Abstract
Objectives
Cognitive and physical functions are both associated with disability and death. Recent studies have addressed the relationship between cognitive declines and physical declines; however, whether various facets of cognition are diversely associated with specific physical functions is yet to be ascertained. The present work examines the longitudinal associations between fluid and crystallized cognitive functions (Gf and Gc) and physical functions.
Methods
The sample consisted of 863 community-dwelling older adults (baseline age 60–79 years) from the National Institute for Longevity Sciences-Longitudinal Study of Aging. The participants were tested on a set of Gf and Gc tests and physical tests (grip strength and gait speed). We ran a series of Multivariate Latent Growth Curve models. Specifically, we tested the relationship between cognitive and physical functions in terms of baseline performance (intercept) and rate of change (slope).
Results
The slope–slope correlations between Gf and physical function were large (grip strength r = 0.64 and gait speed r = 0.68, ps < .001). By contrast, the slope correlations between Gc and physical functions were weak (rs ≤ 0.31) and barely or marginally significant (ps ≤ .06).
Discussion
The results show that distinct domains of cognitive functions have different associations with physical functions. Namely, the aging-associated declines in the tested physical functions are robustly correlated with the declines in Gf, but are only weakly correlated with the declines in Gc. Therefore, Gc measures may be poor proxies for the patient’s frailty and should be considered with caution in clinical assessment.
Keywords: Cognition, Dementia, Frailty, Quantitative Methods
Preserving cognitive function and physical function is paramount to the quality of life in older adults. Cognitive and physical health is essential for the senior citizen’s daily activities and contributes to alleviating the societal burden posed by long-term care. Investigating aging-associated cognitive and physical declines throughout the lifespan is thus a priority for geriatrics research (Hoogendijk et al., 2019; Livingston et al., 2020; Lövdén et al., 2020).
It has been suggested that cognitive and noncognitive aging-associated declines stem from the same seed. For example, the deterioration of the physiology of the entire organism due to senescence may be responsible for the decline of a wide variety of abilities. Such explanations involving a common factor subtending the downturn of multiple intellective and nonintellective skills are often subsumed under the umbrella term common-cause hypothesis (Christensen et al., 2001).
The study of the relationship between cognitive declines and physical declines is a particular case of the common-cause hypothesis. Overall, cognitive aging-associated declines often correlate with physical declines (Hackett et al., 2018; Wu et al., 2015; Zammit et al., 2021). The combination of cognitive and physical declines, usually referred to as cognitive frailty or physio-cognitive decline syndrome, has been suggested to be a potential correlate of aging-related neurodegenerative processes (Kelaiditi et al., 2013; Liu et al., 2020).
However, cognition is not a unidimensional trait. General cognitive ability consists of two conceptually, albeit correlated, distinct constructs (McGrew et al., 1997). Crystallized functions (hereafter Gc) pertain to those cognitive abilities one gains and develops in their social environment. Gc thus encompasses skills such as literacy, numeracy, and domain-specific knowledge in general. By contrast, fluid functions (hereafter Gf) refer to cognitive abilities such as processing speed and working memory.
Gc and Gf exhibit different trajectories across the lifespan. Gc appears to be less affected by age compared to Gf (Rönnlund et al., 2005; Schaie, 1994). Conversely, Gf often shows steeper aging-associated declines (Nishita et al., 2013). Additionally, Gf seems to be less malleable to training than Gc (Gobet & Sala, 2023; Ritchie et al., 2015; Simons et al., 2016). It is thus essential to discriminate between Gf and Gc when examining the relationship between physical declines and cognitive declines. Nonetheless, empirical studies usually do not distinguish the domains of cognitive function associate with physical declines (Bai et al., 2021; Chen et al., 2018; Chou et al., 2019; Huang et al., 2020; Wu et al., 2015).
