Abstract
Peste des petits ruminants (PPR) is a contagious viral disease of small ruminants that is of economic importance in Africa, the Middle East, and Asia. We developed a rapid competitive enzyme-linked immunosorbent assay (rapid c-ELISA) for the diagnosis and surveillance of PPR. This assay detects PPR virus (PPRV) antibodies in serum samples by quantifying the amount of monoclonal antibody (MAb) P-3H12 after 30 min of incubation of a serum-MAb conjugate mixture on plates coated with a PPRV recombinant nucleocapsid protein (rPPRV-N). We tested 249 PPRV-positive serum samples and 733 PPRV-negative serum samples from field ruminants. The threshold of percent inhibition (PI) was determined to be <50 on the basis of the mean PI plus 3 standard deviations for sera from PPRV-negative ruminants. The relative specificity and sensitivity of the rapid c-ELISA were 98.5% (722 of 733 serum samples) and 93.4% (234 of 249 serum samples), respectively. The rapid c-ELISA sensitively detected PPRV antibodies in hyperimmune sera (virus neutralization test [VNT] titer, >512), even at dilutions ≥512 in normal goat serum, and as early as 6 to 13 days postinfection from 12 goats, each of which was infected with one of the four PPRV lineages. Hyperimmune sera from animals experimentally vaccinated with rinderpest virus gave positive results by the rapid c-ELISA when the rinderpest virus VNT titers were >512, although the rapid c-ELISA titers were very low (2 to 16). However, the rapid c-ELISA was negative when the rinderpest virus VNT titer was ≤128. The rapid c-ELISA developed in the present work provides a short turnaround time and could be a useful tool for the diagnosis of PPR and screening for PPRV in the field.
Peste des petits ruminants (PPR) is an acute and highly contagious viral disease of small ruminants, such as sheep and goats, with high rates of morbidity and sometimes high rates of mortality. Economically, it has been the most important disease of these species in sub-Saharan Africa, the Middle East, and southwest Asia since it was first described in West Africa in 1942 (20). It also results in subclinical infection in large ruminants, which act as carriers of infection to small ruminants (1, 14). The PPR virus (PPRV) belongs to the genus Morbillivirus in the family Paramyxoviridae and is closely related to rinderpest virus (RPV) (2, 16). At least four distinct genetic lineages (lineages I, II, III, and IV) of PPRV with different geographical distributions circulate among small ruminants in regions of endemicity (6, 20).
A vigorous program for PPR eradication has been carried out in these regions, including vaccination, seromonitoring, serosurveillance, and the destruction of infected animals and those that have been in contact with them. Rapid detection of infected animals is very important for PPR controls to be effective. Severe cases in which animals show clinical signs in the field can easily be detected through clinical surveillance and the detection of antigen in clinical samples, while the diagnosis of PPRV infection in subclinically infected animals can be achieved by serological surveillance. However, the test prescribed for the detection of PPRV antibody, a virus neutralization test (VNT), is laborious and expensive and requires infectious virus. For these reasons, VNT is not ideal for large-scale routine testing.
Due to their simplicity, high sensitivity, and economy, several competitive enzyme-linked immunosorbent assays (c-ELISAs) have been recognized as suitable systems for use for diagnosis and seroepidemiological surveillance. They target the hemagglutinin (H) protein (19, 21) or nucleocapsid (N) protein (4, 9, 11, 12, 13, 14). The c-ELISA procedure consists of at least four incubation steps, including adsorption of the antigen onto a solid phase, competitive binding of a serum-monoclonal antibody (MAb) mixture to the antigen, detection of the MAb bound to the antigen, and the substrate reaction. Here, we describe a rapid c-ELISA with a short turnaround time (≤1 h), This rapid c-ELISA is a simple, fast, reliable, and inexpensive tool for diagnostic and epidemiological purposes.
MATERIALS AND METHODS
Preparation of antigen-coated ELISA plates.
