV to normalize our recordings. Current steps of 100 msec duration, sufficient to produce four reliable spikes, were used to measure the AHP. We averaged 10 consecutive traces for each cell (10-sec interval between measurements). The same intensity current step of 800 msec duration was used to measure accommodation.
All recordings from CA1 pyramidal cells were accomplished within ∼30 min after membrane rupture due to known rundown in Ca2+ and AHP currents. Cells with input resistance <40 MΩ and resting membrane potential >-55 mV were excluded from the experiment. KMeSO4 was purchased from ICN. All other drugs were obtained from Sigma.
Data acquisition and analysis
Current-clamp recordings were performed by using Axopatch-200B (Axon Instruments). The recorded voltage/current was low-pass filtered at 5 kHz and digitized at 10 kHz with a PCI-MIO-16E-4 board (National Instruments). All data were stored on a PC computer with custom-made software using C++ Builder 5.0 (Borland) and an NI DAQ 6.5 driver (National Instruments).
Data analyses were done by using Clampfit 9 (Axon Instruments) or custom-written software. All statistical values were evaluated with StatView 5 (Abacus Concepts, Inc.) or Origin 7 (MicroCal Software). Values are presented as mean ± SEM. Statistical differences were established at P ≤ 0.05 using one-way ANOVA and two-tailed independent t-tests.
Acknowledgments
We thank M.M. Oh and W. Tseng for technical assistance. Support was provided by National Institutes of Health AG008796 (J.F.D.) and the Max Planck Society (J.S.).
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.89005.
References
- Berger, T.W., Alger, B., and Thompson, R.F. 1976. Neuronal substrate of classical conditioning in the hippocampus. Science 192: 483-485. [DOI] [PubMed] [Google Scholar]
- Berger, T.W., Rinaldi, P., Weisz, D.J., and Thompson, R.F. 1983. Single unit analysis of different hippocampal cell types during classical conditioning of the rabbit. J. Neurophysiol. 50: 1197-1219. [DOI] [PubMed] [Google Scholar]
- Beylin, A.V. and Shors, T.J. 1998. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav. Neurosci. 112: 1327-1338. [DOI] [PubMed] [Google Scholar]
- ———. 2003. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm. Behav. 43: 124-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, T., Nijholt, I., Eckart, K., and Spiess, J. 2002. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J. Neurosci. 22: 3788-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, T., Nijholt, I., Grammatopoulos, D.K., Randeva, H.S., Hillhouse, E.W., and Spiess, J. 2003a. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: Role in neuronal excitability and associative learning. J. Neurosci. 23: 700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, T., Nijholt, I., Kye, M.J., Radulovic, J., and Spiess, J. 2003b. Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat. Neurosci. 6: 911-912. [DOI] [PubMed] [Google Scholar]
- Bond, C.T., Maylie, J., and Adelman, J.P. 1999. Small-conductance calcium-activated potassium channels. Ann. N.Y. Acad. Sci. 868: 370-378. [DOI] [PubMed] [Google Scholar]
- Bremner, J.D. 2003. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol. Bull. 37: 6-25. [PubMed] [Google Scholar]
- Brewin, C.R. 2001. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav. Res. Ther. 39: 373-393. [DOI] [PubMed] [Google Scholar]
- Chen, L., Bao, S., and Thompson, R.F. 1999. Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell–degeneration mutant mice. Behav. Neurosci. 113: 204-210. [DOI] [PubMed] [Google Scholar]
- Conrad, C.D., Jackson, J.L., Wieczorek, L., Baran, S.E., Harman, J.S., Wright, R.L., and Korol, D.L. 2004. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol. Biochem. Behav. 78: 569-579. [DOI] [PubMed] [Google Scholar]
- Cordero, M.I., Venero, C., Kruyt, N.D., and Sandi, C. 2003. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: Evidence for a role of corticosterone. Horm. Behav. 44: 338-345. [DOI] [PubMed] [Google Scholar]
- Coulter, D.A., Lo Turco, J.J., Kubota, M., Disterhoft J.F., Moore, J.W., and Alkon, D.L. 1989. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J. Neurophysiol. 61: 971-981. [DOI] [PubMed] [Google Scholar]
- Daum, I., Schugens, M.M., Ackermann, H., Lutzenberger, W., Dichgans, J., and Birbaumer, N. 1993. Classical conditioning after cerebellar lesions in humans. Behav. Neurosci. 107: 748-756. [DOI] [PubMed] [Google Scholar]
- de Jonge, M.C., Black, J., Deyo, R.A., and Disterhoft, J.F. 1990. Learning-induced afterhyperpolarization reductions in hippocampus are specific for cell type and potassium conductance. Exp. Brain Res. 80: 456-462. [DOI] [PubMed] [Google Scholar]