Furthermore, investigating multivariate aging-associated declines, such as the relation between cognitive function and physical function trajectories in old age, poses a methodological challenge. To reach a statistical power sufficient to detect deviations in longitudinal changes, three elements are paramount: numerous assessment time points (to reduce measurement error), large samples (e.g., N > 500), and extensive time spans (e.g., decades) that permit recording of significant aging-related declines (Brandmaier et al., 2018; Hertzog et al., 2006). All these conditions have been rarely (if ever) met (Béland et al., 2018; Lu et al., 2021; Zaninotto et al., 2018).
The present work fills these gaps. We here examine the differential longitudinal relationships between physical function and domains of cognitive functions (Gf vs Gc). Importantly, we employ data that meet all the above necessary requirements to obtain an adequate statistical power.
Method
Participants
The data were a subset of the National Institute for Longevity Sciences-Longitudinal Study of Aging (NILS-LSA; Shimokata et al., 2000). The NILS-LSA is a population-based prospective cohort study about aging and age-related diseases. The participants (N = 2,267 at baseline) were sex- and age-stratified random samples of Japanese community-dwelling adults aged from 40 to 79 years at baseline (Wave 1: 1997–2000). They were followed up every 2–6 years (Wave 2: 2000–2002, Wave 3: 2002–2004, Wave 4: 2004–2006, Wave 5: 2006–2008, Wave 6: 2008–2010, Wave 7: 2010–2012, Wave 8: 2013–2016, Wave 9: 2018–2022). The study was approved by the Ethics Committee of Human Research at the National Center for Geriatrics and Gerontology, Japan (No. 899-6; 1351-2). The participants provided written informed consent.
This study included those participants (N = 863) that satisfied the following conditions: (a) at least one data point in addition to baseline assessment (N = 360 excluded); (b) no history of dementia at baseline (Wave 1; N = 4 excluded) assessment; (c) no missing data in any of the covariates at baseline assessment (N = 11 excluded); and (d) older than 60 years old (N = 1,029 excluded). The ratio of participants older than 60 years of age with only one data point (N = 251) to the total population over 60 (i.e., N = 863 plus N = 251) was 0.225 (or 22.5%).
The mean time between first and last assessments was 10.17 (standard deviation [SD] = 6.47). The mean number of assessments was 3.68 (SD = 2.48).
Model
Variables
Cognitive assessment
Cognitive performance was assessed with three subscales of the Japanese version of the Wechsler Adult Intelligence Scale Revised Short Form (Wechsler, 1981): the Information test, the Similarities test, and the Digit Symbol Substitution Test (DSST). The Information test assesses declarative knowledge of commonly known facts. Participants are asked to answer general knowledge questions covering people, places, and events (29 items, score range 0–29). The Similarities test assesses logical, abstract thinking by asking participants to state how two things are similar (14 items, score range 0–28). Finally, the DSST assesses one’s processing speed. Participants need to write as many symbols as possible that correspond to a given number in 90 s (score range 0–93). In the three tests, higher scores indicate better performance. A composite score (average of z-scores) of Information and Similarities served as a proxy for Gc; the DSST (z-score) was utilized as a proxy for Gf.
Physical assessment
Physical function was assessed with the gait speed test and the grip strength test, which are proxies for lower-body and upper-body strength, respectively. Participants were told to walk on an 11-m straight walkway at a comfortable speed (including 1 m for acceleration and deceleration). Light sensors (Yagami, Aichi, Japan) were used to record the time taken to walk 10 m at the start and end points. Gait speed was measured in meters per second (m/s). Grip strength was measured in kilograms (kg) with a handgrip dynamometer (Takei Co., Niigata, Japan; Kozakai et al., 2016). The participants were asked to stand and extend their elbows to hold the dynamometer. Two trials per hand were ran, and the maximum value was employed in the analysis.
Intercepts and slopes
The latent intercepts and slopes were estimated from the observed variables (i.e., Gc, Gf, gait speed, and grip strength). The mean slopes indicated whether cognitive function increased or decreased on average over time. The mean intercepts represented the scaled estimated average baseline performance. The intercept variance and the slope variance showed between-subject differences at baseline and in aging-associated change, respectively.
