Abstract
A cross-sectional study that involved secondary analysis of data collected from 681 pregnant women and 183 miners (94 men and 89 women; ratio of men to women, 1:0.95) in Jos, Nigeria, was carried out to determine the reference ranges for CD4+-cell counts in healthy HIV-negative adult Nigerians. The main results of interest were CD4+-cell counts and odds ratios (ORs) of low CD4+-cell counts, defined as below 350 cells per μl. CD4+-cell counts were similar in men and nonpregnant women, with a mean (standard deviation) of 828 (203) cells per μl, but pregnant women had a lower value of 771 (250) cells per μl. None of the factors assessed was related to the odds of having a low CD4+-cell count among men and nonpregnant women, but age, age of marriage, and alcohol usage were significant predictors in pregnant women. Compared to pregnant women less than 20 years old, older women had significantly lower odds of a low CD4+-cell count (ORs were 0.06 for women aged 20 to 29 years and 0.22 for those aged 30 to 39 years). When compared with those pregnant women who were married before 20 years of age, those who married at 20 to 29 years and 30 to 39 years had odds ratios of 6.41 and 9.40, respectively. Previous alcohol use was also associated with low CD4+-cell counts (OR, 5.15). The 95% confidence interval for CD4+-cell counts in healthy adult Nigerians is 547 to 1,327 cells per μl, and this is the first time this has been determined.
CD4+-cell counts, alongside other parameters, are of central importance in the monitoring of immune function. In Nigeria, although country-specific reference ranges for some hematologic measures have been determined earlier (1), CD4+-cell reference values are not available from West African populations. Rather, values from textbooks and other publications based on studies in Western countries are largely employed for clinical decision making. However, there is evidence in the literature of significant geographical and racial differences in these parameters. For example, lower CD4+-cell counts have been recorded for Asians (10) than for Caucasians, and studies in African populations have shown mean CD4+-cell counts in healthy Ethiopians (16) that are markedly lower than those in Ugandans (17) and Tanzanians (11).
The adult prevalence of human immunodeficiency virus (HIV) infection in Nigeria is currently estimated to be 5% (6), which translates to approximately 6 million infected persons in a country with a population of 120 million. Fortunately, both local and international efforts, especially the U.S. President's Emergency Plan for AIDS Relief and the Global Fund, have recently been directed towards the provision of antiretroviral therapy for the estimated 1.5 million AIDS cases in the country. As access to treatment increases, the need to determine local reference values for CD4+-cell counts and factors that may affect it, for accurate monitoring of responses to therapy and other treatment outcomes, becomes more urgent.
This study involves secondary analysis of data obtained from two studies in collaboration with Plateau State Specialist Hospital. The objective is to describe CD4+-cell reference ranges for normal adult Nigerians, based on data obtained from 183 healthy HIV-negative adults and an additional 681 HIV-negative pregnant women.
MATERIALS AND METHODS
Study subjects.
This cross-sectional study involved secondary analysis of data from two larger HIV surveillance studies aimed at determining the seroprevalence of HIV type 1 infection and the risk factors among high-risk groups in the population. Between October 2001 and April 2002, women attending the antenatal clinic at Plateau State Specialist Hospital in Jos, Nigeria, were asked to participate in the study, which involved HIV counseling, collection of basic information on HIV knowledge and behavior, and collection of biological specimens for HIV screening and other tests. In the second study, miners were recruited on-site by study staff members working from a temporary interviewing tent and mobile clinic, as many of the miners were reluctant to leave their work sites to visit nearby clinics for participation in the study. Recruited miners were also offered HIV counseling, administered a questionnaire, and asked to provide blood samples. As it was thought that subjects could opt for only one level of participation or the other, separate informed consents were obtained from subjects for questionnaire administration and blood collection.
Data collection, management, and analysis.
Demographic information and basic knowledge of HIV and risk patterns were collected from each consenting participant by using questionnaires administered by trained interviewers. The questionnaires for both groups of participants were similar, but the antenatal questionnaire included pregnancy-related questions. The data and laboratory test results were double entered centrally into a Microsoft Access database, and where discrepancies were found, manual verification and correction were performed. The data were analyzed with Stata 8 software (14). A two-sided P value of ≤0.05 was considered significant.
