Significance
Lipid nanoparticles (LNP) as a promising delivery system posed several limitations, including intricate preparation process by four-component, the highly inflammatory and cytotoxic adverse events, suboptimal internalization into cytoplasm and gene transfection efficiency due to the egress of LNP through recycling pathways, and unintended targets to specific tissue beyond the liver. With this study, we showed that strong hydrogen bonds can be applied for delivery system to complex with mRNA, abandoning the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation. A noncationic lipid nanoparticle was constructed for mRNA delivery with simple, convenient, and repeatable preparation technology, negligible inflammatory and cytotoxicity side effects, high gene transfection efficiency, and spleen-targeting delivery to induce immunity for disease treatments.
Keywords: LNP, noncationic, immunotherapy, mRNA delivery, spleen targeting
Abstract
Although the tremendous progress has been made for mRNA delivery based on classical cationic carriers, the excess cationic charge density of lipids was necessary to compress mRNA through electrostatic interaction, and with it comes inevitably adverse events including the highly inflammatory and cytotoxic effects. How to develop the disruptive technologies to overcome cationic nature of lipids remains a major challenge for safe and efficient mRNA delivery. Here, we prepared noncationic thiourea lipids nanoparticles (NC-TNP) to compress mRNA by strong hydrogen bonds interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA, abandoning the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation. NC-TNP was a delivery system for mRNA with simple, convenient, and repeatable preparation technology and showed negligible inflammatory and cytotoxicity side effects. Furthermore, we found that NC-TNP could escape the recycling pathway to inhibit the egress of internalized nanoparticles from the intracellular compartment to the extracellular milieu which was a common fact in mRNA-LNP (lipid nanoparticles) formulation. Therefore, NC-TNP-encapsulated mRNA showed higher gene transfection efficiency in vitro and in vivo than mRNA-LNP formulation. Unexpectedly, NC-TNP showed spleen targeting delivery ability with higher accumulation ratio (spleen/liver), compared with traditional LNP. Spleen-targeting NC-TNP with mRNA exhibited high mRNA-encoded antigen expression in spleen and elicited robust immune responses. Overall, our work establishes a proof of concept for the construction of a noncationic system for mRNA delivery with good inflammatory safety profiles, high gene transfection efficiency, and spleen-targeting delivery to induce permanent and robust humoral and cell-mediated immunity for disease treatments.
The mRNA-based therapeutics have been developed as a promising strategy for numerous biological and therapeutic applications, mainly including protein replacement therapy, cancer or other diseases immunotherapy, and genome editing (1–6). For clinical applications, using naked mRNA in vivo is challenging due to the obstacles such as instability and easy degradation by extracellular RNases and the negative charge and large size (300–5,000 kDa) nature of mRNA inhibiting transportation across the cell membrane (7–9). Therefore, an appropriate delivery system was required to unlock the potential of mRNA drug for disease therapy. Current mRNA delivery system for encapsulation with protection against endonucleases and transportation to intracellular compartment focused on the usage of four-component lipid nanoparticles (LNP) containing cationic lipids or ionizable lipids, phospholipids, cholesterol, and PEG conjugated lipids (10–15). Although promising, LNP posed several limitations, including intricate preparation process by four-component, the highly inflammatory and cytotoxic adverse events, suboptimal internalization into the cytoplasm and low gene transfection efficiency due to the egress of LNP through recycling pathways, and unintended targets to specific tissue beyond the liver (12, 16–21). These limitations were mainly caused by the requirement for the excess cationic charge density of lipids to compress mRNA through electrostatic interaction. The mutually conflicting cationic charge requirements for mRNA delivery system induced the development of LNP fall into a dilemma. How to develop the disruptive technologies to abandon the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation remains a major challenge for safe and efficient mRNA delivery.
Here, we introduced thiourea groups as hydrogen bond donors to the lipids for the preparation of noncationic thiourea lipids nanoparticles (NC-TNP) with T-shaped hydroxy groups as assistant bond donors to complex with mRNA by strong hydrogen-bond interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA, abandoning the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation. In vitro gel retardation assay showed that our cation-free NC-TNP could efficiently encapsulate siRNA, pDNA, and mRNA to persistently protect from degradation by nucleases, highlighting the versatility of this approach. Importantly, compared with famous cationic LNP formed by cumbersome four components, NC-TNP as a simple delivery system possessed a superior mRNA transfection efficiency in vitro and in vivo due to the fact that NC-TNP could escape the recycling pathway to inhibit the egress of internalized nanoparticles from intracellular compartment to extracellular milieu which was a common phenomenon in mRNA-LNP formulation. Furthermore, unlike cationic or ionizable mRNA-LNP nanomedicines with positive charge–associated safety concerns, NC-TNP exhibited negligible in vivo inflammatory and cytotoxicity adverse effects. Unexpectedly, in vivo distribution and mRNA transfection results indicated that NC-TNP-encapsulated mRNA could selectively transport to spleen tissue with higher accumulation ratio (spleen/liver) and stronger mRNA-encoding proteins expression than traditional mRNA-LNP formulation. Intravenous mRNA delivery by the NC-TNP system elicited higher mRNA-encoding antigen-specific CD8+ T cells and comparable antibody responses compared with LNP platform. Overall, our work establishes a proof of concept for the construction of a noncationic system for mRNA delivery with good inflammatory safety profiles, high gene transfection efficiency, and spleen-targeting delivery to induce permanent and robust humoral and cell-mediated immunity for disease treatments.
Results
Preparation and Characterization of Noncationic mRNA Delivery System.
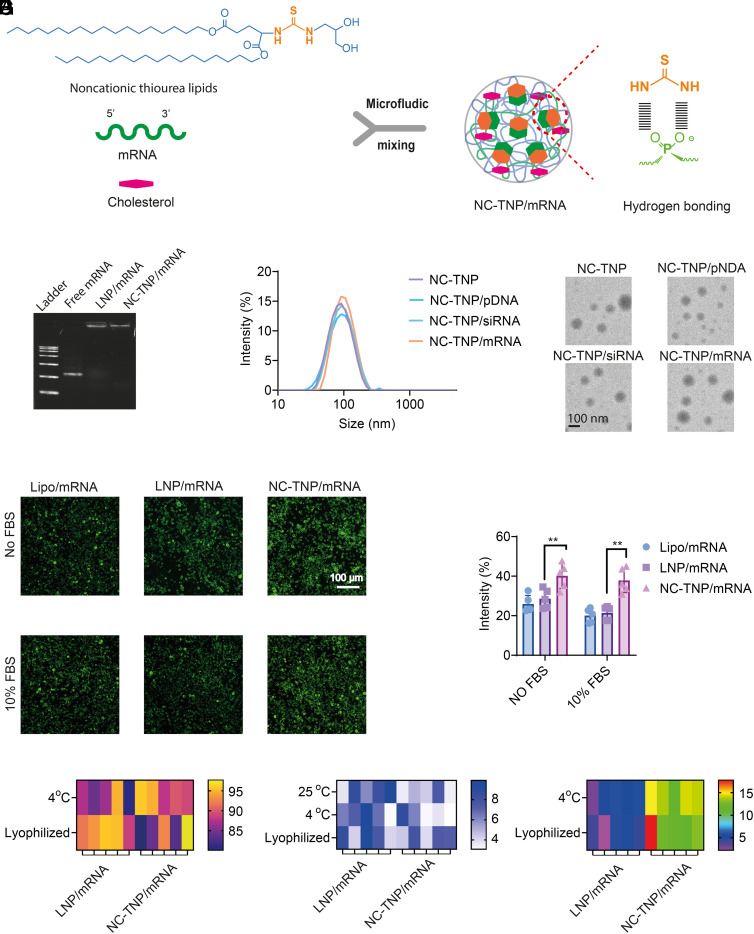
