Abstract
A strain of Pseudomonas putida isolated from activated sewage grew aerobically on the xenoestrogen precursor, nonylphenol polyethoxylate (NPEOx, where x is the number of ethoxylate units) as sole carbon source. Comparative growth yields on NPEOav6, NPEOav9, and NPEOav20 (mixtures with average ethoxylate numbers as indicated) were consistent with utilization of all but two ethoxylate units, and the final accumulating metabolite was identified by gas chromatography-mass spectroscopy as nonylphenol diethoxylate (NPEO2). There was no growth on nonylphenol or polyethylene glycols, and there was no evidence for production of carboxylic acid analogs of NPEOx. Biodegradation kinetics measured by high-pressure liquid chromatography (HPLC) for each component in NPEOx mixtures showed that biodegradation proceeded via successive exoscission of the ethoxylate chain and not by direct scission between the second and third ethoxylate residues. The NPEOx-degrading activity was inducible by substrate, and cell extracts of NPEOav9-induced cells were also active on the pure alcohol ethoxylate, dodecyl octaethoxylate (AEO8), producing sequentially, under either aerobic or anaerobic conditions, AEO7, AEO6, AEO5, etc., thus demonstrating that the pathway involved removal of single ethoxylate units. HPLC analysis of 2,4-dinitrophenylhydrazone derivatives revealed acetaldehyde (ethanal) as the sole aldehydic product from either NPEOav9 or AEO8 under either aerobic or anaerobic conditions. We propose a mechanism for biotransformation which involves an oxygen-independent hydroxyl shift from the terminal to the penultimate carbon of the terminal ethoxylate unit of NPEOx and dissociation of the resulting hemiacetal to release acetaldehyde and the next-lower homolog, NPEOx−1, which then undergoes further cycles of the same reaction until x = 2.
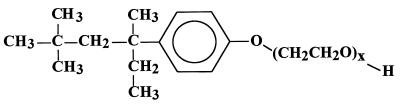
There is growing concern that the perceived reductions in the human male sperm count in the second half of this century, the increased incidence of testicular cancer (5, 32, 35), and the feminization of species inhabiting aquatic freshwater environments (30), may be attributable to endocrine-disrupting chemical pollutants in the environment. In addition to the identified need for studies on epidemiology and for toxicity-testing strategies and methods (6, 27), environmental risk assessments will be required for products from which endocrine disrupters (xenoestrogens) may be derived. Among the sources of putative xenoestrogens are the nonylphenol polyethoxylates (NPEOx [see below]) (Fig. 1), which are commercially important surfactants with industrial, agricultural, and domestic applications. They are comprised of a phenol nucleus that is o, m, or p substituted with one of a variety of hydrophobic, branched, isomeric nonyl moieties, and with a hydrophilic polyethylene glycol (ethoxylate) chain ether linked at the phenolic oxygen. As a result of the mode of synthesis from ethylene oxide monomers, the ethoxylate chains in commercial preparations are polydisperse, with lengths ranging from 1 to typically 50 or more ethylene glycol units; this variation is reflected in the designation NPEOx where x is the number of ethylene glycol (EO) units in the molecule and NP stands for nonylphenol. The combined annual production for the United States, western Europe, and Japan is estimated at about 0.35 Mt, and thus these compounds are common constituents of municipal wastewaters (2). While the higher homologs of NPEOx lack estrogenic activity, the shorter NPEOx homologs and nonylphenol itself are known to exert estrogenic effects in aquatic organisms (18, 19, 39) and in mammals and birds (10, 33, 39).
FIG. 1.
Typical structure of a highly branched isomer of NPEOx in which x may range from 2 to about 50. Commercial mixtures may also contain, in addition to the structure shown, other isomeric C9 branched chains as well as o- and m-substitution patterns.
Primary biodegradation of NPEOx in wastewater treatment plants is relatively efficient, but the resulting intermediates are more recalcitrant and ultimate degradation efficiency is significantly lower (2), so that degradation products, predominantly nonylphenol and lower homologs of NPEOx, are found in sewage treatment effluents (11, 14, 17), rivers (3, 14), estuaries (21, 22), and coastal waters (25). Thus, the biodegradation process converts the relatively benign surfactants into the more endocrine-disruptive components, and an understanding of this process will be an important component in the formulation of environmental risk assessments for this group of products.
Bacteria have been credited with three broad strategies for primary biodegradation of nonionic (polyethoxylate) surfactants (36): separation at the ether bond joining hydrophilic to hydrophobic moieties (central fission); ω/β-oxidative degradation of the hydrophobic group; and successive exoscission of the hydrophilic group. In mixed cultures, the first and second strategies fail for NPEOx due to, respectively, the proximity of the aryl nucleus and the presence of branching in the nonyl chain, leaving only the last mechanism (36). This is consistent with the observed accumulation of residues with intact nonylphenol moieties in natural environments.
Laboratory studies with mixed cultures (20, 23, 31) and pure cultures (15, 24, 26) have demonstrated that biodegradation results in the conversion of NPEOx to shorter homologs, usually nonylphenol diethoxylate (NPEO2) and/or its carboxylic acid analog, and it has been tacitly assumed that this is achieved by sequential removal of C2 ethoxylate units from the end of the chain. However, definitive evidence for the operation of this pathway in pure culture is lacking, and the nature of the ether scission reaction which it requires is unknown. The work now described was undertaken to isolate bacteria capable of growth on NPEOx surfactants, to confirm that growth was at the expense of only the ethoxylate moiety, and to elucidate the pathway and mechanism for ethoxylate removal.
MATERIALS AND METHODS
Materials and reagents.