To assess bias from missing values, we compared the subjects for whom we did not have CD4+-cell counts with those for whom we had CD4 counts, with respect to age and gender distributions, by using chi-square tests. We also conducted sensitivity analyses on our final results to evaluate the impact of the exclusion of those subjects on our conclusions. Means and standard deviations of CD4+-cell count were computed by subgroup (male miners, female miners, and pregnant women). Group variances for continuous variables (age and CD4+-cell counts) were compared with the variance ratio test and group means with two-sample t tests. For categorical variables, group proportions were compared with chi-square tests. The relationships between social, demographic, and behavioral characteristics and CD4 cell counts were examined by using linear regression and logistic regression models. In the multiple linear regression analysis, CD4+-cell counts were used as a continuous dependent variable and the independent variables included age, sex, marital status, circumcision, alcohol use, history of sexually transmitted diseases in the previous 12 months, religion (Muslim or Christian), and socioeconomic status (measured by two proxy variables: ownership of a family car and the subject's level of education).
To determine which covariates reliably predicted a low CD4+-cell count, the CD4+-cell counts were dichotomized into low, defined as less than 350 cells per microliter, and not low. The dichotomized CD4+-cell count was the outcome variable in the logistic regression analysis.
Blood sample collection and testing.
Blood, urine, and cervical/urethral swabs were obtained from consenting individuals. For the antenatal clinic patients, this was done at a central laboratory where biological samples are routinely collected for the hospital. For the miners, the samples were collected at the on-site mobile clinics. Ten milliliters of venous blood was collected in EDTA vacutainer tubes by venipuncture from each volunteer. For pregnant women, blood samples were collected in the central blood bank located within the hospital and then transported to the International Center for Scientific Collaboration-World Laboratory, currently expanded into the Plateau State Human Virology Research Center for HIV antibody testing, within 2 h of collection. For the miners, blood samples were collected in the field, with temperatures much higher than the air-conditioned central blood bank. As such, samples obtained from the miners were transported to the laboratory in ice coolers. Ice packs were placed inside the cooler boxes, and blood samples were placed in racks which were arranged in a way that prevented direct contact of the sample tubes with the ice packs. Samples were transported to the laboratory and processed within 2 h of collection. The samples were screened for HIV infection with the Organon-Technika Vironoska (Biomerieux) enzyme-linked immunosorbent assay (ELISA) kit. Samples that screened positive by ELISA were confirmed by Western blot (Immunetics, Boston, Massachusetts). Indeterminate confirmatory tests were considered negative according to World Health Organization (WHO) guidelines. CD4+-cell counts were determined for each sample by using the manual Coulter (Cytosphere) technique (Beckman Coulter, Inc., Fullerton, California), while the FACSCount (Becton Dickinson, San Jose, California) technique was used for 5% of samples analyzed for quality control. Plasma and T cells were stored in liquid nitrogen for further analysis.
Since the CD4 measurements were conducted over a period of 7 months (October 2001 to April 2002), several measures were put in place to ensure the quality of the data obtained. Such measures included the specific development and use of a standard operating procedure for CD4 enumeration during the study, the use of an access database with error check features for data management, and the keeping of temperature logs on refrigerators to ensure that reagents were optimally preserved. In addition, control tests were run, and results were considered valid only when the runs with controls were successful.
Ethical considerations.
Prior to starting the study, separate approvals of the study protocol were obtained from the Institutional Review Boards of the University of Maryland, Plateau State Specialist Hospital, and the Plateau State Hospitals Management Board. Before enrollment, separate consents were obtained from subjects for questionnaire administration and blood collection. Additionally, although all participants were given a group health talk that covered HIV knowledge, among other topics, those who consented for blood collection and HIV testing were also pretest counseled individually. All consent forms were maintained locked in secure files in the research office. Confidentiality was ensured through secure data management, and no personal identifiers were in the computer system: data and samples were labeled with anonymous identification numbers. Test results were confidentially disclosed to the subjects following posttest counseling. HIV-positive pregnant women were also offered perinatal nevirapine to prevent vertical transmission.
