Abstract
The substance P (SP)-preferring receptor, neurokinin-1 receptor (NK-1R), has an important role in inflammation, immune regulation, and viral infection. We applied a newly developed real-time reverse transcription (RT)-PCR assay to quantify NK-1R mRNA in human neuronal cell line (NT-2N), a human B-cell line (IM9), monocyte-derived macrophages (MDM), peripheral blood lymphocytes (PBL), and human astroglioma cells (U87 MG). The NK-1R real-time RT-PCR assay has a sensitivity of 100 mRNA copies, with a dynamic range of detection between 102 and 107 copies of NK-1R gene transcripts per reaction. This assay is highly reproducible, with an intraassay coefficient variation of threshold cycle (Ct) of less than 1.9%. The NK-1R real-time RT-PCR is highly sensitive for quantitative determination of NK-1R mRNA in human immune cells (MDM and PBL) that express low levels of NK-1R mRNA. In addition, the assay has the ability to accurately quantitate the dynamic changes in NK-1R mRNA expression in interleukin-1β-stimulated U87 MG. These data indicate that the NK-1R real-time RT-PCR has potential for a wide application in investigation of NK-1R expression at the mRNA level under physiological and pathological conditions in both the central nervous system and the immune system.
Substance P (SP), the most extensively studied and potent member of the tachykinin family, is a modulator of neuroimmunoregulation, in particular, the immune functions of mononuclear phagocytes (10). SP specifically activates NF-κB, a transcription factor involved in the control of cytokine expression (22, 26), and stimulates human peripheral blood monocytes to produce inflammatory cytokines including interleukin-1 (IL-1), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α) (11, 19, 24). SP was initially considered a peptide of neural origin (28, 31). SP has also been identified in nonneuronal cell types, including murine macrophages (28, 31), human endothelial cells (23, 27), eosinophils (1), Leydig cells in human and mouse testis (4), and human lymphocytes and monocytes/macrophages (10, 12, 18). SP is secreted by human immune cells, participates in immunoregulation in an autocrine fashion (3, 30), and has a role in the pathogenesis of immune-mediated diseases, including neuroimmunologic diseases and AIDS (9, 16, 21).
The biologic responses to SP are mediated by the neurokinin-1 receptor (NK-1R), the SP preferring receptor, which is a G-protein-coupled receptor bearing seven transmembrane domains (33). NK-1R is present on T cells (36), including CD4+ and CD8+ T cells, B lymphocytes (36), monocyte/macrophages (25), and mast cells (35). Using a competitive PCR technique, NK-1R mRNA has been detected in lipopolysaccharide (LPS)-activated murine macrophages (2). In our study of the role of SP and NK-1R in immunoregulation of the immune cells, we demonstrated that monocyte/macrophages, T lymphocytes, and microglia express NK-1R mRNA as determined by reverse transcription (RT)-PCR (10, 12, 18). The RT-PCR assay, however, is not only laborious and time-consuming, but it also has potential variation and contamination due to post-PCR manipulation. Furthermore, the RT-PCR assay lacks the capability to accurately quantitate NK-1R mRNA. In the present study, we developed a simple, sensitive, rapid, and reproducible real-time RT-PCR assay in order to quantitate NK-1R mRNA in human neuronal and immune cells.
MATERIALS AND METHODS
Cells and treatment.
Peripheral blood was obtained from three healthy normal adult donors. The Institutional Research Board of our institution approved this investigation. The blood samples were identified as human immunodeficiency virus type 1 (HIV-1) antibody negative by anonymous testing with the enzyme-linked immunosorbent assay (ELISA) method (Coulter Immunology, Hialeah, FL). Informed consent was obtained from these subjects. Monocytes were purified according to our previously described technique (6, 7). Freshly isolated monocytes were plated in 24-well plates at a density of 106 cells/well in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS). The total length of time in culture for monocyte-derived macrophages (MDM) was 7 to 10 days. The viability of MDM was monitored by trypan blue exclusion and cell adherence to the wells. Nonadherent peripheral blood lymphocytes (PBL) were collected from gelatin-coated flasks and washed three times with phosphate-buffered saline (PBS). PBL viability was measured by a cell proliferation assay. IM9 (human B lymphoblasts), which expresses NK-1R (29), was obtained from the American Type Culture Collection (ATCC) (Rockville, MD). Human neuronal (NT-2N) cells were derived from Ntera2/cl.D1 (NT2) cells, a human teratocarcinoma cell line (32). Both IM9 and NT-2N were used as positive controls for NK-1R mRNA (20). Human astroglioma cells (U87 MG) were obtained from ATCC and maintained in DMEM with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat inactivated fetal bovine serum. In order to quantitate NK-1R mRNA expression changes, UG87 MG cultured in 24-well plates (105 cells/well) were incubated with or without IL-1β (4 ng/ml) as we previously described (5).
