Abstract
Background
Early detection of colorectal cancer (CRC) is crucial for survival. Primary care, the first point of contact in most cases, needs supportive risk assessment tools. We aimed to replicate the Swedish Colorectal Cancer Risk Assessment Tool (SCCRAT) for non-metastatic CRC in primary care and examine if risk factor patterns depend on sex and age.
Methods
2,920 adults diagnosed with non-metastatic CRC during the years 2015–2019 after having visited a general practitioner the year before the diagnosis were selected from the Swedish Cancer Register and matched with 11,628 controls, using the same inclusion criteria except for the CRC diagnosis. Diagnostic codes from primary care consultations were collected from a regional health care database. Positive predictive values (PPVs) were estimated for the same 5 symptoms and combinations thereof as in the baseline study.
Results
The results for patients aged ≥50 years old in the present study were consistent with the results of the SCCRAT study. All symptoms and combinations thereof with a PPV >5% in the present study had a PPV >5% in the baseline study. The combination of bleeding with abdominal pain (PPV 9.9%) and bleeding with change in bowel habit (PPV 7.8%) were the highest observed PPVs in both studies. Similar risk patterns were seen for all ages and when men and women were studied separately.
Conclusion
This external validation of the SCCRAT for non-metastatic CRC in primary care replicated the baseline study successfully and identified patients at high risk for CRC.
Keywords: colorectal cancer, diagnosis, general practice, replication, risk assessment tool, Sweden
Key messages.
Colorectal cancer identified at an early stage is important for the prognosis.
Validated diagnostic prediction tools are warranted.
A risk assessment tool for non-metastatic colorectal cancer has now been validated.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer death.1 Europe has approximately half a million cases yearly and a quarter of a million deaths.2
Early detection of CRC is crucial for the patient’s prognosis. Even though Sweden has high survival rates, CRC still has a poor prognosis when discovered at an advanced stage: a 15% 5-year-relative survival rate when diagnosed in stage IV compared with 95%, 89%, and 68% when diagnosed in stages I, II, and III, respectively.3
Most cancer investigations start in primary health care (PHC) due to symptoms and signs. Like the United Kingdom, several European countries have implemented urgent referral pathways for suspected cancer. In 2016, urgent referrals for suspected CRC (standardized cancer patient pathways) were introduced in Sweden.4
The standardized cancer patient pathways are not designed to estimate the individual patient’s cancer risk but contain symptoms that should lead to the suspicion of cancer. This is challenging for general practitioners (GPs), who must assess the likelihood of CRC and prioritize investigations and treatments among large groups of patients seeking care with often focal, unspecific and/or common symptoms, each with a possible link to cancer. Diagnostic prediction tools (DPTs) for cancer, i.e. tools that translate epidemiological risk markers to applicable individual patient assessments, are lacking in Swedish PHC. Several types of cancer diagnostic tools for CRC based on patients’ symptom presentation have been developed in Europe, primarily in the United Kingdom.5–10 There is, however, insufficient evidence to suggest that cancer DPTs in PHC affect the clinical outcome11,12 and no cancer DPT has yet been included in UK guidelines.13 To create a solid evidence-base for DPTs for early detection of CRC in PHC, patient populations in many health care systems need to be studied, and the different DPTs externally validated to a higher extent14 due to variations in health care system organizations, cancer-related risk factors, symptom presentation, and documentation traditions. This should include exploratory as well as prospective confirmative studies.
In 2016 Ewing et al.15 published a case–control study based on regional health care databases from the southwestern Swedish Region Västra Götaland (1.7 million inhabitants) and the National Swedish Cancer Register (SCR), resulting in a Swedish CRC Risk Assessment Tool (SCCRAT) for non-metastatic CRC in individuals aged ≥50 years old for use in PHC.
The aim of the present study was to validate the SCCRAT on patients with CRC by replicating the already developed risk algorithm in a population in a different region, Region Stockholm, Sweden as well as examine if the risk marker patterns diverge depending on sex and age. We also aimed to compare the results from Region Stockholm with Region Västra Götaland.