LNP formulations for mRNA delivery was limited to cationic nature which was necessary to compress mRNA through electrostatic interaction, and with it comes inevitably adverse events including the highly inflammatory and cytotoxic effects. We introduced thiourea groups as hydrogen bond donors to the lipids for the preparation of NC-TNP with T-shaped hydroxy groups as assistant bond donors to complex with mRNA by strong hydrogen-bond interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA, abandoning the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation. We synthesized a series of noncationic lipids with thiourea group as a linker to connect carbon chains containing various lengths (C6, C12, and C18) with T-shaped hydroxy head groups (SI Appendix, Fig. S1). In our noncationic lipid, thiourea groups as hydrogen bond donors complexed with phosphate groups of mRNA to induce the mRNA-NC-TNP formulation. Meantime, T-shaped hydroxyl groups contributed to the enhanced transfection efficiency (22). The structure of noncationic lipids was confirmed by 1H-NMR spectra and 13C-NMR spectra (SI Appendix, Figs. S2–S19). NC-TNP-encapsulated mRNA was prepared through microfluidics-mixing technology (Fig. 1A). We also attempted to evaluate whether our NC-TNP could compress pDNA or siRNA. Agarose-gel electrophoresis results indicated that the mobility of the mRNA, pDNA, and siRNA in the formulation of NC-TNP and LNP (containing 1-octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6-(undecyloxy) hexyl]amino]-octanoate (SM-102), 1,2-Distearoyl-sn-glycero-3-phosphorylcholine (DSPC), cholesterol, and 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG), 50:10:38.5:1.5 mol%) was significantly retarded compared with free genes (Fig. 1B and SI Appendix, Fig. S20). Furthermore, we performed the acid-based titration assay and 2-(p-toluidino)-6-naphthalenesulfonic acid (TNS) fluorescence assay to measure whether our NC-TNP binding with mRNA was charged-based. The titration profile of noncationic thiourea group was similar to that of control group, indicating the absence of pH sensitivity of our noncationic thiourea lipid (SI Appendix, Figs. S21 and S22). Meantime, the fluorescence signal of NC-TNP was similar to the control group (just free TNS solution with various pH values), indicating that NC-TNP was permanently uncharged at acid or base situation (SI Appendix, Figs. S23 and S24). We also prepared a neutral lipid imitating our noncationic thiourea lipid but without the thiourea group (SI Appendix, Figs. S25–S27). Agarose-gel electrophoresis results indicated that the mobility of the mRNA in the formulation of a neutral lipid nanoparticle was similar with the free mRNA without retardation, indicating that the neutral lipid nanoparticles prepared by a neutral lipid without thiourea group didn’t have mRNA condensation capability (SI Appendix, Fig. S28). Those results also confirmed the advantage of thiourea groups which was hydrogen bond donors in our noncationic system to complex with mRNA by strong hydrogen-bond interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA. The dynamic light scattering (DLS) and transmission electron microscopy (TEM) results showed the average diameter size of free NC-TNP or genes-loaded NC-TNP was about 100 nm (Fig. 1C and SI Appendix, Fig. S29). To access the mRNA transfection ability by NC-TNP, the DC2.4, B16, and 4T1 cells were seeded in 48-well plate and incubated with mRNA-loaded NC-TNP (mRNA encoding EGFP, 1 µg/mL) for 48 h in the cell medium with or without fetal bovine serum (FBS). The EGFP fluorescence images and quantitative results showed that the transfection efficiency in the group of NC-TNP was higher than that of LNP with or without FBS (Fig. 1D and SI Appendix, Figs. S30 and S31). As shown in SI Appendix, Fig. S32, the mean fluorescence intensity of NC-TNP-transfected DC2.4 cells with or without FBS was higher than that of LNP/mRNA, which was consistent with the fluorescence imaging results. We next evaluated the long-term stability and bioactivity of NC-TNP/mRNA as liquid state and lyophilized powder state at 4 °C for 30 d (23, 24). The long-term bioactivity of mRNA delivery system was measured by in vitro transfection experiments on DC2.4 cells after being incubated with NC-TNP/mRNA (mRNA encoding EGFP, 1 µg/mL) before and after 30 d storage at 4 °C in the state of liquid phase and lyophilized power (Fig. 1E). To better explore the long-term bioactivity of the mRNA delivery system in vivo, C57BL/6 mice were subcutaneously injected by freshly prepared LNP/mRNA and NC-TNP/mRNA (mRNA encoding luciferase, 0.1 mg/kg) and after 30 d storage at 4 °C in the state of liquid phase or lyophilized power. Forty-eight hours postinjection, mice were imaged by an in vivo imaging system (IVIS) (SI Appendix, Fig. S33). The bioluminescence imaging results showed that the mice treated with NC-TNP/mRNA after 30 d storage exhibited similar luciferase expression in the injection site, compared with the treatment of freshly prepared NC-TNP/mRNA. Those results showed that NC-TNP/mRNA can maintain the bioactivity of mRNA very well after long-term storage. Compared with the LNP/mRNA group, slightly changed size distribution and mRNA integrity were observed in the group of NC-TNP/mRNA, indicating the improved stability of NC-TNP/mRNA (Fig. 1 F and G and SI Appendix, Fig. S34). Furthermore, we also found that NC-TNP prepared by noncationic thiourea lipids containing carbon tail of C18 has higher stability than those of carbon tail of C6 or C12. Therefore, NC-TNP prepared by noncationic thiourea lipids with carbon tail of C18 was selected as a representative formulation in the further studies.
Fig. 1.
Design and characterization of noncationic mRNA delivery system. (A) Schematic illustration showing the process of the cargo mRNA formulated into the core of NC-TNP through microfluidic mixing of mRNA in distilled deionized water and noncationic thiourea lipids in an ethanol phase. The thiourea groups in NC-TNP with strong hydrogen bonding activity could interact with phosphate groups of mRNA to form NC-TNP-encapsulated mRNA platform with high stability. (B) Gel electrophoresis of free mRNA, LNP-encapsulated mRNA platform, and NC-TNP-encapsulated mRNA platform to show the mRNA condensation condition. NC-TNP was formulated by the noncationic thiourea lipids with carbon chains of C18, which were named as NC-TNP. (C) The size distributions (Left) and representative transmission electron microscope (TEM) images of free NC-TNP, NC-TNP-encapsulated siRNA, pDNA and mRNA, respectively. (D) LNP and NC-TNP complexed with mRNA encoding enhanced green fluorescent protein (EGFP) incubated with DC2.4 cells to evaluate the EGFP protein expression by confocal microscopy images (Left). Quantitative results of EGFP expression intensity measured by Image J software (Right). Data represent the means ± SD (n = 5 biologically independent samples). One-way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001). (E) For evaluation, the mRNA-encoding proteins expression in a storage environment, LNP-encapsulated mRNA (encoding EGFP), and NC-TNP-encapsulated mRNA were stored at 4 °C in the state of liquid phase and lyophilized power for 30 d. The relative mRNA-encoding EGFP proteins expression intensity was calculated by the ratio of EGFP expressions before and after 30 d. (F) The size of LNP-mRNA formulation and NC-TNP-mRNA formulation was measured before and after 30 d at 25 °C and 4 °C (in the state of liquid phase and lyophilized power), respectively. The percent of change in size before and after 30 d were calculated to evaluate the stability. (G) Changes in the mRNA integrity in the group of LNP-encapsulated mRNA and NC-TNP-encapsulated mRNA in the state of liquid phase and lyophilized power after incubation at 4 °C for 30 d.
Cellular Trafficking.