RNA extraction.
Total RNA was extracted from MDM, PBL, IM9, NT-2N (1 × 106 cells) and U87 MG (4 × 105 cells) and using Tri-Reagent (Molecular Research Center, Cincinnati, OH) as instructed by the manufacturer. In brief, total RNA was extracted by a single step, guanidinium thiocyanate-phenol-chloroform extraction. After centrifugation at 13,000 × g for 15 min, RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and resuspended in 50 μl of RNase-free water.
Cloning of NK-1R cDNA fragment.
The NK-1R mRNA fragment was cloned and identified with the human NK-1R primer pairs (HSPR3/HSPR4) from IM9 cells as reported earlier (14, 15). Briefly, the PCR products amplified by these primers were separated on a 2% agarose gel and then purified with Wizard PCR Preps DNA Purification System (Promega, Madison, WI). The purified NK-1R cDNA fragment was then cloned into a plasmid using the Eukaryotic TA Cloning Kit (Invitrogen Corporation, San Diego, CA). The cloned plasmid containing the NK-1R cDNA fragment was purified with Wizard Plus Minipreps DNA Purification System (Promega, Madison, WI). The presence and orientation of the NK-1R cDNA insert was determined by restriction analysis using EcoRV digestion and DNA sequencing. The purified plasmid was linearized by EcoRI restriction enzyme digestion and purified by phenol-chloroform extraction and alcohol precipitation. This plasmid containing the NK-1R cDNA fragment was used as a template to synthesize mRNA in vitro in order to evaluate the sensitivity and the reproducibility of the real-time RT-PCR assay.
In vitro mRNA synthesis.
NK-1R mRNA transcripts were obtained by transcribing the linearized plasmid containing the NK-1R cDNA insert with MEGAshortscript kit (Ambion, Austin, TX). After digestion with RNase-free DNase (Promega), the resulting RNA transcripts were purified with phenol-chloroform extraction and alcohol precipitation as previously reported (14, 15). The purified RNA transcript was used to construct a standard curve in order to quantitatively measure NK-1R mRNA levels in MDM, PBL, and U87 MG by real-time RT-PCR with the primer pair of NK-1R.
Design of TaqMan probe and primers.
The PCR primers and TaqMan probe used were designed using Primer Express software (PE Biosystems). The primer pair of NK-1R sense and antisense (sense: 5′-CACACTATGGGCCAGTGAGATC-3′; antisense: 5′-GCACACCACGACAATCATCATT-3′) was specific for a 109-bp fragment of NK-1R transcripts. The TaqMan probe sequence was 5′-TCTCTGCCAAG-CGCAAGGTGGTC-3′. The length of the TaqMan probe for NK-1R was designed such that the annealing temperature was 10°C higher than that needed for NK-1R primers. The probe was labeled at the 5′ end with 6-carboxyfluorescein (6-FAM) and at the 3′ end with black hole quencher-1. The sequence of the primer pair for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense); 5′-GTTGCTGTAGCCA-AATTCGTTGT-3′ (antisense). The primers and probe resuspended in Tris-EDTA (TE) buffer were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA), and stored at −30°C.
Reverse transcription.
Total RNA (1 μg) and NK-1R RNA standard were subjected to reverse transcription. Both the random primers and the specific NK-1R primer (antisense) were used in the same reaction. The random primers were used to prime GAPDH. The final reaction mixture (20 μl) contained the following elements: 5 mM MgCl2, 1× RT buffer, 500 μM each deoxynucleoside triphosphates (dNTPs), 1 unit/μl recombinant RNasin, 10 to 15 units of AMV reverse transcriptase (Promega), 50 ng random primers, and 0.1 μM NK-1R-specific antisense primer. The RT was performed at 42°C for 1 h. The reaction was terminated by holding the reaction mixture at 99°C for 5 min. One-tenth (2 μl) of the resulting cDNA was used as a template for real-time PCR amplification.
