Abstract
Many actinomycete strains are able to convert nitrate or nitrite to nitrous oxide (N2O). As a representative of actinomycete denitrification systems, the system of Streptomyces thioluteus was investigated in detail. S. thioluteus attained distinct cell growth upon anaerobic incubation with nitrate or nitrite with concomitant and stoichiometric conversion of nitrate or nitrite to N2O, suggesting that the denitrification acts as anaerobic respiration. Furthermore, a copper-containing, dissimilatory nitrite reductase (CuNir) and its physiological electron donor, azurin, were isolated. This is the first report to show that denitrification generally occurs among actinomycetes.
Denitrification is a biological process that plays an important role in the global nitrogen cycle, because it completes the nitrogen cycle as the reverse reaction of nitrogen fixation. More attention is now being paid to N2O, an intermediate of biological denitrification, because it exhibits a potent greenhouse effect and its concentration in the atmosphere is increasing rapidly. Denitrification is thought to be one of the main sources of N2O that is emitted into the atmosphere (8). By contrast, biological denitrification is at present the most effective process to remove fixed nitrogen pollutants from aqueous ecosystems, in which they cause eutrophication. Therefore, increased knowledge about denitrification has become more important in global environment issues.
Although denitrification has been found to occur in many eubacteria and in a few archaebacteria (1, 2, 11), the list of denitrifiers lacks a unique taxon of gram-positive bacteria, the actinomycetes. Now that denitrifiers have been found even among eukaryotic microorganisms (fungi) (3, 6, 7, 10) and since actinomycetes are a dominant microflora in soils in which temporal or local reduction of oxygen supply should frequently occur, there is no reason to postulate that actinomycetes constitute a nondenitrifying exception among dominant microorganisms in the ecosystem. Here we report our finding of denitrifiers among actinomycetes.
MATERIALS AND METHODS
Microorganisms.
Actinomycete strains were obtained from the type culture collection of the Japanese Collection of Microorganisms (JCM), RIKEN, Saitama, Japan.
Culture and the medium.
A seed culture of each actinomycete was grown aerobically at 28°C for 3 days on a rotary shaker (120 rpm) in a 500-ml Erlenmeyer flask containing 250 ml of medium consisting of 1.36 g of KH2PO4, 30 ml of glycerol, 2.0 g of peptone, 0.2 g of MgSO4 · 7H2O, and 1 ml of trace element solution per 1,000 ml of tap water (pH 7.5). The trace element solution contained, per 1,000 ml of distilled water, 0.2 g of FeSO4 · 7H2O, 1.0 g of CoCl2 · 6H2O, 0.38 g of CuSO4 · 6H2O, 8.6 mg of Na2MoO4 · 7H2O, and 0.2 g of CaCl2. A portion (50 ml, about 150 mg of dry matter) of the seed culture was inoculated into 150 ml of the fresh medium supplemented with 1.5 mmol of sodium nitrate or nitrite and assayed for denitrifying activity by incubation at 28°C for 2 days as reported previously (10). The Erlenmeyer flask (500-ml volume, with two side arms) was sealed after replacing the headspace air with helium (anaerobic) or sealed without replacing the air (O2 limited). Denitrification products were analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), as reported previously (6, 7). 15N-nitrate and 15N-nitrite (99 atom%) were obtained from Shoko-Tsusho (Tokyo, Japan).
Purification of Nir and azurin from Streptomyces thioluteus JCM 4844.
Nitrite reductase (Nir) activity was assayed as reported previously (4). The buffer used for purification was potassium phosphate (pH 6.0) or morpholineethanesulfonic acid (MES) (pH 5.5) with various concentrations of 10% glycerol, 0.25 mM phenylmethylsulfonyl fluoride, and 5 μM CuSO4. Cells (100 g [wet weight]) incubated with nitrite for 6 h were suspended in a 1.5-fold-excess 100 mM phosphate buffer on ice and disrupted by sonication (Branson Sonifier 250; 180 W, 20 min). The disrupted cells were centrifuged at 10,000 × g for 20 min, and Emulgen 913 (nonylphenylethoxylate; Kao, Tokyo, Japan) was gradually added to the resulting supernatant with stirring to make up the final concentration of 1% (wt/vol). The mixture was incubated for an additional 3 h below 5°C and then centrifuged at 100,000 × g for 90 min. The supernatant was dialyzed against 10 mM phosphate buffer containing 0.1% Emulgen 913 and applied to a CM-cellulose CM52 (Whatman, Maidstone, United Kingdom) column (bed, 70 ml) equilibrated with the same buffer. After being washed, the column was eluted with a linear gradient of 0 to 100 mM KCl. Active fractions were collected, concentrated, and subjected to gel filtration with a fast-protein liquid chromatograph (FPLC) (Pharmacia, Uppsala, Sweden) equipped with a Superdex 200HR 10/30 column that was equilibrated with 50 mM phosphate buffer containing 150 mM KCl. The Nir fraction resulting from the gel filtration was used as the purified preparation.