Materials and methods
Study design and setting
This population-based matched case–control study utilized the Stockholm regional health care administration database (VAL)16 for identifying the entire population of Region Stockholm, comprising the Swedish capital Stockholm and surrounding areas (2.4 million inhabitants), and the SCR for identifying cases. All medical data in Region Stockholm, from public and private health care providers, are automatically transferred to the VAL database, which is used for health care planning, practice remuneration, and quality assessment.16
The SCR, established in 1958, is one of the oldest disease registers in Sweden and has high validity.17 All physicians, including pathology laboratories, in Sweden are obliged by law to report all incident cases of cancer from patients to the SCR.
Study population and data sources
The selection of cases and controls emulated the process of the baseline study by Ewing et al.15 as closely as possible. Inclusion criteria for cases were:
Diagnosed in Region Stockholm with CRC (ICD-10 codes C18–20) stages I–III during the years 2015–2019 (identified in the SCR),
Alive and aged ≥18 years old at the date of the CRC cancer diagnosis (index date), and
Having visited a GP in Region Stockholm during the year before the index date.
Exclusion criteria were:
No controls available, or
Having a previous cancer diagnosis (except non-melanocytic skin cancer) registered in the SCR during the 20 years before the index date.
The latter group was deliberately omitted to avoid GP consultations being a control of or concern over a previous cancer diagnosis. Controls were selected from the VAL database based on the index date of the matched case (date of cancer diagnosis of the case), using the same inclusion criteria as for the cases, except for the CRC diagnosis. For each case, up to 4 controls were matched on age, sex, and PHC unit visited, resulting in a total study population of 2,920 cases and 11,628 controls, with 2,890 (99.0%) of the cases having 4 controls. Data on GP visits during the year before the index date were obtained from the VAL database.
The Swedish CRC Risk Assessment Tool
The SCCRAT is based on 5 symptoms and signs, identified by ICD-10 codes18 and KSH97-P codes (an abbreviated version of ICD-10 adapted to Swedish PHC).19 The KSH97-P coding system was, however, not used in the VAL database during the years of the present study and our study is thus based on ICD-10 coding. Table 1 gives the ICD-10 codes for the SCCRAT used in the present study.
Table 1.
ICD-10 codes for symptoms included in the SCCRAT.
| Change in bowel habit | Bleeding | Weight loss | Abdominal pain | Anaemia |
|---|---|---|---|---|
| K590 | K625 | R630 | R100 | D500 |
| K591 | K921 | R634 | R101 | D508 |
| R194 | K922 | R636 | R102 | D509 |
| R589 | R638 | R103 | D641 | |
| R104 | D648 | |||
| D649 |
ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th revision.
The symptoms and signs are: change in bowel habit, (rectal) bleeding, weight loss, abdominal pain, and anaemia. These 5 symptoms were a result of a merging process of all collected ICD-10 codes, after which the codes underwent univariable conditional regression. Variables associated with cancer entered multivariable analyses, after which a list of statistically significant variables associated with CRC was compiled and a positive predictive value (PPV) was calculated for each variable.15
For each symptom and combination of 2 symptoms, SCCRAT gives the PPVs for CRC, under an assumed prevalence of 0.25% for the disease in the studied group, when having at least 1 GP visit with the symptom in question, at least 2 different GP visits with the same symptom, or 2 different symptoms at 1 or more GP visits. For reasons of comparability, this assumed prevalence is the same as in the baseline study by Ewing et al.15
Statistical analyses
Categorical data are reported as frequencies and percentages, n (%), while discrete and continuous data are given as means with accompanying SDs. The associations between the 5 symptoms (predictors) and the outcome CRC diagnosis (yes/no) were calculated using adjusted and unadjusted conditional logistic regression analysis, with the adjusted analyses including all 5 risk factors simultaneously. The results are presented as odds ratios with accompanying 95% confidence intervals (CIs). SCCRAT is evaluated by calculating PPVs with accompanying 95% CIs for each combination of symptoms in the model,20 separately for all ages, individuals aged ≥50 and ≥70 years old, respectively, as well as men and women regardless of age. PPVs are presented as percentages (%). All statistical analyses were performed in R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) with 2-sided P values <0.05 considered statistically significant.