Real-time PCR assay.
The ABI Prism 7000 Sequence Detection System (ABI 7000 SDS) was used for real-time PCR analysis. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescent measurements were recorded during each annealing step. At the end of each PCR run, data were automatically analyzed by the system and amplification plots were obtained. For each PCR, 2 μl of cDNA template was added to 48 μl of PCR Master mixture (5 μl of 1× PCR buffer II, 5 mM MgCl2, 250 μM dNTPs, 400 nM of each primer, 1.5 u of AmpliTaq Gold DNA polymerase, 400 nM of TaqMan probe, and 24.7 μl of water). The PCR buffer contained 5-carboxy-X-rhodamine (5-ROX) (500 nM) as the reference dye for normalization of the reactions. Any possible fluctuations in 5-ROX signals were used to correct the sample signal. The master mixture was prepared freshly for each real-time PCR amplification. In order to generate a NK-1R RNA standard curve to quantify NK-1R mRNA in human immune cells, known amounts of the NK-1R RNA standard were serially diluted 10-fold and amplified in the same plate under the identical conditions. The quantity of NK-1R mRNA in the samples was automatically calculated by the ABI 7000 SDS based on the data obtained from the standard curve. All amplification reactions were performed in duplicate, and average copy numbers of the duplicates were presented in this report, unless otherwise specified. In order to control the integrity of RNA and normalize NK-1R mRNA levels in MDM, PBL, and U87 MG, a GAPDH mRNA fragment in these cells was also amplified using our established real-time RT-PCR with Brilliant SYBR green QPCR Master Mix (Stratagene, La Jolla, CA) as previous reported (17). In order to normalize the NK-1R mRNA levels, the NK-1R mRNA copy numbers in MDM, PBL, and U87 MG samples were divided by the total RNA (ng) determined by the GAPDH real-time RT-PCR in the same sample and then multiplied by 1,000 in order to convert the unit to NK-1R mRNA copy numbers per microgram (μg) of total RNA. The levels of NK-1R mRNA in these cells are expressed as the mean copy number of NK-1R mRNA per μg of total RNA.
RESULTS
Sensitivity of the real-time RT-PCR.
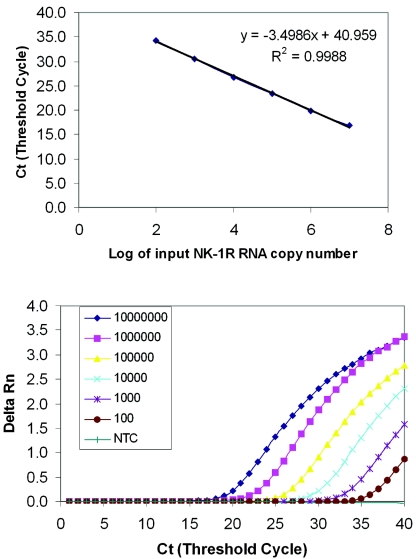
The analytical sensitivity of the real-time RT-PCR was determined using a serial dilution of NK-1R RNA transcripts containing 10, 102, 103, 104, 105, 106, and 107 copies of the transcripts and tested four times, each in duplicate. The real-time RT-PCR detected NK-1R mRNA copy numbers as low as 10 molecules, with the detection rate of 37.5% (3 out of 8 replicates) (data not shown). The detection rate, however, was 100% with NK-1R mRNA copy numbers of 100 or higher (8 out of 8 replicates). The detection limit, therefore, was set at 100 RNA molecules per reaction. A representative result is shown in Fig. 1.
FIG. 1.
