Abstract
Background
Autonomic Dysreflexia (AD) is a crucial emergency complication of cervical and upper thoracic spinal cord injury (SCI). Although there are several treatment options for AD, unfortunately, there is no consensus on the treatment of AD.
This study aimed to present Clinical Practice Guidelines (CPG) development for AD in SCI in different conditions.
Methods
The project was carried out by an executive team of general practitioners and neurosurgeons. A national multidisciplinary panel of experts performed the decision-making step, which consisted of deciding on the final list of recommendations and articulating novel recommendations regarding the infrastructure and fundamental elements necessary for managing patients suffering from AD. Four appraisers evaluated the guidelines using the Appraisal of Guidelines for Research and Evaluation (AGREE II) tools.
Results
A total of 575 articles were found after searching different databases. After the primary screening, title, abstract, and full-text screening were performed, which yielded 9 records. Five were excluded after the AGREE II evaluation. The source guidelines’ recommendations were tabulated as possible scenarios for 15 patient/population, intervention, comparison, and outcomes clinical questions. Based on the expert panel’s opinion, all the recommendations were adaptable. Finally, the suggestions were transformed into a protocol for managing patients suffering from autonomic dysreflexia.
Conclusion
This guideline presented the treatment and pharmacotherapy of autonomic dysreflexia. However, the treatment is being updated. We suggest more educational multimedia for health care professionals, primarily in the emergency department.
Keywords: Autonomic Dysreflexia, Care Protocol, Spinal Cord Injuries, Guideline
↑What is “already known” in this topic:
The emergency complication of autonomic dysreflexia (AD) in cervical and upper thoracic spinal cord injury (SCI) is significant and vital. Despite the fact that there are numerous treatments for AD, there is regrettably no agreed-upon approach.
→What this article adds:
This guideline presented the treatment and pharmacotherapy of AD; however, the treatment is being updated. Although AD usually improves by removing the triggers and drug administration is not recommended in the early stages, it can reduce the symptoms and complications. We suggest more educational multimedia for healthcare professionals, especially in the emergency department/.
Introduction
Autonomic dysreflexia (AD) is a vital emergency complication of cervical and upper thoracic spinal cord injury (SCI)—usually at the level of T6 or above ( 1-3). This occurs in reply to nervous system stimulation under the level of injury as a sudden and uncontrollable sympathetic response (4). After SCI, the spinal tract and sympathetic nervous system may disrupt, and supraspinal control of the spinal autonomic course may be limited. Various stimulation motivates AD, but bladder and colon stimulations are the 2 most common stimuli (5). The other common causes of AD are presented in Table 1 ( 1, 6-8).
Table 1. The etiologies of AD in patients with SCI.
| The etiology of AD | |
|---|---|
| Urinary System | Bladder distension Urinary tract infection Catheterization urinary catheter obstruction Kidney or bladder stones diagnostic or therapeutic Urological procedures (such as cystoscopy, lithotripsy) vibratory stimulation |
| Gastrointestinal System | Constipation Hemorrhoids Anal fissure Acute abdominal pain Digital Rectal examination Diagnostic procedures Peptic Ulcers Gastroesophageal reflux Appendicitis cholelithiasis |
| Skin | Tight clothing Decubitus ulcers or contact with hard or sharp objects Burns, sunburn or frostbite Insect bites Ingrown toenail or paronychia Blisters |
| Reproductive System | Menstruation Pregnancy Labor Sexual intercourse Ejaculation Sexual stimulation by electroejaculation or vibratory stimulation Vaginitis Epididymitis / prostatitis Scrotal compression (sitting on scrotum). |
| Respiratory system | Pulmonary embolism Auxiliary cough maneuver |
| others | Deep vein thrombosis Fractures Drugs such as pseudoephedrine Muscle spasm / spasticity Charcot joint Syringomyelia below the level of injury Postoperative pain Hyperthyroidism Intramuscular injection Excessive consumption of alcohol, substances, caffeine or diuretics Immersion in cold water |
Although AD may seem simple, it can induce life-threatening conditions, including arrhythmias or systolic hypertension up to 300 mm Hg (5). Misdiagnosed and mismanagement of these patients can lead to serious consequences, such as cerebral hemorrhage, retinal detachment, seizures, and death ( 9-12). Recent studies showed cardiovascular and circulatory disease as the most prevalent etiology of death in AD (13-15). Wan et al reported the effects of AD on hemodynamics, and they showed that the most common cause of death was central nerves system-related (hemorrhage and ischemic attacks) (72%) and cardiovascular-related (22%) (12, 13). The symptoms vary greatly, from a simple headache and skin rash to severe hypertension and cerebral hemorrhage (Table 2) (1, 16-18). A patient with SCI often has a blood pressure range of 90 to 110 mm Hg. Blood pressure in AD may only increase by 20 mm Hg. Therefore, it is necessary to compare the current blood pressure with the usual pressure (6, 19). In addition, AD can present with no other symptom except a slight increase in blood pressure, called silent AD (6).
