Abstract
The role of the tripeptide glutathione in the growth and survival of Escherichia coli cells has been investigated. Glutathione-deficient mutants leak potassium and have a reduced cytoplasmic pH. These mutants are more sensitive to methylglyoxal than the parent strain, indicating that in the absence of glutathione-dependent detoxification, acidification of the cytoplasm cannot fully protect cells. However, increasing the intracellular pH of the glutathione-deficient strain resulted in enhanced sensitivity to methylglyoxal. This suggests that acidification of the cytoplasm can provide some protection to E. coli cells in the absence of glutathione. In the presence of the Kdp system, glutathione-deficient mutants are highly sensitive to methylglyoxal. This is due to the higher intracellular pH in these cells. In the absence of methylglyoxal, the presence of the Kdp system in a glutathione-deficient strain also leads to an extended lag upon dilution into fresh medium. These data highlight the importance of glutathione for the regulation of the K+ pool and survival of exposure to methylglyoxal.
The tripeptide glutathione is the major low-molecular-weight thiol in Escherichia coli cells, where it can accumulate up to concentrations exceeding 10 mM (8, 17). The ability of bacteria to accumulate high concentrations of this tripeptide implies that it must have an important physiological function(s). From the analysis of cells of a glutathione-deficient strain of E. coli, created by chemical mutagenesis, it was proposed that this tripeptide protects against an array of toxic compounds, including the naturally occurring electrophile methylglyoxal (2). Glutathione is required for the glyoxalase I and II enzymes that detoxify methylglyoxal to d-lactate via the formation of two metabolites, hemithiolacetal and S-lactoylglutathione (4). In E. coli cells, the primary route of methylglyoxal production is from the glycolytic intermediate, dihydroxyacetone phosphate, by the action of methylglyoxal synthase (13, 25). Elevated levels of methylglyoxal are produced in cells when there is an accumulation of dihydroxyacetone phosphate coupled with low-phosphate pools. Under certain environmental conditions, bacteria produce so much methylglyoxal that millimolar quantities are excreted into the medium (1).
In addition to detoxifying methylglyoxal, glutathione is a negative regulator of the KefB and KefC potassium channels of E. coli such that, in the absence of glutathione, K+ leaks out of these channels (5, 18, 19). The extent of this leak is determined by the concentration of K+ in the medium; elevating the potassium concentration to 10 mM can substantially reduce the leak. Full activation of the KefB and KefC systems requires the formation of glutathione metabolites (5, 11, 12). During the glutathione-dependent detoxification of methylglyoxal, the formation of S-lactoylglutathione activates the KefB and KefC systems (11, 15). This activation results in the rapid loss of potassium from the cell accompanied by a decrease in the intracellular pH (pHi) (10, 12). The decrease in the pHi protects E. coli cells against the toxic effects of methylglyoxal. Consistent with this, E. coli cells are sensitized toward methylglyoxal-induced cell death by conditions that either elevate pHi or reduce the decrease in pHi that occurs upon activation of KefB and KefC (9, 10).
It has been demonstrated previously that cells expressing the Kdp K+ uptake system have a higher pHi and consequently an increased sensitivity toward methylglyoxal (9). Kdp is a high-affinity (Km in the micromolar range), P-type ATPase, induced when intracellular turgor cannot be maintained by the activity of the lower-affinity, constitutive potassium uptake systems (6, 14, 16, 22, 24). Previous work has demonstrated that the growth rates of cells of two glutathione-deficient mutants of E. coli were significantly reduced in low-potassium medium (18). This implies that the Kdp system could not totally compensate for the K+ leak caused by the absence of glutathione in these mutant strains.
In this study, we set out to assess the relative importance of glutathione versus acidification of the cytoplasm in the protection of E. coli cells against methylglyoxal. We have found that glutathione plays an essential role in protecting cells against methylglyoxal. In the absence of this tripeptide, acidification of the cytoplasm can provide only limited protection against methylglyoxal and cells can no longer rapidly metabolize this toxic metabolite. We also found that glutathione plays a vital role in maintaining intracellular K+ pools, such that in the absence of this tripeptide, cells expressing Kdp exit stationary phase very slowly.
Analysis of growth and viability.
