Abstract
BACKGROUND
Optimizing retention in human immunodeficiency virus (HIV) treatment may require sequential behavioral interventions based on patients’ response.
METHODS
In a sequential multiple assignment randomized trial in Kenya, we randomly assigned adults initiating HIV treatment to standard of care (SOC), Short Message Service (SMS) messages, or conditional cash transfers (CCT). Those with retention lapse (missed a clinic visit by ≥14 days) were randomly assigned again to standard-of-care outreach (SOC-Outreach), SMS+CCT, or peer navigation. Those randomly assigned to SMS or CCT who did not lapse after 1 year were randomly assigned again to either stop or continue the initial intervention. Primary outcomes were retention in care without an initial lapse, return to the clinic among those who lapsed, and time in care; secondary outcomes included adjudicated viral suppression. Average treatment effect (ATE) was calculated using targeted maximum likelihood estimation with adjustment for baseline characteristics at randomization and certain time-varying characteristics at rerandomization.
RESULTS
Among 1809 participants, 79.7% of those randomly assigned to CCT (n=523/656), 71.7% to SMS (n=393/548), and 70.7% to SOC (n=428/605) were retained in care in the first year (ATE: 9.9%; 95% confidence interval [CI]: 5.4%, 14.4% and ATE: 4.2%; 95% CI: −0.7%, 9.2% for CCT and SMS compared with SOC, respectively). Among 312 participants with an initial lapse who were randomly assigned again, 69.1% who were randomly assigned to a navigator (n=76/110) returned, 69.5% randomly assigned to CCT+SMS (n=73/105) returned, and 55.7% randomly assigned to SOC-Outreach (n=54/97) returned (ATE: 14.1%; 95% CI: 0.6%, 27.6% and ATE: 11.4%; 95% CI: −2.2%, 24.9% for navigator and CCT+SMS compared with SOC-Outreach, respectively). Among participants without lapse on SMS, continuing SMS did not affect retention (n=122/180; 67.8% retained) versus stopping (n=151/209; 72.2% retained; ATE: −4.4%; 95% CI: −16.6%, 7.9%). Among participants without lapse on CCT, those continuing CCT had higher retention (n=192/230; 83.5% retained) than those stopping (n=173/287; 60.3% retained; ATE: 28.6%; 95% CI: 19.9%, 37.3%). Among 15 sequenced strategies, initial CCT, escalated to navigator if lapse occurred and continued if no lapse occurred, increased time in care (ATE: 7.2%, 95% CI: 3.7%, 10.7%) and viral suppression (ATE: 8.2%, 95% CI: 2.2%, 14.2%), the most compared with SOC throughout. Initial SMS escalated to navigator if lapse occurred, and otherwise continued, showed similar effect sizes compared with SOC throughout.
CONCLUSIONS
Active interventions to prevent retention lapses followed by navigation for those who lapse and maintenance of initial intervention for those without lapse resulted in best overall retention and viral suppression among the strategies studied. Among those who remained in care, discontinuation of CCT, but not SMS, compromised retention and suppression. (Funded by National Institutes of Health grants R01 MH104123, K24 AI134413, and R01 AI074345; ClinicalTrials.gov number, NCT02338739.)
Introduction
Human immunodeficiency virus (HIV) treatment programs must overcome a challenge common to many chronic health conditions: a substantial proportion of patients do not stay in care consistently over time. In the United States and globally, 20 to 30% of patients with HIV experience at least one retention lapse — a period during which they may not consistently take antiretroviral medications — within 2 years of treatment initiation.1-3 Some evidence suggests that people who have experienced treatment lapses comprise most deaths related to acquired immunodeficiency syndrome and HIV transmissions worldwide.4,5 Retention is a challenge not only in HIV treatment. As many as half of persons with diabetes, mental illness, and hypertension — even where services are available — do not stay in care consistently over time,6,7 underscoring the broad challenge of long-term engagement in health care.
Substantial research has focused on testing single interventions to improve retention in HIV treatment. However, many interventions, although more effective than typical standard of care, are either not needed by all or do not work for all those in need. For example, in the WelTel study, Short Message Service (SMS) messages8,9 improved viral suppression from 48 to 57%, suggesting that SMS was unnecessary for about half of patients (who achieved suppression in the control group) and was sufficient to lead to suppression for only about 9% of the remaining participants (who were helped by SMS). In a randomized trial in Tanzania, conditional cash transfers (CCT) improved combined retention in care and viral suppression 6 months after treatment initiation from 73 to 83%, implying that the majority did not need the incentive and only a fraction of those in need benefited.10 Studies of navigation for HIV treatment success find similar effects. For example, a systematic review identified 17 studies in which peer navigators (i.e., lay health care workers) modestly improved treatment outcomes.11,12 For complex behavioral challenges such as retention in HIV care, current evidence, therefore, suggests that available single interventions are neither optimally efficient nor effective.
A novel approach to advance both the efficiency and effectiveness of retention methods is to develop and test sequential adaptive strategies that are composed of a series of single interventions, in which the sequence of interventions delivered depends on the individual patient’s response. Adaptive approaches can thus be considered “personalized” approaches.13,14 Linking the intervention offered to the response minimizes interventions for those for whom the initial intervention is sufficient (optimizing efficiency) while enabling intensification for those needing additional or alternative help (optimizing effectiveness). Research on such adaptive strategies can use novel designs such as sequential randomization.15 We conducted a sequential multiple assignment randomized trial (SMART) in Kenya to evaluate 15 adaptive sequences using previously established interventions including counseling, SMS messages, CCT, and peer navigators.
Methods
STUDY DESIGN
The SMART design consisted of four nested single-time-point randomized trials (Fig. 1) among persons receiving HIV care at five facilities in Nyanza, Kenya. The study was approved by the institutional review boards of the University of California, San Francisco and the Kenya Medical Research Institute and was registered on ClinicalTrials.gov (number NCT02338739).
