Abstract
Malnutrition in children is associated with an increased risk of infection and death. Multiple abnormalities in the immune response, including cytokine production, in protein energy-malnourished children have been described and could account for the increased severity and frequency of infections. In this study, we used flow cytometry to investigate the effects of malnutrition on the production of cytokines (interleukin-2 [IL-2], gamma interferon [IFN-γ], IL-4, and IL-10) in CD4+ and CD8+ cells and the activation capability (as indicated by CD69+ and CD25+ cells). CD4+ and CD8+ cells from malnourished children showed increased production of IL-4 and IL-10 cytokines and decreased production of IL-2 and IFN-γ cytokines compared to that in cells from well-nourished, uninfected and well-nourished, infected children. In addition, malnourished children showed impaired activation capability, since the fluorescence intensity of CD69+ and CD25+ cells was lower than that in cells from well-nourished, uninfected and well-nourished, infected children. These results indicate that malnutrition alters the capacity of CD4+ and CD8+ cells to produce IL-2, IFN-γ, IL-4, and IL-10 in response to stimulus. We concluded that both cytokine production and activation capacity were impaired in malnourished children. This functional impairment may be involved in the failure to develop a specific immune response and the predisposition to infection in these children.
Malnutrition remains one of the most common causes of morbidity and mortality among children throughout the world (1). It is estimated that, in developing countries, more than one-quarter of all children younger than 5 years of age are malnourished (37). Malnutrition has been identified as an important risk factor for predisposition to infections leading to death (36). The strong association between malnutrition and infections has been established through epidemiologic studies conducted in several different countries. The severity of malnutrition determines the risk of death and/or severity of infections (17).
Multiple abnormalities in the immune response, including T-cell number, ratio of T-cell subsets, NK cell activity, and cytokine production, have been described in connection with protein energy malnutrition. Nevertheless, results of studies investigating these topics are controversial (13, 23, 29). Several studies on the effects of malnutrition at the immunological level have been carried out with humans and experimental animals. These studies indicate that malnutrition decreases T-cell function, cytokine production, and the ability of lymphocytes to respond appropriately to cytokines (8, 20, 6).
The characterization of T-cell responses as either Th1-type responses (dominated by the production of gamma interferon [IFN-γ] as associated with cell-mediated immunity) or Th2-type responses (characterized by production of interleukin-4 [IL-4] and IL-5 and associated with humoral immunity) is important because it provides a basis for understanding how T cells contribute to resistance or susceptibility to different infections (15). This characterization is based on the division of CD4+ T lymphocytes into two subsets first established by Mosmann et al. (26). Th1 cells produce IL-2, IFN-γ, and tumor necrosis factor alpha and mediate immunity to viral and bacterial pathogens, whereas Th2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13 and are involved in allergic diseases as well as in defense against parasitic infections (2). Moreover, Th1 and Th2 cells amplify and shape the immune responses that mediate protection of the host during infectious disease (16). The purpose of this study was to assess by flow cytometry the effects of malnutrition on the production of cytokines (IL-2, IFN-γ, IL-4, and IL-10) in CD4+ and CD8+ cells and on the ability of these cells to be activated (as indicated by expression of CD69+ and CD25+).
MATERIALS AND METHODS
Heparinized peripheral blood samples were obtained in the “Hospital General Gustavo Baz Prada” and “Hospital Infantil Iztapalapa.” The study was approved by the Medical Ethics Committee of the General Direction of Medical Services.
Subjects.
Group 1, comprising well-nourished, uninfected (WN) children, included 11 well-nourished children (eight boys and three girls) with no evidence of infection whose ages ranged from 18 to 60 months. All had adequate height and weight according to age (Table 1).
TABLE 1.
Clinical characteristics and nutritional status of WN, WNI, and MNI children
| Study group (n) | Mean age in mos (range) | Mean weight in kg (range) | Mean height in cm (range) | Mean weight deficit (%) | Type of infection (n) |
|---|---|---|---|---|---|
| WN (11) | 40.6 (18-60) | 15.1 (9.5-21) | 93.1 (72-108) | <10 | Not infected |
| WNI (12) | 21.5 (6-48) | 11.7 (7.3-18.5) | 85.4 (67-109) | <10 | Respiratory (11) |
| Sepsis (1) | |||||
| MNIb (12) | 23.7 (6-60) | 7.6 (4.1-13.6) | 79.7 (62-111) | 25.2-55.0 | Gastrointestinal (4) |
| Respiratory (7) | |||||
| Urinary (1) | |||||
| Mixeda (1) |
Gastrointestinal and respiratory infections.