A recombinant N protein of PPRV (rPPRV-N) was prepared as described previously (4) in Spodoptera frugipera (Sf9) cells infected with a recombinant baculovirus (Bacmid/PPRV-N) that expresses the PPRV N protein. rPPRV-N was expressed as a fusion protein with a six-histidine tag on its amino-terminal end so that, by using an anti-His antibody, the antigen could be easily purified from cell lysates or quantified to minimize batch-to-batch variations. Briefly, Sf9 cells were infected with Bacmid/PPRV-N at a multiplicity of infection of 5, washed once, resuspended in a 1/20 volume of a lysis buffer (0.01 M phosphate-buffered saline [PBS] containing 1% Nonidet P-40, 0.05% Tween 20, and protease inhibitors), sonicated briefly, and clarified by centrifugation at 500 × g for 20 min at 4°C. The supernatant was used as the antigen in the ELISA. If necessary, the antigen was further purified by an affinity chromatography method with a ProBond purification system (Invitrogen, Carlsbad, Calif.), according to the instructions of the manufacturer.
Each well of the ELISA plates (MaxiSorp; Nunc, Roskilde, Denmark) was coated with 50 μl of the rPPRV-N antigen of predetermined concentration in 0.01 M PBS (pH 7.2) for 1 h at 37°C with constant shaking. After a brief wash, each well of the plates was incubated with 50 μl of 1× Biostab immunoassay stabilizer solution (Sigma-Aldrich, St. Louis, Mo.) diluted in a blocking buffer (0.01 M PBS containing 5% skim milk) for 1 h at room temperature, shaken off, dried in a vacuum dryer, sealed, and stored at 4°C until use for the rapid c-ELISA.
Peroxidase-labeled MAb.
MAb P-3H12 was purified with an ImmunoPure (A/G) immunoglobulin G purification kit (Pierce) and then peroxidase labeled with a peroxidase labeling kit (Roche, Mannheim, Germany), according to the instructions of the manufacturer. Briefly, 0.3 ml of purified MAb (4.0 mg/ml) in sodium carbonate-hydrocarbonate buffer (100 mM; pH 9.8) was coupled to 0.1 ml of activated peroxidase solution (16 mg/ml) for 2 h at 25°C. The reaction was stopped by the addition of 40 μl of 2 M triethanolamine solution (pH 9.8) for 30 min at 4°C and then with an additional 25 μl of the same solution for 2 h at 4°C. After the addition of 10 μl of 1 M glycine solution (pH 7.0), the MAb conjugate was allowed to dialyze extensively and was then suspended in the same volume of stabilizing reagent (included with the kit). The MAb conjugate solution was stored at 4°C for short periods or was lyophilized for longer-term storage at 4°C.
Sera.
Two bovine serum samples with anti-RPV antibodies against RPV lineages I (αRPV-I) and II (αRPV-II) were kindly supplied by H. M. Wamwayi (Kenya Agriculture, Research Institute [KARI], Muguga, Kenya). Four goat serum samples with anti-PPRV antibodies against PPRV lineages I (αPPRV-I), II (αPPRV-II), III (αPPRV-III), and IV (αPPRV-IV) were kindly supplied by E. Couacy-Hymann (Laboratoire National d'Appui au Développement Agricole, Laboratoire Central de Pathologie Animale [LANADA/LCPA], Bingerville, Ivory Coast). Rabbit serum with anti-RPV antibodies against the RPV-RBOK vaccine strain (αRPV-RBOK) was kindly supplied by J. Anderson (Institute for Animal Health, Pirbright, United Kingdom). Serum samples (samples K9061, K9062, and Rb001 to Rb010) from 12 cattle vaccinated with the RPV LATC strain (103.0 to 104.0 50% tissue culture infective doses per dose) were prepared in our laboratory. Two of the serum samples (samples K9061 and K9062) were collected from two animals that had received two inoculations, with a 3-week interval between inoculations for each animal; and the other serum samples (samples Rb001 to Rb010) were collected from cattle that had been vaccinated once. All cattle were bled 3 weeks after their last vaccination.
Control serum samples included in a reference c-ELISA kit for PPR serology (14) were used to optimize the rapid c-ELISA in this study; these included strongly positive, weakly positive, and negative sera. E. Couacy-Hymann (LANADA/LCPA) kindly supplied a total of 70 serum samples from 12 goats experimentally infected with the four PPRV lineages (lineages I, II, III, and IV; three goats were infected with each PPRV lineage).