Results
Characteristics of the 2,920 CRC cases and 11,628 matched controls included in the present study are given in Table 2. Of the cases 1,343 (46%) had a stage III CRC, 1,483 (50.8%) were males, and 1,602 (54.9%) were between 50 and 70 years old at the date of the CRC diagnosis, with a mean (SD) age of 70.7 (12.6) years. During the year before the index date, 1,457 (49.9%) of the cases had visited a GP at least once with at least 1 of the 5 symptoms. Among the controls, the corresponding number was 906 (7.8%). The present validation is a replication of a previous baseline study and includes almost 6 times as many patients as the baseline study in Region Västra Götaland.
Table 2.
Characteristics of CRC cases and matched controls visiting a GP during the year before the index date.
| Variable | Cases | Controls | P valuea |
|---|---|---|---|
| n = 2,920 | n = 11,628 | ||
| TNM stage | |||
| I | 731 (25.0) | – | – |
| II | 846 (29.0) | – | – |
| III | 1,343 (46.0) | – | – |
| Men, n (%) | 1,483 (50.8) | 5,901 (50.7) | – |
| Age at index date (years), mean (SD) | 70.7 (12.6) | 70.6 (12.5) | – |
| <50 years, n (%) | 211 (7.2) | 846 (7.3) | – |
| ≥50 to <70 years, n (%) | 1,602 (54.9) | 6,333 (54.5) | – |
| ≥70 years, n (%) | 1,107 (37.9) | 4,449 (38.3) | – |
| Time from first GP visit to index date (days), mean (SD) | 232 (118) | 262 (96) | <0.001 |
| Total number of GP visits, mean (SD) | 5.3 (6.0) | 4.6 (5.4) | <0.001 |
| Number of GP visits with at least 1 symptom | |||
| At least 1 visit, n (%) | 1,457 (49.9) | 906 (7.8) | <0.001 |
| At least 2 visits, n (%) | 485 (16.6) | 286 (2.5) | <0.001 |
| Number of GP visits with change in bowel habit | |||
| At least 1 visit, n (%) | 439 (15.0) | 249 (2.1) | <0.001 |
| At least 2 visits, n (%) | 101 (3.5) | 56 (0.5) | <0.001 |
| Number of GP visits with bleeding | |||
| At least 1 visit, n (%) | 220 (7.5) | 47 (0.4) | <0.001 |
| At least 2 visits, n (%) | 34 (1.2) | 6 (0.1) | <0.001 |
| Number of GP visits with weight loss | |||
| At least 1 visit, n (%) | 28 (1.0) | 19 (0.2) | <0.001 |
| At least 2 visits, n (%) | 8 (0.3) | 5 (0.04) | 0.001 |
| Number of GP visits with abdominal pain | |||
| At least 1 visit, n (%) | 449 (15.4) | 405 (3.5) | <0.001 |
| At least 2 visits, n (%) | 126 (4.3) | 97 (0.8) | <0.001 |
| Number of GP visits with anaemia | |||
| At least 1 visit, n (%) | 528 (18.1) | 241 (2.1) | <0.001 |
| At least 2 visits, n (%) | 184 (6.3) | 110 (0.9) | <0.001 |
| Total number of different symptoms observed | <0.001 | ||
| None, n (%) | 1,463 (50.1) | 10,722 (92.2) | |
| One, n (%) | 1,265 (43.3) | 852 (7.3) | |
| Two, n (%) | 178 (6.1) | 53 (0.5) | |
| Three, n (%) | 13 (0.4) | 1 (0.009) | |
| Four, n (%) | 1 (0.03) | 0 (0.0) | |
Only GP visits occurring during the year before the index date are included. Symptoms considered were: change in bowel habit, bleeding, weight loss, abdominal pain, and anaemia. Index date is defined as the date of CRC diagnosis for the matched case patient.
a P values from unadjusted conditional logistic regression analyses for the outcome non-metastatic CRC stages I–III.
For all symptoms, GP visits were more common among cases than controls. Anaemia was the most common symptom, which had been observed at a GP visit for 528 (18.1%) of the cases, compared with only 241 (2.1%) of the controls, while weight loss was least common, observed among only 28 (1.0%) of the cases and 19 (0.2%) of the controls. The observed differences were in all cases statistically significant (all P values ≤0.001, Table 2).