RESULTS
This analysis was restricted to HIV-seronegative individuals for whom CD4+-cell counts were available. A total of 762 pregnant women and 529 miners (297 men and 232 women) were recruited. Of these, 22 male miners, 8 female miners and 79 pregnant women were HIV type 1 positive and were excluded from our analysis. An additional 46 male and 54 female miners had missing HIV test results and were also excluded from this analysis. CD4+-cell counts were not available for 135 men, 87 female miners, and 2 pregnant women, and they were also excluded from the analysis.
The subjects excluded on account of missing CD4+-cell counts were similar to those included in the study with respect to gender and age (P = 0.17) distributions, suggesting no significant selection bias. Six subjects (two male miners and four female miners) had indeterminate Western blot results and were considered to be HIV negative and included in the analyses. Their CD4 profiles were similar to those of the subjects that were known to be truly negative.
Study population.
Of the 864 participants included in these analyses, 94 (10.9%) were male miners, 89 (10.3%) were female miners, and 681 (78.8%) were pregnant women. The mean ages (± standard deviations [SD]) for the three subgroups were 36.8 (± 11.7), 37.5 (± 11), and 27.3 (± 6.0) years, respectively. The mean (SD) CD4+-cell count for all participants was 783 (± 241) cells per microliter, and the corresponding values for the three groups were 838 (± 193), 818 (± 213) and 771 (± 250), respectively. The CD4+-cell count distributions are summarized in Table 1, along with demographic characteristics of the study subjects.
TABLE 1.
Demographic characteristics and CD4+-cell counts of the three subgroups of study participantsa
| Characteristic | No. (%) of or parameter for miners
|
No. (%) of or parameter for pregnant women (n = 681) | |||
|---|---|---|---|---|---|
| Men (n = 94) | Women (n = 89) | P-valueb | Men and women (n = 183) | ||
| Sex | |||||
| Male | 94 (100) | 94 (51.4) | |||
| Female | 89 (100) | 89 (48.6) | 681 (100) | ||
| Married | 84 (89.4) | 57 (64.0) | <0.0001 | 141 (77.1) | 653 (96.7) |
| Education | <0.01 | ||||
| <1 yrc | 25 (26.6) | 54 (60.7) | 79 (43.9) | 6 (0.9) | |
| Primary (1-6 yrs) | 35 (36.2) | 31 (34.8) | 65 (34.8) | 185 (28.5) | |
| Junior secondary (7-9 yrs) | 11 (11.7) | 2 (2.2) | 13 (7.1) | 112 (17.2) | |
| Senior secondary (10-12 yrs) | 18 (19.2) | 1 (1.1) | 19 (10.4) | 204 (31.4) | |
| Tertiary (13-16 yrs) | 6 (6.4) | 1 (1.1) | 7 (3.8) | 137 (21.1) | |
| Unspecified | 6 (0.9) | ||||
| Takes alcohol | 0.62 | ||||
| Yes | 6 (37.5) | 5 (29.4) | 11 (33.3) | 37 (5.5) | |
| No | 10 (62.5) | 12 (70.6) | 22 (66.7) | 633 (94.5) | |
| Circumcised | 0.38 | ||||
| Yes | 37 (42.1) | 40 (48.8) | 77 (45.3) | 48 (7.5) | |
| No | 51 (57.9) | 42 (51.2) | 93 (54.7) | 590 (92.5) | |
| Owns family car | 0.92 | ||||
| Yes | 4 (4.4) | 4 (4.7) | 8 (4.5) | 156 (23.2) | |
| No | 87 (95.6) | 81 (95.3) | 168 (95.5) | 518 (76.8) | |
| Had STD last year | 0.13 | ||||
| Yes | 21 (25.3) | 12 (15.6) | 33 (20.6) | 101 (15.4) | |
| No | 62 (74.7) | 65 (84.4) | 127 (79.4) | 555 (84.6) | |
| Religion | 0.33 | ||||
| Christian | 92 (98.9) | 86 (100) | 178 (99.4) | 518 (76.1) | |
| Muslim | 1 (1.1) | 0 (0.0) | 1 (0.6) | 163 (23.9) | |
| Age (yrs) | 0.59 | ||||
| 10-19 | 1 (1.1) | 0 (0.0) | 1 (0.6) | 53 (7.8) | |
| 20-29 | 23 (24.5) | 17 (19.1) | 40 (21.9) | 386 (56.7) | |
| 30-39 | 34 (36.2) | 28 (31.5) | 62 (33.9) | 204 (30.00) | |
| 40-49 | 14 (14.9) | 19 (21.4) | 33 (18.0) | 18 (2.6) | |
| 50-59 | 10 (10.6) | 9 (10.1) | 19 (10.4) | 0 (0.00) | |
| 60-69 | 12 (12.8) | 16 (18) | 28 (15.3) | 20 (2.9) | |
| ± test resultd | |||||
| Mean CD4+ cell count (SD) | 838 (193) | 818 (213) | 0.51 | 828 (203) | 771 (250) |
| Reference range | 528-1330 | 497-1310 | 547-1327 | 321-1314 | |