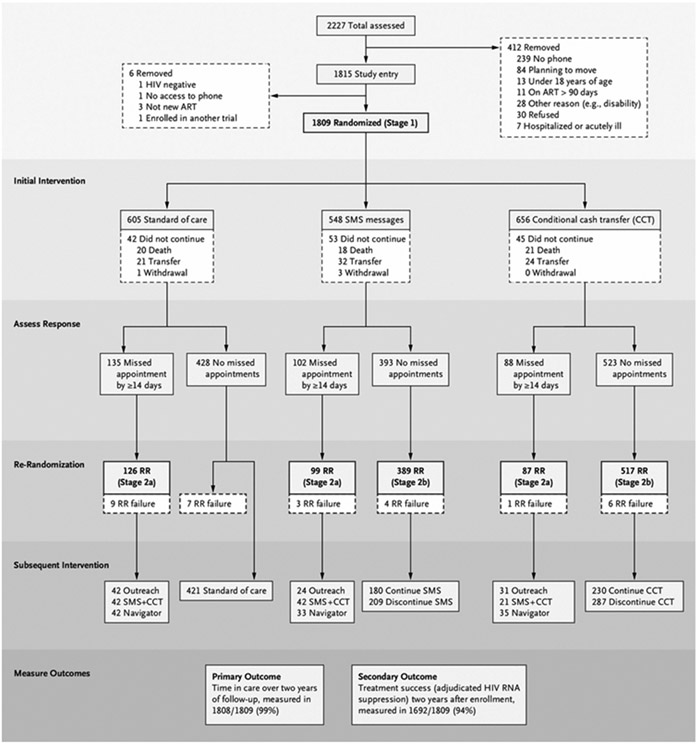
Figure 1. Trial Profile and Consort Diagram, Reflecting SMART Design.
In Stage 1 (n=1809), we compared standard of care (SOC, routine education and counseling) with the addition of Short Message Service (SMS) text messages or of conditional cash transfers (CCT) of 400 Kenyan shillings for making appointments. In Stage 2a (n=312), we compared standard-of-care outreach (composed of phone and/or in-person contact attempts within the first 72 hours of missing a visit) with addition of either SMS+CCT or assignment to a navigator. In Stage 2b, those without a lapse in the first year while receiving SMS (n=389) and CCT (n=517) were randomly assigned again to stop or continue. We evaluated effects on retention and HIV ( human immunodeficiency virus) RNA (ribonucleic acid) outcomes. ART denotes antiretroviral therapy; RR, rerandomization; and SMART, sequential multiple assignment randomized trial.
PARTICIPANTS
We enrolled adults (≥18 years of age) living with HIV who had initiated antiretroviral therapy within 90 days. Additional inclusion criteria were no plan to relocate outside the province, cell phone and SMS message access, and willingness to be contacted upon missing an appointment. Persons who were hospitalized or enrolled in another trial were excluded. All participants provided written informed consent.
RANDOMIZATION
In Stage 1, we randomly assigned all participants to one of three groups: standard of care (SOC), which included routine education and counseling, SMS text messages, or CCT for each clinic appointment attended within three business days of the appointment. In Stage 2a, participants who were 14 days or more late to a scheduled visit during the first year of follow-up (a “retention lapse”) were randomly assigned again at time of lapse to standard-of-care outreach (SOC-Outreach), SMS+CCT, or peer navigation (i.e., a lay health care worker assigned to address individual patient barriers to care). In Stage 2b, participants receiving SMS or CCT in Stage 1 who completed 1-year follow-up without a retention lapse were randomly assigned again to either stop or continue the initial intervention. Participants were followed for 2 years or until the occurrence of death or study withdrawal.
The combined Stage 1, 2a, and 2b interventions yielded 15 adaptive strategies (Fig. 1 and Table S1 in the Supplementary Appendix), each composed of an initial intervention, a reengagement intervention (triggered for patients not retained in care for the first year), and a deescalation decision (stop or continue, triggered if retained in care during the first year while on CCT or SMS). For example, 1 of the 15 adaptive strategies is to start with CCT; if a lapse occurs in the first year, escalate to navigator or, if no lapse occurs in the first year, stop CCT. A comparison of the entire strategy with SOC accounts for both the sequential effects of the interventions (e.g., benefits from initial CCT vs. SOC, combined with benefits of navigator vs. SOC-Outreach among those who lapse) and possible differential effects of later interventions resulting from initial interventions (e.g., due to the characteristics of patients who lapse on CCT or to lasting effects of CCT). For example, initial receipt of CCT might inure patients who lapse to the benefits of subsequent peer navigation or, equally plausibly, might potentiate navigator effects through habit formation and thereby improve navigator effectiveness.
Treatment assignments were computer generated using equal probability randomization by a nonstudy statistician. For each participant, three sealed, opaque envelopes (one for each random assignment) were prepared and labeled with study ID and Stage. Stage 1 assignments only were unblinded by participants and recorded by study staff at the time of enrollment. Access to Stage 2a or 2b random assignment, if and when indicated, was through a custom Open Data Kit application, with envelopes as a backup. All study staff and participants were blinded to second-stage assignment until criteria for rerandomization were met (either due to a lapse or at 1 year of follow-up for those consistently retained and enrolled in SMS or CCT).
PROCEDURES
In Stage 1, participants assigned to SOC11 received group patient education at each visit, individual or group counseling at treatment initiation, and additional counseling as needed. Participants assigned to SMS were sent messages conveying greetings and encouragement once weekly. Participants could respond through SMS, including requesting a call for additional support. SMS message content was developed through prebaseline qualitative research; participants could choose messages tailored to their preferences.12 Participants assigned to CCT received 400 Kenyan shillings (approximately 3 USD) from study staff each time they made a visit within three business days of a scheduled appointment. The amount was based on estimated median two-way transportation and opportunity costs to attend a clinic visit.