Sensitivity and linearity analysis of the NK-1R real-time RT-PCR. A reading of change in fluorescence (Rn) as a function of cycle numbers is demonstrated for a range of known input copy numbers of the NK-1R RNA transcript derived from the plasmid containing NK-1R cDNA fragment. Tenfold serial dilutions of the NK-1R RNA starting from 102 to 107 molecules per reaction were amplified by the real-time RT-PCR. (A) The standard curve of the serial dilutions of the NK-1R RNA with a correlation coefficient (R2) of 0.9988. (B) Amplification plot of the serial dilutions of NK-1R RNA. The dynamic detection range is 5 orders of magnitude from 102 to 107 molecules, and the detection sensitivity is 100 NK-1R mRNA copies per reaction. NC, negative control which lacked PCR-amplified product when reverse transcriptase was omitted from the RT reaction using 107 molecules of NK-1R RNA standard.
Linearity, range of quantification, and precision.
Amplification of NK-1R RNA transcripts at different concentrations showed the linearity over a range of 5 orders of magnitude (Fig. 1) with the correlation coefficient R2 = 0.99. In order to determine the variation of repetitive measurements of real-time PCR between different runs, 10-fold serial dilutions of NK-1R cDNA (ranging from 102 to 107 copies per reaction) were examined by the real-time PCR in four different experiments. The coefficient of variation (CV) of threshold cycle (Ct) values within an assay was 1.9% (Table 1), and the interassay variation of Ct is comparable to that of the intraassay variation (Table 2).
TABLE 1.
Intra-assaya accuracy of NK-1R real-time RT-PCR
| Replicate | Threshold cycle of input copies
|
|||||
|---|---|---|---|---|---|---|
| 10,000,000 | 1,000,000 | 100,000 | 10,000 | 1,000 | 100 | |
| 1 | 16.9 | 19.7 | 23.4 | 26.8 | 30.2 | 33.9 |
| 2 | 16.9 | 20.1 | 23.3 | 26.6 | 30.4 | 33.5 |
| 3 | 16.8 | 19.6 | 23.1 | 27.0 | 30.4 | 34.4 |
| 4 | 16.4 | 20.0 | 23.2 | 27.0 | 31.0 | 35 |
| Mean (± SD) | 16.7 (± 0.2) | 19.8 (± 0.2) | 23.3 (± 0.1) | 26.7 (± 0.2) | 30.5 (± 0.4) | 34.2 (± 0.7) |
| CVb (%) | 1.38 | 1.11 | 0.56 | 0.75 | 1.18 | 1.9 |
Four replicate samples from each dilution were amplified in the same plate.
CV, coefficient of variation.
TABLE 2.
Inter-assaya accuracy of NK-1R real-time RT-PCR
| Replicate | Threshold cycle of input copies
|
|||||
|---|---|---|---|---|---|---|
| 10,000,000 | 1,000,000 | 100,000 | 10,000 | 1,000 | 100 | |
| 1 | 16.78 | 19.83 | 23.27 | 26.74 | 30.49 | 34.18 |
| 2 | 16.99 | 19.49 | 23.09 | 27.09 | 31.19 | 34.43 |
| 3 | 16.89 | 19.77 | 23.09 | 26.97 | 30.96 | 34.34 |
| 4 | 17.22 | 20.06 | 23.24 | 27.66 | 30.4 | 34.92 |
| Mean (± SEM) | 16.97 (± 0.09) | 19.79 (± 0.12) | 23.17 (± 0.05) | 27.12 (± 0.20) | 30.76 (± 0.19) | 34.47 (± 0.16) |
The data are generated from four separate assays performed on different days.
Real-time RT-PCR quantification of NK-1R mRNA.
Human monocytes, macrophages, lymphocytes, and U87 MG express NK-1R mRNA as demonstrated using conventional RT-PCR (5, 10, 12). We first examined the feasibility of the real-time RT-PCR for quantification of NK-1R mRNA in MDM and PBL isolated from three subjects. NK-1R mRNA levels in NT-2N cells and IM9 cells were also quantitated by the real-time RT-PCR. The reproducibility of the assay with four repetitions was excellent, with a variation of less than 15% for all the samples tested except IM9 (23.69%) (Table 3). NK-1R mRNA levels varied in immune cells isolated from three different donors. As expected, the neuronal cells (NT-2N) and IM9 expressed significantly higher levels of NK-1R mRNA than human immune cells (Table 3). In order to further determine the accuracy and specificity of the assay, we examined the dynamic changes in NK-1R mRNA in IL-1β-treated U87 MG. IL-1β (4 ng/ml) significantly induced NK-1R mRNA expression in U87 MG (Table 4). The highest levels of NK-1R mRNA were observed in U87 MG cells one hour after IL-1β stimulation (Table 4). The changes in the levels of NK-1R mRNA were quantitated accurately and reproducibly by the real-time RT-PCR (Table 4).