NPEOx surfactant mixtures Ethylan 77, Ethylan BCP, and Ethylan 20, kindly donated by Ackross Chemicals Ltd., Manchester, United Kingdom, contained branched-chain nonylphenols attached to polydisperse polyethylene glycol chains averaging 6, 9, and 20 glycol units and were designated NPEOav6, NPEOav9, and NPEOav20, respectively. Pure dodecanol ethoxylates C12EO5, C12EO7, and C12EO8 were obtained from Nikkol Chemical Company, Tokyo, Japan. All other reagents, including high-pressure liquid chromatography (HPLC)-grade n-hexane, isopropanol, methanol, and acetonitrile, were from Fisher Scientific UK, Loughborough, United Kingdom.
Isolation, maintenance, and growth of bacteria.
Activated sewage sludge from Dyffryn Sewage Treatment Works, South Glamorgan, United Kingdom, was used to inoculate sterile basal salts medium (pH 7) containing 0.1% (wt/vol) NPEOav9 as the sole added carbon source. Basal salts contained (grams/liter) K2HPO4 (3.5), KH2PO4 (1.5), NH4Cl (0.5), NaCl (0.5), MgCl2 · 6H2O (0.15), and Na2SO4 · 2H2O (0.34) plus trace elements (1 ml/liter) containing (grams/liter) NaHCO3 · 10H2O (2.0), MnSO4 · 4H2O (0.3), Zn(CH3CO2)2 · 2H2O (0.2), (NH4)Mo7O24 · 4H2O (0.02), CuSO4 · 5H2O (0.5), Al2(SO4)3 (0.2), CoCl2 · 6H2O (0.5), and Fe(NH4)2(SO4)2 (0.5). Cultures were agitated at 30°C and 150 rpm. After several transfers to fresh medium, individual colonies were isolated on plates containing the same medium solidified with Noble agar. Axenic strains were maintained on agar slants of the same medium.
Bacterial isolates were shown to be gram negative (29) and further identified by using BIOLOG GN multiwell plates (BIOLOG Inc., Haywood, Calif.).
Bacteria were grown routinely in liquid basal salts medium supplemented with surfactants as required. Growth was measured by removing 1-ml samples from culture flasks, adding methanol (50% [vol/vol], final concentration) to dissolve insoluble hydrophobic products of biodegradation, and then measuring optical density at 600 nm. Separate experiments demonstrated that cells were unaffected by the presence of this concentration of methanol.
Preparation and use of cell lysate.
Pseudomonas putida was grown in 2-liter flasks containing 500-ml batches of 0.1% (wt/vol) NPEOav9 in basal salts medium and incubated with shaking (150 rpm) at 30°C for 24 h. Cells from 2 liters of culture medium were harvested by centrifugation at 5,200 × gav for 10 min in a Sorvall RC-5B Super Refrigerated centrifuge and washed free from residual NPEOx by resuspension in 2 liters of 0.1% (wt/vol) AEO8 (the alcohol polyethoxylate dodecyl octaethoxylate) in phosphate buffer (3.5 g of K2HPO4 and 1.5 g of KH2PO4 per liter [pH 7]) and recentrifugation. The AEO8 was subsequently removed by three further washes in phosphate buffer, followed by a final wash in 20 mM Tris-HCl buffer (pH 7) to remove phosphate. The combined final cell pellets were resuspended in a total of 40 ml of Tris-HCl buffer (pH 7, 4°C) and disrupted by passage three times through a French pressure cell (Aminco, Bethesda, Md.) operating at 126 MPa. The lysate was centrifuged at 8,300 × gav in a Sorvall RC-5B centrifuge for 15 min, and the supernatant was filtered through a 0.2-μm-pore size filter (Sartorious Minisart) to remove any residual cells and debris.
Aliquots (1 ml) of lysate were mixed with 1 ml of 0.2% (wt/vol) of the appropriate surfactant in minimal basal medium and incubated at 25°C. Samples were removed for either thin-layer chromatography (TLC) analysis of the ethoxylate moiety or derivatization and HPLC analysis for the presence of aldehydes. Control incubations without the added surfactant were included to check for false-positive results arising from carryover of surfactant in the lysate. For anaerobic experiments, cell lysate and surfactant solutions were separately sparged with nitrogen for 30 min. Lysate (1 ml) was placed in 10-ml vials fitted with septum caps, and the headspace was purged with dinitrogen before injection of test surfactant.
Cobalt thiocyanate active substance (CTAS) assay.
The assay for nonionic surfactants described by Crabb and Persinger (13) was modified to enable a rapid assessment of the NPEOx biodegradation in culture media. Aliquots (1 ml) were transferred at the desired time from culture flasks into acid-washed optically matched Pyrex test tubes (100 by 12 mm). Immediately, 1.25 ml of the ammonium cobalt thiocyanate solution (522 g of ammonium thiocyanate and 28 g of cobalt nitrate dissolved in 1 liter of pure water) and 1.5 ml of chloroform were added. The mixture was vortex mixed for eight 5-s bursts to extract the cobalt thiocyanate-ethoxylate complexes into the chloroform layer, which was then separated by centrifugation at 3,200 rpm for 5 min in an MSE Centaur 2 bench centrifuge. The absorbance of the chloroform extract was measured at 620 nm on a Novaspec II spectrophotometer. This assay was useful for assessing the overall biodegradation of mixed longer NPEOx surfactants but was insensitive toward the shorter (x < 6) homologs.
TLC.