In addition to the experimental sera described above, a total of 982 field serum samples were used to evaluate the rapid c-ELISA. PPR-positive caprine and ovine sera (n = 249) from West Africa, where PPR is endemic, were obtained from the Département d'Élevage et Médecine Vétérinaire, Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD-EMVT), Montpellier, France. PPRV-negative sera (n = 733) were collected from Korean flocks and herds of goats (n = 409) and cattle (n = 324) of various sizes. All sera were confirmed to be positive or negative by a VNT for PPRV.
Indirect ELISA.
Indirect ELISAs were used to titrate the rPPRV-N protein to the concentrations needed for the preparation of ELISA plates and the peroxidase-labeled MAb needed for competition with serum antibodies. Briefly, ELISA plates coated with 25 μl of serial dilutions of rPPRV-N antigen were incubated with 25 μl of serial dilutions of peroxidase-labeled MAb P-3H12 in a blocking buffer (0.01 M PBS, 5% skim milk, 0.05% Tween 20) for 30 min at 37°C. After the plates were washed, they were incubated with 50 μl of o-phenylenediamine substrate (Sigma-Aldrich) for 10 min at room temperature, and the colorimetric reaction was stopped by adding 50 μl of 1.25 M sulfuric acid. The optical density (OD) of each well was measured at a wavelength of 492 nm.
Rapid c-ELISA.
Antigen-coated plates were incubated with 50 μl of a mixture of equal volumes of the MAb P-3H12 conjugate (final dilution, 1:800 in blocking buffer) and the test serum sample (final dilution, 1:20 in blocking buffer) for 30 min at 37°C. Strongly positive, weakly positive, and negative control sera were included. In each run, all sera, including the serum controls, were tested in duplicate. The amount of MAb conjugate bound to the antigen was quantified by using the o-phenylenediamine substrate described above. The OD of each well was converted to the percent inhibition (PI) induced by the competition between the MAb and serum antibodies by using the following formula: [1 − (OD of serum-MAb mixture/OD of MAb alone)] × 100. This test was repeated three times.
VNT.
A previously described (15) microtiter VNT technique was used, with some modifications, to detect neutralizing antibodies in sera. Prior to the test, all sera were heat inactivated at 56°C for 30 min. A twofold deletion series was created by starting with a 1:10 final dilution; 25 μl of each dilution was added in duplicate, followed by incubation with 100 50% tissue culture infective doses of PPRV strain Nig75/1 (7) or RPV strain LATC (3) at 37°C for 45 min. One hundred microliters of Vero cells (American Type Culture Collection, Manassas, Va.) at a concentration of 1.5 × 105 cells/ml was added to each well. The wells without virus served as controls. The plates were monitored for the cytopathic effects of PPRV for 7 days. The VNT titer was defined as the highest dilution of serum that inhibited the cytopathic effect by 50%. Sera with VNT titers of ≤1:10 were considered negative.
Reference c-ELISA kit.
A PPRV c-ELISA kit developed at CIRAD-EMVT, a Food and Agriculture Organization reference laboratory for PPRV, was used as the reference kit in the present work. All procedures were carried out according to the instructions in the manual included with the kit. Wells with PI values ≥50 were considered positive.
RESULTS
Optimization of rapid c-ELISA.
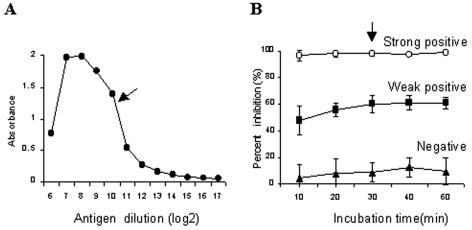
In the present work, a rapid c-ELISA was designed to test serum samples by quantifying the amount of MAb bound after a short incubation with a mixture of serum and the MAb on antigen-coated plates. The recombinant nucleocapsid protein rPPRV-N was used as an antigen for the rapid c-ELISA: 10 ml of the rPPRV-N antigen was prepared from 200 ml of Sf9 cells (2 × 106 cells per ml) infected with the Bacmid/PPRV-N recombinant baculovirus in a spinner apparatus and titrated with unlabeled MAb P-3H12 to determine the optimal dilution of the antigen required to coat the plates (Fig. 1A). The optimal dilution of the crude protein was determined to be 1:1,600, which corresponded to approximately 3.6 μg of purified rPPRV-N protein per ml. MAb P-3H12 was used as a competing antibody. MAb P-3H12 recognizes an immunodominant epitope within the amino-terminal half of the N protein, as described previously (5). A total of approximately 6 mg of the MAb purified from mouse ascitic fluid was coupled with 8 mg of activated peroxidase. The optimal dilution of the MAb conjugate for the rapid c-ELISA (1:400) was determined by using the endpoint titration method in the rPPRV-N-coated plates. The optimal incubation time for the serum-MAb conjugate mixture (30 min) was determined by using reference serum controls (strongly positive, weakly positive, and negative serum samples) (Fig. 1B). All serum samples were tested at a final dilution of 1:20. To examine the stability of the antigen, ELISA plates coated with rPPRV-N were stored at 4°C for various periods and then tested with the reference serum controls. No reduction in the ELISA reactivity of the antigen was observed within 6 months, the longest time that the plates were stored.
