Abstract
The pathogenesis of multiple sclerosis (MS) suggests that, in genetically susceptible subjects, T lymphocytes undergo activation in the peripheral compartment, pass through the BBB, and cause damage in the CNS. They produce pro-inflammatory cytokines; induce cytotoxic activities in microglia and astrocytes with the accumulation of reactive oxygen species, reactive nitrogen species, and other highly reactive radicals; activate B cells and macrophages and stimulate the complement system. Inflammation and neurodegeneration are involved from the very beginning of the disease. They can both be affected by oxidative stress (OS) with different emphases depending on the time course of MS. Thus, OS initiates and supports inflammatory processes in the active phase, while in the chronic phase it supports neurodegenerative processes. A still unresolved issue in overcoming OS-induced lesions in MS is the insufficient endogenous activation of the Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) pathway, which under normal conditions plays an essential role in mitochondria protection, OS, neuroinflammation, and degeneration. Thus, the search for approaches aiming to elevate endogenous Nrf2 activation is capable of protecting the brain against oxidative damage. However, exogenous Nrf2 activators themselves are not without drawbacks, necessitating the search for new non-pharmacological therapeutic approaches to modulate OS. The purpose of the present review is to provide some relevant preclinical and clinical examples, focusing on certain exogenous and endogenous Nrf2 activators and the modulation of therapeutic plasma exchange (TPE). The increased plasma levels of nerve growth factor (NGF) in response to TPE treatment of MS patients suggest their antioxidant potential for endogenous Nrf2 enhancement via NGF/TrkA/PI3K/Akt and NGF/p75NTR/ceramide-PKCζ/CK2 signaling pathways.
Keywords: multiple sclerosis, oxidative stress modulation, Nuclear Factor Erythroid 2-Related Factor 2 pathway activation, exogenous, endogenous, nerve growth factor, therapeutic plasma exchange
1. Introduction
Multiple sclerosis (MS) is an autoimmune multifocal inflammatory disease of the central nervous system (CNS) characterized by chronic inflammation, demyelination, axonal damage, and subsequent gliosis. The CNS regions that are commonly affected by MS include the periventricular region, the subcortical region, the optic nerve, the spinal cord, the brain stem, and the cerebellum. According to the natural course of the disease, it is categorized as relapsing–remitting, secondary progressive, and primary progressive [1]. The etiology of MS presumes that, in genetically pre-disposed patients, T lymphocytes become activated in the peripheral compartment, pass through the blood–brain barrier (BBB), and cause tissue damage in the CNS compartment. They produce proinflammatory cytokines; induce cytotoxic functions in microglia and astrocytes with the accumulation of reactive oxygen species (ROS), reactive nitrogen species (RNS), and other superoxide radicals; stimulate B cells and macrophages; and activate the complement system [2]. The imbalance between ROS accumulation and clearance underlies oxidative stress (OS), and this plays a critical role in regulating cell and tissue physiological and pathological processes [3]. Inflammation and neurodegeneration are involved from the very beginning of the disease [4]. They can both be affected by OS with different emphases depending on the time course of MS. Thus, in the acute phase OS initiates inflammatory processes, while in the chronic phase, it supports neurodegeneration [5]. A still unresolved issue in overcoming OS-induced lesions in MS is the failure to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, which basically plays a critical role in preventing mitochondrial failure, OS, neuroinflammation, and degeneration [6]. The level of endogenous stimulation of the Nrf2 pathway is not sufficient to counteract OS overload. Thus, the application of exogenous approaches that are capable of elevating endogenous Nrf2 stimulation can contribute to protecting the brain tissue against oxidative damage [7]. However, exogenous Nrf2 activators themselves are not without drawbacks [8,9,10], necessitating the search for new non-pharmacological approaches to modulate OS. The purpose of this review is to present some relevant preclinical and clinical examples, focusing on certain exogenous and endogenous Nrf2 activators and the therapeutic plasma exchange (TPE) modulation of OS in MS.
In this focus review, along with a short summary of OS and the Nrf2 pathway in MS, we explore some relevant natural and synthetic compounds, nerve growth factor (NGF) and TPE in the context of their role in Nrf2 activation, and OS modulation.
2. Methodological Approaches
A search in the literature was performed through September 2023 of MEDLINE, EMBASE, and Cochrane Library based on the Medical Subject Heading (MeSH) of “oxidative stress”, “OS”, “reactive oxygen species”, “ROS”, “reactive nitrogen species”, “RNS”, “superoxide radicals”, “oxidants”, “antioxidants”, “enzymatic”, “non-enzymatic”, “balance”, “modulation”, “nuclear factor erythroid 2-related factor 2”, “Nrf2”, “Keap1/Nrf2/ARE”, “Nrf2/HO-1”, “signaling”, “pathway”, “activation”, “activators”, “exogenous”, “endogenous”, “natural”, “synthetic”, “pharmacological”, “non-pharmacological”, “multiple sclerosis”, “MS”, “acute”, “chronic”, “relapsing remitting”, “secondary progressive”, “primary progressive”, “aggressive”, “attacks”, “exacerbations”, “relapses”, “central nervous system”, “CNS”, “inflammation”, “neuro-inflammation”, “demyelination”, “degeneration”, “neuro-degeneration”, “neurodegenerative”, “diseases”, “neuroprotection”, “nerve growth factor”, “NGF”, “plasma levels”, “neurotrophins”, “receptors”, “tropomyosin receptor kinase A”, “TrkA”, “p75 neurotrophin receptor”, “p75NTR”, “therapeutic plasma exchange”, “nanomembrane-based”, “plasmapheresis”, and “apheresis”, as well as through a manual search in the local database (National Library of Saints Cyril and Methodius, Sofia, Bulgaria). The search had no language restrictions.
3. OS in MS
OS plays a key role in neurodegenerative processes by inducing oxidative damage to lipid, protein, and deoxyribonucleic acid (DNA) molecules. Oxidative destruction of proteins makes them alter their active configuration and form oligomers with different functions or molecular fragments that cause pro-inflammatory processes that aggravate OS. Mitochondrial DNA also undergoes alterations caused by ROS and RNS, leading to misfunctions in the key proteins involved in key cellular metabolic processes. The impairment of mitochondrial activity leads to the accumulation of ROS and activation of pro-apoptotic pathways. Lipid peroxidation caused by ROS destroys the structure, asymmetry, and permeability of cell membranes’ bilayer, leading to an enhanced inflammatory response, changes in calcium homeostasis, and neuronal death [11,12,13]. Moreover, the degree of oxidative attack towards proteins, lipids, and DNA frequently is sufficient to generate neoepitopes due to a loss of immunological tolerance, leading to the development of autoimmunity [14,15,16]. The latter implication increases the relevance of using TPE as a means of both modulating OS and ameliorating its adverse consequences.
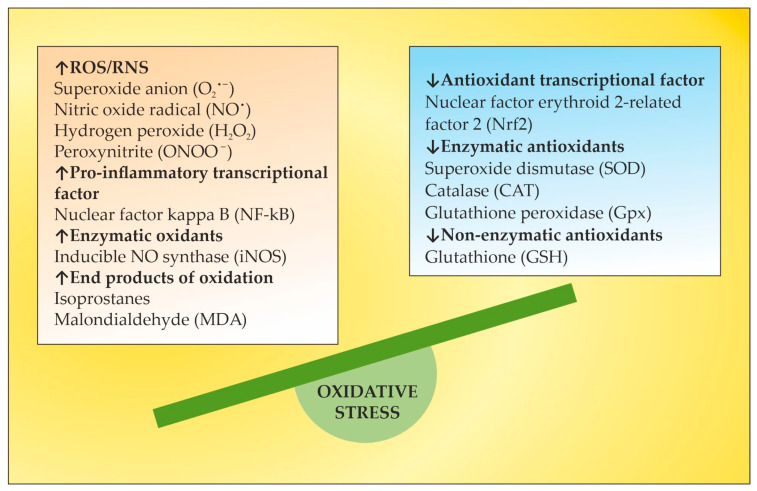
OS is a well-recognized pathogenic factor in the onset and development of a vast variety of neurodegenerative pathologies, including MS. This neurodegenerative disease with a pronounced neuroinflammatory component is characterized by different symptoms and clinical manifestations, which are basically accompanied by inflammation-related damage to the CNS and different levels of disturbed motor activity in affected patients [17]. Inflammation elevates the content of free radicals, causing a higher number of oxidative stress biomarkers [18]. OS is generally an impaired oxidant/antioxidant balance that can cause neuroinflammation and neurodegeneration [19]. The imbalance is characterized by increases in ROS/RNS, the pro-inflammatory transcriptional factor, enzymatic oxidants, and oxidation end products at the expense of decreases in the antioxidant transcriptional factor and enzymatic and non-enzymatic antioxidants (Figure 1). Since OS may be associated with neurodegenerative disorders, an antioxidant-related therapeutic approach might be a promising perspective [20]. OS has also been assumed to be a significant pathogenic factor underlying the etiology of MS [21].
Figure 1.
Major OS markers and factors of redox imbalance implicated in MS pathogenesis. Adapted from [22] and modified.
OS, mitochondrial damage, and energy are possibly implicated in plaque formation and neurological degeneration in lesions of white and grey matter [23,24,25]. The brain tissue is a target of oxidative attack because of the high oxygen requirements for its functional activity, significant amount of polyunsaturated fatty acids (PUFAs), and comparatively low activity and level of antioxidant enzyme systems [26]. ROS and RNS play an essential role in MS occurrence and development by contributing to the impairment of oligodendroglia functions [27]. According to the literature, the relapsing–remitting and progressive phenotypes of MS are determined by different mechanisms. For instance, focal inflammatory processes are associated with the development of relapses, while axonal degeneration may be associated with disease progression. OS in MS is presumed to be associated with both processes in MS patients [26,28]. ROS play a multifunctional role in the formation of MS lesions [29]. ROS are known to accumulate due to the interaction between monocytes and the brain endothelium, the latter leading to tight-junction modifications and disturbance of the blood–brain barrier’s integrity, which induces the migration of leukocytes into the CNS [29,30]. In addition, the infiltrated leukocytes induce an accumulation of higher amounts of ROS, which underlie myelin degradation [31], destruction of the oligodendroglia [32], and finally neuronal and axonal impairment [33].
The major sources of ROS include the mitochondria, peroxisomes, and phagocytic cells. Macrophages occur as an important source of ROS [34] due to high oxygen consumption [35,36]. Recent studies in experimental models of MS have reported that the rapid response of resident immune cells in the CNS, most notably the pro-inflammatory polarization of microglia [37] and astrocytes [38], is associated with the expression and release of reactive molecules associated with OS [39,40]. They have a significant effect on chronic myelin loss and inhibit the remyelination and repair of local CNS lesions [41]. Thus, activated microglia and astrocytes are often considered a major source of ROS and RNS in the CNS [42].
Myelin, which surrounds the axons and also acts as a major target of immune attacks in MS, consists of about 30% protein and 70% lipids. Malondialdehyde (MDA) is a marker of lipid peroxidation and is a result of PUFA peroxidation [18]. This aldehyde provides information on the level of OS by evaluating the oxidative damage of lipids [18]. Isoprostanes, which also serve as markers of lipid oxidative damage, are prostaglandin F-like products of the peroxidation of unsaturated acyl chains, predominantly of arachidonic acid [43,44]. The content of isoprostanes in plasma have shown to be elevated in MS, including in relapsing–remitting MS and secondary progressive MS types [45,46]. There are reports showing that isoprostanes and MDA are increased in the plasma of MS patients compared with controls and support their role as indicators of disease development, disability progression, or therapeutic response [47]. Along with altering ROS plasma levels, they reflect the success or failure of antioxidant treatment in MS patients [17].