Covariates
The models’ estimates were adjusted by including several numeric and binary covariates. These covariates included the participant’s education in years (numeric), baseline age in years (numeric), sex (male or female), living arrangements (living alone or with others), previously or currently smoking (yes or no), apolipoprotein E (APOE) genotype (carrying the ε4 allele or not), and having a history (present or none) of stroke, diabetes, or hypertension.
Statistical Analysis
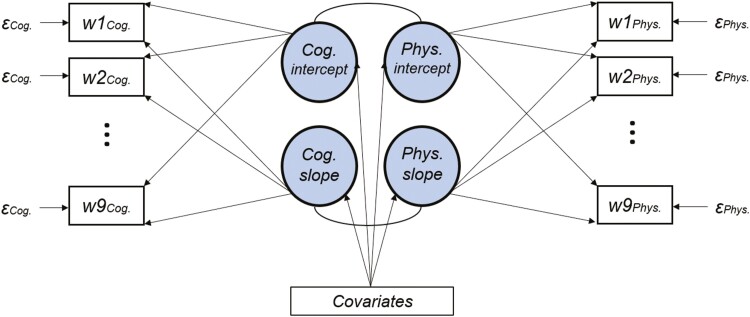
A set of Multivariate Latent Growth Curve (MLGC) models were run (Robitaille et al., 2012). Two latent intercepts and two latent slopes were estimated for all the combinations of cognitive (Gf vs Gc) and physical (gait speed vs grip strength) variables (2 × 2 design for a total of four models). The factor loadings were fixed to 1 for the intercepts. Age-associated declines were allowed to be nonlinear. Thus, the slope loadings were freely estimated (except the factor loadings of the first wave and last wave, which were fixed to 0 and 1 to set the scale). A set of time-invariant covariates were added to the model as predictors of the latent variables. The residuals were constrained to be equal across the waves to facilitate model convergence. The slopes and the intercepts were allowed to correlate. The numeric variables were all centered and scaled. Figure 1 depicts the whole model.
Figure 1.
The schematic of the Multivariate Latent Growth Model. The four latent factors (circles), the two intercept and the slope, predicted the observed cognitive (cog) variables and physical (phy) variables (ts; waves from 3 to 8 were omitted for the sake of clarity). Residuals (ɛs) were constrained to be equal across waves.
As expected, attrition (i.e., the loss of participants) over the nine waves of the survey was substantial (from Wave 2 to Wave 9, 822 [95%], 679 [79%], 588 [68%], 488 [57%], 410 [48%], 339 [39%], 262 [30%], and 140 [16%] participants, respectively). Full Information Maximum Likelihood (FIML) was employed to handle missing data due to attrition. Unlike listwise deletion, FIML uses all the available data, including cases with missing values, to estimate model parameters. FIML thus maximizes statistical power, leading to more efficient and precise parameter estimates. FIML is employed when data are missing completely at random (MCAR; i.e., when missingness does not depend on any systematic factors) and when data are missing at random (MAR; i.e., when missingness depends on any systematic factors that are included in the model, as baseline age and baseline cognitive and physical frailty). We thus assumed data to be mostly MCAR and MAR. Nonetheless, it is worth mentioning that, as in any longitudinal survey, there may be unobserved variables affecting the participants’ dropout patterns.
We ran the analyses with the lavaan R package (R Core Team, 2021; Rosseel, 2012). All the details regarding the models’ parameters, fit indexes, correlation matrices, and residual matrices are reported at https://osf.io/3b4uh/.
Results
The baseline descriptive statistics are summarized in Table 1. Means and SDs are reported for the numeric variables. Counts and percentages are reported for the binary covariates.
Table 1.
