Abstract
Enterohemorrhagic Escherichia coli (EHEC) exhibits a pattern of localized adherence to host cells, with the formation of microcolonies, and induces a specific histopathological phenotype collectively known as the attaching and effacing lesion. The genes encoding the products responsible for this phenotype are located on a 35-kb pathogenicity island designated the locus of enterocyte effacement, which is also shared by enteropathogenic E. coli. We have identified an open reading frame (ORF) which is located upstream of the espA, espB, and espD genes on the complementary strand and which exhibits high homology to the genes spiB from Salmonella, yscD from Yersinia, and pscD from Pseudomonas. Localization studies showed that the encoded product is present in the cytoplasmic and inner membrane fractions of EHEC. The construction and characterization of a recombinant clone containing an in-frame deletion of this ORF demonstrated that the encoded product is a putative member of a type III system required for protein secretion. Disruption of this ORF, designated pas (protein associated with secretion), abolished the secretion of Esp proteins. The mutant adhered only poorly and lost its capacities to trigger attaching and effacing activity and to invade HeLa cells. These results demonstrate that Pas is a virulence-associated factor that plays an essential role in EHEC pathogenesis.
The incidence of food-borne infections caused by enterohemorrhagic Escherichia coli (EHEC) has increased since the description of this pathogen in the early 1980s (48). EHEC can cause severe and potentially life-threatening diseases, being the major cause of bloody diarrhea and acute renal failure (6, 25). Up to 20% of infected patients develop hemolytic-uremic syndrome or hemorrhagic colitis (25). Typically, children and elderly people are the most susceptible, with high mortality rates (10% of hemolytic-uremic syndrome cases) (1). The low infective dose of EHEC (7) favors the development of epidemic outbreaks, such as that recently described in Japan, which affected more than 9,000 school children (59).
In many aspects, the pathogenesis of EHEC resembles that of enteropathogenic E. coli (EPEC); however, one distinctive feature of EHEC is the capacity to produce Shiga toxins. These powerful cytotoxins attack endothelial cells of blood vessels and appear to be involved in the increased incidence of complications experienced by infected patients (42, 56). In the early stages of the infection process, bacteria attach to the surface of eukaryotic cells, forming small localized colonies; then, they trigger the attaching and effacing lesion. This event is characterized by intimate bacterial contact, localized destruction of microvilli, and reorganization of cytoskeletal proteins beneath the attached bacteria.
A pathogenicity island known as the locus of enterocyte effacement (LEE) encodes the bacterial products required for the production of the attaching and effacing lesion (39). The eaeA gene codes for the outer membrane protein intimin, which is required for intimate bacterial attachment and which is essential for the infectious phenotype (12). The eaeA gene is located upstream of a gene cluster encoding several proteins (EspA, EspD, and EspB) secreted by a type III secretion system (13, 15, 20, 32, 36). The production of these proteins is temperature and medium dependent (17, 32) and triggers, in an as-yet-unknown manner, the inositol triphosphate signal transduction cascade that leads to microvillus disruption and rearrangement of the cytoskeleton (4, 49). Type III secretion systems are widespread in a variety of pathogenic bacteria and are encoded by at least 20 genes (for reviews, see references 5 and 21). The Yop (Yersinia outer proteins) secretion apparatus, partly composed of Ysc (Yersinia secretion) proteins, is the prototype of these systems (5, 21). In EHEC, four secretion apparatus genes, sepABCD, are located in the left half of the LEE, upstream of eaeA (30). Disruption of each of these genes abolishes the signal transduction events that are required for bacterial interactions with eukaryotic cells during the infection process (20, 49).
In this work, we identified and characterized a novel gene, designated pas (protein associated with secretion), which is located between the eaeA and espA genes. The encoded product is essential for the secretion of Esp proteins. An EHEC derivative containing an in-frame deletion in the pas gene was highly impaired in attachment and lost the capacities to trigger attaching and effacing activity and to invade eukaryotic cells.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The strains and plasmids used in this study are described in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (50), on LB agar plates, and in serum-free Dulbecco’s modified Eagle medium (DMEM; GIBCO, Karlsruhe, Germany) supplemented with 100 mM HEPES (pH 7.4). Plasmids were maintained in E. coli DH5α, and the INVαF′ strain was used as a recipient for cloning of fragments amplified by PCR into the pCR2.1 vector. Media were supplemented with chloramphenicol (50 μg ml−1), ampicillin (200 μg ml−1), or nalidixic acid (50 μg ml−1) when required.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant genotype, phenotype, or serotype | Source or reference |
|---|---|---|
| Strains | ||
| EDL933 | Prototypic O157:H7 EHEC strain | 42 |
| E32511/0 | O157 EHEC strain | 62 |
| E32511/0 Nalr | Nalr spontaneous derivative of E32511/0 | This study |
| 413.89-1 | O26:H− STEC strain | 61 |
| EDL933 Δpas | EDL933 derivative containing an in-frame deletion of the pas gene | This study |
| DH5α | endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 φ80d lacZΔM15 Δ(lacZYA-argF)U169 | 50 |
| INVαF′ | F′ endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 relA1 φ80 lacZΔM15 Δ(lacZYA-argF)U169 deoR λ− | Invitrogen |
| S17-1λpir | Tpr SmrrecA thi pro hsdRM+ RP4:2-Tc:Mu:Km Tn7 λpir | 10 |
| M15(pREP4) | Nals Strs Rifslac ara gal mtl F−recA+ uvr+ | Qiagen |
| Plasmids | ||
| pQE30 | Apr; expression vector | Qiagen |
| pCR2.1 | Apr Kmr; high-copy-number vector for cloning PCR products | Invitrogen |
| pMAK700oriT | Cmr; thermosensitive positive-selection suicide vector | 57 |
| pKSC1 | Cmr; pMAK700oriT derivative containing an 875-kb PCR fragment generated with primers ANK36 and ANK39 and encompassing the pas gene with a 960-bp internal deletion | This study |
| pKSC2 | Apr Kmr; pCR2.1 derivative containing a 1,830-bp PCR fragment generated with primers ANK36 and ANK39 and encompassing the pas gene with upstream and downstream regions | This study |
| pANK84 | Apr Kmr; pCR2.1 derivative containing a PCR fragment generated with primers ANK7191 and AE19 and encompassing the region between eaeA and espB | This study |
| pQE30-PasEE | Apr; pQE30 derivative containing a 1,221-bp PCR fragment generated with primers ANK49 and ANK50 and carrying a histidine-tagged Pas fusion protein | This study |
DNA manipulations.
Plasmid DNA isolation, restriction endonuclease digestion, ligation, transformation, agarose gel electrophoresis, and other standard DNA techniques were carried out as described by Sambrook et al. (50). Oligonucleotides (Table 2) were synthesized by GIBCO. Colony PCR, extraction of PCR products, and cloning experiments were performed in accordance with standard protocols (50). DNA sequencing was performed by the method of Sanger et al. (51) with a Taq DyeDeoxy Terminator Cycle Sequencing Kit and an automated model 373A DNA sequencer (Applied Biosystems) according to the manufacturer’s instructions. Restriction and modification enzymes were purchased from New England BioLabs, Schwalbach, Germany. Electroporation was carried out with a gene pulser (Bio-Rad Laboratories) as described by O’Callaghan and Charbit (43). Searches in databases for nucleotide and amino acid sequence homologies were performed with the BLASTP (2), BLASTP + BEAUTY (2, 64), NNPP (47), and PSORT (41) algorithms.