FIG. 1.
Determination of optimal conditions for the rapid c-ELISA. (A) Titration of antigen coated on the ELISA plates; (B) PI values for strongly positive, weakly positive, and negative sera for various incubation times. Arrows, optimal condition.
Threshold, specificity, and sensitivity.
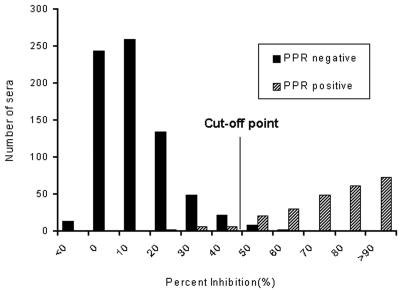
The positive-negative threshold (PI value) of the rapid c-ELISA was arbitrarily set by adding 3 standard deviations to the mean PI value for known PPRV-negative serum samples (n = 733). Here, the rapid c-ELISA gave a mean PI value of 15.8 and a standard deviation of 11.5. Consequently, the threshold PI value was determined to be 50 [(15.8 ± 11.5) × 3] (Fig. 2). On the basis of the threshold (PI > 50), 1.6% of the PPRV-negative serum samples showed nonspecific positive reactions in the rapid c-ELISA, resulting in a relative specificity of 98.5% (722 of 733 serum samples) (Fig. 2).
FIG. 2.
Distribution of PI values for 249 PPR-positive serum samples and 733 PPR-negative serum samples obtained by the rapid c-ELISA. The threshold cutoff value of a PI of 50 was determined on the basis of the distribution of the PPR-negative serum samples (mean PI plus 3 standard deviations).
PPRV-positive ruminant sera (n = 249) with various VNT titers for PPRV were then subjected to the rapid c-ELISA. Of the 249 serum samples, 93.4% (234 of 249 serum samples) gave positive results by the rapid c-ELISA (Fig. 2). All of the positive sera with negative rapid c-ELISA results (n = 15) were also negative with the reference c-ELISA kit, although they were all positive for PPRV by VNT (VNT titers, ≥1:40) (Table 1). Therefore, the relative sensitivity of the rapid c-ELISA compared with the results of VNT was 93.4%.
TABLE 1.
PI values for VNT-positive and rapid c-ELISA-negative serum samples obtained by the rapid c-ELISA and with the reference c-ELISA kit
| Serum sample (species) | PPRV VNT titer | PI value
|
|
|---|---|---|---|
| Reference c-ELISA kit | Rapid c-ELISA | ||
| 2-50 (caprine) | 1:80 | 27 | 31 |
| 2-57 (ovine) | 1:80 | 38 | 35 |
| 2-63 (ovine) | 1:113 | 38 | 45 |
| 2-88 (caprine) | 1:113 | 32 | 17 |
| 3-5 (ovine) | 1:80 | 47 | 31 |
| 3-13 (ovine) | 1:56 | 26 | 31 |
| 3-56 (ovine) | 1:80 | 36 | 38 |
| 4-32 (caprine) | 1:40 | 30 | 32 |
| 4-50 (caprine) | 1:113 | 48 | 37 |
| 5-51 (ovine) | 1:113 | 24 | 25 |
| 5-56 (ovine) | 1:40 | 22 | 29 |
| 5-88 (caprine) | 1:113 | 38 | 47 |
| 5-89 (caprine) | 1:113 | 32 | 46 |
| 7-17 (ovine) | 1:56 | 35 | 42 |
| 7-56 (ovine) | 1:56 | 41 | 41 |
To determine if the rapid c-ELISA detects cross-reactive RPV antibodies, we tested RPV-positive sera with various VNT titers as well as hyperimmune RPV-positive caprine sera. All serum samples were serially diluted with normal bovine serum, and the endpoint titers were determined by the rapid c-ELISA. All PPRV-positive caprine sera (αPPRV-I, αPPRV-II, αPPRV-III, and αPPRV-IV) had ELISA titers ≥512, similar to the VNT titers for PPRV. Hyperimmune RPV sera αRPV-I, αRPV-II, and αRPV-RBOK (with VNT titers of ≥512 for RPV) had titers of 16, 8, and 2, respectively, by the rapid c-ELISA, indicating the presence of cross-reactive RPV antibodies. Meanwhile, 12 serum samples from RPV-vaccinated cattle with VNT titers ≤1:256 gave negative results by the rapid c-ELISA (Table 2).