In Stage 2a, patients with a retention lapse were randomly assigned again to one of the following interventions: SOC-Outreach, which involved phone and in-person contact attempts in the first 72 hours after a missed appointment, carried out by lay health care workers using standard clinic practices; combined SMS and CCT, each component delivered as in Stage 1; or peer navigation. Peer navigators were lay health care workers with contextual knowledge of social ties, geography, and the health system, as well as, in many cases, lived experience with HIV. Navigators received a single 3-day training focused on relationship with the patient, helping patients rather than enforcing rules, and respect for the client based loosely on principles of motivational interviewing. Peer navigators were embedded within the lay health worker cadre, were hired through existing human resources mechanisms, reported to program management structure, and made the same salaries as their nonresearch peers. A single mentor worked with navigators during the study through weekly rounds. The navigator intervention was intentionally “light touch” to be scalable if effective.
At the time of initial retention lapse (≥14 days late for an appointment), study staff attempted to contact patients through phone and physical tracing over 14 additional days (i.e., days 15 through 28 after they missed an appointment). Patients successfully contacted during this period were randomly assigned again and encouraged to return to the clinic. If contact attempts were unsuccessful, participants were randomly assigned again without contact on day 14 of the tracing period (28 days after a missed appointment). Participants randomly assigned again to SOC-Outreach without contact received no further study interventions. Those randomly assigned again to SMS+CCT without contact were sent a text message informing them of the availability of a CCT on return to the clinic. Those randomly assigned again to a navigator without contact continued to be traced, now as a part of the navigator intervention. In Stage 2b, patients who experienced no lapse during the first year on CCT or SMS were randomly assigned again at 1 year of follow-up to either continue or discontinue the initial intervention in order to evaluate sustainability.
Sociodemographic and clinical measures were collected at the time of enrollment, at rerandomization (for those contacted), and after 1 and 2 years of follow-up using an Open Data Kit application. Clinic appointments and visits were captured by electronic medical records (with study verification). Evaluations of HIV ribonucleic acid (RNA) plasma levels were performed in the regional Clinical Laboratory Improvement Amendments-certified laboratory using the Roche COBAS platform. To avoid artificial impacts on retention, study staff did not intervene to obtain HIV RNA outcomes during the study period.
OUTCOMES
Four single-time-point trials were nested within the SMART design. For Stage 1, among all randomly assigned participants, the primary outcome was retention in care without lapse (≥14 days late for an appointment) for 1 year following initial randomization. For Stage 2a, among those with a retention lapse, the primary outcome was return to clinic by 1 year following rerandomization. In Stage 2b, among those with no lapse in year 1 while in either the SMS or CCT group the primary outcome was retention in care without lapse for 1 year following rerandomization. The primary outcome for comparison of the 15 adaptive strategies, which used the full SMART design combining all stages, was proportion of time spent actively engaged in care, defined as proportion of days during 2-year follow-up that a patient was alive and had not been 14 days or more late for a scheduled appointment. The originally planned primary end point was plasma HIV RNA suppression at 24 months; this was modified midway through the study as a result of the introduction of more consistent viral load monitoring and a more aggressive switch to second-line therapy in clinical programs that could make a single viral load measurement at 24 months less representative of cumulative treatment success.
For Stage 1, a secondary outcome was a composite of viral suppression and retention at the end of 1 year. For Stage 2a (among those with a lapse by end of 1 year), 2b (among those without a lapse by end of 1 year), and the full SMART, adjudicated viral suppression 2 years after enrollment was a secondary outcome (referred to below as “treatment success”). In this approach, participants were classified according to HIV RNA level at 2 years (success defined as HIV RNA ≤1000 copies/ml); persons without an HIV RNA measurement were classified as either treatment failure or missing using a standardized multidisciplinary adjudication process blinded to treatment group. Final outcomes at 2 years were determined by the study team and included efforts to ascertain and verify care visits at facilities outside of original study clinics.
STATISTICAL ANALYSIS
We first analyzed each of the four stage-specific nested randomized trials separately. In Stage 1, we compared cumulative probability of retention (no lapse by 1 year) among SOC, SMS, and CCT groups using the Kaplan–Meier estimator and Greenwood’s formula16 for standard error estimates. We compared the probability of 1-year composite viral suppression and retention among groups using targeted maximum likelihood estimation (TMLE) to adjust for covariates at the time of random assignment (sex, age, World Health Organization stage, CD4+ T cell level, alcohol use, pregnancy, and site).17 Analysis of Stage 2 nested trials used analogous methods; we further evaluated effect modification of Stage 2a intervention effects by Stage 1 assignment. Effects, estimated with targeted maximum likelihood, were reported on an absolute scale as average treatment effects (ATE).
We estimated mean counterfactual outcomes for each of the 15 adaptive strategies using longitudinal TMLE, with adjustment for retention lapse during the first year and for the same covariates at the time of first random assignment along with time-varying characteristics at the time of second random assignment (time to rerandomization, pregnancy at rerandomization, time-updated plasma HIV RNA level, contact at the time of rerandomization, and missing status). Death was considered a competing risk. We further conducted four prespecified hypothesis tests that contrasted the standard-of-care strategy (SOC, with SOC-Outreach if a lapse occurred) with each “fully active” adaptive strategy (SMS or CCT, each intensified to a navigator following a lapse and continued if no lapse occurred, and SMS or CCT, each intensified to SMS+CCT following a lapse and continued if no lapse occurred). An additional prespecified test compared SOC with time-limited CCT (CCT transitioned to SOC-Outreach if a retention lapse occurred and discontinued after 1 year if no lapse occurred). Further post hoc exploratory comparisons were conducted and are noted as such. Throughout the article, 95% confidence intervals (CIs) reported are unadjusted for multiplicity.