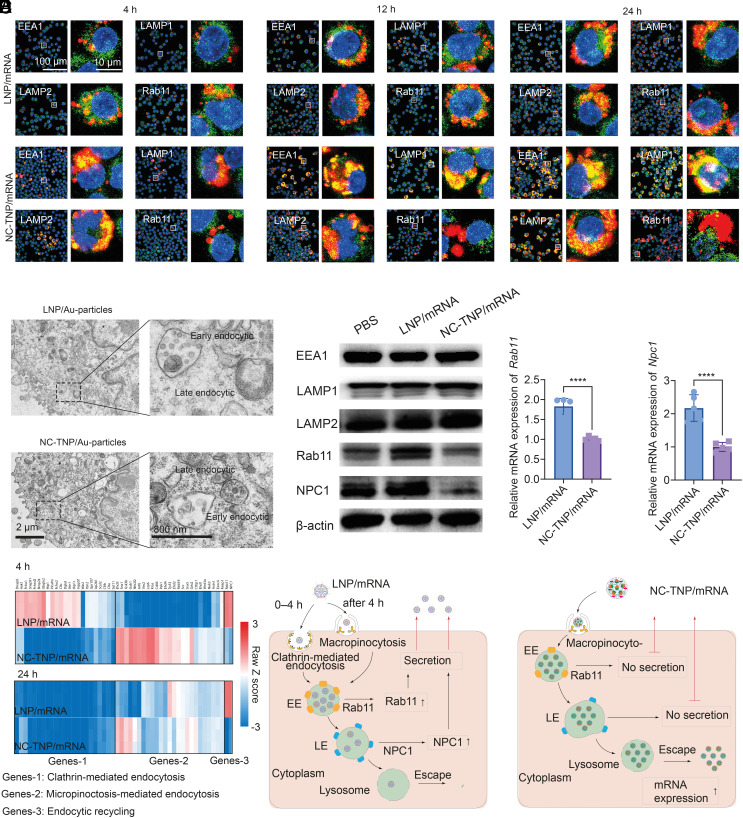
The higher transfection efficiency of NC-TNP/mRNA than that of LNP/mRNA motivated us to reveal the underlying mechanism. A systematic research had been done on the different mechanisms of NC-TNP and LNP internalization, intracellular trafficking, and endosomal escape. We chose three different cell lines DC2.4, B16, and 4T1 as the representative research objects in the following experiments. To address whether the internalization pathways of NC-TNP were different from LNP, we used siRNA to down-regulate the endocytic regulators, including micropinocytosis regulators (RAC1, CTBP1, and Rabankyrin-5), clathrin-mediated endocytosis regulators (CLTC, LDLR, and LRP1), and caveolin-mediated endocytosis regulators (CAV1 and CDC42) (SI Appendix, Figs. S35–S37) (19, 25–27). Before 4 h of incubation, the uptake efficiency of LNP was significantly decreased in the presence of clathrin-mediated endocytosis regulators, whereas the micropinocytosis regulators reduced the accumulation efficiency of NC-TNP. After 4 h of incubation, we observed no difference between NC-TNP and LNP in the behaviors of uptake after different inhibition treatments. Downregulation of micropinocytosis regulators (RAC1, CTBP1, and Rabankyrin-5) induced about 55% and 60% reduction of NC-TNP and LNP internalization into DC2.4, respectively, confirming that the micropinocytosis-mediated entrance pathway was required for the uptake of NC-TNP and LNP. The similar results were obtained after incubation with B16 and 4T1 tumors which showed the cellular uptake of NC-TNP and LNP was micropinocytosis-mediated pathway in the second stage (after 4 h incubation) (SI Appendix, Figs. S36 and S37). Then, the intracellular trafficking of Cy5 labeled NC-TNP and LNP was evaluated to find the mechanisms that induced the different transfection efficiency between NC-TNP and LNP. After incubation with DC2.4 for different times, the cells were stained with early endosome marker (EEA1), late endosome marker (LAMP1), lysosome marker (LAMP2), and endocytic recycling pathways marker (Rab11) to analyze the transport of NC-TNP and LNP in different endocytic compartments through colocalization studies. As shown in Fig. 2A, there is higher intracellular accumulation of NC-TNP in DC2.4 within different treatment times than that of LNP. Furthermore, we observed higher colocalization between Rab11 (endocytic recycling compartment marker) and Cy5 labeled LNP than that of NC-TNP. These results indicated that the decreased intracellular accumulation of LNP than NC-TNP may be a result of increased exocytosis through egress of LNP by endocytic recycling mediated pathway. The similar phenomenon was observed in B16 and 4T1 cells (SI Appendix, Fig. S38). To further evaluate the nature of cellular trafficking of NC-TNP and LNP, the intracellular distribution of nanoparticles at ultrastructural level in DC2.4 after incubation with NC-TNP/gold-particles and LNP/gold-particles were measured by TEM. Ultrastructural analyses showed that the majority of LNP/gold-particles were trapped in early endosome compartment and extremely difficult to find in late endosome or cytoplasm, whereas NC-TNP/gold-particles were clearly detected within late endosome and cytoplasm (Fig. 2B). Some literature studies have reported that the formation of late endosome during internalization process was an essential way for delivery of exogenously mRNA (28). Furthermore, ultrastructural analyses also showed that the amount of LNP/gold-particles accumulated in early endosome compartment decreased with increasing the incubation time, which was consistent with the results fluorescence colocalization analysis (SI Appendix, Fig. S39). Those results confirmed that LNP with positive charge rather than noncationic NC-TNP could be egressed by endocytic recycling mediated pathway. DC2.4, B16, and 4T1 cells treated with LNP showed the higher level of Rab11 and NPC1 protein expression, compared with NC-TNP (Fig. 2C and SI Appendix, Fig. S40). Then, quantitative reverse transcription PCR (qRT-PCR) was used to exam the relative mRNA levels of Rab11, and NPC1 in DC2.4, B16, and 4T1 cells after incubation with LNP and NC-TNP. The levels of Rab11, Npc1 in the group of LNP was higher than that of NC-TNP (Fig. 2D and SI Appendix, Figs. S41 and S42). The effect of LNP and NC-TNP on endocytosis pathway–related gene regulation was calculated in a heatmap by cluster analysis (Fig. 2E). The gene expressions of clathrin-mediated pathway in the cells treated with LNP for 4 h were higher than that of NC-TNP. After incubation with 24 h, the gene expressions of the micropinocytosis-mediated pathway in the cells treated with LNP was similar to NC-TNP. Those results further indicated the internalization mechanism of LNP was a clathrin-mediated pathway before 4 h and micropinocytosis-mediated pathway after 4 h and the entranceway of NC-TNP was a perpetual micropinocytosis-mediated pathway. Furthermore, the gene expression of recycling pathways in the cells treated with LNP was significantly higher than that of NC-TNP. Overall, the cellular internalization and trafficking behaviors of LNP and NC-TNP were summarized in Fig. 2F. Endosomal escape was an essential and rate-limiting step to obtain the satisfactory nucleic acid therapeutic effects. To mimic the intracellular endosome/lysosome, we prepared the anionic liposomes labeled with fluorescence resonance energy transfer (FRET) pairs. We assessed the interactions between LNP/mRNA or NC-TNP/mRNA with endosome/lysosome-mimicking liposomes through FRET-dependent membrane mixing assay at pH 5.0 (simulate endosomal/lysosomal condition). Once the destabilization or rupture of endosome/lysosome-mimicking liposomes occurred, the increased distance between the two FRET probes induced the donor emission signal increase and acceptor fluorescence signal decrease (SI Appendix, Fig. S43A). Compared with incubation with NC-TNP/mRNA, the endosome/lysosome mimicking liposomes mixing with LNP/mRNA showed a higher donor fluorescence signal (SI Appendix, Fig. S43B). The results confirmed that LNP/mRNA has a stronger ability to induce the destruction or destabilization of endosomal/lysosomal membranes. Therefore, LNP/mRNA with better membrane destabilization should have higher ability of endosomal escape than NC-TNP/mRNA. However, the above speculation was not consistent with the gene transfection efficiency result (Fig. 1E), which inspired us to explore the underlying mechanism. We speculated whether the effect of endosome/lysosome membrane on the NC-TNP/mRNA was different with the LNP/mRNA. We prepared LNP/mRNA and NC-TNP/mRNA labeled with FRET pairs to mix with endosome/lysosome mimicking liposomes (SI Appendix, Fig. S43C). For LNP/mRNA and NC-TNP/mRNA alone, the strong fluorescence signal at 590 nm wavelength showed the FRET efficiency was very high. After incubation with endosome/lysosome mimicking liposomes, the emission intensity of LNP/mRNA at 590 nm was significantly decreased and the emission intensity at 530 nm was increased compared with LNP/mRNA alone due to the LNP dissociation inducing the separation distance between the FRET pairs. However, the FRET efficiency in the group of NC-TNP/mRNA after incubation with endosome/lysosome mimicking liposomes was slightly decreased than the station of NC-TNP/mRNA alone (SI Appendix, Fig. S43 D and E). Those results indicated that LNP/mRNA could maintain the intact nanostructure without destroy or dissociation during the process of the endosome/lysosome escape into cytosol. After incubation with endosome/lysosome mimicking liposomes, the percentage of released mRNA form LNP was about fivefold higher than that from NC-TNP (SI Appendix, Fig. S43F). Inevitably, a part of mRNA released from cationic or ionizable LNP was detained in lysosome, which suffered from the degradation by lysosome. After internalization, NC-TNP/mRNA enhanced the endosome membrane permeabilization and destabilization due to the hydrogen-bond interaction between thiourea groups of NC-TNP and the phosphate groups of lipids in the endosome/lysosome membrane. Then, NC-TNP/mRNA with intact nanostructure and protection effect to prevent mRNA degradation was escaped from the ruptured endosome/lysosome into cytoplasm. mRNA was released from NC-TNP due to the spontaneous disassembly of nanoparticles in the cytoplasmic dilution environment. Compared with LNP/mRNA, most of the NC-TNP/mRNA could maintain the whole nanostructure without destabilization or disassembly in the endosome/lysosome compartment to escape from lysosome to cytoplasm (SI Appendix, Fig. S43G).
Fig. 2.
