Abstract
We evaluated the performance of Western blot (WB) analysis using commercially available antigen strips and compared the results with those of indirect hemagglutination (IHA) and indirect immunofluorescence (IFAT) for the serodiagnosis of human schistosomiasis. The antigen preparation was a crude extract of Schistosoma mansoni. The WB profile characteristics of schistosomiasis were characterized by comparing the results for 58 serum samples from patients with parasitologically proven S. mansoni (n = 12) and S. haematobium (n = 46) infections and 37 individuals with probable cases of schistosomiasis but with only positive serology results. The specificity of WB analysis was assessed by testing 12 serum samples from healthy subjects, 67 serum samples from patients with other proven helminthic and protozoan infections, and 16 serum samples from patients with autoantibodies. Six immunodominant bands (65, 70, 80, 95, 110, and 120 kDa) were revealed with sera from patients with schistosomiasis. The presence of three or more bands in the range 65 to 120 kDa, with the exception of the 100-kDa band, was considered diagnostic for Schistosoma infection and had a specificity of 100% in our series. In patients with proven schistosomiasis, the sensitivity of WB analysis was 84.5%, whereas those of IFAT and IHA were 65.5 and 72.9%, respectively. For serologically proven cases, the sensitivity of WB analysis was 97.3%. The overall sensitivity and specificity for both groups of patients were 89.5 and 100%, respectively, with positive and negative predictive values of 100 and 91.3%, respectively. We conclude that WB analysis is a useful technique for the immunological diagnosis of schistosomiasis.
Schistosomiasis remains a serious public health problem worldwide, infecting more than 200 million people, mostly in tropical regions, and is endemic in 74 developing nations (13). Cases of schistosomiasis imported by immigrants and tourists from areas of endemicity are on the increase (3). Some of these patients are asymptomatic or have nonspecific biological or clinical signs (17). Diagnosis is usually based on clinical data associated with the detection of eggs in stool, urine, and/or rectal and bladder biopsy specimens (4). However, in lightly infected individuals with low levels of egg production and excretion, the diagnosis might be inaccurate. Many antibody assay techniques have been developed (e.g., indirect immunofluorescence [IFAT], enzyme-linked immunosorbent assay [ELISA], and indirect hemagglutination [IHA]). However, few serological tests are commercially available, and preparation of the antigen requires the maintenance of a complete parasite cycle and homemade antigen extraction. In the last decade, the rate of introduction of immunoblotting into the repertoire of assays for the serodiagnosis of parasitic infections has been increasing. Several investigators have reported on the usefulness of Western blot (WB) analysis for differentiating between recent and chronic Schistosoma infections (17) and different Schistosoma species (15) and for recognizing the isotype in infected children (12), but no industrial kit was available, until recently. The present study describes the development, sensitivity, and specificity of industrially produced strips for WB analysis made with crude Schistosoma mansoni antigens.
MATERIALS AND METHODS
Patients and sera.
Serum samples from 58 patients with parasitologically confirmed schistosomiasis (S. mansoni, n = 12; S. haematobium, n = 46) were used to identify specific Schistosoma antigens. For all of these patients, the diagnosis of schistosomiasis was confirmed by the demonstration of eggs in stools by using the Kato-Katz thick smear technique (8) or by the demonstration of eggs in urine. Sera from 37 patients considered to have probable schistosomiasis on the basis of positive serological test results by both IFAT and IHA were used to complete the assessment of the performance of WB analysis compared to those of IHA and IFAT.
Cross-reactivity was assessed by selecting sera from 12 healthy French patients who had not traveled to areas of endemicity and 67 patients with other proven parasitic diseases confirmed by blood smears, stool examinations, positive specific serological test results, echography, or computed tomography scanning: amoebiasis (4 patients), malaria (4 patients), toxoplasmosis (4 patients), visceral leishmaniasis (6 patients), Ascaris infection (2 patients), hydatidosis (5 patients), filariasis (26 patients), toxocariasis (9 patients), and trichinellosis (7 patients). Additional sera from 16 patients with autoimmune disorders were also used in order to evaluate the risk of nonspecific reactions related to systemic disorders, including those that produce autoantibodies (8 patients) and rheumatoid factor (8 patients).
Methods.