Conditional logistic regression analyses
Results from adjusted and unadjusted conditional logistic regression analyses are given in Table 3. All symptoms were significantly associated with the outcome CRC stages I–III, in both adjusted and unadjusted analyses (all P values <0.001). Notably, having had a GP visit with the symptom (rectal) bleeding was associated with a 27.5 times higher risk of being diagnosed with CRC stages I–III in the adjusted analysis, while anaemia was associated with a 12.7 times higher risk.
Table 3.
Results from adjusted and unadjusted conditional logistic regression analyses for the outcome non-metastatic CRC stages I–III in cases and controls matched on age and sex.
| Symptom | Unadjusted | P value | Adjusteda | P value | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Change in bowel habit | 8.2 | 6.9–9.8 | <0.001 | 9.3 | 7.7–11.3 | <0.001 |
| Bleeding | 19.8 | 14.3–27.4 | <0.001 | 27.5 | 19.3–39.2 | <0.001 |
| Weight loss | 6.1 | 3.3–11.1 | <0.001 | 3.6 | 1.7–7.5 | <0.001 |
| Abdominal pain | 5.2 | 4.5–6.1 | <0.001 | 5.9 | 5.0–7.0 | <0.001 |
| Anaemia | 10.8 | 9.1–12.8 | <0.001 | 12.7 | 10.5–15.3 | <0.001 |
OR, odds ratio.
aAdjusted for all other variables in the table.
Validation of the SCCRAT: individuals aged ≥50 years old
Figure 1 gives the SCCRAT separately for all ages, individuals aged ≥50 years old and individuals aged ≥70 years old, respectively. Figure 1 shows the PPVs for individuals aged ≥50 years old and serves as a validation of the results in the baseline study.15 Notably, most of the observed PPVs in Fig. 1 are higher than the corresponding baseline PPVs, with only 5 PPVs being lower: The combination of change in bowel habit and bleeding, which with a PPV of 13.7% was the highest observed PPV in the baseline study, had a PPV of 7.8% in the present study. This value is, however, still the next highest PPV for this age group in Fig. 1. Likewise, the combination of bleeding and abdominal pain, with the next highest PPV of 12.2% in the baseline study, was 9.9% in the present study. This is, however, the highest PPV for this age group in the present study. Finally, having at least 2 different GP visits with weight loss, the combination of weight loss