TABLE 2.
Oligonucleotides used for PCR and sequencing
| Name | Nucleotide sequence (5′-3′) |
|---|---|
| ANK36 | GTGGATCCCCAGGCTTAGCCCTTCAATCGTTTC |
| ANK37 | CGTACCGCCCCGGGCGTTATCTTCCGTACCTAG |
| ANK38 | CCCGGGGCGGTACGATGAAGAATCCAAC |
| ANK39 | GTGGATCCCACCCCGGCTAAAATATGTATTG |
| ANK49 | GCAGGATCCATGTTATCCTCATATAAAATAAAC |
| ANK50 | CCAGGTACCTTAATACGACAGTGGAATATG |
| ANK7191 | GCTTTATTCTGGCTCTCAAAAACG |
| AE19a | CAGGTCGTCGTGTCTGCTAAA |
| KSCeaeA | CAGACATCTAGTGAGCAGC |
| ANK292 | GGATCCCGGGAGCAGCTTCTCGATTGTCG |
This oligonucleotide has been described elsewhere (23).
Cloning of the EHEC chromosomal region between the eaeA and espB genes.
Primers AE19 (sense) and ANK7191 (antisense), which are homologous to positions 1962 to 1979 and positions 263 to 240 of the EHEC strain EDL933 eaeA and espB published sequences, respectively (EMBL database accession no. Z11541 and X96953, respectively), were used to amplify by PCR the region between the eaeA and espB genes. The resulting 5,583-bp fragment was cloned into the pCR2.1 vector, generating pANK84. Unidirectional digestion from each strand was performed with a double-stranded nested deletion kit (Pharmacia, Freiburg, Germany), and both strands of the resulting clones were sequenced with vector-specific universal primers.
Construction of a nonpolar mutation.
Overlap extension PCR (29) was used to generate an in-frame deletion in the pas gene. Two PCR fragments were obtained by use of an Expand High Fidelity kit (Boehringer Mannheim GmbH, Mannheim, Germany) with the primer pairs ANK36-ANK37 (452 bp) and ANK38-ANK39 (437 bp). The resulting products contained the first 100 bp and the last 161 bp of the pas open reading frame (ORF), respectively. A 14-bp overlap in the sequences allowed the amplification of an 875-bp fragment during a second PCR with the primer pair ANK36-ANK39. The resulting product, which encompassed a pas gene containing an internal 960-bp deletion, was digested with BamHI and cloned into BclI-digested pMAK700oriT (57), generating pKSC1. This plasmid was transformed into the S17-1λpir strain and then transferred by conjugation (27) into the recipient EHEC strain E32511/0 Nalr. Plasmid pKSC1 was recovered from E32511/0 and subsequently electroporated into EHEC strain EDL933. Cointegration and excision of the suicide vector were performed as previously described (57). The in-frame deletion contained in the EDL933 Δpas mutant resulting from the allelic exchange was confirmed by Southern blot analysis and DNA sequencing of a PCR product obtained with the primers KSCeaeA and ANK292, which have homology with adjacent external sequences (data not shown). The primers ANK36 and ANK39 were used to amplify the full-length pas gene and 340 bp of the region located upstream of the start codon; the product was subsequently cloned into pCR2.1, generating pKSC2, which was used for complementation studies.
Production of a Pas-specific antiserum.
The pas gene was amplified by PCR with the primer pair ANK49-ANK50. The resulting product was digested with BamHI/KpnI, ligated with predigested pQE30 (Qiagen), and subsequently transformed into E. coli strain M15(pREP4). The resulting pQE30-PasEE plasmid carries a histidine-tagged Pas fusion protein. Overexpression and purification of the recombinant protein were performed in accordance with the manufacturer’s recommendations (Qiagen) under denaturing conditions. Mice were immunized intraperitoneally (50 μg of protein with Freund’s incomplete adjuvant) and given a booster after 15 days. Blood was collected 15 days following the last immunization, and the Pas-specific antiserum (MαPas) was separated and stored at −20°C.
Detection of secreted proteins.
To enhance the expression and secretion of Esp proteins, bacteria were grown in DMEM-HEPES until they reached an absorbance at 600 nm of 0.6 (31). The proteins present in supernatant fluids were precipitated by the addition of 10% (vol/vol) trichloroacetic acid, overnight incubation at 4°C, and subsequent centrifugation at 4,000 × g for 30 min. The dry pellet was resuspended in 1.5 M Tris (pH 8.8). To obtain whole-cell extracts, bacteria were pelleted and, after resuspension in electrophoresis sample buffer (50), boiled at 100°C for 10 min. Bacteria were fractionated to obtain periplasmic, cytoplasmic, and outer and inner membrane extracts in accordance with standard protocols (52). Proteins (30 μg/lane) were fractionated by discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with a 12.5% separating gel (50). Proteins were transferred onto a positively charged Biodyne B nylon membrane (Pall, Dreieich, Germany) by use of a semidry device (Bio-Rad). Nonspecific binding sites were saturated with 5% (vol/vol) low-fat milk (1.5%) in phosphate-buffered saline (PBS)–Tween 20 (0.1%, vol/vol). The EspB, EspE, and Pas proteins were detected with mouse monoclonal antibodies specific for EspB (mabMαEspB) and EspE (mabMαEspE) (9, 17) and the Pas-specific antiserum (MαPas) as primary antibodies and horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) and IgM as secondary antibodies (Bio-Rad). Antigen-antibody complexes were visualized by chemiluminescence with an ECL kit (Amersham Life Science, Braunschweig, Germany).
Analysis of the interactions between Pas and proteins secreted by EHEC.
To study the interactions between Pas and Esp proteins, proteins present in supernatant fluids were concentrated, fractioned by SDS-PAGE, and blotted onto a nitrocellulose membrane (Sartorius, Göttingen, Germany) as described above. Single lanes were cut and individually treated. Nonspecific binding sites were blocked with 5% (wt/vol) bovine serum albumin in PBS-Tween 20 (0.5%, vol/vol) at room temperature (RT) for 1 h. Strips were incubated at 4°C overnight with 0.5% (wt/vol) bovine serum albumin in PBS-Tween 20 containing either purified recombinant Pas or cytoplasmic or inner membrane fractions from strain EDL933. Strips were washed with PBS-Tween 20 for 15 min, blocked at RT for 1 h, washed, and incubated with the Pas-specific antiserum (MαPas) diluted 1:1,000 for 1 h at RT. After being washed, the strips were further incubated for 1 h at RT with horseradish peroxidase-conjugated rabbit anti-mouse IgG and IgM (Bio-Rad) and washed, and antigen-antibody complexes were visualized by chemiluminescence with the ECL kit. To detect Esp proteins, control strips were incubated overnight at 4°C with monoclonal antibodies specific for EspA, EspB, EspD, and EspE (9, 17), and the strips were processed as described above.