Some of the row totals do not add up to the total figure of 864 because of missing data. Subjects with missing values were left out of the analysis for each variable.
Values are for the chi-square test comparing proportions and t test comparing means, between male and female miners.
All but four subjects in this category (one male miner and three female miners) had no education at all.
Variance ratio test P value was 0.58 (male miners versus female miners). Reference ranges were determined using the 2.5 and 97.5 percentiles of CD4+-cell count within each group.
CD4+-cell counts.
On initial bivariate analysis of the complete data (including all three groups), age by decades, gender, marital status, education, circumcision, and interaction terms between age and pregnancy and between age and gender appeared to be significantly related to the CD4 count. However, correction for pregnancy in a multivariate model removed any significance of these variables. Their significance in the crude analysis reflected only sociodemographic differences between the pregnant women and the miners (the pregnant women had significantly lower CD4+-cell counts, and they were younger and more likely to be married and had more education, on average). The only consistently statistically significant covariate was pregnancy (P < 0.001). When pregnancy had been accounted for, there was no significant difference in mean CD4+-cell counts between men and women. Based on the final linear regression model, pregnant women had 57 CD4+ cells per microliter of blood fewer than nonpregnant women and men. Because of this difference, stratified analyses looking separately at each group of subjects were conducted.
Male and female miners.
Although men had, on average, higher CD4+-cell counts than women and CD4+-cell counts decreased slightly with each decade increase in age, when the pregnant women were excluded from the analysis, none of the examined covariates was significantly related to CD4 levels.
Pregnant women only.
A separate analysis of the data on pregnant women also showed no significant relationships between CD4+-cell counts and any of the covariates evaluated. The slight variation observed with CD4+-cell counts by trimester was also not significant. The mean (SD) CD4+-cell counts for women in their first, second, and third trimesters were 790 (± 251), 763 (± 246), and 798 (± 264) cells per microliter, respectively.
While none of the miners had a low CD4+-cell count (<350 cells/microliter), 25 (3.67%) of the pregnant women did. The logistic regression analysis was therefore restricted to the 681 pregnant women for whom we had CD4+-cell counts. Based on existing literature and the crude odds ratios obtained, potential predictor variables that were examined include alcohol use, history of sexually transmitted infections in the previous 12 months, history of genital discharge, marital status, male and female circumcision (assessed separately by analysis stratified by gender), age by decades, religion (Christian or Muslim), age at marriage by decades, parity (≤4 versus >4), whether or not the current pregnancy was the first, trimester of pregnancy, and polygamous relationships (whether or not the husband had more than one wife). Socioeconomic status was again evaluated with two proxy variables: family car ownership and education. Effect modifications among the covariates were also evaluated using interaction terms in the models. A number of different approaches were employed in covariate selection for our models. Forward, backward, forward stepwise, and backward stepwise techniques all gave the same result, with alcohol use and the indicator variables for age by decades and marriage age by decades being the only risk factors significantly related to the odds of having a low CD4+-cell count (Table 2).