All sera from patients with schistosomiasis were tested by one or two techniques besides WB analysis. The IFAT technique was performed with 4-μm-thick cryostat sections of adult S. mansoni parasites by the technique of Ambroise-Thomas and Andrews (2). Serum samples with equally distributed fluorescence through the worm tissue and a titer ≥100 were considered positive. Focal or spotty fluorescence was not considered in the selection of positive sera. The IHA schistosomiasis kit sold by Fumouze Laboratories (Levallois-Perret, France) was used according to the instructions of the manufacturer. The results were evaluated by use of a cutoff titer of 1:160.
Antigens for WB analysis were obtained from an adult S. mansoni worm (a strain from Guadeloupe, West Indies, France) recovered from experimentally infected Swiss mice (Charles River, Les Oncins, France). Adult worms were washed three times in physiological saline containing protease inhibitors (leupeptin, 0.5 μg/ml; phenylmethylsulfonyl fluoride, 170 μg/ml; and pepstatin, 0.7 μg/ml). The suspension was ground in a mortar at 4°C, submitted to three cycles of freezing and thawing in liquid nitrogen, and sonicated (six times for 1 min each time). The crude extract was centrifuged at 100,000 × g for 1 h, and the supernatant was filtered (pore size, 0.22 μm) and stored at −80°C. The protein content was quantified by the technique of Lowry et al. (10).
The antigens were delivered to LD-BIO Diagnostics (Lyon, France), which produced the WB strips. Briefly, the antigen solution was electrophoresed in 13% acrylamide gels with the discontinuous sodium dodecyl sulfate buffer system described by Laemmli (9), with slight modifications. A mixture of biotinylated and prestained proteins (myosin, β-galactosidase, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, trypsin inhibitor, lysozyme, and aprotinin) obtained from Bio-Rad Laboratories (Hercules, Calif.) was used as a molecular weight standard. The gels were run until the 30-kDa trypsin inhibitor (which had been prestained blue) reached the bottoms of the gels. The proteins were then transferred to nitrocellulose sheets as described by Towbin et al. (14), with slight modifications. The nitrocellulose sheets were coated with Tris-NaCl (pH 7.4) containing 5% nonfat milk. The blots were washed twice with Tris-NaCl, dried, and cut into 4-mm-wide strips. The antigen strips were provided by LD-BIO in a kit containing all the reagents needed for WB analysis. WB assays were performed according to the instructions of the manufacturer. Briefly, the strips were incubated with sera diluted 1:50 in Tris-NaCl sample buffer for 90 min. After a washing step with Tris-NaCl washing buffer, the strips were incubated with an anti-human immunoglobulin G-alkaline phosphatase conjugate for 60 min. After another washing step, the protein fractions recognized by the sera were revealed by the corresponding substrate-chromogenic solution containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate. The reaction was stopped by washing the strips with distilled water. The strips were dried and glued to paper for reading and storage. Positive and negative controls were tested in each assay.
Repeatability, reproducibility, and interference.
Repeatability and reproducibility were assessed by testing four serum samples (three positive serum samples and one negative serum sample) three times with the same reagent, by testing the same four serum samples in four different assays with the same reagent, and by testing eight serum samples (six positive and two negative serum samples) two times by the same assay with reagents from two different batches. We used serial dilutions (1/50, 1/100, and 1/160) of sera with high and low titers with the classical serological techniques, and we added a positive control to each series. The results obtained with the different dilutions showed no differences in sensitivity or background readings. All serum samples used in this study were tested at a dilution of 1/50.
Interference was analyzed by testing sera containing hemoglobin (n = 2), lipids (n = 2), or bilirubin (n = 2).
Statistical analysis.
The sensitivity of the test was defined as the number of patients who gave a positive test result as a proportion of the total number of patients who had parasitologically proven schistosomiasis. The specificity of the test was defined as the number of patients who gave a negative test result as a proportion of the total number of control patients (patients with other infections, patients with autoimmune antibodies, and healthy donors).
RESULTS
The repeatability and reproducibility of WB analysis were very good. The dilution of sera used in the present study (1:50) achieved a good sensitivity, with very few background readings observed. In addition, no interference with hemoglobin, bilirubin, or lipids was observed.