Tissue culture methods and analysis by immunofluorescence microscopy.
HeLa cells (ATCC CCL2) were maintained in DMEM supplemented with 25 mM HEPES, 10% (vol/vol) fetal calf serum (FCS), and glutamine (GIBCO) in an atmosphere containing 5% CO2 at 37°C. To study the reorganization of cellular actin underneath bacteria upon EHEC infection, cells were seeded onto 12-mm-diameter glass coverslips (InterMed Nunc, Wesbaden-Biebrich, Germany) at a concentration of approximately 5 × 104 per well in 24-well Nunclon Delta tissue culture plates (InterMed Nunc). Cell monolayers were infected with overnight-grown bacteria resuspended in DMEM-HEPES at a cell/bacterium ratio of 1:100. After 6 h of incubation, the monolayers were washed to remove unattached bacteria, fixed with 3.7% (vol/vol) p-formaldehyde in PBS, and permeabilized with 0.2% Triton X-100 in PBS, and bacteria were stained with a rabbit polyclonal antiserum against EHEC O157:K− (Behring, Marburg, Germany). Coverslips were washed, the primary antibody was labelled with fluorescein isothiocyanate-conjugated goat anti-rabbit antibodies (Dianova, Hamburg, Germany), and F actin was stained (34) with tetramethyl-rhodamine-isothiocyanate-labelled phalloidin (Sigma, Deisenhofen, Germany). Coverslips were washed and mounted, and cells were examined by epifluorescence with a Zeiss Axiophot microscope (Carl Zeiss, Jena, Germany).
Scanning and transmission electron microscopy.
For scanning electron microscopy studies, infected cells grown on round 12-mm Thermanox glass coverslips were fixed in cacodylate buffer (0.1 M cacodylate, 0.01 M MgCl2, 0.01 M CaCl2, pH 6.9) containing 3% (vol/vol) glutaraldehyde and 5% (vol/vol) p-formaldehyde for 45 min on ice, washed with PBS, dehydrated in a graded series of acetone, and subjected to critical-point drying with CO2. Samples were sputtered with a 10-nm gold film and examined with a Zeiss DSM 982 Gemini field-emission scanning electron microscope. Transmission electron microscopy was used to visualize internalized bacteria. At 6 h postinfection, the monolayers were washed twice with PBS and fixed in cacodylate buffer (see above) for 45 min on ice. Postfixation was performed for 1 h at RT with 1% (wt/vol) aqueous OsO4 in cacodylate buffer, and the monolayers were dehydrated in a graded series of acetone (10, 30, and 50%, vol/vol). In-block staining was performed with 2% (wt/vol) uranyl acetate in 70% acetone overnight. After complete dehydration in 90 and 100% (vol/vol) acetone, the samples were incubated in a mixture of 1 part acetone/1 part Spurr’s resin (54) overnight, followed by 1 part acetone/2 parts Spurr’s resin. Subsequently, the samples were placed in pure Spurr’s resin, with several changes. After polymerization of the resin at 70°C for 8 h, the samples were trimmed and cut with a glass knife (Ultracut S; Leica, Bensheim, Germany). Ultrathin sections were collected onto Formvar-covered 300-mesh copper grids and poststained with uranyl acetate for 30 min at 4°C and lead citrate for 3 min at 20°C with an Ultrostainer (Leica). Sections were examined with a Zeiss EM 910 transmission electron microscope at an acceleration voltage of 80 kV at calibrated magnifications.
Quantitative determination of bacterial attachment and invasion.
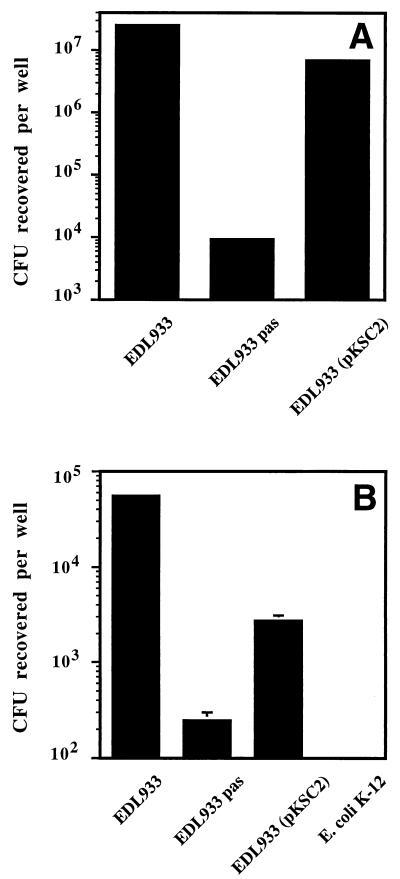
HeLa cells were seeded into 24-well plates (5 × 104 cells/well) and grown overnight in DMEM-HEPES with 10% FCS. Prior to infection, each well was washed and the medium was replaced with DMEM-HEPES supplemented or not supplemented with FCS. Cell monolayers were infected at a bacterium/cell ratio of 100:1 for 3.5 h. Supernatant fluids were subsequently discarded, the wells were washed with PBS to remove nonadherent bacteria, DMEM supplemented with gentamicin (100 μg ml−1) was added, and HeLa cells were further incubated for 2.5. The wells were washed with PBS, HeLa cells were lysed by the addition of 500 μl of 0.25% (vol/vol) Triton X-100, and the number of CFU recovered from each well was determined by plating of appropriate dilutions on LB agar plates with a Spiral Plater (Autoplate Model 3000; Bio-Sys, Karben, Germany). For the quantification of attached bacteria, cells were infected for 6 h with antibiotic-free DMEM-HEPES (during this period, monolayers were washed several times to remove nonadherent bacteria). The cells were washed and lysed, and the total number of bacteria recovered per well was determined. Values were corrected by subtracting the number of viable intracellular bacteria, as determined with matching controls pretreated with gentamicin. Reported results are mean values of three independent experiments ± standard errors of the mean. The statistical significance of the results obtained was evaluated by Student’s t test; differences were considered significant at a P value of ≤0.05.
Nucleotide sequence accession numbers.
The nucleotide sequences reported here (pas, orf1, espA, espD, and espB) will appear in the EMBL database under accession no. Y13068 and Y13859 (EDL933 and 413.89-1, respectively).
RESULTS AND DISCUSSION
Identification of a gene in the LEE of EHEC encoding a protein with homology to the SpiB protein of Salmonella typhimurium.