TABLE 2.
Endpoint antibody titration by the rapid c-ELISA and VNT of sera from animals experimentally immunized with PPRV or RPV
| Serum sample | VNT titera | c-ELISA titerb |
|---|---|---|
| PPRV hyperimmune serum | ||
| αPPRV-I | >1:512 | 1:512 |
| αPPRV-II | >1:512 | 1:512 |
| αPPRV-III | >1:512 | >1:512 |
| αPPRV-IV | >1:512 | >1:512 |
| RPV hyperimmune serum | ||
| αRPV-I | >1:512 | 16 |
| αRPV-II | >1:512 | 8 |
| αRPV-RBOK | 1:1,280 | 2 |
| RPV-vaccinated serumc | ||
| K9061 | 1:256 | <2 |
| K9062 | 1:180 | <2 |
| Rb001 | 1:22 | <2 |
| Rb002 | 1:16 | <2 |
| Rb003 | 1:45 | <2 |
| Rb004 | 1:64 | <2 |
| Rb005 | 1:32 | <2 |
| Rb006 | 1:32 | <2 |
| Rb007 | 1:22 | <2 |
| Rb008 | 1:32 | <2 |
| Rb009 | 1:16 | <2 |
| Rb010 | 1:16 | <2 |
VNT was with homologous viruses PPRV Nig75/1 for PPRV and RPV LATC for RPV.
The titer represents the highest serum dilution showing PI values of ≥50.
Two cattle (animals K9061 and K9062) were vaccinated twice, with a 3-week interval between inoculations, and the other animals (animals Rb001 to Rb010) received one inoculation each. All cattle were bled 3 weeks after the last vaccination. RPV strain LATC was used.
Measurement of anti-N antibody titers in sera of goats experimentally infected with PPRV.
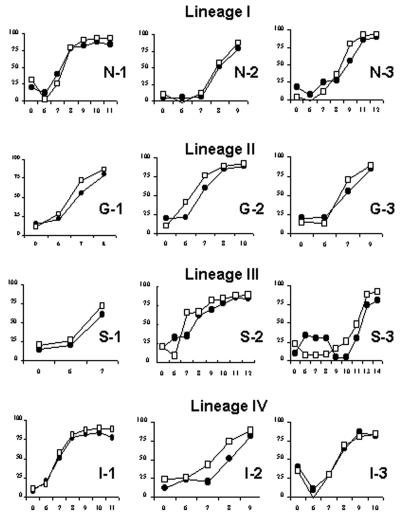
We next compared the dynamics of the humoral immune responses to PPRV infection obtained by the rapid c-ELISA and with the reference c-ELISA kit. We tested a total of 70 serum samples that had been collected sequentially from experimentally infected goats following PPRV infection with the four lineage groups (three goats per group). As determined by the rapid c-ELISA, seroconversion was detected in all goats between 6 and 13 days after infection, as shown in Fig. 3. Most animals seroconverted on the same day in both tests, but some individuals (animals S-2, S-3, and I-2) were found to be positive 1 day sooner by the rapid c-ELISA than with the reference c-ELISA kit. Overall, however, there was no indication that the rapid c-ELISA was capable of detecting seropositive animals sooner after infection than the reference c-ELISA kit, since the dynamics of the antibody responses were similar in both tests.
FIG. 3.