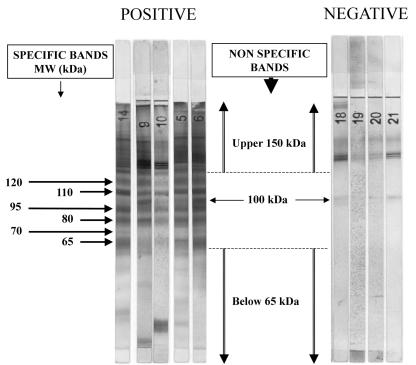
The WB profiles obtained with sera from patients with confirmed schistosomiasis yielded numerous bands between 40 and 200 kDa. Bands of >120 and <65 kDa were considered nonspecific, as they were also found in some control serum samples. Among the bands ranging from 65 to 120 kDa, a band of 100 kDa was observed for most case and control serum samples. Therefore, only the remaining bands of 65, 70, 80, 95, 110, and 120 kDa were considered for diagnosis. These bands were usually large, and sometimes, some of the bands appeared as a close doublet (Fig. 1). The bands were observed at a frequency of 47.7 to 91.9% with 95 serum samples from patients with confirmed or probable Schistosoma infection (Table 1). By contrast, these bands were absent from most of the 91 control serum samples from healthy patients (n = 12) or patients with other parasitic infections (n = 63) or autoimmune diseases (n = 16). The exceptions were a serum sample from a patient positive for rheumatoid factor with a band of 65 kDa and serum samples from three patients with a band at 70 kDa. The latter patients comprised two patients with hydatidosis and one patient with toxocariasis (data not shown). Therefore, we assumed that the presence of three or more bands in the range of 65 to 120 kDa, with the exception of the 100-kDa band, was diagnostic for Schistosoma infection, since this allowed us to achieve a specificity of 100% in our series.
FIG. 1.
Specific bands for schistosomiasis-positive sera. MW, molecular mass.
TABLE 1.
Frequency of characteristic bands for patients with confirmed and probable schistosomiasis
| Patient group | No. (%) of serum samples with bands of the following size by WB analysis:
|
|||||
|---|---|---|---|---|---|---|
| 65 kDa | 70 kDa | 80 kDa | 95 kDa | 110 kDa | 120 kDa | |
| Confirmed schistosomiasis (n = 58) | 47 (81.0) | 34 (58.6) | 48 (82.8) | 46 (79.3) | 47 (81.0) | 47 (81.0) |
| Probable schistosomiasis (n = 37) | 25 (67.6) | 13 (58.6) | 31 (83.2) | 32 (86.5) | 32 (86.5) | 34 (91.9) |
| Total (n = 95) | 72 (75.8) | 47 (49.5) | 79 (83.2) | 78 (82.1) | 79 (83.2) | 81 (85.3) |
By use of this criterion of positivity, the sensitivity of WB analysis was compared with those of IHA and IFAT with samples from patients with proven schistosomiasis (Table 2). On the basis of the criterion described above, 36 of 46 (78.3%) patients with proven S. haematobium infection and 11 of 12 (91.7%) patients with S. mansoni infection were found to be positive by WB analysis. This corresponds to a global sensitivity with this series of patients of 84.5%, whereas the sensitivities of IFAT and IHA were 65.5 and 72.9%, respectively. It is noteworthy that the eight S. haematobium-infected patients who were found to be negative by WB analysis were also negative by IHA and IFAT. For patients with probable (serologically proven) schistosomiasis, the sensitivity of WB analysis was 97.3% (36 of 37 serum samples). When both serologically and parasitologically positive patients were considered, the global sensitivity of WB analysis was 89.5% (85 of 95 serum samples), with positive and negative predictive values of 100% and 91.3%, respectively.
TABLE 2.