The Baseline Descriptive Statistics of the Participants
| Variable | Mean | SD | N | % |
|---|---|---|---|---|
| Age (year) | 68.64 | 5.38 | ||
| Education (year) | 10.68 | 2.64 | ||
| Information (point) | 13.35 | 5.59 | ||
| Similarities (point) | 11.48 | 5.53 | ||
| DSST (point) | 42.48 | 11.22 | ||
| Grip strength (kg) | 29.47 | 9.06 | ||
| Gait speed (m/s) | 1.26 | 0.19 | ||
| Males | 409 | 47.4 | ||
| Living alone | 64 | 7.4 | ||
| Smoking | 379 | 43.9 | ||
| APOE4 carrier | 171 | 19.8 | ||
| Stroke | 33 | 3.8 | ||
| Hypertension | 316 | 36.6 | ||
| Diabetes | 102 | 11.8 |
Notes: DSST = Digit Symbol Substitution Test; SD = standard deviation.
The goodness of fit indexes (the Comparative Fit Index [CFI], the Standardized Root Mean Square Residual [SRMR], and the Root Mean Square Error of Approximation [RMSEA]) indicated an acceptable fit in all the models (all CFIs ≥ 0.96, SRMRs ≤ 0.08, and RMSEAs ≤ 0.04). The models’ estimates can be deemed trustworthy (for the details, see Supplementary Table S1). Furthermore, the models, whose slopes’ factor loadings were allowed to estimate nonlinear longitudinal change, exhibited a better fit compared to linear models (ps < 10−11; Satorra–Bentler test for nested models). Table 2 reports the factor loadings sorted by type of outcome.
Table 2.
The Slope Factor Loadings Sorted by Latent Factor and Model
| Model | Cognitive slope loadings | Physical slope loadings |
|---|---|---|
| Gf & gait speed | [0, 0.07, 0.07, 0.12, 0.21, 0.27, 0.37, 0.59, 1] | [0, 0.01, 0.06, 0.14, 0.18, 0.29, 0.48, 0.69, 1] |
| Gf & grip strength | [0, 0.07, 0.06, 0.11, 0.20, 0.26, 0.36, 0.59, 1] | [0, 0.20, 0.23, 0.41, 0.48, 0.62, 0.71, 0.67, 1] |
| Gc & gait speed | [0, −0.01, 0.14, 0.20, 0.37, 0.38, 0.48, 0.69, 1] | [0, 0.00, 0.05, 0.16, 0.20, 0.30, 0.50, 0.72, 1] |
| Gc & grip strength | [0, −0.02, 0.13, 0.19, 0.34, 0.34, 0.45, 0.68, 1] | [0, 0.21, 0.24, 0.43, 0.49, 0.64, 0.73, 0.68, 1] |
The decrease in Gf over the two decades of the survey was large. The standardized mean slopes (i.e., the mean change over the whole survey in SDs) were −1.33 and −1.41, when estimated with gait speed and grip strength, respectively. The decrease in Gc was less pronounced, but still substantial (the standardized mean slopes were −0.88 and −0.93). Analogously, both gait speed and grip strength exhibited steep declines in all the four models (−1.33, −2.11 and −1.35, −2.14, in the Gf and Gc models, respectively). The comparability between standardized mean sloped was possible thanks to the consistent standardization of the slope factor loadings (i.e., Wave 1 and Wave 9 factor loadings are fixed to 0 and 1 in all the models).
The model-estimated correlations between the latent slopes of the two measures of physical functions and Gf (0.68 and 0.64 for gait speed and grip strength, respectively; both ps < .001) were greater than the ones with Gc (0.31 and 0.28; p = .046 and p = .057). That is, Gf declines shared a much larger amount of variance with physical declines than Gc declines (see also Supplementary Figure S1). The intercept–intercept correlations were small or close to zero (rs ≤ 0.16; Table 3). Supplement 2 reports all the models’ parameters in detail.
Table 3.