Included second-degree malnourished (5), marasmic (6), and kwashiorkor (1) children.
Group 2, comprising well-nourished, infected (WNI) children, included 12 well-nourished children (six girls and six boys) hospitalized because of respiratory or gastrointestinal bacterial infections. Their ages ranged from 6 to 48 months, and all had adequate weight/height ratios according to age (Table 1).
Group 3, comprising malnourished, infected (MNI) children, included five girls and seven boys whose ages ranged from 6 to 60 months. Five children had second-degree malnutrition (weight/height deficits of >25% and <40% according to age). Seven had severe (third-degree) malnutrition, six with marasmus and one with kwashiorkor. They were hospitalized because of gastrointestinal or respiratory bacterial infections (Table 1).
In all cases, the severity of malnutrition was assessed according to clinical signs and symptoms of malnutrition, as well as weight/height deficits determined according to the established values for Mexican children (30).
Antibodies.
The antibodies utilized were peridinin chlorophyll protein (PerCP)-anti-CD4, PerCP-anti-CD8, fluorescein isothiocyanate (FITC)-anti-IL-2, FITC-anti-IFN-γ, phycoerythrin (PE)-anti-IL-4, allophycocyanin-anti-IL-10, PE-anti-CD25, and allophycocyanin-anti-CD69 (Becton Dickinson Immunocytometry Systems, San José, CA).
Cell preparation and in vitro culture.
Whole blood was prepared for cytokine stimulation as described previously (19). Cells were cultured for 5 h at 37°C and 5% CO2 in RPMI 1640 medium without l-glutamine and fetal bovine serum. Cells were stimulated with phorbol 12-myristate 13-acetate (25 ng/ml; Sigma Chemical Company, St. Louis, Mo.) and ionomycin (1 μg/ml; Sigma) in the presence of brefeldin A (10 μg/ml; Sigma). Activated cultures were aliquoted for staining.
Stain of cell surface antigens.
Specific staining of the respective cell surface molecules was performed with anti-human CD4-PerCP or anti-human CD8-PerCP. One hundred microliters of cell suspension was incubated with 10 μl of a fluorescence-conjugated antibody for 30 min at room temperature. As controls, FITC- and PE-labeled nonspecific mouse immunoglobulin G1 antibodies were used to establish background fluorescence. After incubation, cells were washed with 1% bovine serum albumin prepared in phosphate-buffered saline.
Detection of intracellular cytokines.
After cell surface antigen staining, fluorescence-activated cell sorter lysing solution was added to each tube. After further incubation, samples were centrifuged and treated with fluorescence-activated cell sorter permeabilizing solution (Becton Dickinson) for 10 min at room temperature. Samples were washed with 1% bovine serum albumin in phosphate-buffered saline and incubated with fluorescence-labeled anti-cytokine antibodies for 30 min. After incubation, cells were washed and, finally, were fixed in 1% paraformaldehyde prior to analysis.
Flow cytometry.
After fixation, four-color cytometry was done using a FACSCalibur flow cytometer. A minimum of 10,000 cell-gate events were acquired and analyzed with CELL Quest (Becton Dickinson) software.
Statistical analysis.
Results are expressed as arithmetic means ± standard errors. Differences between groups were analyzed using the Mann-Whitney U test for unpaired samples. Differences with P values of <0.05 were considered significant.