4. Nrf2 Pathway in MS
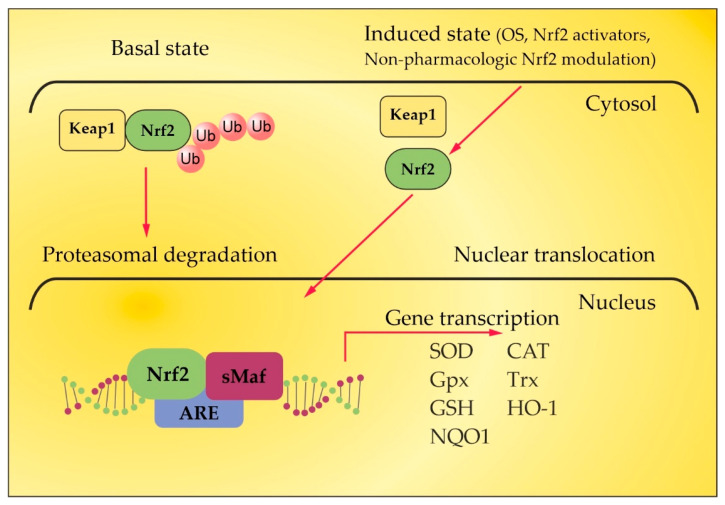
Under homeostatic conditions, Nrf2 is localized in the cytosol through its negative regulator, kelch-like ECH-associated protein 1 (Keap1), and is subject to proteasomes disintegration through a couple of ubiquitin ligase systems [48,49]. Thus, the Keap1/Nrf2 interaction underlies a low expression of Nrf2-regulated genes. OS or Nrf2 activators, along with non-pharmacologic Nrf2 modulation, break the complex between Nrf2 and Keap1, inducing the translocation of Nrf2 towards the nucleus. There, Nrf2 heterodimerizes with small musculoaponeurotic fibrosarcoma (sMaf) proteins and binds to antioxidant response elements (ARE) in the promoter region of target genes [48,49,50,51,52]. This region encodes several antioxidant enzymes that neutralize ROS and electrophiles, involving superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, glutathione reductase, and heme oxygenase-1 (HO-1) (Figure 2) [53,54].
Figure 2.
Keap1/Nrf2/ARE signaling pathway. Adapted from [52] and modified.
Autopsy specimens of MS patients show the upregulation of Nrf2 and the Nrf2-responsive genes HO-1 and NQO-1, in and near to the active lesions in spinal cord and brain samples [55,56], as an integral part of the cellular anti-oxidative response. The increased expression of Nrf2 has been observed in astrocytes and macrophages in active lesions [54] and in oligodendrocytes at the lesion’s edges [55]. However, the level of Nrf2 expression is cell-type-specific [57]. In the CNS, astrocytes harbor a more efficient anti-oxidative potential compared to neurons. The expression of Nrf2 is lower in neurons, even when they are surrounded by Nrf2-positive glia [56]. Consistent with the essential role of Nrf2 for ROS detoxification, Nrf2-deficient cells are more susceptible to oxidative destruction, etc. [58]. This may underlie a limited capacity of neurons to cope with OS.
In experimental autoimmune encephalomyelitis (EAE), an established model for MS, Nrf2-deficient mice show a more rapid onset, an exacerbated clinical severity, an increased number of lesions and infiltrating immune cells, greater microglial activation, and visual dysfunction [59,60,61]. Additionally, and importantly, the oligodendrocytes damaged in the EAE lesions have relatively low levels of Nrf2. Therefore, low levels of Nrf2 or the impaired activation of Nrf2 in oligodendrocytes may account for selective susceptibility in neuroinflammatory conditions due to the high vulnerability of these oligodendrocytes to OS [62].
Dysfunctions in the Nrf2 signaling pathway result in the impairment of redox homeostasis, which leads to ROS/RNS overload [63] and the prevalence of other redox-sensitive transcription factors such as activator protein 1 and the pro-inflammatory nuclear factor kappa-light chain-enhancer of activated B cells (NF-kB). The latter stimulates the expression of certain genes involved in MS pathogenesis, such as tumor necrosis factor α (TNF-α), iNOS, interleukin 1α/β (IL-1α/β), and some growth factors [64]. There is a crosstalk between the Nrf2 and NF-ĸB pathways. Nrf2 binds with its transcriptional cofactor cAMP-response-element-binding protein (CBP) to initiate ARE-driven gene expression. When NF-ĸB binds with CBP in a competitive way, it hinders the binding between CBP and Nrf2, the latter leading to inhibition of Nrf2 activation. Thus, NF-kB and Nrf2 compete for CBP, which promotes DNA binding [65]. Given that OS and inflammation are closely related processes in MS, the activation of the Nrf2 pathway interferes with both processes in a complex manner [66].
HO-1, one of the most important antioxidant enzymes downstream of Nrf2, plays an important role for the anti-inflammatory activity in EAE and MS [67]. Decreased expression of HO-1 was found in peripheral blood mononuclear cells (PBMCs) of MS patients, and a significant downregulation of this enzyme was observed during disease exacerbations [68]. These findings indicate a higher likelihood of relapse in patients with reduced HO-1 expression in PBMC and are confirmed by the results of a microarray meta-analysis [68]. In this context, HO-1 inducers may occur as important factors in the treatment of MS.
A thorough understanding of Nrf2-mediated HO-1 gene transactivation requires taking into account the cooperation or competition with other transcriptional factors at ARE and ARE-like sites [69]. The activating transcription factor 4 (ATF4) can dimerize with Nrf2 at ARE to promote the expression of HO-1, whereas the transcription repressor BTB and CNC homology 1 (BACH1), which are critical in the regulation of the HO-1 gene, compete with Nrf2 at the ARE binding sites, and Nrf2-induced expression requires the inactivation of BACH1 sites [69]. In addition, the expression of HO-1 is downregulated by BACH1 when the heme content is low, but higher heme levels inhibit BACH1–DNA binding and promote BACH1 exportation and degradation [70]. TPE techniques using peripheral venous access and high transmembrane pressure cause hemolysis during the procedure [71], which could contribute to BACH1 inhibition as well. Likewise, the significant decrease in plasma proteins and other plasma constituents observed during TPE [72] could contribute to amino acid losses, a well-known inducer of ATF4 [73]. Thus, TPE could modulate HO-1 expression indirectly at multiple levels.
In summary, OS and antioxidant Nrf2 pathways are important players in the pathophysiology of MS and represent a promising target for approved or investigational pharmacological and non-pharmacological therapies of MS [57].
5. The Role of Certain Natural and Synthetic Compounds as Exogenous Nrf2 Activators
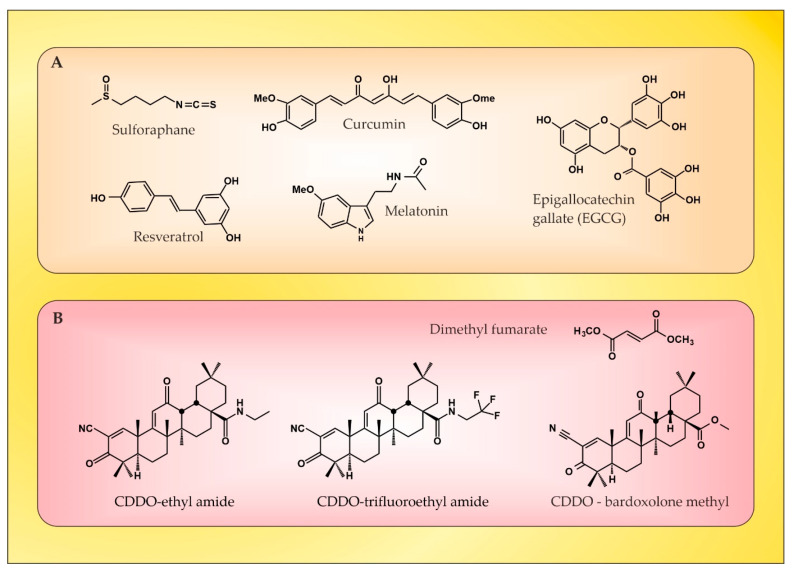
The redox homeostasis in the brain tissue is under the control of Nrf2, and there has been a growing interest in identifying natural or synthetic compounds (Figure 3) that are able to modify Nrf2 activity, for example, in MS, in which ROS/RNS have a recognized function [74].
Figure 3.
Chemical structures of certain natural (A) and synthetic (B) Nrf2 activators. Adapted from [10] and modified.
Curcumin (Figure 3A) has been tested as a component of the therapeutic protocol of MS [75,76,77]. Its immunomodulatory and anti-inflammatory properties are mainly mediated by its capacity to inhibit COX-2, iNOS, and also the NF-kB transcription pathway [78]. It reduces the clinical severity of EAE, the content of inflammatory cells infiltrating the spinal cord, the production of IL-17, and the generation of TNF-α, accompanied by the upregulation of Treg cells, suggesting that curcumin enhances Th2/Treg responses in EAE [79,80]. Only one study revealed the role of curcumin in increasing the expression of Nrf2/HO-1 in an EAE model of MS [81]. However, some significant drawbacks, such as poor solubility, low bioavailability, and dose-dependent pro-oxidant activity, hinder its wider clinical use.
Resveratrol (Figure 3A) affects various targets through different pathways, most notably Nrf2, silent information regulator T1 (SIRT1), and NF-κB [66]. In preclinical animal studies, it decreases the disease severity [82,83], the inflammatory infiltrates [84], and the NF-kB signaling [85] and improves the antioxidant potential through Nrf2 activation [85]. However, one study reveals the exacerbation of disease severity. The authors suggest that the worsening of EAE may be a result of the increased migration of inflammatory cells across the BBB, since resveratrol acts as a vasodilator, resulting in improved endothelial cell function, increased blood flow volume, and decreased blood flow velocity [86]. Like curcumin, resveratrol has poor bioavailability. Few clinical studies have evaluated the efficacy of resveratrol as a component of the therapeutic protocol of neurodegenerative diseases (Alzheimer’s disease and Friedreich Ataxia) [87,88], but none have addressed its potential in MS.
Epigallocatechin gallate (EGCG) (Figure 3A) is the most investigated flavonoid that occurs that has the greatest capacity for improving MS and EAE. There are studies showing beneficial effects of this flavonoid related to the reduced migration of immune cells beyond the BBB, reduced demyelination in the spinal cord, and decreased secretion of pro-inflammatory cytokines [89,90,91,92,93,94,95]. No clinical studies have addressed the significance of Nrf2 as a mechanism of anti-inflammatory and antioxidant activity of EGCG. However, EGCG has been shown to activate Nrf2 in animal models of Alzheimer’s disease [96] and ischemic stroke [97]. The results of clinical trials investigating the neuroprotective potential of EGCG are inconclusive [66].
Sulforaphane (Figure 3A) is a potent Nrf2 inducer [98]. It is 13.5 times more potent than curcumin and 105 times more potent compared to resveratrol, as evaluated through the induction of NQO-1 activity [99], with much better tissue penetration, absorption, and bioavailability compared to curcumin and resveratrol [51]. Studies on the mechanism of action of sulforaphane have revealed that it enhances the expression of HO-1 and NQO1 via upregulation of the Nrf2 pathway, thereby reducing the levels of OS. The direct effect of sulforaphane on microglia is manifested by increasing Nrf2 DNA-binding activity and the expression of Nrf2 downstream genes (NQO-1 and HO-1) [100]. It also reduces T-cell-mediated immunity and inflammation. Moreover, it reduces demyelination and CNS infiltration and inhibits the production of metalloproteinase 9 (related to the degradation of the extracellular matrix), which contributes to the preservation of the BBB [95]. Thus, it inhibits the development of EAE in mice through its antioxidant and anti-autoimmune inflammatory properties [101,102].