TABLE 2.
Significant risk factors for low CD4+-cell counts in the pregnant womena
| Subject parameter | Crude parameter
|
Adjusted parameterb
|
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Alcohol taker | ||||
| No | 1.00 | 1.00 | ||
| Yes | 3.72 | 1.20-11.49 | 5.15 | 1.48-17.97 |
| Age (yrs) | ||||
| <20 | 1.00 | 1.00 | ||
| 20-29 | 0.7 | 0.19-2.64 | 0.06 | 0.01-0.52 |
| 30-39 | 2.0 | 0.55-7.18 | 0.22 | 0.03-1.78 |
| Marriage age (yrs) | ||||
| <20 | 1.00 | 1.00 | ||
| 20-29 | 2.69 | 0.87-8.26 | 6.41 | 1.37-29.89 |
| 30-39 | 7.59 | 1.30-44.4 | 9.40 | 1.15-76.64 |
OR, odds ratio; CI, confidence interval.
Adjusted for the other covariates in the table.
DISCUSSION
Our study looked at the values of CD4+-cell counts in 183 healthy HIV-negative adults and 681 HIV-negative pregnant women in Jos, Nigeria. The pregnant women were found to have significantly higher levels of education, lower rates of circumcision, and higher family car ownership rates than the miners (Table 1). These indices all suggest that the pregnant women were of higher socioeconomic status than the miners, which is to be expected, as the pregnant women enrolled in the study were mostly middle-class Jos city dwellers, while the miners were mostly poorer village dwellers who worked menial jobs in the mines.
Not surprisingly, CD4 levels in pregnant women were significantly lower than those in nonpregnant women and men. This is consistent with the findings of most studies that examined the effect of pregnancy on CD4+-cell counts. An Indian study (4) among HIV-negative people found absolute CD4+-cell counts to be significantly lower in pregnant women than in nonpregnant women. Similarly, an earlier study among African women demonstrated reduced absolute values of CD4+, CD8+, and total lymphocytes in pregnancy (2). There is evidence that this effect of pregnancy on the CD4+-cell count may be modified by HIV infection, though. Van Benthem and his team (22) did not find any significant differences in CD4+-cell counts in HIV-infected pregnant women and nonpregnant controls. The mean CD4+-cell count among pregnant women in our study was 771 cells/microliter, which is similar to findings in Indian women (4), who had a mean value of 764 cells/microliter.
The documented relationship between gestational age and CD4+-cell levels in pregnant women varies in the literature. While Temmerman et al. (15) found no relationship between gestational age and CD4+-cell counts in both HIV-positive and HIV-negative women in Kenya, Tuomala et al. (18) found an increase in CD4+-cell counts of 2.76 cells/microliter per week of pregnancy during serial measurements of CD4+-cell counts in pregnant women. Our study found no association of gestational age with CD4+-cell counts in our study population. Neither the indicator variables for the trimester nor their quadratic terms were significant in a linear regression model, suggesting the absence of even a nonlinear relationship.
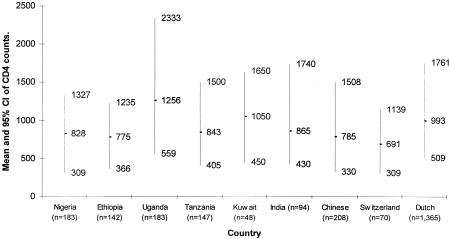
When we combined the data for men and nonpregnant women in our study, the mean (SD) of the CD4+-cell counts was 828 (203) cells/microliter, which is higher than the values reported for healthy adult Ethiopians (16), Chinese (9), and Swiss (3). The values were comparable to those in Tanzanians (20) and Indians (19) and markedly lower than those reported for Ugandans (17), Kuwaitis (8), and Dutch (16). Figure 1 shows the values from our study in relation to findings from three other African countries (Ethiopia, Uganda, and Tanzania), Kuwait, India, China, Switzerland, and The Netherlands. The relatively small variability in CD4+-cell counts in our study may be because this result is based on a highly homogenous community of miners, while some of the other studies recruited more diverse subjects from multiple study sites.