TABLE 3.
Intra-assay accuracy of NK-1R real-time RT-PCR for the specimensa
| Replicate | NK-1R mRNA (copy no.)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| D1
|
D2
|
D3
|
Cell line
|
|||||
| PBL | MDM | PBL | MDM | PBL | MDM | IM9 | NT-2N | |
| 1 | 3,090 | 1,865 | 9,362 | 1,846 | 20,136 | 3,241 | 171,472 | 1,717,887 |
| 2 | 2,421 | 1,640 | 8,448 | 1,522 | 16,607 | 3,139 | 106,608 | 1,435,145 |
| 3 | 3,012 | 1,361 | 9,856 | 1,858 | 18,763 | 3,478 | 110,087 | 1,363,270 |
| 4 | 2,843 | 1,424 | 9,606 | 1,419 | 16,607 | 3,733 | 120,444 | 1,425,958 |
| Mean (± SD) | 2,842 (± 299) | 1,573 (± 229) | 9,318 (± 614) | 1,661 (± 224) | 18,022 (± 1,730) | 3,398 (± 265) | 127,153 (± 3,0125) | 1,485,565 (± 158,40) |
| CVb (%) | 10.52 | 14.55 | 6.58 | 13.48 | 9.60 | 7.80 | 23.69 | 1.10 |
D, donor; healthy peripheral blood. Abbreviations: PBL, peripheral blood lymphocytes; MDM, monocyte-derived macrophages; IM9, human B lymphoblasts; NT-2N, human neuronal cells derived from Ntera2/cl.D1 (NT2) cells.
CV, coefficient of variation.
TABLE 4.
Quantitation of NK-1R mRNA in IL-1β-treated U87 MG
| Expt | NK-1R mRNA (copy no.) post-IL-1β stimulation (h)
|
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 9 | 24 | |
| 1 | 24,515 | 264,423 | 75,697 | 61,054 | 33,433 | 27,036 |
| 2 | 26,824 | 321,514 | 88,219 | 75,193 | 37,037 | 33,116 |
| Mean | 25,669 | 292,969 | 81,958 | 68,124 | 35,235 | 30,076 |
| Fold | 1 | 11.4 | 3.19 | 2.65 | 1.37 | 1.17 |
DISCUSSION
NK-1R has been identified not only on neuronal cells, but also on the immune cells, including monocytes, macrophages, and T-cells (10, 12). NK-1R has important roles in the immunoregulation of immune cells and in inflammatory diseases. Using a conventional RT/nested PCR assay, we showed that human immune cells express NK-1R (10, 12). Because of the low levels of NK-1R expression in the immune cells, it is difficult to quantitate NK-1R mRNA in these cells. Thus, the establishment of a highly quantitative and sensitive assay for NK-1R mRNA becomes imperative.
Real-time RT-PCR analysis has been employed successfully for both basic research and clinical applications (8, 37). Since PCR amplification is an exponential assay, a very small change in the amplification efficiency produces a dramatic difference in the amount of final products (34). The monitoring of the entire process of PCR (real time), rather than merely the end product, permits precise quantitation. More importantly, real-time PCR, which uses both primers and a probe, significantly increases the specificity of the assay. Since the NK-1R real-time RT-PCR has a wide dynamic detection range (102 to 107 copies per reaction), sample dilution or concentration is not required, which is one of the problems encountered in competitive RT-PCR (13). It is not necessary to run the PCR-amplified products on agarose gel after real-time PCR, which not only eliminates post-PCR procedure, but also avoids variation and contamination caused by post-PCR manipulation.