Melatonin (Figure 3A) acts as antioxidant and immunomodulatory agent, with either pro- or anti-inflammatory functions in a context-dependent way [103]. It offers potent protection against the DNA oxidative damage through a variety of mechanisms, including an increase in antioxidant enzymes [104]. The activation of antioxidant enzymes results predominantly from the stimulation of Nrf2 and its downstream proteins HO-1, NQO-1, and SOD [104,105,106,107]. The majority of preclinical studies show the beneficial effect of melatonin in treating EAE, but only one study shows its association with the upregulation of the Nrf2/HO-1 pathway [108]. In a small clinical trial, melatonin supplementation led to a statistically significant increase in SOD and GPx in the erythrocytes of secondary progressive MS patients. There was a positive correlation between SOD levels and the Expanded Disability Status Scale (EDSS) score both before and after melatonin treatment [109], indicating the importance of antioxidant protection in controlling disability in MS.
Oleanolic acid (CDDO) has been used as a source for the synthesis of several novel synthetic derivatives with improved neuroprotective properties due to Nrf2 activation. Among them, ethyl amide (CDDO-EA) (Figure 3B), methyl-amide (CDDO-ME or Bardoloxene Methyl) (Figure 3B), and trifluoroethyl amide (CDDO-TFEA) (Figure 3B) have shown the capacity to attenuate the clinical severity of EAE models, decrease Th1 and Th17 cytokines, diminish expression of iNOS in basal conditions, and upregulate HO-1 expression (over 3-fold relative to control conditions), which is consistent with the Nrf2 pathway upregulation [110,111].
Dimethyl fumarate (DMF) (Figure 3B) is a prodrug that is converted to monomethyl fumarate (MMF) [112], which is able to induce Nrf2 in different models, such as in astrocytes (over 15-fold increase in HO-1-mRNA levels at 10 μM) [113]. The administration of DMF resulted in the increased expression of NQO-1 and HO-1 genes in ex-vivo-stimulated peripheral blood mononuclear cells (PBMCs) as well [114]. Its mechanism of action is related to the direct modification of the Nrf2 inhibitor, Keap-1 [61], activating Nrf2 and thereby protecting neurons and oligodendrocytes from oxidative damage [61], as well as to its ability to increase glutathione (GSH) levels to inhibit NF-κB translocation, thereby reducing the expression of NF-κB-dependent genes (proinflammatory cytokines, chemokines, and adhesion molecules) [115]. DMF inhibits leukocyte migration and thereby the infiltration of immune cells in the CNS [116,117]. It could also directly mediate a shift toward Th2 instead of Th1 differentiation [118]. In the EAE model, DMF is reported to reduce symptom severity and preserve myelin and axon density [61]. Clinical trials have demonstrated that DMF significantly reduces the number of lesions [119], the annualized relapse rate, the risk of confirmed disability progression, the new or enlarging hypertense lesions, and the gadolinium-enhanced lesions [120,121]. Since 2013, DMF has been approved by the FDA and the EMA as a first-line therapy for relapsing–remitting MS [122].
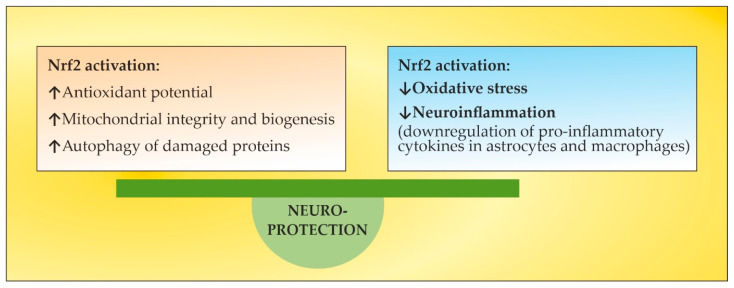
However, DMF has relatively low efficacy and specificity [10]. The clinical use of DMF has been associated with the occurrence of a number of adverse effects, such as abdominal pain, nausea, and treatment-related progressive multifocal leukoencephalopathy along with long-lasting, severe lymphocytopenia [112,123,124,125]. In a clinical trial of DMF in patients with relapsing–remitting MS, the induction of the NRF2 transcriptional target NQO1 was found to negatively correlate with the age of the patient [126]. All these disadvantages of DMF may shift the focus to the use of other approved drugs or new non-pharmacological therapeutic approaches with similar Nrf2-inducing potential. Fingolimod (a classical S1PR modulator) regulates mitochondrial OS in neuronal cells by enhancing Nrf2 activity with increased HO-1 and NQO1 gene expression [127]. Siponimod (a novel S1PR modulator) activates Nrf2 while hampering NFκB in human astrocytes, suggesting direct anti-inflammatory and antioxidant pharmacological effects [128] (Figure 4). Another clinically approved drug that has also been reported to activate Nrf2, together with other anti-inflammatory mechanisms of action, is natalizumab, an antibody targeting the cell adhesion molecule α-4 integrin [129]. The role of TPE in the context of Nrf-2 activation will be discussed below.
Figure 4.
Neuroprotective effects of Nrf2 activation. Adapted from [10] and modified.
6. The Role of NGF as an Endogenous Nrf2 Activator
NGF is the most recognized neurotrophin, and it was described in 1952 by Levi-Montalcini [130]. NGF activates two types of membrane receptors, tropomyosin receptor kinase A (TrkA) and p75 neurotrophin receptor (p75NTR) [131]. Depending on the NGF-specific receptors, signaling pathways associated with neuronal differentiation, maturation and survival, axonal and dendrite development, or apoptosis can be stimulated. When TrkA and p75NTR are co-expressed, they comprise a two-receptor heterotetrameric complex that binds to NGF, resulting in the activation of various signaling pathways [132,133].
In neurological disorders with neuroinflammation, almost all resident cells of the CNS overexpress NGF [134]. Moreover, NGF can cross the BBB when the BBB becomes permeable in pathological conditions, such as MS [135]. It is noteworthy that NGF levels influence glial physiology. There is evidence that in an in vivo mouse model [136], the depletion of NGF causes pro-inflammatory astrocyte activation and neurotoxicity. Conversely, the upregulation of NGF directs microglia to an anti-inflammatory phenotype [137], thereby leading to neuroprotection. The latter is of particular importance in the context of OS in MS, considering the role of pro-inflammatory microglia and astrocytes in the generation of ROS/RNS.
Previous studies have observed the significant effects of the intracranial administration of NGF on the downregulation of cytokines (important factors in OS) that are specific to the CNS parenchyma and not detected in the periphery [138]. In the case of MS, during acute attacks, patients show increased levels of NGF in CSF compared to healthy individuals, which can be seen as an attempt to protect the CNS tissue against inflammation [139]. There, observations imply the relevance of NGF-related therapeutic schemes in patients with inflammatory and neurodegenerative pathologies [136]. Last but not least, NGF antibodies have been observed to exacerbate the neuropathological signs of EAE [140]. This suggests not only the importance of NGF in decreasing the EAE lesions [141], but also offers new possibilities for increasing the anti-inflammatory potential of NGF in MS patients by removing these antibodies using TPE [142,143].
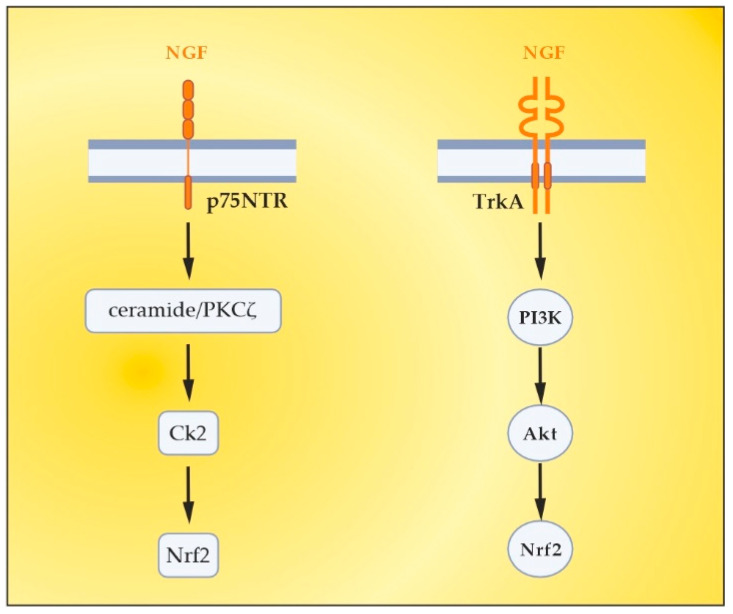
Besides the already-mentioned anti-inflammatory potential, NGF plays an important role in antioxidant protection in experimental and clinical settings. As early as the 1990s, preclinical studies outlined the role of NGF in reducing OS [144,145]. The activation of the MAPK pathway appears to be required for this action of NGF (namely acute suppression of neuronal ROS production by NGF), implicating TrkA signaling in defense against oxidative damage [145,146]. Other research from the late 1990s and the early next decade revealed the role of NGF in the upregulation of enzymatic antioxidants (SOD, HO-1, GPx, and catalase) through phosphoinositide-3-kinase (PI3K)/Akt and NF-kB signaling [22,147,148,149]. More recent studies have shown that the activation of TrkA by NGF induces the nuclear translocation of Nrf2 and subsequently induces the transcription of ARE-containing genes [150,151,152]. Moreover, the activated Nrf2 can in turn up-regulate NGF gene expression and a positive feedback loop between Nrf2 and NGF can be formed [150,151], which further strengthens the evidence for the potential of NGF as an Nrf2-inducer. New evidence suggests that NGF is able to activate p75NTR/ceramide-protein kinase C-ζ (PKCζ)/casein kinase 2 (CK2) signaling pathways through direct interaction with p75NTR in order to mediate its activation of Nrf2 [153] (Figure 5).
Figure 5.
NGF-mediated activation of Nrf2 transcriptional factor. Adapted from [143] and modified.
Most interesting in view of future clinical benefits is a recent preclinical study whose results provide evidence that the depletion of peripheral GSH pools increases peripheral circulating NGF, which orchestrates a neuroprotective response in the CNS, at least in the striatum, via the NGF/TrkA/Akt/Nrf2 pathway [154]. A plausible explanation for the role of NGF after systemic OS suggests an increased synthesis of NGF in peripheral tissues, followed by secretion and increased levels in the systemic circulation. Peripheral NGF then reaches the cerebral circulation and activates the TrkA pathway in brain endothelial cells, which induces the transcription of sulfhydryl AAs L-cys/L-cys2 transporters in neurons and glial cells. Given that brain GSH synthesis is limited by the presence of the sulfhydryl AAs L-cys and L-cys2 [155], it has been suggested that the activation of the NGF/TrkA/Akt/Nrf2 signaling pathway in the striatum may be related to the transcriptional regulation of amino acid transporter genes associated with the presence of L-cys and L-cys2 in the brain. In addition, NGF synthesis is elevated in brain endothelial cells, and NGF is secreted into the brain parenchyma, activating the NGF/TrkA/PI3K/Akt/Nrf2 pathway, which stimulates the expression of antioxidant genes in the CNS. Another possibility is that peripheral NGF enters the CNS directly, as suggested in the 1980s by Levi-Montalcini [156], which may occur with a permeable BBB, as is the case in acute exacerbations of MS [135,143]. Translating the results of this preclinical model to the real clinical setting is achievable through the application of TPE in the treatment of acute exacerbations of MS. Through this non-pharmacological therapeutic approach, both an increase in circulating levels of NGF and a modulation of OS can be achieved [17,143]. Evidence for this will be presented in the next section.