Sensitivities of IFAT, IHA, and WB techniques with sera from patients with parasitologically confirmed cases of schistosomiasis
| Parameter | IFAT (n = 58) | IHA (n = 48) | WB analysis (n = 58) |
|---|---|---|---|
| No. of serum specimens: | |||
| Positive | 38 | 35 | 49 |
| Negative | 20 | 13 | 9 |
| Sensitivity (%) | 65.5 | 72.9 | 84.5 |
DISCUSSION
Imported cases of schistosomiasis in areas of nonendemicity are frequent among populations of immigrants and travelers returning from the tropics (19). Most of these patients do not excrete eggs or excrete only a few eggs in an uneven fashion (20), and these cases are often unrecognized (6). Diagnosis of schistosomiasis by detection of specific antibodies is likely to be more sensitive than diagnosis by the traditional parasitological techniques (7). Doenhoff et al. (5) suggest that according to the current state of the art, people who are parasitologically negative and/or circulating antigen negative cannot be assumed to be noninfected. However, positive serological test results do not necessarily prove an infection, as imperfect techniques and cross-reactions may result in false-positive results. Weak positivity may be difficult to interpret; and false-negative reactions also occur, especially with S. haematobium. In order to incorporate serodiagnosis into routine clinical laboratory practice, an easy-to-use, sensitive, and specific serological test is needed. Unfortunately, only a few serological tests for the diagnosis of schistosomiasis are commercially available. IHA has a good sensitivity and an excellent specificity, especially when a cutoff of 160 is used; is easy to use; and can be used to monitor patients (18). However, false-positive results may occur with Fasciola hepatica-infected patients (unpublished observation). In this study IHA yielded false-negative results for 10 serum samples that were positive by WB analysis. The reference test (IFAT) requires the maintenance of a cycle, which is fastidious and time-consuming and which gives false-negative results in some cases for patients infected with S. haematobium. These drawbacks tend to restrict serodiagnosis to specialized research centers.
In the present study, we used commercially available strips prepared by a well-standardized procedure. The sensitivity of WB analysis in this study was 97.3% for sera from patients with serologically proven cases and 84.5% with sera from patients with proven schistosomiasis, with an overall sensitivity and specificity for both groups of patients of 89.5 and 100%, respectively. Reproducible WB profiles were obtained over repeated experiments and allowed the identification of six well-defined bands that were characteristic of Schistosoma infection. The masses of these bands ranged from 65 to 120 kDa. In contrast to the findings of Valli et al. (17), we found that bands <65 kDa were poorly specific and could not be used for diagnosis. These discrepancies might be due to the use of different antigenic preparations. No cross-reactions were observed with sera from patients with other helminth infections or other control sera, as long as bands in the zone between 65 and 120 kDa were used to read the results. Our study included eight serum samples that were from patients with parasitologically proven S. haematobium infection but with negative serologies by IFAT and IHA and that also yielded negative results by WB analysis, suggesting that a negative result for Schistosoma by WB analysis does not exclude the possibility of an S. haematobium infection. There was no difference in the patterns of bands for sera from patients infected with S. mansoni or S. haematobium. Thus, the kit is not species specific. In comparison, Tsang et al. (15) used homologous adult microsomal antigens, which allowed the detection of the 30-kDa band highly specific for infection with S. mansoni (1) and the detection of the 23-kDa band specific for infection with S. haematobium (15). The WB technique with crude antigen from adult worms is essentially qualitative and cannot be used for longitudinal studies for the evaluation of treatment
Purified antigens of adult worms allowed the use of microsomal antigens of S. mansoni (MAMA) and S. haematobium (HAMA) and the development of rapid, highly specific, and sensitive assays, such as Falcon Assay Screening Test (FAST)-ELISA (11, 16) and WB analysis as screening and confirmatory assays, respectively, for the detection of the species causing the infection (1). The WB technique with HAMA resulted in a sensitivity of 94% for S. haematobium egg-positive patients; the sensitivity increased to 100% when the WB technique was combined with FAST-ELISA (1). The ELISA with MAMA was positive with 53 to 83% of sera from patients infected with S. haematobium (1, 11). Detection of specific antibodies to MAMA and HAMA was found to be 100% specific for the detection of S. mansoni and S. haematobium, respectively, when they were used in the FAST-ELISA and immunoblot assays (1).
In conclusion, WB analysis is an interesting tool for the detection or confirmation of schistosomiasis. The ease of use, good sensitivity, and excellent specificity of the WB technique described in this study make it a useful test for the immunological diagnosis of schistosomiasis.
