Abstract
BACKGROUND
We sought to determine whether ongoing taste disturbance in the postacute sequelae of coronavirus disease 2019 period is associated with persistent virus in primary taste tissue.
METHODS
We performed fungiform papillae biopsies on 16 patients who reported taste disturbance lasting more than 6 weeks after molecularly determined severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Then, on multiple occasions, we rebiopsied 10 of those patients who still had taste complaints for at least 6 months postinfection. Fungiform papillae obtained from other patients before March 2020 served as negative controls. We performed hematoxylin and eosin staining to examine fungiform papillae morphology and immunofluorescence and fluorescence in situ hybridization to look for evidence of persistent viral infection and immune response.
RESULTS
In all patients, we found evidence of SARS-CoV-2, accompanying immune response and misshapen or absent taste buds with loss of intergemmal neurite fibers. Six patients reported normal taste perception by 6 months postinfection and were not further biopsied. In the remaining 10, the virus was eliminated in a seemingly random fashion from their fungiform papillae, but four patients still, by history, reported incomplete return to preinfection taste perception by the time we wrote this report.
CONCLUSIONS
Our data show a temporal association in patients between functional taste, taste papillae morphology, and the presence of SARS-CoV-2 and its associated immunological changes. (Funded by Intramural Research Program/National Institute on Aging/National Institute of Allergy and Infectious Diseases/National Institutes of Health; ClinicalTrials.gov numbers NCT03366168 and NCT04565067.)
Introduction
We have previously reported that angiotensin-converting enzyme 2, one of the known receptors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is present on type II taste receptor cells within taste buds embedded in taste papillae.1 SARS-CoV-2 is known to infect and replicate within these cells during the acute phase of coronavirus disease 2019 (Covid-19), likely accounting for complete loss of taste (ageusia), partial loss of taste (hypogeusia), and/or distorted sense of taste (dysgeusia) during the early stages of infection.1 However, symptoms of altered taste perception may be present in people for several months and in some patients for more than a year following SARS-CoV-2 infection.2 To study whether the pathology underlying persistent taste symptoms still resides in the primary taste organs, we performed fungiform papillae biopsies from patients who had both a positive polymerase chain reaction (PCR+) for SARS-CoV-2 and acute taste disturbance that was followed by lingering taste symptoms. None required hospitalization, and none were prescribed monoclonal antibodies to SARS-CoV-2 or antiviral treatment for their infection. In the biopsy samples, we looked for the presence of virus and hallmarks of pathology that might be linked to taste disturbance. In 10 patients with lingering taste symptoms, we obtained fungiform papillae on multiple occasions allowing us to investigate the pathophysiology underlying their altered taste perception and observe any changes occurring within the fungiform papillae that might correlate with taste alterations. Taste perception in 6 of those 10 patients returned to pre–SARS-CoV-2 levels by the time of their last biopsy.
Materials and Methods
FUNGIFORM PAPILLAE BIOPSIES
All biopsies were performed at the National Institute on Aging Clinical Research Unit, MedStar Harbor Hospital, Baltimore. Randomly selected complete fungiform papillae, fewer than or equal to eight per participant mostly from the center of the tongue close to the midline, were obtained with institutional review board approval (IRB/NIH #2018-AG-N010: NCT03366168). Patients with taste dysfunction were biopsied between August 2020 and December 24, 2021 (IRB/NIH #2020-000140, 2018-AG-N322: NCT04565067) (Table S1 in the Supplementary Appendix). Patients provided written consent. The technique is described in text and visual media format by the Monell Chemical Senses Institute and the University of Pennsylvania groups.3 All biopsies were performed within a 5-minute time frame by J.M.E., 4 minutes after applying topical 20% benzocaine gel to the area for fungiform papillae excision. Bleeding was minimal to none, and there was little to no nontaste epithelium removed (Fig. 1A). At each visit with a scheduled biopsy, if more than 50 fungiform papillae could be visually identified, papillae were excised. Circumvallate papillae were not biopsied, even though they contain about half of the total taste bud number in humans. We took this approach because, unlike fungiform papillae, we did not find definitive published evidence that circumvallate papillae regenerate in humans, and the participants in this study already had taste dysfunction. Photographs of a tongue and excised fungiform papillae are shown in Figure 1A1 and 1A2, along with a hematoxylin and eosin (H&E) staining of a 10-μm section through fungiform papillae showing the location of taste buds and their cell layers (Figs. 1A3 and 3B1). Of note, taste buds occupy less than 1% volume of a fungiform papilla. Excised fungiform papillae were placed in 4% paraformaldehyde overnight at 4°C and then cryoprotected by dehydration at 4°C in sequentially increasing concentrations of sucrose of 10%, 20%, and 30%. Next, fungiform papillae were frozen in optimal cutting temperature media (O.C.T. Compound, Sakura Finetek) and stored at −80°C. They were then cryosectioned (10 μm) using a Leica CM 1950 cryostat and sections stored at −80°C. PCR was performed on RNA extracts from nasal swabs for SARS-CoV-2 detection (ARUP Laboratories, Salt Lake City, UT, until December 2, 2021, and then Reopen Diagnostics, Pandemic Response Lab, Rockville, MD, to present).