FIG. 1.
Comparison of CD4 cell counts in the study population with published values from other populations (3, 7, 9, 11, 16, 17, 19, 20).
Among the miners (men and nonpregnant women), there was no significant gender difference with regard to CD4+-cell counts. This is consistent with results of studies conducted in Central African Republic (12) and Kuwait (8), both of which found no significant gender differences in CD4+-cell counts. By contrast, the Ethiopian study (10) reported significantly higher CD4+-cell counts in women than in men, as did studies among Indians (19) and Ugandans (17). It is not clear whether there are true variations across countries in the relationship between gender and CD4+-cell counts or these results are due to confounding factors.
The cutoff point of 350 cells per microliter was chosen because of its implications for decision making in HIV clinical management. The current WHO guidelines for the initiation of antiretroviral therapy in adults and adolescents in resource-limited settings is to treat patients infected with stage III or worse disease and with a CD4+-cell count of less than 350 cells/microliter (23), while in the United States, it is recommended that any patients with CD4+-cell counts below 350 cells/microliter or viral loads above 55,000 copies/ml be treated (21).
Our results show no effect of age on CD4+-cell counts among healthy men and nonpregnant women. This is consistent with previously reported findings in India (19), Kuwait (7), and Central African Republic (12), in which no significant changes in CD4+-cell counts with regard to age were found in adulthood. In pregnant women, however, the odds of having a low CD4+-cell count were significantly related to both age by decades and age of marriage by decades. While older women had significantly lower odds of low CD4+-cell counts, women who married at later ages had significantly higher odds when alcohol use had been taken into account. While the effect of marriage age may be due to prolonged exposure to sexually transmitted diseases and other infections, with development of chronic lymphocytosis, in women who got married earlier, there is a need to look further into the interactions between the effects of age and pregnancy on CD4+-cell counts.
Our results indicate that alcohol use in pregnant women may be associated with low CD4+-cell counts, increasing the odds five times. However, due to the small number of women with information on alcohol use and the lack of quantitative data on alcohol use, we would exercise caution in interpreting this finding. The study conducted in India (19) reported no significant effect of alcohol on CD4+-cell counts of normal adults.
This study is not without limitations. Subjects were enrolled based on negative ELISA results for HIV antibodies, and it is possible that some of them were newly infected and still in the window period of their disease. Also, the validity of the comparison of CD4+-cell counts between studies will depend on the comparability of the techniques used in estimating the cell counts and on the expertise of the laboratory technicians. Other factors that could conceivably affect results are the durations and temperatures of sample storage, which could differ significantly between studies. The implementation of low-cost techniques that have been shown to work well in suboptimal conditions will be of immense importance in the global strategy for the treatment of AIDS, as it will allow easy and effective monitoring of patients in resource poor settings (5, 13). The CD4+/CD8+ ratios and the proportions of T-cell subsets are more robust measures of immune function but could not be computed in this study because available data was limited to CD4+-cell counts due to the manual technique used. Finally, Nigeria is a very heterogeneous country, with ethnic and regional variations in population characteristics, which may necessitate comparing the reference values presented here with those from other regions of the country.
The main significance of this paper is in providing baseline CD4+ values for Nigeria. The fact that these baseline values are not identical to those from Western countries and even other African countries suggests that the current WHO guidelines for ARV therapy initiation in developing countries (23) may sometimes need to be adapted to country-specific CD4+-cell count reference values. In the absence of any established country-specific reference values for CD4+-cell counts, we recommend these values for use by in-country clinicians.