7. The Role of TPE as an OS Modulator
TPE is a therapeutic approach that involves extracorporeal blood removal, as well as an exchange of blood plasma or components. It basically removes a significant volume of plasma (1 to 1.5 of the patient’s total plasma volume (TPV) per treatment) which is accompanied with corresponding volume replacement using colloid solutions or a combination of crystalloid/colloid solutions [157]. The aim of TPE is to remove potentially pathogenic substances with high molecular weight (>150 kDA), involving autoantibodies, pro-inflammatory mediators, lipids, and other molecules or molecular fragments from the intravascular space [157,158]. However, the mechanism of TPE action in inflammatory and neurodegenerative disorders involves more than just a removal of potentially a number of pathogenic molecules. It should be noted that TPE may also modulate cellular immunological response by changing the ratio between T helper type-1 (Th-1) and type-2 (Th-2) cells. Th-2 cells regulate the humoral immune response by facilitating B-cell autoantibody production and play an important role in the development of neurodegenerative autoimmune pathologies. By shifting the balance between peripheral T cells from Th-2 to Th-1 predominance, TPE modulates the pathological immune response and thus might induce a beneficial effect [159].
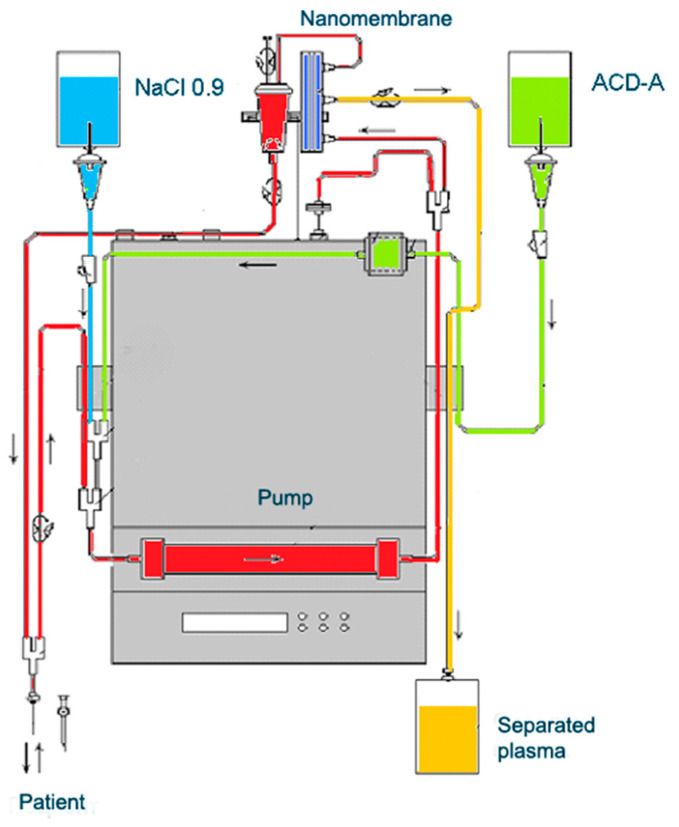
Our experience in the TPE treatment of MS exclusively involves the use of nanomembrane-based TPE (Figure 6) [17]. This innovative method involves passage of the patient’s blood through nanomembranes designed to filter out definite molecules [18,142,160,161,162,163,164]. There are pores in the nanomembrane with a diameter of 30–50 nm, which allow the filtration of molecules with a molecular weight below 40 kDa. The device has a volume of up to 70 mL [165]. The most widely used replacement is saline (NaCl 0.9), which is cheap and has no adverse effects, even when 25% of the circulating plasma is replaced [162]. The use of saline to replace the removed plasma is in agreement with the so-called low-volume plasma exchange (LVPE), which ranges from 350 mL to 2 l plasma volume removal for each separate procedure [166,167,168,169].
Figure 6.
Diagram of nanomembrane-based TPE device.
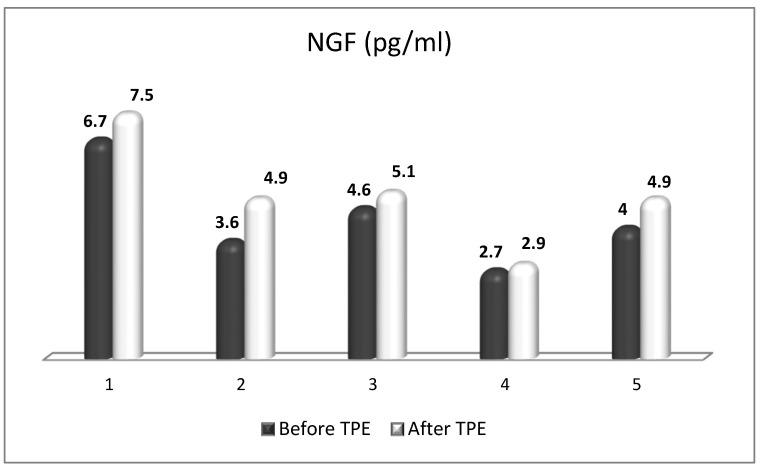
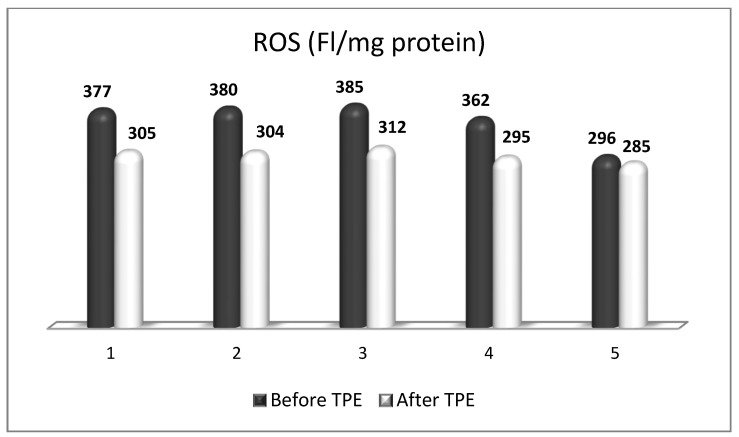
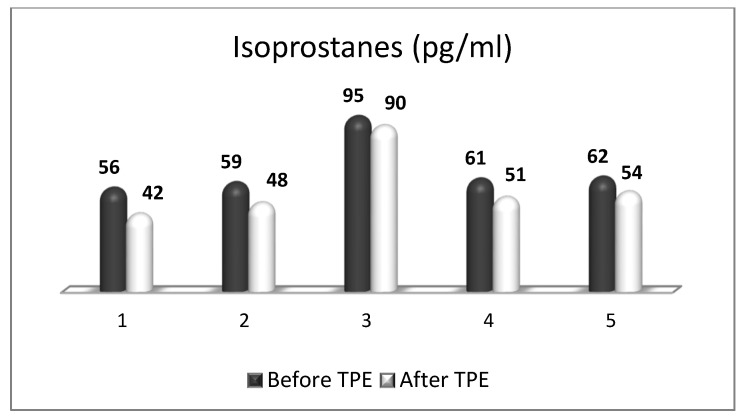
We performed a nanomembrane-based TPE treatment in steroid-refractory MS in five patients with a relapsing–remitting MS [18,143,164]. Most patients received three TPE procedures in LVPE mode (patients 1, 2, 3, 5), whereas one of them received four nanomembrane-based TPE procedures in LVPE mode (patient 4). We observed an elevation in NGF plasma levels in all patients (Figure 7) [143]. The measurement of OS markers (ROS and isoprostanes) showed that the application of TPE leads to a reduction in the OS level (Figure 8 and Figure 9) [17], which is favorable for the general and neurological status of patients.
Figure 7.
Changes in the level of NGF of patients (1, 2, 3, 4, 5) with MS before and after TPE.
Figure 8.
Changes in the ROS content of patients (1, 2, 3, 4, 5) with MS before and after TPE. (FI/mg protein = fluorescence intensity/mg protein).
Figure 9.
Changes in the level of isoprostanes of patients (1, 2, 3, 4, 5) with MS before and after TPE (pg/mL).
It has been found that TPEs, mainly in patients with a lower degree of disability (Kurtzke EDSS ≤ 5 points for the RR form of MS [19], i.e., with predominant inflammation in the relapse occurring, not neurodegenerative), have a relatively favorable effect on clinical symptoms, which leads to an overall stabilization in the course of the disease. A significant decrease of 0.5 points on Kurtzke EDSS was assumed to establish the responder rate [168]. Only one patient (patient 3) had no improvement in the evaluated clinical disability (Kurtzke EDSS before TPE 8.5 points, after TPE 8.5 points). In these cases, also related to measurement of the OS before and after TPE [18,164], an improvement (according to Kurtzke EDSS, Table 1 [17]) of about 80% was found, which was consistent with the 74% improvement in the same type of patients (the relapsing–remitting form of MS) published by other authors [170].
Table 1.
The effect of plasmapheresis on OS (ROS, isoprostanes) and disability scores (Kurtzke EDSS) in patients with MS (ROS—reactive oxygen species; EDSS—expanded disability status scale).
| Patient Number | ROS (FI/mg Protein) | Isoprostanes (pg/mL) | Kurtzke EDSS | |||
|---|---|---|---|---|---|---|
| Before TPE | After TPE | Before TPE | After TPE | Before TPE | After TPE | |
| 1. | 377 | 305 | 56 | 42 | 6.5 | 6.0 |
| 2. | 380 | 304 | 59 | 48 | 6.5 | 6.0 |
| 3. | 385 | 312 | 95 | 90 | 8.5 | 8.5 |
| 4. | 362 | 295 | 61 | 51 | 3.0 | 2.5 |
| 5. | 296 | 285 | 62 | 54 | 6.0 | 5.5 |
TPE is presumed to affect NGF in many different ways. The most probable explanation for the reported increase in NGF levels in the plasma of our MS patients could be related to the removal of autoantibodies against NGF-producing cells and NGF itself [143]. The elevated NGF content after TPE could either contribute to or occur as a consequence of augmented NGF levels in the CNS (considering the NGF’s ability to pass the BBB in accordance with its gradient) [135]. Whatever the explanation, the observed results can be considered as a beneficial anti-inflammatory effect induced by the decreased inflammatory level (autoantibodies, immune complexes, cytokines, etc.) related to discarded plasma.
In addition, we observed a decrease in the plasma levels of sphingosine-1-phosphate (S1P) due to its direct loss with the discarded plasma as well [143]. It is believed that S1P has antioxidant effects that occur by potentiating the antioxidant enzymes SOD and catalase [171]. Thus, by reducing the S1P-like systemic GSH depletion mentioned above, we may postulate another possible mechanism in order to explain the elevated circulatory levels of NGF found by others in preclinical settings [154]. Long before us, however, other authors investigated the parameters of OS after low-density lipid apheresis and found an increase in plasma GSH levels [172]. While GSH can be removed via hemodialysis due to its low molecular weight, this is clearly not valid for low-density lipid apheresis [172]. The particular disease for which the corresponding blood purification technique is applied really matters.