and anaemia, and the combination of abdominal pain and anaemia, with PPVs of 1.6%, 3.8%, and 3.5%, respectively, were slightly lower than the corresponding baseline PPVs of 2.9%, 5.6%, and 4.2%. Finally, it should be noted that all PPVs with a value >5% in the present study had a PPV >5% also in the baseline study. Likewise, all PPVs with a value >2.5% in the present study, except for the combination of change in bowel habit and abdominal pain, had a PPV >2.5% also in the baseline study.
Fig. 1.
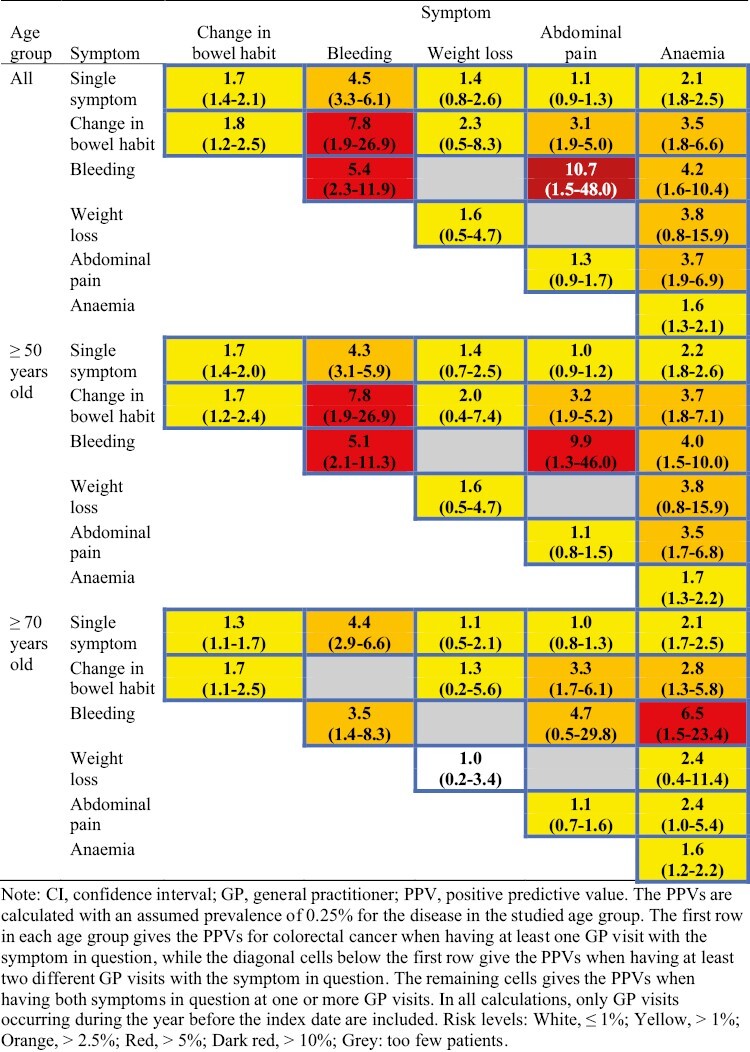
Swedish colorectal cancer risk assessment tool (SCCRAT) in the Stockholm Region for non-metastatic colorectal cancer stage I–III among patients according to age. Each cell gives the PPV (%) with 95% CI for the symptom(s) in question (for colour figure refer to online version).
SCCRAT for other ages and stratified on sex
For the other age groups in Fig. 1, the differences compared with the group aged ≥50 years old are in general small. A notable difference is, however, that the PPV of 6.5% for the combination of bleeding and anaemia in the age group ≥70 years old is considerably higher than the corresponding PPVs of 4.2% for the group of all ages and 4.0% for the group aged ≥50 years old. In contrast, the PPV of 4.7% for the combination of bleeding and abdominal pain in the age group ≥70 years old is considerably lower than the corresponding PPVs of 10.7% for the group of all ages and 9.9% for the group aged ≥50 years old.
Figure 2 gives the SCCRAT separately for men and women. Although the risk patterns overall are similar regardless of sex, a noticeably difference is the more than 3 times higher PPV for the combination of change in bowel habit and abdominal pain among men compared with women, 7.2% vs. 2.1%. Likewise, the PPV of 7.8% for having at least 2 different GP visits with bleeding observed for men is almost double the corresponding PPV of 4.1% for women.
Fig. 2.
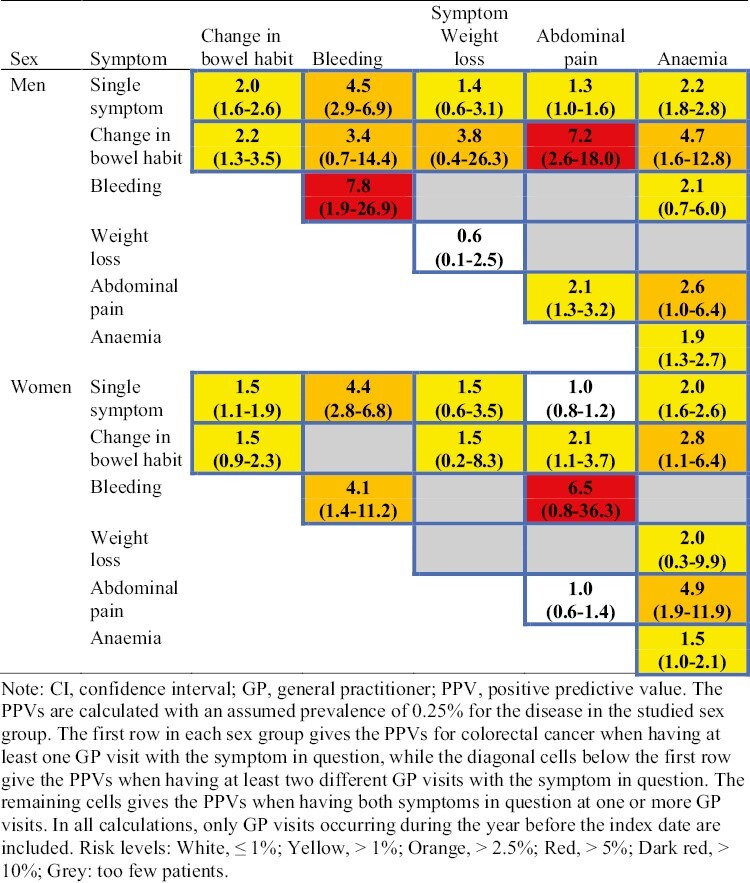
Swedish colorectal cancer risk assessment tool (SCCRAT) for non-metastatic colorectal cancer stage I–III among patients according to sex. Each cell gives the PPV (%) with 95% CI for the symptom(s) in question (for colour figure refer to online version).