Kinetics of early antibody development determined by the rapid c-ELISA and with the reference c-ELISA kit in goats (n = 12) with induced PPRV infections (three goats infected with each PPRV lineage). The cutoff PI value was set at 50. Open squares, results obtained by the rapid c-ELISA; closed squares, results obtained with the reference c-ELISA kit.
DISCUSSION
The objective of this study was to develop an ELISA for PPRV serology that retained the sensitivity of currently available commercial c-ELISA kits while being faster and easier to use. The rapid c-ELISA described here fulfills these objectives. The procedure with the commercial c-ELISA kit consists of four reaction steps: adsorption of the antigen onto a solid phase, competition between the serum and an MAb, detection of the MAb bound to the antigen, and a substrate reaction. Therefore, a turnaround time of at least 3 h is needed for these c-ELISA kits. For our rapid c-ELISA, we used antigen (recombinant N protein)-coated plates, a peroxidase-labeled MAb (MAb P-3H12), and a 30-min competition between serum and the MAb. Our rapid c-ELISA thus comprises two reaction steps, a 30-min incubation of the serum-MAb mixture and a substrate reaction, providing fewer reaction steps and a shorter turnaround time than the commercial c-ELISA kits. This made it possible to test serum samples several times during the workday, so our rapid c-ELISA would be suitable for use for diagnostic and epidemiological purposes.
The rPPRV-N antigen coated on the plates for the rapid c-ELISA was prepared with a baculovirus expression system. This system makes it economically possible to produce several hundred times more recombinant antigen than the amount that can be produced by the conventional means of preparation of target antigen from whole virus, as previously described for other morbilliviruses (4, 8, 11, 23). Recombinant N protein is normally prone to rapid degradation by cellular components (such as cellular proteases) or mechanical factors (such as freezing-thawing), so it is generally purified to prevent such degradation. Nevertheless, we were able to use crude cell lysates in the presence of protease inhibitors and a protein stabilizer without any purification, and the activity of the peroxidase and the antigenicity of the protein on the plates were not affected when the recombinant N protein was stored for at least 6 months at 4°C. However, without protease inhibitors and a protein stabilizer, the rPPRV-N antigen should be purified to prevent degradation from cellular factors. rPPRV-N was expressed as a fusion protein with a six-histidine tag on its amino-terminal end so that, by using an anti-His antibody, the antigen could be easily purified from cell lysates or quantified to minimize batch-to-batch variations.
The MAb used in the rapid c-ELISA, MAb P-3H12, showed competition (a positive antibody reaction) with sera from all goats infected experimentally with four lineages of PPRV, although the times to seroconversion measured by the rapid c-ELISA varied. This result indicates that the epitope of MAb P-3H12 is immunodominant and is present on the N proteins of all lineages of PPRV. In particular, the rapid c-ELISA detected seroconversion in most of the animals between 6 and 8 days after infection. Considering the rapid dynamics of the antibody responses to infection, the rapid c-ELISA can be used for diagnostic purposes when virus isolation and antigen detection are not available, for example, when sample conditions are inappropriate, sampling times are insufficient, or the laboratory capacity is inadequate.
In the present work, the rapid c-ELISA failed to detect the PPRV antibody in 15 PPRV-positive field serum samples (n = 249). These ELISA-negative serum samples (n = 15) were also all negative with the reference c-ELISA kit, which also targets antibodies against the PPRV N protein. Libeau et al. (14) reported that the response of the anti-N antibody measured with the c-ELISA kit (up to 90 days) did not last as long as the response of the neutralizing antibody (up to 120 days) in kids born to vaccinated mothers. Similar observations have been reported for RPV (10). Therefore, the lower sensitivity of the rapid c-ELISA compared with that of VNT might be due to some degree to the different kinetics between neutralizing antibodies and the N protein, although the actual histories of the animals from which the field sera were collected are not known.