The E. coli strains used in this study were derivatives of E. coli K-12 as follows: Frag1 (F− thi rha lacZ), MJF355 [Frag1 gshA::Tn10 (kan)], Frag5 (Frag1 ΔkdpABC), Frag56 [Frag5 gshA::Tn10 (kan)]. For all experiments, Kx medium (where x is the millimolar concentration of potassium) supplemented with 0.2% (wt/vol) glucose as the carbon source was used (7). Kx minimal buffer lacked all growth supplements except glucose. For the growth experiments, cells were grown overnight in Kx medium as defined in the text, centrifuged (4,500 × g for 15 min), washed twice with K0.2 buffer, and diluted 10-fold into K0.2 medium, and the growth was monitored. Cells for viability experiments were grown to mid-exponential phase (optical density at 650 nm = 0.4), centrifuged (4500 × g for 15 min), and resuspended in fresh prewarmed Kx medium as defined in the text. Measurements was conducted exactly as described previously (10, 11). Samples were prepared and the methylglyoxal disappearance assay was performed as previously described (9, 10). For the pHi and potassium measurements, cells were grown to late exponential phase (optical density at 650 nm = 0.8), filtered (Whatman, 0.45-μm pore size), and resuspended in Kx minimal buffer. Measurements were conducted exactly as described previously (10, 11). Methylglyoxal and glutathione were added from 0.65 M and 100 mM aqueous stock solutions, respectively. All experiments were conducted at least twice. The data shown are a representative set, and the error bars represent the standard deviations from the means for one experiment.
Glutathione plays a vital role in the survival of E. coli cells against methylglyoxal.
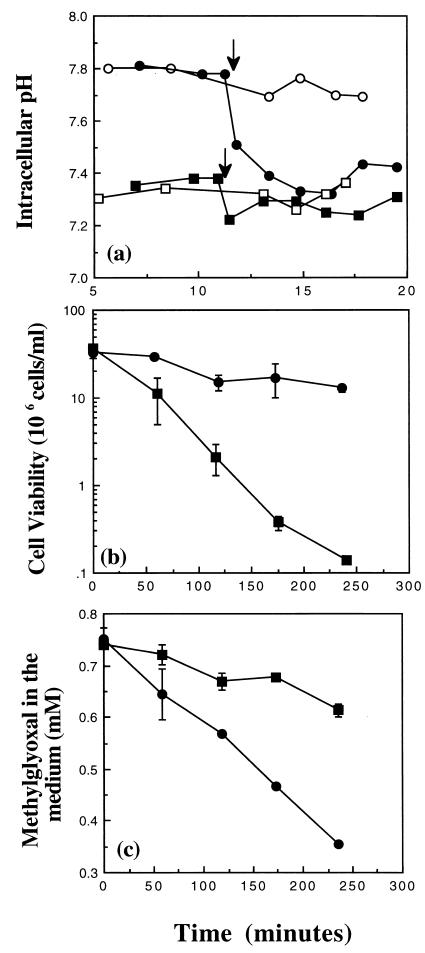
It has been demonstrated previously that upon resuspension in low-potassium medium, cells of the glutathione-deficient E. coli strain Frag56 [gshA::Tn10 (kan) ΔkdpABC] rapidly leak K+ through the KefB and KefC systems (5, 19). In E. coli cells, the transport of potassium and the regulation of the cytoplasmic pH are linked (3). Consistent with the observed leak of K+ in cells of Frag56, we found that the pHi in medium containing 0.2 mM K+ (K0.2) was substantially lower than in the parent strain, Frag5 ([Fig. 1a]; the pHi values were 7.35 and 7.8 ± 0.05 for Frag56 and Frag5, respectively). Upon the addition of 3 mM methylglyoxal, the pHi of Frag56 decreased slightly and then remained at a level similar to that of the untreated cells. In contrast, the pHi of cells of Frag5 decreased to 7.35 ± 0.05 within 4 min. Previously, it has been shown that a reduction of the pHi below 7.4 protects E. coli cells against methylglyoxal (9, 10). However, detoxification of methylglyoxal by the glutathione-dependent glyoxalase system has also been found to be a major determinant of sensitivity to methylglyoxal (15). To assess the relative importance of glutathione versus acidification of the cytoplasm in protection against methylglyoxal, exponential-phase cells of Frag5 and Frag56 were exposed to 0.7 mM methylglyoxal in K0.2 medium (Fig. 1b). Cells of Frag56 were much more sensitive to methylglyoxal than were cells of the parent strain, Frag5; only 0.4% of the former versus 38% of the latter survived 4 h of exposure. In the absence of glutathione, the detoxification of methylglyoxal was also greatly reduced compared with that of the parent strain (Fig. 1c). Methylglyoxal reacts spontaneously with glutathione to form hemithiolacetal (4, 15). Therefore, it was not possible to examine the effect of supplementing the growth medium of cells of Frag56 with glutathione on methylglyoxal sensitivity and detoxification. However, the inclusion of 1 mM glutathione during the growth of Frag56 cells to early exponential phase, prior to resuspension in glutathione-free medium, significantly enhanced the survival and detoxification capacity in the presence of methylglyoxal (data not shown). These data provide evidence that glutathione plays a vital role in the survival of E. coli cells in the presence of methylglyoxal. They also suggest that acidification of the cytoplasm cannot totally compensate for the loss of glutathione.