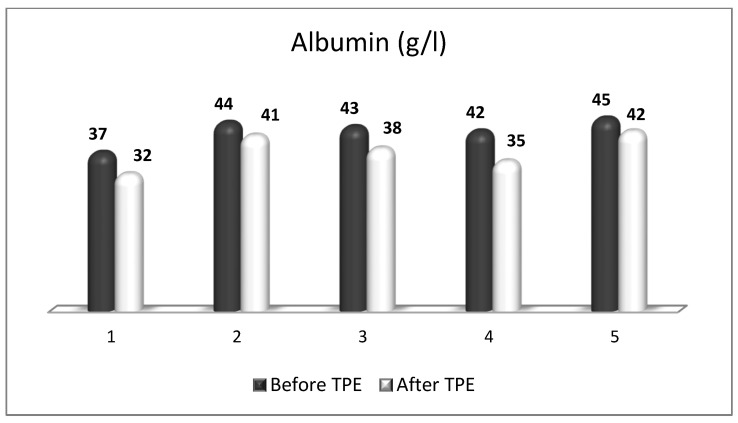
There is another factor that may influence OS during TPE treatment in a context-dependent manner. In the field of neuroscience, the level of plasma albumin is mainly studied as a measure of OS at different levels of disease activity [173]. Consistent with this, a recent meta-analysis identified hypoalbuminemia in MS as strong clinical evidence of increased OS [173]. However, there are controversial data on cohorts without hypoalbuminemia in MS patients as well [174,175]. So far, there is only one study that longitudinally analyzed the effect of plasmapheresis or immunoabsorption (IA) on albumin quantity and redox state in patients with autoimmune-mediated neurological disorders [176]. The study showed that plasmapheresis induced a severe and long-lasting change in endogenous irreversibly oxidized albumin levels during the course of treatment and 12 days after the last plasmapheresis, which was not the case in patients treated with IA [176]. Nevertheless, they found a mean decrease in albumin concentration of about 4.7 g/l during one IA treatment with the proportion of increased irreversibly oxidized albumin over five IA treatments within the normal range [176]. It should be noted that IA is an apheresis technique where commercial albumin is not administered as a replacement fluid (just as is the case with our nanomembrane-based TPE). Interestingly, in our five MS patients presented above (all with reduced OS, Figure 7 and Figure 8), we found almost the same mean decrease in albumin concentration of about 4.6 g/l during a single treatment with nanomembrane TPE (Figure 10) [177]. Different apheresis techniques probably matter. As for the difficult question of to what extent the technological improvement in the apheresis membrane could improve outcomes, only future large-scale studies could provide an evidence-based answer.
Figure 10.
Changes in the level of albumin (normal range 35–50) of patients (1, 2, 3, 4, 5) with MS before and after TPE (g/l).
8. Summary and Conclusions
Redox homeostasis in the brain is controlled by Nrf2, and we have witnessed increasing interest in identifying natural or synthetic compounds to modify Nrf2 activity [74]. However, their application is associated with some significant limitations, such as poor solubility, low bioavailability, and dose-dependent pro-oxidant activity [66]. Basically, Nrf2 activation plays a beneficial role under physiological conditions, but it promotes cancer development and metastasis, and also anticancer drug resistance after cancer is established [3]. In addition, exogenous antioxidants are also susceptible to oxidation, and therefore, their use as foods or supplements should be carefully considered [178]. The administration of high doses of antioxidants is considered to potentially expose the body to additional risks, as there is increasing evidence of some harmful effects, such as the risk of cancer, involving Nrf2 activation [66]. An important limitation in taking drugs with proven anti-inflammatory and antioxidant effects is their limited therapeutic range and safety profile. For example, corticosteroids are established as first-line drugs for the treatment of acute demyelinating attacks/exacerbations of MS [17,143,179,180]. Antioxidant effects have been established in MS patients, such as increased SOD activity and decreased ROS and MDA levels after methylprednisolone treatment [47,181,182]. However, if patients do not respond to corticosteroid therapy, which occurs in 20–25% of MS cases, a second corticosteroid pulse therapy in combination with TPE is recommended after an interval of 10–14 days [169,180,183], or switching to TPE entirely when the adverse effects of corticosteroids are presented. The latter would have its clinical rationale in terms of affecting OS as well. The same applies to DMF in the event of progressive multifocal leukoencephalopathy, severe lymphocytopenia, or a faster depletion of its antioxidant effect, as is the case in elderly patients [112,123,124,125,126].
TPE is an effective and relatively safe method that can be applied in the treatment of neurodegenerative diseases [17,184]. However, the use of TPE in the management of acute relapsing MS is still modest [184] and also has inherent limitations in terms of local specificities related to the population under investigation, experience, availability, and insurance coverage [17,165].
The most important limitations and advantages of the various Nrf2 activators are summarized in Table 2 [10,17,112,123,124,125,150,151,152,153,165,185,186].
Table 2.
Limitations and advantages of different Nrf2 activators.
| Nrf2 Activators | Limitations | Advantages |
|---|---|---|
| Natural exogenous | Poor drug solubility Low oral bioavailability Increased first-pass metabolism Quick biotransformation and elimination Low plasma concentrations Non-specific effects (can act on other signaling pathways, especially at high doses) |
Ingestion with food Available as dietary supplements Affordable due to fair price Increasing clinical trial experience for the treatment of neurodegenerative and neuro-inflammatory CNS disorders (including MS) |
| Synthetic exogenous | Lymphocytopenia, leukoencephalopathy | Clinically approved in MS treatment |
| Endogenous | Experimental preclinical evidence Preliminary investigational clinical evidence Less affordable due to high price of TPE |
No need for pharmacokinetic (drug delivery) optimization Antioxidant, anti-inflammatory, and immunomodulatory rapid clinical improvement in MS patients |
Nrf2 is reported to either suppress or induce inflammation and immunity in a cell-type- and disease-dependent manner [69]. Nrf2 activation alters the balance between Th1/Th2 in preclinical disease models by altering Th1-driven responses and shifts T helper lymphocytes toward Th2 differentiation. On the other hand, the increased production of Nrf2 in macrophages downregulates the expression of inflammatory genes through inhibition of the NF-kB signaling pathway [69]. TPE can alter the immunological response of cells as well, however, only by shifting the Th1/Th2 balance toward Th-1 predominance [159] along with a decrease in OS and pro-inflammatory processes [143]. To what extent the combined approach of the administration of exogenous activators, together with the application of TPE and the resulting endogenous stimulation of Nrf2, may contribute to a better clinical response in MS will be the subject of future profound investigations.
In conclusion, our preliminary findings from the shared clinical experience with nanomembrane-based TPEs outline new perspectives in OS modulation. The increased plasma levels of NGF in response to TPE treatment of MS patients suggest their antioxidant potential for endogenous Nrf2 enhancement via NGF/TrkA/PI3K/Akt and NGF/p75NTR/ceramide-PKCζ/CK2 signaling pathways.
Author Contributions
Both authors (D.T. and A.M.) made corresponding contributions to the conception, methodology, results interpretation, and drafting the manuscript. Both authors (D.T. and A.M.) agreed to be accountable for all aspects of the work and gave their approval of this version to be published. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available under request to corresponding author Dimitar Tonev (dgtsofia@abv.bg).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was financially supported by the Bulgarian Ministry of Education and Science: Contract DO1-154/28/08/2018/Scientific Infrastructure in Cell Technologies in Biomedicine (SICTB); agreement DO1-178/2022.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lublin F.D., Reingold S.C., Cohen J.A., Cutter G.R., Sørensen P.S., Thompson A.J., Wolinsky J.S., Balcer L.J., Banwell B., Barkhof F., et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H., Brück W., Lucchinetti C.F. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R., Liang L., Matsumoto M., Iwata K., Umemura A., He F. Reactive Oxygen Species and NRF2 Signaling, Friends or Foes in Cancer? Biomolecules. 2023;13:353. doi: 10.3390/biom13020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 5.Adamczyk B., Adamczyk-Sowa M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxid. Med. Cell Longev. 2016;2016:1973834. doi: 10.1155/2016/1973834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado P.P., Guevara C., Olesen M.A., Orellana J.A., Quintanilla R.A., Ortiz F.C. Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways. Antioxidants. 2022;11:1146. doi: 10.3390/antiox11061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan L., Johnson J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta. 2014;1842:1208–1218. doi: 10.1016/j.bbadis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Amoroso R., Maccallini C., Bellezza I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants. 2023;12:778. doi: 10.3390/antiox12030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova A.T., Kostov R.V., Kazantsev A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Guo C., Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012;7:376. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004;10:18. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 14.Maes M., Mihaylova I., Kubera M., Leunis J.C., Geffard M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: New pathways that underpin the inflammatory and neuroprogressive pathophysiology. J. Affect. Disord. 2011;135:414–418. doi: 10.1016/j.jad.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Maes M., Kubera M., Mihaylova I., Geffard M., Galecki P., Leunis J.C., Berk M. Increased autoimmune responses against autoepitopes modified by oxidative and nitrosative damage in depression: Implications for the pathways to chronic depression and neuroprogression. J. Affect. Disord. 2013;149:23–29. doi: 10.1016/j.jad.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Nunes S., Vargas H., Prado E., Barbosa D., de Melo L., Moylan S. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci. Biobehav. Rev. 2013;37:1336–1345. doi: 10.1016/j.neubiorev.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Tonev D.G., Momchilova A.B. Therapeutic Plasma Exchange in Certain Immune-Mediated Neurological Disorders: Focus on a Novel Nanomembrane-Based Technology. Biomedicines. 2023;11:328. doi: 10.3390/biomedicines11020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsonchev Z., Alexandrov A., Momchilova A., Pankov R., Orozova M., Georgieva R., Georgiev S., Alexandrov S., Voinov V., Anaya F., et al. Therapeutic Apheresis with Nanotechnology Membrane for Human Diseases. Volume 2. Bulgarian Academy of Science Prof. Marin Drinov Publishing House; Sofia, Bulgaria: 2020. pp. 1–163. [Google Scholar]