The mechanism of TC-TNP-encapsulated mRNA internalization and intracellular transport. (A) Representative confocal microscopy images of DC2.4 cells incubated with Cy5 labeled mRNA-LNP and mRNA-NC-TNP formulations for 4 h, 12 h, and 24 h, respectively. After incubation, the treated DC2.4 cells were washed by PBS and stained by anti-EEA1 antibody for early endosomes markers, anti-LAMP1 antibody for late endosomes markers, anti-LMAP2 antibody for lysosomes markers, and anti-Rab11 antibody for endocytic recycling compartment. The cell nuclei were stained with Hoechst33342. (B) Ultrastructure of DC2.4 cells after 4 h exposure to LNP/gold-nanoparticles and NC-TNP/gold-nanoparticles. Large amounts of LNP/gold-nanoparticles were found inside the early endocytic compartment. However, the amount of LNP/gold-nanoparticles accumulated in later endocytic compartment was significantly lower than that of NC-TNP/gold-nanoparticles. (C) DC2.4 cells treated with mRNA-LNP and mRNA-NC-TNP (mRNA encoding EGFP, 1 µg/mL) for 24 h. Western blot analysis the proteins of EEA1, LAMP1, LAMP2, Rab11, and NPC1 levels after treatments. (D) Quantitative of mRNA levels of Rab11 and NPC1 genes determined by qRT-PCR in DC2.4 cells after incubation with LNP/mRNA and NC-TNP/mRNA. Data represent the means ± SD (n = 5 biologically independent samples). One-way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001). (E) Heatmap of cellular uptake related gene expression changes in DC2.4 cells after incubation with LNP/mRNA and NC-TNP/mRNA. (F) Schematic showing the cellular internalization and trafficking behaviors of LNP/mRNA and NC-TNP/mRNA. For LNP/mRNA, DC2.4 uptake of LNP/mRNA was based on the caveolin mediated entry mechanism before 4 h incubation. Then, cellular internalization of LNP/mRNA was about micropinocytosis mediated pathway after 4 h incubation. Upon internalization into DC2.4, most of LNP/mRNA was trapped in the early endocytic compartment. A small fraction of LNP/mRNA moved to late endosome and following escape from late endosome/lysosome to cytoplasm. Large amounts of LNP/mRNA were egressed from intracellular environment to extracellular milieu through endocytic recycling mediated pathway. For NC-TNP/mRNA, the internalization pathway is about micropinocytosis before and after 4 h. The internalized NC-TNP/mRNA could be easily transported from early endosomes to late endosomes. Then, NC-TNP/mRNA could escape from the endosome/lysosome compartment to cytoplasm for mRNA expressions without recycling pathways.
Inflammation-Related Side Effects and Cytotoxic Effects.
In the above studies, the control group of LNP was ionizable LNP containing typical four components, ionizable cationic lipid, phosphatidylcholine, cholesterol, and polyethylene glycol-lipid. Some literature studies and clinical trials reported both ionizable LNP and cationic LNP (containing dioleoyl-3-trimethylammonium propane (DOTAP), DSPC, cholesterol, and DMG-PEG, 50:10:38.5:1.5 mol%) with the potential liability to induce high inflammatory and unwanted side effects (17, 18, 21, 29, 30). We detailedly examined whether our noncationic NC-TNP has the advantage of biological safety without inflammation-related side effects and cytotoxic effects than cationic LNP (c-LNP) and ionizable LNP (i-LNP). We subcutaneously injected empty c-LNP, i-LNP, and NC-TNP (20 µg per mouse) into C57BL/6 mice (Fig. 3A). C57BL/6 mice were sacrificed and the skin samples (about 1 cm2) at the injection site were harvested at different time points after injection. As shown in SI Appendix, Fig. S44, the empty c-LNP and i-LNP injection groups induced the redness and swelling of skin samples at the injection site, whereas the skin samples in the group of NC-TNP exhibited little inflammatory signs. The representative hematoxylin and eosin (H&E) images of skin samples from mice after 24 h injection showed the obvious inflammation signs in the groups of c-LNP and i-LNP, whereas the skin samples from mice treated with NC-TNP and PBS were normal without inflammation (SI Appendix, Fig. S45). Flow cytometry results revealed the amount of CD45+ leucocytic infiltrate and the percentages of neutrophils in CD45+ cells in the group of c-LNP and i-LNP were significantly higher than that of NC-TNP (Fig. 3B). Furthermore, Luminex analysis of tissues from mice treated with c-LNP and i-LNP showed notable higher levels of inflammatory chemokines and cytokines than that of NC-TNP (SI Appendix, Figs. S46 and S47). The RNA-seq analysis showed that the inflammatory genes expressions in the skin samples from mice treated with c-LNP and i-LNP were higher than that of NC-TNP (SI Appendix, Fig. S48). Meantime, we also employed intramuscular injection of c-LNP, i-LNP and NC-TNP into mice to evaluate the inflammation-related side effects and cytotoxic effects (Fig. 3C). Similar to the subcutaneous injection, intramuscular c-LNP and i-LNP led to high level of inflammatory cytokines (GM-CSF, IL-1β, and IL-6) (Fig. 3D). By comparison, the treatment of NC-TNP only induced negligible changes of inflammatory cytokines. The RNA-seq and qRT-PCR analysis also revealed that c-LNP and i-LNP are higher inflammatory than our noncationic NC-TNP (Fig. 3E and SI Appendix, Fig. S49). To further study the impact of c-LNP, i-LNP, and NC-TNP on the activation of systemic inflammatory responses, we intravenously injected c-LNP, i-LNP, and NC-TNP into mice (Fig. 3F). At 3 d postinjection, blood was harvested and analyzed using enzyme-linked immunosorbent assay (ELISA) to measure the level of inflammatory chemokines and cytokines. c-LNP and i-LNP induced robust upregulation of systemic proinflammatory cytokines, while the NC-TNP showed a slight increase in induction of serum cytokines (Fig. 3G). Additionally, the mice were killed and the heart was isolated for H&E staining to examine the histopathological changes. As shown in Fig. 3H and SI Appendix, Fig. S50 c-LNP and i-LNP triggered visible cardiomyocyte apoptosis, while NC-LNP treatment revealed no obvious histopathological damage. We also evaluated the relative gene expressions of proinflammatory cytokines in liver and spleen from mice after different treatments by qRT-PCR. c-LNP and i-LNP induced about 5-fold upregulation of the pro-inflammatory cytokines-related genes expressions, compared with PBS group. However, the relative genes expressions of proinflammatory cytokines were similar with the PBS group (Fig. 3I and SI Appendix, Fig. S51). Furthermore, we also prepared the ionizable lipids (S-ionizable lipid-1 and S-ionizable lipid-2) and cationic lipids (S-cationic lipid) with the similar structure with our noncationic thiourea lipids to examine whether our noncationic NC-TNP has the advantage of biological safety without inflammation-related side effects and cytotoxic effects than cationic LNP and ionizable LNP (SI Appendix, Figs. S52–S60). The results proved that the ionizable LNP prepared by S-ionizable lipid-1 or S-ionizable lipid-2 and cationic LNP containing S-cationic lipid have the positive charge–associated adverse effects (SI Appendix, Fig. S61). Thus, our findings indicated that noncationic NC-TNP induced negligible inflammatory and cytotoxicity side effects either in intradermal, intramuscular, or intravenous administration manner, superior to c-LNP or i-LNP that elicited robust inflammation-related side effects and cytotoxic effects.
Fig. 3.
The highly inflammatory and cytotoxic effects. (A) Schematic illustration of the experiment design. C57BL/6 mice were subcutaneously injected c-LNP, i-LNP, and NC-TNP (20 µg per mouse). After different times, the skin samples from the injection site were collected for analysis. (B) The skin samples were harvested and lysed to single-cell suspension for flow analysis. Quantitative percentages of CD45+ cells (Left) and the proportion of neutrophils (CD45+CD11b+Ly6G+) in CD45+ cells (Medium). C57BL/6 mice were subcutaneously injected LNP and NC-TNP (20 µg per mouse). After 1 d, 3 d, 5 d, and 7 d, the skin samples from the injection site were collected for analysis. The curve showed the changes of quantitative percentages of CD45+ cells at different times (Right). (C) C57BL/6 mice were intramuscularly injected LNP and NC-TNP (20 µg per mouse). After 3 d, the muscle samples from the injection site were collected for analysis. (D) After 3 d, the muscle samples from the injection site were harvested and the inflammatory chemokines and cytokines were measured by Luminex analysis. (E) Heatmap of inflammatory related genes expression changes of muscle samples from the mice after different treatments. (F) Schematic illustration of the experimental schedule. C57BL/6 mice were intravenously injected LNP and NC-TNP (20 µg per mouse). After 3 d, blood, spleen, liver, and heart were collected for further analysis. (G) The level of inflammatory cytokines in serum from mice after different treatments. (H) H&E staining images of heart from the mice after different treatments to evaluate the inflammation responses. (I) Liver and spleen tissues were harvested from the mice after different treatments and lysed to measure the level of inflammatory cytokines by qRT-PCR. Data represent the means ± SD (n = 5 biologically independent samples). One-way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).