Table 2. Signs and symptoms of patients with AD.
| Signs and symptoms of AD |
|---|
| Hypertension: more than 20 to 40 mm Hg above baseline Severe Pounding headache Pupil dilation Bradycardia relative to baseline Anxiety Nasal congestion Hot flashes, redness and excessive sweating top of the level of the injury Cold and pale skin under the level of injury Piloerection, Thermodysregulation or Hyperhidrosis Blurred vision Respiratory distress arrhythmia Convulsion Cerebral hemorrhage |
A remarkable problem related to AD is the lack of knowledge about the disease in patients and health care professionals, which can be very destructive. In a study by Jackson and Acland, 29 of 70 medical staff could not answer any questions about AD, and only 16 had received previous training about the disease. This shows the great need to provide the necessary education about this disease (20). Although there are several treatment options for AD, unfortunately, there is no consensus for its treatment and pharmacotherapy, mainly due to the lack of appropriate clinical trials (21). The latest study in Iran in 2016 reported that AD (37%) was one of the most common complications among 1137 patients with spinal cord injuries. This study showed that the prevalence of AD is even more common than neuropathic pain (37% vs 31%) and similar to urinary tract infections (37% vs 37.5%) among spinal cord patients (22). AD, a potentially serious condition, can be easily prevented and treated if health care professionals and patients have enough awareness and knowledge about this disease. If physicians consider AD in dealing with SCI patients, they can reduce the community’s dangerous complications and burden. In this regard, we also adopted a quality-of-care assessment tool for patients with traumatic SCI (23). This study aimed to present clinical practice guidelines (CPGs) development for AD in SCI in different conditions. CPGs are developed through a systematic review of the evidence and an assessment of the potential benefits and harms of the recommendations.
Methods
An executive team of general practitioners and neurosurgeons carried out the project. All members of the executive committee had work and research experience in the relevant field. All committee members also received training on the guideline adaption process. The executive committee performed all steps except the decision-making. The decision-making step was performed by a national team composed of multidisciplinary experts from all over the country, including experts active in managing AD. The decision-making step consisted of deciding on the final list of recommendations and articulating novel recommendations regarding the infrastructure and fundamental elements necessary for managing patients suffering from AD in Iran.
Initially, databases of clinical guidelines were thoroughly searched using the general keywords AD and SCI. Databases consist of National Guidelines Clearinghouse (NGC), Guidelines International Network (G-I-N), National Institute for Clinical Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), NHMRC Clinical Guideline Portal, TRIP database, World Health Organization, National Clinical Guideline Centre, Canadian Medical Association, Royal College of Nursing (RCN) (UK), National Electronic Library for Health (UK), New Zealand Guidelines Group, Academy of Neurologic Communication Disorders and Sciences, Australian State Departments of Health and Ageing, Guidelines Advisory Committee (Canada), Institute for Clinical Systems Improvement (ICSI), International Council of Nurses (ICN), Registered Nurses Association of Ontario, Medical Journal of Australia Clinical Guidelines, Royal Australian College of General Practitioners and The Canadian Coordinating Office for Health Technology Assessment. Published literature was initially searched using search terms related to AD and SCI until June 2018, then updated to June 2021.
Then, the initial screening of the guides was performed according to the following criteria:
- Availability of the full version of the guideline,
- Being in the group of guidelines, guidance, and systematic reviews,
- Credibility.