Samples (typically 10 μl) were spotted onto preactivated normal-phase K60F silica plates (Whatman) and eluted in ethyl acetate-glacial acetic acid-water in the proportions 4:3:3 by volume (to separate the surfactants NPEOx and C12EOx from polyethylene glycols) or 70:16:15 by volume (to separate surfactant homologs). Spots were revealed by spraying with modified Burger reagent (11), which binds to molecules containing ethoxylate groups to form an orange-brown complex. The stock Burger reagent contained (i) 1.7 g of bismuth oxynitrate dissolved in 220 ml of glacial acetic acid and (ii) 40 g of potassium iodide, made up to 1 liter with pure water. Ten milliliters of the stock reagent was added to 1 ml of orthophosphoric acid, 10 ml of ethanol, and 5 ml of 20% (wt/vol) barium chloride to make the spray reagent. While not fully quantitative, this TLC method was invaluable for rapidly assessing biodegradability and evaluating metabolite patterns arising from commercial mixtures and pure surfactants.
HPLC measurement of parent surfactants and metabolites.
HPLC for analysis of NPEOx homologs was performed essentially by the method of Ahel and Giger (1), using a normal-phase Lichrosorb 10-μm irregular packed NH2-bonded silica column (4.6 mm [inside diameter] by 250 mm; Phenomenex, Macclesfield, United Kingdom). The automated HPLC system comprised Milton-Roy Consta Metric III metering pumps, an LDC/Milton-Roy/Spectromonitor D detector set at 277 nm, and a 50-μl injection loop connected to a 100-sample automated injection device and accessory control module controlled by a Commodore CBM model 8050 processor with an MP3000 integrator. Elution was achieved at the flow rate of 1.5 ml/min with solvents A (n-hexane–isopropanol, 9:1 [vol/vol]) and B (isopropanol/ ultrapure water, 9:1 [vol/vol]) in linear gradients between the following limits: at t = 0, A = 93% and B = 7%; at t = 60 min, A = 37% and B = 63%; at t = 62 min, A = 93% and B = 7%; at t = 67 min, A = 93% and B = 7%. Surfactant samples from biodegradation experiments were prepared for injection by centrifuge microfiltration in 0.75-ml, 0.2-μm-pore-size microfiltration centrifuge tubes (Lida Manufacturing Corp., Kenosha, Wis.) and centrifuged for 5 min at 3,000 rpm on a bench microcentrifuge.
Ahel and Giger (1) showed that the molar extinction coefficients were identical for all the NPEOx homologs, including nonylphenol itself (x = 0). Therefore, from the molar extinction coefficient of nonylphenol and the HPLC peak areas derived from standard solutions, the molar amounts of each NPEOx homolog were calculated.
HPLC of glycolaldehyde and acetaldehyde.
The method of Benassi et al. (9) for the detection of formaldehyde as its 2,4-dinitrophenylhydrazone, was modified for use with acetaldehyde and glycolaldehyde. To derivatize the aldehydes, 1-ml aliquots of standard or test samples were added to 9 ml of tetrahydrofuran and 0.8 ml of 2,4-dinitrophenylhydrazine (0.1% [wt/vol] in 1.4 M HCl), vortex mixed for 20 s, and allowed to settle for 2 min on ice before the addition of 0.8 ml of 0.1 M phosphate buffer (pH 7) and 1.4 ml of 1 M NaOH. Derivatized samples were stored on ice prior to HPLC analysis. Derivatized glycolaldehyde and acetaldehyde were separated on a Lichrosorb RP18 column (4.6 mm [inside diameter] by 250 mm; PhaseSep, Deeside, United Kingdom) installed in a Dionex 300 ion chromatograph fitted with a Dionex 4400 integrator (Dionex UK, Camberley, United Kingdom) and an LDC Milton Roy Spectromonitor IV detector (354 nm). Samples were injected via a 50-μl injection loop and eluted isocratically in acetonitrile-water (2:3 [vol/vol]) at a flow rate of 1 ml/min.
Assay for acetaldehyde.
Acetaldehyde in solution was determined by reaction with 3-methyl-2-benzothiazolone hydrazone in the presence of ferric chloride according to the method of Barary et al. (8).
GC-MS of biodegradation end products.
A sample (1 ml) of spent culture fluid from the biodegradation of NPEOav9 by P. putida was acidified to pH 2 in 1 M HCl (HPLC grade; Fisher) and extracted into chloroform (HPLC grade) by vortex mixing for 20 s. The lower chloroform layer was removed, dried over anhydrous sodium sulfate (HPLC grade), and analyzed by gas chromatography-mass spectrometry (GC-MS) using a Hewlett-Packard 5890 HP Series II gas chromatograph fitted with an Automated Hewlett-Packard Injector 7673 and a silica HP5 column (30 m by 0.25 mm) and interfaced with a Hewlett-Packard 5971A mass spectrometer. The conditions were as follows: 1-μl injection volume; carrier gas, helium (0.5 ml/min); temperature gradient, 50 to 170°C at 30°C/min, 170 to 240°C at 5°C/min, and 240 to 280°C at 2°C/min, isothermal for 8 min; total run time, 46 min. The ionization energy and ionizer temperature for electron impact ionization were 70 eV and 285°C, respectively.
RESULTS AND DISCUSSION
Isolation of bacteria.
Enrichment cultures yielded two strains of the genus Pseudomonas, each of which was confirmed for its ability to utilize, as sole source of carbon and energy for growth in liquid culture, NPEOav6, NPEOav9, and NPEOav20 but not polyethylene glycol or nonylphenol. In view of their close similarity in these respects, results are presented here only for one strain, which was further identified as P. putida type 1A1 (degrees of similarity with BIOLOG database were 0.910 and 0.935 for 4- and 24-h readings of color development in BIOLOG GN plates).