FIG. 1.
The importance of glutathione in protection against methylglyoxal. Cells were grown overnight in K10 medium. After outgrowth into exponential phase, cells were resuspended in either fresh prewarmed K0.2 buffer or medium as defined in the text. Cell viability, pHi, and methylglyoxal disappearance measurements were conducted exactly as described previously (9–11). (a) pHi measurements in strains Frag5 (kdpABC [•, ○]) and Frag56 [gshA::Tn10 (kan) kdpABC; ■, □] in buffer supplemented with (closed symbols) or without (open symbols) 3 mM methylglyoxal at the time indicated by the arrows. (b) Cell viability of strains Frag5 (•) and Frag56 (■) in medium supplemented with 0.7 mM methylglyoxal at time zero. (c) Methylglyoxal disappearance assay (symbols are the same as in panel b).
Acidification of the cytoplasm can protect in the absence of glutathione.
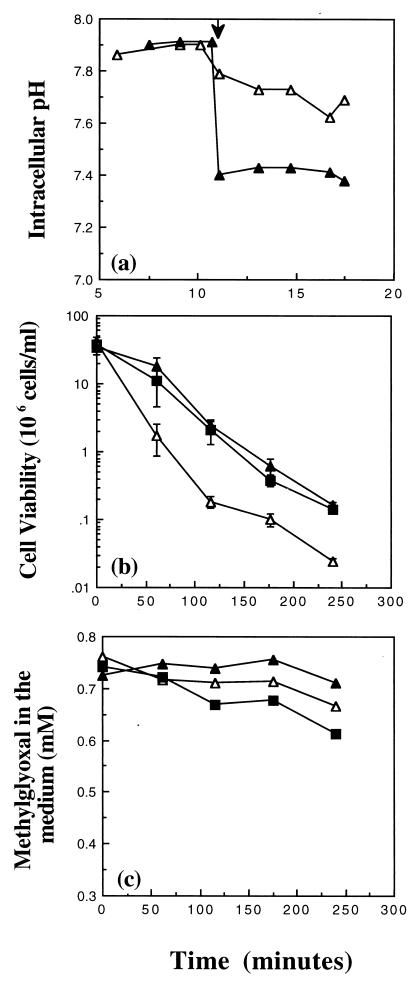
To determine whether acidification of the cytoplasm was providing any protection against methylglyoxal in the absence of glutathione, cells of Frag56 [gshA::Tn10 (kan) ΔkdpABC] were suspended in medium containing 10 mM K+ (K10). In K10 medium, the pHi of Frag56 cells after the addition of methylglyoxal was 7.7 ± 0.1 compared with 7.35 ± 0.05 for cells of the same strain in K0.2 (Fig. 2a and 1a, respectively). The higher pHi for Frag56 cells in K10 correlated with an increased sensitivity to 0.7 mM methylglyoxal (Fig. 2b). These data suggested that the lower pHi in cells of the glutathione-deficient strain was able to provide some protection to cells against methylglyoxal. To confirm this, the pHi of Frag56 cells in K10 was reduced by the addition of 25 mM sodium acetate and the effect on cell survival was assessed (Fig. 2a and b, respectively). Sodium acetate is a weak acid and can traverse the bacterial membrane in its undissociated form. Once inside the cell, the weak acid will dissociate and liberate protons, resulting in cytoplasmic acidification (3, 23). The addition of 25 mM sodium acetate reduced the pHi immediately from 7.85 to 7.35 ± 0.05, increasing resistance to 0.7 mM methylglyoxal (Fig. 2a and b, respectively). The level of protection was similar to that of Frag56 cells incubated in K0.2 medium, providing evidence that the decrease in the pHi was responsible (Fig. 2b). Acidification of the cytoplasm in cells lacking glutathione did not protect by enhancing the rate of detoxification of methylglyoxal (Fig. 2c). These data suggest that acidification of the cytoplasm can protect cells against methylglyoxal, even in the absence of glutathione, and that this defense mechanism is separate from detoxification.