In the next step, the guidelines were evaluated by 4 appraisers according to the Appraisal of Guidelines for Research and Evaluation (AGREE II) tools. In the AGREE tool, 23 items are organized within 6 domains—scope and purpose; stakeholder involvement; rigor of development; clarity of presentation; applicability; and editorial independence—followed by 2 global rating items for an overall assessment. A specific aspect of guideline quality is shown by each domain and scored between 1 (completely opposite/non-observance of the desired criterion) to 7 (completely agree/complete observance of the selected criterion). The score of each section was calculated by adding the score given to the criteria and standardizing the total score according to the maximum and minimum score that can be obtained in that section. A minimum score of 60% on the critical AGREE domain, Rigor of Development, was utilized for inclusion based on the AGREE.
At this stage, the recommendations of the selected clinical guidelines were extracted in the form of clinical scenarios and tabulated, answering the same patient/population, intervention, comparison and outcomes (PICO) questions. Recommendations answering the same question were combined to form a single question and recommendation, whereas recommendations that included different interventions or populations were presented as separate questions.
At the end of this stage, the evidence supporting each recommendation was determined based on the reference listed in the specified clinical guidance, and the result section was extracted. The expert panel mentioned and considered the side effects and benefits other than the main consequences of the intervention.
Evidence of each recommendation was evaluated. New evidence was added for recommendations, where
A. If, for a question, there were more than 1 recommendation, which was inconsistent with each other;
B. If the level of evidence for recommendations is low, high-level evidence is considered to include systematic review studies or one of the following: evidence for treatment: clinical randomized trials; diagnostic evidence: cross-sectional studies or clinical prediction rules; evidence for side effects: cohort studies; prognostic evidence: inception cohorts.
The information about the clinical advantage of interventions and measures was sent to each of the panel members, and they were asked to state their final judgment regarding the clinical advantage (low, high, and moderate). Also, if the members used evidence other than the available evidence, the results of those studies were sent to other members. A questionnaire on each recommendation was sent to members to evaluate the adaptability of each recommendation (low, high, and moderate). The amount of agreement on the views of the panel members was reviewed. The recommendations that were agreed upon were considered as the final recommendation. Recommendations on which there was no complete agreement were examined in the attendance session. At the meeting, the remaining recommendations were discussed, each member stated their reasons based on existing evidence and mentioned criteria for adapting guidelines, and the recommendations were rescored.
Results
A total of 575 articles were found after searching different databases. Two members of the executive committee screened the records considering the inclusion criteria. After the primary screening, title, abstract, and full-text screenings yielded 10 records. Five were excluded after the AGREE II evaluation. The remaining 5 records were selected as source guidelines for data extraction:
1. “A systematic review of the management of autonomic dysreflexia after spinal cord injury” (21).
2. “Chronic spinal cord injury: management of patients in acute hospital settings” (24)
3. “Treatment of autonomic dysreflexia for adults & adolescents with spinal cord injuries” (25)
4. “Acute management of autonomic dysreflexia: individuals with spinal cord injury presenting to health Care Facilities” (6)
5. “Evaluation and management of autonomic dysreflexia and other autonomic dysfunctions: preventing the highs and lows: management of blood pressure, sweating, and temperature dysfunction” (26)
The recommendations of source guidelines were classified as possible scenarios for 15 PICO clinical questions. Based on the expert panel’s opinion, all the recommendations were adaptable. Finally, the recommendations were transformed into a protocol for managing patients suffering from AD (Figure 1).
Figure 1.