In Vivo Transfection and Spleen-Specific Targeting.
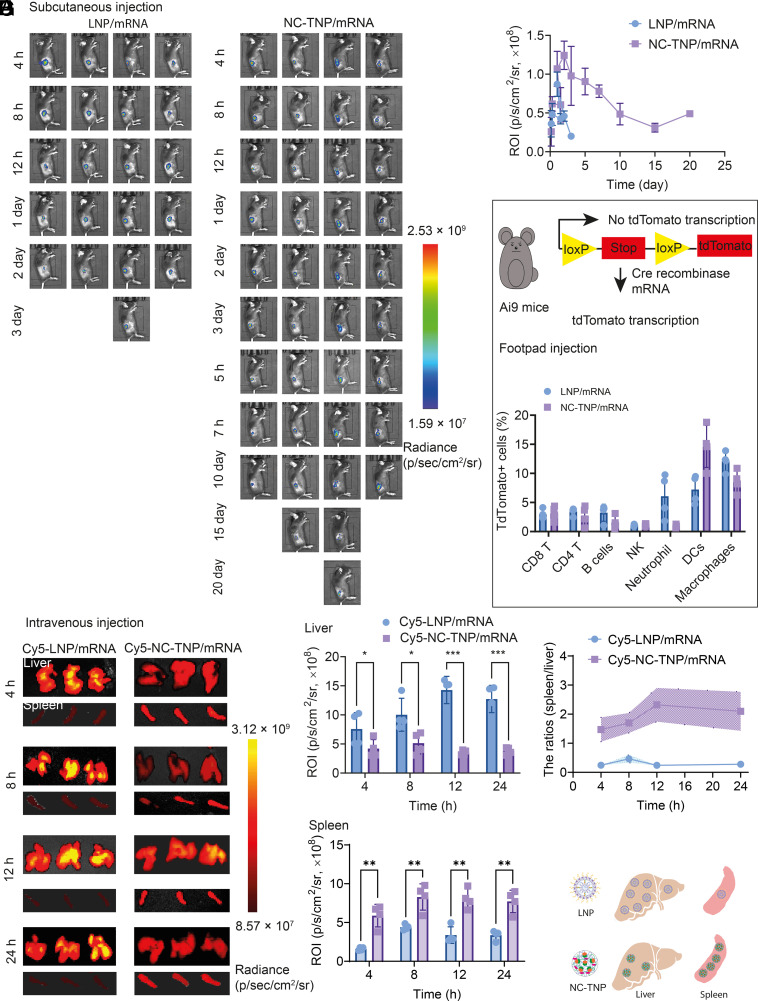
Inspired by the superiority of in vitro mRNA transfection efficiency and negligible systemic inflammatory responses of our noncationic mRNA delivery system, we evaluated in vivo transfection efficiency and systematic delivery behavior. In the following studies, the control group of LNP was ionizable LNP containing typical four components, ionizable cationic lipid, phosphatidylcholine, cholesterol, and polyethylene glycol-lipid. To assess in vivo duration of protein expression, LNP and NC-TNP/mRNA (mRNA encoding luciferase, 0.5 mg/kg) were subcutaneously injected into C57BL/6 mice via an insulin needle syringe. Representative luminescence images showed that the luciferase protein expression in the injection site form the mice treated with LNP/mRNA persisted through at most 3 d (Fig. 4A). However, NC-TNP/mRNA was shown to translate luciferase proteins with significant fluorescence signals on day 20 postinjection. Translational kinetics of LNP/mRNA and NC-TNP showed that the translation half-life of NC-TNP was about 10-fold higher than that of LNP (Fig. 4B). These results demonstrated that NC-TNP could protect mRNA from degradation and enhance the gene transfection duration in vivo, which was consistent with the in vitro cellular trafficking results. The lymph nodes as the sites for eliciting immune responses, we also exam the intranodal delivery efficiency and distribution of LNP/mRNA and NC-TNP/mRNA (mRNA encoding Cre, 0.1 mg/kg) using the Ai9 mouse (Fig. 4C). After 48 h injection, the lymph nodes were isolated to measure the transfected immune cells with tdTomato expression. Over 14% DCs were transfected following injection of NC-TNP/mRNA, which was about twofold higher than that of LNP/mRNA. Some literature studies reported that mRNA delivery with positive charge tended to predominantly accumulate in the lung and liver of mice not in the spleen. However, the distribution of mRNA delivery system with negative charge shifted progressively from lung and liver toward the spleen (20, 31). We speculated that our noncationic mRNA delivery system, NC-TNP, has the advantage to target to the spleen, compared with traditional LNP. C57BL/6 mice were injected intravenously with Cy5 labeled LNP/mRNA and NC-TNP/mRNA and the fluorescence signals of ex vivo organs were measured by IVIS at different times postinjection. The Cy5-labeled NC-TNP/mRNA was shown to induce significant fluorescence signals in spleen, which was higher than the LNP-treated group (Fig. 4 D and E). However, the accumulation of NC-TNP/mRNA in liver was lower than that of LNP. Furthermore, the spleen/liver fluorescence ratios of Cy5-labeled NC-TNP/mRNA were about 20-fold compared to the group of Cy5-labeled LNP/mRNA (Fig. 4F). All those results confirmed that our noncationic delivery system targeted accumulation in the spleen rather than the liver (Fig. 4G). Furthermore, we also evaluated whether the accumulated NC-TNP in the spleen tissue causes the inflammation-related side effects and cytotoxic effects. The spleen histological results revealed no obvious histopathological damage in the group of NC-LNP (SI Appendix, Fig. S62).
Fig. 4.
In vivo mRNA transfection and the targeted delivery NC-TNP/mRNA to spleen. (A) To evaluate the duration pattern of in vivo mRNA-encoding proteins expression based on different delivery system, mRNA loaded LNP and NC-TNP were subcutaneously injected to C57BL/6 mice (mRNA encoding luciferase, 0.5 mg/kg). The bioluminescence imaging at different times postinjection was obtained by IVIS spectrum imaging system. (B) Bioluminescent intensity quantification of mRNA-encoding proteins expression measured in C57BL/6 mice after different treatments. (C) Schematic representation of experimental workflow on Ai9 mice. LNP and NC-LNP-encapsulated mRNA (mRNA encoding Cre, 0.1 mg/kg) were subcutaneously injected into the footpad of Ai9 mice. Forty-eight hours postinjection, the lymph nodes were harvested and lysed to single-cell suspensions to evaluate the percentages of tdTomato+ cells in CD8+ T cells (tdTomato+CD3+CD8+), CD4+ T cells (tdTomato+CD3+CD4+), B cells (tdTomato+CD45+CD3-CD19+), NK (tdTomato+CD45+NK1.1+), Neutrophil (tdTomato+CD45+CD11b+Ly6G+), DCs (tdTomato+CD45+CD11c+), and Macrophages (tdTomato+CD45+CD11b+F4/80+) by flow analysis. (D) mRNA loaded Cy5 labeled LNP and NC-TNP (mRNA encoding luciferase, 0.5 mg/kg) were intravenously injected to C57BL/6 mice. After different times of injection, the liver and spleen tissues were harvested for fluorescence imaging. Ex vivo liver and spleen imaging showed that NC-TNP has the ability to increase the specific accumulation in spleen compared with the treatment of LNP. (E) Fluorescence intensity quantification in liver and spleen from the mice after different treatments. Data represent the means ± SD (n = 5 biologically independent samples). One-way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001). (F) The fluorescence ratios intensity quantification of spleen to liver in (E). (G) Schematic illustration of the mechanism of spleen targeting delivery.
In Vivo Immune Responses.