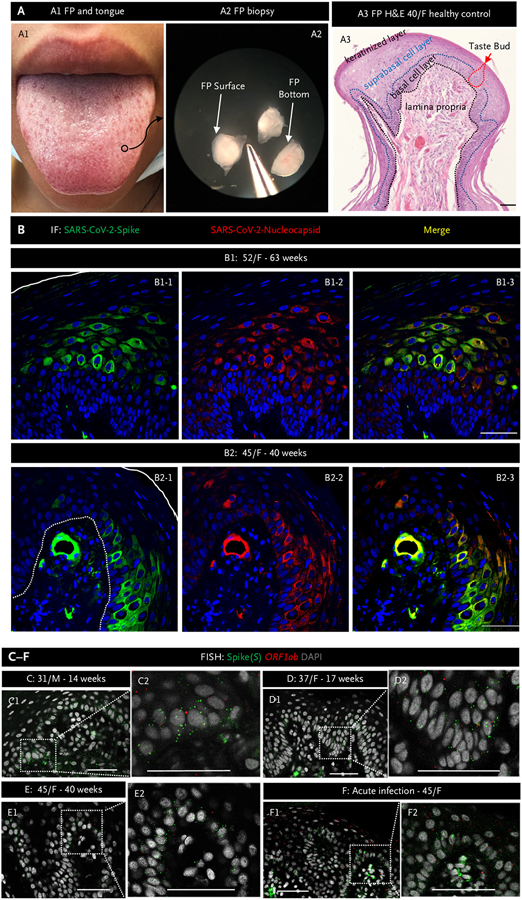
Figure 1. Severe Acute Respiratory Syndrome Coronavirus 2 Persists in Fungiform Papillae from Postacute Sequelae of Covid-19 Patients.
Panel A1 shows a human tongue with a fungiform papilla outlined in a black circle. Panel A2 shows excised fungiform papillae (FP) under a dissection microscope. Panel A3 shows hematoxylin and eosin (H&E) staining of an fungiform papilla. The black dotted line delineates the epithelial layer from the lamina propria, whereas the blue dotted line delineates the epithelial suprabasal from basal cell layers. The red dotted line outlines a taste bud. The surface of the fungiform papilla is covered by a keratinized cell layer. Panel B presents representative images of immunofluorescent staining for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (green) and nucleocapsid (red) proteins in FP epithelium from two patients experiencing chronic taste disturbances post–SARS-CoV-2. Nuclei are stained in blue using 4′,6-diamidino-2-phenylindole (DAPI). The solid white line indicates the surface of FP, and the white dotted line demarcates the epithelium from lamina propria. Note consistent colocalization of spike (S) and nucleocapsid proteins, both of which are present in the FP epithelium and in a blood vessel in the lamina propria in Panel B2. Panels C to F, fluorescence in situ hybridization (FISH), demonstrate the presence of viral RNA using a probe for S (green dots) and a probe for the SARS-CoV-2 open reading frame 1 ab (ORF1ab) negative-strand RNA, produced when the virus is replicating (red dots). Inserts show a higher magnification of the areas marked by rectangles. In all instances, note the presence of S. The presence of ORF1ab indicates replication of the virus in FP. Scale bars indicate 50 μm. F denotes female; and M, male.