- de Kloet, E.R., Oitzl, M.S., and Joels, M. 1999. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 22: 422-426. [DOI] [PubMed] [Google Scholar]
- de Quervain, D.J.-F., Roozendaal, B., and McGaugh, J.L. 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394: 787-790. [DOI] [PubMed] [Google Scholar]
- Diamond, D.M. and Rose, G.M. 1994. Stress impairs LTP and hippocampal-dependent memory. Ann. N.Y. Acad. Sci. 746: 411-414. [DOI] [PubMed] [Google Scholar]
- Disterhoft, J.F., Coulter, D.A., and Alkon, D.L. 1986. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc. Natl. Acad. Sci. 83: 2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, F.A., Konnerth, A., Sakmann, B., and Takahashi, T. 1989. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 414: 600-612. [DOI] [PubMed] [Google Scholar]
- Foy, M.R., Stanton, M.E., Levine, S., and Thompson, R.F. 1987. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural. Biol. 48: 138-149. [DOI] [PubMed] [Google Scholar]
- Haug, T. and Storm, J.F. 2000. Protein kinase A mediates the modulation of the slow Ca2+-dependent K+ current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J. Neurophysiol. 83: 2071-2079. [DOI] [PubMed] [Google Scholar]
- Joels, M. and de Kloet E.R. 1991. Effect of corticosteroid hormones on electrical activity in rat hippocampus. J. Steroid Biochem. Mol. Biol. 40: 83-86. [DOI] [PubMed] [Google Scholar]
- Joels, M. and Vreugdenhil, E. 1998. Corticosteroids in the brain: Cellular and molecular actions. Mol. Neurobiol. 17: 87-108. [DOI] [PubMed] [Google Scholar]
- Karst, H. and Joels, M. 1991. The induction of corticosteroid actions on membrane properties of hippocampal CA1 neurons requires protein synthesis. Neurosci. Lett. 130: 27-31. [DOI] [PubMed] [Google Scholar]
- Kim, J.J. and Yoon, K.S. 1998. Stress: Metaplastic effects in the hippocampus. Trends Neurosci. 21: 505-509. [DOI] [PubMed] [Google Scholar]
- Knuttinen, M.-G., Gamelli, A.E., Weiss, C., Power, J.M., and Disterhoft, J.F. 2001. Age-related effects on eyeblink conditioning in the F344 × BN F1 hybrid rat. Neurobiol. Aging 22: 1-8. [DOI] [PubMed] [Google Scholar]
- Köhler, M., Hirschberg, B., Bond, C.T., Kinzie, J.M., Marrion, N.V., Maylie, J., and Adelman, J.P. 1996. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709-1714. [DOI] [PubMed] [Google Scholar]
- Kye, M., Spiess, J., and Blank, T. 2004. Expression of the murine SK2 gene is modulated by corticosteroids and nuclear factor κB, program no. 1000.1. Abstract Viewer/Itinerary Planner, Society for Neuroscience, Washington, DC.
- Leuner, B., Falduto, J., and Shors, T.J. 2003. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 23: 659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon, I., Britton, J.C., and Phan, K.L. 2003. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress 6: 151-156. [DOI] [PubMed] [Google Scholar]
- Mark, G.P., Rada, P.V., and Shors, T.J. 1996. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74: 767-774. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D. and Disterhoft, J.F. 1997. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J. Neurophysiol. 78: 1030-1044. [DOI] [PubMed] [Google Scholar]
- McEwen, B.S. 2001. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N.Y. Acad. Sci. 933: 265-277. [DOI] [PubMed] [Google Scholar]
- Moyer Jr., J.R. and Disterhoft, J.F. 1994. Nimodipine decreases calcium action potentials in rabbit hippocampal CA1 neurons in an age-dependent and concentration-dependent manner. Hippocampus 4: 11-17. [DOI] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Deyo, R.A., and Disterhoft, J.F. 1990. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104: 243-252. [DOI] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Thompson, L.T., and Disterhoft. J.F. 1996. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 16: 5536-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Power, J.M., Thompson, L.T., and Disterhoft, J.F. 2000. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J. Neurosci. 20: 5476-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt, D.J. and Malizia, A.L. 2004. Structural and functional brain changes in posttraumatic stress disorder. J. Clin. Psychiat. 65(Suppl 1): 11-17. [PubMed] [Google Scholar]
- Oh, M.M., Power, J.M., Thompson, L.T., and Disterhoft, J.F. 2000. Apamin increases excitability of CA1 hippocampal pyramidal neurons. Neurosci. Res. Comm. 27: 135-142. [DOI] [PubMed] [Google Scholar]