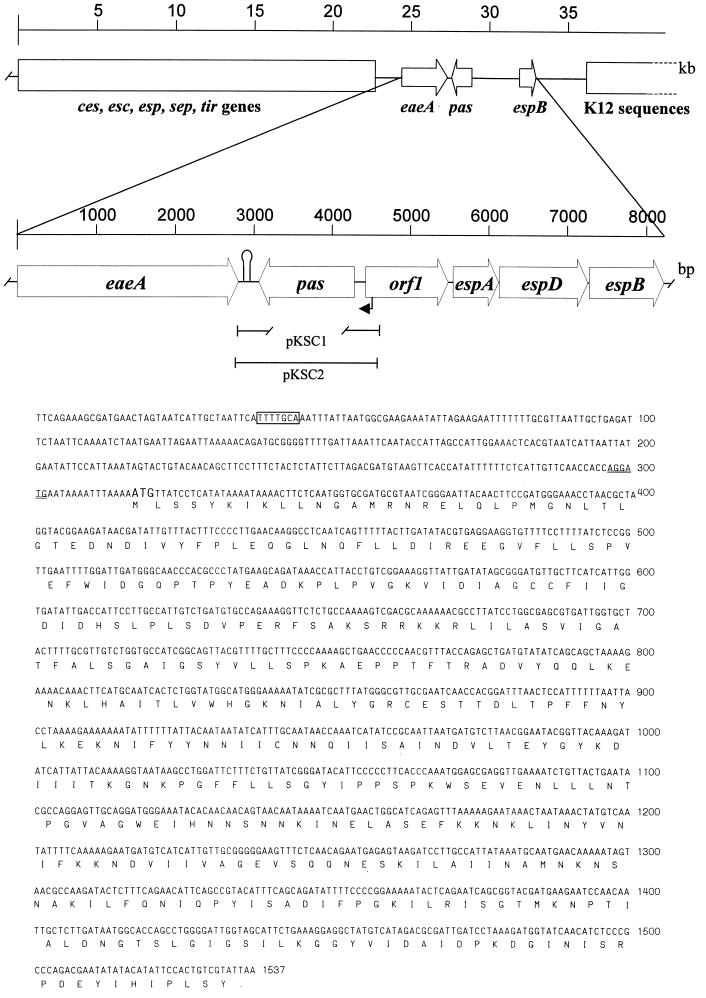
To date, a database sequence for the region between eaeA nd espB is available only for EPEC strain E2348/69 (19) (EMBL accession no. AF022236) and shows six ORFs with the following arrangement: eaeA, escD, sepL, espA, espD, and espB. Earlier studies had shown that EHEC strain EDL933 contains an espB gene which exhibits 75% similarity to espB from EPEC strain E2348/69 (17). In this work, we generated a plasmid (pANK84) which contains a PCR-amplified fragment encompassing the region between eaeA and espB of EHEC strain EDL933 (for details, see Materials and Methods). As expected, the insert contained in pANK84 exhibited a high degree of homology to the EPEC sequences available in the database. An analysis of the nucleotide sequence from pANK84 led to the identification of four ORFs with the following arrangement: pas, orf1, espA, and espD. The start codon for pas is located 1,484 bp downstream of eaeA in the complementary strand. Using the NNPP promoter prediction algorithm (47), we found 273 bp upstream of the pas start codon a potential nitrogen-regulated promoter with a predicted probability of 1.00 (Fig. 1). The 1,221-bp ORF encodes a 406-amino-acid product (Pas) with a predicted molecular mass of 45.3 kDa and a pI of 6.96. A sequence resembling a rho-independent terminator was found 221 to 253 bp downstream of the TAA stop codon, suggesting that pas is monocistronically transcribed.
FIG. 1.
Map of the chromosomal region between the eaeA and espB genes from EHEC strain EDL933. Genes and ORFs are shown as arrows. sep genes required for type III secretion are shown as a large closed box, sequences common to E. coli K-12 are shown by an open box, the stem-loop between eaeA and pas is shown as a hairpin symbol, a potential nitrogen-regulated promoter is shown as a black arrow in the map and boxed within the nucleotide sequence, and a potential Shine-Dalgarno sequence is shown by double underlining within the nucleotide sequence. The fragment of the pas gene deleted in pKSC1 is shown as a broken line below the pas box.
Using the Bestfit algorithm (11), we found that the translated product of pas exhibited 97.5% identity and 97.8% similarity to the homologous sequence of EPEC, EscD. So far, the biological role of the putative product encoded by the escD locus from EPEC has not been characterized. The polypeptides which are encoded by sequences from rabbit EPEC strains (EMBL accession no. U59502, U59503, and U59504) and which correspond to the COOH-terminal part of Pas (amino acids 85 to 406) exhibit a high degree of homology (96 to 97% identity and 98% similarity) to the Pas protein. To assess whether Pas is conserved among EHEC strains, the chromosomal regions encompassing the pas genes from EHEC strain E32511/0 and Shiga toxin-producing E. coli (STEC) strain 413.89-1 were amplified, cloned, sequenced, and compared to that of strain EDL933. The sequence analysis showed that the encoded proteins exhibited 97.3 and 99.5% identities and 98 and 99.5% similarities, respectively, to the EDL933 Pas protein. These data confirm the strong conservation of Pas within EHEC.
When the sequence of the pas-encoded product was analyzed with the PSORT algorithm (41), no typical NH2-terminal signal peptide was detected, and the predicted topology of the protein was the inner membrane. Analysis with the BLASTP + BEAUTY algorithm (2, 64) showed that Pas exhibits the highest homology to translocation proteins SpiB of S. typhimurium (24% identity and 44% similarity in a 324-amino-acid overlap), YscD of Yersinia enterocolitica and Yersinia pestis (24 and 26% identities and 45% similarity in 178- and 115-amino-acid overlaps, respectively), and PscD of Pseudomonas aeruginosa (21% identity and 41% similarity in a 405-amino-acid overlap). Although the SpiB sequence available in the database (EMBL accession no. U51927) lacks the COOH terminus, the full-length translated product (sequence kindly provided by M. Hensel [25a]) has a similar degree of homology (22.8% identity and 35.8% similarity in a 339-amino-acid overlap). Pas also exhibits significant homology to components of type III secretion systems from plant pathogenic bacteria, such as HrpQ from Erwinia amylovora, HrpJ3 from Pseudomonas syringae, and HrpW from Burkholderia solanacearum (EMBL accession no. L25828, U07346, and Z14056). SpiB has been suggested to be part of a second type III secretion system in Salmonella spp. which is required for bacterial survival within phagocytic cells (44); however, reports are contradictory. Ochman et al. (44) found that spi mutants lacked a modified form of flagellin, whereas Hensel et al. (26) reported that mutations in spi/ssa genes resulted in the abolition of protein secretion but did not affect flagellin production (26). YscD is a component of a type III secretion system located in the inner membrane, where it mediates the transport of virulence factors through the bacterial membrane (46).