Acknowledgments
The study research team for this AIDS Prevention Initiative in Nigeria at the Plateau State Specialist Hospital includes Pam Datong, Lawrence Ayuba, Comfort Daniyam, Silas Gurumdi, Edwina Mang, Jelpe Dadik, Ibrahim Vandi, Ruth Guyit, Ndam Lar, Bitrus Matawal, Keziah Best, Esther Beka, Ruth Dalyop, Chundung Gyang, Ibraheem Garba, Patricia Paul, Demas, Luka Othniel, Simon Cartier (Plateau State Specialist Hospital, Jos, Nigeria); Alash'le Abimiku, Robert Gallo, William Blattner, John Vertefeuille, Manhattan Charurat, Anne Sill, Pacha Villalba-Diebold, Anuli Ajene, Olumuyiwa Aina (Institute of Human Virology, Baltimore, Maryland); Phyllis Kanki, Jean-Louis Sankalé, and Oluwole Odutolu (Harvard University, Boston, Massachusetts).
We thank Peter O'Driscoll, previously at the Institute of Human Virology and currently at Johns Hopkins Bloomberg School of Public Health, for database construction and management and the study volunteers at Plateau State Specialist Hospital and the mining camp in rural Plateau.
This study was carried out as part of the AIDS Prevention Initiative in Nigeria in collaboration with the Harvard School of Public Health and with funding from the Bill and Melinda Gates Foundation.
REFERENCES
- 1.Azikiwe, A. N. 1984. Platelet count values in healthy Nigerian medical students in Jos. East Afr. Med. J. 61:482-485. [PubMed] [Google Scholar]
- 2.Bisalinkumi, E., P. Nawrocki, A. Chao, M. Bulterys, A. Dushimimana, E. Mugabo, and A. Saah. 1992. T-cell subset changes during and after pregnancy in a cohort of HIV-1 sero(+) and sero(−) African mothers. VIIIth Int. Conf. AIDS, abstr. no. PoA 2086. [Online.] http://www.aegis.com/aidsline/1992/dec/M92C4967.html.
- 3.Bisset, L. R., Lung, T. L., M. Kaelin, E. Ludwig, and R. W. Dubs. 2004. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy population in Switzerland. Eur. J. Haematol. 72:203-212. [DOI] [PubMed] [Google Scholar]
- 4.Dayama, A., S. Pandit, R. Mudaliar, R. Bharadwaj, A. N. Shrotri, and S. Joshi. 2003. A pilot study on CD4 and CD8 cell counts in healthy HIV seronegative pregnant women. Indian J. Med. Res. 117:198-200. [PubMed] [Google Scholar]
- 5.Diagbouga, S., C. Chazallon, M. D. Kazatchkine, P. Van de Perre, A. Inwoley, S. M'Boup, M. P. David, A. T. Tenin, R. Soudre, J. P. Aboulker, and L. Weiss. 2003. Successful implementation of a low-cost method for enumerating CD4+ T lymphocytes in resource-limited settings: the ANRS 12-26 study. AIDS 17:2201-2208. [DOI] [PubMed] [Google Scholar]
- 6.Federal Ministry of Health. 2004. 2003 national HIV sero-prevalence sentinel survey; technical report: April 2004. Federal Ministry of Health, Abuja, Nigeria.