RESULTS
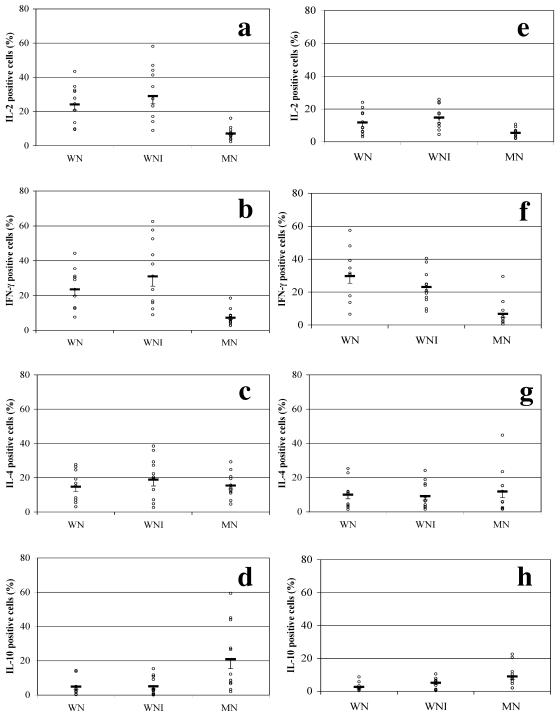
The percentages of CD4+ and CD8+ cells marking positive for IL-2, IFN-γ, IL-4, and IL-10 are shown in Fig. 1. The percentage of CD4+ IL-2-positive cells in malnourished children (7.14% ± 0.74%) was significantly diminished compared to those in WN (24.15% ± 3.33%) and WNI (29.07% ± 4.55%) children (Fig. 1a) (P < 0.005).
FIG. 1.
Effect of malnutrition on percentages of cytokine-positive cells. The percentages of IL-2-, IFN-γ-, IL-4-, and IL-10-producing CD4+ cells from WN (n = 11), WNI (n = 12), and malnourished, infected (MN; n = 12) children are shown on the left. The percentages of IL-2-, IFN-γ-, IL-4-, and IL-10-producing CD8+ cells from the same groups are shown on the right. Data are based upon flow cytometric analysis of 10,000 events and are means ± standard errors. a, P < 0.005 (MN versus WN and WNI groups); b, P < 0.005 (MN versus WN and WNI groups); d, P < 0.005 (MN versus WN group) and P < 0.05 (MN versus WNI group); e, P < 0.05 (MN versus WN group) and P < 0.005 (MN versus WNI group); f, P < 0.005 (MN versus WN group) and P < 0.01 (MN versus WNI group); h, P < 0.005 (MN versus WN group) and P < 0.05 (MN versus WNI group).
Figure 1b shows that the percentages of IFN-γ-positive cells were significantly higher in the WNI (30.99% ± 5.62%) and WN (23.53% ± 3.48%) groups than in MNI children (7.18% ± 1.32%; P < 0.005).
The MNI children showed a decreased percentage (15.44% ± 2.05%) of IL-4-positive cells compared to WN (14.77% ± 2.68%) and WNI (18.85% ± 3.60%) children. The percentages of CD4+ IL-4-positive cells showed no statistical differences among groups (Fig. 1c). In contrast, the percentage of IL-10-positive cells was significantly higher in the MNI group (21.12% ± 5.59%) than in the WN (4.89% ± 1.54%) and WNI (5.09% ± 1.53%) groups (Fig. 1d) (P < 0.005).
Decreased production of type 1 cytokines was observed in CD8+ cells from malnourished children (IL-2-positive cells, 5.38% ± 0.81%, and IFN-γ-positive cells, 6.78% ± 2.32%; P < 0.05) compared with that in cells from well-nourished, uninfected (11.75% ± 2.16% and 29.68% ± 4.42%, respectively) and well-nourished, infected (14.78% ± 2.02% and 23.10% ± 2.92%, respectively) children (Fig. 1e and f).
Even though the percentage of CD8+ IL-4-producing cells tended to be greater in malnourished children than in WN and WNI children (11.85% ± 3.61%, 10.04% ± 2.43%, and 9.17% ± 2.21%, respectively), the difference was not statistically significant (Fig. 1g). In contrast, the percentage of CD8+ IL-10-positive cells was significantly higher in MNI children (8.97% ± 1.95%) than in WN (2.64% ± 0.81%; P < 0.005) and WNI (5.14% ± 1.15%; P > 0.05) children, as shown in Fig. 1h.