Sample-size calculations for primary longitudinal analyses were based on simulations informed by regional electronic medical record–based clinical data (which anticipated a higher frequency of initial lapses than occurred). Calculations assumed strategy-specific mean outcomes ranging from 75 to 90%. Sample size was based on precision for the mean outcomes of the 15 adaptive strategies rather than on hypothesis testing because the a priori objective was to provide effectiveness estimates for a range of possible sequenced strategies. With a target sample size of 1900 persons, the average 95% CI width was anticipated to range from 3.6 to 4.9%. There was no data-monitoring committee. Analyses were conducted using R version 4.1 and the ltmle package.18
ROLE OF THE FUNDING SOURCE
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. Author contributions are reported in the Supplementary Appendix.
Results
STUDY POPULATION AND OUTCOME ASCERTAINMENT
Of the 2227 persons approached, 1845 met the eligibility criteria and 1815 were enrolled and initially randomly assigned between March 11, 2015, and October 24, 2017 (Fig. 1, Table 1, and Table S2). The study population is broadly representative of persons living with HIV in Africa by age, sex, and other sociodemographic features (Table S3). Six persons were subsequently found to not meet the inclusion criteria and were excluded from all analyses. Of the remaining 1809 participants, 325 (18.0%) experienced a retention lapse in the first year of observation; of these, 312 of 325 (96.0%) were appropriately randomly assigned again and included in Stage 2a analyses.
Table 1.
Baseline Characteristics of the Trial Populations.*
| Total Study Population by Assignment to Stage 1 Interventions |
||||
|---|---|---|---|---|
| Total | SOC | CCT | SMS | |
| n | 1809 | 605 | 656 | 548 |
| Age — yr, median (25th to 75th percentile) | 31.5 (11.9) | 31.7 (11.5) | 31.5 (13.7) | 31.3 (10.5) |
| Male sex — no. (%) | 623 (34.4) | 213 (35.2) | 229 (34.9) | 181 (33.0) |
| Occupation — no. (%) | ||||
| Farmer | 195 (10.8) | 62 (10.2) | 80 (12.2) | 53 (9.7) |
| Teacher | 39 (2.2) | 11 (1.8) | 19 (2.9) | 9 (1.6) |
| Fisherfolk | 16 (0.9) | 8 (1.3) | 1 (0.2) | 7 (1.3) |
| Housework | 59 (3.3) | 19 (3.1) | 19 (2.9) | 21 (3.8) |
| Small business owner/trader | 626 (34.6) | 218 (36.0) | 229 (34.9) | 179 (32.7) |
| Driver | 72 (4.0) | 20 (3.3) | 27 (4.1) | 25 (4.6) |
| Professional | 50 (2.8) | 19 (3.1) | 17 (2.6) | 14 (2.6) |
| Security | 33 (1.8) | 10 (1.7) | 13 (2.0) | 10 (1.8) |
| Casual labor | 163 (9.0) | 69 (11.4) | 54 (8.2) | 40 (7.3) |
| Student | 39 (2.2) | 14 (2.3) | 14 (2.1) | 11 (2.0) |
| Unemployed | 231 (12.8) | 66 (10.9) | 74 (11.3) | 91 (16.6) |
| Other | 281 (15.5) | 89 (14.7) | 107 (16.3) | 85 (15.5) |
| Missing | 5 (0.3) | — | 2 (0.3) | 3 (0.5) |
| Education level — no. (%) | ||||
| None | 48 (2.7) | 14 (2.3) | 11 (1.7) | 23 (4.2) |
| Some primary | 1076 (59.5) | 362 (59.8) | 376 (57.3) | 338 (61.7) |
| Some secondary | 526 (29.1) | 175 (28.9) | 211 (32.2) | 140 (25.5) |
| Some college/university | 155 (8.6) | 54 (8.9) | 56 (8.5) | 45 (8.2) |
| Missing | 4 (0.2) | — | 2 (0.3) | 2 (0.4) |
| Date of ART initiation, median date (25th percentile to 75th percentile in number of days) | 5/25/2016 (192 days) | 5/31/2016 (184 days) | 5/23/2016 (197 days) | 5/16/2016 (201 days) |
| Date of study enrollment, median date (25th percentile to 75th percentile in number of days) | 5/31/2016 (191 days) | 6/6/2016 (183 days) | 5/30/2016 (195 days) | 5/24/2016 (195 days) |
| Site — no. (%) | ||||
| Ahero County Hospital | 131 (7.2) | 48 (7.9) | 55 (8.4) | 28 (5.1) |
| Lumumba Hospital | 704 (38.9) | 235 (38.8) | 246 (37.5) | 223 (40.7) |
| Pandiperiri Clinic | 145 (8.0) | 47 (7.8) | 55 (8.4) | 43 (7.8) |
| Migori District Hospital | 439 (24.3) | 147 (24.3) | 158 (24.1) | 134 (24.5) |
| Rongo District Hospital | 390 (21.6) | 128 (21.2) | 142 (21.6) | 120 (21.9) |
| CD4 at ART initiation — median (25th percentile to 75th percentile) | 362.5 (321.2)† | 349 (318.75)‡ | 384 (324)§ | 342 (315)¶ |
| WHO stage at ART initiation — no. (%) | ||||
| Stage 1 | 1093 (60.4) | 363 (60.0) | 401 (61.1) | 329 (60.0) |
| Stage 2 | 531 (29.4) | 198 (32.7) | 180 (27.4) | 153 (27.9) |
| Stages 3 and 4 | 185 (10.2) | 44 (7.3) | 75 (11.4) | 66 (12.0) |
| Pregnant at ART initiation — no. (%) | 121 (10.3) | 36 (9.2) | 54 (12.7) | 32 (8.7) |
| PHQ-9 >2 at ART enrollment — no. (%) | 159 (8.8) | 58 (9.6) | 59 (9.0) | 42 (7.7) |
| MOS — median (25th percentile to 75th percentile) | 3 (2) | 3 (2) | 3 (2) | 3 (3) |
| Food insecurity — median (25th percentile to 75th percentile) | 6 (10) | 7 (10) | 6 (10) | 6 (10) |
ART denotes antiretroviral therapy; CCT, conditional cash transfers for on-time visits; food insecurity, food insecurity assessed by a modified six-item Household Food Insecurity Access Scale (score range 0 to 18, with lower scores indicating less food insecurity); MOS, Medical Outcomes Study instrument assessing social support short form (scale 0 to 4, with 0 as least support); PHQ-9, Patient Health Questionnaire-9 (score range 0 to 27 with lower scores indicating less depression); SMS, Short Message System text message intervention; SOC, standard of care with routine education and counseling; and WHO, World Health Organization.19,20
Data missing for n=377.