- 7.Kaaba, S. A., S. Al Fadhli, M. Burhamah, H. Al Jafar, and A. Khamis. 2004. Lymphocyte subsets in healthy adult Kuwaiti Arabs with relative benign ethnic neutropenia. Immunol. Lett. 91:49-53. [DOI] [PubMed] [Google Scholar]
- 8.Kaaba, S. A., S. Al Fadhli, and A. Khamis. 2002. Reference values of lymphocyte subsets in the normal healthy adult Kuwaiti Arab population. Immunol. Lett. 81:199-203. [DOI] [PubMed] [Google Scholar]
- 9.Kam, K. M., W. L. Leung, M. Y. Kwok, M. Y. Hung, S. S. Lee, and W. P. Mak. 1996. Lymphocyte subpopulation reference ranges for monitoring human immunodeficiency virus-infected Chinese adults. Clin. Diagn. Lab. Immunol. 3:326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, B. W., H. K. Yap, F. T. Chew, T. C. Quah, K. Prabhakaran, G. S. Chan, S. C. Wong, and C. C. Seah. 1996. Age and sex related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry 26:8-15. [DOI] [PubMed] [Google Scholar]
- 11.Levin, A., G. Brubaker, J. S. Shao, D. Kumby, T. R. O'Brien, J. J. Goedert, K. W. Strauss, W. A. Blattner, and I. Hannet. 1996. Determination of T-lymphocyte subsets on site in rural Tanzania: results in HIV-1 infected and non-infected infected individuals. Int. J. STD AIDS 7:288-291. [DOI] [PubMed] [Google Scholar]
- 12.Menard, D., M. J. Mandeng, M. B. Tothy, E. K. Kelembho, G. Gresenguet, and A. Talarmin. 2003. Immunohematological reference ranges for adults from Central African Republic. Clin. Diagn. Lab. Immunol. 10:443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pattanapanyasat, K., S. Lerdwana, H. Shain, E. Noulsri, C. Thepthai, V. Prasertsilpa, A. Eksaengsri, and K. Kraisintu. 2003. Low-cost CD4 enumeration in HIV-infected patients in Thailand. Asian Pac. J. Allergy Immunol. 21:105-113. [PubMed] [Google Scholar]
- 14.Statacorp. 2002. Stata statistical software, release 8. Statacorp, College Station, Tex.
- 15.Temmerman, M., N. Nagelkerke, J. Bwayo, E. N. Chomba, J. Ndinya-Achola, and P. Piot. 1995. HIV-1 and immunological changes during pregnancy: a comparison between HIV-1 seropositive and HIV-1 seronegative women in Nairobi, Kenya. AIDS 9:1057-1060. [PubMed] [Google Scholar]
- 16.Tsegaye, A., T. Messele, T. Tilahun, E. Hailu, T. Sahlu, R. Doorly, A. L. Fontanet, and T. Rink de Wit. 1999. Immunohematological reference ranges for adult Ethiopians. Clin. Diagn. Lab. Immunol. 6:410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tugume, S. B., E. M. Piwowar, T. Lutalo, P. N. Mugyenyi, R. M. Grant, F. W. Mangeni, K. Pattishall, and E. Katongole-Mbidde. 1995. Hematological reference ranges among healthy Ugandans. Clin. Diagn. Lab. Immunol. 2:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuomala, R. E., A. Kalish, C. Zorilla, H. Fox, W. Shearer, A. Landay, S. Vermund, S. Landesman, and D. Burns. 1997. Changes in total, CD4+ and CD8+ lymphocytes during pregnancy and 1 year post partum in human immunodeficiency virus infected women. Obstet. Gynecol. 89:967-974. [DOI] [PubMed] [Google Scholar]
- 19.Uppal, S. S., S. Verma, and P. S. Dhot. 2003. Normal values of CD4 and CD8 lymphocyte subsets in healthy Indian adults and the effects of sex, age, ethnicity and smoking. Cytometry 52B:32-36. [DOI] [PubMed] [Google Scholar]
- 20.Urassa, W. K., E. M. Mbena, A. B. Swai, H. Gaines, F. S. Mhalu, and G. Biberfeld. 2003. Lymphocyte subset enumeration in HIV seronegative and HIV seropositive adults in Dar es Salaam, Tanzania: determination of reference values in males and females and comparison of two flow cytometric methods. J. Immunol. Methods 277:65-74. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. 2004. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, D.C.
- 22.Van Benthem, B. H. B., P. Vernazza, R. A. Coutinho, and M. Prins. 2002. The impact of pregnancy and menopause on CD4 lymphocyte counts in HIV infection in women and the Swiss HIV Cohort Study. AIDS 16:919-924. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2004. Scaling up antiretroviral therapy in resource limited settings: treatment guidelines for a public health approach. W.H.O., Geneva, Switzerland.