In the malnourished group, the number of IL-4-expressing cells markedly exceeded the number of IFN-γ-expressing cells. The production of the type 1 cytokine IFN-γ in malnourished children was depressed in comparison with that in well-nourished, uninfected and well-nourished, infected children. These data indicate an alteration in the balance of type 1/type 2 cytokine responses related to malnutrition.
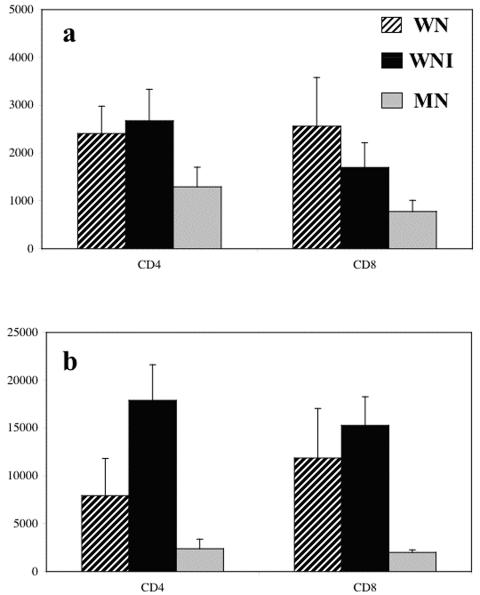
The level of expression, indicated by the fluorescence intensity (FI), of activation markers CD69 and CD25 was analyzed. FIs of CD25+ cells were similar in WN and WNI children. The FIs of CD4+ CD25+ cells and CD8+ CD25+ cells were significantly higher in both WN and WNI groups than in MNI children (Fig. 2a) (P of <0.01 and <0.05, respectively).
FIG. 2.
Expression of activation antigens CD25 (a) and CD69 (b) by CD4+ and CD8+ cells from activated peripheral blood cells. Cells were activated with phorbol 12-myristate 13-acetate-ionomycin for 5 h at 37°C. Cells were stained and analyzed as described in Materials and Methods. Data are based upon flow cytometric analysis of 10,000 events and are means ± standard errors. Results are expressed as FI. (a) CD4, P < 0.01 (MN versus WN and WNI groups; CD8), P < 0.001 (MN versus WN and WNI groups). (b) CD4, P < 0.001 (MN versus WNI group) and P < 0.05 (WNI versus WN group); CD8, P < 0.001 (MN versus WN and WNI groups).
When FIs of CD69+ cells were compared, they were clearly higher in both WN and WNI groups. Additionally, it was clear that the malnourished children showed an impaired activation response. FIs for CD4+ activated cells and CD8+ activated cells were significantly lower for the MNI group (Fig. 2b) (P < 0.001).
DISCUSSION
Malnutrition in children is associated with increased risk of infection and death. Multiple abnormalities in the immune response in malnourished children have been described and could account for increased severity and frequency of infections. The generation of protective T-cell responses against infectious agents is a complex process in which cytokines and costimulatory molecules provide signals that direct the development of adaptive immunity (15). In this study, the production of type 1 cytokines (IL-2 and IFN-γ) and type 2 cytokines (IL-4 and IL-10) in CD4+ and CD8+ cells was evaluated to determine whether the impaired immunological responses observed during malnutrition were associated with alterations in cytokine production.
We analyzed three groups of children: well-nourished, uninfected (WN), well-nourished, infected (WNI), and malnourished, infected (MNI) children, in order to identify possible infection-related and malnutrition-related alterations. In general, malnourished children had severe infections; therefore, the inclusion of a group of well-nourished, infected children also hospitalized with severe infections was considered important. This group may indicate changes in cytokine expression and activation related to infections. However, the comparison between WNI and MNI groups revealed differences that may be related to malnutrition.
Peripheral blood CD4+ and CD8+ cells from malnourished children showed reduced production of type 1 cytokines (IL-2 and IFN-γ) compared with that in cells from well-nourished, uninfected and well-nourished, infected children. In contrast, an increase in the production of type 2 cytokines (IL-4 and IL-10) was found. The decreases in IL-2 and IFN-γ production observed in malnourished children are in agreement with results of previous studies (6, 24).