- 19.Ljubisavljevic S., Stojanovic I., Cvetkovic T., Vojinovic S., Stojanov D., Stefanovic N., Pavlovic D. Erythrocytes’ antioxidative capacity as a potential marker of oxidative stress intensity in neuroinflammation. J. Neurol. Sci. 2014;337:8–13. doi: 10.1016/j.jns.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Pentón-Rol G., Cervantes-Llanos M., Martínez-Sánchez G., Cabrera-Gómez J.A., Valenzuela-Silva C.M., Ramírez-Nuñez O., Casanova-Orta M., Robinson-Agramonte M.A., Lopategui-Cabezas I., López-Saura P.A. TNF-α and IL-10 downregulation and marked oxidative stress in Neuromyelitis Optica. J. Inflamm. 2009;6:18. doi: 10.1186/1476-9255-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jana A., Pahan K. Oxidative Stress Kills Human Primary Oligodendrocytes Via Neutral Sphingomyelinase: Implications for Multiple Sclerosis. J. Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bono S., Feligioni M., Corbo M. Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Mol. Neurodegener. 2021;16:71. doi: 10.1186/s13024-021-00479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahad D., Lassmann H., Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008;34:577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer M.T., Sharma R., Lim J.L., Haider L., Frischer J.M., Drexhage J., Mahad D., Bradl M., van Horssen J., Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haider L., Fischer M.T., Frischer J.M., Bauer J., Höftberger R., Botond G., Esterbauer H., Binder C.J., Witztum J.L., Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz G.G., Pacheco-Moisés F.P., Bitzer-Quintero O.K., Ramírez-Anguiano A.C., Flores-Alvarado L.J., Ramírez-Ramírez V., Macias-Islas M.A., Torres-Sánchez E.D. Immunology and Oxidative Stress in Multiple Sclerosis: Clinical and Basic Approach. Clin. Dev. Immunol. 2013;2013:708659. doi: 10.1155/2013/708659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amorini A.M., Petzold A., Tavazzi B., Eikelenboom J., Keir G., Belli A., Giovannoni G., Di Pietro V., Polman C., D’Urso S., et al. Increase of uric acid and purine compounds in biological fluids of multiple sclerosis patients. Clin. Biochem. 2009;42:1001–1006. doi: 10.1016/j.clinbiochem.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Bjartmar C., Trapp B.D. Axonal and neuronal degeneration in multiple sclerosis: Mechanisms and functional consequences. Curr. Opin. Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 29.van der Goes A., Wouters D., van der Pol S.M.A., Huizinga R., Ronken E., Adamson P., Greenwood J., Dijkstra C.D., de Vries H.E. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- 30.Schreibelt G., Musters R.J.P., Reijerkerk A., de Groot L.R., van der Pol S.M.A., Hendrikx E.M.L., Döpp E.D., Dijkstra C.D., Drukarch B., de Vries H.E. Lipoic Acid Affects Cellular Migration into the Central Nervous System and Stabilizes Blood-Brain Barrier Integrity. J. Immunol. 2006;177:2630–2637. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- 31.van der Goes A., Brouwer J., Hoekstra K., Roos D., Berg T.K.V.D., Dijkstra C.D. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J. Neuroimmunol. 1998;92:67–75. doi: 10.1016/S0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 32.van Meeteren M.E., Hendriks J., Dijkstra C.D., van Tol E.A. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem. Pharmacol. 2004;67:967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Hendriks J.J., Teunissen C.E., de Vries H.E., Dijkstra C.D. Macrophages and neurodegeneration. Brain Res. Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Gonsette R.E. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voet S., Prinz M., van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 2019;25:112–123. doi: 10.1016/j.molmed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Linnerbauer M., Wheeler M.A., Quintana F.J. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobore T.O. Oxidative/Nitroxidative stress and multiple sclerosis. J. Mol. Neurosci. 2021;71:506–514. doi: 10.1007/s12031-020-01672-y. [DOI] [PubMed] [Google Scholar]
- 40.Pegoretti V., Swanson K.A., Bethea J.R., Probert L., Eisel U., Fischer R. Inflammation and oxidative stress in multiple sclerosis: Consequences for therapy development. Oxid. Med. Cell. Longev. 2020;2020:7191080. doi: 10.1155/2020/7191080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varas R., Ortiz F.C. Neuroinflammation in demyelinating diseases: Oxidative stress as a modulator of glial Cross-Talk. Curr. Pharm. Des. 2019;25:4755–4762. doi: 10.2174/1381612825666191216125725. [DOI] [PubMed] [Google Scholar]
- 42.Milatovic D., Zaja-Milatovic S., Gupta R.C., Yu Y., Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halliwell B., Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993;57:715S–724S; discussion 724S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B. Lipid peroxidation, antioxidants and cardiovascular disease: How should we move forward? Cardiovasc. Res. 2000;47:410–418. doi: 10.1016/S0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 45.Teunissen C.E., Sombekke M., van Winsen L., Killestein J., Barkhof F., Polman C.H., Dijkstra C.D., Blankenstein M.A., Pratico D. Increased plasma 8,12-iso-iPF2alpha- VI levels in relapsing multiple sclerosis patients are not predictive of disease progression. Mult. Scler. 2012;18:1092–1098. doi: 10.1177/1352458511433306. [DOI] [PubMed] [Google Scholar]
- 46.Miller E., Mrowicka M., Saluk-Juszczak J., Ireneusz M. The level of isoprostanes as a non-invasive marker for in vivo lipid peroxidation in secondary progressive multiple sclerosis. Neurochem. Res. 2011;36:1012–1016. doi: 10.1007/s11064-011-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibitoye R., Kemp K., Rice C., Hares K., Scolding N., Wilkins A. Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark. Med. 2016;10:889–902. doi: 10.2217/bmm-2016-0097. [DOI] [PubMed] [Google Scholar]
- 48.Liddell J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants. 2017;6:65. doi: 10.3390/antiox6030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsuoka F., Yamamoto M. Small Maf proteins (MafF, MafG, MafK): History, structure and function. Gene. 2016;586:197–205. doi: 10.1016/j.gene.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carvalho A.N., Firuzi O., Gama M.J., Horssen J.V., Saso L. Oxidative Stress and Antioxidants in Neurological Diseases: Is There Still Hope? Curr. Drug Targets. 2017;18:705–718. doi: 10.2174/1389450117666160401120514. [DOI] [PubMed] [Google Scholar]
- 52.Ohl K., Tenbrock K., Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016;277:58–67. doi: 10.1016/j.expneurol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cores A., Piquero M., Villacampa M., León R., Menéndez J.C. NRF2 regulation processes as a source of potential drug targets against neurodegenerative diseases. Biomolecules. 2020;10:904. doi: 10.3390/biom10060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cores Á., Carmona-Zafra N., Clerigué J., Villacampa M., Menéndez J.C. Quinones as Neuroprotective Agents. Antioxidants. 2023;12:1464. doi: 10.3390/antiox12071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Licht-Mayer S., Wimmer I., Traffehn S., Metz I., Brück W., Bauer J., Bradl M., Lassmann H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. 2015;130:263–277. doi: 10.1007/s00401-015-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Horssen J., Drexhage J.A., Flor T., Gerritsen W., van der Valk P., de Vries H.E. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic. Biol. Med. 2010;49:1283–1289. doi: 10.1016/j.freeradbiomed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Lee D.H., Gold R., Linker R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: Therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012;13:11783–11803. doi: 10.3390/ijms130911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J.M., Li J., Johnson D.A., Stein T.D., Kraft A.D., Calkins M.J., Jakel R.J., Johnson J.A. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 59.Johnson D.A., Amirahmadi S., Ward C., Fabry Z., Johnson J.A. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol. Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larabee C.M., Desai S., Agasing A., Georgescu C., Wren J.D., Axtell R.C., Plafker S.M. Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis. Mol. Vis. 2016;22:1503–1513. [PMC free article] [PubMed] [Google Scholar]
- 61.Linker R.A., Lee D.H., Ryan S., van Dam A.M., Conrad R., Bista P., Zeng W., Hronowsky X., Buko A., Chollate S., et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 62.Juurlink B.H., Thorburne S.K., Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(SICI)1098-1136(199804)22:4<371::AID-GLIA6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Zgorzynska E., Dziedzic B., Walczewska A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021;22:9592. doi: 10.3390/ijms22179592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winyard P.G., Blake D.R. Antioxidants, redox-regulated transcription factors, and inflammation. Adv. Pharmacol. 1997;38:403–421. doi: 10.1016/s1054-3589(08)60993-x. [DOI] [PubMed] [Google Scholar]
- 65.Liddell J.R. Interplay between Nrf2 and NF-κB in Neuroinflammatory Diseases. J. Clin. Cell. Immunol. 2017;8:489. doi: 10.4172/2155-9899.1000489. [DOI] [Google Scholar]
- 66.Michaličková D., Hrnčíř T., Canová N.K., Slanař O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 2020;873:172973. doi: 10.1016/j.ejphar.2020.172973. [DOI] [PubMed] [Google Scholar]
- 67.Schluesener H.J., Seid K. Heme oxygenase-1 in lesions of rat experimental autoimmune encephalomyelitis and neuritis. J. Neuroimmunol. 2000;110:114–120. doi: 10.1016/S0165-5728(00)00352-0. [DOI] [PubMed] [Google Scholar]
- 68.Fagone P., Patti F., Mangano K., Mammana S., Coco M., Touil-Boukoffa C., Chikovani T., Di Marco R., Nicoletti F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neuroimmunol. 2013;261:82–86. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 69.He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X., Guo J., Wei X., Niu C., Jia M., Li Q., Meng D. Bach1: Function, Regulation, and Involvement in Disease. Oxid. Med. Cell Longev. 2018;2018:1347969. doi: 10.1155/2018/1347969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeh J.H., Chen W.H., Chiu H.C., Bai C.H. Hemolysis in double-filtration plasmapheresis. Am. J. Clin. Pathol. 2007;127:76–80. doi: 10.1309/F8XMTALTTCE5H37U. [DOI] [PubMed] [Google Scholar]
- 72.Srinivas D., Kamath Sriganesh K., Chakrabarti D., Venkateswaran P. Effect of Therapeutic Plasma Exchange on Plasma Constituents in Neurointensive Care Unit Patients: A Retrospective Study. J. Neuroanaesthesiol. Crit. Care. 2021;8:197–202. doi: 10.1055/s-0041-1734412. [DOI] [Google Scholar]
- 73.Ameri K., Harris A.L. Activating transcription factor 4. Int. J. Biochem. Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Paladino S., Conte A., Caggiano R., Pierantoni G.M., Faraonio R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell Physiol. Biochem. 2018;47:1951–1976. doi: 10.1159/000491465. [DOI] [PubMed] [Google Scholar]
- 75.Gao S., Duan X., Wang X., Dong D., Liu D., Li X., Sun G., Li B. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem. Toxicol. 2013;59:739–747. doi: 10.1016/j.fct.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 76.Sahin K., Orhan C., Tuzcu Z., Tuzcu M., Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012;50:4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Zeng C., Zhong P., Zhao Y., Kanchana K., Zhang Y., Khan Z.A., Chakrabarti S., Wu L., Wang J., Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell Cardiol. 2015;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 79.Xie L., Li X.K., Funeshima-Fuji N., Kimura H., Matsumoto Y., Isaka Y., Takahara S. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int. Immunopharmacol. 2009;9:575–581. doi: 10.1016/j.intimp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 80.Kanakasabai S., Casalini E., Walline C.C., Mo C., Chearwae W., Bright J.J. Differential regulation of CD4(+) T helper cell responses by curcumin in experimental autoimmune encephalomyelitis. J. Nutr. Biochem. 2012;23:1498–1507. doi: 10.1016/j.jnutbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Mohajeri M., Sadeghizadeh M., Najafi F., Javan M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology. 2015;99:156–167. doi: 10.1016/j.neuropharm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Singh N.P., Hegde V.L., Hofseth L.J., Nagarkatti M., Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol. Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imler T.J., Jr., Petro T.M. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(−) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int. Immunopharmacol. 2009;9:134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 84.Gandy K.A.O., Zhang J., Nagarkatti P., Nagarkatti M. Resveratrol (3,5,4’-Trihydroxy-trans-Stilbene) Attenuates a Mouse Model of Multiple Sclerosis by Altering the miR-124/Sphingosine Kinase 1 Axis in Encephalitogenic T Cells in the Brain. J. Neuroimmune Pharmacol. 2019;14:462–477. doi: 10.1007/s11481-019-09842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghaiad H.R., Nooh M.M., El-Sawalhi M.M., Shaheen A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017;54:3219–3229. doi: 10.1007/s12035-016-9891-5. [DOI] [PubMed] [Google Scholar]