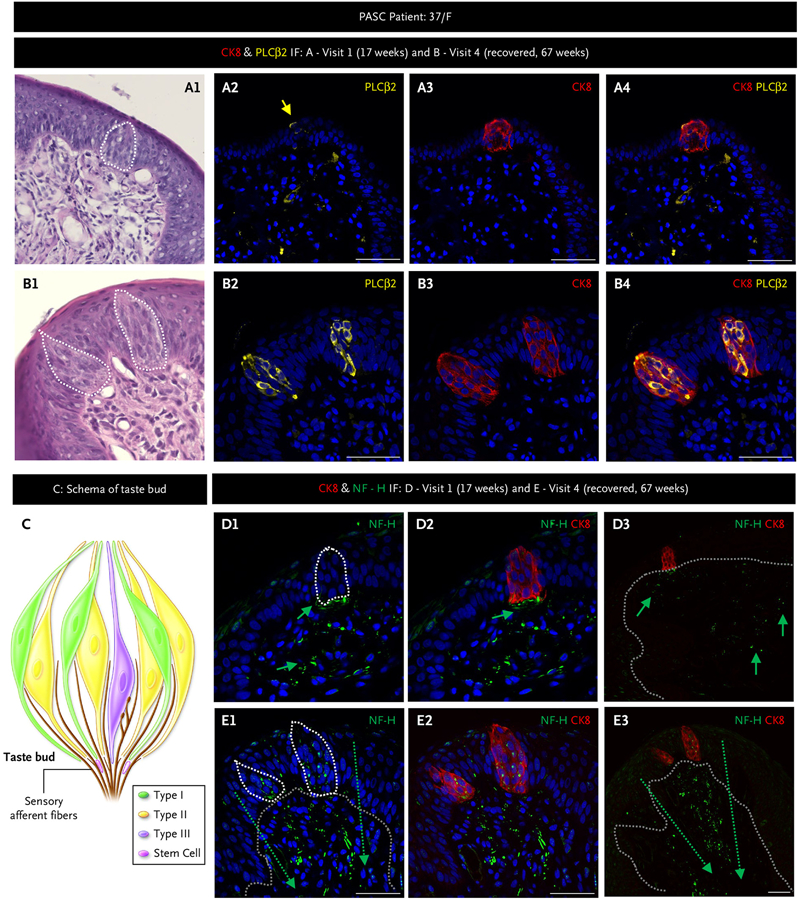
Figure 3. Taste Bud and Sensory Fiber Architecture Are Disrupted in Postacute Sequelae of Coronavirus Disease 2019 That Can Resolve Spontaneously.
We followed a 37-year-old female (F) with multiple biopsies from 17 weeks postinfection through to full recovery by 67 weeks using hematoxylin and eosin and immunofluorescent (IF) staining on adjacent fungiform papillae for the taste receptor cells marker cytokeratin 8 (CK8, red) present on all taste cell receptors; the type II taste cell receptor marker, phospholipase C beta-2 (PLCβ2, yellow); the taste cell receptor subtype that also has angiotensin-converting enzyme 2 (Panels A and B); and the nerve fiber marker neurofilament heavy (NF-H, green) (Panels D and E). Panel C schema shows taste cell receptor cell types and their afferent nerve fibers. Taste buds are composed of discreet clusters of taste cell receptors that include type I, II (the most numerous cell type in human fungiform papillae), and III, as well as a few stem cells that differentiate into mature taste cell receptors. The white dotted lines (A1, B1, D1, and E1) outline taste buds, and grey dotted lines (D3, E1, and E3) delineate the epithelial layer from the lamina propria. Nerve fibers run between taste cell receptors in taste buds, converging in the plexus beneath the taste bud and, from there, relay tastant information to the brain. At 17 weeks, a solitary disordered taste bud in 1/8 fungiform papillae was present, and it contained just one type II taste cell receptor (Panel A). By 67 weeks, fungiform papillae contains normal-appearing taste buds and type II taste cell receptors, normal orientation of the basal cell layer (Panel B), and nerve fibers that were disrupted and lacking in the taste bud at 17 weeks (Panel D) were now reinnervating the taste buds as well as the fungiform papillae lamina propria (Panel E). Scale bars indicate 50 μm. PASC denotes postacute sequelae of coronavirus disease 2019.