- Oh, M.M., Kuo, A.G., Wu, W.W., Sametsky, E.A., and Disterhoft, J.F. 2003. Watermaze learning enhances excitability of CA1 pyramidal neurons. J. Neurophysiol. 90: 2171-2179. [DOI] [PubMed] [Google Scholar]
- Power, J.M., Oh, M.M., and Disterhoft. J.F. 2001. Metrifonate decreases sI(AHP) in CA1 pyramidal neurons in vitro. J. Neurophysiol. 85: 319-322. [DOI] [PubMed] [Google Scholar]
- Sah, P. 1996. Ca2+-activated K+ current in neurons: Types, physiological roles and modulation. Trends Neurosci. 19: 150-154. [DOI] [PubMed] [Google Scholar]
- Scoville, W.B. and Milner, B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurolog. Neurosurg. Psychiat. 20: 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors, T.J. 1999. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport 10: 2811-2815. [DOI] [PubMed] [Google Scholar]
- ———. 2001. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol. Learn. Mem. 75: 10-29. [DOI] [PubMed] [Google Scholar]
- Shors, T.J. and Servatius, R.J. 1997. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 68: 92-96. [DOI] [PubMed] [Google Scholar]
- Shors, T.J., Weiss, C., and Thompson, R.F. 1992. Stress-induced facilitation of classical conditioning. Science 257: 537-539. [DOI] [PubMed] [Google Scholar]
- Shors, T.J., Beylin, A.V., Wood, G.E., and Gould, E. 2000. The modulation of Pavlovian memory. Behav. Brain Res. 110: 39-52. [DOI] [PubMed] [Google Scholar]
- Shors, T.J., Chua, C., and Falduto, J. 2001a. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 21: 6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors, T.J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. 2001b. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R., Vander Schaaf, E.R., Thompson, R.F., and Weisz, D.J. 1986. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 100: 729-744. [DOI] [PubMed] [Google Scholar]
- Stackman, R.W., Hammond, R.S., Linardatos, E., Gerlach, A., Maylie, J., Adelman, J.P., and Tzounopoulos, T. 2002. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 22: 10163-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, M. and Pedarzani, P. 2000. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell. Neurosci. 15: 476-493. [DOI] [PubMed] [Google Scholar]
- Stuart, G.J., Dodt, H.U., and Sakmann, B. 1993. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 423: 511-518. [DOI] [PubMed] [Google Scholar]
- Takehara, K., Kawahara, S., Takatsuki, K., and Kirino, Y. 2002. Time-limited role of the hippocampus in the memory for trace eyeblink conditioning in mice. Brain Res. 951: 183-190. [DOI] [PubMed] [Google Scholar]
- Tseng, W., Guan, R., Disterhoft, J.F., and Weiss, C. 2004. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus 14: 58-65. [DOI] [PubMed] [Google Scholar]
- Vergara, C., Latorre, R., Marrion, N.V., and Adelman, J.P. 1998. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8: 321-329. [DOI] [PubMed] [Google Scholar]
- Warren, D.A., Castro, C.A., Rudy, J.W., and Maier, S.F. 1991. No spatial learning impairment following exposure to inescapable shock. Psychobiology 19: 127-134. [Google Scholar]
- Weiss, C., Kronforst-Collins, M.A., and Disterhoft, J.F. 1996. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus 6: 192-209. [DOI] [PubMed] [Google Scholar]
- Weiss, C., Bouwmeester, H., Power, J.M., and Disterhoft, J.F. 1999a. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. 99: 123-132. [DOI] [PubMed] [Google Scholar]
- Weiss, C., Knuttinen, M.-G., Power, J.M., Patel, R.I., O'Connor, M.S., and Disterhoft, J.F. 1999b. Trace eyeblink conditioning in the freely moving rat: Optimizing the conditioning parameters. Behav. Neurosci. 113: 1-6. [DOI] [PubMed] [Google Scholar]
- Weiss, C., Preston, A.R., Oh, M.M., Schwarz, R.D., Welty, D., and Disterhoft, J.F. 2000. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J. Neurosci. 20: 783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C., Venkatasubramanian, P.N., Aguado, A.S., Power, J.M., Tom, B.C., Li, L., Chen, K.S., Disterhoft, J.F., and Wyrwicz, A.M. 2002. APP overexpression impairs delay eyeblink conditioning and decreases hippocampal volume in mice. Neurobiol. Dis. 11: 425-433. [DOI] [PubMed] [Google Scholar]
- Wilson, M.A. and McNaughton, B.L. 1993. Dynamics of the hippocampal ensemble code for space. Science 261: 1055-1058. [DOI] [PubMed] [Google Scholar]
- Xu, L., Anwyl, R., and Rowan, M.J. 1997. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 387: 497-500. [DOI] [PubMed] [Google Scholar]
- Zola, S.M. and Squire, L.R. 2001. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus 11: 92-98. [DOI] [PubMed] [Google Scholar]