Utilization of NPEOx as growth substrate.
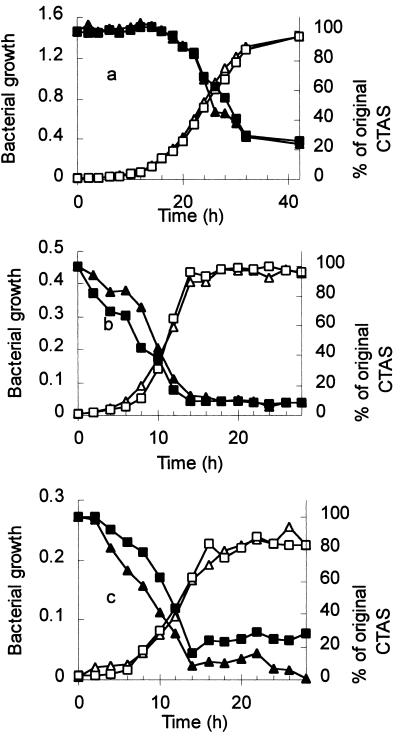
Figure 2 shows the growth curves and surfactant disappearance as measured by CTAS for P. putida growing separately on each of the three surfactants NPEOav6, NPEOav9, and NPEOav20. For the shortest homolog (Fig. 2c), the disappearance of surfactant (CTAS) was simultaneous with growth. In contrast, for the longest homolog tested (NPEOav20 [Fig. 2a]), extensive growth occurred before there was any depletion of CTAS. The CTAS assay responds well to, but is unable to discriminate among, the longer homologs, and it is progressively less sensitive to shorter polyethylene glycol chains. Thus, whereas cleavage near the phenyl group would result in immediate loss of CTAS response for all homologs, sequential exoscission of terminal glycol units from longer homologs would result in no change in CTAS response until the chains had been shortened significantly. Therefore, these data provided preliminary evidence that P. putida degraded NPEOx via successive exoscission of the polyethoxylate chain rather than by central cleavage.
FIG. 2.
Utilization of the commercial surfactants NPEOav20 (a), NPEOav9 (b), and NPEOav6 (c) as growth substrates for P. putida. Growth was assessed by optical density measurements (open symbols) of cultures treated with methanol (50% [vol/vol], final concentration) to solubilize metabolites. The initial concentration of surfactant was 1.62 mM, based on UV absorbance, and residual concentrations (filled symbols) were measured as CTAS (620 nm). Different symbol shapes indicate duplicate independent experiments.
The final cell yields (Fig. 2) increased in the order NPEOav6 < NPEOav9 < NPEOav20, and there was no growth on nonylphenol itself. These results strongly implied that the carbon for growth was derived exclusively from the ethoxylate chain, a conclusion confirmed (i) by measurements of the UV absorbance of cell-free culture media which remained constant throughout growth and (ii) by HPLC analysis (with UV detection) of intermediates formed during biodegradation, which showed that as the ethoxamers were degraded to shorter homologs, the total peak area remained constant throughout. Available carbon for growth from each surfactant mixture (NPEOav6, NPEOav9, and NPEOav20) was calculated from HPLC analysis of each mixture, first, on the basis that degradation proceeded to NPEO1. On this basis, the growth yields in stationary phase were correlated with the available carbon with r2 = 0.9998. When available carbon was recalculated on the basis that degradation proceeded to NPEO2, NPEO3, NPEO4, or NPEO5, the value of r2 was 0.9998, 0.9977, 0.9916, or 0.9814, respectively. The highest (and indistinguishable) values of r2 for NPEO1 and NPEO2 provided evidence that degradation proceeded at least to the diethoxylate.
GC-MS analysis of end product of biodegradation of NPEOav9.
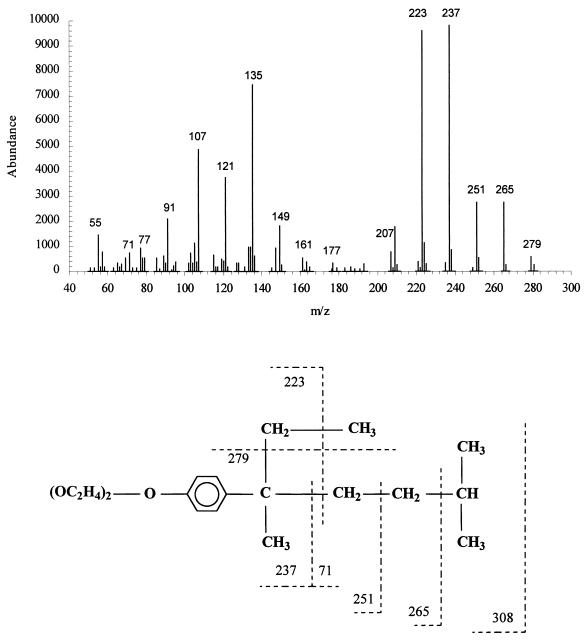
To determine the structure of the end product of the biodegradation of NPEOav9, this surfactant was degraded to completion by P. putida, and the end product was analyzed by GC-MS. The gas chromatogram showed three peaks (a to c) eluting close together (retention times of 21.55 [a], 21.94 [b], and 22.30 [c] min). In spectrum a, the molecular ion was 308, corresponding to NPEO2. Confirmation of this identity was provided by the fragmentation pattern which centered largely around fragmentation of the nonyl chain. Thus, the major ions with m/z values of 279, 237, 223, and 209 correspond to [(M + 1)−n(CH2)], where n = 2, 5, 6, and 7. Spectrum b contained the same ions and additionally m/z = 251, corresponding to n = 4. Spectra a and b were closely similar to those obtained by Maki et al. (24) for (1,3,3-trimethyl-1-ethyl butyl)phenol diethoxylate and (1,1,4,4-tetramethyl pentyl)phenol diethoxylate, respectively. Spectrum c (Fig. 3) shares these same major ions and also contains the m/z = 265 ion corresponding to loss of a three-carbon fragment from NPEO2; we suggest this is a third isomer, (1,4-dimethyl-1-ethyl pentyl)phenol diethoxylate (Fig. 3). Whatever the isomeric structures of the nonyl group, the major conclusion from these data is that the diethoxylate NPEO2 accumulates as the major end product of NPEOav9 biodegradation in P. putida.