IMMUNOFLUORESCENCE OF HUMAN FUNGIFORM PAPILLAE SECTIONS
Slides were rinsed three times in tris-buffered saline (TBS, pH 7.4; Quality Biologicals). Antigen retrieval was performed using sodium citrate buffer (pH 6.0; Sigma-Aldrich) at 96°C for 30 minutes. Slides were allowed to cool to room temperature for 30 minutes and then rinsed three times in TBS. Sections were permeabilized with Triton-X 100 (0.3%) in TBS for 5 minutes at room temperature and then incubated with blocking buffer consisting of goat serum (5%), bovine serum albumin (5%), gelatin (0.1%), Tween 20 (0.05%), and sodium azide (0.05%) (all from Sigma-Aldrich) in TBS for 1 hour at room temperature. They were then incubated at 4°C overnight with primary antibodies (Table S2) diluted in blocking buffer. The tissue sections were rinsed with TBS containing Tween 20 (0.1%) and incubated for 1 hour with fluorescently labeled secondary antibodies (Table S3). After nuclear staining with 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich), sections were mounted with Fluoromount-G (SouthernBiotech). Appropriate noprimary-antibody and host immunoglobulin G controls (Table S4) were prepared with each batch of slides. Confocal fluorescent images were captured using Zen 3.1 software on a Zeiss LSM-880 confocal microscope (Carl Ziess LLC); brightness and contrast were adjusted globally by Fiji-win64 (Life-Line versions).4
FLUORESCENCE IN SITU HYBRIDIZATION
Fluorescence in situ hybridization (FISH) was performed as we described previously1 using the fixed frozen tissue protocol for the RNAscope Multiplex Fluorescent Detection Reagents v2 kit (Cat# 323110, Advanced Cell Diagnostics). Cataloged RNAscope probes were from Advanced Cell Diagnostics (Table S5). The probes to SARS-CoV-2 spike (S) antisense detecting the presence of the viral particles and open reading frame 1ab (ORF1ab) sense detecting the replicating virus were validated on sections from healthy fungiform papillae biopsied before March 2020, which showed no amplification or fluorescence.1 Sections incubated with the positive control probes targeting RNA polymerase II subunit A (POLR2A), peptidylprolyl isomerase B (PPIB), and ubiquitin C (UBC) and the negative control probe targeting the bacterial gene bacillus subtilis dihydrodipicolinate reductase (Dapb) from Advanced Cell Diagnostics were included in each FISH experiment.
QUANTIFICATION
The FISH-IF V2.1.5 algorithm in the HALO image analysis software platform (HALO platform V3.3; Indica Labs) was used to quantify the number of cells positive for the cluster of differentiation (CD)8 T cell markers, with probes to CD3 and CD8α, and cells positive for interleukin 1β (IL-1β) with probe to IL-1β. CD8 T cells in two to four sections from each of three fungiform papillae per individual were counted. One-way analysis of variance with the Tukey multiple comparisons was performed using GraphPad Prism 9 (GraphPad Software Inc.). Data are presented as mean ±standard error.
Results
CHARACTERISTICS OF THE PARTICIPANTS
We biopsied 16 patients (ages 20 to 70 years) who reported normal taste before contracting Covid-19 and then ongoing taste symptoms that negatively impacted their hedonic/affective responses to food more than 6 weeks after first testing positive for SARS-CoV-2; 10 of those with ongoing symptoms returned for additional biopsies. Taste perception information was obtained from their medical history (patient characteristics are displayed in Table S1). Additionally, we biopsied four individuals more than 6 weeks after their infection who were ageusic during the acute phase of SARS-CoV-2 but who subsequently fully recovered their previral taste perception and general well-being within 4 to 6 weeks of testing positive by PCR for SARS-CoV-2. Furthermore, we biopsied fungiform papillae in three patients who were ageusic during their acute infection period (PCR+ <14 days). We had fungiform papillae cryosections from 98 individuals ages 20 to 91 years without taste symptoms before the start of SARS-CoV-2 pandemic in Maryland in March 2020 that served as true negative controls, and we biopsied nine participants between March 2020 and December 2021 who, by history, were never infected with SARS-CoV-2 (Table S1).
EVIDENCE OF SARS-COV-2 PERSISTENCE IN TASTE PAPILLAE
SARS-CoV-2 was not present in taste buds or in any cell layers of fungiform papillae in different patients biopsied before March 2020 or in the nine participants without medical history of SARS-CoV-2 (Fig. S1A and S1B). By contrast, we found evidence of the virus in the epithelial layer (basal and suprabasal cell layers) and lamina propria of fungiform papillae from all individuals who were experiencing taste deficits from 6 to 63 weeks after testing PCR+. We show fungiform papillae sections from two patients: a 52-year-old female at 63 weeks and a 45-year-old female at 40 weeks after testing PCR+ (Fig. 1B1 and 1B2), whose epithelial cells contained SARS-CoV-2 spike and nucleocapsid proteins. Note the absence of staining when the immunoglobulin G control is used in place of the primary antibody in the patients (Fig. S1C) and absence of staining for spike and nucleocapsid proteins in a healthy control fungiform papilla (Fig. S1A). An RNAscope antisense probe specific to the genomic positive-strand RNA (for proof of viral infection) of the spike protein sequence and a sense probe specific to the ORF1ab negative-strand RNA (for proof of viral replication) indicated the presence of replicating virus in three patients at 14, 17, and 40 weeks after testing PCR+ (Fig. 1C, 1D, and 1E, respectively), although at lower levels than during acute infection (Fig. 1F, day 14 after PCR+).1 ORF1ab and S signal are absent in healthy controls (Fig. S1B).