The Latent Factors’ Correlations Sorted by Model
| Model | Coefficient | Estimate | SEM | p Value | Std. Est |
|---|---|---|---|---|---|
| Gf & gait speed | intercept–intercept | 0.08 | 0.02 | <.001 | 0.16 |
| slope–slope | 0.49 | 0.13 | <.001 | 0.68 | |
| Gf & grip strength | intercept–intercept | 0.05 | 0.02 | .001 | 0.12 |
| slope–slope | 0.26 | 0.07 | <.001 | 0.64 | |
| Gc & gait speed | intercept–intercept | 0.07 | 0.02 | <.001 | 0.16 |
| slope–slope | 0.10 | 0.05 | .046 | 0.31 | |
| Gc & grip strength | intercept–intercept | 0.02 | 0.01 | .147 | 0.05 |
| slope–slope | 0.06 | 0.03 | .057 | 0.28 |
Notes: Estimate = unstandardized model coefficient (covariance); p Value = statistical significance; SEM = standard error of mean; Std. Est = standardized model coefficient (correlation).
Finally, alternative ways to address longitudinal attrition did not produce meaningfully different results. Partial listwise deletion (e.g., keeping only those participants who took part in at least five waves; N = 491), instead of FIML, provided the same pattern of results. The correlations between Gf declines and physical declines were substantial (rs = 0.65; ps < .001). Conversely, the correlations between Gc declines and physical declines were weak (rs = 0.27; ps > .05). These results upheld the idea that there was a decoupling between Gf and Gc declines irrespective of the mechanisms driving the participants’ dropouts.
Discussion
This work has examined the relationship between aging-associated cognitive declines and physical declines. We have collected data from a sample of Japanese older adults over nine waves spanning approximately 20 years. Two dimensions of cognitive function and two dimensions of physical function have been evaluated in four MLGC models and included a set of covariates to control for potential confounding effects. Several studies have been conducted to explore the relationship between physical and cognitive function in the older adults. For instance, Huang et al. (2020) have shown that mobility subtype frailty (characterized by low grip strength and low walking speed) is associated with decreased DSST scores. However, like other similar investigation, this study does not estimate the correlation between trajectories of physical and cognitive functions, implements a relatively short follow-up (which reduces the statistical power necessary to detect longitudinal change), and focuses solely on one domain of cognitive function (i.e., without collecting any Gc measures).
The results in present study show a pronounced difference between Gf- and Gc-related outcomes. While the correlation between aging-associated physical declines and Gf declines (slope–slope correlations) is substantial, Gc declines are only loosely related to physical declines. Interestingly, this pattern of results is independent of the type of physical function tested (lower- or upper-body strength). Thus, these outcomes are unlikely to be the mere byproduct of one’s physical inability to perform complex paper-and-pencil cognitive tests (e.g., the DSST). Furthermore, baseline cognitive and physical performances are, at most, only barely related (near-zero intercept–intercept correlations). It is therefore reasonable to suppose that physical prowess (or the lack thereof) can hardly be a serious confounding factor.
In brief, aging-related physical and cognitive declines can be reasonably seen as two dimensions of a more general phenomenon, as suggested in the cognitive frailty framework (Cesari et al., 2020). Nevertheless, the relationship exhibits some specifics of interest. Namely, it seems to be more appreciable in decline rates rather than baseline performances and, most notably, when fluid cognitive functions (Gf) rather than crystallized cognitive functions (Gc) are involved.
The decoupling of Gf and Gc declines in their relationship with physical declines is probably the consequence of the nature of these constructs. Along with physical declines, Gf declines express a more general phenomenon, that is, one’s overall aging-associated decline (as theorized in the cognitive frailty framework and similar frameworks). In fact, Gf is a measure of core cognitive mechanisms. The deterioration of such mechanisms is likely to share the same causes with physical declines (e.g., neurodegenerative processes). By contrast, Gc, which consists of domain-specific knowledge acquired throughout the lifespan, does not produce the same pattern of results.