Discussion
The results of the present study are consistent with the results in the baseline study by Ewing et al.15 When we applied an earlier calculated risk algorithm based on 5 symptoms and signs to a different population, they were found to be independently associated with non-metastatic CRC, and several combinations of these had high predictive value for non-metastatic CRC. In particular, various combinations of change in bowel habit, bleeding, anaemia, and/or abdominal pain had high PPVs. Moreover, all PPVs with a value >5% in the present study had a PPV >5% also in the baseline study. Similar risk patterns were seen for all ages and when men and women were studied separately.
Strengths and limitations
The main strength of the present study is that it is based on the entire population of a region with 2.4 million inhabitants and all models are based on a real-world health care situation, which provides the opportunity for generalizable conclusions in relation to early cancer detection. Both the present replication study and the baseline study in Region Västra Götaland had nearly no missing cases of CRC, as all subsidized health care was included. Although the populations showed small differences as regards age and cancer stage the risk algorithms constructed based on the Stockholm populations were very similar. The large sample size in the present study made it possible to reliably validate the results of the baseline study as well as to construct separate SCCRATs for different age groups and for both sexes.
The use of unique personal identification numbers enabled the linking of data on diagnoses registered in Region Stockholm with high-quality national data on all CRC diagnoses, with stage at diagnosis verified by pathologists from the SCR.
The use of ICD-10 codes can be considered both a strength and a limitation. The risk of both selection and recall bias is small as the information is collected prospectively on all patients. However, this presupposes that all presented symptoms have been registered correctly. It has been described in previous studies that GPs do not code for all symptom diagnoses presented in the consultation. Important information about symptoms can instead be stated, e.g. in free text in the medical record, information that may be lost in this study.21 To investigate whether the inclusion of symptoms and signs given in free text could affect the results of the SCCRAT would be of great value.
The SCCRAT is based on 5 symptoms and signs known to be associated with CRC. Other known factors of importance for CRC such as family history of CRC are not coded in PHC and could therefore not be included in the SCCRAT. Only patients who consulted their physician in the year before CRC diagnosis were included in this study, leaving out patients diagnosed after only consulting the emergency room or found through screening. However, most patients diagnosed with cancer present with symptoms in primary care, and it is the GP that initiates the diagnostic pathway of patients later diagnosed with cancer.22–24 Our way of validation was done with the presupposition that the SCCRAT was complete. If we had chosen a broader approach other diagnoses and symptoms may have turned up as predictors of cancer.
Results in perspective
Although several other CRC DPTs, similar to SCCRAT, has been developed based on registry data,14 these have not yet been deemed fully useful.12 At this stage of development, it is important to investigate the reproducibility of DPTs in various health care systems. The Swedish and UK populations face the same medical issues such as an ageing population, lifestyle-related conditions, cancer, cardiovascular diseases, and comorbidity. Patients in Sweden consult their GP less often than patients in England but the consultation last longer, on average 20 min compared with 10 min in England.25 Both countries have primarily tax-based health systems. In Sweden, most GPs are publicly employed, whereas in the United Kingdom they are mostly self-employed.26
A qualitative study that investigated potential prediagnostic differences that could explain some of the differences in cancer survival between the United Kingdom and Sweden, did not observe any differences in how symptoms were experienced and in awareness of what the symptoms might mean.27 However, there were differences in patients’ willingness to visit and revisit their GP.27 This would suggest that DPT could be transferrable between United Kingdom and Sweden but should be tested in both countries first.