FIG. 3.
Mass spectrum and fragmentation pattern of the end product of degradation of NPEOav9 by P. putida. Extracts of acidified spent culture medium were analyzed by GC-MS. Spectra were obtained for three isomeric components eluting with retention times of 21.55 (a), 21.94 (b), and 22.30 (c) min. The spectrum shown is for component c. See text for details.
Biodegradation kinetics of component homologs of NPEOx.
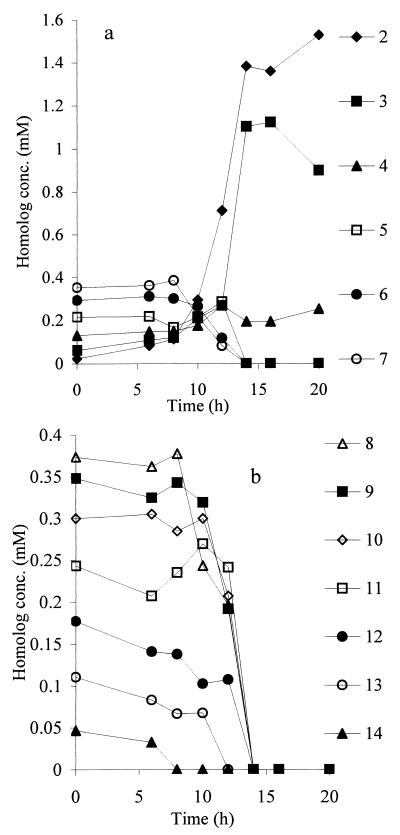
The identification by GC-MS of NPEO2 as the accumulating end product of biodegradation of NPEOx enabled the corresponding HPLC peak to be identified. It is reasonable to assume that successively eluting peaks in the HPLC chromatogram differed by single ethylene glycol units, thereby enabling assignment of all the higher homologs in the series.
Availability of a fully standardized HPLC system enabled quantitative determination of the biodegradation kinetics of each component homolog in a commercial mixture. The observed pattern for biodegradation of NPEOav9 by P. putida (Fig. 4) showed the longest homologs (NPEO13 and NPEO14) disappearing progressively from the outset, while intermediate homologs were more persistent and NPEO2 accumulated as the major end product. The accumulation of NPEO2 could occur by either (i) cleavage of all homologs at the ether bond between the second and third glycols from the phenyl ring (EO2-3 cleavage), (ii) sequential removal of single glycol units so that each homolog is eroded to the NPEO2 structure (exoscission), or (iii) scission at ether links within the polyethylene glycol chain (endoscission). For exclusive EO2–3 cleavage, all homologs would decrease simultaneously, albeit possibly at different rates, but there would be no increases in amounts of any but the end product itself. In contrast, for sequential exocleavage of single glycol units or endoscission, the longest homolog could decrease only in amount, but intermediate-length homologs would be undergoing formation from longer ones and degradation to shorter ones and thus may achieve a steady state for a while, or even increase in amounts if the rate of formation exceeded the rate of biodegradation. This condition would persist until the homolog became the longest remaining, and then it would decrease in amount. It is clear from Fig. 4 that intermediate homologs frequently showed small increases, for example, at 8 and 10 h for NPEO11, at 10 h for NPEO10, at 8 h for NPEO9, NPEO8, and NPEO7, at 6 h for NPEO6, and at 10 and 12 h for NPEO5, NPEO4, and NPEO3. Similar results were obtained with NPEOav6 and NPEOav20. This pattern eliminated the EO2–3 scission mechanism, leaving endo- or exoscission of the polyethoxylate chains as remaining possibilities. Because the organism did not utilize free polyethylene glycols, endoscission of NPEOx would have resulted in the accumulation of polyethylene glycols. No such accumulation was ever detected on TLC plates with the Burger reagent (12), which indicates that the mechanism of polyethoxylate degradation was by exoscission.
FIG. 4.
Biodegradation kinetics of individual homologs NPEO2–7 (a) and NPEO8–14 (b) during growth of P. putida on NPEOav9. Samples from culture flasks (Fig. 2b) were analyzed by HPLC as described in the text. Numbers alongside the symbols indicate the ethoxylate number of each homolog.
Activity of NPEOx degraders toward AEOx.
The difficulty in interpreting the data in Fig. 4 is that the immediate product of exo-glycol cleavage of a particular component was already present in the mixture and itself undergoing biodegradation. Although the pure NPEOx components needed to resolve this problem were unavailable, pure AEOx components were available and were used as follows. Cells of P. putida grown on NPEOav9, harvested and reincubated with the growth substrate, achieved complete biodegradation as assessed by TLC analysis. However, broth-grown cells were completely without activity. This finding indicated that the ether scission system was inducible by substrate or a transient metabolite. Moreover, NPEOav9-grown cells, but not broth-grown cells, degraded the pure alcohol ethoxylate AEO8, which indicated that the ether scission system induced by and active on NPEOav9, was also active on AEO8. This allowed the use of the pure compounds AEO8, AEO7, and AEO5 in mechanistic studies, thus avoiding the complications from homolog mixtures.