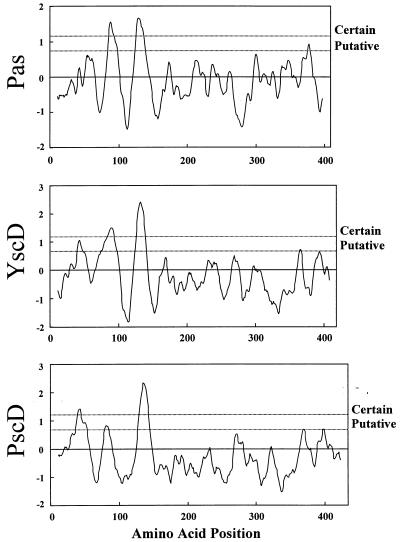
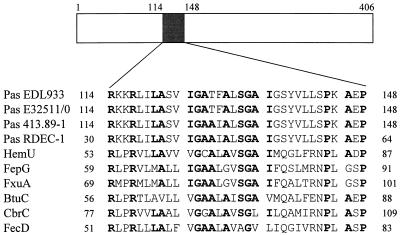
The low degree of homology between related proteins from different species is a common feature among components of type III secretion systems (37). However, Pas, PscD, and YscD exhibit a highly conserved hydrophobicity pattern (Fig. 2), suggesting the presence of shared structural features that may be required to accomplish similar tasks in the secretion process. Interestingly, the BLASTP algorithm (2) revealed similarities between Pas and the Y. enterocolitica HemU protein, a permease that is required for the transport of hemin across the cytoplasmic membrane (55). Alignment with other bacterial permeases showed a highly conserved motif [R(x)2R(x)2LA(x)2IGAA(x)1A(x)SGAI(x)7P(x)A(x)P] in the NH2-terminal region of the Pas protein (Fig. 3). This consensus motif is within the second predicted transmembrane domain (8, 53) of Pas and Pas homologs, suggesting that it may be required for the biological activity of such proteins.
FIG. 2.
Hydrophobicity plots for Pas and the Pas-homologous proteins YscD and PscD. The amino acid sequences of Pas, PscD (EMBL accession no. U56077), and YscD (EMBL accession no. M74011) were analyzed with the program TopPred II and the Kyte-Doolittle algorithm (8, 35).
FIG. 3.
Comparison between Pas and bacterial permeases. The NH2-terminal region of the protein contains two predicted transmembrane domains (positions 78 to 98 and positions 118 to 138). The numbers on the left and right sides indicate the amino acid positions. Conserved residues are shown in boldface letters. Pas EDL933, EHEC EDL933; Pas E32511/0, EHEC E32511/0; Pas 413.89-1, STEC 413.89-1; Pas RDEC-1, rabbit EPEC RDEC-1 (EMBL accession no. U59503); HemU, Y. enterocolitica (SPTREMBL accession no. P74980); FepG, E. coli (SWISSPROT accession no. P23877); FxuA, Mycobacterium smegmatis (SPTREMBL accession no. Q50376); BtuC, E. coli (SWISSPROT accession no. P06609); CbrC, Erwinia chrysanthemi (SWISSNEW accession no. Q47086); FecD, E. coli (SWISSPROT accession no. P15029).
Subcellular localization of the product encoded by the pas gene.
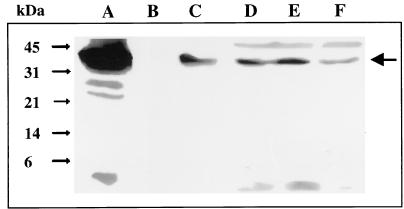
Strain EDL933 was grown in DMEM-HEPES, and the efficient secretion of Esp proteins was confirmed by Western blot analysis. Bacterial cultures were then fractionated into supernatant, outer membrane, periplasmic, inner membrane, and cytoplasmic fractions, and the resulting extracts were separated by SDS-PAGE and analyzed by immunoblotting with a Pas-specific antiserum to determine the topology of Pas. One major band and two more weakly reacting bands of approximately 45 (major), 51, and 59 kDa were detected in the cytoplasmic fraction (Fig. 4). The electrophoretic mobility of the major product corresponded to the predicted molecular mass of Pas (45.3 kDa). Therefore, Pas seems to be located in the cytoplasm of actively secreting EHEC; this location appears to be inconsistent with its predicted topology and homology to inner membrane proteins. However, the Pas-specific antiserum also detected a weakly reacting band and two major bands of 45 (weak), 51, and 59 kDa in the inner membrane fraction. Extended boiling and iodoacetamide treatment did not modify the observed patterns (data not shown), suggesting that the lower-mobility bands did not result from the formation of either heterodimers or intramolecular disulfide bonds.
FIG. 4.
Subcellular localization of the Pas protein. Bacterial cultures were fractionated, and protein extracts were separated by SDS-PAGE (0.6 μg of protein in lane A and 30 μg in all other lanes) and analyzed by immunoblotting with a Pas-specific antiserum (MαPas) to determine the topology of the pas-encoded product. Lane A, recombinant histidine-tagged Pas protein; lane B, supernatant fraction; lane C, outer membrane fraction; lane D, inner membrane fraction; lane E, periplasmic fraction; lane F, cytoplasmic fraction. The molecular masses of the main protein products are indicated by arrows.
The presence of two bands with lower mobilities suggests posttranslational modification of Pas, leading to the major forms detected in the inner membrane fraction. In fact, phosphorylation and acetylation of bacterial proteins may account for alterations in the electrophoretic mobility pattern. Therefore, studies were performed in an attempt to characterize the nature of the putative posttranslational modification of Pas. Pretreatment of the inner membrane fraction with phosphatase (2 h at 37°C) did not modify the electrophoretic mobility, suggesting that the observed pattern was not caused by phosphorylation (data not shown). Fatty acids can be linked to acyl proteins through an ester or thioester bond that is labile in the presence of hydroxylamine (24). However, pretreatment of the inner membrane fraction with 1 M hydroxylamine (pH 10) did not alter the intensity of the observed bands (data not shown). Therefore, if a fatty acid is added to Pas, it should be through a hydroxylamine-resistant bond such as an amide linkage (almost always myristate). In agreement with this hypothesis, the scanning of Pas for pattern occurrence (3) showed seven predicted myristoylation sites (positions 12 to 17, 29 to 34, 127 to 132, 134 to 139, 234 to 239, 367 to 372, and 390 to 395).
The product encoded by the pas gene is required for bacterial attachment to and invasion of HeLa cells.
The pathogenicity island LEE, which includes the gene encoding the Pas protein, is essential for the virulence of both EPEC and EHEC. In addition, it has been shown that the integration of LEE into a nonpathogenic E. coli strain is sufficient to promote attaching and effacing activity (38). Therefore, to determine the role of pas in the pathogenesis of infections caused by EHEC, a mutant containing a 960-bp in-frame deletion in this gene was generated as described in Materials and Methods. The deletion disrupts pas 100 bp downstream of the start codon, generating a 261-bp truncated version of pas. The mutated pas allele was cloned into the thermosensitive suicide vector pMAK700oriT (57), generating pKSC1. Since we were unable to introduce this plasmid into EDL933 directly either by conjugation or by electroporation, probably due to the presence of restriction systems (15), EHEC strain E32511/0 was used as an intermediate bacterial host. Plasmid DNA isolated from this strain was transferred by electroporation into EDL933; cointegration and excision of the vector were performed as previously described (57). The recombinant clone resulting from the allelic exchange, EDL933 Δpas, was hemolytic on sheep blood agar plates; PCR analysis confirmed the presence of the hly gene, which is located on the megaplasmid, as well as of the stx-1 and stx-2 genes (data not shown). No differences in growth were observed between the mutant and the wild-type strain in either LB broth or DMEM. The disruption present in the Δpas mutant resulted in abolition of the expression of Pas, as determined by Western blot analysis with a Pas-specific antiserum (data not shown).