FIG. 2.
Acidification of the cytoplasm can provide some protection against methylglyoxal in the absence of glutathione. Cells of Frag56 [gshA::Tn10 (kan) kdpABC] were grown overnight in K10 medium. After outgrowth into exponential phase, cells were resuspended in either fresh prewarmed medium or buffer as defined in the text. Cell viability, pHi, and methylglyoxal disappearance measurements were conducted exactly as described previously (9–11). (a) pHi measurements in K10 buffer in the presence of 3 mM methylglyoxal added at 10 min (▵) and 25 mM sodium acetate added at the time indicated by the arrow (▴). (b) Cell viability after the addition of 0.7 mM methylglyoxal at time zero. Symbols: K10 (▵), K0.2 (■), or K10 medium supplemented with 25 mM sodium (▴) added immediately prior to methylglyoxal. (c) Methylglyoxal disappearance (symbols are the same as in panel b).
Kdp enhances the sensitivity of a glutathione-deficient mutant toward methylglyoxal.
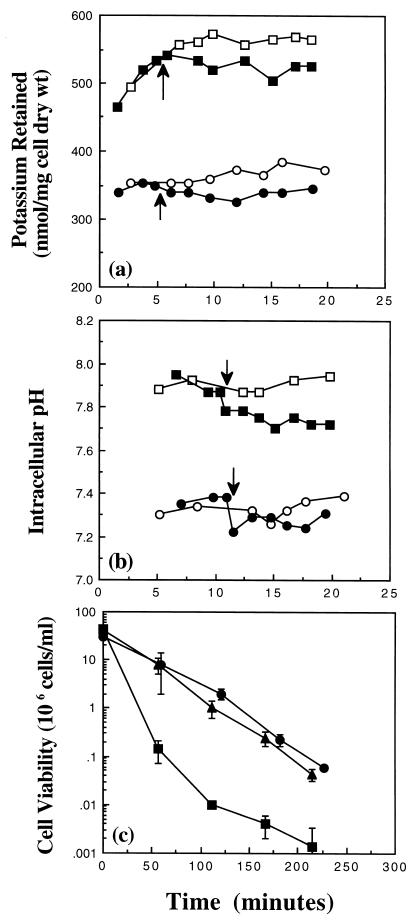
The work presented in this study so far was done with cells that lack the high-affinity, potassium uptake system, Kdp. However, wild-type E. coli cells possess the Kdp system, and when their intracellular K+ pool cannot be maintained, this system is induced (6, 14, 15, 22, 24). Cells of MJF355 [gshA::Tn10 (kan)] in K0.2 have a higher K+ pool and pHi compared with cells of Frag56 [gshA::Tn10 (kan) ΔkdpABC] under the same conditions (Fig. 3a and b, respectively). Increased sensitivity to methylglyoxal has been demonstrated previously in Kdp+ cells, due to a higher pHi (9). Consistent with this finding, cells of MJF355 had an increased sensitivity to 0.7 mM methylglyoxal in relation to cells of Frag56 in K0.2 (Fig. 3c). Acidification of the cytoplasm of MJF355 cells, by the addition of 25 mM sodium acetate, increased the protection against 0.7 mM methylglyoxal to a level similar to that of Frag56 (Fig. 3c). These data confirmed that lowering of the pHi can protect cells against methylglyoxal, even in the absence of glutathione.
FIG. 3.