The rapid c-ELISA cross-reacted with hyperimmune RPV-positive sera that had VNT titers >512, although the ELISA titers of the RPV-positive sera were very low compared to those of the hyperimmune PPRV-positive sera. Meanwhile sera (n = 12) from animals that had been vaccinated against RPV and that had VNT titers of ≤1:256 were negative by the rapid c-ELISA. Thus, the rapid c-ELISA can detect cross-reactive RPV antibodies in the sera of ruminant animals in regions where RPV had been endemic in the past or where RPV vaccination (in particular, repeated vaccinations) had been performed. If so, the results for samples from these regions positive by the rapid c-ELISA should be confirmed by a secondary immunoassay (i.e., a PPRV- or RPV-specific c-ELISA) or cross VNTs for PPRV and RPV. In addition, the RPV vaccination histories of the animals should be taken into consideration. Fortunately, in most regions of endemicity in the world, RPV has already been eradicated and RPV vaccination has stopped (17, 18). Under some circumstances, endpoint titration by the rapid c-ELISA might be a feasible tool for differentiating field RPV infection from RPV vaccination, because the rapid c-ELISA can detect cross-reactive RPV antibodies with high VNT titers (>1:512). Conversely, the results may indicate that the rapid c-ELISA failed in practice to differentiate PPRV-infected animals from those that had recovered from virulent RPV infection. Nevertheless, rapid detection of animals infected with or exposed to either PPRV or RPV may be a useful tool for the timely implementation of emergency control programs, since both diseases are highly contagious.
Unlike rinderpest, PPR is spreading from areas of endemicity in Africa and Asia to neighboring countries and has devastated the livestock industry. Countries free of the disease, especially countries or regions neighboring areas of endemicity, require extensive surveillance to prevent the introduction of the disease to naïve animal populations. The capability of the rapid c-ELISA to deal with a large number of samples at a time and its short turnaround time may better serve the needs of surveillance and control programs. Therefore, although the test remains to be further evaluated extensively in the field, the rapid c-ELISA described in this report may be a useful serological tool.
Acknowledgments
We thank G. Libeau (CIRAD-EMVT), J. Anderson (Institute for Animal Health), H. M. Wamwayi (Kari NRVC), and E. Couacy-Hymann (LANADA/LCPA) for supplying us with diagnostic reagents and field sera. Chan-jin Lee and Sang-Mi Kang are also gratefully acknowledged for excellent technical assistance.
This work was supported by a grant from the National Veterinary Research and Quarantine Service, Korean Ministry of Agriculture and Fishery.
REFERENCES
- 1.Anderson, E. C., M. Jago, T. Mlengeya, C. Timms, A. Payne, and K. Hirji. 1990. A serological survey of rinderpest antibody in wildlife and sheep and goats in northern Tanzania. Epidemiol. Infect. 105:203-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, T., I. K. Visser, L. Mamaev, L. Goatley, M. F. van Bressem, and A. D. Osterhaus. 1993. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 193:1010-1012. [DOI] [PubMed] [Google Scholar]
- 3.Choi, K. S., C. H. Kwon, C. U. Choi, J. G. Lee, and Y. B. Kang. 1998. Biological properties of attenuated rinderpest virus (LATC strain) adapted in Vero cell. RDA. J. Vet. Sci. 40:61-70. [Google Scholar]
- 4.Choi, K. S., J. J. Nah, C. U. Choi, Y. J. Ko, H. J. Sohn, G. Libeau, S. Y. Kang, and Y. S. Joo. 2003. Monoclonal antibody-based competitive ELISA for simultaneous detection of rinderpest virus and peste des petits ruminants virus antibodies. Vet. Microbiol. 96:1-16. [DOI] [PubMed] [Google Scholar]
- 5.Choi, K. S., J. J. Nah, K. Y. Ko, S. Y. Kang, K. Yoon, and N. I. Jo. 2005. Antigenic and immunogenic investigation of B-cell epitopes in the nucleocapsid protein of peste des petits ruminants virus. Clin. Diagn. Lab. Immunol. 12:114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhar, P., B. P. Sreenivasa, T. Barrett, M. Corteyn, R. P. Singh, and S. K. Bandyopadhyay. 2002. Recent epidemiology of peste des petits ruminants virus (PPRV). Vet. Microbiol. 88:153-159. [DOI] [PubMed] [Google Scholar]