These findings bear practical implications concerning clinical assessment with geriatric patients. Gf measures should be preferred over Gc measures when evaluating the patient’s frailty. In fact, if cognitive frailty is defined as the combination of cognitive and physical declines, and Gc decline does not go along with physical decline, Gc is, ipso facto, not a reliable proxy for cognitive frailty. This does not necessarily imply that Gc-related performance examination is of no use in older patients. Nonetheless, Gc appears to be a weak proxy for aging-associated general declines. Thus, the patient’s good performance in Gc tests should not be regarded as a sufficient criterion to exclude the possibility of frailty-associated conditions.
Finally, it is worth mentioning the present study’s main limitations. First, Gf and Gc were assessed with only one and two tests, respectively. We thus suggest testing the relationship between physical and cognitive declines by employing a broader range of cognitive tests (e.g., nonverbal and verbal working memory). Moreover, this sample consisted of relatively healthier older adults compared to the general population. For example, the participants autonomously traveled to the venue of the data collection, which required a certain degree of functional independence. Although the stratified random sampling probably mitigates this potential issue, examining the relationship between physical and cognitive declines in less active older adults would be a valid extension of our findings.
Conclusion
Declines in fluid cognitive functions (Gf) are strongly correlated with both gait speed and grip strength declines (slope–slope correlations). Conversely, declines in crystallized cognitive functions (Gc) are weakly correlated with declines in physical performance. While corroborating the cognitive frailty theory, these results highlight the need to distinguish between fluid and crystallized cognitive declines in connection with physical declines.
Supplementary Material
Contributor Information
Giovanni Sala, Department of Psychology, Institute of Population Health, University of Liverpool, Liverpool, UK; Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Yukiko Nishita, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Chikako Tange, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Shu Zhang, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Fujiko Ando, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan; Faculty of Health and Medical Sciences, Aichi Shukutoku University, Nagakute, Aichi, Japan.
Hiroshi Shimokata, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan; Graduate School of Nutritional Sciences, Nagoya University of Arts and Sciences, Nisshin, Aichi, Japan.
Rei Otsuka, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Hidenori Arai, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan.
Shevaun D Neupert, (Psychological Sciences Section).
Funding
This work was supported by the Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan (grant number 21-18) and the JSPS KAKENHI grant number 21H05349.
Conflict of Interest
None.
Data Availability
The data and the R codes to reproduce the results are publicly available at https://osf.io/3b4uh/. This study was not preregistered.
Author Contributions
G. Sala: Conceptualization, methodology, formal analysis, original draft preparation, review and editing. Y. Nishita: Conceptualization, methodology, investigation, original draft preparation, review and editing. C. Tange: Investigation, review and editing. Z. Shu: Investigation, review and editing. F. Ando: Investigation, review and editing. H. Shimokata: Investigation, review and editing. R. Otsuka: Investigation, review and editing. H. Arai: Conceptualization, review and editing.
References