The presence of Kdp greatly sensitizes a glutathione-deficient strain toward methylglyoxal. Cells were grown overnight in K10 medium, and outgrowth into exponential phase was performed in either K10 or K0.2 medium for strains Frag56 [kdpABC gshA::Tn10 (kan)] and MJF355 [gshA::Tn10 (kan)], respectively. Cells were then resuspended in either K0.2 medium or buffer as defined in the text, and potassium, pHi, and viability measurements were conducted exactly as described previously (10, 11). (a) Potassium measurements of strain Frag56 (•, ○) and MJF355 (■, □) in buffer supplemented either with (closed symbols) or without (open symbols) 3 mM methylglyoxal at the times indicated by the arrows. (b) pHi measurements in strain Frag56 (data are the same as in Fig. 1a) and MJF355 (symbols same as in panel a). (c) Cell viability in medium supplemented with 0.7 mM methylglyoxal at time zero. Symbols: Frag56 (•), MJF355 (■), and MJF355 supplemented with 25 mM sodium acetate (▴) added immediately prior to methylglyoxal.
Glutathione is required to prevent slow emergence from stationary phase in cells possessing Kdp.
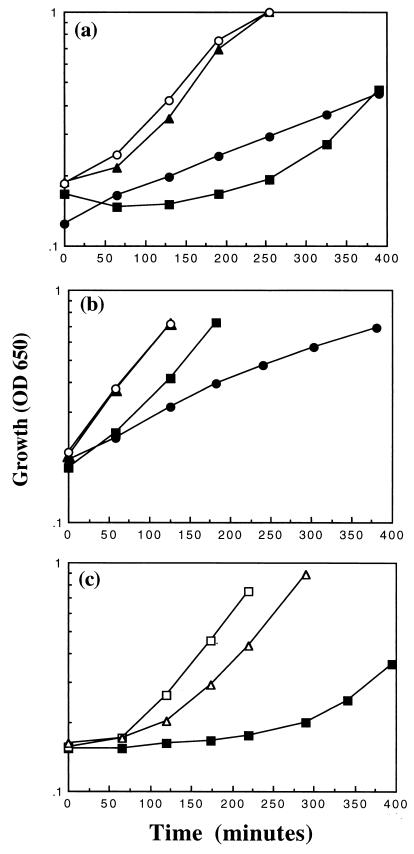
While preparing cultures for the pHi and viability experiments, we observed that prior to the establishment of the normal growth rate (Fig. 4a), an overnight culture of MJF355 [gshA::Tn10 (kan)] in K0.2 medium was able to grow in fresh K0.2 medium only after an extended lag. The inability of MJF355 cells to grow immediately in fresh K0.2 medium was not due to cell death in the overnight culture, since the viability was not significantly affected (data not shown). In contrast, cells of Frag56 [gshA::Tn10 (kan) ΔkdpABC], despite a reduced growth rate in this medium compared with that of cells of Frag5 (ΔkdpABC), were able to grow after dilution of an overnight culture into fresh K0.2 medium (Fig. 4a). Cells of the parent strain Frag1 did not exhibit this extended lag upon resuspension in K0.2 medium when grown overnight in K0.2 medium, indicating that the presence of Kdp per se was not responsible. These data suggested that in the absence of glutathione, the leak of K+ via KefB and KefC coupled with recapture by the Kdp system impairs the ability of cells to emerge from the stationary phase.
FIG. 4.
Glutathione is required to prevent the extended lag in cells possessing the Kdp system in low-potassium medium. Cells were grown overnight and resuspended in medium as defined in the text. Growth experiments were conducted exactly as described. (a) Cells of Frag1 (▴), MJF355 [gshA::Tn10 (kan); ■], Frag5 (kdpABC; ○), and Frag56 [gshA::Tn10 (kan) kdpABC; •] were grown overnight in K0.2 medium and resuspended in fresh K0.2 medium, and the growth was monitored. (b) Cells were grown overnight in K10 medium and resuspended in K0.2 medium, and the growth was monitored (symbols are the same as in panel a). (c) Cells of MJF355 were grown overnight in K0.2 medium in either the presence (□) or absence (■, ▵) of 1 mM glutathione. The overnight cultures were then resuspended in K0.2 medium in either the presence (▵) or absence (□, ■) of 1 mM glutathione, and the growth was monitored.