- 86.Sato F., Martinez N.E., Shahid M., Rose J.W., Carlson N.G., Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am. J. Pathol. 2013;183:1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moussa C., Hebron M., Huang X., Ahn J., Rissman R.A., Aisen P.S., Turner R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017;14:1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yiu E.M., Tai G., Peverill R.E., Lee K.J., Croft K.D., Mori T.A., Scheiber-Mojdehkar B., Sturm B., Praschberger M., Vogel A.P., et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015;262:1344–1353. doi: 10.1007/s00415-015-7719-2. [DOI] [PubMed] [Google Scholar]
- 89.Aktas O., Prozorovski T., Smorodchenko A., Savaskan N.E., Lauster R., Kloetzel P.M., Infante-Duarte C., Brocke S., Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 90.Herges K., Millward J.M., Hentschel N., Infante-Duarte C., Aktas O., Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE. 2011;6:e25456. doi: 10.1371/journal.pone.0025456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen A., Fiebiger S., Bros H., Hertwig L., Romero-Suarez S., Hamann I., Chanvillard C., Bellmann-Strobl J., Paul F., Millward J.M., et al. Treatment of Chronic Experimental Autoimmune Encephalomyelitis with Epigallocatechin-3-Gallate and Glatiramer Acetate Alters Expression of Heme-Oxygenase-1. PLoS ONE. 2015;10:e0130251. doi: 10.1371/journal.pone.0130251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semnani M., Mashayekhi F., Azarnia M., Salehi Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017;55:199–205. doi: 10.5114/fn.2017.70484. [DOI] [PubMed] [Google Scholar]
- 93.Sun Q., Zheng Y., Zhang X., Hu X., Wang Y., Zhang S., Zhang D., Nie H. Novel immunoregulatory properties of EGCG on reducing inflammation in EAE. Front. Biosci. 2013;18:332–342. doi: 10.2741/4104. [DOI] [PubMed] [Google Scholar]
- 94.Wang J., Pae M., Meydani S.N., Wu D. Green tea epigallocatechin-3-gallate modulates differentiation of naïve CD4⁺ T cells into specific lineage effector cells. J. Mol. Med. 2013;91:485–495. doi: 10.1007/s00109-012-0964-2. [DOI] [PubMed] [Google Scholar]
- 95.Wu D. Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis. J. Investig. Med. 2016;64:1213–1219. doi: 10.1136/jim-2016-000158. [DOI] [PubMed] [Google Scholar]
- 96.Chesser A.S., Ganeshan V., Yang J., Johnson G.V. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016;19:21–31. doi: 10.1179/1476830515Y.0000000038. [DOI] [PubMed] [Google Scholar]
- 97.Bai Q., Lyu Z., Yang X., Pan Z., Lou J., Dong T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav. Brain. Res. 2017;321:79–86. doi: 10.1016/j.bbr.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 98.Boddupalli S., Mein J.R., Lakkanna S., James D.R. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: Perspectives in maintaining the antioxidant activity of vitamins a, C, and e. Front. Genet. 2012;3:7. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Houghton C.A., Fassett R.G., Coombes J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016;2016:7857186. doi: 10.1155/2016/7857186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Innamorato N.G., Rojo A.I., García-Yagüe A.J., Yamamoto M., de Ceballos M.L., Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 101.Li B., Cui W., Liu J., Li R., Liu Q., Xie X.H., Ge X.L., Zhang J., Song X.J., Wang Y., et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013;250:239–249. doi: 10.1016/j.expneurol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Yoo I.H., Kim M.J., Kim J., Sung J.J., Park S.T., Ahn S.W. The Anti-Inflammatory effect of sulforaphane in mice with experimental autoimmune encephalomyelitis. J. Korean Med. Sci. 2019;34:e197. doi: 10.3346/jkms.2019.34.e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hardeland R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019;20:1223. doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galano A., Tan D.X., Reiter R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules. 2018;23:530. doi: 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng Y., Zhu J., Mi C., Xu B., Jiao C., Li Y., Xu D., Liu W., Xu Z. Melatonin antagonizes Mn-induced oxidative injury through the activation of keap1-Nrf2-ARE signaling pathway in the striatum of mice. Neurotox. Res. 2015;27:156–171. doi: 10.1007/s12640-014-9489-5. [DOI] [PubMed] [Google Scholar]
- 106.Ding K., Wang H., Xu J., Li T., Zhang L., Ding Y., Zhu L., He J., Zhou M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 107.Negi G., Kumar A., Sharma S.S. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on NF-κB and Nrf2 cascades. J. Pineal. Res. 2011;50:124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 108.Long T., Yang Y., Peng L., Li Z. Neuroprotective Effects of Melatonin on Experimental Allergic Encephalomyelitis Mice Via Anti-Oxidative Stress Activity. J. Mol. Neurosci. 2018;64:233–241. doi: 10.1007/s12031-017-1022-x. [DOI] [PubMed] [Google Scholar]
- 109.Miller E., Walczak A., Majsterek I., Kędziora J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J. Neuroimmunol. 2013;257:97–101. doi: 10.1016/j.jneuroim.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Pareek T.K., Belkadi A., Kesavapany S., Zaremba A., Loh S.L., Bai L., Cohen M.L., Meyer C., Liby K.T., Miller R.H., et al. Triterpenoid modulation of IL-17 and Nrf-2 expression ameliorates neuroinflammation and promotes remyelination in autoimmune encephalomyelitis. Sci. Rep. 2011;1:201. doi: 10.1038/srep00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei H.J., Pareek T.K., Liu Q., Letterio J.J. A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE. Sci. Rep. 2017;7:9886. doi: 10.1038/s41598-017-06907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mrowietz U., Morrison P.J., Suhrkamp I., Kumanova M., Clement B. The Pharmacokinetics of Fumaric Acid Esters Reveal Their In Vivo Effects. Trends Pharmacol. Sci. 2018;39:1–12. doi: 10.1016/j.tips.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Lin S.X., Lisi L., Dello Russo C., Polak P.E., Sharp A., Weinberg G., Kalinin S., Feinstein D.L. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3:e00055. doi: 10.1042/AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gopal S., Mikulskis A., Gold R., Fox R.J., Dawson K.T., Amaravadi L. Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies. Mult. Scler. 2017;23:1875–1883. doi: 10.1177/1352458517690617. [DOI] [PubMed] [Google Scholar]
- 115.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., León R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Dehmel T., Döbert M., Pankratz S., Leussink V.I., Hartung H.P., Wiendl H., Kieseier B.C. Monomethylfumarate reduces in vitro migration of mononuclear cells. Neurol. Sci. 2014;35:1121–1125. doi: 10.1007/s10072-014-1663-2. [DOI] [PubMed] [Google Scholar]
- 117.Rubant S.A., Ludwig R.J., Diehl S., Hardt K., Kaufmann R., Pfeilschifter J.M., Boehncke W.H. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J. Investig. Dermatol. 2008;128:326–331. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- 118.Litjens N.H., Rademaker M., Ravensbergen B., Rea D., van der Plas M.J., Thio B., Walding A., van Dissel J.T., Nibbering P.H. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur. J. Immunol. 2004;34:565–575. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- 119.Schimrigk S., Brune N., Hellwig K., Lukas C., Bellenberg B., Rieks M., Hoffmann V., Pöhlau D., Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: An open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006;13:604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 120.Fox R.J., Miller D.H., Phillips J.T., Hutchinson M., Havrdova E., Kita M., Yang M., Raghupathi K., Novas M., Sweetser M.T., et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 121.Gold R., Kappos L., Arnold D.L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M.T., Yang M., Sheikh S.I., et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 122.Xu Z., Zhang F., Sun F., Gu K., Dong S., He D. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst. Rev. 2015;4:CD011076. doi: 10.1002/14651858.CD011076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ermis U., Weis J., Schulz J.B. PML in a patient treated with fumaric acid. N. Engl. J. Med. 2013;368:1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- 124.Nieuwkamp D.J., Murk J.L., van Oosten B.W., Cremers C.H., Killestein J., Viveen M.C., Van Hecke W., Frijlink D.W., Wattjes M.P., PML in Dutch MS Patients Consortium PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N. Engl. J. Med. 2015;372:1474–1476. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 125.Rosenkranz T., Novas M., Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N. Engl. J. Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 126.Hammer A., Waschbisch A., Kuhbandner K., Bayas A., Lee D.H., Duscha A., Haghikia A., Gold. R., Linker R.A. The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann. Clin. Transl. Neurol. 2018;5:668–676. doi: 10.1002/acn3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martín-Montañez E., Pavia J., Valverde N., Boraldi F., Lara E., Oliver B., Hurtado-Guerrero I., Fernandez O., Garcia-Fernandez M. The S1P mimetic fingolimod phosphate regulates mitochondrial oxidative stress in neuronal cells. Free Radic. Biol. Med. 2019;137:116–130. doi: 10.1016/j.freeradbiomed.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 128.Colombo E., Bassani C., De Angelis A., Ruffini F., Ottoboni L., Comi G., Martino G., Farina C. Siponimod (BAF312) Activates Nrf2 While Hampering NFκB in Human Astrocytes, and Protects from Astrocyte-Induced Neurodegeneration. Front. Immunol. 2020;11:635. doi: 10.3389/fimmu.2020.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tasset I., Bahamonde C., Agüera E., Conde C., Cruz A.H., Pérez-Herrera A., Gascón F., Giraldo A.I., Ruiz M.C., Lillo R., et al. Effect of natalizumab on oxidative damage biomarkers in relapsing-remitting multiple sclerosis. Pharmacol. Rep. 2013;65:624–631. doi: 10.1016/S1734-1140(13)71039-9. [DOI] [PubMed] [Google Scholar]
- 130.Levi-Montalcini R., Skaper S.D., Dal Toso R., Petrelli L., Leon A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 131.Barbacid M. Neurotrophic factors and their receptors. Curr. Opin. Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 132.Chao M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 133.Chao M.V., Hempstead B.L. p75 and Trk: A two-receptor system. Trends Neurosci. 1995;18:321–326. doi: 10.1016/0166-2236(95)93922-K. [DOI] [PubMed] [Google Scholar]
- 134.Lorenzini L., Baldassarro V.A., Stanzani A., Giardino L. Nerve Growth Factor: The First Molecule of the Neurotrophin Family. Adv. Exp. Med. Biol. 2021;1331:3–10. doi: 10.1007/978-3-030-74046-7_1. [DOI] [PubMed] [Google Scholar]
- 135.Loy R., Taglialatela G., Angelucci L., Heyer D., Perez-Polo R. Regional CNS uptake of blood-borne nerve growth factor. J. Neurosci. Res. 1994;39:339–346. doi: 10.1002/jnr.490390311. [DOI] [PubMed] [Google Scholar]
- 136.Tiberi A., Carucci N.M., Testa G., Rizzi C., Pacifico P., Borgonovo G., Arisi I., D’onofrio M., Brandi R., Gan W.-B., et al. Reduced levels of NGF shift astrocytes toward a neurotoxic phenotype. Front. Cell Dev. Biol. 2023;11:1165125. doi: 10.3389/fcell.2023.1165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rizzi C., Tiberi A., Giustizieri M., Marrone M.C., Gobbo F., Carucci N.M., Meli G., Arisi I., D’Onofrio M., Marinelli S., et al. NGF steers microglia toward a neuroprotective phenotype. Glia. 2018;66:1395–1416. doi: 10.1002/glia.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Villoslada P., Genain C.P. Role of nerve growth factor and other trophic factors in brain inflammation. Prog. Brain Res. 2004;146:403–414. doi: 10.1016/S0079-6123(03)46025-1. [DOI] [PubMed] [Google Scholar]