REFERENCES
- 1.Al-Sherbiny, M. M. A., A. M. Osman, K. Hancock, A. M. Deelder, and V. C. W. Tsang. 1999. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am. J. Trop. Med. Hyg. 60:960-966. [DOI] [PubMed] [Google Scholar]
- 2.Ambroise-Thomas, P., and P. Andrews. 1976. Development of fluorescent antibodies directed against larval stages, eggs and adults of Schistosoma mansoni in mice harbouring unisexual or bisexual infections. Tropenmed. Parasitol. 27:483-488. [PubMed] [Google Scholar]
- 3.Day, J. H., A. D. Grant, J. F. Doherty, P. L. Chiodini, and S. G. Wright. 1996. Schistosomiasis in travellers returning from sub-Saharan Africa. BMJ 313:268-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deelder, A. M., and W. W. Duchenne. 1989. Serodiagnostik van Schistosoma-infecties. Ned. Tijdschr. Geneeskd. 133:154-156. [PubMed] [Google Scholar]
- 5.Doenhoff, M. J., P. L. Chiodini, and J. V. Hamilton. 2004. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 20:35-39. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahim, A., H. El Morshedy, E. Omer, S. El Daly, and R. Barakat. 1997. Evaluation of the Kato-Katz thick smear and formol ether sedimentation techniques for quantitative diagnosis of Schistosoma mansoni infection. Am. J. Trop. Med. Hyg. 57:706-708. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton, J. V., M. Klinkert, and J. M. Doenhoff. 1998. Diagnosis of schistosomiasis: antibody detection, with notes on parasitological and antigen detection methods. Parasitology 117:S41-S57. [DOI] [PubMed] [Google Scholar]
- 8.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397-400. [PubMed] [Google Scholar]
- 9.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 10.Lowry, O. H., N. J. Rosenbrough, A. L. Farr, and R. J. Randal. 1951. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 193:265-271. [PubMed] [Google Scholar]
- 11.Maddison, S. E., S. B. Slemenda, V. C. Tsang, and R. A. Pollard. 1985. Serodiagnosis of Schistosoma mansoni with microsomal adult worm antigen in an enzyme-linked immunosorbent assay using a standard curve developed with a reference serum pool. Am. J. Trop. Med. Hyg. 34:484-494. [DOI] [PubMed] [Google Scholar]
- 12.Noya, O., Z. Fermin, B. Alarcon de Noya, S. Losada, C. Colmenares, and T. Hermoso. 1995. Humoral immune response of children with chronic schistosomiasis isotype recognition of adult worm antigens. Parasite Immunol. 17:319-328. [DOI] [PubMed] [Google Scholar]
- 13.Savioli, L., E. Renganathan, A. Montresor, A. Davis, and K. Behbehani. 2002. Schistosomiasis and soil-transmitted helminth infections: forging control efforts. Trans. R. Soc. Trop. Med. Hyg. 96:577-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang, V. C., K. Hancock, S. E. Maddison, A. L. Beatty, and D. M. Moss. 1984. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J. Immunol. 132:2607-2613. [PubMed] [Google Scholar]
- 16.Tsang, V. C. W., and P. P. Wilkins. 1991. Immunodiagnosis of schistosomiasis: screen with FAST-ELISA and confirm with immunoblot. Clin. Lab. Med. 11:1029-1039. [PubMed] [Google Scholar]
- 17.Valli, L. C. P., H. Y. Kanamura, R. M. Da Silva, R. Ribeiro-Rodrigues, and A. D. R. Dietze. 1999. Schistosomiasis mansoni: immunoblot analysis to diagnose and differentiate recent and chronic infection. Am. J. Trop. Med. Hyg. 61:302-307. [DOI] [PubMed] [Google Scholar]
- 18.van Gool, T., H. Vetter, T. Vervoort, M. J. Doenhoff, J. Wetsteyn, and D. Overbosch. 2002. Serodiagnosis of imported schistosomiasis by a combination of a commercial indirect hemagglutination test with Schistosoma mansoni adult worm antigens and an enzyme-linked immunosorbent assay with S. mansoni egg antigens. J. Clin. Microbiol. 40:3432-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitty, C. J., D. C. Mabey, M. Armstrong, S. G. Wright, and P. L. Chiodini. 2000. Presentation and outcome of 1107 cases of schistosomiasis from Africa diagnosed in a non-endemic country. Trans. R. Soc. Trop. Med. Hyg. 94:531-534. [DOI] [PubMed] [Google Scholar]
- 20.Ye, X. P., C. A. Donnelly, R. M. Anderson, Y. L. Fu, and A. M. Agnew. 1998. The distribution of Schistosoma japonicum eggs in faeces and the effect of stirring faecal specimens. Ann. Trop. Med. Parasitol. 92:181-185. [DOI] [PubMed] [Google Scholar]