Previously, alterations in the capacity to produce some cytokines in malnourished children have been reported. González et al. (10) observed that lymphocytes were unable to secrete normal quantities of cytokines or to achieve adequate immunological function and proposed that the altered physiology of lymphocytes may be related mainly to the impairment of the immunological response observed in malnourished children.
Previous findings indicate that in CD8+ T cells, diminished IL-2 production induces anergy (i.e., failure to proliferate to antigen) that inhibits autocrine IL-2 production (21). In our laboratory, it has been demonstrated that malnourished children show a lower proportion of memory cells (CD45RO+) than well-nourished children (27). A previous study showed that the reduced proportion of IL-2-producing cells is predominantly associated with a decreased proportion of IL-2-producing CD45RO+ cells (4). Therefore, the reduction in IL-2-expressing cells in malnourished children may be due to the reduced number of CD45RO+ cells and/or to the reduced capacity of CD45RO+ cells to produce cytokines.
The data obtained in the present study showed a significant decrease in IL-2 production by CD4+ and CD8+ cells from malnourished children, and this may be an important factor related to the increased susceptibility to infections observed in malnourished children. Additionally, results obtained in the present study showed a significant decrease in IFN-γ production by CD4+ and CD8+ cells. IFN-γ is a key cytokine in the development of type 1 immune responses, which are required for the elimination of pathogens (38). Also, IFN-γ induces differentiation and activation of monocytes/macrophages and enhances their microbicidal effector functions (5). Moreover, IL-2 stimulates IFN-γ production (24), and therefore reduced IL-2 expression further inhibits IFN-γ synthesis. The decreased IFN-γ production may be also related to the impaired cell-mediated immunity shown in children with malnutrition.
Data revealed that the mean percentages of CD4+ and CD8+ IL-4-expressing cells in malnourished children were increased in relation to those in well-nourished children. A previous study showed that when CD8+ T cells are activated in the presence of IL-4 they lose their cytotoxic functions. Frequent and/or prolonged production of IL-4 could contribute to the elevated serum immunoglobulin levels reported in undernourished children (31). The higher IL-4 production observed in this study may contribute to the decreased immune responses shown in malnourished children.
An important increase in the percentages of CD4+ and CD8+ IL-10-expressing cells was evident in malnourished children. Type 2 cytokine IL-10 is widely regarded as a suppressor factor for type 1 responses (32, 25).
Induction of higher levels of IL-10, and a reciprocal inhibition of IL-2 production by innate cells, has been reported in connection with a large number of pathogens (22). Moreover, IL-10 has a direct effect on CD4+ T cells, the suppression of IL-2, and IFN-γ secretion (9, 33).
In addition, it has been reported that IL-10 can decrease the cytotoxic functions of CD8+ T cells when it is added before or at the time of activation (12). Similar results were obtained for CD4+ T cells (11). IL-10 is a suppressive cytokine that may contribute to the decreased production of IL-2 and IFN-γ observed in this study. Therefore, IL-10 may be an important immunosuppressive factor related to the impaired immune response observed in malnourished children.
CD69 and CD25 antigen expression.
In the present study, we demonstrated that the activation capability of cells from malnourished children is decreased. The cells from MNI children showed a reduced level of expression of activation markers, both CD69, an early activation marker (18), and CD25, which is expressed later in response to stimuli (3).
Other authors have previously described a decreased response to mitogens in T cells from malnourished children (35, 6, 14), and interestingly, activation and proliferation of T cells in the presence of IL-10 can induce unresponsiveness/anergy (7, 11, 34). These results agree with the data observed here. In a previous study, it was found that the activation capability of T-lymphocyte subsets (CD4+ CD69+and CD8+ CD69+) is considerably decreased in malnourished children. These results suggest that the peripheral blood lymphocytes from malnourished children are unable to start their activation process, and this finding might help to explain some of the T-cell immunoregulatory abnormalities observed in these children (28).
No differences were observed according to the degree of malnutrition and type of infection. Further studies including a greater number of patients will be necessary to address the relationship between these factors.