- 139.Guarnieri G., Sarchielli E., Comeglio P., Herrera-Puerta E., Piaceri I., Nacmias B., Benelli M., Kelsey G., Maggi M., Gallina P., et al. Tumor necrosis factor α influences phenotypic plasticity and promotes epigenetic changes in human basal forebrain cholinergic neuroblasts. Int. J. Mol. Sci. 2020;21:6128. doi: 10.3390/ijms21176128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Micera A., Properzi F., Triaca V., Aloe L. Nerve growth factor antibody exacerbates neuropathological signs of experimental allergic encephalomyelitis in adult Lewis rats. J. Neuroimmunol. 2000;104:116–123. doi: 10.1016/S0165-5728(99)00272-6. [DOI] [PubMed] [Google Scholar]
- 141.Villoslada P., Hauser S.L., Bartke I., Unger J., Heald N., Rosenberg D., Cheung S.W., Mobley W.C., Fisher S., Genain C.P. Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of t helper cell type 1 and 2 cytokines within the central nervous system. J. Exp. Med. 2000;191:1799–1806. doi: 10.1084/jem.191.10.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kenarov P., Petrov N., Voinov V., Daskalov M., Anaya F., Russo G., Momchilova A. A new approach using nanomem-brane—Based therapeutic plasmapheresis for treatment of patients with multiple sclerosis. A case report. J. Pharmacol. Clin. Toxicol. 2014;2:1031. [Google Scholar]
- 143.Tonev D., Momchilova A. Therapeutic Plasma Exchange and Multiple Sclerosis Dysregulations: Focus on the Removal of Pathogenic Circulatory Factors and Altering Nerve Growth Factor and Sphingosine-1-Phosphate Plasma Levels. Curr. Issues Mol. Biol. 2023;45:7749–7774. doi: 10.3390/cimb45100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Satoh T., Sakai N., Enokido Y., Uchiyama Y., Hatanaka H. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J. Biochem. 1996;120:540–546. doi: 10.1093/oxfordjournals.jbchem.a021447. [DOI] [PubMed] [Google Scholar]
- 145.Dugan L.L., Creedon D.J., Johnson E.M., Jr., Holtzman D.M. Rapid suppression of free radical formation by nerve growth factor involves the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA. 1997;94:4086–4091. doi: 10.1073/pnas.94.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang L.-L., Wang R., Tang X.-C. Huperzine A Protects SHSY5Y Neuroblastoma Cells against Oxidative Stress Damage via Nerve Growth Factor Production. Eur. J. Pharmacol. 2005;519:9–15. doi: 10.1016/j.ejphar.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 147.Sampath D., Perez-Polo R. Regulation of antioxidant enzyme expression by NGF. Neurochem. Res. 1997;22:351–362. doi: 10.1023/A:1027387105882. [DOI] [PubMed] [Google Scholar]
- 148.Salinas M., Diaz R., Abraham N.G., Ruiz de Galarreta C.M., Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 149.Rojo A.I., Salinas M., Martín D., Perona R., Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J. Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mimura J., Kosaka K., Maruyama A., Satoh T., Harada N., Yoshida H., Satoh K., Yamamoto M., Itoh K. Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J. Biochem. 2011;150:209–217. doi: 10.1093/jb/mvr065. [DOI] [PubMed] [Google Scholar]
- 151.Kosaka K., Mimura J., Itoh K., Satoh T., Shimojo Y., Kitajima C., Maruyama A., Yamamoto M., Shirasawa T. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J. Biochem. 2010;147:73–81. doi: 10.1093/jb/mvp149. [DOI] [PubMed] [Google Scholar]
- 152.Su R., Su W., Jiao Q. NGF protects neuroblastoma cells against β-amyloid-induced apoptosis via the Nrf2/HO-1 pathway. FEBS Open Bio. 2019;9:2063–2071. doi: 10.1002/2211-5463.12742. Erratum in: FEBS Open Bio 2020, 10, 288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Ishii T., Warabi E., Mann G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019;133:169–178. doi: 10.1016/j.freeradbiomed.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 154.Valdovinos-Flores C., Limón-Pacheco J.H., León-Rodríguez R., Petrosyan P., Garza-Lombó C., Gonsebatt M.E. Systemic L-Buthionine -S-R-Sulfoximine Treatment Increases Plasma NGF and Upregulates L-cys/L-cys2 Transporter and γ-Glutamylcysteine Ligase mRNAs Through the NGF/TrkA/Akt/Nrf2 Pathway in the Striatum. Front. Cell Neurosci. 2019;13:325. doi: 10.3389/fncel.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shih A.Y., Erb H., Sun X., Toda S., Kalivas P.W., Murphy T.H. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Levi-Montalcini R., Aloe L. Differentiating effects of murine nerve growth factor in the peripheral and central nervous systems of Xenopus laevis tadpoles. Proc. Natl. Acad. Sci. USA. 1985;82:7111–7115. doi: 10.1073/pnas.82.20.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Connelly-Smith L., Alquist C.R., Aqui N.A., Hofmann J.C., Klingel R., Onwuemene O.A., Patriquin C.J., Pham H.P., Sanchez A.P., Schneiderman J., et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J. Clin. Apher. 2023;38:77–278. doi: 10.1002/jca.22043. [DOI] [PubMed] [Google Scholar]
- 158.Redant S., De Bels D., Ismaili K., Honoré P.M. Membrane-based therapeutic plasma exchange in intensive care. Blood Purif. 2021;50:290–297. doi: 10.1159/000510983. [DOI] [PubMed] [Google Scholar]
- 159.Reeves H.M., Winters J.L. The mechanisms of action of plasma exchange. Br. J. Haematol. 2014;164:342–351. doi: 10.1111/bjh.12629. [DOI] [PubMed] [Google Scholar]
- 160.Yamakova Y., Ilieva V.A., Petkov R., Yankov G. Nanomembrane-Based Therapeutic Plasmapheresis after Non-Invasive Ventilation Failure for Treatment of a Patient with Acute Respiratory Distress Syndrome and Myasthenia Gravis: A Case Re-port. Blood Purif. 2019;48:382–384. doi: 10.1159/000502078. [DOI] [PubMed] [Google Scholar]
- 161.Alexandrov A., Vassileva P., Momchilova A., Tsonchev Z., Kirilova Y., Ivanova R., Sapundzhiev P., Petkova D., Tzoneva R., Daskalov M., et al. A new approach using nanomembrane-based therapeutic plasmapheresis for treatment of patients with multiple sclerosis and neuromyelitis optica. Comptes Rendus L’academie Bulg. Sci. 2016;69:373–384. [Google Scholar]
- 162.Momchilova A., Tsonchev Z., Hadzhilazova M., Tzoneva R., Alexandrov A., Nikolakov D., Ilieva V., Pankov R. Sphin-golipid Metabolism Is Dysregulated in Erythrocytes from Multiple Sclerosis Patient. Comptes Rendus L’academie Bulg. Sci. 2020;73:426–432. [Google Scholar]
- 163.Sapundzhiev P., Momchilova A., Vassileva P., Kirilova Y., Ivanova R., Bozhilova M., Orozova M., Staneva G., Krastev P., Pankov R., et al. Plasmapheresis Affects Ophthalmological Parameters and Oxidative Stress in Patients with Multiple Sclerosis and Neuromyelitis Optica. Arch. Biomed. Eng. Biotechnol. 2021;5:000617 [Google Scholar]
- 164.Tenchov B., Koynova R., Antonova B., Zaharinova S., Abarova S., Tsonchev Z., Komsa-Penkova R., Momchilova A. Blood plasma thermal behavior and protein oxidation as indicators of multiple sclerosis clinical status and plasma exchange therapy progression. Thermochim. Acta. 2019;671:193–199. doi: 10.1016/j.tca.2018.12.001. [DOI] [Google Scholar]
- 165.Tonev D., Georgieva R., Vavrek E. Our Clinical Experience in the Treatment of Myasthenia Gravis Acute Exacerbations with a Novel Nanomembrane-Based Therapeutic Plasma Exchange Technology. J. Clin. Med. 2022;11:4021. doi: 10.3390/jcm11144021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Escolar G., Páez A., Cid J. Conventional Therapeutic Plasma Exchange Versus Low Volume Plasma Exchange in Chronic Pathologies: Potential Benefit in Alzheimer’s Disease. Plasmatology. 2022;16:26348535221131685. doi: 10.1177/26348535221131685. [DOI] [Google Scholar]
- 167.Klingele M., Allmendinger C., Thieme S., Baerens L., Fliser D., Jan B. Therapeutic apheresis within immune-mediated neurological disorders: Dosing and its effectiveness. Sci. Rep. 2020;10:7925. doi: 10.1038/s41598-020-64744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dorst J., Fillies F., Dreyhaupt J., Senel M., Tumani H. Safety and Tolerability of Plasma Exchange and Immunoadsorption in Neuroinflammatory Diseases. J. Clin. Med. 2020;9:2874. doi: 10.3390/jcm9092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Padmanabhan A., Connelly-Smith L., Aqui N., Balogun R.A., Klingel R., Meyer E., Pham H.P., Schneiderman J., Witt V., Wu Y., et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 170.Lipphardt M., Mühlhausen J., Kitze B., Heigl F., Mauch E., Helms H.-J., Müller G.A., Koziolek M.J. Immunoadsorption or plasma exchange in steroid-refractory multiple sclerosis and neuromyelitis optica. J. Clin. Apher. 2019;34:381–391. doi: 10.1002/jca.21686. [DOI] [PubMed] [Google Scholar]
- 171.Chawla S., Sahni C., Tulsawani R., Singh M., Saraswat D., Bansal A., Saxena S. Exogenous sphingosine 1-phosphate protects murine splenocytes against hypoxia-induced injury. Lipids. 2014;49:191–202. doi: 10.1007/s11745-013-3860-9. [DOI] [PubMed] [Google Scholar]
- 172.Schettler V., Methe H., Staschinsky D., Schuff-Werner P., Müller G.A., Wieland E. Review: The oxidant/antioxidant balance during regular low density lipoprotein apheresis. Ther. Apher. 1999;3:219–226. doi: 10.1111/j.1091-6660.1999.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 173.Zhang S.Y., Gui L.N., Liu Y.Y., Shi S., Cheng Y. Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 2020;14:823. doi: 10.3389/fnins.2020.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.LeVine S.M. Albumin and multiple sclerosis. BMC Neurol. 2016;16:47. doi: 10.1186/s12883-016-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xue H., Yang Z., Wang L., Jiang Y., Li J., Wu M., Wang G., Zhang Y., Zhang M. Factors Influencing the Degree of Disability in Patients with Multiple Sclerosis. Front. Neurol. 2021;12:714631. doi: 10.3389/fneur.2021.714631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boss K., Stettner M., Szepanowski F., Mausberg A.K., Paar M., Pul R., Kleinschnitz C., Oettl K., Kribben A. Severe and long-lasting alteration of albumin redox state by plasmapheresis. Sci. Rep. 2022;12:12165. doi: 10.1038/s41598-022-16452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alexandrov A., Momchilova A., Orozova M., Alexandrov S., Krastev P., Stanev G., Nikolova B., Tsonchev Z. Therapeutic Apheresis. Bulgarian Academy of Science Prof. Marin Drinov Publishing House; Sofia, Bulgaria: 2022. pp. 1–120. [Google Scholar]
- 178.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jacob S., Mazibrada G., Irani S.R., Jacob A., Yudina A. The Role of Plasma Exchange in the Treatment of Refractory Autoimmune Neurological Diseases: A Narrative Review. J. Neuroimmune Pharmacol. 2021;16:806–817. doi: 10.1007/s11481-021-10004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Queiroz A.L.G., Soares Neto H.R., Kobayashi T.T., Silva S.M.C.A. Plasma exchange in inflammatory demyelinating disorders of the central nervous system: Reasonable use in the clinical practice. Arq. Neuropsiquiatr. 2023;81:296–307. doi: 10.1055/s-0042-1758447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tanaka M., Vécsei L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines. 2020;8:406. doi: 10.3390/biomedicines8100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang P., Xie K., Wang C., Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014;72:249–254. doi: 10.1159/000363515. [DOI] [PubMed] [Google Scholar]
- 183.Cortese I., Chaudhry V., So Y.T., Cantor F., Cornblath D.R., Rae-Grant A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76:294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Das J., Chauhan V.D., Mills D., Johal N.J., Tan M., Matthews R., Keh R., Lilleker J.B., Gosal D., Sharaf N. Therapeutic plasma exchange in neurological disorders: Experience from a tertiary neuroscience centre. Transfus. Apher. Sci. 2019;58:102654. doi: 10.1016/j.transci.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 185.Grilc N.K., Sova M., Kristl J. Drug Delivery Strategies for Curcumin and Other Natural Nrf2 Modulators of Oxidative Stress-Related Diseases. Pharmaceutics. 2021;13:2137. doi: 10.3390/pharmaceutics13122137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tastan B., Arioz B.I., Genc S. Targeting NLRP3 Inflammasome with Nrf2 Inducers in Central Nervous System Disorders. Front. Immunol. 2022;13:865772. doi: 10.3389/fimmu.2022.865772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available under request to corresponding author Dimitar Tonev (dgtsofia@abv.bg).