Another blue fraction was separated from the Nir-containing fraction as the result of the CM-cellulose column chromatography procedure described above. This blue fraction was applied again to a CM-cellulose column equilibrated with 10 mM MES buffer (without Emulgen 913) and eluted with a linear gradient of 0 to 100 mM NaCl. The blue fraction was collected and applied to an SP-Sepharose Fast Flow column (Pharmacia) equilibrated with the same buffer and eluted with a 0 to 100 mM NaCl gradient. The fraction was finally subjected to gel filtration with a FPLC as described above. Blue eluate was collected and used as purified azurin.
Other methods.
Absorption spectra were measured with a Beckman Instruments (Fullerton, Calif.) DU7500 spectrophotometer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted according to the method of Laemmli (5).
RESULTS
Screening of denitrifying actinomycetes.
Actinomycetes of various genera were assayed for denitrifying activity. The strains that evolved N2O at just stoichiometric or near stoichiometric amounts (i.e., with 100% yield) under at least one condition among four types of conditions tested are shown in Table 1. We could not, however, find a denitrifier that evolved dinitrogen (N2) as the final product.
TABLE 1.
Denitrifying actinomycetes
| Strain | N2O evolution (μmol) froma:
|
|||
|---|---|---|---|---|
| Nitrate
|
Nitrite
|
|||
| Anaerobic | O2 limited | Anaerobic | O2 limited | |
| Streptomyces lavendulae subsp. lavendulae JCM 4664 | 600 | 705 | 751 | 45 |
| S. thioluteus JCM 4844 | 762 | 730 | 700 | 721 |
| S. coelicolor JCM 4357 | 771 | 505 | —b | — |
| S. flavotricini JCM 4371 | 15 | 451 | 403 | — |
| S. cavourensis subsp. cavourensis JCM 4555 | 409 | 64 | 25 | 306 |
| S. cinnamoneus JCM 4633 | 40 | 73 | 25 | 254 |
| S. endus JCM 4636 | 10 | 604 | — | — |
| S. zelensis JCM 5024 | 354 | 340 | 82 | 711 |
| S. akiyoshiensis JCM 4970 | 137 | 82 | 739 | 619 |
| S. glaucus JCM 6922 | 157 | 577 | 550 | 562 |
| S. aureofaciens JCM 4624 | 44 | 128 | 19 | 630 |
| Streptosporangium roseum JCM 3005 | 28 | 760 | 28 | 60 |
| Micromonospora chalcea JCM 3031 | — | 15 | — | 750 |
| Microtetraspora glauca JCM 3300 | 20 | — | 21 | 121 |
| Saccharothrix australiensis JCM 3370 | — | 78 | 13 | 745 |
| Spirillospora albida JCM 3041 | — | 398 | 613 | 246 |
| Dactylosporangium aurantiacum JCM 3083 | 96 | 746 | 30 | 86 |
| Pilimelia anulata JCM 3090 | 151 | 112 | 132 | 681 |
| Saccharomonospora caesia JCM 3098 | 88 | 79 | 38 | 770 |
| Kineosporia aurantiaca JCM 3230 | 636 | 100 | 37 | 658 |
| Dermatophilus congolensis JCM 3081 | 137 | 571 | 77 | 151 |
| Nocardia salmonicida JCM 4826 | 53 | 750 | 38 | 47 |
Each flask contained 1.5 mmol of nitrate or nitrite. Other details are described in Materials and Methods.
—, below 10 μmol. The following strains did not evolve N2O under the same conditions: Streptomyces balcacci (JCM 4272), S. lividans (JCM 4783), S. phaerofaciens (JCM 4814), S. griseolus (JCM 4042), S. griseus (JCM 4047), S. diastaticus (JCM 4128), S. albus (JCM 4177), S. californicus (JCM 4567), S. wedmorensis (JCM 4937), S. hygroscopicus (JCM 4772), Amycolatopsis orientalis (JCM 4600), Glycomyces harbinensis (JCM 7347), Rothia dentocariosa (JCM 3067), Oerslovia turbata (JCM 3160), Nocardia asteroides (JCM 3384), Saccharopolyspora erythraea (JCM 4026), Excellospora viridilutea (JCM 7346), Actinomyces naeslundii (JCM 8349).
Denitrification by S. thioluteus JCM 4844.