Flow chart of Management Protocol for patients with AD
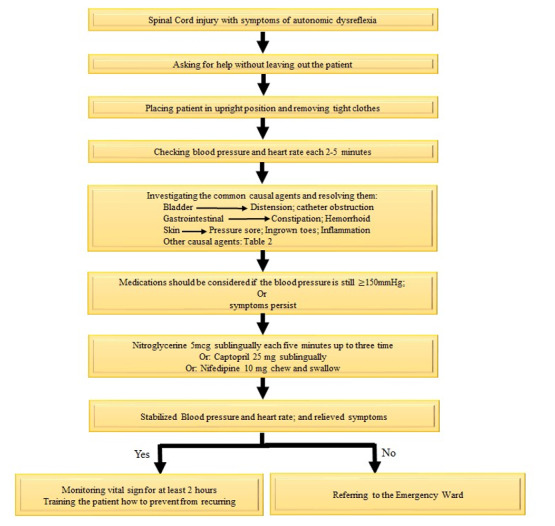
1.1. Clinical Recommendation
a) Never leave the patient alone when managing patients with AD
b) Sit the patient upright and lower the legs to decrease the blood pressure
c) Remove tight clothes, shoes, socks, and belts
d) During AD, the blood pressure will likely change, thus, it should be checked every 2 to 5 minutes (1, 6 )
e) Ask the patient or companions about the history of AD and its previous cause
f) Identify and resolve the cause of AD (Table 1). Start with the most common factors: (26)
1.2. Urinary System
a) Consider the patient’s urinary status and bladder emptying—intermittent catheterization, permanent catheterization, suprapubic catheterization. Is the urine output proportional to the fluid intake? (1)
b) Catheterize the patient if a catheter is not in place. Be careful because catheterization can exacerbate the symptoms. Thus, it is better to inject 2% lidocaine gel into the urethra and wait 2 minutes. Then, perform catheterization. (6)
c) If the patient is catheterized, check the catheter for sediment, blockage, fold, inflammation, dark urine, or other signs of a urinary tract infection. If the catheter is blocked, you can rinse it with 10 to 15 mL saline (physiological serum) at the body temperature. Avoid manually compressing the bladder. If the pressure increases without catheter output, remove it and place a new catheter for the patient.
d) Monitor the blood pressure and vital signs during bladder drainage (6).
1.2.1. Gastrointestinal System
a) Ask about the patient’s gastrointestinal habits. Is there a new change? Is constipation, impaction, or fecal or bowel distension possible?
b) If the patient’s systolic blood pressure is ˂150 mm Hg, perform the following steps to examine the patient’s rectum (6):
c) Wear gloves.
d) Anesthetize the anal area using 2% lidocaine gel.
e) Wait 2 minutes If possible.
f) Insert your finger gently into the rectum.
g) Look for the stool with your finger dipped in the lubricant gel and remove it if possible.
h) If the patient’s symptoms worsen, stop the procedure. Inject more anesthetic gel and wait for 20 minutes. Recheck the rectum for stool.
i) If the systolic blood pressure is ˃150 mm Hg, first use a short-acting antihypertensive drug to decrease the blood pressure (See Pharmacotherapy section).
1.2.2. Monitor the Patient for Uncommon Causes
a) Examine the skin for compression, sores, or inflammation
b) Sexual activity and stimulations in men and women with SCI may provoke AD.
c) Self-induced AD (boosting) to improve daily sports performance or daily practice is an uncommon and dangerous cause that can result in uncontrollable hypertension (26).
d) Check other causes (Table 1).
1.3. Pharmacotherapy
AD usually improves with the removal of the triggers. Drug administration in the early stages is not recommended, but it can reduce symptoms and complications. It is allowed only if the etiology of AD has been eliminated and the pressure stays ˃150-170 mm Hg (21). In this case, rapid-onset and short-acting antihypertensive drugs are recommended.
• Sublingual nitroglycerin (0.4 mg, every 5-10 min up to 3 times) can be used (1, 21, 25). Ask the patients for consumption of phosphodiesterase 5 inhibitors drugs (Sildenafil, Tadalafil, etc) in the last 48-24 hours (27).
• If the patients take the phosphodiesterase 5 inhibitors, sublingual captopril (25 mg) or nifedipine (10 mg) can be administered instead of nitroglycerin. Although nifedipine has typically side effects such as hypotension and should be used cautiously, it can prevent dangerous hypertensive reactions in SCI patients with AD (7, 21). It is therefore essential to closely monitor the patient. (1)
• There are other options for long-term treatments, such as terazosin (1 mg per night, gradually increasing to 5 mg) and prazosin (0.5-1 mg for 2-3 times a day).
• Orthostatic hypotension may occur in patients with AD. Nonpharmacological interventions should be considered to maintain baseline BP, and then pharmacological interventions are recommended when nonpharmacological interventions prove ineffective (26).