In the present study, we successfully utilized real-time PCR for quantification of NK-1R mRNA levels in human immune cells (MDM, PBL, and IM9) and neuronal cells (NT-2N). We also examined the accuracy and reproducibility of the real-time RT-PCR for quantifying the dynamic changes in NK-1R mRNA in IL-1β-treated human astroglioma cells (U87 MG). Using conventional RT-PCR we previously showed that IL-1β induced the expression of NK-1R mRNA in U87 MG (5). In the current study, we demonstrated that the real-time RT-PCR assay is capable of demonstrating the changes in NK-1R mRNA levels in U87 MG with high accuracy and specificity. In addition, the real-time RT-PCR is more sensitive than conventional RT-PCR, since the latter requires a nested PCR amplification (85 cycles) (10, 12). In conclusion, the NK-1R real-time RT-PCR is highly sensitive, precise, and reproducible. The assay is particularly useful for quantitation of NK-1R mRNA in nonneuronal cells that express low levels of NK-1R mRNA. Thus, the real-time RT-PCR assay is an important tool for the investigation of the role of NK-1R in inflammation, immune regulation and viral infections.
Acknowledgments
This work was supported by NIH-MH 49981 to S.D.D. and NIH-DA112815 to W.Z.H.
REFERENCES
- 1.Aliakbari, J., S. P. Sreedharan, C. W. Turck, and E. J. Goetzl. 1987. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem. Biophys. Res. Commun. 148:1440-1445. [DOI] [PubMed] [Google Scholar]
- 2.Bost, K. L., S. A. Breeding, and D. W. Pascual. 1992. Modulation of the mRNAs encoding substance P and its receptor in rat macrophages by LPS. Reg. Immunol. 4:105-112. [PubMed] [Google Scholar]
- 3.Castagliuolo, I., M. Riegler, A. Pasha, S. Nikulasson, B. Lu, C. Gerard, N. P. Gerard, and C. Pothoulakis. 1998. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J. Clin. Investig. 101:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiwakata, C., B. Brackmann, N. Hunt, M. Davidoff, W. Schulze, and R. Ivell. 1991. Tachykinin (substance-P) gene expression in Leydig cells of the human and mouse testis. Endocrinology 128:2441-2448. [DOI] [PubMed] [Google Scholar]
- 5.Guo, C. J., S. D. Douglas, Z. Gao, B. A. Wolf, J. Grinspan, J. P. Lai, E. Riedel, and W. Z. Ho. 2004. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia 48:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan, N. F., D. E. Campbell, and S. D. Douglas. 1986. Purification of human monocytes on gelatin-coated surfaces. J. Immunol. Methods 95:273-276. [DOI] [PubMed] [Google Scholar]
- 7.Hassan, N. F., J. R. Cutilli, and S. D. Douglas. 1990. Isolation of highly purified human blood monocytes for in vitro HIV-1 infection studies of monocyte/macrophages. J. Immunol. Methods 130:283-285. [DOI] [PubMed] [Google Scholar]
- 8.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 9.Ho, W. Z., J. P. Lai, Y. Li, and S. D. Douglas. 2002. HIV enhances substance P expression in human immune cells. FASEB J. 16:616-618. [DOI] [PubMed] [Google Scholar]
- 10.Ho, W. Z., J. P. Lai, X. H. Zhu, M. Uvaydova, and S. D. Douglas. 1997. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 159:5654-5660. [PubMed] [Google Scholar]
- 11.Kincy-Cain, T., and K. L. Bost. 1997. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 158:2334-2339. [PubMed] [Google Scholar]
- 12.Lai, J. P., S. D. Douglas, and W. Z. Ho. 1998. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 86:80-86. [DOI] [PubMed] [Google Scholar]
- 13.Lai, J. P., S. D. Douglas, and W. Z. Ho. 2002. Mimic-based RT-PCR quantitation of substance P mRNA in human mononuclear phagocytes and lymphocytes. Methods Mol. Biol. 193:129-147. [DOI] [PubMed] [Google Scholar]
- 14.Lai, J. P., S. D. Douglas, E. Rappaport, J. M. Wu, and W. Z. Ho. 1998. Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J. Neuroimmunol. 91:121-128. [DOI] [PubMed] [Google Scholar]