Data missing for n=121.
Data missing for n=143.
Data missing for n=113.
Among participants initially randomly assigned to the SMS or CCT interventions, 393 of 548 participants (71.7%) and 523 of 656 participants (79.7%), respectively, were observed to have no retention lapse within the first year; of these, 389 of 393 participants without lapse on SMS (99.0%) and 517 of 523 participants without lapse on CCT (98.9%) were appropriately randomly assigned again to a discontinuation decision and included in Stage 2b analyses. At each stage of randomization, patient characteristics were similar across study groups (Table 1 and Table S2). Primary end points for Stages 1, 2a, and 2b were assessed among all participants in each nested trial; the primary end point for the sequential trial was assessed among 1808 of 1809 participants (99.9%). Follow-up was completed on November 26, 2019; outcome data collection, including adjudication for care status and treatment failure, continued until November 30, 2020.
INTERVENTION FIDELITY
Among persons assigned to SMS and CCT (pooled across stages), 99.3% of intended cash transfers were dispensed and 97.1% of intended SMS messages were delivered. Among participants without a retention lapse in Stage 1 who were randomly assigned again to discontinue SMS, 17 of 209 (8.1%) received more than four subsequent messages; no cash transfers were dispensed to persons randomly assigned again to discontinue CCT. Among persons randomly assigned again to navigation, a navigator attempted to contact 107 of 110 (97.3%) and successfully contacted 102 of 110 (92.7%) a median of 6 days (interquartile range: 0, 76) after rerandomization, and the proportion of patients who were successfully contacted by a navigator was similar across first-stage study groups (i.e., first-stage treatment did not affect delivery of navigator intervention).
EFFECTS OF INITIAL PREVENTION INTERVENTIONS
In Stage 1, 523 of 656 participants initially randomly assigned to CCT (79.7%) were retained in care for 1 year compared with 428 of 605 randomly assigned to SOC (70.7%) (ATE of CCT vs. SOC: 9.9%; 95% CI: 5.4%, 14.4%) and 393 of 548 randomly assigned to SMS (71.7%) (ATE of CCT vs. SMS: 5.7%; 95% CI: 1.3%, 10.1%). In SMS versus SOC, ATE was 4.2% (95% CI: −0.7%, 9.2%) (Fig. 2 and Tables S4 and S5). Compared with SOC, composite retention and suppression at 1 year was higher among those randomly assigned to CCT (ATE CCT vs. SOC: 10.6%; 95% CI: 5.6%, 15.5%) and SMS (ATE SMS vs. SOC: 5.7%; 95% CI: 0.3%, 11.1%).
Figure 2. Kaplan–Meier Plots of Stage 1 and Stage 2 Outcomes.
Panel A shows time to first retention lapse (clinic visit missed by 14 or more days) after initial random assignment among all participants (Stage 1, n=1809). Panel B shows time to return to clinic after rerandomization among those with a retention lapse in year 1 (Stage 2a, n=312). Lower panels show time to first lapse after rerandomization among participants with no lapse in year 1 on Short Message Service (SMS) text intervention (Panel C; Stage 2b, n=389) or conditional cash transfers for on-time visit (CCT) intervention (Panel D; Stage 2b, n=517). Effect estimates shown are absolute differences in cumulative incidence of event by 1 year. The widths of the confidence intervals (CIs) have not been adjusted for multiplicity; therefore, the CIs should not be used to reject or not reject effects. SOC denotes initial standard of care (routine education and counseling); and SOCO, standard-of-care outreach (standard of care following a retention lapse).