- 7.Diallo, A., W. P. Taylor, P. C. Lefevre, and A. Provost. 1989. Atténuation d'une souche de virus de la peste des petits ruminants: candidat pour un vaccin homologue vivant. Rev. Elev. Med. Vet. Pays Trop. 42:311-319. [PubMed] [Google Scholar]
- 8.Fooks, A. R., J. R. Stephenson, A. Warnes, B. A. Dowsett, B. K. Rima, and G. W. G. Wilkinson. 1993. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J. Gen. Virol. 74:1439-1444. [DOI] [PubMed] [Google Scholar]
- 9.Hummel, K. B., D. D. Erdman, J. Heath, and W. J. Bellini. 1992. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J. Clin. Microbiol. 30:2874-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institut d'Elevage et de Médecine Vétérinaire des PaysTropicaux. 1966. Connaissances acquises récemment sur la peste bovine et son virus. Rev. Elev. Med. Vet. Pays Trop. 19:365-413. [PubMed] [Google Scholar]
- 11.Ismail, T., S. Ahmad, M. D'Souza-Ault, M. Bassiri, J. Saliki, C. Mebus, and T. Yilma. 1994. Cloning and expression of the nucleocapsid gene of virulent Kabete O strain of rinderpest virus in baculovirus: use in differential diagnosis between vaccinated and infected animals. Virology 198:138-147. [DOI] [PubMed] [Google Scholar]
- 12.Kamata, H., S. Ohkubo, Y. Matsuura, Y. Kamata, K. Tsukiyama-Kohara, K. Imaoka, C. Kai, Y. Yoshikawa, and K. Yamanouchi. 1993. Expression in baculovirus vector system of the nucleocapsid protein gene of rinderpest. J. Virol. Methods 43:159-166. [DOI] [PubMed] [Google Scholar]
- 13.Libeau, G., A. Diallo, D. Calvez, and P. C. Lefevre. 1992. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet. Microbiol. 31:147-160. [DOI] [PubMed] [Google Scholar]
- 14.Libeau, G., C. Préhaud, R. Lancelot, F. Colas, L. Guerre, D. H. L. Bishop, and A. Diallo. 1995. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res. Vet. Sci. 58:50-55. [DOI] [PubMed] [Google Scholar]
- 15.Mariner, J. C., J. A. House, C. A. Mebus, and M. C. van den Ende. 1993. The use of thermostable Vero cell-adapted rinderpest vaccine as a heterologous vaccine against peste des petits ruminants. Res. Vet. Sci. 54:212-216. [DOI] [PubMed] [Google Scholar]
- 16.Mitra-Kaushik, S., R. Nayak, and M. S. Shaila. 2001. Identification of a cytotoxic T-cell epitope on the recombinant nucleocapsid proteins of rinderpest and peste des petits ruminants viruses presented as assembled nucleocapsids. Virology 279:210-220. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay, A. K., W. P. Taylor, and P. L. Roeder. 1999. Rinderpest: a case study of animal health emergency management. Rev. Sci. Tech. 18:164-178. [DOI] [PubMed] [Google Scholar]
- 18.Roeder, P. L., and W. P. Taylor. 2002. Rinderpest. Vet. Clin. N. Am. Food. Anim. Pract. 18:515-547. [DOI] [PubMed] [Google Scholar]
- 19.Saliki, J. T., G. Libeau, J. A. House, C. A. Mebus, and E. J. Dubovi. 1993. Monoclonal antibody-based blocking enzyme-linked immunosorbent assay for specific detection and titration of peste-des-petits-ruminants virus antibody in caprine and ovine sera. J. Clin. Microbiol. 31:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaila, M. S., D. Shamaki, M. A. Forsyth, D. Diallo, L. Goatley, R. P. Kitching, and T. Barrett. 1996. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 43:149-153. [DOI] [PubMed] [Google Scholar]
- 21.Singh, R. P., B. P. Screenivasa, P. Dhar, R. N. Roy, and S. K. Brandyopadhyay. 2000. Development and evaluation of a monoclonal antibody based competitive enzyme-linked immunosorbent assay for the detection of rinder-pest virus antibodies. Rev. Sci. Tech. 19:754-763. [DOI] [PubMed] [Google Scholar]
- 22.von Messling, V., T. C. Harder, V. Moennig, P. Rautenberg, I. Nolte, and L. Haas. 1999. Rapid and sensitive detection of immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnes, A., A. R. Fooks, A. B. Dowsett, G. W. Wilkinson, and J. R. Stephenson. 1995. Expression of the measles virus nucleoprotein gene in Escherichia coli and assembly of nucleocapsid-like structures. Gene 160:173-178. [DOI] [PubMed] [Google Scholar]