Further support for this proposal was obtained from cultures of cells in K10 medium. Strains MJF355 [gshA::Tn10 (kan)] and Frag56 [gshA::Tn10 (kan) ΔkdpABC] were grown overnight in K10 medium to reduce the K+ leak, via the KefB and KefC systems, and the growth was monitored upon resuspension in K0.2 medium (Fig. 4b). Cells of MJF355 were able to grow better than Frag56 cells, without the extended lag, under these conditions. To confirm the importance of glutathione, cells of MJF355 were grown overnight in K0.2 medium in the presence of 1 mM glutathione and then resuspended in fresh K0.2 medium, and the growth was monitored (Fig. 4c). The inclusion of glutathione in the overnight culture greatly reduced the lag phase of MJF355 cells upon resuspension in K0.2 medium. The lag phase of MJF355 cells was also substantially reduced when cells were grown overnight in K0.2 medium and then resuspended in fresh K0.2 medium supplemented with 1 mM glutathione (Fig. 4c). However, the inclusion of glutathione in the overnight culture led to a greater reduction in the lag phase of MJF355 cells. Increasing the glutathione concentration above 1 mM did not reduce the lag phase further (data not shown). These data suggest that the extended lag can be prevented by reducing the K+ leak in either the overnight culture or after resuspension into fresh medium. It should be noted that cells of the parent strain, Frag1, initially grew slightly more slowly than cells of Frag5 (ΔkdpABC) upon resuspension in K0.2 medium when grown overnight in K0.2 medium (Fig. 4a). However, there was no difference in the growth of Frag1 and Frag5 when cells were grown overnight in K10 medium (Fig. 4b). These results suggest that the activity of the Kdp system itself can pose a slight growth disadvantage to cells during extended growth in low-K+ medium. This disadvantage is increased in glutathione-deficient strains.
The data presented in this study demonstrate that glutathione plays an important role in the survival of E. coli cells. In the presence of the glutathione, cells rapidly metabolize methylglyoxal and are protected against the toxic effects of this electrophile. In contrast, in cells lacking glutathione, the metabolism of methylglyoxal is greatly reduced and cells rapidly lose viability. The slow metabolism of methylglyoxal in the absence of glutathione suggests that the glutathione-independent detoxification pathways play only a minor physiological role in E. coli cells (20, 21). Alternatively, it is possible that in the absence of glutathione, cellular enzymes are more susceptible to methylglyoxal attack, such that their functions are impaired. If such detoxification systems were susceptible to high methylglyoxal concentrations, this would further underline the importance of glutathione. In addition, the activities of glyoxalase I and II would be important, since they would allow the regeneration of glutathione from hemithiolacetal, the spontaneous reaction product of methylglyoxal with glutathione.
In this study, we set out to address the importance of glutathione versus acidification of the cytoplasm in protection of E. coli cells against methylglyoxal. We have shown that acidification of the cytoplasm, consequent upon the leak of K+ by the KefB and KefC systems, can provide some protection to cells in the absence of glutathione against methylglyoxal. However, complete protection against methylglyoxal could occur only in glutathione-replete cells. These data demonstrate that for maximal survival in the presence of methylglyoxal, E. coli cells require both glutathione and an acidification of the cytoplasm. In addition, our data suggest that it is important for E. coli cells to carefully regulate their glutathione levels to enable them to rapidly exit stationary phase.
Acknowledgments
We acknowledge the support of the Wellcome Trust for the award of a Toxicology Fellowship to G.P.F. and a Research Leave Fellowship to I.R.B.
Thanks also to Debra McLaggan for the critical reading of the manuscript and to Vanessa Santana for technical assistance.