EVIDENCE FOR ONGOING IMMUNE RESPONSE IN FUNGIFORM PAPILLAE WHEN VIRUS IS PRESENT
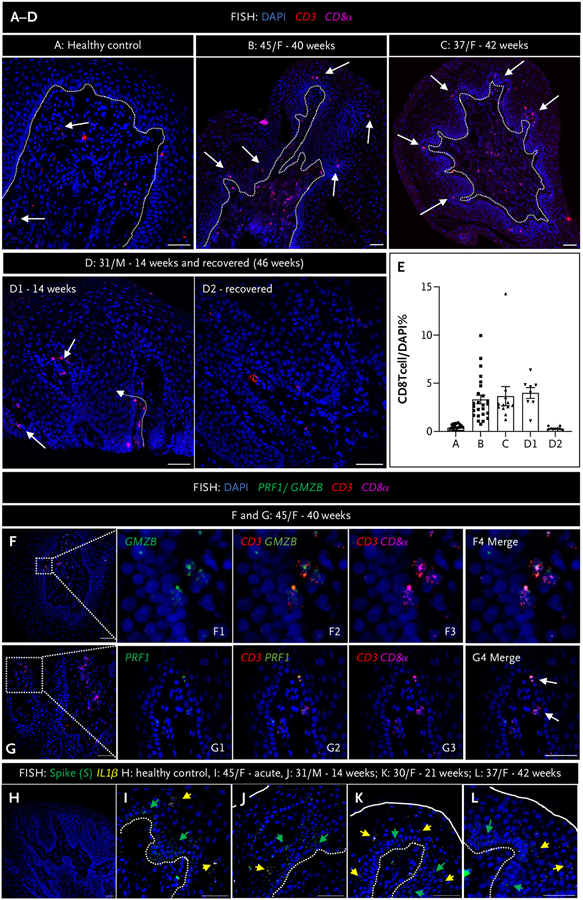
Patients with olfactory and taste disorders in the acute phase develop highly functional virus-specific CD4 and CD8 T cells.5 In addition to virus, we found ongoing immune response as indicated by the increased presence of cytotoxic CD8 T cells and the inflammatory cytokine, IL-1β, in fungiform papillae from 11 of 16 of our patients (Fig. 2). The epithelium of a normal healthy human fungiform papilla typically contains approximately 0.4% CD8 T cells of the total number of a fungiform papilla section cells (Fig. 2A and 2E and Fig. S2A).6,7 In the case of postacute sequelae Covid-19 (PASC) patients, there was an increase in the number of CD8 T cells present in the epithelium of their fungiform papillae. Specifically, the numbers of intraepithelial CD8 T cells were nearly 10 times higher compared with the healthy individuals. This increase is illustrated in Figure 2B to 2E. For example, in case B, the percentage of the intraepithelial CD8 T cells was 3.3%. Similarly, in case C, it was 3.7%, and in case D1, it was 3.99%. These values indicate a substantial elevation in the presence of CD8 T cells within the epithelium of the patients’ fungiform papillae compared with the normal healthy levels. These CD8 T cells were functionally active, as indicated by the expression of cytotoxic perforin (PRF1) or granzyme B (GMZB) (Fig. 2F and 2G). Furthermore, these cytotoxic CD8 T cells were absent in patients’ fungiform papillae when taste symptoms abated (Fig. 2D2 and Fig. S2B and S2C). IL-1β was not present in fungiform papillae of healthy controls (Fig. 2H). However, in acutely virus-infected fungiform papillae (Fig. 2I), the percentage of IL-1β positive cells was 9.1% of the papilla section. Additionally, in PASC patients’ virus-infected fungiform papillae (Fig. 2J to 2L), the percentages of IL-1β–positive cells per fungiform papilla section were as follows: case J, 11.3%; case K, 16.9%; and case L, 14.6%. These findings indicate that even in PASC patients who experience prolonged symptoms following a Covid-19 infection, there is a significant presence of IL-1β in the infected papillae. Although the antigenic specificity of these infiltrated CD8 T cells remains to be determined, these findings show evidence of ongoing immune responses during SARS-CoV-2 persistence.