It is worth noting that the above findings confirmed the advantage of our noncationic delivery system as an alternative to traditional cationic or ionizable LNP. Those benefits include increased intracellular accumulation of NC-TNP without endocytic recycling mediated egress, avoided positive charge associated safety concerns, improved gene transfection efficiency and duration, and realized spleen-specific targeting. Inspired by those superiorities, we try to determine whether NC-TNP/mRNA could be used as vaccines to induce robust immune responses for disease treatment. C57BL/6 mice were subcutaneously immunized by LNP/mRNA and NC-TNP/mRNA (mRNA encoding OVA, 1 mg/kg) on day 0 (Fig. 5A). On day 14, blood, spleen and lymph nodes were harvested for further analysis. In contrast with the LNP/mRNA group, the percentages of CD80+ and CD86+ DCs were significantly higher within the lymph nodes (Fig. 5B). The amount of SIINFEKL-MHCI specific DCs in NC-TNP/mRNA was about 0.5-fold greater than that of LNP/mRNA. After immunization, peripheral blood was harvested from mice with different treatments for flow analysis. The results showed that the frequency of SIINFEKL-MHCI-specific CD8+ T cells were significantly higher compared to LNP/mRNA (Fig. 5C). Spleen were collected from immunized mice and lysed to single-cell suspension which was restimulated with OVA257–264 peptides. The splenocyte proliferation assay showed that the splenocytes in the group of NC-TNP/mRNA proliferated more efficiently than that of LNP/mRNA (Fig. 5D). Based on the ability of NC-TNP on spleen targeting, we further evaluated whether our mRNA delivery system NC-TNP could induce robust immune responses through intravenous injection (Fig. 5E). Seven days post-injection, mice were killed to collect spleen and blood. Spleen was harvested and lysed to single-cell suspensions for flow analysis. We found that NC-TNP/mRNA induced a higher proportion of maturated pDCs and cDCs than LNP/mRNA (Fig. 5F). Furthermore, the activated NK, CD4+, and CD8+ T cells in the group of NC-TNP were much higher than that in LNP/mRNA (Fig. 5G). The percentage of SIINFEKL-MHCI specific CD8+ T cells in splenocytes from mice treated with NC-TNP was significantly higher than in the mice injected with LNP (Fig. 5H). After stimulation with OVA257–264, the percentage of IFN-γ+ CD8+ T cells and IFN- γ +CD4+ T cells in NC-TNP was higher than that of LNP (Fig. 5I). We also investigated the CD8+ T cells in peripheral blood mononuclear cells (PBMCs) isolated from the mice after different treatments. The NC-TNP treatment resulted in increased in the percentage of CD8+ T cells (Fig. 5J). To further investigate the long-term immune responses elicited by NC-TNP/mRNA, C57BL/6 mice were immunized with NC-TNP/mRNA and LNP/mRNA (mRNA encoding OVA, 1 mg/kg) on day 0, 7 and 14 (Fig. 5K). Compared with LNP/mRNA group, the proportion of CD8+ T cells and SIINFKL-MHCI specific CD8+ T cells was higher from day 10 to day 60 (Fig. 5K and SI Appendix, Fig. S63). Then, the immune memory effects elicited by NC-TNP/mRNA and LNP/mRNA were assessed. Compared with LNP/mRNA group, the proportion of central memory T cells (CD8+CD44+CD62L+) and effector memory T cells (CD8+CD44+CD62L−) in NC-TNP/mRNA group showed an increase (SI Appendix, Fig. S64).
Fig. 5.
In vivo permanent and targeting immune responses. (A) Schematic illustration showed the timeline of subcutaneous immunization. C57BL/6 mice were subcutaneously injected with LNP/mRNA and NC-TNP/mRNA (mRNA encoding OVA, 1 mg/kg) on day 0. After 14 d immunization, mice were killed and the lymph nodes, blood, and spleen were harvested for further evaluation by flow analysis. (B) For lymph nodes. On day 14, the lymph nodes were isolated and lysed to prepare single-cell suspensions. The percentage of CD11c+CD80+ DCs (Left), CD11c+CD86+ DCs (Medium), and CD11c+SIINFEKL-MHCI+ DCs (Right) in whole lymph nodes were measured by flow analysis. (C) For blood. On day 14, blood was collected and centrifuged to obtain peripheral blood mononuclear cells (PBMC). Representative flow cytometry plots (Left) and quantitative results from flow cytometry (Medium) to show the percentages of SIINFEKL specific CD8+ T cells. Quantitative result to show the frequency of IFN-γ+CD8+ T. (D) For spleen. On day 14, spleen was collected and lysed to prepare single-cell suspensions of splenocyte. The splenocyte was seeded into 96-well plates and restimulated ex vivo with OVA257–264 (50 µg/mL). After 72 h incubation, the proliferation profile was measured by CCK-8. (E) Schematic illustration showed the timeline of intravenous immunization. C57BL/6 mice were intravenously injected with LNP/mRNA and NC-TNP/mRNA (mRNA encoding OVA, 1 mg/kg) on day 0. After 14 d immunization, mice were killed and the blood, and spleen were harvested for further evaluation by flow analysis. For spleen (F–I). (F) On day 14, the spleen was isolated and lysed to prepare single-cell suspensions. The percentages of CD11c+CD86+ DCs (Left) and CD11c+CD86+ DCs (Right) in the whole cDC and pDC measured by flow analysis and the quantified mean fluorescence intensity was calculated by ImageJ software. (G) The activation CD69 marker expression in NK, B cells, CD4+ T cells, and CD8+ T cells form spleen. (H) Representative flow cytometry plots (Left) and quantitative results from flow cytometry (Medium) to show the percentages of SIINFEKL specific CD8+ T cells. (I) Quantitative result to show the frequency of IFN-γ+CD8+ T cells and IFN-γ+CD4+ T cells. (J) For blood. On day 14, blood was collected and centrifuged to obtain peripheral blood mononuclear cells (PBMC). Representative flow cytometry plots to show the percentages of CD8+ T cells. (K) Schematic illustration showed the design of experiment to evaluate the long-term immune memory effect elicited by LNP/mRNA and NC-TNP/mRNA. C57BL/6 mice were intravenously immunized by different mRNA formulations (mRNA encoding OVA, 1 mg/kg) on day 0, 7, and 14. The kinetics of the frequency of SIIFNEKL specific CD8+ T cells in CD8+ T cells after different times post immunization was measured. Data represent the means ± SD (n = 5 biologically independent samples). One-way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Discussion
Due to the rapid production of intreated proteins in the targeted cell cytoplasm without inserting into the genome, mRNA has immense potential for applications in protein replacement, immunotherapy, and gene editing. However, the physicochemical properties of naked mRNA (instability, large molecular weight, and negative charge) limited its internalization to obtain therapeutic purposes. Therefore, the application of delivery vectors to overcome the above barriers has been the most important approach to gain access to the cell interior. The encapsulation of mRNA into the delivery carriers protected mRNA from degradation. In addition, mRNA delivery platform should be enable to deliver mRNA to specific organs or cells and facilitate endosomal escape to enhance protein expressions. LNP, as an ideal delivery system, has been widely applied across the pharmaceutical industry. Although the tremendous progress has been made for mRNA delivery based on classical LNP, the excess cationic charge density of lipids was necessary to compress mRNA through electrostatic interaction, and with it comes inevitably adverse events including the highly inflammatory and cytotoxic effects. The mRNA delivery with positive charge tended to predominantly accumulate in the lung and liver of mice not in the spleen. However, the distribution of mRNA delivery system with decreased positive charge shifted progressively from lung and liver towards the spleen. The spleen, the largest secondary immune organ, is the site for eliciting immune responses. How to design delivery system for spleen targeted transportation was essential to induction of immune responses for the application of mRNA in vaccine. The mutually conflicting cationic charge requirements for mRNA delivery system induced the development of LNP fall into a dilemma. How to develop the disruptive technologies to abandon the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation remains a major challenge for safe and efficient mRNA delivery. Inspired by the literature using intercalation as a noncationic interaction to bind double-stranded siRNA, we try to find a similar method to bind single-stranded mRNA. Nanoparticles modified with intercalating groups showed strong siRNA binding ability for gene delivery (32, 33).
Here, we have shown that strong hydrogen bonds can be applied for delivery system to complex with mRNA, abandoning the hidebound and traditional electrostatic force to construct mRNA-cationic lipids formulation. The agarose-gel electrophoresis and stability analysis results showed that NC-TNP compressed mRNA by strong hydrogen bonds interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA to avoid the degradation. Unexpected, The EGFP expression level in NC-TNP/mRNA was higher than LNP/mRNA. This exciting result inspired us to find the underlying mechanism. We detailedly evaluated the intracellular transportation behavior of NC-TNP and LNP in DC2.4, B16, and 4T1 cells. The cellular internalization of LNP was about micropinocytosis mediated pathway after 4 h incubation. Large amounts of LNPs were egressed from intracellular environment to extracellular milieu through endocytic recycling mediated pathway. However, the internalized NC-TNP could be easily transported from early endosomes to late endosomes. Then, NC-TNP could escape from the endosome/lysosome compartment to cytoplasm for mRNA expressions without recycling pathways.