In conclusion, the results obtained in this study show that malnutrition severely impairs IL-2 and IFN-γ production. In contrast, data show an increase of IL-4 and IL-10 production by CD4+ and CD8+ cells in malnourished children. These findings show that malnutrition alters the balance of type 1 and type 2 responses. In addition, the activation capability of CD4+ and CD8+ cells is considerably decreased. These alterations may contribute to the reduced immunological capacity and to the increased sensitivity to infection associated with malnutrition.
Acknowledgments
This study was partially supported by CONACyT, México (grant 95819), and FOMES, México (grant 98-35-28).
We thank Patricia Birot and Violeta Villadozola, from “Hospital General Gustavo Baz Prada,” for their help in collecting blood samples, Edith Cortés for her technical assistance, and Humberto González-Márquez for editing of graphics.
REFERENCES
- 1.Anonymous. 1999. Management of severe malnutrition: a manual for physicians and other senior health workers. World Health Organization, Geneva, Switzerland.
- 2.Biller, H., B. Bade, H. Matthys, W. Luttmann, and C. Virchow. 2001. Interferon-γ secretion of peripheral blood CD8+ T lymphocytes in patients with bronchial asthma: in vitro stimulus determines cytokine production. Clin. Exp. Immunol. 126:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso, A., S. Licenziati, M. Corulli, A. D. Canaris, M. A. de Francesco, S. Fiorentini, L. Peroni, F. Fallacara, F. Dima, A. Balsari, and A. Turano. 1997. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 27:71-76. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers, I. M. H., G. Janossy, M. Contreras, and C. Navarrete. 1998. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood 92:11-18. [PubMed] [Google Scholar]
- 5.Chan, J., K. Tanaka, C. Mannion, D. Carroll, M. Tsang, Y. Xing, C. Lowenstein, and B. Bloom. 1997. Effects of protein calorie malnutrition on mice infected with BCG. J. Nutr. Immunol. 5:11-19. [Google Scholar]
- 6.Chandra, R. K. 1991. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am. J. Clin. Nutr. 53:1087-1101. [DOI] [PubMed] [Google Scholar]
- 7.Chernoff, A. E., E. V. Granowitz, L. Shapiro, E. Vannier, G. Lonnemann, J. B. Angel, J. S. Kennedy, A. R. Rabson, S. M. Wolff, and C. A. Dinarello. 1995. A randomized, controlled trial of IL-10 in humans: inhibition of inflammatory cytokine production and immune responses. J. Immunol. 154:5492-5496. [PubMed] [Google Scholar]
- 8.Dai, G., and D. N. McMurray. 1998. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect. Immun. 66:3562-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt, R., H. Yssel, and J. E. de Vries. 1993. Direct effects of IL-10 on subsets of human CD4C T cell clones and resting T cells. J. Immunol. 150:4754-4765. [PubMed] [Google Scholar]
- 10.González, C., L. Rodríguez, E. Bonilla, M. Betancourt, N. Siller, E. Zumano, and R. Ortiz. 1997. Electrophoretic analysis of plasmatic and lymphocyte secreted proteins in malnourished children. Med. Sci. Res. 25:643-646. [Google Scholar]
- 11.Groux, H., M. Bigler, J. E. de Vries, and M. G. Roncarolo. 1996. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4 T cells. J. Exp. Med. 184:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groux, H., M. Bigler, J. E. de Vries, and M. G. Roncarolo. 1998. Inhibitory and stimulatory effects of IL-10 on human CD8 T cells. J. Immunol. 160:3188-3193. [PubMed] [Google Scholar]
- 13.Hoffman-Goetz, L. 1988. Lympokines and monokines in protein-energy malnutrition, p. 9-23. In R. K. Chandra (ed.), Nutrition and immunology. Alan R. Liss, Inc., New York, N.Y.