REFERENCES
- 1.Ackerman R S, Cozzarelli N R, Epstein W. Accumulation of toxic concentrations of methylglyoxal by wild-type Escherichia coli K-12. J Bacteriol. 1974;119:357–362. doi: 10.1128/jb.119.2.357-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apontoweil A, Berends W. Isolation and characterisation of glutathione-deficient mutants of Escherichia coli. Biochim Biophys Acta. 1975;399:10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- 3.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper R A. Metabolism of methylglyoxal in micro-organisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- 5.Elmore M J, Lamb A J, Ritchie G Y, Douglas R M, Munro A, Gajewska A, Booth I R. Activation of potassium efflux from Escherichia coli by glutathione metabolites. Mol Microbiol. 1990;4:405–412. doi: 10.1111/j.1365-2958.1990.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 6.Epstein W. Osmoregulation by potassium transport in Escherichia coli. FEMS Microbiol Rev. 1986;39:73–78. [Google Scholar]
- 7.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahey R C, Brown W C, Adams W B, Worsham M B. Occurrence of glutathione in bacteria. J Bacteriol. 1978;133:1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson G P, Chacko A D, Lee C, Booth I R. The activity of the high-affinity K+ uptake system Kdp sensitizes cells of Escherichia coli to methylglyoxal. J Bacteriol. 1996;178:3957–3961. doi: 10.1128/jb.178.13.3957-3961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson G P, McLaggan D, Booth I R. Potassium channel by glutathione-S-conjugates in Escherichia coli: protection against methylglyoxal is mediated by cytoplasmic acidification. Mol Microbiol. 1995;17:1025–1033. doi: 10.1111/j.1365-2958.1995.mmi_17061025.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson G P, Munro A W, Douglas R M, McLaggan D, Booth I R. Activation of potassium channels during metabolite detoxification in Escherichia coli. Mol Microbiol. 1993;9:1297–1303. doi: 10.1111/j.1365-2958.1993.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson G P, Nikolaev Y, McLaggan D, Booth I R. Survival during exposure to the electrophilic reagent N-ethylmaleimide in Escherichia coli: role of KefB and KefC potassium channels. J Bacteriol. 1997;179:1007–1012. doi: 10.1128/jb.179.4.1007-1012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopper D J, Cooper R A. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J. 1972;128:321–329. doi: 10.1042/bj1280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–469. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLean M J, Ness L S, Ferguson G P, Booth I R. The role of glyoxalase I in the detoxification of methylglyoxal and the activation of the KefB K+ efflux system in Escherichia coli. Mol Microbiol. 1998;27:563–571. doi: 10.1046/j.1365-2958.1998.00701.x. [DOI] [PubMed] [Google Scholar]
- 16.Malli, R., and W. Epstein. Expression of the Kdp ATPase is consistent with regulation by turgor. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 17.McLaggan D, Logan T M, Lynn D G, Epstein W. Involvement of γ-glutamyl peptides in osmoadaptation of Escherichia coli. J Bacteriol. 1990;172:3631–3636. doi: 10.1128/jb.172.7.3631-3636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meury J, Kepes A. Glutathione and the gated potassium channels of E. coli. EMBO J. 1982;1:339–343. doi: 10.1002/j.1460-2075.1982.tb01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller S, Douglas R M, Carter P, Booth I R. Mutations in the glutathione-gated KefC K+ efflux system of Escherichia coli that cause constitutive activation. J Biol Chem. 1997;272:24942–24947. doi: 10.1074/jbc.272.40.24942. [DOI] [PubMed] [Google Scholar]
- 20.Misra K, Banjerjee A B, Ray S, Ray M. Glyoxalase III from Escherichia coli: a single novel enzyme for the conversion of methylglyoxal into D-1 acetate without reduced glutathione. Biochem J. 1995;305:999–1003. doi: 10.1042/bj3050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra K, Banjerjee A B, Ray S, Ray M. Reduction of methylglyoxal in Escherichia coli K12 by an aldehyde reductase and alcohol dehydrogenase. Mol Cell Biochem. 1996;156:117–124. doi: 10.1007/BF00426333. [DOI] [PubMed] [Google Scholar]
- 22.Rhoads D B, Epstein W. Cation transport in Escherichia coli. IX. Regulation of K+ transport. J Gen Physiol. 1978;72:283–295. doi: 10.1085/jgp.72.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmond C V, Kroll R G, Booth I R. The effect of food preservatives on pH homeostasis. J Gen Microbiol. 1984;130:2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- 24.Siebers A, Altendorf K. K+-translocating Kdp-ATPases and other bacterial P-type ATPases. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1992. pp. 225–252. [Google Scholar]
- 25.Tötemeyer S, Booth N A, Nichols W W, Dunbar B, Booth I R. From famine to feast: the role of methylglyoxal production in Escherichia coli. Mol Microbiol. 1998;27:553–562. doi: 10.1046/j.1365-2958.1998.00700.x. [DOI] [PubMed] [Google Scholar]