1.4. Outpatient Management
After the disappearance of symptoms with normal blood pressure, it is necessary to monitor the blood pressure and vital signs for at least 48 hours, depending on the severity of the symptoms. Two reasons are mentioned: (1) possibility of hypotension and (2) recurrent AD. This monitoring can be done at home depending on the severity of the symptoms and the knowledge and awareness of the patients and their companions (1).
1.5. Refer to the Hospital
• If the cause of AD is not found or the blood pressure is uncontrolled, the patient should be referred to the emergency room. Also, if AD symptoms are diagnosed without hypertension and other known etiology, the patient should be directed to the emergency room. (1, 6 )
• It is advised that at-risk patients carry an identity card that details the history, cause, and treatment of the condition so that it can be used when appropriate by the majority of medical professionals managing critically ill AD patients.
1.6. Outpatient Treatment Protocol in the Pregnant Woman With AD
Women with SCI during pregnancy, labor, delivery, and breastfeeding are at risk for AD (26). Blood pressure disturbances during pregnancy are not uncommon in the general population. AD can occur throughout pregnancy, but labor is more sensitive than ever. (16, 28, 29) The symptoms of AD in pregnancy are similar to normal women. (6) The most important differential diagnosis of AD in SCI pregnant women is preeclampsia, characterized by the classic triad of hypertension, edema, and proteinuria. (6) Cooccurrence of AD and preeclampsia may complicate diagnosis and treatment. (6, 16) Both are usually diagnosed during labor pain, thus, a midwife or gynecologist is needed to confirm the condition and the possibility of starting labor and check the fetal heart. Also, postpartum breastfeeding, breast engorgement, or mastitis in women with SCI may trigger AD (26).
Due to the pressure on the vena cava in pregnant women in the supine position, hypotension is possible. In this case, lateral tilt or upright position can help the patient recover. (6)
The initial steps in gravid women are equivalent to nongravid women. Pregnant women with suspected AD should be referred to a gynecology and obstetrics service center in the cases mentioned below (6):
• The first episode of AD during pregnancy
• The third trimester of pregnancy
• Vaginal bleeding
• Doubt about the onset of labor
• Failure to resolve symptoms despite taking initial steps
• Resistant hypertension after the resolution of the initial episode of AD
• Deciding on pharmacotherapy and choosing an antihypertensive drug
• Hypotension
• Suspicious or uncertain diagnosis, cause, and symptoms, even if the pressure is normal
Discussion
This study aimed to present CPG development for AD in SCI among Iranian patients in different conditions. AD is a severe condition, but there are limited clinical trials and guidelines in the literature review. The most effective way to prevent AD is to train health care providers and patients about the cause and management of AD. Still, recognition of particular triggers for AD should be noticed to manage this condition (17).
AD can be treated by various nonpharmacological and pharmacological strategies (30). In addition to known pharmacologic strategies, some new techniques were expressed in recent studies. Fabro et al (31) showed that using baclofen intrathecally in SCI patients significantly reduced the symptomatic episodes of AD. Botulinum toxin subtype A is suggested as an effective treatment for hyper-reflexive bladders and detrusor-sphincter dyssynergia in patients with SCI (32). Walter et al reported that intradetrusor onabotulinum toxin A injections as a safe strategy in AD could recover lower urinary tract action (33). Also, capsaicin represents the reduction of the severity of the hyperreflexia by a selective inhibitory effect on sensory C-fibers in patients with AD. Anti CD-11 d antibody as another agent may reduce colorectal distention-induced AD by at least 50%, which persisted for 6 weeks and the magnitude of the hypertensive reflex during colorectal distention within the first 6 hours of an injury. Cell transplantation to restore sensory/motor function has been studied recently, but there are limited data about its ability to improve autonomic deficits in patients with SCI (32).
The treatment and medication for AD were discussed in this guideline. Although the symptoms and problems of AD can be reduced and drug administration is not advised in the early stages, AD normally gets better by removing the triggers (21 ).
Limitation: The reliability and validity of AD management have not been evaluated. As another limitation, only English articles and published papers were studied.