- Bai, A., Xu, W., Sun, J., Liu, J., Deng, X., Wu, L., Zou, X., Zuo, J., Zou, L., Liu, Y., Xie, H., Zhang, X., Fan, L., & Hu, Y. (2021). Associations of sarcopenia and its defining components with cognitive function in community-dwelling oldest old. BMC Geriatrics, 21(1), 1–11. 10.1186/s12877-021-02190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béland, F., Julien, D., Bier, N., Desrosiers, J., Kergoat, M.-J., & Demers, L. (2018). Association between cognitive function and life-space mobility in older adults: Results from the FRéLE longitudinal study. BMC Geriatrics, 18(1), 1–15. 10.1186/s12877-018-0908-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandmaier, A. M., Von Oertzen, T., Ghisletta, P., Lindenberger, U., & Hertzog, C. (2018). Precision, reliability, and effect size of slope variance in latent growth curve models: Implications for statistical power analysis. Frontiers in Psychology, 9, 294. 10.3389/fpsyg.2018.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari, M., Sloane, P. D., & Zimmerman, S. (2020). The controversial condition of cognitive frailty: What it is, what it should be. Journal of the American Medical Directors Association, 21(2), 146–148. 10.1016/j.jamda.2019.12.013 [DOI] [PubMed] [Google Scholar]
- Chen, S., Honda, T., Narazaki, K., Chen, T., Kishimoto, H., Haeuchi, Y., & Kumagai, S. (2018). Physical frailty is associated with longitudinal decline in global cognitive function in non-demented older adults: A prospective study. The Journal of Nutrition, Health & Aging, 22(1), 82–88. 10.1007/s12603-017-0924-1 [DOI] [PubMed] [Google Scholar]
- Chou, M.-Y., Nishita, Y., Nakagawa, T., Tange, C., Tomida, M., Shimokata, H., Otsuka, R., Chen, L.-K., & Arai, H. (2019). Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatrics, 19(1), 1–11. 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, H., Mackinnon, A. J., Korten, A., & Jorm, A. F. (2001). The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychology of Aging, 16(4), 588–599. 10.1037//0882-7974.16A588 [DOI] [PubMed] [Google Scholar]
- Gobet, F., & Sala, G. (2023). Cognitive training: A field in search of a phenomenon. Perspectives on Psychological Science, 18(1), 125–141. 10.1177/17456916221091830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, R. A., Davies-Kershaw, H., Cadar, D., Orrell, M., & Steptoe, A. (2018). Walking speed, cognitive function, and dementia risk in the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society, 66(9), 1670–1675. 10.1111/jgs.15312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog, C., Lindenberger, U., Ghisletta, P., & von Oertzen, T. (2006). On the power of multivariate latent growth curve models to detect correlated change. Psychological Methods, 11(3), 244. 10.1037/1082-989X.11.3.244 [DOI] [PubMed] [Google Scholar]
- Hoogendijk, E. O., Afilalo, J., Ensrud, K. E., Kowal, P., Onder, G., & Fried, L. P. (2019). Frailty: Implications for clinical practice and public health. Lancet (London, England), 394(10206), 1365–1375. 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- Huang, S. T., Tange, C., Otsuka, R., Nishita, Y., Peng, L. N., Hsiao, F. Y., Tomida, M., Shimokata, H., Arai, H., & Chen, L. K. (2020). Subtypes of physical frailty and their long-term outcomes: A longitudinal cohort study. Journal of Cachexia, Sarcopenia and Muscle, 11, 1223–1231. 10.1002/jcsm.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelaiditi, E., Cesari, M., Canevelli, M., van Kan, G. A., Ousset, P.-J., Gillette-Guyonnet, S., Ritz, P., Duveau, F., Soto, M. E., Provencher, V., Nourhashemi, F., Salvà, A., Robert, P., Andrieu, S., Rolland, Y., Touchon, J., Fitten, J. L., Vellas, B., & IANA/IAGG. (2013). Cognitive frailty: Rational and definition from an (IANA/IAGG) international consensus group. The Journal of Nutrition, Health & Aging, 17(9), 726–734. 10.1007/s12603-013-0367-2 [DOI] [PubMed] [Google Scholar]
- Kozakai, R., Ando, F., Kim, H. Y., Yuki, A., Otsuka, R., & Shimokata, H. (2016). Sex-differences in age-related grip strength decline: A 10-year longitudinal study of community-living middle-aged and older Japanese. The Journal of Physical Fitness and Sports Medicine, 5(1), 87–94. 10.7600/jpfsm.5.87 [DOI] [Google Scholar]