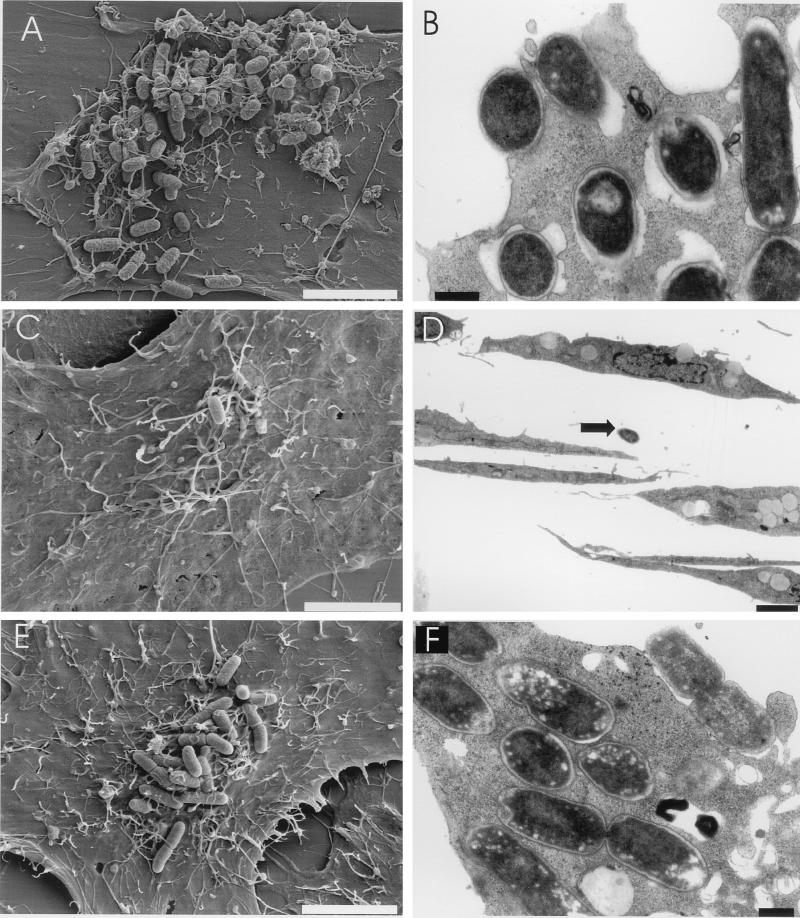
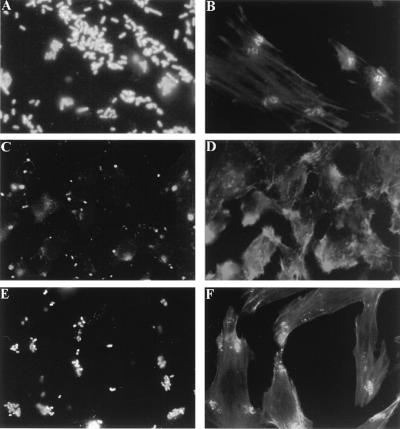
The attachment of the EDL933 Δpas mutant to HeLa cells was initially analyzed by scanning electron microscopy. Parental strain EDL933 attached very efficiently to HeLa cells, often forming microcolonies (approximately 50 to 90 bacteria/cell), whereas the Δpas derivative exhibited very poor attachment (≤0.1 bacterium/cell) and was rarely found, if at all, attached to HeLa cells (Fig. 5A, C, and D). These experiments were expanded by quantitative adhesion assays. The results obtained confirmed that the Δpas derivative attached very poorly to HeLa cells (Fig. 6A). Ultrathin sections of infected cells analyzed by transmission electron microscopy revealed that strain EDL933 was also able to efficiently invade HeLa cells. Wild-type bacteria were present either within a vacuolar compartment or free in the cytoplasm (Fig. 5B). Quantitative invasion studies (Fig. 6B) further confirmed that EDL933 was able to invade eukaryotic cells. Bacterial invasion of HeLa cells was FCS independent, since infection studies performed with DMEM-HEPES not supplemented with FCS or supplemented with FCS, either inactivated or not, gave similar results (data not shown). The capacity to penetrate HeLa cells was abolished in the Δpas derivative, which exhibited an invasion rate similar to that of a control E. coli K-12 strain (Fig. 6B). The number of intracellular bacteria (approximately 1% of the inoculum after 3.5 h of infection) increased during the first 4 to 10 h of infection (data not shown); however, after 24 h, the number of viable microorganisms recovered per well was dramatically reduced (20 times). It seems unlikely that the reduction in the number of recovered bacteria can be attributed to an altered viability of HeLa cells, since similar percentages of viable cells were observed in infected and uninfected monolayers by trypan blue staining (data not shown). Interestingly, previous studies performed by Oelschlaeger et al. showed that EHEC can efficiently invade certain cell lines, such as T24 and HCT-8 cells, when the infection time is prolonged, whereas it is not taken up by INT407 or HEp-2 cells (45). These findings may explain the difference between the impressive number of intracellular bacteria detected by electron microscopy and previous reports in which the poor invasiveness of EHEC was highlighted (40). These contrasting observations suggest the importance of performing studies to define the real role of invasion during the natural infection process.
FIG. 5.
Analysis of bacterial attachment to and invasion of HeLa cells. Attachment and invasion of EHEC were analyzed by scanning (A, C, and E) and transmission (B, D, and F) electron microscopy after 6 h of infection with strains EDL933 (A and B), EDL933 Δpas (C and D), and EDL933 Δpas(pKSC2) (E and F). A single nonattached Δpas mutant cell is indicated by an arrow in panel D. Bars, 5 μm (A, C, and E), 0.5 μm (B and F), and 2.5 μm (D).
FIG. 6.
Attachment of and invasion by the EDL933 Δpas mutant. The ability of EHEC strain EDL933, EDL933 Δpas derivative, and complemented EDL933 Δpas(pKSC2) to attach to (A) and invade (B) HeLa cells after 6 h of infection was analyzed by determining the total number of attached bacteria (A) and gentamicin-resistant internalized bacteria (B) as described in Materials and Methods. The differences between the EDL933 Δpas mutant and both the EDL933 parental strain and the EDL933 Δpas(pKSC2) derivative were statistically significant (P ≤ 0.05).