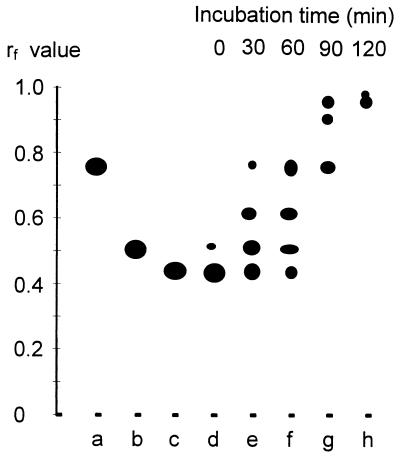
Extracts of NPEOav9-grown cells were incubated with AEO8, and samples were removed at intervals and analyzed by TLC using the Burger reagent to reveal polyethylene glycol-containing compounds (Fig. 5). Samples of AEO8, AEO7, and AEO5 were also chromatographed as standards. The first sample was removed immediately after mixing cells with substrate; during the time taken to process this sample, a small amount of conversion occurred to give a single new spot corresponding to AEO7. After 30 min of incubation, AEO7, AEO6, and small amounts of AEO5 were present, but shorter homologs did not appear until the 90-min sample. The facts that no short homologs were formed initially and that AEO7, AEO6, and AEO5 showed clear precursor-product relationships confirmed that degradation proceeded by stepwise exoscission of single glycol units and not by endoscission. The same results were obtained under aerobic and anaerobic conditions.
FIG. 5.
TLC analysis of intermediates produced during biodegradation of pure AEO8 by extracts of NPEOav9-grown cells of P. putida. Standards used were AEO5 (a), AEO7 (b), and AEO8 (c). Samples were removed from incubation mixtures at 0 (d), 30 (e), 60 (f), 90 (g), and 120 (h) min.
Acetaldehyde production from NPEOav9 in cell extracts.
The two most likely mechanisms for liberation of glycol units from NPEOx are (38) (i) hydroxyl (HO) shift from the terminal −CH2OH to the penultimate carbon to yield the hemiacetal −O−CH(OH)CH3 followed by spontaneous decomposition of the hemiacetal to acetaldehyde and (ii) insertion of oxygen via monooxygenation to produce the hemiacetal −O−CH(OH)−CH2OH, which on decomposition yields glycolaldehyde. Thus, identification of the aldehyde product by HPLC is a suitable discriminator for these mechanisms. With 2,4-dinitrophenylhydrazine used to derivatize the aldehydes and HPLC to separate and identify the resulting UV-absorbing hydrazones, no aldehydes were detectable in the culture medium containing whole cells biodegrading NPEO9. This was not surprising because it is normal for cells which generate toxic aldehydes to keep the steady-state concentration very low by having efficient systems for aldehyde removal (7). In contrast, cell extracts in which onward metabolism was disrupted were capable of significant biodegradation of NPEOav9 (monitored by TLC analysis), and moreover they produced abundant acetaldehyde but neither glycolaldehyde nor any other aldehyde (by HPLC analysis of 2,4-dinitrophenylhydrazones). The same results were obtained under aerobic conditions and after purging all solutions with nitrogen.
To confirm that oxygen was not required for acetaldehyde production, cell extracts were sparged with nitrogen and then incubated under a nitrogen atmosphere with nitrogen-sparged AEO8 solution (final concentration of approximately 2 mM). Samples were removed at intervals and assayed for acetaldehyde by the method of Barary et al. (8). The results showed that the acetaldehyde concentration reached 3.5 mM after 2 h. This is much higher than the oxygen concentration present even in air-saturated water and therefore much higher still than in the nitrogen-sparged solution actually used. Thus, the acetaldehyde must be liberated in a mechanism which is independent of oxygen.
Mechanism of biotransformation.
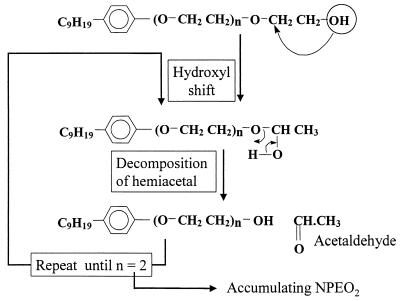
The combined evidence presented in this paper, viz. (i) analysis of metabolite patterns from NPEOx mixtures, (ii) precursor-product relationships for biodegradation of the pure ethoxamer of AEO8 via AEO7, AEO6, and AEO5, and (iii) liberation of acetaldehyde, shows unequivocally that in P. putida, NPEOx was degraded via sequential removal of glycol units (as acetaldehyde) to the ethoxamer NPEO2, which then accumulated. This pathway (Fig. 6) contrasted sharply with the central ether scission pathway commonly observed for alcohol ethoxylate degraders (34, 37) and reflected the influence of the aromatic phenyl group in inhibiting such pathways in NPEOx (36).
FIG. 6.
Proposed pathway and mechanism for the biodegradation of NPEOx by P. putida.
The proposed nonoxidative hydroxyl shift mechanism of ether scission (Fig. 6) accounts for the oxygen-independent liberation of acetaldehyde. This mechanism is dependent on the availability, in the polyethoxylate, of a terminal hydroxyl group which is effectively transferred to the penultimate carbon to produce the labile hemiacetal. The anaerobic Pelobacter venetianus and Bacteroides strain PG1 (16) degrade polyethylene glycol to acetaldehyde by a similar hydroxyl shift mechanism. To account for a similar oxygen-independent degradation of polyethylene glycol to acetaldehyde by Acinetobacter, Pearce and Heydeman (28) proposed a mechanism involving dehydration of the terminal glycol unit and hydrolysis (without hemiacetal formation) to yield the enol form of acetaldehyde and the next-lower polyethylene glycol homolog. Although this mechanism is possible, the data are also consistent with the hydroxyl shift mechanism.