Figure 2. Fungiform Papillae in Postacute Sequelae Covid-19 Patients Are Characterized by the Presence of Activated CD8 T Cells and Cytokine Expression.
Panels A to D show fluorescence in situ hybridization (FISH) using probes for CD3 (red dots) and CD8α (purple dots) probes for cytotoxic CD8 T cells, in fungiform papillae (FP) of a healthy control (Panel A), and in patients with chronic taste disturbances (Panels B to D1) and after recovery (D2). White arrows indicate CD8 T cells located in the epithelial layer and lamina propria. The dotted white line demarcates the epithelial layer from the lamina propria. Panel E shows CD8 T cell quantification from FP sections of a healthy control (A) and three patients (B to D). Although formal morphometry was not done, there were visually greater numbers of CD8 T cells in the epithelial layer of FP from patients compared with panel A, whereas there was a return to control numbers after recovery (D1 vs. D2), for example. Panels F and G show FISH using probes to either granzyme B (GMZB) or perforin shown in green (where indicated) and CD3 (red dots) and CD8α (purple dots) in a 45-year-old female patient (F) at 40 weeks postinfection. Rectangles in panels F and G indicate the areas shown in higher magnification in F1 to F4 and G1 to G4, respectively. Panels H to L show FISH using probes for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S, green dots) and the cytokine IL-1β (yellow dots) in FP of a healthy control (H), a patient with acute taste disturbances (I), and postacute sequelae of coronavirus disease 2019 (PASC) patients (J–L). The solid white line indicates the surface of FP, and the white dotted line demarcates the epithelial layer from the lamina propria. The green arrows indicate cells positive for S, and the yellow arrows indicate cells positive for IL-1β. Healthy FP controls do not express either S or IL-1β, whereas both are expressed in FP during acute infection and in PASC patients. Scale bars indicate 50 μm.
EVIDENCE OF RECOVERY AND SARS-COV-2 ELIMINATION OVER TIME
In 9 of the 16 (56%) patients, we did not find any taste buds on visit 1 in the fungiform papillae we biopsied. In 7 of the 16 (44%) patients, where taste buds were present, their numbers were decreased, with approximately half of the fungiform papillae containing one taste bud instead of the more typical one to three per healthy fungiform papilla. When taste buds were present in fungiform papillae on visit 1, their morphology was altered, as noted in the following discussion, but they did not contain virus, unlike in the acute stage.1 The buds were smaller, had irregular shapes, and contained fewer taste receptor cells that were also smaller compared with normal taste receptor cells (Fig. 3A1, example of H&E staining). The basal cells were not orderly configured and were of varying sizes with atypical nuclei (Fig. 3A1). Type II taste receptor cells are the sweet-, umami-, and bitter-sensing cells, defined by the presence of phospholipase C beta 2, and are the most numerous taste receptor cells in human taste buds in fungiform papillae (Fig. 3C and Fig. S3A). They secrete adenosine triphosphate that initiates the taste signal for all five prototypic tastes.8 Where fungiform papillae contained a taste bud on visit 1, the type II taste receptor cells were low in number and misshapen (Fig. 3A2 to 3A4 and Fig. S3B). As some of our patients recovered their taste sensation, their fungiform papillae then contained normal-appearing taste buds and a broad connective tissue core covered by a thin epithelium. Type II taste receptor cells were the most abundant cell type in the taste bud once more and had the typical spindle shape with a characteristic large, round nucleus (Fig. 3B2 to 3B4 and 3C). These morphologic changes were detected in patients of both sexes and all ages examined (Fig. S4). Furthermore, fungiform papillae basal cell orientation and numbers were normal in the recovery stage (Fig. 3B1 and Fig. S4).