- Liu, L.-K., Chou, K.-H., Hsu, C.-C. H., Peng, L.-N., Lee, W.-J., Chen, W.-T., Lin, C.-P., Chung, C.-P., Wang, P.-N., & Chen, L.-K. (2020). Cerebellar-limbic neurocircuit is the novel biosignature of physio-cognitive decline syndrome. Aging (Albany NY), 12(24), 25319–25336. 10.18632/aging.104135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén, M., Fratiglioni, L., Glymour, M. M., Lindenberger, U., & Tucker-Drob, E. M. (2020). Education and cognitive functioning across the life span. Psychological Science in the Public Interest, 21(1), 6–41. 10.1177/1529100620920576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S., Liu, Y., Guo, Y., Ho, H. C., Song, Y., Cheng, W., Chui, C. H. K., Chan, O. F., Webster, C., Chiu, R. L. H., & Lum, T. Y. S. (2021). Neighbourhood physical environment, intrinsic capacity, and 4-year late-life functional ability trajectories of low-income Chinese older population: A longitudinal study with the parallel process of latent growth curve modelling. EClinicalMedicine, 36, 100927. 10.1016/j.eclinm.2021.100927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew, K. S., Flanagan, D. P., Keith, T. Z., & Vanderwood, M. (1997). Beyond g: The impact of gf-gc specific cognitive abilities research on the future use and interpretation of intelligence tests in the schools. School Psychology Review, 26(2), 189–210. 10.1080/02796015.1997.12085858 [DOI] [Google Scholar]
- Nishita, Y., Tange, C., Tomida, M., Ando, F., & Shimokata, H. (2013). Does high educational level protect against intellectual decline in older adults?: A 10-year longitudinal study. Japanese Psychological Research, 55(4), 378–389. 10.1111/jpr.12028 [DOI] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing.https://www.R-project.org/
- Ritchie, S. J., Bates, T. C., & Deary, I. J. (2015). Is education associated with improvements in general cognitive ability, or in specific skills? Developmental Psychology, 51(5), 573. 10.1037/a0038981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille, A., Muniz, G., Piccinin, A. M., Johansson, B., & Hofer, S. M. (2012). Multivariate longitudinal modeling of cognitive aging. GeroPsych, 25(1), 15–24. 10.1024/1662-9647/a000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund, M., Nyberg, L., Bäckman, L., & Nilsson, L.-G. (2005). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging, 20(1), 3. 10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Rosseel, Y. (2012). lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software, 48(2), 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Schaie, K. W. (1994). The course of adult intellectual development. The American Psychologist, 49(4), 304. 10.1037//0003-066x.49.4.304 [DOI] [PubMed] [Google Scholar]
- Shimokata, H., Ando, F., & Niino, N. (2000). A new comprehensive study on aging the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA). Journal of Epidemiology, 10(1 Suppl.), 1–9. 10.2188/jea.10.1sup_1 [DOI] [PubMed] [Google Scholar]
- Simons, D. J., Boot, W. R., Charness, N., Gathercole, S. E., Chabris, C. F., Hambrick, D. Z., & Stine-Morrow, E. A. (2016). Do “brain-training” programs work? Psychological Science in the Public Interest, 17(3), 103–186. 10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1981). Manual for the Wechsler Adult Intelligence Scale—Revised (WAIS-R). The Psychological Corporation. [Google Scholar]
- Wu, Y.-H., Liu, L.-K., Chen, W.-T., Lee, W.-J., Peng, L.-N., Wang, P.-N., & Chen, L.-K. (2015). Cognitive function in individuals with physical frailty but without dementia or cognitive complaints: Results from the I-Lan Longitudinal Aging Study. Journal of the American Medical Directors Association, 16(10), 899.e9–899.e16. 10.1016/j.jamda.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Zammit, A. R., Piccinin, A. M., Duggan, E. C., Koval, A., Clouston, S., Robitaille, A., Brown, C. L., Handschuh, P., Wu, C., Jarry, V., Finkel, D., Graham, R. B., Muniz-Terrera, G., Praetorius Björk, M., Bennett, D., Deeg, D. J., Johansson, B., Katz, M. J., Kaye, J., … Hofer, S. M. (2021). A coordinated multi-study analysis of the longitudinal association between handgrip strength and cognitive function in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(2), 229–241. 10.1093/geronb/gbz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto, P., Batty, G. D., Allerhand, M., & Deary, I. J. (2018). Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. Journal of Epidemiology and Community Health, 72(8), 685–694. 10.1136/jech-2017-210116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and the R codes to reproduce the results are publicly available at https://osf.io/3b4uh/. This study was not preregistered.