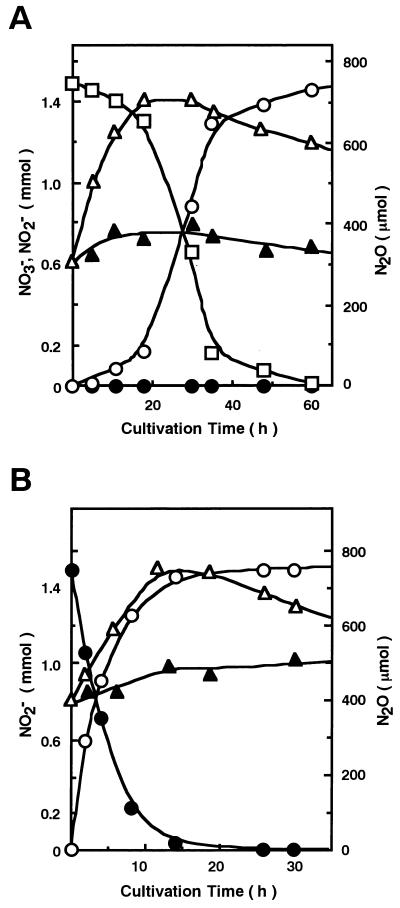
Among the distinct denitrifiers found (Table 1), S. thioluteus was selected, and its denitrifying system was investigated in more detail. Figure 1A and B shows time-dependent evolution of N2O during anaerobic incubation of S. thioluteus with nitrate and nitrite, respectively; both substrates were converted to N2O stoichiometrically. Both nitrogen atoms in the N2O molecule were shown by GC-MS to be derived from nitrate or nitrite by use of 14N- and 15N-nitrate or -nitrite (data not shown). It is interesting that N2O evolved from nitrate after a time lag, whereas it evolved from nitrite immediately, suggesting that the dissimilation system for nitrite is constitutive. The denitrification accompanied distinct cell growth. Replacement of nitrate or nitrite with ammonium ions resulted in a marked decrease in cell growth, suggesting that nitrate or nitrite was utilized for respiration but not as a nutritional nitrogen source.
FIG. 1.
N2O evolution from nitrate or nitrite by intact cells. S. thioluteus was incubated with sodium nitrate (A) or nitrite (B) under anaerobic conditions, and the amounts per flask of N2O (○), nitrate (□), nitrite (•), and dry cell matter (▵) were determined at each cultivation time. Replicate experiments for dry cell matter (▴) were also examined in which nitrate or nitrite was replaced with the same amount of ammonium ions (ammonium sulfate).
Purification of Nir and azurin.
We could detect in the cell extract Nir and nitric oxide reductase (Nor) activities by use of NADH-phenazine methosulfate as the electron donor (data not shown). Both Nir and Nor activities seemed to be membrane bound. Nir was solubilized and purified (Table 2). Its Mr value was estimated as 41,000 by SDS-PAGE and as 83,000 by gel filtration under nondenaturing conditions (data not shown). We subsequently isolated an azurin-like blue protein (Table 3). Both purified preparations of Nir and azurin gave a single band on SDS-PAGE.
TABLE 2.
Purification of Nir from S. thioluteus
| Purification step | Total protein (mg) | Total activity of (μmol of NO/min) | Sp act (μmol of NO/min/mg) | Recovery (%) |
|---|---|---|---|---|
| Solubilized extracts | 4,218 | 3,260 | 0.76 | 100 |
| CM-cellulose | 49 | 2,320 | 47 | 71 |
| Gel filtration | 8.6 | 1,104 | 128 | 33 |
TABLE 3.
Purification of azurin from S. thioluteus
| Purification step | Total protein (mg) | Azurin (μmol) | Purificationa | Recovery (%) |
|---|---|---|---|---|
| CM-cellulose | 47.6 | 1.59 | 0.08 | 100 |
| Second CM-cellulose | 21.3 | 1.13 | 0.33 | 71 |
| SP-Sepharose | 13.7 | 0.83 | 0.43 | 52 |
| Gel filtration | 7.5 | 0.61 | 0.48 | 38 |
A620/A280.
Properties of CuNir and azurin.
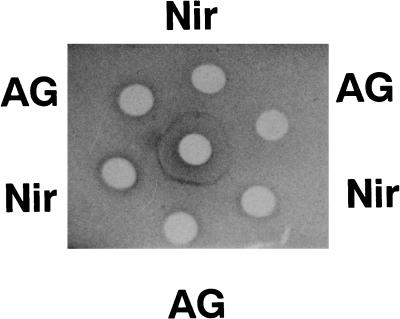
The absorption spectrum of Nir (data not shown) exhibited a single peak at 600 nm (ɛ = 4.0 mM−1 cm−1 per monomer), showing that it is a blue CuNir. The presence of copper is also expected from the inhibition by diethyldithiocarbamic acid (data not shown) and by the similarity of its partial amino acid sequence to those of other bacterial CuNir (unpublished data). It is rather surprising that Nir of S. thioluteus reacted with the antibody raised against Nir of Fusarium oxysporum (3), forming a precipitate line that fused without spurs with that due to the antigen (Fig. 2). This indicates a close structural similarity between the fungal Nir and the actinomycete Nir. The absorption spectrum of azurin (data not shown) showed a peak at 620 nm (ɛ = 4.3 mM−1 cm−1). The spectrum and the Mr value estimated from SDS-PAGE (16,600) along with the amino-terminal amino acid sequence (9) closely resembled those of azurins known so far. Anaerobic incubation of reduced azurin with purified Nir in the presence of nitrite resulted in its oxidation, whereas it was not oxidized in the absence of nitrite (data not shown), indicating that the azurin is a physiological electron donor of Nir.