EFFECTS OF REENGAGEMENT INTERVENTIONS
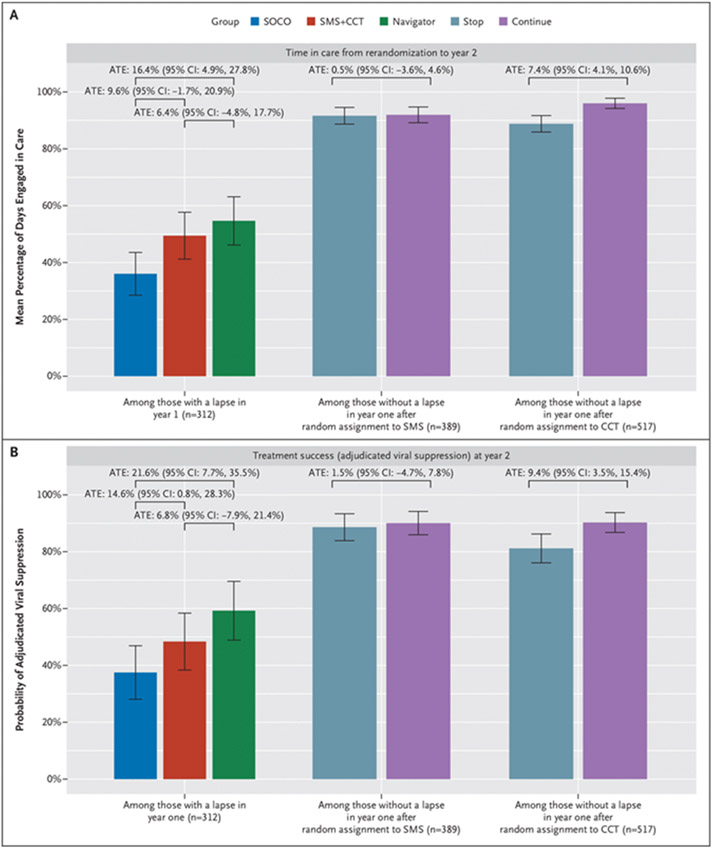
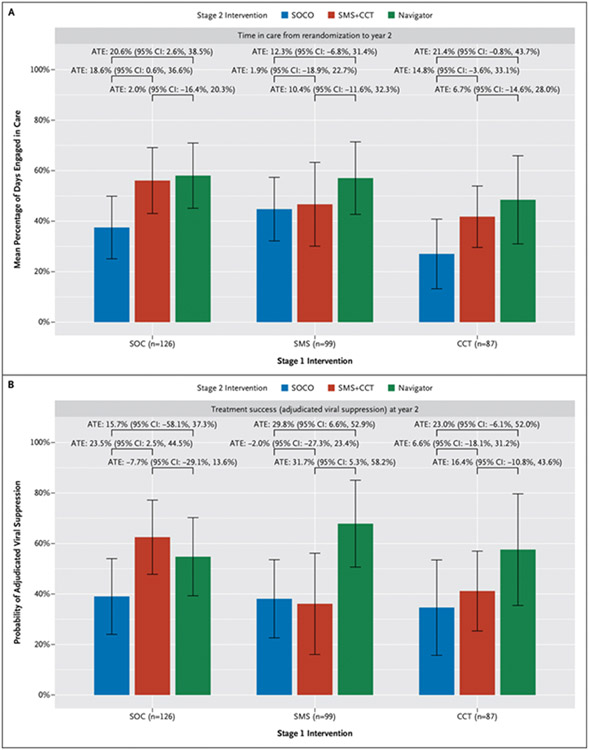
In Stage 2a analyses of reengagement among those with a lapse during year 1 (n=312), 76 of 110 participants assigned to a navigator (69.1%) returned to care within a year, compared with 54 of 97 randomly assigned again to SOC-Outreach (55.7%) (ATE navigator vs. SOC-Outreach: 14.1%; 95% CI: 0.6%, 27.6%); 73 of 105 participants (69.5%) returned to care in the SMS + CCT group (ATE SMS 1 CCT vs. SOC-Outreach: 11.4%; 95% CI: −2.2%, 24.9%) (Figs. 2 and 3 and Tables S6 and S7). Both treatment success (ATE: 21.6%; 95% CI: 7.7%, 35.5%) and proportion of follow-up time engaged in care (ATE: 16.4%; 95% CI: 4.9%, 27.8%) were higher among persons randomly assigned again to a navigator compared with those assigned to SOC-Outreach. Treatment success (ATE: 6.8%; 95% CI: −7.9%, 21.4%) and proportion of time in care (ATE: 9.6%; 95% CI: −1.7%, 20.9%) among persons randomly assigned again to SMS1 CCT were more similar to those among persons assigned to SOC-Outreach (Fig. 4).
Figure 3. Stage 2a and 2b Outcomes.
Figure shows mean proportion of follow-up time engaged in care from rerandomization to study close at year 2 (Panel A) and proportion with treatment success (adjudicated viral suppression) at study close (Panel B) among participants with a retention lapse in year 1 (Stage 2a, n=312) and among participants with no lapse in the first year on the Short Message Service (SMS) text message intervention (Stage 2b, n=389) and on the conditional cash transfers for on-time visits (CCT) intervention (Stage 2b, n=517). Effect estimates shown are absolute differences in mean outcomes (average treatment effect [ATE] adjusted for baseline characteristics and thus not equal to crude differences). The widths of the confidence intervals (CIs) have not been adjusted for multiplicity; therefore, the CIs should not be used to reject or not reject effects. SOCO denotes standard-of-care outreach (standard of care following a retention lapse).
Figure 4. Stage 2a Outcomes Stratified by Stage 1 Interventions.
Figure shows mean proportion of follow-up time engaged in care from rerandomization to study close at year 2 (Panel A) and proportion with treatment success (adjudicated viral suppression) at study close (Panel B) among participants with a retention lapse in year 1 (Stage 2a, n=312), stratified by initial (Stage 1) randomized treatment assignment. Effect estimates shown are absolute differences in mean outcomes (average treatment effect [ATE] adjusted for baseline characteristics and thus not equal to crude differences). The widths of the confidence intervals (CIs) have not been adjusted for multiplicity; therefore, the CIs should not be used to reject or not reject effects. CCT denotes conditional cash transfers for on-time visits; SMS, Short Message Service text message intervention; SOC, initial standard of care (routine education and counseling); and SOCO, standard-of-care outreach (standard of care following a retention lapse).
EFFECTS OF INTERVENTION DISCONTINUATION
In Stage 2b analyses of initial lapse among participants with no retention lapse on CCT during year 1 and successfully randomly assigned again (n=517), those randomly assigned to continue CCT had increased retention in care for an additional year (n=192/230; 83.5%) compared with those randomly assigned to discontinuing CCT (n=173/287, 60.3%; ATE: 28.6%; 95% CI: 19.9%, 37.3%). Treatment success at study close (ATE: 9.4%; 95% CI: 3.5%, 15.4%) and time engaged in care (ATE: 7.4%; 95% CI: 4.1%, 10.6%) were also higher among those who continued CCT. In contrast, among participants with no retention lapse on SMS during year 1 and who were successfully randomly assigned again (n=389), retention in care for an additional year was not clearly different between persons randomly assigned again to continue (n=122/180, 67.8%) versus discontinue (n=151/209, 72.2%) SMS (ATE: −4.4%; 95% CI: −16.6%, 7.9%), nor was treatment success or time engaged in care (Figs 2 and 3 and Tables S8 and S9).