Conclusion
we suggest providing more educational multimedia and software for health care professionals, especially emergency departments because they can limit the burden of AD through prevention and early nonpharmacological management.
Conflict of Interests
The authors declare that they have no competing interests.
Funding
This work was funded by Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences (grant number is 97-02-38-38856).
Acknowledgment
This work was funded by Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences (grant number is 97-02-38-38856).
Ethical Approval
The Ethics Committee of Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, approved the study (Ref No.: is 97-02-38-319).
Cite this article as : Ebrahimi H, Maroufi SF, Abdollahzadegan S, Rahimi-Movaghar V. Clinical Practice Guideline Development for Autonomic Dysreflexia in Spinal Cord Injury. Med J Islam Repub Iran. 2023 (9 Oct);37:109. https://doi.org/10.47176/mjiri.37.109
References
- 1.Milligan J, Lee J, McMillan C, Klassen H. Autonomic dysreflexia: recognizing a common serious condition in patients with spinal cord injury. Can Fam Physician. 2012;58(8):831. [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson A. Autonomic dysreflexia. Spinal Cord. 1999;37(6):383. doi: 10.1038/sj.sc.3100867. [DOI] [PubMed] [Google Scholar]
- 3.Lindan R, Joiner E, Freehafer A, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18(5):285. doi: 10.1038/sc.1980.51. [DOI] [PubMed] [Google Scholar]
- 4.Hagen E. Acute complications of spinal cord injuries. World J Orthop. 2015;6(1):17–23. doi: 10.5312/wjo.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrest G. Atrial fibrillation associated with autonomic dysreflexia in patients with tetraplegia. Arch Phys Med Rehabil. 1991;72(8):592. [PubMed] [Google Scholar]
- 6.Organizations CfSCMM. Acute management of autonomic dysreflexia: individuals with spinal cord injury presenting to health-care facilities. J Spinal Cord Med. 2002;25:S67. [PubMed] [Google Scholar]
- 7.Furlan J. Autonomic dysreflexia: a clinical emergency. J Trauma Acute Care Surg. 2013;75(3):496–500. doi: 10.1097/TA.0b013e31829fda0a. [DOI] [PubMed] [Google Scholar]
- 8.Courtois F, Rodrigue X, Côté I, Boulet M, Vézina JG, Charvier K. et al. Sexual function and autonomic dysreflexia in men with spinal cord injuries: how should we treat. Spinal Cord. 2012;50(12):869. doi: 10.1038/sc.2012.83. [DOI] [PubMed] [Google Scholar]
- 9.Dolinak D, Balraj E. Autonomic dysreflexia and sudden death in people with traumatic spinal cord injury. Am J Forensic Med Pathol. 2007;28(2):95. doi: 10.1097/PAF.0b013e3180600f99. [DOI] [PubMed] [Google Scholar]
- 10.Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Paraplegia. 1992;30(5):355. doi: 10.1038/sc.1992.82. [DOI] [PubMed] [Google Scholar]
- 11.Vallès M, Benito J, Portell E, Vidal J. Cerebral hemorrhage due to autonomic dysreflexia in a spinal cord injury patient. Spinal Cord. 2005;43(12):738. doi: 10.1038/sj.sc.3101780. [DOI] [PubMed] [Google Scholar]
- 12.Yarkony G, Katz R, Wu Y. Seizures secondary to autonomic dysreflexia. Arch Phys Med Rehabil. 1986;67(11):834. [PubMed] [Google Scholar]
- 13.Wan D, Krassioukov A. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med. 2014;37(1):2–10. doi: 10.1179/2045772313Y.0000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord. 2001;39(6):310. doi: 10.1038/sj.sc.3101162. [DOI] [PubMed] [Google Scholar]
- 15.Garshick E, Kelley A, Cohen S, Garrison A, Tun C, Gagnon D. et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camune B. Challenges in the management of the pregnant woman with spinal cord injury. J Perinat Neonatal Nurs. 2013;37(3):225. doi: 10.1097/JPN.0b013e31829ca83f. [DOI] [PubMed] [Google Scholar]
- 17.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med. 2012;22(1):39–45. doi: 10.1097/JSM.0b013e3182420699. [DOI] [PubMed] [Google Scholar]