To confirm the role of the pas-encoded product in the observed phenotype, a fragment encompassing the full-length pas gene and 340 bp of the region located upstream of the ATG codon was amplified by PCR with primers ANK36 and ANK39. The PCR-amplified fragment was subsequently cloned into the pCR2.1 vector, generating pKSC2. Plasmid pKSC2 was transformed into EDL933 Δpas. The provision of the pas gene in trans partially restored the attachment and invasiveness of EDL933 Δpas, resulting in partial complementation of the mutant phenotype (Fig. 5E and F and Fig. 6A and B). The lack of full complementation may be explained by the lower levels of secreted proteins observed in the complemented EDL933 Δpas mutant than in the wild-type strain (see below).
The product encoded by the pas gene is required for actin accumulation underneath adherent bacteria.
Since the reorganization of cytoskeletal proteins plays an important role in the attachment of EHEC, studies were performed to assess whether this activity was impaired in the mutant strain. Immunofluorescence microscopic studies involving staining of F actin with fluorochrome-labelled phalloidin revealed that in contrast to parental strain EDL933, mutant strain EDL933 Δpas was deficient in inducing actin accumulation (Fig. 7A to D). The ability of bacteria to direct actin reorganization and accumulation was restored after the introduction of plasmid pKSC2, containing the pas gene, into EDL933 Δpas (Fig. 7E and F). The lack of full complementation, as indicated by the lower number of microcolonies formed on the surface of HeLa cells, may be explained, at least in part, as a gene dosage effect or by variations in transcriptional efficiencies related to the degree of supercoiling on the plasmid (16, 22, 28). However, the overall microscopic staining pattern of the actin accumulated underneath adherent bacteria was the same as that observed after infection with the wild-type strain.
FIG. 7.
Immunofluorescence microscopy of HeLa cells infected with EHEC strain EDL933. HeLa cells after 6 h of infection with strains EDL933 (A and B), EDL933 Δpas (C and D), and EDL933 Δpas(pKSC2) (E and F) are shown. Bacteria were labelled with O157-specific antiserum (A, C, and E), and actin was labelled with phalloidin (B, D, and F), both as described in Materials and Methods.
The pas gene is essential for the secretion of Esp proteins.
Esp proteins, which are secreted by a type III secretion system, are required for the signal transduction events leading to cytoskeletal reorganization. Therefore, we analyzed bacterial production and secretion of Esp proteins by immunoblotting. EspB was detected in the supernatant fluids of EDL933 cultures, whereas no protein was observed in the supernatant fluids of EDL933 Δpas cultures (Fig. 8). These results indicated that in the mutant strain, either the expression or the secretion of EspB was disrupted. Similar results were obtained when antibodies specific for EspA and EspD were used (data not shown).
FIG. 8.
Expression and secretion of EspB by the EDL933 Δpas mutant. The presence of EspB was determined in bacterial culture supernatants (lanes A to C) and whole-cell lysates (lanes D to F) by Western blotting as described in Materials and Methods. Lanes A and D, EDL933; lanes B and E, EDL933 Δpas; lanes C and F, EDL933 Δpas(pKSC2). The main protein product is indicated by an arrow on the right.
The deletion contained in EDL933 Δpas is located upstream of the espB gene. Although the mutation generated in the recombinant strain is a nonpolar in-frame deletion, to rule out any potential effect in the expression of espB, whole-cell extracts were examined for intracellular pools of Esp proteins. These immunoblotting experiments with a monoclonal antibody specific for the EspB protein demonstrated that the EDL933 Δpas mutant was able to produce EspB. Therefore, the effect of the pas mutation is at the level of secretion of EspB. In support of this hypothesis, the provision of the pas gene in trans to the EDL933 Δpas mutant partially reestablished the export of the EspB protein (Fig. 8).
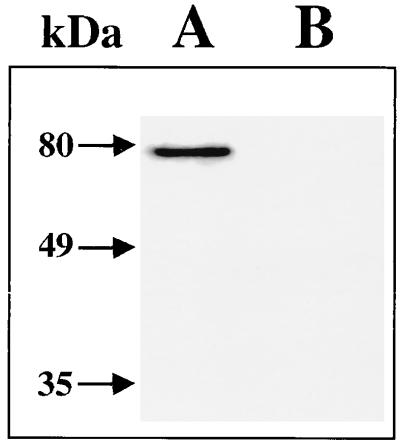
A critical event in the triggering of actin accumulation is intimate bacterial attachment to host cells, which is mediated by the surface protein intimin. EPEC and STEC strains synthesize receptors for intimin (Tir and EspE, respectively), which are subsequently transferred by the bacteria into the target cells (9, 33). Therefore, to analyze whether the impaired attachment exhibited by the pas mutant can be due, in part, to an indirect effect on EspE production, the secretion of EspE was studied by Western blotting. The EspE protein was not observed in supernatants of the EDL933 Δpas mutant (Fig. 9).
FIG. 9.
Expression and secretion of EspE by the EDL933 Δpas mutant. Culture supernatants of EHEC EDL933 (lane A) and its Δpas derivative (lane B) were analyzed by Western blotting with a monoclonal antibody specific for EspE (mabMαEspE) as described in Materials and Methods. Molecular masses are indicated by arrows.
Interactions between Pas and proteins secreted by EHEC.
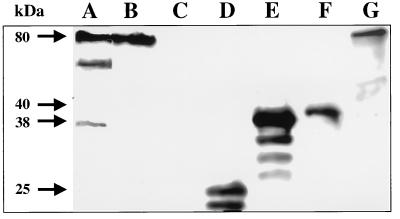
The potential involvement of Pas in the translocation process for Esp proteins and the localization of the putative modified Pas in the inner membrane prompted us to investigate whether Pas can bind to Esp proteins. Filter binding assays were performed with recombinant Pas (see Materials and Methods). However, since histidine-tagged Pas was purified under denaturating conditions, experiments were also performed with cytoplasmic (major 45-kDa band) and inner membrane (major 51- and 59-kDa bands) fractions of strain EDL933. The protein samples were incubated with membrane strips containing immobilized secreted proteins from EHEC, and Pas binding was detected with a Pas-specific antiserum. The results obtained suggested that Pas binds to the EspE protein and to another secreted protein of approximately 53 kDa, whose identity is unknown (Fig. 10, lanes A, B, and G). Under our experimental conditions, Pas binding to either EspA or EspD was not detected. However, a weak band corresponding to the electrophoretic mobility of EspB was detected (Fig. 10, lanes A and E), suggesting that Pas may also interact with EspB. Interestingly, only samples containing the higher-mobility forms of Pas (recombinant protein and cytoplasmic fraction) reacted with the secreted proteins (Fig. 10, lanes A and B), whereas the inner membrane fraction, which contains the putative modified form of Pas, did not bind (Fig. 10, lane C). The results obtained suggested that in order to carry out its biological activity Pas requires alternating cycles of insertion and deinsertion in the inner membrane (18) and that because only the cytoplasmic form of Pas is able to bind Esp proteins.
FIG. 10.