FIG. 2.
Double immunodiffusion test with nitrite reductase. The wells contained the antibodies against Nir of F. oxysporum (center) (3, 4), antigen (Nir of F. oxysporum) (AG), and Nir of S. thioluteus (Nir).
DISCUSSION
It is rather surprising that so many actinomycetes tested exhibited distinct denitrifying activities (Table 1), because this phenomenon has not been reported. We isolated CuNir and azurin from such an actinomycete, namely, S. thioluteus. They were found to be very similar to the counterparts of denitrifying bacteria known so far. We also observed that denitrification by S. thioluteus accompanied cell growth, suggesting the coupling of it to generation of ATP. These results demonstrated for the first time that denitrification is also generally distributed among actinomycetes. So our previous (3, 6, 7, 10) and present results have extended occurrence of denitrification to new taxa, fungi and actinomycetes. Comparison of these new systems with those of other eubacteria should be of evolutionary interest (9).
All of the systems of new denitrifiers (fungi and actinomycetes) that we have found to date are incomplete in that they cannot reduce N2O to N2 and thus evolve N2O as the denitrification product. On a per-molecule basis, N2O has more than 200 times the greenhouse effect of carbon dioxide. Actinomycetes such as Streptomyces spp. belong to the dominant microflora in soils and sludges or scums in sewage. Denitrifying fungi such as fusaria (6) are also widely distributed in soil. It therefore seems reasonable to postulate that the recent increase of N2O in the atmosphere originates, at least in part, from nitrogen-containing fertilizer that is used in great quantities to feed the increasing human population (8) and, as a result, from denitrification by these incomplete systems. Therefore, further understanding of these systems is very important not only for biochemical progress in this field but also for taking preventive measures against aggravation in the global environment.
ACKNOWLEDGMENTS
This work was supported by the Bio-oriented Technology Research Advancement Institution (BRAIN) and the TARA Sakabe Project of the University of Tsukuba and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, and Sports of Japan.
REFERENCES
- 1.Averill B A. Dissimilatory nitrite and nitric oxide reductases. Chem Rev. 1996;96:2951–2964. doi: 10.1021/cr950056p. [DOI] [PubMed] [Google Scholar]
- 2.Berks B C, Ferguson S J, Moir J W B, Richardson D J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232:97–173. doi: 10.1016/0005-2728(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Matsuo Y, Takimoto A, Suzuki S, Maruo F, Shoun H. Denitrification, a novel type of respiratory metabolism in fungal mitochondrion. J Biol Chem. 1996;271:16263–16267. doi: 10.1074/jbc.271.27.16263. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Shoun H. The copper-containing dissimilatory nitrite reductase involved in the denitrifying system of the fungus Fusarium oxysporum. J Biol Chem. 1995;270:4146–4151. doi: 10.1074/jbc.270.8.4146. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Shoun H, Kim D-H, Uchiyama H, Sugiyama J. Denitrification by fungi. FEMS Microbiol Lett. 1992;94:277–282. doi: 10.1016/0378-1097(92)90643-3. [DOI] [PubMed] [Google Scholar]
- 7.Shoun H, Tanimoto T. Denitrification by the fungus Fusarium oxysporum and involvement of cytochrome P-450 in the respiratory nitrite reduction. J Biol Chem. 1991;266:11078–11082. [PubMed] [Google Scholar]
- 8.Smil, V. 1997. Global population and the nitrogen cycle. Sci. American, July, 76–81.
- 9.Takaya N, Kobayashi M, Shoun H. Fungal denitrification, possible occurrence of a natural horizontal gene transfer from prokaryote to eukaryote. In: Syvanen M, Kado C, editors. Horizontal gene transfer. London: Chapman and Hall; 1998. pp. 321–327. [Google Scholar]
- 10.Usuda K, Toritsuka N, Matsuo Y, Kim D-H, Shoun H. Denitrification by the fungus Cylindrocarpon tonkinense: anaerobic cell growth and two isozyme forms of cytochrome P-450nor. Appl Environ Microbiol. 1995;61:883–889. doi: 10.1128/aem.61.3.883-889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]