An important question is whether the tools can affect detection at earlier cancer stage at diagnosis. What distinguishes SCCRAT from those developed in the United Kingdom is that it shows combinations of symptoms and signs in patients with non-metastatic CRC, thus focussing on discovery of CRC in stages with better survival.
In the United Kingdom, the patient’s probability of having an undiagnosed cancer based on symptoms and signs are getting more attention. In the PHC guidelines for referral, the National Institute for Health and Care Excellence (NICE) threshold for urgent referral was set at a 3% risk of undiagnosed cancer 2015.13 A threshold for urgent referral has not been decided in Sweden but is needed if DPTs are to be implemented into clinical practise.
Implications for research and clinical practice
Identifying CRC via symptoms and signs is the major clinical pathway for diagnosis, even in a setting with CRC screening programmes. Implementing a useful DPT could help GPs identify patients at high risk of non-metastatic CRC.
The research presented here indicates that the SCCRAT for non-metastatic CRC in individuals aged >50 years for use in PHC is valid also in Region Stockholm. It remains to investigate the SCCRAT prospectively and its effects on time to diagnosis, cancer stage at diagnosis, and other health-related outcomes in diagnosed patients.
Conclusion
The present study is a successful external replication of a previously published risk assessment tool for non-metastatic CRC in PHC. Furthermore, we also identified patients at high risk with the use of a simple tool based on symptom registration. Tools were also created for different age groups and for men and women separately. We call for DPTs that are based on free text symptoms from PHC records that make use of the information at hand in the clinical situation in PHC.
Supplementary Material
Contributor Information
Elinor Nemlander, Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Solna, Sweden; Academic Primary Health Care Centre, Region Stockholm, Stockholm, Sweden.
Andreas Rosenblad, Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Solna, Sweden; Regional Cancer Centre Stockholm-Gotland, Region Stockholm, Stockholm, Sweden; Department of Medical Sciences, Division of Clinical Diabetology and Metabolism, Uppsala University, Uppsala, Sweden.
Eliya Abedi, Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Solna, Sweden; Academic Primary Health Care Centre, Region Stockholm, Stockholm, Sweden.
Jan Hasselström, Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Solna, Sweden; Academic Primary Health Care Centre, Region Stockholm, Stockholm, Sweden.
Annika Sjövall, Division of Coloproctology, Department of Pelvic Cancer, Karolinska University Hospital, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Axel C Carlsson, Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Solna, Sweden; Academic Primary Health Care Centre, Region Stockholm, Stockholm, Sweden.
Marcela Ewing, Institute of Medicine, Department of Community Medicine and Public Health, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Funding
This work was supported by the Regional Cancer Centre Stockholm Gotland, Sweden and the Einar Belvén foundation.
Ethical approval
This study was approved by the Swedish Ethical Review Authority (Dnr. 2020-06037; 2021-06567-02).
Conflict of interest
None declared.