OTHER FINDINGS IN FUNGIFORM PAPILLAE
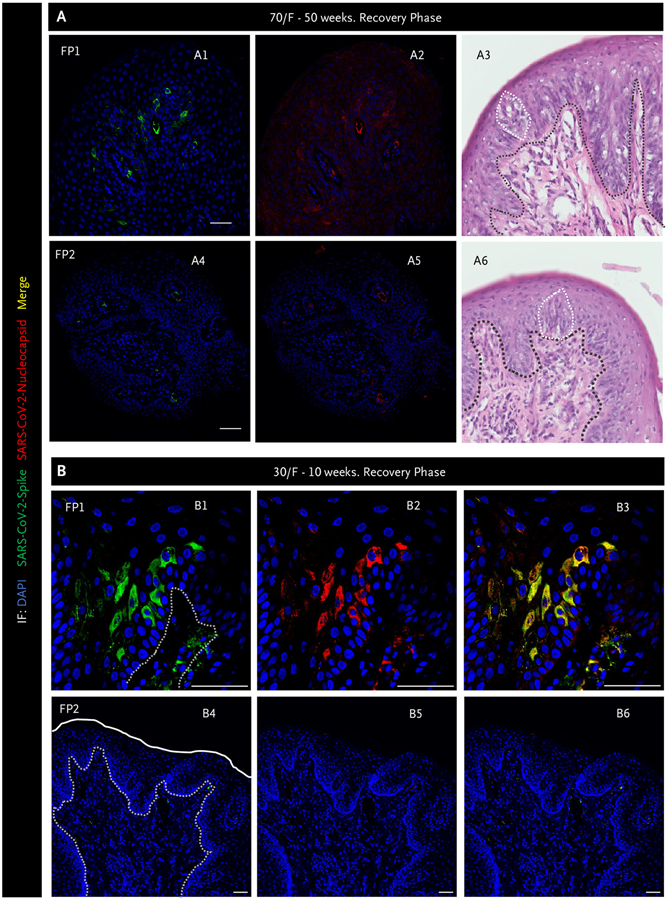
Neurites in taste buds are characterized by their expression of neurofilament heavy.9 We observed disruption of neurites in the lamina propria as well as their absence within misshapen taste buds from fungiform papillae of patients (Fig. 3D). In the recovery stage, we observed the reconstruction of neurites forming connections between taste buds and neurites in the lamina propria and the presence of neurites within taste buds (Fig. 3E and Fig. S5). On follow-up biopsies of some patients’ fungiform papillae, viral persistence was not uniform (i.e., one fungiform papilla may contain SARS-CoV-2, whereas an adjacent would not) (Fig. 4). For example, virus and accompanying basal cell layer disruption was evident in fungiform papillae taken from a 70-year-old female who reported taste (and smell) loss at 34 weeks post-PCR+ (Fig. S6). At 50 weeks post-PCR+, her taste sensation had improved, and we found spike and nucleocapsid protein in just one of her fungiform papillae with abnormal basal cell and taste bud morphology (Fig. 4A1 to 4A3). Mean-while, a neighboring fungiform papilla without virus contained a taste bud with typical morphology, aligned and contiguous basal cells (Fig. 4A4 to 4A6). In another example (Fig. 4B), a 30-year-old woman reported complete taste loss during the acute phase of SARS-CoV-2 infection and for several weeks thereafter. Figure 4B1 to 4B3 shows a fungiform papilla taken from this patient as her taste perception began to return, at 10 weeks post-PCR+, that contained virus, whereas Figure 4B4 to 4B6 shows an adjacent fungiform papilla without virus.
Figure 4. Severe Acute Respiratory Syndrome Coronavirus 2 Is Still Present in Some Fungiform Papillae as Taste Perception Recovers.
Immunofluorescence (IF) images for spike (green) and nucleocapsid (red) proteins and hematoxylin and eosin staining from two separate fungiform papillae (FP) (Panels A1 to A6). The black dotted line indicates the separation of the epithelial layer from lamina propria, whereas the white dotted line outlines taste buds. Panels A1 and A2 show one fungiform papilla (FP1) that still has virus present and has a disordered taste bud and basal cell layer (Panel A3), whereas Panels A4 and A5 show FP2 that does not contain virus and has a normal-appearing taste bud and stereotypical basal cell layer (Panel A6). Panel B has similar findings to Panel A. FP1 (Panels B1 to B3) contains virus, whereas the neighboring FP2 does not (Panel B4 to B6). The white line outlines the surface of the FP, and the white dotted lines delineate the epithelial layer from the lamina propria. However, no taste buds were present in either fungiform papilla. Note clearance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) does not occur simultaneously across all FP in an individual recovering taste perception but rather can occur randomly. Scale bars indicate 50 μm. DAPI denotes 4′,6-diamidino-2-phenylindole; and F, female.