While the hydroxyl shift mechanism shown in Fig. 6 clearly has precedents in bacterial metabolism of polyethylene glycols, this is the first report of the occurrence of such a mechanism in the biodegradation of nonionic polyethoxylate surfactants. Moreover, the P. putida enzyme system must be quite distinct from the previously reported systems because it lacks any activity toward polyethylene glycol itself.
The sequential exoscission of glycol units from NPEOx is consistent with the study of Kveštak and Ahel (20), who showed the formation of shortened but unoxidized NPEOx homologs by mixed estuarine cultures. On the other hand, some workers (4, 24) have interpreted the frequent occurrence of oxidized intermediates (carboxylic acid analogs) in mixed culture and environmental samples as indicative of the importance of oxidative mechanisms in NPEOx biodegradation. A parallel situation exists in the biodegradation of polyethylene glycols, for which there is evidence for their oxidation to carboxylic acids but no evidence on which to assess whether oxidation is a precursor to, or a component of, the ether scission step (38). It may be that nonoxidative ether scission dominates NPEOx (and polyethylene glycol) biodegradation to shorter homologs and that the longer-lived intermediates undergo hydroxyl group oxidation as a side reaction, possibly mediated by alcohol dehydrogenases known to occur ubiquitously in bacteria.
In environmental samples, although NPEO2 accumulates significantly, its carboxylic acid analog and the more endocrine-disrupting nonylphenol are also found (3, 4, 11, 14, 17, 21, 22, 25). These transformations were not observed in P. putida, and so other organisms or possibly abiotic processes must be involved to achieve these conversions in the environment.
The progressive shortening of ethoxylate chains during biodegradation will make the molecules more lipophilic, which is expected to increase adsorption of these surfactants to organic-rich sediments and to decrease affinity for more polar mineral components in natural sediments. Adsorption may in turn influence the bioavailability of the biodegradation intermediates to further biodegradation by bacteria. Thus, the successive exoscission mechanism established in this study is likely to have significant implications in assessing the environmental fate of this group of surfactants.
ACKNOWLEDGMENTS
We thank the UK Natural Environment Research Council for the award of a studentship to D.M.J. and Procter and Gamble for financial support.
REFERENCES
- 1.Ahel M, Giger W. Determination of nonionic surfactants of the alkylphenol polyethoxylate type by high-performance liquid chromatography. Anal Chem. 1995;57:2584–2590. [Google Scholar]
- 2.Ahel M, Giger W, Koch M. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment. I. Occurrence and transformation in sewage treatment. Water Res. 1994;28:1131–1142. [Google Scholar]
- 3.Ahel M, Giger W, Schaffner C. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment. II. Occurrence and transformation in rivers. Water Res. 1994;28:1143–1152. [Google Scholar]
- 4.Ahel M, Hršak D, Giger W. Aerobic transformation of short-chain alkylphenol ethoxylates by mixed bacterial cultures. Arch Environ Contam Toxicol. 1994;26:540–548. [Google Scholar]
- 5.Anonymous. Male reproductive health and environmental oestrogens. Lancet. 1995;345:933–935. . (Editorial.) [PubMed] [Google Scholar]
- 6.Ashby J, Houthoff E, Kennedy S J, Stevens J, Bars R, Jekat F W, Campbell P, Van Miller J, Carpanini F M, Randall G L P. The challenge posed by endocrine-disrupting chemicals. Environ Health Perspect. 1997;105:164–169. doi: 10.1289/ehp.97105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attwood M M, Quayle J R. Proceedings of the 4th International Symposium on Microbial Growth on C1 Compounds 1984. Washington D.C: American Society for Microbiology; 1984. Formaldehyde as a central intermediary metabolite of methylotrophic metabolism; pp. 315–323. [Google Scholar]
- 8.Barary M H, El-Yazbi F A, Bedair M A. Spectrophotometric determination of aldehydes in alcohols. Anal Lett. 1991;24:857–869. [Google Scholar]
- 9.Benassi C A, Semenzato A, Zacoaria F. High performance liquid chromatography of the free formaldehyde in cosmetics preserved with Dowicil 200. J Chromatogr. 1990;502:193–200. [Google Scholar]
- 10.Bicknell R J, Herbison A E, Sumpter J P. Estrogenic activity of an environmentally persistent alkylphenol in the reproductive-tract but not the brain of rodents. J Steroid Biochem Mol Biol. 1995;54:7–9. doi: 10.1016/0960-0760(95)00118-j. [DOI] [PubMed] [Google Scholar]
- 11.Brunner P H, Capri S, Marcomini A, Giger W. Occurrence and behaviour of linear alkylbenzenesulphonates, nonylphenol, nonylphenol mono- and nonylphenol diethoxylates in sewage and sewage sludge treatment. Water Res. 1988;22:1465–1472. [Google Scholar]
- 12.Burger K. Methods for the microdetermination and trace detection of surfactants. III. Trace detection and determination of surface-active polyethoxylate compounds and polyethylene glycols. Z Anal Chem. 1963;196:251–259. [Google Scholar]
- 13.Crabb N, Persinger H. A determination of the apparent molar absorption coefficients of the cobalt thiocyanate complexes of nonylphenol ethylene oxide adducts. J Am Oil Chem Soc. 1968;45:611–615. [Google Scholar]