In addition, local and systemic inflammation responses analysis showed that noncationic NC-TNP-induced negligible inflammatory and cytotoxicity side effects either in intradermal, intramuscular, and intravenous administration manner, superior to c-LNP or i-LNP that elicited robust inflammation-related side effects and cytotoxic effects. At the beginning, cationic LNP has been widely developed to compress mRNA for delivery. However, the excess cationic charge density of LNP always induced the highly inflammatory and cytotoxic effects. Then, ionizable LNP was developed to reduce the unwanted safety side effect. Even so, more and more preclinical studies showed that the potential inflammatory and cytotoxic effects of ionizable LNP were also existed (18). In this study, we also observed the inflammatory responses induced by ionizable LNP. Compared with famous cationic or ionizable LNP formed by cumbersome four components with high inflammation-related side effects and cytotoxic effects, NC-TNP was a delivery system for mRNA with simple, convenient, and repeatable preparation technology and showed negligible inflammatory and cytotoxicity side effects.
Furthermore, the ex vivo imaging showed the ability of NC-TNP with spleen-selective accumulation. The targeting delivery mRNA to specific organs was also a big challenge. Several previous reports have confirmed that the relationship between the charge of delivery systems and the organ targeting. The mRNA delivery system with positive charge trended to accumulate in liver and lung. The distribution of mRNA delivery system shifted progressively from lung and liver toward the spleen. It is regrettable that the positive charge nature of LNP as a delivery system complexed with mRNA through electrostatic interaction induced to accumulate in lung and liver, not spleen which was an important immune organ. Spleen-targeted NC-TNP/mRNA should be a promising candidate for application as vaccines to elicit robust immune responses to treat cancer or other diseases.
Overall, our work establishes a proof of concept for the construction of a noncationic system for mRNA delivery with good inflammatory safety profiles, high gene transfection efficiency, and spleen-targeting delivery to induce durable and robust humoral and cell-mediated immunity for disease treatments.
Materials and Methods
Preparation of NC-TNP-Encapsulated mRNA.
Noncationic thiourea lipids and cholesterol at a molar ratio of 60:40 were added in ethanol (6 mg/mL). mRNA was diluted in distilled deionised water (1 mg/mL). Then, lipids ethanol solution and mRNA aqueous solution were rapidly injected into the microfluidics chips with two syringe pumps at ethanol to aqueous volume ratio of 1/3 (speed ratio was ethanol to aqueous was 0.1/0.3 mL/min). The mixtures solution was dialyzed against PBS buffer with a dialysis tube (Cut-off MWCO: 10 kDa) at 4 °C overnight.
The empty NC-TNP was prepared with the similar method. We just injected lipids ethanol solution and distilled deionized water into the microfluidics.
Preparation of LNP-Encapsulated mRNA.
We used the dioleoyl-3-trimethylammonium propane (DOTAP) as representative cationic lipids and 1-octylnonyl-8-[(2-hydroxyethyl)[6-oxo-6-(undecyloxy) hexyl]amino]-octanoate (SM-102) as representative ionizable lipids to form the cationic and ionizable LNPs, respectively.
DOTAP or SM-102, 1,2-Distearoyl-sn-glycero-3-phosphorylcholine (DSPC), cholesterol, and 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG) (about 50:10:38.5:1.5 mol%) were added in ethanol (10 mg/mL). mRNA was diluted in 50 mM citrate buffer with pH 4.0 (1 mg/mL). Then, lipids ethanol solution and mRNA aqueous solution were mixed in a microfluidics chip with ethanol to aqueous volume ratio of 1/3 (speed ratio was ethanol to aqueous was 0.1/0.3 mL/min). The mixture solution was dialyzed against PBS buffer with a dialysis tube (Cut-off MWCO: 10 kDa) at 4 °C overnight.
The empty cationic or ionizable LNP was prepared with the similar method. We just injected lipids ethanol solution and distilled deionized water into the microfluidics.
Transfection.
DC2.4, B16, or 4T1 cells were seed at 10,000 cells per well in 24-well and cultured with complete medium overnight. NC-TNP/mRNA, LNP/mRNA and lipo/mRNA (mRNA encoding EGFP, 1 µg per well) were added to the 48-well cultured with medium with 10% FBS for incubation 48 h. Subsequently, the medium was removed and washed by PBS and analyzed by confocal microscopy of flow cytometer.
Uptake.
DC2.4, B16, or 4T1 cells were seed in confocal dishes with complete medium overnight. Then, Cy5 labeled NC-TNP and LNP were added to the dishes with complete medium for incubation with different time points. Subsequently, the cells were washed by PBS and fixed by 4% paraformaldehyde. The fixed cells were blocked by 5% bovine serum albumin (BSA) for 30 min. The primary antibodies dilutions (anit-EEA1 body, anti-LAMP1 antibody, anti-LAMP2 antibody, and anti-Rab11 antibody) according to the instruction were added for incubation 2 h at room temperature. The primary antibodies solution was removed and washed by PBS for three times; the cells were incubated with second antibodies for 1 h. After washing three times with PBS, the cells were stained by DAPI and analyzed by confocal microscopy.
Ultrastructure of Cell.
DC2.4, B16, or 4T1 cells were seeded in 6-well plates with complete medium overnight. Then, gold particles–loaded NC-TNP and LNP were added to the well with complete medium for incubation. The treated cells were collected and fixed with ferrocyanide-reduced osmium. After gradient dehydration by ethanol, cells were embedded with resin. Then, 80-nm sections were cut and stained with azure blue and toluidine blue for TEM observation.
Western Blot Analysis.
DC2.4 were seeded into 6-well plates with complete medium overnight. Then, the cells were incubated with NC-TNP and LNP for 24 h. After treatments, the cells were washed and lysed by RIPA buffer (100 mM Tris–HCl, pH 8, 250 mM NaCl, 0.5% Nonidet P-40, 1 × cocktail of protease inhibitor). The obtained proteins were loaded on 10% polyacrylamide gels and transferred to nitrocellulose membranes, which were blocked by 5% BSA solution for 30 min. The primary antibodies dilutions (anit-EEA1 body, anti-LAMP1 antibody, anti-LAMP2 antibody, anti-Rab11 antibody, anti-NPC1, and β-actin) according to the instruction were added for incubation 2 h at room temperature. The primary antibodies solution was removed and washed by PBS-T for three times, the membranes were incubated with second antibodies for 1 h. After washing three times with PBS-T, the protein bands were detected after treatment with detection kit using imaging system.
qRT-PCR.
We used RNA isolation kit to extract total RNA from the treated cells or isolated tissues samples from mice after different treatments. The concentration and integrity of extracted RNA was measured by a Nanodrop (Thermo) at 260/280 nm. Complementary DNA (cDNA) was prepared from the total RNA by reverse-transcription using a Strand cDNA Synthesis Kit. The level of relative targeted messenger RNA (mRNA) was detected with qRT-PCR using the SYBR Green plus reagent kit. Each reaction was repeated for three times. Detailed information about the relative primers is showed in SI Appendix, Table S2. The levels of the mRNA expression were measured based on the 2−ΔΔCT approach. The relative mRNA expression was normalized to β-actin expression.
In Vivo Inflammatory.
The in vivo inflammatory reactions in C57BL/6 induced by LNP and NC-TNP (20 µg/per mouse) were investigated through intradermal, intramuscular, or intravenous injection. After 3 d of injection, the skin samples were harvested to prepare single cells solution for flow analysis. The quantitative percentages of inflammatory cells were measured, including Neutrophils (CD45+CD11b+Ly6G+). Representative flow cytometry gating strategies for experiments are showed in SI Appendix, Figs. S65–S71. For the intramuscular delivery, the tissue on injection site was harvested to measure the percentages of inflammatory cells and inflammatory cytokine. For intravenous injection, LNP and NC-TNP were intravenously injected into C57BL/6 mice. Three days post administration, the inflammatory cytokine in blood was evaluated and the heart was harvested for H&E staining.
ELISA.
The process of ELISA was performed according to the instructions of manufacturer. First, 90-well ELISA plates were coated with mouse antibody (depending on the measurement of cytokines) with a concentration of 1 µg/mL at 4 °C. At 48 h after coating, the ELISA plates were washed three time by PBS containing 0.05% Tween 20. Then, we used 1.5% bovine serum albumin (BSA) solution in PBS to block the plates for 2 h. The supernatant from treated cells or serum samples and cytokine standard dilutions were diluted tenfold with PBS containing 1.5% BSA and added into the plate well. After 12 h incubation at 4 °C, the plates were washed three times by PBS before adding biotin-conjugated detection antibody (1:1000 dilution) for 2 h at room temperature. The plates were washed by PBS containing 0.05% Tween 20 and incubated with horseradish peroxidase streptavidin in PBS containing 1.5% BSA for 30 min at 25 °C. After a three times wash, we added tetramethylbenzidine (TMB) solution into each well. A few minutes later, H2SO4 solution (2.5 N) was added as stop solution. The absorbance was detected by a microplate reader. The concentration of cytokines in samples were calculated according to the standard curve.