Discussion
Here, we present evidence for ongoing pathology consisting of persistence of SARS-CoV-2, immune response, and loss of intergemmal neurite fibers with taste bud architecture disruption in fungiform papillae from PASC patients with taste disturbance. We did not observe this pathology in fungiform papillae biopsied from healthy participants, in participants who fully recovered their taste perception within 6 weeks of infection, or in any fungiform papillae obtained from participants in our fungiform papillae biopsy study between January 2018 and March 2020. Restoration of taste perception was characterized by random presence of virus in some fungiform papillae, whereas neighboring fungiform papillae did not contain virus. Most importantly, we observed the recovery of normal fungiform papilla cytoarchitecture in 6 of 16 instances and reported normal taste function even in patients who had experienced more than 11 months of taste dysfunction after testing PCR+. We speculate that the cause of taste perception changes in PASC is due to pathology within taste papillae stemming directly from prolonged local presence of virus and its associated immune response.
Here, we provide evidence that human fungiform papillae retain SARS-CoV-2 in basal and suprabasal cell layers even out to 63 weeks after an acute infection. When virus was present, neurite fibers were disrupted in the fungiform papillae core and epithelial layer. However, when the virus was no longer present, the neurites we observed had normal-appearing structure and innervated new taste buds. Taste bud formation and regeneration in mammals are dependent on afferent nerves.10 Neuronal-derived R-spondin and sonic hedgehog are secreted proteins known to support taste stem cell differentiation.11,12 We further speculate that ongoing infection and the cytotoxic immune response mediated by CD8 T cells in taste papillae could be affecting these pathways with consequent loss of taste bud renewal as we saw in some of our patients.
In summary, we provide evidence from a small number of patients that a contributor to PASC symptoms is persistent reservoirs of SARS-CoV-2. In our small patient sample, spontaneous taste perception recovery occurred even when symptoms had persisted for more than a year.
Supplementary Material
Footnotes
Disclosures
Supported by the Intramural Research Program/National Institute on Aging/National Institute of Allergy and Infectious Diseases/National Institutes of Health.
Author disclosures and other supplementary materials are available at evidence.nejm.org.
We thank the trial participants and their families and the nursing staff at MedStar Harbor Hospital. We thank Marc Raley in Visual Media, National Institute on Aging/National Institutes of Health for help with constructing Figure 3C.
References
- 1.Doyle ME, Appleton A, Liu Q-R, Yao Q, Mazucanti CH, Egan JM. Human type II taste cells express angiotensin-converting enzyme 2 and are infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Pathol 2021;191:1511–1519. DOI: 10.1016/j.ajpath.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robineau O, Zins M, Touvier M, et al. Long-lasting symptoms after an acute Covid-19 infection and factors associated with their resolution. JAMA Netw Open 2022;5:e2240985. DOI: 10.1001/jamanetworkopen.2022.40985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spielman AI, Pepino MY, Feldman R, Brand JG. Technique to collect fungiform (taste) papillae from human tongue. J Vis Exp 2010(42): e2201. DOI: 10.3791/2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. DOI: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mele D, Calastri A, Maiorano E, et al. High frequencies of functional virus-specific CD4+ T cells in SARS-CoV-2 subjects with olfactory and taste disorders. Front Immunol 2021;12:748881. DOI: 10.3389/fimmu.2021.748881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng P, Yee KK, Rawson NE, Feldman LM, Feldman RS, Breslin PAS. Immune cells of the human peripheral taste system: dominant dendritic cells and CD4 T cells. Brain Behav Immun 2009;23:760–766. DOI: 10.1016/j.bbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann M, Allers K, Heldt C, et al. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8+ T cells. Mucosal Immunol 2021;14:1381–1392. DOI: 10.1038/s41385-021-00437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnamon S, Finger T. The role of ATP and purinergic receptors in taste signaling. Handb Exp Pharmacol 2022;275:91–107. DOI: 10.1007/164_2021_518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayedi Y, Michlig S, Park M, Koch A, Lumpkin EA. Somatosensory innervation of healthy human oral tissues. J Comp Neurol 2021;529:3046–3061. DOI: 10.1002/cne.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan D, Chettouh Z, Consalez GG, Brunet J-F. Taste bud formation depends on taste nerves. Elife 2019;8:e49226. DOI: 10.7554/eLife.49226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu W-J, Mann RK, Nguyen A, et al. Neuronal delivery of Hedgehog directs spatial patterning of taste organ regeneration. Proc Natl Acad Sci U S A 2018;115:E200–E209. DOI: 10.1073/pnas.1719109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, Lu C, Ohmoto M, et al. R-spondin substitutes for neuronal input for taste cell regeneration in adult mice. Proc Natl Acad Sci U S A 2021;118:e2001833118. DOI: 10.1073/pnas.2001833118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.