- 14.Field J A, Reed R L. Nonylphenol polyethoxy carboxylate metabolites of nonionic surfactants in U.S. paper mill effluents, municipal sewage treatment plant effluents, and river waters. Environ Sci Technol. 1996;30:3544–3550. [Google Scholar]
- 15.Frassinetti S, Isoppo A, Corti A, Vallini G. Bacterial attack of non-ionic aromatic surfactants: comparison of degradative capabilities of new isolates from nonylphenol polyethoxylate polluted wastewaters. Environ Technol. 1996;17:199–213. [Google Scholar]
- 16.Frings J, Schramm E, Schink B. Enzymes involved in anaerobic polyethylene glycol degradation by Pelobacter venetianus and Bacteroides strain PG1. Appl Environ Microbiol. 1992;58:2164–2167. doi: 10.1128/aem.58.7.2164-2167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giger W, Brunnen P H, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225:623–625. doi: 10.1126/science.6740328. [DOI] [PubMed] [Google Scholar]
- 18.Jobling R, Sheahan D, Osborne J A, Matthiessen P, Sumpter J P. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic compounds. Environ Toxicol Chem. 1996;15:194–202. [Google Scholar]
- 19.Jobling R, Sumpter J P. Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol. 1993;27:361–372. [Google Scholar]
- 20.Kveštak R, Ahel M. Biotransformation of nonylphenol polyethoxylate surfactants by estuarine mixed bacterial cultures. Arch Environ Contam Toxicol. 1995;29:551–556. [Google Scholar]
- 21.Kveštak R, Ahel M. Occurrence of toxic metabolites from nonionic surfactants in the Krka River estuary. Ecotoxicol Environ Saf. 1994;28:25–34. doi: 10.1006/eesa.1994.1031. [DOI] [PubMed] [Google Scholar]
- 22.Kveštak R, Terzic S, Ahel M. Input and distribution of alkylphenol polyethoxylates in a stratified estuary. Mar Chem. 1994;46:89–100. [Google Scholar]
- 23.Maki H, Fujita M, Fujiwara Y. Identification of final biodegradation product of nonylphenol ethoxylate (NPE) by river microbial consortia. Bull Environ Contamin Toxicol. 1996;57:881–887. doi: 10.1007/s001289900272. [DOI] [PubMed] [Google Scholar]
- 24.Maki H, Masuda N, Fujiwara Y, Ike M, Fujita M. Degradation of alkylphenol ethoxylates by Pseudomonas sp. strain TR01. Appl Environ Microbiol. 1994;60:2265–2271. doi: 10.1128/aem.60.7.2265-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcomini A, Pavoni B, Sfriso A, Orio A A. Persistent metabolites of alkylphenol polyethoxylates in the marine environment. Mar Chem. 1990;29:307–323. [Google Scholar]
- 26.Nguyen M H, Sigoillot J-C. Isolation of coastal sea water and characterisation of bacterial strains involved in non-ionic surfactant degradation. Biodegradation. 1997;7:369–375. doi: 10.1007/BF00056420. [DOI] [PubMed] [Google Scholar]
- 27.Nimrod A C, Benson W H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996;26:335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- 28.Pearce B A, Heydeman M T. Metabolism of di(ethylene glycol)[2-(2′-hydroxyethoxy)ethanol] and other short poly(ethylene glycol)s by Gram negative bacteria. J Gen Microbiol. 1980;118:21–27. [Google Scholar]
- 29.Powers E M. Efficacy of the nonstaining KOH technique for rapidly determining Gram reactions of food-borne and waterborne bacteria and yeasts. Appl Environ Microbiol. 1995;61:3756–3758. doi: 10.1128/aem.61.10.3756-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raloff J. The gender benders: are environmental “hormones” emasculating wildlife? Sci News. 1994;145:24–27. [Google Scholar]
- 31.Rudling L, Solyom P. The investigation of biodegradability of branched nonyl phenol ethoxylates. Water Res. 1974;8:115–119. [Google Scholar]
- 32.Sharpe R M, Skakkebaek N E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 33.Soto A M, Justicia H, Wray J W, Sonnenschein C. p-Nonyl phenol: an estrogenic xenobiotic released from modified polystyrene. Environ Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tidswell E C, Russell N J, White G F. Ether-bond scission in the biodegradation of alcohol ethoxylate surfactants by Pseudomonas sp. strain SC25A. Microbiology. 1996;142:1123–1131. doi: 10.1099/13500872-142-5-1123. [DOI] [PubMed] [Google Scholar]
- 35.Toppari J, Larsen J C, Christiansen P, Giwercman A, Grandjean P, Guillette L J, Jegou B, Jensen T K, Jouannet P, Keiding N, Leffers H, McLachlan J A, Meyer O, Muller J, Rajpertdemeyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek N E. Male reproductive health and environmental estrogens. Environ Health Perspect. 1996;104:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White G F. Bacterial biodegradation of ethoxylated surfactants. Pestic Sci. 1993;37:159–166. [Google Scholar]
- 37.White G F, Higgins T P, John D M. Proceedings of the International Symposium Environmental Biotechnology, Oostende, Belgium 1997. Antwerp, Belgium: Technologisch Institut; 1997. Multiple mechanisms for biodegradation of non-ionic surfactants in bacteria; pp. 265–274. [Google Scholar]
- 38.White G F, Russell N J, Tidswell E C. Bacterial scission of ether bonds. Microbiol Rev. 1996;60:216–232. doi: 10.1128/mr.60.1.216-232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White R, Jobling S, Hoare S A, Sumpter J P, Parker M G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135:175–183. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]