In Vivo Imaging.
All animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University (Approval No.: 20210377).
C57BL/6 mice were subcutaneously injected with LNP/mRNA and NC-TNP (mRNA encoding firefly luciferase). Whole-body bioluminescence was measured at different times after injection using an in vivo Imaging System (IVIS) Lumina system (PerkinElmer). Mice were intraperitoneally injected by D-luciferin, and the imaging was obtained after 5 min postinjection.
Then, 6–8 week old tdTomato transgenic Ai9 mice (express tdTomato upon Cre-mediated recombination) were injected with LNP and NC-TNP loaded mRNA (Cre-mRNA, 0.1 mg/kg) through footpad. After 24 h, Ai9 mice were killed and the inguinal lymph nodes were isolated. The lymph nodes were mechanically fragmented by scissors followed by digestion (hank’s solution containing 1 mg/mL collagenase IV and 50 IU/mL DNase) for 1 h at 37 °C under shaking. The red blood cells were removed by ACK lysing buffer and the cells suspension was filtered through 40-µm cell strainers and centrifugalized at 1,500 rpm for 3 min. The obtained single-cell suspensions were stained with fluorescence antibodies to detect the percentage of tdTomato positive cells across different types of immune cells (CD3+CD4+ T cells, CD3+CD8+ T cells, CD45+CD11c+ DCs, CD45+NK1.1+ NK cells, CD45+CD11b+F4/80+ Macrophages, CD45+CD3−CD19+ B cells) in lymph nodes by Flow cytometry. Representative flow cytometry gating strategies for experiments are showed in SI Appendix, Figs. S65–S71.
C57BL/6 mice were intravenously injected with Cy5 labeled LNP and NC-TNP. After different times of injection, the mice were killed and the major organs were isolated for ex vivo imaging using an IVIS Lumina system (PerkinElmer). Quantitative results of fluorescence intensity were obtained by calculating the average radiance (photon s−1 cm−2 sr−1) in a region of interest.
In Vivo Immune Responses.
C57BL/6 mice were vaccinated by LNP/mRNA and NC-TNP/mRNA (OVA-mRNA, 1 mg/kg) through subcutaneous injection on day 0. Then, the mice were killed on day 14, and the blood, inguinal lymph nodes, and spleen were collected for further analysis.
C57BL/6 mice were vaccinated by LNP/mRNA and NC-TNP/mRNA (OVA-mRNA, 1 mg/kg) through intravenous injection on day 0. Then, the mice were killed on day 7, and the blood, and spleen were collected for further analysis.
Peripheral blood mononuclear cells (PBMC) were collected and centrifuged at 1,500 rpm for 3 min at 25 °C. The down pellet was collected and resuspended with ACK buffer to remove red blood cells. Then, 1 mL cold PBS was added into the tube to stop the lysis process. The preventive PBMC were stained with fluorescence antibodies to detect the percentage of CD8+ T cells (CD3+CD45+CD8+), CD8+INF-γ+ T cells (CD3+CD45+CD8+IFN-γ+) and OVA tetramer+CD8+ T (CD3+CD45+CD8+SIINFEKL-MHCI+) in PBMC by flow cytometry. Representative flow cytometry gating strategies for experiments are showed in SI Appendix, Figs. S65–S71.
The obtained lymph nodes were mechanically fragmented by scissors followed by digestion (Hank’s solution containing 1 mg/mL collagenase IV and 50 IU/mL DNase) for 1 h at 37 °C under shaking. The red blood cells were removed by ACK lysing buffer and the cell suspension was filtered through a 40-µm cell strainers and centrifugalized at 1,500 rpm for 3 min. The obtained single-cell suspensions were stained with fluorescence antibodies to detect the percentage of CD8+ T cells (CD3+CD45+CD8+) by flow cytometry. Representative flow cytometry gating strategies for experiments are showed in SI Appendix, Figs. S65–S71.
Spleen was harvested from mice after vaccination with LNP/mRNA and NC-TNP/mRNA and lysed with ACK buffer to remove red blood cells. The mixture cells solution was filtrated through 40-μm cell strainers. The splenocytes were seed in 96-well plate at a density of 104 cells per well and restimulated with OVA peptides (SIINFEKL) (50 µg/mL) for 60 h. The splenocytes proliferation was measured by CCK-8 method. For detection of IFN-γ secretion, the supernatant from the restimulated splenocytes were collected for the evaluation of ELISA.
Statistical Analysis.
Statistical evaluations were completed using Prism 9 (GraphPad software). One way ANOVA was used for the comparisons (NS, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported by National Natural Science Foundation of China projects (Nos. 32171320, 81371667, 82302384, and 32201173), Xi'an Association for Science and Technology young Talent Promotion Plan (095920221341), Natural Science Foundation of Shaanxi Province of China (2023-JC-YB-196), Qinchuangyuan cited the high-level innovation and entrepreneurship talent program (QCYRCXM-2022-27 and QCYRCXM-2022-232), Science and Technology Projects in Guangzhou (2023A04J1510), and the Fundamental Research Funds for the Central Universities (No. QTZX23058). Thanks for Prof. Zhihai Qin from Zhenzhou University supported the Ai9 mice.
Author contributions
C.Z. and H.D. designed research; C.W., C.Z., W.W., X.L., and H.D. performed research; C.W., C.Z., and H.D. contributed new reagents/analytic tools; C.W., C.Z., W.W., and H.D. analyzed data; and C.W., C.Z., and H.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. D.G.A. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Sahin U., Karikó K., Türeci Ö., mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Zhang Y., Dong Y., Lipid nanoparticle–mRNA formulations for therapeutic applications. Acc. Chem. Res. 54, 4283–4293 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melamed J. R., et al. , Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv. 9, eade1444 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S., et al. , Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rurik J. G., et al. , CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangi L., et al. , Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31, 898–907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam M. A., et al. , Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2, 850–864 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowalski P. S., Rudra A., Miao L., Anderson D. G., Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaabani E., et al. , Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 29, 871–899 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou X., Zaks T., Langer R., Dong Y., Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., et al. , Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., et al. , Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. U.S.A. 119, e2207841119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu M., et al. , Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. U.S.A. 118, e2020401118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Akiva E., et al. , Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl. Acad. Sci. U.S.A. 120, e2301606120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng L., Bandara S. R., Tan Z., C., Leal Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc. Natl. Acad. Sci. U.S.A. 120, e2301067120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D., et al. , One-component multifunctional sequence-defined ionizable amphiphilic Janus dendrimer delivery systems for mRNA. J. Am. Chem. Soc. 143, 12315–12327 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Tahtinen S., et al. , IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Ndeupen S., et al. , The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahay G., et al. , Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653–658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q., et al. , Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You Y., et al. , Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 7, 887–900 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Majeti B. K., Karmali P. P., Reddy B. S., Chaudhuri A., In vitro gene transfer efficacies of N, N-dialkylpyrrolidinium chlorides: A structure−activity investigation. J. Med. Chem. 48, 3784–3795 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Zhao H., et al. , Long-term stability and protection efficacy of the RBD-targeting COVID-19 mRNA vaccine in nonhuman primates. Signal Transduct. Target. Ther. 6, 438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crommelin D. J. A., Anchordoquy T. J., Volkin D. B., Jiskoot W., Mastrobattista E., Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 110, 997–1001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y., et al. , Caveolae-mediated endocytosis drives robust siRNA delivery of polymeric nanoparticles to macrophages. ACS Nano 15, 8267–8282 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Gilleron J., et al. , Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Jung J.-U., Cobb M. H., WNK1 controls endosomal trafficking through TRIM27-dependent regulation of actin assembly. Proc. Natl. Acad. Sci. U.S.A. 120, e2300310120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S., et al. , Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 17, 5711–5718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrams M. T., et al. , Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: Effect of dexamethasone co-treatment. Mol. Ther. 18, 171–180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv H., Zhang S., Wang B., Cui S., Yan J., Toxicity of cationic lipids and cationic polymers in gene delivery. J. Controlled Release 114, 100–109 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Kranz L. M., et al. , Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Zhou K., et al. , Intercalation-mediated nucleic acid nanoparticles for siRNA delivery. Chem. Commun. 52, 12155–12158 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Huang X., et al. , Intercalation-driven formation of siRNA nanogels for cancer therapy. Nano Lett. 21, 9706–9714 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.