- 18.Blackmer J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ. 2003;169(9):931. [PMC free article] [PubMed] [Google Scholar]
- 19.Guttmann L, Frankel H, Paeslack V. Cardiac irregularities during labour in paraplegic women. Paraplegia. 1965;3(2):144. doi: 10.1038/sc.1965.14. [DOI] [PubMed] [Google Scholar]
- 20.Jackson C, Acland R. Knowledge of autonomic dysreflexia in the emergency department. Emerg Med J. 2011;28(10):866. doi: 10.1136/emj.2009.085159. [DOI] [PubMed] [Google Scholar]
- 21.Krassioukov A, Warburton DE, Teasell R, Eng JJ;. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90(4):682. doi: 10.1016/j.apmr.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derakhshanrad N, Yekaninejad M, Vosoughi F, Fazel FS, Saberi H. Epidemiological study of traumatic spinal cord injuries: experience from a specialized spine center in Iran. Spinal Cord. 2016;54(10):901. doi: 10.1038/sc.2016.10. [DOI] [PubMed] [Google Scholar]
- 23.Ghodsi Z, Jazayeri SB, Pourrashidi A, Sadeghi-Naini M, Azadmanjir Z, Baigi V. et al. Development of a comprehensive assessment tool to measure the quality of care for individuals with traumatic spinal cord injuries. Spinal Cord Ser Cases. 2023;9(1):12. doi: 10.1038/s41394-023-00569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gall A, Turner-Stokes L, Group GD. Chronic spinal cord injury: management of patients in acute hospital settings. Clin Med (Lond) 2008;8(1):70. doi: 10.7861/clinmedicine.8-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Middleton J Treatment of autonomic dysreflexia for adults & adolescents with spinal cord injuries. A medical emergency targeting health professionals. Chatswood, NSW: NSW State Spinal Cord Injury Service; 2010 ; Available from: www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155149/Auto_Dysreflexia_Fact_sheet.pdf
- 26.Krassioukov A, Linsenmeyer TA, Beck LA, Elliott S, Gorman P, Kirshblum S, Vogel L, Wecht J, Clay S. Evaluation and Management of Autonomic Dysreflexia and Other Autonomic Dysfunctions: Preventing the Highs and Lows: Management of Blood Pressure, Sweating, and Temperature Dysfunction. Top Spinal Cord Inj Rehabil. 2021 Spring;27(2):225–290. doi: 10.46292/sci2702-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabchevsky A, Kitzman P. Latest approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics. 2011;8(2):274. doi: 10.1007/s13311-011-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker E, Cardenas D. Pregnancy in spinal cord injured women. Arch Phys Med Rehabil. 1996;77(5):201. doi: 10.1016/s0003-9993(96)90041-6. [DOI] [PubMed] [Google Scholar]
- 29.Westgren N, Levi R. Motherhood after traumatic spinal cord injury. Paraplegia. 1994;32(8):517. doi: 10.1038/sc.1994.83. [DOI] [PubMed] [Google Scholar]
- 30.Eldahana KC, Rabchevsky AG. Autonomic Dysreflexia after Spinal Cord Injury: Systemic Pathophysiology and Methods of Management. Auton Neurosci. 2018;209:59–70. doi: 10.1016/j.autneu.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabro ASD, Mejia M, Nemunaitis G. An investigation of the relationship between autonomic dysreflexia and intrathecal baclofen in patients with spinal cord injury. J Spinal Cord Med. 2018;41(1):102. doi: 10.1080/10790268.2017.1314878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharif H, Hou S. Autonomic dysreflexia: a cardiovascular disorder following spinal cord injury. Neural Regen Res. 2017;12(9):1390–1400. doi: 10.4103/1673-5374.215241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter M, Kran SL, Ramirez AL, Rapoport D, Nigro MK, Stothers L. et al. Intradetrusor OnabotulinumtoxinA Injections Ameliorate Autonomic Dysreflexia while Improving Lower Urinary Tract Function and Urinary Incontinence-Related Quality of Life in Individuals with Cervical and Upper Thoracic Spinal Cord Injury. J Neurotrauma. 2020;37(18):2023. doi: 10.1089/neu.2020.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]