EFFECTS OF SEQUENCED ADAPTIVE STRATEGIES
Using the full SMART design, we estimated the mean proportion of time spent engaged in care (among 1808/1809 participants with the primary outcome measured) and probability of treatment success (among 1692/1809 participants with treatment success measured) after 2 years of follow-up under each of the 15 adaptive strategies (Fig. 5, Fig. S1, and Table S10). In five prespecified hypothesis tests, people randomly assigned to three fully active strategies (SMS with intensification to a navigator following a retention lapse, CCT with intensification to a navigator following a lapse, and CCT with intensification to SMS+CCT following a lapse, all with initial interventions continued if no lapse occurred) had increased time in care (with effect sizes ranging from 5.5 to 7.2%) and treatment success (effect sizes ranging from 6.5 to 8.2%) compared with SOC. The strategy of initial CCT followed by escalation to a navigator if retention lapse occurred and continuation of CCT if no lapse occurred resulted in the highest proportion of total time in care (90.4% of follow-up days; 95% CI: 87.3%, 93.5%; ATE vs. SOC: 7.2%; 95% CI: 3.7%, 10.7%) and the highest probability of treatment success (83.0% success; 95% CI: 79.0%, 87.0%; ATE vs. SOC: 8.2%; 95% CI: 2.2%, 14.2%).
Figure 5. Effects of the 14 Active Sequenced Adaptive Strategies Compared with SOC.
Figure shows standard of care (SOC) routine education and counseling initially, escalation to standard-of-care outreach (SOCO [standard of care following a retention lapse]) or those with a lapse, and maintenance of SOC for those with no lapse during the first year of follow-up (SOC > SOCO/Continue). Effects on mean proportion of follow-up time engaged in care are shown in Panel A and probability of treatment success (adjudicated viral suppression) at the end of follow-up year 2 in Panel B. Each strategy is expressed as three stages: the initial intervention, the intervention assigned in event of a lapse in year 1, and the intervention assigned at the end of year 1 if no lapse occurs. For example, conditional cash transfers for on-time visits (CCT) > SOCO > STOP refers to an initial CCT intervention, followed by SOCO should a lapse occur, and by discontinuation of the cash transfer should no lapse occur in the first year. Point estimates of the average treatment effects (markers) with 95% confidence intervals (bars) are shown. The widths of the confidence intervals have not been adjusted for multiplicity; therefore, they should not be used to reject or not reject effects. SMS denotes Short Message Service text message intervention.
Patients randomly assigned to the fourth fully active strategy (SMS with intensification to SMS+CCT following a lapse and continued if no lapse occurred) had increased time in care compared with SOC (ATE: 4.4%; 95% CI: 0.6%, 8.2%) but did not have an increase in treatment success (ATE: 2.9%; 95% CI: −3.6%, 9.5%). A strategy of time-limited CCT did not improve time in care (ATE: 2.2%; 95% CI: −1.4%, 5.9%) or treatment success (ATE: −2.0%, 95% CI: −8.2%, 4.2%) compared with SOC.
In post hoc analyses, patients randomly assigned to sequenced “rescue” approaches consisting of initial SOC with escalation to CCT or a navigator if a lapse occurred had modestly higher time in care (ATE: 3.4%; 95% CI: 0.6%, 6.3% and ATE: 4.1%; 95% CI: 1.2%, 6.9%, respectively) and treatment success (ATE: 5.0%; 95% CI: 0.2%, 9.8% and ATE: 4.3%; 95% CI: −0.2%, 8.8%, respectively) compared with SOC throughout. Among all strategies in which persons with no lapse in the first year received SOC subsequently, a strategy of initial SMS with escalation to a navigator if a lapse occurred had the greatest effect on treatment success compared with SOC (ATE: 6.2%; 95% CI: 0.2%, 12.2%).
We observed no adverse events deemed a result of any of the trial interventions (Table S11); a total of 94 deaths occurred during the study, with no notable differences in mortality rate by Stage 1 treatments (rate ratio of SMS vs. SOC: 0.88; 95% CI: 0.46, 1.7; CCT vs. SOC: 0.81; 95% CI: 0.44, 1.50).
Discussion
Among persons starting HIV treatment in Kenya, random assignment to a sequence of individually efficacious interventions for retention, in which the sequence depended on patient response, revealed insights unavailable in standard single-intervention studies. Although initial use of either CCT or SMS led to meaningful improvements 1 year later, approximately one in four patients remained in need of additional support, implying that prevention does not obviate reengagement efforts. Among participants who experienced a lapse in retention, only navigators meaningfully improved treatment success (adjudicated viral suppression), and effects were consistent across the initial prevention interventions. Resumption of SOC among participants who remained in treatment increased the risk of lapse for those on CCT but had no effect for those on SMS, suggesting a greater durability of SMS effects, even if those effects were initially smaller. Overall, active prevention of retention lapse with either CCT or SMS, accompanied by escalation to a peer navigator for those with a lapse and continuation of initial prevention intervention among those with no lapse, yielded the best outcomes of the strategies studied in this trial.
In addition to demonstrating the effect of SMS on initial treatment outcomes, this SMART design showed that among those doing well on SMS, cessation of SMS after 1 year did not reduce retention compared with continuation. These findings suggest that SMS may have created a connection to care and habit formation that was durable, offering support for their continued expansion in practice. The SMART design also showed that stopping CCT among those doing well while receiving it reduced success.21 This suggests both that CCT effects were transient and because the absolute number among those succeeding on CCT who lapsed after discontinuation was larger than the number benefiting initially, the possibility that people not initially helped by CCT were harmed by cessation of the intervention. Among those with a treatment lapse, CCT+SMS motivated return to the clinic similarly to assignment to a navigator but yielded less improvement in viral load suppression, supporting the concern that incentives may act through extrinsic motivation.22
Our findings also underscore the role of peers in improving health services in sub-Saharan Africa.23 Peers acted through encounters with patients that occurred in the community. This community-rooted approach may be important for reengagement interventions and requires the social, geographical, and interpersonal knowledge in which peers have expertise. In addition, peers may be uniquely positioned by their lived experience to demonstrate to patients, particularly those struggling to stay in care, the benefits of retention. We found benefits of peer navigation following a lapse in retention. Peer navigation and SMS+CCT had similar effects on return to care; however, the 22% absolute increase in treatment success achieved by peer navigators was higher than the 7% absolute increase of CCT+SMS, each compared with SOC-Outreach.