- 15.Lai, J. P., S. D. Douglas, F. Shaheen, D. E. Pleasure, and W. Z. Ho. 2002. Quantification of substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin. Diagn. Lab. Immunol. 9:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai, J. P., W. Z. Ho, G. X. Zhan, Y. Yi, R. G. Collman, and S. D. Douglas. 2001. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc. Natl. Acad. Sci. USA 98:3970-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai, J. P., J. H. Yang, S. D. Douglas, X. Wang, E. Riedel, and W. Z. Ho. 2003. Quantification of CCR5 mRNA in human lymphocytes and macrophages by real-time reverse transcriptase PCR assay. Clin. Diagn. Lab. Immunol. 10:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai, J. P., G. X. Zhan, D. E. Campbell, S. D. Douglas, and W. Z. Ho. 2000. Detection of substance P and its receptor in human fetal microglia. Neuroscience 101:1137-1144. [DOI] [PubMed] [Google Scholar]
- 19.Laurenzi, M. A., M. A. Persson, C. J. Dalsgaard, and A. Haegerstrand. 1990. The neuropeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand. J. Immunol. 31:529-533. [DOI] [PubMed] [Google Scholar]
- 20.Li, Y., S. D. Douglas, D. E. Pleasure, J. Lai, C. Guo, P. Bannerman, M. Williams, and W. Ho. 2003. Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression. J. Neurosci. Res. 71:559-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Y., S. D. Douglas, L. Song, S. Sun, and W. Z. Ho. 2001. Substance P enhances HIV-1 replication in latently infected human immune cells. J. Neuroimmunol. 121:67-75. [DOI] [PubMed] [Google Scholar]
- 22.Lieb, K., B. L. Fiebich, M. Berger, J. Bauer, and K. Schulze-Osthoff. 1997. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J. Immunol. 159:4952-4958. [PubMed] [Google Scholar]
- 23.Linnik, M. D., and M. A. Moskowitz. 1989. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides 10:957-962. [DOI] [PubMed] [Google Scholar]
- 24.Lotz, M., J. H. Vaughan, and D. A. Carson. 1988. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 241:1218-1221. [DOI] [PubMed] [Google Scholar]
- 25.Lucey, D. R., J. M. Novak, V. R. Polonis, Y. Liu, and S. Gartner. 1994. Characterization of substance P binding to human monocytes/macrophages. Clin. Diagn. Lab. Immunol. 1:330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marriott, I., M. J. Mason, A. Elhofy, and K. L. Bost. 2000. Substance P activates NF-kappaB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J. Neuroimmunol. 102:163-171. [DOI] [PubMed] [Google Scholar]
- 27.Milner, P., V. Ralevic, A. M. Hopwood, E. Feher, J. Lincoln, K. A. Kirkpatrick, and G. Burnstock. 1989. Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia 45:121-125. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi, S. 1987. Substance P precursor and kininogen: their structures, gene organizations, and regulation. Physiol. Rev. 67:1117-1142. [DOI] [PubMed] [Google Scholar]
- 29.Parnet, P., M. Mitsuhashi, C. W. Turck, B. Kerdelhue, and D. G. Payan. 1991. Tachykinin receptor cross-talk. Immunological cross-reactivity between the external domains of the substance K and substance P receptors. Brain Behav. Immun. 5:73-83. [DOI] [PubMed] [Google Scholar]
- 30.Pascual, D. W., and K. L. Bost. 1990. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 71:52-56. [PMC free article] [PubMed] [Google Scholar]
- 31.Pernow, B. 1983. Substance P. Pharmacol. Rev. 35:85-141. [PubMed] [Google Scholar]
- 32.Pleasure, S. J., C. Page, and V. M. Lee. 1992. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. 12:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quartara, L., and C. A. Maggi. 1997. The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation. Neuropeptides 31:537-563. [DOI] [PubMed] [Google Scholar]
- 34.Raeymaekers, L. 1993. Quantitative PCR: theoretical considerations with practical implications. Anal. Biochem. 214:582-585. [DOI] [PubMed] [Google Scholar]
- 35.Shanahan, F., J. A. Denburg, J. Fox, J. Bienenstock, and D. Befus. 1985. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J. Immunol. 135:1331-1337. [PubMed] [Google Scholar]
- 36.Stanisz, A. M., R. Scicchitano, P. Dazin, J. Bienenstock, and D. G. Payan. 1987. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J. Immunol. 139:749-754. [PubMed] [Google Scholar]
- 37.Tyagi, S., D. P. Bratu, and F. R. Kramer. 1998. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16:49-53. [DOI] [PubMed] [Google Scholar]