Data availability
Data are available upon reasonable request from the corresponding author. All diagnostic data upon which the study are based can be obtained from rc.datauttag@vgregion.se.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Freddie B. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F.. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3. Swedish Colorectal Cancer Register. Colorectal cancer report 2020. Umeå, Sweden: The Swedish Colorectal Cancer Register; 2021. [accessed 2022 June 14]. https://cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/kvalitetsregister/tjock--och-andtarm-2021/patientrapport.pdf [Google Scholar]
- 4. Wilkens J, Thulesius H, Schmidt I, Carlsson C.. The 2015 National Cancer Program in Sweden: introducing standardized care pathways in a decentralized system. Health Policy. 2016;120(12):1378–1382. [DOI] [PubMed] [Google Scholar]
- 5. Hamilton W, Round A, Sharp D, Peters TJ.. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hippisley-Cox J, Coupland C.. Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2013;63(606):e11–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hippisley-Cox J, Coupland C.. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2013;63(606):e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nørrelund N, Nørrelund H.. Colorectal cancer and polyps in patients aged 40 years and over who consult a GP with rectal bleeding. Fam Pract. 1996;13(2):160–165. [DOI] [PubMed] [Google Scholar]
- 9. Fijten GH, Starmans R, Muris JW, Schouten HJ, Blijham GH, Knottnerus JA.. Predictive value of signs and symptoms for colorectal cancer in patients with rectal bleeding in general practice. Fam Pract. 1995;12(3):279–286. [DOI] [PubMed] [Google Scholar]
- 10. Marshall T, Lancashire R, Sharp D, Peters TJ, Cheng KK, Hamilton W.. The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance. Gut. 2011;60(9):1242–1248. [DOI] [PubMed] [Google Scholar]
- 11. Price S, Spencer A, Medina-Lara A, Hamilton W.. Availability and use of cancer decision-support tools: a cross-sectional survey of UK primary care. Br J Gen Pract. 2019;69(684):e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medina-Lara A, Grigore B, Lewis R, Peters J, Price S, Landa P, Robinson S, Neal R, Hamilton W, Spencer AE.. Cancer diagnostic tools to aid decision-making in primary care: mixed-methods systematic reviews and cost-effectiveness analysis. Health Technol Assess. 2020;24(66):1–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NICE (National Institute for Health and Care Excellence). Suspected cancer: recognition and referral. 2017. [accessed 2022 June 14]. https://www.nice.org.uk/guidance/ng12/chapter/Introduction
- 14. Grigore B, Lewis R, Peters J, Robinson S, Hyde CJ.. Development, validation and effectiveness of diagnostic prediction tools for colorectal cancer in primary care: a systematic review. BMC Cancer. 2020;20(1):1–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ewing M, Naredi P, Zhang C, Mansson J.. Identification of patients with non-metastatic colorectal cancer in primary care: a case-control study. Br J Gen Pract. 2016;66(653):e880–e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wandell P, Carlsson AC, Wettermark B, Lord G, Cars T, Ljunggren G.. Most common diseases diagnosed in primary care in Stockholm, Sweden, in 2011. Fam Pract. 2013;30(5):506–513. [DOI] [PubMed] [Google Scholar]
- 17. Barlow L, Westergren K, Holmberg L, Talbäck M.. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 18. International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes [accessed 2022 June 14]. Wolfbane.com/icd
- 19. Socialstyrelsen. Klassifikation av sjukdomar och hälsoproblem 1997. Primärvård (KSH97-P) [Classification of diseases and health problems 1997. Primary care (KSH97-P)]. Stockholm: Socialstyrelsen; 1997. [Google Scholar]
- 20. Fagerland MW, Lydersen, S, Laake P.. Statistical analysis of contingency tables. 1st ed. New York: Chapman and Hall/CRC; 2017. [Google Scholar]
- 21. Ford E, Nicholson A, Koeling R, Tate A, Carroll J, Axelrod L, Smith HE, Rait G, Davies KA, Petersen I, et al. . Optimising the use of electronic health records to estimate the incidence of rheumatoid arthritis in primary care: what information is hidden in free text? BMC Med Res Methodol. 2013;13:1–12. doi: 10.1186/1471-2288-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones R, Latinovic R, Charlton J, Gulliford MC.. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334(7602):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. 2009;59(563):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheel BI, Holtedahl K.. Symptoms, signs, and tests: the general practitioner’s comprehensive approach towards a cancer diagnosis. Scand J Prim Health Care. 2015;33(3):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Organisation for Economic Co-operation and Development. Health at a Glance 2015. Paris: OECD; 2015. [Google Scholar]
- 26. Brown S, Castelli M, Hunter DJ, Erskine J, Vedsted P, Foot C, Rubin G.. How might healthcare systems influence speed of cancer diagnosis: a narrative review. Soc Sci Med. 2014;116(100):56–63. doi: 10.1016/j.socscimed.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacArtney J, Malmström M, Overgaard Nielsen T, Evans J, Bernhardson BM, Hajdarevic S, Chapple A, Eriksson LE, Locock L, Rasmussen B, et al. . Patients’ initial steps to cancer diagnosis in Denmark, England and Sweden: what can a qualitative, cross-country comparison of narrative interviews tell us about potentially modifiable factors? BMJ Open. 2017;7(11):e018210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request from the corresponding author. All diagnostic data upon which the study are based can be obtained from rc.datauttag@vgregion.se.