- 14.Hughes, D. A. 1998. The influence of the diet on the maturation of the immune system. Allergy 55:26-28. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, C. A., and S. L. Reiner. 2000. Cytokines and T cells in host defense. Curr. Opin. Immunol. 12:413-418. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic, D., L. Zhugong, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 17.Kuvibidila, S., J. A. Mark, R. P. Wagner, L. Yu, and D. Ode. 1995. Soluble transferrin receptor as an index of iron status in Zaïrian children with malaria. Am. J. Trop. Med. Hyg. 98:373-378. [PubMed] [Google Scholar]
- 18.Maino, V. C., M. A. Suni, and J. J. Ruitenberg. 1995. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry 20:127-133. [DOI] [PubMed] [Google Scholar]
- 19.Maino, V. C. 1998. Rapid assessment of antigen induced cytokine expression in memory T cells by flow cytometry. Vet. Immunol. Immunopathol. 63:199-207. [DOI] [PubMed] [Google Scholar]
- 20.Malavé, I., M. Vethercourt, R. Chacón, D. Quiñones, C. Rebrij, and G. Bolívar. 1998. Production of interleukin-6 in cultures of peripheral blood mononuclear cells from children with primary protein-calorie malnutrition and from eutrophic controls. Ann. Nutr. Metab. 42:266-273. [DOI] [PubMed] [Google Scholar]
- 21.Malek, T. R. 2002. T helper cells, IL-2, and the generation of cytotoxic T-cell responses. Trends Immunol. 23:465-467. [DOI] [PubMed] [Google Scholar]
- 22.McGuirk, P., and. H. G. Mills Kingston. 2002. Review: pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 23.McMurray, D. N. 1984. Cell-mediated immunity in nutritional deficiency. Prog. Food Nutr. Sci. 8:193-228. [PubMed] [Google Scholar]
- 24.Mengheri, E., F. Nobili, G. Crocchioni, and J. Lewis. 1992. Protein starvation impairs the ability of activated lymphocytes to produce interferon-γ. J. Interferon Res. 12:17-21. [DOI] [PubMed] [Google Scholar]
- 25.Moore, K. W., R. DeWaal Malefyt, L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Gredlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 27.Nájera, O., C. González, G. Toledo, L. López, E. Cortés, M. Betancourt, and R. Ortiz. 2001. CD45RA and CD45RO isoforms in infected malnourished and infected well-nourished children. Clin. Exp. Immunol. 126:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nájera, O., C. González, G. Toledo, E. Cortés, L. López, M. Betancourt, and R. Ortiz. 2001. Early activation of T, B and NK lymphocytes in infected malnourished and infected well-nourished children. J. Nutr. Immunol. 5:85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nájera, O., C. González, G. Toledo, L. López, and R. Ortiz. 2004. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin. Diagn. Lab. Immunol. 11:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-Galván, R. 1976. Somatometría pediátrica. Arch. Investig. Med. (Mexico) 6(Suppl. 1):5. [PubMed]
- 31.Reddy, V., N. Raghutamulu, and P. Bhaskaram. 1976. Secretory IgA in protein calorie malnutrition. Arch. Dis. Child. 51:871-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher, A., and R. L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385-409. [DOI] [PubMed] [Google Scholar]
- 33.Taga, K., H. Mostowski, and G. Tosato. 1993. Human interleukin-10 can directly inhibit T-cell growth. Blood 81:2964-2967. [PubMed] [Google Scholar]
- 34.Taga, K., and G. Tosato. 1991. IL-10 inhibits human T cell proliferation and Il-2 production. J. Immunol. 148:1143-1148. [PubMed] [Google Scholar]
- 35.Touraine, J., and G. Gay. 1981. Déficit immunitaire secondaire à la malnutrition. Gastroenterol. Clin. Biol. 5:835-838. [PubMed] [Google Scholar]
- 36.Tupasi, T. E., M. A. Velmonte, M. E. G. Sanvictores, L. Abramham, L. E. D. Leon, S. A. Tan, C. A. Miguel, and M. C. Saniel. 1988. Determinants of morbidity and mortality due to acute respiratory infections: implications and intervention. J. Infect. Dis. 157:615-623. [DOI] [PubMed] [Google Scholar]
- 37.United Nations Administrative Committee on Coordination. 2000. Fourth report on the world nutrition situation. United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition, Geneva, Switzerland.
- 38.Young, H. A., and K. J. Hardy. 1995. Role of interferon-γ in immune cell regulation. J. Leukoc. Biol. 58:373-381. [PubMed] [Google Scholar]