This trial has limitations. Even though the trial design allowed us to examine adaptations, not all sequenced strategies of potential value were included. For example, we could have assigned navigators as first-line interventions and randomly assigned patients who succeeded to discontinuation, thus evaluating the role and sustainability of navigation as an initial intervention. An additional limitation is the fact that this study was conducted before the widespread use of integrase inhibitors, which may influence results.
In conclusion, we evaluated a set of sequenced retention strategies in which the intervention selected depended on an individual’s response and is therefore personalized. “Light touch” interventions such as CCT and SMS prevented some retention lapses; however, monitoring and intensified reengagement interventions were important for those who lapsed. Persons who lapsed did not fare well with current SOC-Outreach but were responsive to intensified interventions, most strongly to peer-based navigation. Such strategies may also be needed to optimize engagement in care for other chronic medical conditions.
Supplementary Material
Disclosures
Supported by grants (R01 MH104123, K24 AI134413, and R01 AI074345) from the National Institutes of Health.
We thank the Family AIDS Care and Education Services program operating HIV services in western Kenya, as well as the patients in these communities and the front-line health care workers in the region.
Footnotes
Author disclosures and other supplementary materials are available at evidence.nejm.org.
A data sharing statement provided by the authors is available at evidence.nejm.org.
References
- 1.Holmes CB, Sikazwe I, Sikombe K, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: findings from a multistage sampling-based survey. PLoS Med 2018;15:e1002489. DOI: 10.1371/journal.pmed.1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sikazwe I, Eshun-Wilson I, Sikombe K, et al. Retention and viral suppression in a cohort of HIV patients on antiretroviral therapy in Zambia: regionally representative estimates using a multistage-sampling–based approach. PLoS Med 2019;16:e1002811. DOI: 10.1371/journal.pmed.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015;2:e107–e116. DOI: 10.1016/S2352-3018(15)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haachambwa L, Kandiwo N, Zulu PM, et al. Care continuum and postdischarge outcomes among HIV-infected adults admitted to the hospital in Zambia. Open Forum Infect Dis 2019;6:ofz336. DOI: 10.1093/ofid/ofz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Purcell DW, Sansom SL, Hayes D, Hall HI. Vital signs: HIV transmission along the continuum of care — United States, 2016. MMWR Morb Mortal Wkly Rep 2019;68:267–272. DOI: 10.15585/mmwr.mm6811e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wozniak G, Khan T, Gillespie C, et al. Hypertension control cascade: a framework to improve hypertension awareness, treatment, and control. J Clin Hypertens (Greenwich) 2016;18:232–239. DOI: 10.1111/jch.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MK, Bullard KM, Gregg EW, del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014;161:681–689. DOI: 10.7326/M14-0019. [DOI] [PubMed] [Google Scholar]
- 8.Odeny TA, Bukusi EA, Cohen CR, Yuhas K, Camlin CS, McClelland RS. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS 2014;28:2307–2312. DOI: 10.1097/QAD.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376:1838–1845. DOI: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 10.Fahey CA, Njau PF, Katabaro E, et al. Financial incentives to promote retention in care and viral suppression in adults with HIV initiating antiretroviral therapy in Tanzania: a three-arm randomised controlled trial. Lancet HIV 2020;7:e762–e771. DOI: 10.1016/S2352-3018(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang LW, Kagaayi J, Nakigozi G, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One 2010;5:e10923. DOI: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno Y, Higa DH, Leighton CA, Roland KB, Deluca JB, Koenig LJ. Is HIV patient navigation associated with HIV care continuum outcomes? AIDS 2018;32:2557–2571. DOI: 10.1097/QAD.0000000000001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol 2012;8:21–48. DOI: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Holmes CB, Moshabela M, Sikazwe I, Petersen ML. Personalized public health: an implementation research agenda for the HIV response and beyond. PLoS Med 2019;16:e1003020. DOI: 10.1371/journal.pmed.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med 2014;4:260–274. DOI: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood M A report of the natural duration of cancer. 33rd ed. London: H.M.S.O., 1926. [Google Scholar]
- 17.Rose S, van der Laan M. Targeted learning. New York: Springer, 2011: 83–100. DOI: 10.1007/978-1-4419-9782-1_5. [DOI] [Google Scholar]
- 18.Petersen M, Schwab J, Gruber S, Blaser N, Schomaker M, van der Laan M. Targeted maximum likelihood estimation for dynamic and static longitudinal marginal structural working models. J Causal Inference 2014;2:147–185. DOI: 10.1515/jci-2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. DOI: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–714. DOI: 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- 21.Galárraga O, Sosa-Rubí SG. Conditional economic incentives to improve HIV prevention and treatment in low-income and middle-income countries. Lancet HIV 2019;6:e705–e714. DOI: 10.1016/S2352-3018(19)30233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charness G, Gneezy U. Incentives to exercise. Econometrica 2009; 77:909–931. DOI: 10.3982/ECTA7416. [DOI] [Google Scholar]
- 23.Hermann K, Van Damme W, Pariyo GW, et al. Community health workers for ART in sub-Saharan Africa: learning from experience — capitalizing on new opportunities. Hum Resour Health 2009;7:31. DOI: 10.1186/1478-4491-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.