Binding of Pas to secreted proteins from EHEC strain EDL933. Supernatant fluids from EDL933 cultures were concentrated, separated by SDS-PAGE, and blotted onto nitrocellulose membranes. Strips were cut and incubated with either purified histidine-tagged Pas (lane A) or the Pas-containing cytoplasmic (lane B) or inner membrane (lane C) fractions of EDL933. Bound Pas was detected by immunoblotting with a Pas-specific antiserum (MαPas) as described in Materials and Methods. The localization of EspA (lane D), EspB (lane E), EspD (lane F), and EspE (lane G) proteins in matching strips was determined with protein-specific monoclonal antibodies. The molecular masses of the main protein products are indicated by arrows.
To achieve the secretion of Esp proteins and the efficient delivery of Tir/EspE into eukaryotic cells, a functional type III secretion system is required (30, 33, 63). It has been speculated that Tir/EspE is not secreted by a type III system, since secreted Tir lacks the NH2-terminal methionine (33). However, Tir processing may occur within the cytoplasm and may not be coupled with the secretion process. The fact that the Δpas mutant was unable to secrete EspE suggests that the receptor for intimin indeed uses a type III secretion system. Therefore, the phenotype of the Δpas mutant can be explained by (i) blocked secretion of EspA, EspB, and EspD proteins, which results in impaired attaching and effacing activity (13, 32, 36), and (ii) abolished translocation of Tir/EspE into the target cell, which prevents intimate bacterial attachment from occurring.
The altered export of Esp proteins may be due to the disruption of a universal chaperon specific for EspA, EspB, EspD, and EspE. However, it is unlikely that the Pas protein can act as a chaperon for different polypeptides, since type III chaperonins are generally specific for a single protein (60). In addition, Pas does not have common features of chaperons, such as a low molecular weight, an acidic pI, or the presence of an amphipathic α helix near the COOH terminus (60). Finally, two proteins that seem to be chaperonins for EspB and for EspB/EspD were recently described (14, 58). Alternatively, Pas may be a regulator, its inactivation leading to a block in the secretion of Esp proteins by an indirect effect. However, the overall sequence homology with members of type III secretion systems that are directly involved in protein secretion, the presence in Pas of a transmembrane domain that is typical for proteins involved in translocation, the putative location of a modified Pas in the inner membrane, and the altered export of Esp proteins allow us to hypothesize that Pas is the EHEC and EPEC homolog of the translocation proteins SpiB/SsaD, YscD, and PscD. pas is the first gene involved in transport that is located downstream of the eaeA gene on the right-hand end of the LEE. Additional work is required to elucidate the specific role of Pas during the secretion process and to identify other proteins whose transport is Pas dependent. However, the data presented here clearly demonstrate that Pas also should be necessary for these microorganisms to successfully colonize hosts during natural infections.
ACKNOWLEDGMENTS
We gratefully acknowledge E. Medina for help in mouse immunization studies, M. Hensel for providing us with unpublished sequence data for SpiB/SsaD, and K. N. Timmis for generous support and encouragement.
This work was, in part, supported by a Lower Saxony-Israel Cooperation Grant funded by the Volkswagen Foundation (21.45-75/2).
C. Deibel was supported by a doctoral fellowship from the Boehringer Ingelheim Fonds.
REFERENCES
- 1.Acheson D W, Keusch G T. Which Shiga toxin-producing types of Escherichia coli are important? ASM News. 1996;62:302–306. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Appel R D, Bairoch A, Hochstrasser D F. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem Sci. 1994;19:258–260. doi: 10.1016/0968-0004(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdanove A J, Wei Z M, Zhao L, Beer S V. Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 7.Cassels F J, Wolf M K. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol. 1995;15:214–226. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 8.Claros M G, von Heijne G. Prediction of transmembrane segments in integral membrane proteins, and the putative topologies, using several algorithms. CABIOS. 1994;10:685–686. [Google Scholar]
- 9.Deibel C, Krämer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Lai L C, Taylor K A. The locus of enterocyte effacement pathogenicity island of enteropathogenic Escherichia coli encodes secretion functions and remnants of transposons at its extreme right end. Gene. 1997;184:107–114. doi: 10.1016/s0378-1119(96)00581-1. [DOI] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorman C J, Ni Bhriain N, Higgins C F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990;344:789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- 17.Ebel F, Deibel C, Kresse A U, Guzmán C A, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 19.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic E. coli. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 20.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galán J E. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1997;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 22.Galán J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gannon V P, Rashed M, King R K, Thomas E J. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J Clin Invest. 1993;31:1268–1274. doi: 10.1128/jcm.31.5.1268-1274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grand R J. Acylation of viral and eukaryotic proteins. Biochem J. 1989;258:625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 25a.Hensel, M. Unpublished data.
- 26.Hensel M, Shea J E, Raupach B, Monack D, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion system apparatus of Salmonella pathogenicity island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 27.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins C F, Dorman C J, Stirling D A, Waddell L, Booth I R, May G, Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in Salmonella typhimurium and Escherichia coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 29.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 33.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 34.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 36.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 38.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 39.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKee M L, O’Brien A D. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–2074. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien A D, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet. 1983;i:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 43.O’Callaghan D, Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990;223:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- 44.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oelschlaeger T A, Barett T J, Kopecko D J. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect Immun. 1994;62:5142–5150. doi: 10.1128/iai.62.11.5142-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reese M G, Harris N L, Eeckman F H. Large scale sequencing specific neural networks for promoter and splice recognition. In: Hunter L, Klein T, editors. Proceedings of the Pacific Symposium on Biocomputing. 1996. [Google Scholar]
- 48.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 49.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. p. 60. [Google Scholar]
- 53.Shea C M, McIntosh M A. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol. 1991;5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 54.Spurr A R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 55.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 56.Tesh V L, Samuel J E, Perera L P, Sharefkin J B, O’Brien A D. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis. 1991;164:344–352. doi: 10.1093/infdis/164.2.344. [DOI] [PubMed] [Google Scholar]
- 57.Viret J F, Cryz S J, Jr, Favre D. Expression of Shigella sonnei lipopolysaccharide in Vibrio cholerae. Mol Microbiol. 1996;19:949–963. doi: 10.1046/j.1365-2958.1996.435967.x. [DOI] [PubMed] [Google Scholar]
- 58.Wainwright L A, Kaper J B. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol Microbiol. 1998;27:1247–1260. doi: 10.1046/j.1365-2958.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe H, Wada A, Inagaki Y, Itoh K, Tamura K. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan. Lancet. 1996;348:831–832. doi: 10.1016/s0140-6736(05)65257-9. [DOI] [PubMed] [Google Scholar]
- 60.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 61.Wieler L H, Vieler E, Erpenstein C, Schlapp T, Steinruck H, Bauerfeind R, Byomi A, Baljer G. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willshaw G A, Smith H R, Scotland S M, Rowe B. Cloning of genes determining the production of Vero cytotoxin by Escherichia coli. J Gen Microbiol. 1985;131:3047–3053. doi: 10.1099/00221287-131-11-3047. [DOI] [PubMed] [Google Scholar]
- 63.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–156. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 64.Worley K C, Wiese B A, Smith R F. BEAUTY: An enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genet Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]